Herein we show that upon confinement in small volumes, groups of Pseudomonas aeruginosa bacteria, containing as few as one to three cells, are able to initiate quorum sensing (QS) and achieve QS-dependent growth. In addition, we show that, at low numbers of cells, initiation of QS is highly variable within a clonal population. QS pathways are involved in critical functions of microorganisms, such as pathogenesis, development of biofilms, sporulation, acquisition of nutrients, conjugation, motility, and production of secondary metabolites such as antibiotics.[1] Experimental control of QS pathways through confinement may enable fundamental research into the role of QS in small groups of cells and could provide a tool for growing unculturable bacteria or inducing antibiotic production.
QS is by definition a high-density behavior, regulated by the number of cells per unit volume. QS is initiated by the accumulation of released signaling molecules, such as auto-inducers (AIs), and is known to be initiated when the density of cells rises above a threshold level. There are two approaches to activating high-density behavior in cultures: 1) seed a macroscopic volume with bacteria and let them divide until they reach high density, or 2) take a few cells and confine them in a very small volume to enable accumulation of AIs (Figure 1A). Activation of QS through strategy (1) has dominated and has led to the general view that QS is a process to coordinate the collective behavior of large groups of cells,[2,3] and the possibility that small groups of bacteria could initiate QS upon confinement is often overlooked. Nevertheless, strategy (2) is important to consider, because QS pathways are relevant to function, survival, and growth of small numbers of cells—for example, early in biofilm formation, in early stages of infection, or in soils.
Figure 1.
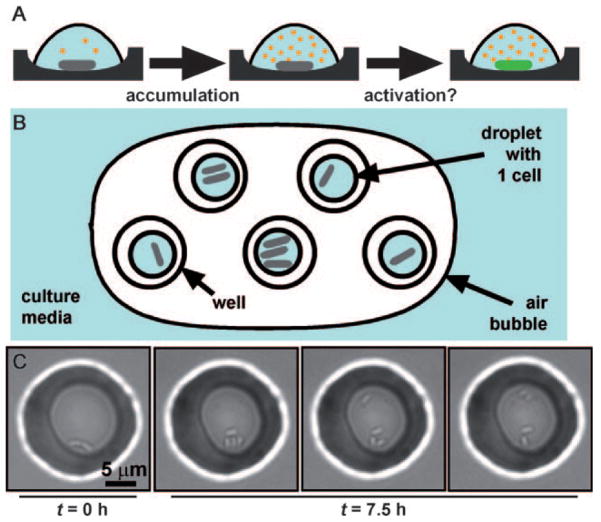
A hypothesis for activation of QS by confinement of single cells and a device for testing this hypothesis. A) Pseudomonas aeruginosa cells (gray rods) excrete auto inducers (AIs, orange circles) that can accumulate in the media. In a confined volume, the AIs do not diffuse away, and thus they reach high concentrations around the cell. We hypothesized that in a small volume, a single cell would be able to accumulate AIs above the critical concentration needed to initiate QS. In these experiments, QS was visualized by using a green-fluorescent protein (GFP) reporter gene for lasB (green rod). B) Schematic drawing of arrays of sub-picoliter droplets that contain confined bacteria covered by air. The entire device is sealed inside a petri dish (not shown). C) Small groups of bacteria can grow and divide when confined in droplets with volumes less than 1 pL. After 7.5 h, the original cell has divided, as shown by the increase in the number of cells in the droplet, and the bacteria are also motile, as indicated by the series of pictures, taken several seconds apart, showing movement of some of the cells in the droplet.
Because confinement influences the diffusion of released signaling molecules, QS has been redefined in terms of diffusion[4] or efficiency[5] sensing. Spatial constraints and transport parameters, such as the flow rate through open systems, have been shown to play a role in the regulation of QS.[6–9] In addition, it has been shown that even small groups of Staphylococcus aureus cells inside a vesicle of a host cell are able to initiate QS,[10,11] and that initiation is required for escape from the vesicle.[11,12] In another example, the restriction of diffusion on dry leaf surfaces reduced the size of a quorum down to groups of dozens of cells.[13] In these experiments the cells were not only confined but they were also exposed to the host environment. It is not known in these systems to what extent host factors or confinement are playing a role in the initiation of QS.
