Abstract
The concept of self-regulation is central to the understanding of human development. Self-regulation allows effective socialization and predicts both psychological pathologies and levels of achievement in schools. What has been missing are neural mechanisms to provide understanding of the cellular and molecular basis for self-regulation. We show that self-regulation can be measured during childhood by parental reports and by self-reports of adolescents and adults. These reports are summarized by a higher order factor called effortful control, which reflects perceptions about the ability of a given person to regulate their behavior in accord with cultural norms. Throughout childhood effortful control is related to children’s performance in computerized conflict related tasks. Conflict tasks have been shown in neuroimaging studies to activate specific brain networks of executive attention. Several brain areas work together at rest and during cognitive tasks to regulate competing brain activity and thus control resulting behavior. The cellular structure of the anterior cingulate and insula contain cells, unique to humans and higher primates that provide strong links to remote brain areas. During conflict tasks, anterior cingulate activity is correlated with activity in remote sensory and emotional systems, depending upon the information selected for the task. During adolescence the structure and activity of the anterior cingulate has been found to be correlated with self-reports of effortful control.
Studies have provided a perspective on how genes and environment act to shape the executive attention network, providing a physical basis for self-regulation. The anterior cingulate is regulated by dopamine. Genes that influence dopamine levels in the CNS have been shown to influence the efficiency of self-regulation. For example, alleles of the COMT gene that influence the efficiency of dopamine transmission are related to the ability to resolve conflict. Humans with disorders involving deletion of this gene exhibit large deficits in self-regulation. Alleles of other genes influencing dopamine and serotonin transmission have also been found to influence ability to resolve conflict in cognitive tasks. However, as is the case for many genes, the effectiveness of COMT alleles in shaping self-regulation depends upon cultural influences such as parenting. Studies find that aspects of parenting quality and parent training can influence child behavior and the efficiency of self-regulation.
During development, the network that relates to self-regulation undergoes important changes in connectivity. Infants can use parts of the self-regulatory network to detect errors in sensory information, but the network does not yet have sufficient connectivity to organize brain activity in a coherent way. During middle childhood, along with increased projection cells involved in remote connections of dorsal anterior cingulate and prefrontal and parietal cortex, executive network connectivity increases and shifts from predominantly short to longer range connections. During this period specific exercises can influence network development and improve self-regulation. Understanding the physical basis of self-regulation has already cast light on individual differences in normal and pathological states and gives promise of allowing the design of methods to improve aspects of human development.
Keywords: Attention, genetic alleles, neural networks, self-regulation
PHYSICAL BASIS OF PSYCHOLOGICAL CONCEPTS
The idea of reducing all psychological concepts to their physical basis is an old theme in philosophical and scientific circles. The paradigm case for this kind of reduction is probably an understanding of “consciousness” [21, 68]. Full reductionism is only one reason for seeking an understanding of psychological concepts at the level of molecules and proteins. Even if full reduction remains only a distant or impossible goal, some illumination of complex concepts like consciousness is possible now. Posner [68] argued that the psychological concept of attention was to an understanding of consciousness as DNA is to an understanding of life. Clearly DNA does not explain all of the mechanisms of life, but no student of life would do without understanding some genetics. The genetics of attention is now important in understanding the physical basis of attentional networks [46, 73]. The genetics of attention provides an opportunity to examine the physical basis of a complex psychological concept related to consciousness
This paper is an example of progress in understanding the physical basis of a complex psychological function. Consciousness can be divided into awareness of the world around us and voluntary control of our own thoughts and behavior. Although there has probably been more effort to understand conscious awareness, particularly of the visual world [58], we have been also concerned with aspects of voluntary control, as they arise early in life. This aspect of voluntary control is often called self-regulation. Every parent is aware of the remarkable transformation from infancy to childhood when children develop the ability to regulate their emotions and to persist in working toward goals in the face of distractions. Even though self-regulation does not deal with all aspects of volition and even less of consciousness, it is of sufficient complexity and centrality to serve as a model system for illuminating a psychological concept by examining its physical basis. Moreover, the results of our examination of volition also cast some light on aspects of awareness (see section on awareness).
Although self-regulation has been seen as primarily an issue in child development, its genetic basis suggests an important evolutionary history. In fact, a number of genes have been identified related to the brain network that we believe underlies self-regulation [72]. Our approach has been to understand the anatomy of self-regulation through use of neuroimaging and then to examine how genes and experience develop this network within individuals. This allows us to discuss evolutionary changes in the network between nonhuman primates and humans, as well as more recent changes that might reflect aspects of human evolution. This paper concerns both these types of evolution.
