Abstract
Women at increased risk for breast cancer are at increased risk for ovarian cancer as well, reflecting common risk factors and intertwined etiology of the two diseases. We previously developed a rat model of elevated breast and ovarian cancer risk, allowing evaluation of dual target cancer prevention strategies. Tamoxifen, a FDA-approved breast cancer chemoprevention drug, has been shown to promote ovarian cysts in premenopausal women; however, the effect of tamoxifen on ovarian cancer risk is still controversial. In the current experiment, Fischer 344 rats (n=8 per treatment group) received tamoxifen (TAM) or vehicle (CONT) in factorial combination with combined breast and ovarian carcinogen (17β-estradiol and 7, 12 dimethylbenza[α]anthracene, respectively). Mammary and ovarian morphologies were normal in CONT and TAM groups. Carcinogen (CARC) treatment induced mammary dysplasia with elevated cell proliferation and reduced estrogen receptor alpha expression and promoted preneoplastic changes in the ovary. In CARC+TAM-treated group, tamoxifen reduced preneoplastic changes and proliferation rate in the mammary gland but not in the ovary compared to rats treated with carcinogen alone. Putative stem cell markers [Oct-4 and aldehyde dehydrogenase-1 (ALDH-1)] were also elevated in the mammary tissue by carcinogen and this expansion of the stem cell population was not reversed by tamoxifen. Our study suggests that tamoxifen prevents early progression to mammary cancer but has no effect on ovarian cancer progression in this rat model.
Keywords: breast cancer, ovarian cancer, cancer prevention, preclinical model, tamoxifen
Introduction
The development of promising breast cancer chemoprevention agents (i.e. selective estrogen receptor modulators (SERMs), aromatase inhibitors and retinoids (1–3)) has been permitted by minimally invasive techniques to access tissue, availability of surrogate biomarkers and relatively high incidence of the disease (4, 5). In contrast, ovarian cancer prevention trials are seldom attempted due to low disease incidence, the absence of accepted disease-specific biomarkers and the invasiveness of sampling for ovarian tissue. Consequently, although most ovarian cancers are diagnosed at advanced stages resulting in high mortality rates, prevention of ovarian cancer remains elusive (6). One practical approach for successful prevention of ovarian cancer may be the development of chemoprevention agents acting simultaneously against both ovarian and breast cancer.
Breast and ovarian adenocarcinoma share numerous risk factors (e.g. estrogen exposure, ovulation, nulliparity, obesity, family history, BRCA1/2 mutations) and women at increased risk for one of these cancers are often also at risk for the other suggesting intertwined disease pathways (7, 8). Recent studies have shown that women receiving hormone replacement therapy are at increased risk for both cancers (9–11). Alternatively, drugs that decrease ovarian cancer risk may actually increase the incidence of breast cancer (e.g. progesterone (12, 13)). To date, no human chemoprevention trials have been designed simultaneously targeting both breast and ovarian cancers despite the promise of such an approach. Indeed, successful human ovarian cancer chemoprevention has only been demonstrated incidentally during the course of breast cancer prevention trials (i.e. fenretinide) (14). To investigate common chemoprevention strategies, our laboratory has developed a preclinical model that exhibits early changes of mammary and ovarian carcinogenesis in the rat (15). This model allows observation of synergistic and antagonistic drug actions against breast and ovarian cancers that are ignored when each cancer is examined in isolation.
Tamoxifen, the most commonly used breast cancer chemoprevention drug, blocks cell proliferation in the breast and has been shown to cause tumor regression and inhibit tumor formation, especially in ER+ breast tumors (16). In the ovary, especially in premenopausal women, tamoxifen has been suggested to promote abnormal ovarian function and cyst formation, a putative ovarian preneoplastic change (17, 18). Tamoxifen and other SERMs have also been used to stimulate ovarian function in subfertile women with some question as to impact on ovarian cancer risk (19, 20).
Tamoxifen prevents 70% of ER+ breast cancers in high risk women, but fails to prevent ER− and some ER+ tumors (3). One possibility for the lack of tamoxifen efficacy on 30% of ER+ cancers may be the presence of an E2-independent breast stem cell population (21). The existence of self-renewing, pluripotent stem cells have been demonstrated both in human breast and rodent mammary glands (22, 23). Following recurrent carcinogen exposure, these long-lived breast stem cells are thought to accumulate mutations leading to tumor formation. The size of the breast stem cell pool has therefore been hypothesized to serve as a determinate of the likelihood for breast cancer incidence. Indeed, several studies have suggested a strong correlation between increased number of breast stem cells and elevated breast cancer risk as well as a possible intervention that targets stem cell for cancer treatment and prevention (24–27). In the current study, we use a combined breast and ovarian cancer model to examine the effect of tamoxifen on markers of cancer risk, stemness and progression in the ovary and mammary gland during carcinogenesis.
Materials and methods
Animals and treatments
Female Fischer 344 rats (Harlan, Indianapolis, IN, n = 8 per treatment group) weighing 50–55 g were housed in a climate and light (12L:12D) controlled environment and received food and water ad libitum. All experimental protocols were approved by the University of Kansas Medical Center Animal Care and Use Committee. Animals were randomly assigned into 4 different treatment groups as shown in Table 1. Rats were anesthetized using ketamine hydrochloride and xylazine (80 and 8 mg/kg, respectively). Hemiovariectomy was performed aseptically to concentrate ovulation upon the remaining ovary and hasten a senescent hormonal milieu (28, 29) as these are risk factors of human ovarian cancer (15, 30, 31). The remaining ovary was treated by passing a 7, 12 dimethylbenza[α]anthracene (DMBA)-impregnated (2.5 mm region dipped in melted DMBA) or vehicle 5-0 silk suture through the ovary twice such that the DMBA or vehicle region was apposed directly and gently secured to the ovarian surface epithelium. Rats receiving ovarian DMBA were subsequently treated with 17β-estradiol (E2, 1.5mg, pellet implant, Hormone Pellet Press, Leawood, KS) (32). Our laboratory has previously shown that this treatment combination promotes progression to simultaneous mammary and ovarian cancer in the rat following 6 months of treatment (15). Rats were further treated with tamoxifen (5 mg, pellet implant, Hormone Pellet Press) or vehicle to test the effect of tamoxifen in early mammary and ovarian cancer (33).