To study high-density behavior of a few cells experimentally without influence from host factors requires the creation of confined spaces. Microfluidics is a useful tool for working with very small volumes,[14,15] and recently has become an attractive tool in microbiology[16–18] partly because of to its ability to control the spatial structure of microbial cultures.[19,20] Microfluidics also enables separation of individual cells,[21,22] which makes it possible to measure heterogeneity in populations of cells at the single-cell level.[23–27]
To investigate the initiation of QS by small groups of cells in the absence of interactions with the host, we adapted a microfluidic technique previously described[28] to create an array of droplets, each approximately 100 fL in volume, in wells made from the biocompatible[29] resin SU-8 (see Supporting Information).[30] Bacteria were added by flowing low-density culture media through a poly(dimethylsiloxane) (PDMS) channel placed over the wells. Bacteria were allowed to settle and therefore concentrate over the wells. We introduced an air bubble over the wells to form individual droplets, which were physically separated from one another (Figure 1B). As the air bubble swept over the wells bacteria were pushed into wells and ended up at a cell density much higher than the initial culture. We found that upon confinement bacteria divided (Figure 1C) and remained motile for over 7 h (Supporting Information; movie S1), indicating that nutrients in the media and gases were not depleted.
To detect QS, we used P. aeruginosa cells containing a fluorescent reporter for the QS-controlled gene lasB.[31] Green fluorescence indicated a high level of lasB induction, which has been shown to correlate closely with extracellular AI concentrations,[31] and is interpreted as activation of QS. Upon confinement in volumes of approximately 100 fL, small groups of P. aeruginosa cells were able to initiate QS (Figure 2). Activation of QS by very small groups of P. aeruginosa cells was not an artifact of the materials used to make the device, as the phenomenon was also observed for small groups of cells confined in droplets on a glass slide covered by air (Supporting Information; Figure S1).
Figure 2.
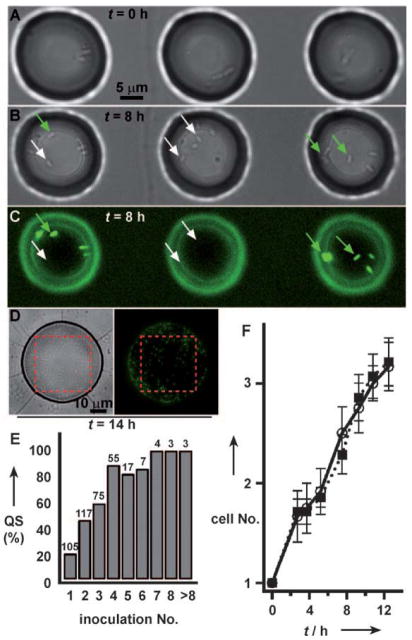
Small populations of clonal cells initiate QS upon confinement (Supporting Information; movie S2), and do so with marked variability. A)–C) Three adjacent droplets, each containing a small population of cells at time zero, show variability in initiation of QS after 8 h. White arrows point to cells that did not initiate QS; green arrows point to cells that initiated QS. D) Cells in a larger droplet (ca. 20 pL) grew to a population of hundreds of cells and show more homogeneous expression of QS, as visualized by the fluorescence reporter. Inside the red dashed boxes, cell counts are 109 total (from bright field, left), and 98 initiated QS (from GFP, right). E) Initiation of QS in droplets, loaded at time zero with 1 to 14 cells, was measured after 10 h, demonstrating increased variability in initiation of QS within small groups of cells. A well was scored as “with QS” if at least one bacterium in the well was fluorescent. Inoculation No. is the initial number of cells; the bar for >8 represents groups of 9, 10, and 14 cells at t =0. Numbers above bars represent the total number of wells N for that inoculation number. F) Initiation of QS in droplets containing single cells at time zero was not correlated with the growth rate of the cells. Bars represent standard error. ○,—: droplets that achieved quorum sensing; ■,•••••: droplets that did not achieve quorum sensing.
In three adjacent droplets each starting with two cells (Figure 2A), there was heterogeneity in initiation of QS both among and within the wells. After 8 h, two droplets had cells that initiated QS (Figure 2B and C, left and right droplets, green arrows), while one droplet had cells that had not initiated QS (Figure 2B and C, center droplet, white arrows). This heterogeneity was observed despite all three droplets showing similar numbers of cells per well after 8 h. Heterogeneity in activation of QS was even observed within the same droplet: in the left droplet in Figure 2B and C, only about half of the cells initiated QS. This heterogeneity within a well also occurred when starting from a single cell (Supporting Information; Figure S2).