SELF-REGULATION AND DEVELOPMENT
During infancy the caregiver provides much of self-regulation. Soothing by holding and rocking or by orienting of attention as common practices for control of distress. Holding the child so as to focus on the external physical environment or the social world of interaction with the caregiver provides a means of raising and lowering sensory stimulation. External controls on arousal, distress and sensory input eventually become internalized as toddlers come to control their own emotional and cognitive levels. This process allows the caretaker to accommodate the child to controls appropriate for a given culture and environment. Success in the development of self-regulation has many advantages for the child’s future, and caregivers can report on their ability to regulate the child’s emotions even from the first days of life.
Starting at about age 3 years parents can answer questions about their child’s ability to control their emotions and behavior. For example, caregivers answer questions such as: when playing alone how often is your child distracted, how often does you child look immediately when you point. Adults may be asked how often do you make plans you do not follow through? The answers are aggregated to form scales measuring attention focusing, inhibitory control, low intensity pleasure, and perceptual sensitivity. These are summarized in a higher order scale called Effortful Control (EC) [80].
Effortful control has been studied in relation to many important achievements of childhood. For example, empathy is strongly related to effortful control, with children high in effortful control showing greater empathy [79]. To display empathy towards others requires that we interpret their signals of distress or pleasure. Imaging work shows that sad faces activate the amygdala. As sadness increases, this activation is accompanied by activity in the anterior cingulate as part of the attention network [8]. It seems likely that the cingulate activity represents the basis for our attention to the distress of others.
Developmental studies find two routes to successful socialization. A strongly reactive amygdala related to fear would provide the signals of distress allowing empathic feelings toward others and leading to hesitancy to perform behaviors that might cause harm. These children are relatively easy to socialize. In the absence of this form of control, development of the cingulate would allow appropriate attention to the signals provided by amygdala activity. Consistent with its influence on empathy, effortful control also appears to play a role in the development of conscience. The internalization of moral principles appears to be facilitated in fearful preschool-aged children, especially when their mothers use gentle discipline [59]. In addition, internalized conscience is facilitated for children high in effortful control [60]. Two separable control systems, one reactive (fear) and one self-regulative (effortful control) appear to regulate the development of conscience.
Individual differences in effortful control are also related to some aspects of the child’s developing knowledge of themselves and others, such as theory of mind, knowing that people’s behavior is guided by their beliefs, desires, and other mental states [13]. Tasks that require the inhibition of a prepotent response are related to theory of mind tasks even when other factors, such as age, intelligence, and working memory are factored out [13]. Effortful control is positively related to success in school, higher levels of empathy and other prosocial behaviors, and lowered risk for pathologies such as conduct disorder, substance abuse and antisocial behavior. The successful acquisition of effortful control involves genetic and temperamental predisposition as well as the social environment provided by caregivers. Indeed, self-regulation is said to be the key mediator between genetic predisposition, early experience and adult functioning [40].
Self-regulation is thus a key ingredient of the change between infancy and childhood. Throughout the lifespan continues to serve the function of voluntary regulation of emotions, thoughts and actions. What is new in this paper is specification of some of the mechanisms involved in this complex process at the systems, cellular and molecular levels, thus obtaining a physical basis for the complex of mental events that comprise self-regulation. The bulk of this chapter is devoted to an exposition of this effort.
ANATOMY OF SELF-REGULATION
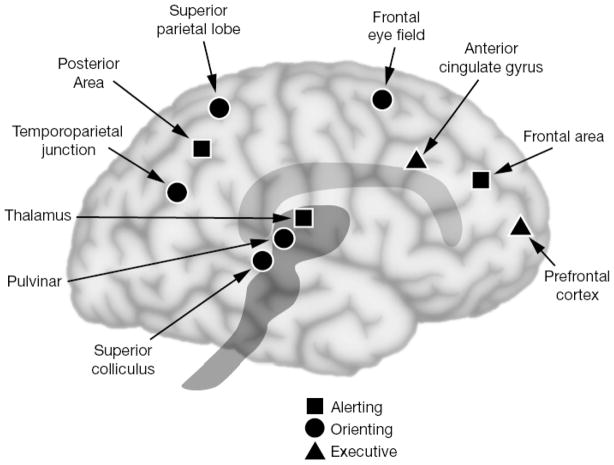
A frontal neural network (see figure 1) including the anterior cingulate and lateral prefrontal cortex active in different tasks that involve attention when conflict is present and/or producing a non-habitual response is required [10,65,75]. One important study [30] examined a wide range of verbal, spatial and object tasks selected from intelligence tests that had in common a strong loading on the factor of general intelligence (g). These items were contrasted with perceptually similar control items that did not require the kind of attention and thought involved in general intelligence. This subtraction led to differential activity in two major areas. One was the anterior cingulate and the second was lateral prefrontal cortex.
Figure 1.