Table 1.
Experimental groups to examine the effect of tamoxifen on the progression towards concurrent mammary and ovarian cancer.
| Treatment Group | CONT | TAM | CARC | CARC+TAM |
|---|---|---|---|---|
| Ovarian treatment | Vehicle | Vehicle | DMBA | DMBA |
| Systemic treatment | Vehicle | Vehicle | E2 | E2 |
| Tamoxifen treatment | Vehicle | Tamoxifen | Vehicle | Tamoxifen |
CONT = vehicle-treated animals, TAM = tamoxifen-treated animals, and CARC = carcinogen-treated animals.
Tissue preparation
Rats were killed by decapitation at 6 months post-treatment and the right thoracic mammary glands were fixed in 4% paraformaldehyde (PFA) and embedded in paraffin. Right abdominal mammary glands were spread onto a glass slide, fixed in 4% PFA overnight, hydrated, infused with alum carmine (4 days), dehydrated, cleared in xylene, and stored in methyl salicylate. Left thoracic mammary glands were snap frozen and stored at −80°C. The ovary was bisected through the site of DMBA application. One half was fixed in 4% PFA and embedded in paraffin while the remainder was snap frozen and stored at −80°C.
Immunohistochemistry
Six-micron sections of mammary glands and ovaries were deparaffinized, rehydrated, and stained with hematoxylin and eosin. Mammary and ovarian sections (midsaggital, at 3 different equidistant levels per tissue) were evaluated for morphological changes associated with early progression to mammary adenocarcinoma (MAC) and epithelial ovarian cancer (EOC) by an observer blinded to treatment group identity (34, 35). Adjacent sections were prepared for immunostaining by antigen retrieval (93°C, 10mM citrate buffer, 25 minutes) and incubation with 0.3% hydrogen peroxide (Lab Vision, Fremont, CA). Non-immune serum or primary antibodies against Ki-67 (1:100; Clone Ki-S5; rabbit monoclonal antibody, Lab Vision), estrogen receptor alpha (ERα; 1:200; MC-20; mouse monoclonal antibody, Santa Cruz), ALDH1A1 (1:150; rabbit polyclonal; Abcam, Cambridge, MA) or Oct-3/4 (1:50, mouse monoclonal, Santa Cruz) were applied and visualized with biotinylated secondary antibodies (Lab Vision) and diaminobenzidine (DAB) chromogen. All incubations were carried out using a Dako LV-1 autostainer (Carpinteria, CA).
Protein isolation and immunoblotting
Samples of mammary gland (n = 4) and ovary (n = 4) from all treatment groups were homogenized in lysis buffer (Cell Signaling Technology, Danvers, MA) with 1 mM phenylmethylsulfonyl fluoride. The lysates were centrifuged at 10,000g for 15 minutes at 4°C and supernatant collected. Protein concentrations were measured using a BCA protein assay kit (Pierce, Rockford, IL). Following boiling for 5 minutes in Laemmli sample buffer (Bio-Rad, Hercules, CA), samples (25µg protein) and ladders (Kaleidoscope prestained standards, Bio-Rad) were run on 10% Tris-HCl Criterion Precast gels (Bio-Rad) under reducing conditions and transferred onto nitrocellulose membrane. Membranes were blocked with 10% milk in Tris-Buffer Saline with Tween-20 (TBST) for 1 hour at room temperature and incubated with antibodies against ALDH1A1 (1µg/ml), cyclooxygenase-2 (COX-2; 2µg/ml, rabbit polyclonal, LabVision), or Oct-3/4 (1:200) at 4°C overnight. Following washing in TBST, blots were incubated in peroxidase-conjugated donkey anti-mouse, anti-rabbit or anti-goat antibodies (Jackson ImmunoResearch, West Grove, PA) for 2 hours at room temperature and washed. Heart tissue lysates were used as positive control (36) and primary antibody omission was used as negative control. Protein signals were visualized using chemiluminescent substrate (Pierce) and protein bands were quantified using GelPro. Equal protein loading was confirmed by stripping (BlotFresh, SignaGen, Gaithersburg, MD) and reprobing the membranes with β-actin antibody (1:20,000, goat polyclonal, sc-1616, Santa Cruz). Data are presented as integrated optical densities.
Quantitative Analysis of Preneoplastic Lesions
Mammary sections stained with H&E were evaluated and each section was assigned a dysplasia score according to the presence of pre-neoplastic and neoplastic lesions associated with breast cancer progression (15, 35). A score of 0 represented normal mammary histology. Preneoplastic changes included mild (score = 1) or severe (2) ductal hyperplasia and/or hyperplasia with atypia (3). Neoplastic changes included ductal carcinoma in situ (DCIS, score = 4) and invasive cancer (5). The sum of scores from all 3 sections from each animal was used as the total dysplasia score; a value ranging from 0 to 15.
Pre-neoplastic changes of the ovary were defined as surface hyperplasia, inclusion cysts, stromal hyperplasia and papilloma, each being a separate histologic parameter (15, 34, 37). For each ovarian section, each parameter was given a score of 0, 1, or 2, based on the severity or prevalence of each pre-neoplastic category (i.e. a score of 0 represented normal histology, a score of 1 corresponded to a moderate prevalence or degree of change and a score of 2 indicated a high incidence or degree of abnormality). Scores for all 4 histologic parameters were added up to give a dysplasia score for each ovarian section. The sum of all 3 dysplasia scores for each animal gave rise to the total dysplasia score, a value ranging from 0 to 24. These preneoplastic criteria are the same as those used by Stewart et al. with this rat model of ovarian adenocarcinoma (34).