In this culture media, cells grew at similar rates in confined volumes whether they turned on QS or not (Figure 2F). There was also no correlation of QS with the volume of the droplet (Supporting Information; Figure S3, distribution of well volumes for QS vs. non-QS cells, two-tailed p = 0.8098, Chi-square test). Thus, the heterogeneity in activation of QS was not due to damage to cells during loading or to another gross problem within the wells, but rather represents inherent variability in P. aeruginosa. We monitored initiation of QS over time in droplets loaded at t =0 with 1 to 14 cells. After 10 h, only 20% of groups starting with single cells initiated QS and 100% of groups starting with seven or more cells initiated QS (Figure 2E). In a larger droplet that grew to nearly one hundred cells (Figure 2D) and in the bulk culture near the outlet of the device (Supporting Information; Figure S4), most of the cells within the droplet had initiated QS, indicating that heterogeneity in initiation of QS was not due to plasmid loss. The behavior of small groups of cells may be more random than large groups because of stochastic effects that arise from small sample sizes.
Time of first division in these experiments occurred at approximately 3 h, and initiation of QS occurred at approximately 8 h (Supporting Information; Figure S5). Because division was generally more rapid than initiation of QS, single cells usually divided before activating QS; rarely, however, individual cells initiated QS before dividing (Figure 3 and Supporting Information S6). This result is in contrast with the view of QS as a collective behavior.
Figure 3.
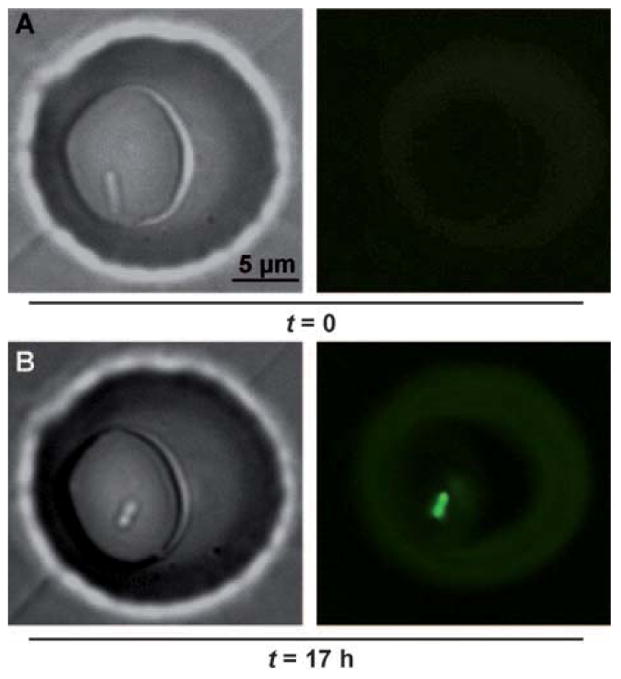
A single Pseudomonas aeruginosa bacterium, originating from a low-density culture, initiated QS after confinement for 17 h in an approximately 200 fL droplet. Bright-field images show the bacterium at 0 h (A) and 17 h (B). Fluorescent images show activation of QS after 17 h, as visualized by the expression of fluorescent reporter for the QS-controlled gene lasB.
Finally, we tested whether confinement of small groups of cells could have functional consequences for those cells. Even though the lasB-GFP strain we were using is exceptionally well characterized,[31] we wished to confirm that our results were not an artifact of the reporter and had functional significance. The growth of P. aeruginosa in media with adenosine as the sole carbon source is QS-dependent; the enzyme needed in the adenosine catabolic pathway, Nuh, is controlled by LasR,[32] one of the regulators of QS and lasB expression. We loaded wells (20 μm in diameter) with 1 to 3 cells in M9 media with 0.01% adenosine. The device was then incubated at 30°C and both growth of cells and activation of QS were monitored over time. After 29 h, 2 out of 14 wells loaded with 1 to 2 cells at t =0 showed both initiation of QS and dense growth within the wells (Figure 4A, green arrows). Initiation of QS was required for growth: no other wells contained cells that had initiated QS or cells that had divided more than once, even after 29 h (Figure 4A, white arrow).
Figure 4.
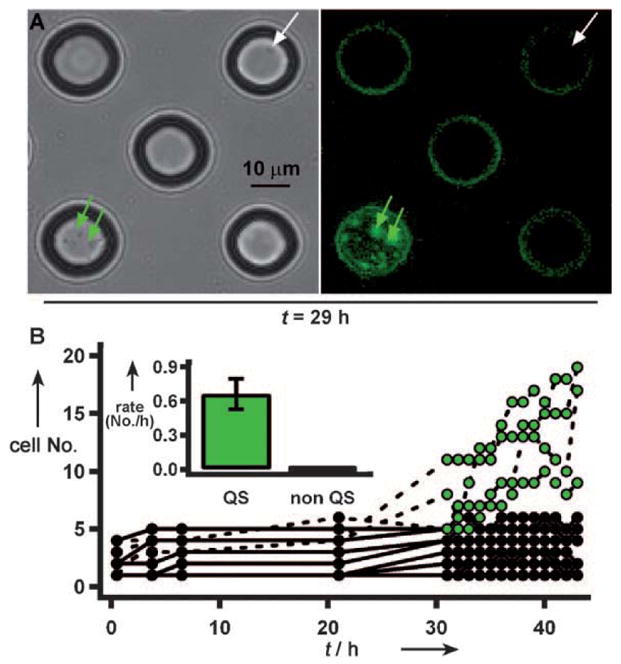
In media with adenosine as the sole carbon source, small groups of bacteria did not divide more than a few times, unless they initiated QS. A) Both wells indicated by arrows started with one cell at t =0. After 29 h, a well with cells that had initiated QS (green arrows) contained a population of tens of cells, whereas in a well with cells which did not initiate QS (white arrow) the cells did not divide. Fluorescence on the edges of wells is an artifact. B) After 31 h, bacteria in 4 of 16 wells (50 μm diameter) initiated QS, (green dots, dashed lines). Inset: the growth rate of the cells which initiated QS, 0.7 cellshour−1, is significantly higher than the growth rate of cells which did not initiate QS, 0.01 cellshour−1 (p <0.0001). Error bars are standard error. Owing to inaccuracies in counting moving cells which occupy multiple imaging planes, counts at any time have an error of approximately 1–4 cells.
To quantify the influence of QS on growth in this media, we monitored division and initiation of QS by P. aeruginosa cells in a different array of wells (50 μm diameter, Figure 4B). Initially, some cells in the wells divided, with populations increasing to at most five cells. Similar weak growth has been reported for cells in starvation conditions in the absence of available carbon sources.[33] Of the 18 wells we monitored, cells in four wells initiated QS between 21 and 31 h. The percentage of wells with initiated cells (ca. 22%) was similar to that observed for single bacteria confined in 100 fL droplets (ca. 20%; Figure 2E). If the cells in a well did not initiate QS by t =31 h, they also did not initiate QS by t = 43 h, nor did the number of cells increase over time (Figure 4B). These results confirmed that the activation of the fluorescent reporter was well correlated with functionally significant, QS-dependent behavior and that there was heterogeneity at the single-cell level for QS-dependent behavior. Thus, QS can be initiated in confined small groups of cells with functional consequences, and this confinement can be used to induce and measure heterogeneity of high-density behavior at the single-cell level.
Herein we demonstrated that confinement alone, in the absence of potential host cell factors, caused a single cell to activate QS pathways, demonstrating the possibility of auto-crine signaling by the QS pathways. These results argue against the commonly stated belief that quorum sensing requires millions of cells, confirming that QS responses are consequences of biomass per unit volume, and that if the volume is small enough, a single cell provides sufficient biomass to initiate QS. These results emphasize that QS-controlled factors may be induced by a few cells in a confined environment and that confinement may play a role in the relationship between QS and virulence in pathogens such as P. aeruginosa. These results also provide support for the views of QS as diffusion-sensing and efficiency-sensing rather than social interactions. Clearly, these results do not provide information on the relative importance of these views of QS. Confinement should be a general mechanism for inducing high-density behavior in small groups of cells and could be relevant to any systems in which accumulation of released signals regulates cellular functions. Microfluidic tools such as the chemistrode[34,35] may provide a convenient way of capturing microbes from the environment directly into confined volumes. Confinement,[36] combined with isolation of bacteria in wells etched at the ends of fiber-optic arrays,[37] could be an attractive method for monitoring single highly confined cells of microorganisms. These results pose several new questions about P. aeruginosa, such as, what is the mechanism of heterogeneity in initiation of QS? Does it arise at the level of signal production, the ability to respond, or both? What are the levels of the AIs in the droplets? Can active transport of AIs play a role? Is the heterogeneity specific for these growth conditions or this strain? These results also open new opportunities for determining the role of confinement-induced QS in other bacterial systems. For example, this approach could be used to ask if there is any difference in gene expression when initiation of QS occurs as a result of confinement, as opposed to reaching high density by growth. In addition, confinement may become a tool to test the initiation of QS pathways of species that cannot be grown to high density in culture, or where such growth is slow or inconvenient. Confinement will likely affect “quorum acting” in similar ways (e.g. in initiation of blood coagulation by bacteria),[38] and is a tool to understand and control autocrine signaling in other systems, and to monitor behavior of single cells.[39] Confinement could potentially be used to induce growth of unculturable microorganisms, to help search for activation of virulence mechanisms, or to initiate production of antibiotics, signaling molecules, and other microbial secondary metabolites.