Ki-67, and ERα expression in the mammary ductal epithelia cells and ovarian surface epithelium was quantified by counting immunoreactive cells and total cells (at least 1000 cells were evaluated per section) and presented as % immunoreactive epithelial cells. Location and distribution of ALDH-1 and Oct-3/4 expression were documented. All data are presented as the 15 mean ± SEM. Protein levels of Ki-67, ERα, COX-2, ALDH-1 and Oct-3/4 expression determined by immunohistochemistry and western blot were analyzed using one-way analysis of variance with treatment type as main effect. Dysplasia scores were analyzed using a nonparametric test (Mann Whitney test). Differences were considered significant when p≤0.05.
Results
Tamoxifen blocks mammary carcinogenesis
Mammary gland whole mounts
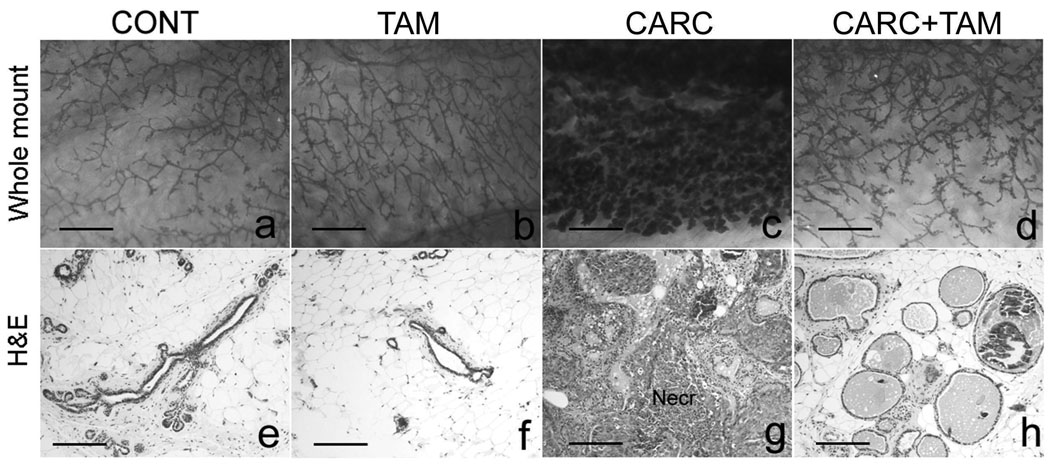
Control and TAM rats had normal mammary morphology (Figure 1a, b). Carcinogen (E2+DMBA) treatment increased area occupied by alveoli (Figure 1c). This effect was markedly reduced by tamoxifen in CARC+TAM group (Figure 1d).
Figure 1.
Whole mounts (a–d, 20x), and H&E sections (e–h, 100x) of the mammary gland. Controls (CONT) and tamoxifen-treated (TAM) animals showed normal mammary histology (a–b, e–f). Carcinogen treatment (CARC) caused preneoplasia and neoplasia (c and g) and this effect was blocked by CARC+TAM (d and h). Secreting mammary glands were observed in CARC+TAM rats. Scale bars = 5 mm (a–d), 200 µm (e–h).
Mammary tissue histology
Controls showed a normal appearance of lobular/acinar units surrounded by abundant adipose tissue (Figure 1e). These units constituted a single layer of myoepithelium and inner mammary epithelial cells. TAM animals also showed normal mammary histology (Table 2; Figure 1f). All CARC animals exhibited pathologic mammary histology ranging from hyperplasia to disseminated DCIS and had higher dysplasia scores when compared to controls (Figure 1g; Table 2, p< 0.0001). In CARC+TAM rats, the number and morphology of lobular units were restored to near normality (Figure 1h). These rats also showed mildly increased ductal branching and enlarged intraductal lumen as compared to controls but no dysplastic foci were present in any of these animals (Table 2).
Table 2.
Mammary and ovarian dysplasia scores.
| Animal group | Mammary Gland | Ovaries |
|---|---|---|
| CONT | 0 | 0.444±0.242 |
| TAM | 0 | 1.375±0.324 |
| CARC | 7.875±0.789* | 7.286±1.085* |
| CARC+TAM | 0 | 5.833±0.543* |
Dysplasia scores are present as mean±SEM for each treatment group. CONT = control, TAM = tamoxifen-treated animals, and CARC = carcinogen-treated animals.
differs significantly from control (p < 0.05). In the ovary, the differences between CONT and TAM as well as CARC and CARC+TAM were not significant (p = 0.202 and 0.085, respectively).
Tamoxifen neither blocks nor accelerates the progression to ovarian cancer in a rat model
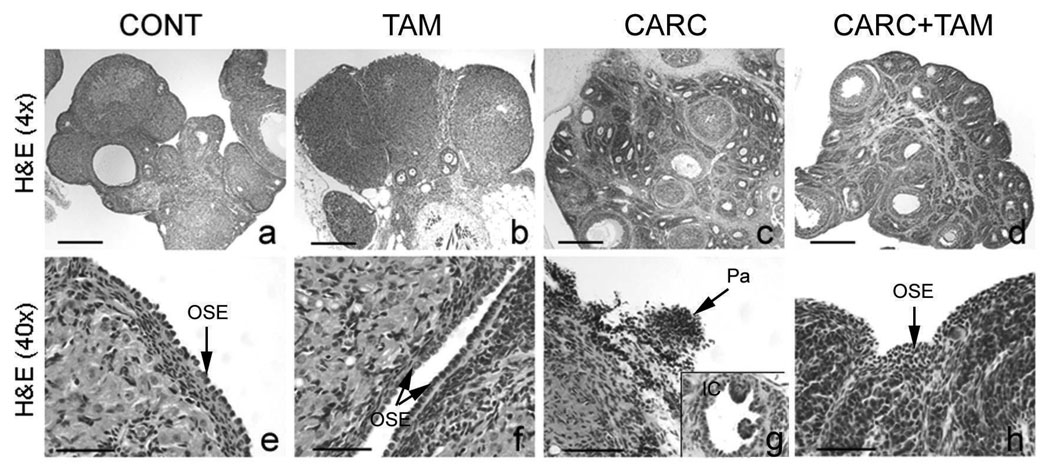
Controls showed normal ovarian morphology with mild inflammatory reaction to suture materials and rare inclusion cysts (Figure 2a, e; Table 2). Compared to controls, TAM rats showed a slight increase in dysplasia score mostly due to the occasional presence of inclusion cysts; however, this difference was not significant (p= 0.202, Figure 2b, f; Table 2). Consistent with our previous findings, CARC rats received higher dysplasia score when compared to controls (p< 0.0001) and showed markedly abnormal ovarian morphology with disorganized granulosal clusters, stromal hyperplasia, epithelial hyperplasia, papilloma and glandular cystic changes resembling inclusion cysts (Figure 2c, g; Table 2). In CARC+TAM group, tamoxifen did not reduce the degree of ovarian preneoplasia following carcinogen treatment (p= 0.0851; Figure 2d, h; Table 2). Interestingly, tamoxifen treatment seemed to increase the number of ovarian follicles when compared to those of the CARC group (data not shown).