Supplementary Material
Footnotes
This research was supported by the NIH Director’s Pioneer Award DP1 OD003584 to R.F.I. We thank S. Molin for generously providing the reporter strain, J. Shapiro and O. Zaborina for helpful discussion, and E. B. Haney for contributions to writing this manuscript.
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.200901550.
References
- 1.Miller MB, Bassler BL. Annu Rev Microbiol. 2001;55:165. doi: 10.1146/annurev.micro.55.1.165. [DOI] [PubMed] [Google Scholar]
- 2.Fuqua WC, Winans SC, Greenberg EP. J Bacteriol. 1994;176:269. doi: 10.1128/jb.176.2.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith RS, Iglewski BH. J Clin Invest. 2003;112:1460. doi: 10.1172/JCI20364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Redfield RJ. Trends Microbiol. 2002;10:365. doi: 10.1016/s0966-842x(02)02400-9. [DOI] [PubMed] [Google Scholar]
- 5.Hense BA, Kuttler C, Mueller J, Rothballer M, Hartmann A, Kreft JU. Nat Rev Microbiol. 2007;5:230. doi: 10.1038/nrmicro1600. [DOI] [PubMed] [Google Scholar]
- 6.Horswill AR, Stoodley P, Stewart PS, Parsek MR. Anal Bioanal Chem. 2007;387:371. doi: 10.1007/s00216-006-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park S, Wolanin PM, Yuzbashyan EA, Silberzan P, Stock JB, Austin RH. Science. 2003;301:188. doi: 10.1126/science.1079805. [DOI] [PubMed] [Google Scholar]
- 8.Parent ME, Snyder CE, Kopp ND, Velegol D. Colloids Surf B. 2008;62:180. doi: 10.1016/j.colsurfb.2007.09.031. [DOI] [PubMed] [Google Scholar]
- 9.Kirisits MJ, Margolis JJ, Purevdorj-Gage BL, Vaughan B, Chopp DL, Stoodley P, Parsek MR. J Bacteriol. 2007;189:8357. doi: 10.1128/JB.01040-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shompole S, Henon KT, Liou LE, Dziewanowska K, Bohach GA, Bayles KW. Mol Microbiol. 2003;49:919. doi: 10.1046/j.1365-2958.2003.03618.x. [DOI] [PubMed] [Google Scholar]
- 11.Qazi SNA, Counil E, Morrissey J, Rees CED, Cockayne A, Winzer K, Chan WC, Williams P, Hill PJ. Infect Immun. 2001;69:7074. doi: 10.1128/IAI.69.11.7074-7082.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jarry TM, Memmi G, Cheung AL. Cell Microbiol. 2008;10:1801. doi: 10.1111/j.1462-5822.2008.01166.x. [DOI] [PubMed] [Google Scholar]
- 13.Dulla G, Lindow SE. Proc Natl Acad Sci USA. 2008;105:3082. doi: 10.1073/pnas.0711723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song H, Chen DL, Ismagilov RF. Angew Chem. 2006;118:7494 – 7516. [Google Scholar]; Angew Chem Int Ed. 2006;45:7336. [Google Scholar]
- 15.Song H, Tice JD, Ismagilov RF. Angew Chem. 2003;115:792. doi: 10.1002/anie.200390203. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2003;42:768. [Google Scholar]
- 16.Weibel DB, DiLuzio WR, Whitesides GM. Nat Rev Microbiol. 2007;5:209. doi: 10.1038/nrmicro1616. [DOI] [PubMed] [Google Scholar]
- 17.Ingham CJ, Vlieg J. Lab Chip. 2008;8:1604. doi: 10.1039/b804790a. [DOI] [PubMed] [Google Scholar]
- 18.Koehler JM, Henkel T. Appl Microbiol Biotechnol. 2005;69:113. doi: 10.1007/s00253-005-0176-6. [DOI] [PubMed] [Google Scholar]
- 19.Kim HJ, Boedicker JQ, Choi JW, Ismagilov RF. Proc Natl Acad Sci USA. 2008;105:18188. doi: 10.1073/pnas.0807935105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keymer JE, Galajda P, Muldoon C, Park S, Austin RH. Proc Natl Acad Sci USA. 2006;103:17290. doi: 10.1073/pnas.0607971103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koester S, Angile FE, Duan H, Agresti JJ, Wintner A, Schmitz C, Rowat AC, Merten CA, Pisignano D, Griffiths AD, Weitz DA. Lab Chip. 2008:1110. doi: 10.1039/b802941e. [DOI] [PubMed] [Google Scholar]
- 22.Boedicker JQ, Li L, Kline TR, Ismagilov RF. Lab Chip. 2008;8:1265. doi: 10.1039/b804911d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faley S, Seale K, Hughey J, Schaffer DK, VanCornpernolle S, McKinney B, Baudenbacher F, Unutmaz D, Wikswo JP. Lab Chip. 2008;8:1700. doi: 10.1039/b719799c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Carlo D, Lee LP. Anal Chem. 2006;78:7918. doi: 10.1021/ac069490p. [DOI] [PubMed] [Google Scholar]
- 25.Rettig JR, Folch A. Anal Chem. 2005;77:5628. doi: 10.1021/ac0505977. [DOI] [PubMed] [Google Scholar]
- 26.Matsunaga T, Hosokawa M, Arakaki A, Taguchi T, Mori T, Tanaka T, Takeyama H. Anal Chem. 2008;80:5139. doi: 10.1021/ac800352j. [DOI] [PubMed] [Google Scholar]
- 27.Zhong JF, Chen Y, Marcus JS, Scherer A, Quake SR, Taylor CR, Weiner LP. Lab Chip. 2008;8:68. doi: 10.1039/b712116d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park MC, Hur JY, Kwon KW, Park SH, Suh KY. Lab Chip. 2006;6:988. doi: 10.1039/b602961b. [DOI] [PubMed] [Google Scholar]
- 29.Voskerician G, Shive MS, Shawgo RS, von Recum H, Anderson JM, Cima MJ, Langer R. Biomaterials. 2003;24:1959. doi: 10.1016/s0142-9612(02)00565-3. [DOI] [PubMed] [Google Scholar]
- 30.Song H, Ismagilov RF. J Am Chem Soc. 2003;125:14613. doi: 10.1021/ja0354566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hentzer M, Riedel K, Rasmussen TB, Heydorn A, Andersen JB, Parsek MR, Rice SA, Eberl L, Molin S, Hoiby N, Kjelleberg S, Givskov M. Microbiol-Sgm. 2002;148:87. doi: 10.1099/00221287-148-1-87. [DOI] [PubMed] [Google Scholar]
- 32.Heurlier K, Denervaud V, Haenni M, Guy L, Krishnapillai V, Haas D. J Bacteriol. 2005;187:4875. doi: 10.1128/JB.187.14.4875-4883.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Givskov M, Eberl L, Moller S, Poulsen LK, Molin S. J Bacteriol. 1994;176:7. doi: 10.1128/jb.176.1.7-14.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu W, Kim HJ, Lucchetta EM, Du W, Ismagilov RF. Lab Chip. 2009 doi: 10.1039/b904958d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen D, Du WB, Liu Y, Liu WS, Kuznetsov A, Mendez FE, Philipson LH, Ismagilov RF. Proc Natl Acad Sci USA. 2008;105:16843. doi: 10.1073/pnas.0807916105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rissin DM, Walt DR. Nano Lett. 2006;6:520. doi: 10.1021/nl060227d. [DOI] [PubMed] [Google Scholar]
- 37.Ahn S, Walt DR. Anal Chem. 2005;77:5041. doi: 10.1021/ac0505270. [DOI] [PubMed] [Google Scholar]
- 38.Kastrup CJ, Boedicker JQ, Pomerantsev AP, Moayeri M, Bian Y, Pompano RR, Kline TR, Sylvestre P, Shen F, Leppla SH, Tang WJ, Ismagilov RF. Nat Chem Biol. 2008;4:742. doi: 10.1038/nchembio.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Molter TW, Holl MR, Dragavon JM, McQuaide SC, Anderson JB, Young AC, Burgess LW, Lidstrom ME, Meldrum DR. IEEE Trans Autom Sci Eng. 2008;5:32. doi: 10.1109/tase.2007.909441. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.