Figure 2.
H&E sections of the ovary. Scale bars = 600 µm (a–d), 50 µm (e–h and insert). CONT = controls, TAM = tamoxifen-treated rats, CARC = E2/DMBA-treated rats, OSE = ovarian surface epithelia, Pa = papilloma, IC = inclusion cyst.
Expression of Ki-67, ERα and COX-2 in mammary gland and ovary under normal or dysplastic conditions
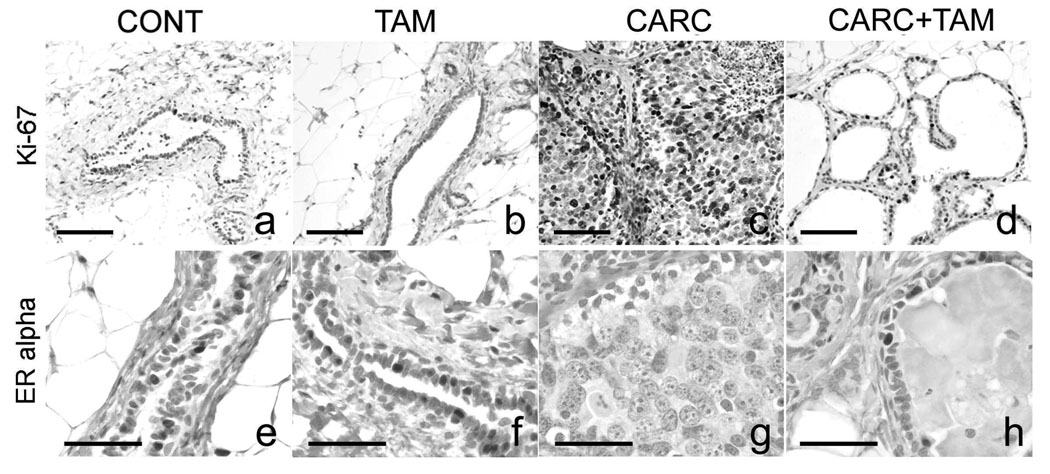
Ki-67 expression was localized in the nucleus of ductal epithelial cells in the mammary gland. Average numbers of Ki-67 positive cells per 100 ductal epithelia were 5.17 ± 2.11, 4.58 ± 1.93, 22.94 ± 3.57, and 8.75 ± 0.79 in CONT, TAM, CARC, and CARC+TAM animals, respectively. These data showed that cellular proliferation was elevated in the mammary gland of CARC-treated animals when compared to CONT (p< 0.0001) and TAM (p< 0.0001) animals. Tamoxifen inhibited carcinogen-induced Ki-67 elevation in CARC+TAM rats when compared to CARC rats (p= 0.0003; Figure 3). Very few ovarian surface epithelial cells expressed Ki-67 (less then 0.2% immunoreactivity) and there was no difference in expression among different treatment groups (p> 0.05).
Figure 3.
Ki-67 and ER alpha immunostaining in the mammary gland. Mammary epithelial proliferation (% Ki-67) was increased above controls in CARC animals (a and c, p<0.05). CARC+TAM treatment reduced this elevation (c and d, p<0.05). TAM alone did not effect % Ki-67 compared to controls (a and b). Carcinogen treatment depleted ER expression in the mammary gland when compared to control and TAM-treated animals (e–g, p<0.05). There was a trend for ERα expression to increase in response to TAM treatment in CARC+TAM animals when compared to CARC group; however, this difference was not significant (g and h, p=0.063). Scale bars = 100 µm (a–d), 50 µm (e–h).
ERα expression
Carcinogen treatment depleted ERα expression (immunoreactivity = 0.25 ± 0.06%; Figure 3) in the mammary gland when compared to control (10.30 ± 1.855%, p< 0.0001) and TAM-treated animals (11.87 ± 0.88%, p< 0.0001). While no significant differences were detected, there was a trend for ERα expression to increase in response to TAM treatment in CARC+TAM animals (3.61 ± 0.25%, p= 0.063) when compared to CARC group. In the ovary, percentages of ERα immunoreactivity in ovarian surface epithelium were 40.97 ± 5.00, 47.45 ± 1.57, 41.69 ± 5.98 and 40.68 ± 6.66 for CONT, TAM, CARC, and CARC+TAM animals, respectively. No change in ERα expression was found in the ovary among different treatment groups.
Inflammation biomarker
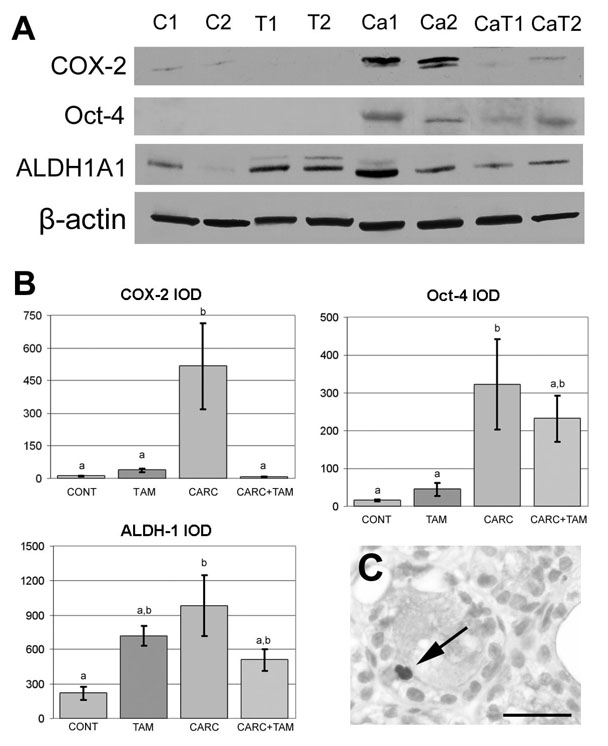
COX-2 protein level was elevated in the mammary gland of CARC rats (IOD = 517.49 ± 197.27) when compared to CONT (11.47 ± 0.56, p= 0.0067) and TAM (37.612 ± 8.28, p= 0.0089) animals. Tamoxifen treatment reduced COX expression in CARC+TAM rats when compared to CARC group (7.60 ± 1.00, p= 0.0065; Figure 4A and 4B). In the ovary, COX-2 expression was not altered by CARC treatment (p> 0.05).
Figure 4.
Cyclooxygenase 2 (COX-2), Oct-4, and aldehyde dehydrogenase 1 (ALDH-1) expression in the mammary gland. A. Representative western blots of mammary glands from rats treated with vehicle (C1, C2), tamoxifen (T1, T2), carcinogen (CA1, CA2), and carcinogen + tamoxifen (CA+T1, CA+T2). β-actin was used as a loading control. B. Quantitative analysis of western blots showing that COX-2, Oct-4 and ALDH-1 levels (y axis represents IOD, Integrated Optical Density) were elevated following carcinogen treatment (CARC) compared to controls (CONT). COX-2 expression was reduced by tamoxifen (TAM) in CARC+TAM group. Letters indicate significant differences among different treatment groups. C. Cytoplasmic ALDH-1 immunostaining was observed in a few luminal epithelial cells in the mammary gland of CARC animals (Arrow). No immunoreactivity was observed in CONT and TAM animals. Scale bars = 5 50 µm.
Levels of putative stem cell markers in the mammary gland and ovary
In the mammary gland, immunoblot analysis showed that Oct-3/4 and ALDH-1 expression were increased in CARC rats compared to controls (Figure 4A and B, p= 0.014 and 0.012, respectively). Surprisingly, while TAM drastically reduced histological progression to breast cancer, TAM had no effect on the induction of stem cell markers by sustained exposure to estrogen. Our results showed that ALDH-1 and Oct-3/4 levels between CARC and CARC+TAM animals and between CONT and TAM animals do not differ (p> 0.05, Figure 4). Immunohistochemistry revealed that while no immunoreactivity was observed in ductal epithelial cells of CONT and TAM animals, ALDH-1-positive cells were present in the cytoplasm of a few lobules in CARC and CARC+TAM animals (Figure 4C). However, immunoreactivity of Oct-3/4 was not observed in the selected mammary gland sections. While the stem cell hypothesis has been explored in breast carcinogenesis, no putative stem cell markers have been suggested to be associated with ovarian carcinogenesis. In the current study, immunohistochemistry data suggest that ALDH-1 and Oct-3/4 are not expressed in ovarian surface epithelia.
Discussion
Mammary gland
Tamoxifen inhibited mammary cancer progression in our preclinical model of breast and ovarian carcinogenesis, consistent with previous data from clinical trials and animal studies (3, 38). Ki-67, a proliferation marker, and COX-2, an inflammation marker, are potential markers of breast cancer risk and have been used as surrogate markers of response in human phase II chemoprevention trials (39). In our rat model, Ki-67 and COX-2 also correlated with progression of mammary carcinoma. Mammary ERα expression is down-regulated in CARC animals consistent with previous studies showing the loss of ERα following E2-initiated cell proliferation (15, 40); however, it is also possible that the loss of ERα is temporary and is caused by ligand-induced receptor degradation (41).
Ovary
The current study is the most detailed experiment investigating the effect of tamoxifen on ovarian physiology and cancer progression. While our study showed that tamoxifen does not retard ovarian cancer, this negative finding is very important and in agreement with the human literature with more intensive biomarker and histopathology data than in human study. Although there seemed to be a slight increase in dysplasia in the ovary of animals treated with tamoxifen alone when compared to controls, this elevation was not significant. While there is a possibility of cancer incidence with longer tamoxifen administration, six months treatment (one quarter of life-span in rats) in the current experiment far exceeds the recommended treatment time for women taking tamoxifen (less or equal to 5 years). Women taking tamoxifen have an increased risk for developing follicular cysts in the ovary (17, 18), and it has been suggested that tamoxifen-induced ovarian cysts may contribute to increased risk of ovarian cancer (42). However, in the current experiment, tamoxifen neither augment nor diminishes preneoplastic lesions induced by carcinogen treatment in the ovary in our high risk model. Our results therefore suggest that tamoxifen, as a common prevention therapy for breast cancer, does not affect ovarian cancer risk in animals at high risk for both mammary and ovarian cancer. While COX-2 levels remained unchanged in difference treatment groups. Recent studies revealed the relevance of COX-1 but not COX-2 expression in ovarian tumors development (43). The role of COX-1 in mammary and ovarian carcinogenesis should be further investigated using this model. In addition, no endometrial neoplasia was observed in our model following 6 months of sustained tamoxifen administration.
Stem cell biomarkers
Oct-4 is a transcriptional factor expressed by early embryonic and germ cells and has been used to identify pluripotent cell populations (44). ALDH-1, an enzyme that is required for the conversion of retinol to retinoic acids, is highly enriched in hematopoietic stem cells and recently, researchers have suggested its presence in breast stem cells as well (45). Our data showed increased expression of both markers in the mammary gland of rats treated with carcinogens. This finding suggests that stem cell populations are expanded during mammary carcinogenesis in our model.
Estrogen is used to induce mammary carcinogenesis in the current experiment. The mechanism by which estrogen acts on stem cell number is still unclear since most studies agree that breast stem cells are ER− (26). However, studies have also shown that dysregulation of breast stem cells, or an increased stem cell pool size, can be induced by exposure to elevated breast epithelial mitogens such as insulin-related growth factor-1 and steroid hormones including estrogens (46, 47). One rationale for this effect of estrogen is via an indirect mechanism or stem cell niche; thus, estrogen acts on ER+ cells surrounding the stem cell and promotes paracrine signaling (26, 48). Interestingly, rats treated with carcinogen+tamoxifen were rescued from progression towards mammary cancer but still exhibited elevated mammary stem cell markers. This observation may suggest that tamoxifen, while retarding breast cancer progression, does not act upon the stem cell population but rather has its effects on the differentiated epithelia. This in turn is consistent with the absence of ER in the breast stem cell (26); however, our understanding of mammary stem cell markers and biology will need to improve to fully answer this question.
Combined model of breast and ovarian cancer prevention
The rat model of breast and ovarian carcinogenesis used here, while allowing us to observe synergistic and antagonistic drug action in our search for a dual target prevention strategy, has some inherent limitations. The human population best modeled by these experiments is probably menopausal women on hormone replacement therapy and the results may be less relevant to other populations. This model is also focused on early changes of breast and ovarian cancer, since these are the intended targets for cancer chemoprevention, rather than following animals to tumor incidence. While this shortens the trials and parallels our human chemoprevention studies (39, 49), it does entail the use of surrogate endpoint biomarkers for cancer with their inherent uncertainties.
Breast and ovarian cancer share similar etiology (endocrine background, risk factors, epithelial origin, etc) reflecting common disease pathways; however, these cancers show discrepancy in terms of development and cancer cell type. This difference in pathology may be due to differences in the cells of origin or the hormonal milieu surrounding them. Despite these differences in the later stages of disease, the initiation factors for breast and ovarian cancer are similar and therefore, it is plausible to target both cancers simultaneously for prevention.
In the present study, we have demonstrated that, while tamoxifen is an effective breast cancer prevention drug for ER+ disease, it does not retard the development of ovarian preneoplasia and therefore is not ideal for simultaneous prevention of breast and ovarian cancer. Our results also suggest that while tamoxifen has been shown to induce ovarian cyst formation, it does not increase ovarian cancer risk in this model. Mechanistically, hormonal mammary carcinogenesis in this model is accompanied with elevated expression of ALDH-1 and Oct-4 and this putative expansion of the ALDH-1- or Oct-4-positive stem cell population is not reversed by tamoxifen cancer chemoprevention. These data also confirm that our combined breast and ovarian cancer model allows the observation of synergistic and antagonistic drug action on the breast and ovary. Simultaneous breast and ovarian cancer prevention is biologically feasible and may offer the best possibility for ovarian cancer prevention. Future studies will include investigation of common mechanism/ disease pathways and evaluation of other candidate drugs for simultaneous chemoprevention of both breast and ovarian cancers using this model.
Acknowledgements
We would like to acknowledge Dr. Ossama Tawfik and Dr. Sara Li for technical assistance. This project has been funded in part with the University of Alabama-Birmingham NCI Breast SPORE (NCI CA089019) and the Kansas Masonic Cancer Research Institute.
Abbreviations
- ALDH-1
aldehyde dehydrogenase-1
- CARC
carcinogens-treated animals
- CONT
vehicle-treated animals
- COX-2
cyclooxygenase-2
- DCIS
ductal carcinoma in situ
- DAB
diaminobenzidine
- DMBA
7, 12 dimethylbenza[α]anthracene
- E2
17β-estradiol
- EOC
epithelial ovarian cancer
- ER
estrogen receptor
- IOD
Integrated Optical Density
- MAC
mammary adenocarcinoma
- PFA
paraformaldehyde
- SERMs
selective estrogen receptor modulators
- TAM
tamoxifen, and
- TBST
Tris-Buffer Saline with Tween-20
References
- 1.Veronesi U, De Palo G, Marubini E, et al. Randomized trial of fenretinide to prevent second breast malignancy in women with early breast cancer. Journal of the National Cancer Institute. 1999;91:1847–1856. doi: 10.1093/jnci/91.21.1847. [DOI] [PubMed] [Google Scholar]
- 2.Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. Jama. 2006;295:2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 3.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. Journal of the National Cancer Institute. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 4.Fabian CJ, Kimler BF, Zalles CM, et al. Short-term breast cancer prediction by random periareolar fine-needle aspiration cytology and the Gail risk model. Journal of the National Cancer Institute. 2000;92:1217–1227. doi: 10.1093/jnci/92.15.1217. [DOI] [PubMed] [Google Scholar]
- 5.Kelloff GJ, Lippman SM, Dannenberg AJ, et al. Progress in chemoprevention drug development: the promise of molecular biomarkers for prevention of intraepithelial neoplasia and cancer--a plan to move forward. Clin Cancer Res. 2006;12:3661–3697. doi: 10.1158/1078-0432.CCR-06-1104. [DOI] [PubMed] [Google Scholar]
- 6.Gershenson DM, Tortolero-Luna G, Malpica A, et al. Ovarian intraepithelial neoplasia and ovarian cancer. Obstetrics and gynecology clinics of North America. 1996;23:475–543. [PubMed] [Google Scholar]
- 7.MacMahon B. Epidemiology and the causes of breast cancer. International journal of cancer. 2006;118:2373–2378. doi: 10.1002/ijc.21404. [DOI] [PubMed] [Google Scholar]
- 8.Brekelmans CT. Risk factors and risk reduction of breast and ovarian cancer. Current opinion in obstetrics & gynecology. 2003;15:63–68. doi: 10.1097/00001703-200302000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Katalinic A, Rawal R. Decline in breast cancer incidence after decrease in utilisation of hormone replacement therapy. Breast cancer research and treatment. 2007 doi: 10.1007/s10549-007-9566-z. [DOI] [PubMed] [Google Scholar]
- 10.Danforth KN, Tworoger SS, Hecht JL, Rosner BA, Colditz GA, Hankinson SE. A prospective study of postmenopausal hormone use and ovarian cancer risk. British journal of cancer. 2007;96:151–156. doi: 10.1038/sj.bjc.6603527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lacey JV, Jr, Brinton LA, Leitzmann MF, et al. Menopausal hormone therapy and ovarian cancer risk in the National Institutes of Health-AARP Diet and Health Study Cohort. Journal of the National Cancer Institute. 2006;98:1397–1405. doi: 10.1093/jnci/djj375. [DOI] [PubMed] [Google Scholar]
- 12.Birrell SN, Butler LM, Harris JM, Buchanan G, Tilley WD. Disruption of androgen receptor signaling by synthetic progestins may increase risk of developing breast cancer. Faseb J. 2007 doi: 10.1096/fj.06-7518com. [DOI] [PubMed] [Google Scholar]
- 13.Risch HA. Hormonal etiology of epithelial ovarian cancer, with a hypothesis concerning the role of androgens and progesterone. Journal of the National Cancer Institute. 1998;90:1774–1786. doi: 10.1093/jnci/90.23.1774. [DOI] [PubMed] [Google Scholar]
- 14.De Palo G, Mariani L, Camerini T, et al. Effect of fenretinide on ovarian carcinoma occurrence. Gynecologic oncology. 2002;86:24–27. doi: 10.1006/gyno.2002.6663. [DOI] [PubMed] [Google Scholar]
- 15.Ting AY, Kimler BF, Fabian CJ, Petroff BK. Characterization of a preclinical model of simultaneous breast and ovarian cancer progression. Carcinogenesis. 2007;28:130–135. doi: 10.1093/carcin/bgl140. [DOI] [PubMed] [Google Scholar]
- 16.Vogel VG. Reducing the risk of breast cancer with tamoxifen in women at increased risk. J Clin Oncol. 2001;19:87S–92S. [PubMed] [Google Scholar]
- 17.Metindir J, Aslan S, Bilir G. Ovarian cyst formation in patients using tamoxifen for breast cancer. Japanese journal of clinical oncology. 2005;35:607–611. doi: 10.1093/jjco/hyi165. [DOI] [PubMed] [Google Scholar]
- 18.Cohen I, Figer A, Tepper R, et al. Ovarian overstimulation and cystic formation in premenopausal tamoxifen exposure: comparison between tamoxifen-treated and nontreated breast cancer patients. Gynecologic oncology. 1999;72:202–207. doi: 10.1006/gyno.1998.5201. [DOI] [PubMed] [Google Scholar]
- 19.Steiner AZ, Terplan M, Paulson RJ. Comparison of tamoxifen and clomiphene citrate for ovulation induction: a meta-analysis. Human reproduction (Oxford, England) 2005;20:1511–1515. doi: 10.1093/humrep/deh840. [DOI] [PubMed] [Google Scholar]
- 20.Laven JS, Fauser BC. What role of estrogens in ovarian stimulation. Maturitas. 2006;54:356–362. doi: 10.1016/j.maturitas.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 21.Song LL, Miele L. Cancer stem cells--an old idea that's new again: implications for the diagnosis and treatment of breast cancer. Expert opinion on biological therapy. 2007;7:431–438. doi: 10.1517/14712598.7.4.431. [DOI] [PubMed] [Google Scholar]
- 22.Proia DA, Kuperwasser C. Reconstruction of human mammary tissues in a mouse model. Nature protocols. 2006;1:206–214. doi: 10.1038/nprot.2006.31. [DOI] [PubMed] [Google Scholar]
- 23.Russo J, Balogh GA, Chen J, et al. The concept of stem cell in the mammary gland and its implication in morphogenesis, cancer and prevention. Front Biosci. 2006;11:151–172. doi: 10.2741/1788. [DOI] [PubMed] [Google Scholar]
- 24.Anderson E, Clarke RB. Epithelial stem cells in the mammary gland: casting light into dark corners. Breast Cancer Res. 1999;1:11–13. doi: 10.1186/bcr5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wicha MS. Identification of murine mammary stem cells: implications for studies of mammary development and carcinogenesis. Breast Cancer Res. 2006;8:109. doi: 10.1186/bcr1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sleeman KE, Kendrick H, Robertson D, Isacke CM, Ashworth A, Smalley MJ. Dissociation of estrogen receptor expression and in vivo stem cell activity in the mammary gland. The Journal of cell biology. 2007;176:19–26. doi: 10.1083/jcb.200604065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 Is a Marker of Normal and Malignant Human Mammary Stem Cells and a Predictor of Poor Clinical Outcome. Cell stem cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anzalone CR, Hong LS, Lu JK, LaPolt PS. Influences of age and ovarian follicular reserve on estrous cycle patterns, ovulation, and hormone secretion in the Long-Evans rat. Biology of reproduction. 2001;64:1056–1062. doi: 10.1095/biolreprod64.4.1056. [DOI] [PubMed] [Google Scholar]
- 29.Chatterjee A, Greenwald GS. The long-term effects of unilateral ovariectomy of the cycling hamster and rat. Biology of reproduction. 1972;7:238–246. doi: 10.1093/biolreprod/7.2.238. [DOI] [PubMed] [Google Scholar]
- 30.Riman T, Nilsson S, Persson IR. Review of epidemiological evidence for reproductive and hormonal factors in relation to the risk of epithelial ovarian malignancies. Acta obstetricia et gynecologica Scandinavica. 2004;83:783–795. doi: 10.1111/j.0001-6349.2004.00550.x. [DOI] [PubMed] [Google Scholar]
- 31.Kreiger N, Sloan M, Cotterchio M, Parsons P. Surgical procedures associated with risk of ovarian cancer. International journal of epidemiology. 1997;26:710–715. doi: 10.1093/ije/26.4.710. [DOI] [PubMed] [Google Scholar]
- 32.Li JJ, Papa D, Davis MF, et al. Ploidy differences between hormone- and chemical carcinogen-induced rat mammary neoplasms: comparison to invasive human ductal breast cancer. Molecular carcinogenesis. 2002;33:56–65. doi: 10.1002/mc.10022. [DOI] [PubMed] [Google Scholar]
- 33.Li SA, Weroha SJ, Tawfik O, Li JJ. Prevention of solely estrogen-induced mammary tumors in female aci rats by tamoxifen: evidence for estrogen receptor mediation. The Journal of endocrinology. 2002;175:297–305. doi: 10.1677/joe.0.1750297. [DOI] [PubMed] [Google Scholar]
- 34.Stewart SL, Querec TD, Ochman AR, et al. Characterization of a carcinogenesis rat model of ovarian preneoplasia and neoplasia. Cancer research. 2004;64:8177–8183. doi: 10.1158/0008-5472.CAN-04-1702. [DOI] [PubMed] [Google Scholar]
- 35.Thompson HJ, Singh M. Rat models of premalignant breast disease. Journal of mammary gland biology and neoplasia. 2000;5:409–420. doi: 10.1023/a:1009582012493. [DOI] [PubMed] [Google Scholar]
- 36.Oyama T, Isse T, Kagawa N, et al. Tissue-distribution of aldehyde dehydrogenase 2 and effects of the ALDH2 gene-disruption on the expression of enzymes involved in alcohol metabolism. Front Biosci. 2005;10:951–960. doi: 10.2741/1589. [DOI] [PubMed] [Google Scholar]
- 37.Crist KA, Zhang Z, You M, et al. Characterization of rat ovarian adenocarcinomas developed in response to direct instillation of 7,12-dimethylbenz[a]anthracene (DMBA) coated suture. Carcinogenesis. 2005;26:951–957. doi: 10.1093/carcin/bgi039. [DOI] [PubMed] [Google Scholar]
- 38.Decensi A, Robertson C, Viale G, et al. A randomized trial of low-dose tamoxifen on breast cancer proliferation and blood estrogenic biomarkers. Journal of the National Cancer Institute. 2003;95:779–790. doi: 10.1093/jnci/95.11.779. [DOI] [PubMed] [Google Scholar]
- 39.Fabian CJ, Kimler BF, Anderson J, et al. Breast cancer chemoprevention phase I evaluation of biomarker modulation by arzoxifene, a third generation selective estrogen receptor modulator. Clin Cancer Res. 2004;10:5403–5417. doi: 10.1158/1078-0432.CCR-04-0171. [DOI] [PubMed] [Google Scholar]
- 40.Cheng G, Weihua Z, Warner M, Gustafsson JA. Estrogen receptors ER alpha and ER beta in proliferation in the rodent mammary gland. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:3739–3746. doi: 10.1073/pnas.0307864100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nawaz Z, Lonard DM, Dennis AP, Smith CL, O'Malley BW. Proteasome-dependent degradation of the human estrogen receptor. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:1858–1862. doi: 10.1073/pnas.96.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spicer DV, Pike MC, Henderson BE. Ovarian cancer and long-term tamoxifen in premenopausal women. Lancet. 1991;337:1414. doi: 10.1016/0140-6736(91)93096-r. [DOI] [PubMed] [Google Scholar]
- 43.Daikoku T, Tranguch S, Trofimova IN, et al. Cyclooxygenase-1 is overexpressed in multiple genetically engineered mouse models of epithelial ovarian cancer. Cancer research. 2006;66:2527–2531. doi: 10.1158/0008-5472.CAN-05-4063. [DOI] [PubMed] [Google Scholar]
- 44.Tai MH, Chang CC, Kiupel M, Webster JD, Olson LK, Trosko JE. Oct4 expression in adult human stem cells: evidence in support of the stem cell theory of carcinogenesis. Carcinogenesis. 2005;26:495–502. doi: 10.1093/carcin/bgh321. [DOI] [PubMed] [Google Scholar]
- 45.Ferrell CM, Dorsam ST, Ohta H, et al. Activation of stem-cell specific genes by HOXA9 and HOXA10 homeodomain proteins in CD34+ human cord blood cells. Stem cells (Dayton, Ohio) 2005;23:644–655. doi: 10.1634/stemcells.2004-0198. [DOI] [PubMed] [Google Scholar]
- 46.Savarese TM, Strohsnitter WC, Low HP, et al. Correlation of umbilical cord blood hormones and growth factors with stem cell potential: implications for the prenatal origin of breast cancer hypothesis. Breast Cancer Res. 2007;9:R29. doi: 10.1186/bcr1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Assis S, Hilakivi-Clarke L. Timing of dietary estrogenic exposures and breast cancer risk. Annals of the New York Academy of Sciences. 2006;1089:14–35. doi: 10.1196/annals.1386.039. [DOI] [PubMed] [Google Scholar]
- 48.Mallepell S, Krust A, Chambon P, Brisken C. Paracrine signaling through the epithelial estrogen receptor alpha is required for proliferation and morphogenesis in the mammary gland. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:2196–2201. doi: 10.1073/pnas.0510974103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fabian CJ, Kimler BF, Zalles CM, et al. Reduction in proliferation with six months of letrozole in women on hormone replacement therapy. Breast cancer research and treatment. 2007 doi: 10.1007/s10549-006-9476-5. [DOI] [PubMed] [Google Scholar]