Brain networks underlying attention. One fronto-parietal network is involved in orienting to sensory events (circles), while a cingulo-opercular network relates to the resolution of conflict among responses (triangles, executive network), a third network is responsible for achieving the alert state involves norepinepherine from the midbrain (squares).
Conflict
Many imaging studies have been conducted using either the Stroop task or variants of it that involve conflict among elements [10,11,12]. The Stroop task requires the person to respond to the color of ink of a target which can be a competing color word. In a numerical version of the Stroop that has been used with trained primates it was found that both humans and macaques took additional time to respond during conflict trials [105]. In fact the increase in RT was about the same for the two species, but while humans rarely made an error, macaques made almost 25% errors in conflict trials even after many hundreds of training trials suggesting that their network for resolving conflict is not as efficient. We examined three conflict tasks, two of which were suitable for children, in a group of adults, using the same MRI scanner to determine areas of activation [34]. We found that all three tasks had a common focus in the anterior cingulate and, in addition, all activated similar areas of the lateral prefrontal cortex.
The more dorsal area of the anterior cingulate has been shown to be active primarily in cognitive tasks like the Stroop. However, when tasks have a more emotional component they activate a more ventral part of the cingulate [12]. We have argued that these two areas are involved in regulation of cognitive and emotional networks. Subsequent studies have found many more subareas of the cingulate, but the generalization that more dorsal areas are related to cognitive control and more ventral areas related to emotional control seems to hold up.
Connectivity
An extensive study of the connectivity of the cingulate has shown that more dorsal areas, related to cognition, connect heavily to frontal and parietal areas, while the more ventral areas related to emotion regulation have their connectivity to limbic emotional areas.
Comparative anatomical studies point to important differences in the evolution of cingulate connectivity between non-human primates and humans. Anatomical studies show a great expansion of white matter, which has increased more in recent evolution than has the neocortex itself [109]. One type of projection cell called the Von Economo neuron, is found only in the anterior cingulate and a related area of the anterior insula [2]. It is thought that this neuron is important in communication between the cingulate and other brain areas. This neuron is not present at all in macaques and expands greatly in frequency between great apes and humans. The two brain areas in which Von Economo neurons are found (cingulate and anterior insula) are also shown to be in close communication, even during the resting state [26]. Moreover, there is some evidence that the number of these neurons increases in development between infancy and later childhood [2]. In our view, this neuron and the rapid and efficient connectivity it provides, is a major reason why self-regulation in adult humans can be so much stronger than in other organisms. The development of this system may also relate to the achievements in self-regulation that we have documented between infancy and age 7–8 years. This form of self-regulation shows the close connection between the dorsal anterior cingulate, the anterior insula [94] and areas of the brain related to perception, language and action. Because of the regulation provide by the brain network involving the cingulate and insula we call this the executive attention network.
It is possible to use fMRI to examine the functional connectivity between brain areas during the performance of a task [26]. Two studies illustrate the use of fMRI to trace the interaction of the anterior cingulate with other brain areas. In one study subjects were required to switch between auditory and visual modalities [16]. The dorsal anterior cingulate was coupled either to visual or auditory sensory areas depending on the selected modality. Another study [32] required subjects to resolve conflict related to negative emotion. Activation of the ventral anterior cingulated was coupled to the amygdala in this form of conflict resolution. Studies requiring control of positive [6] or negative emotional reactions [64] to stimuli have shown strong activation in the anterior cingulate, in comparison to conditions in which the stimuli were presented without the instruction for self-regulation. In another study a close coupling between the anterior insula and cingulate was found during switching between active and passive mental states [94].
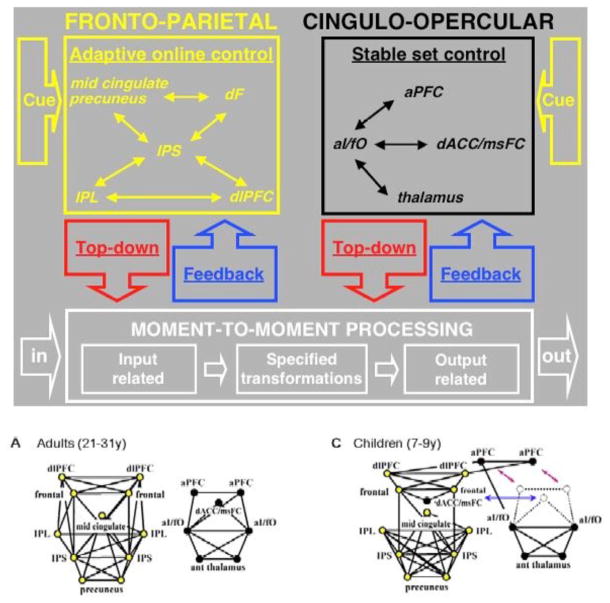
Neural areas found active in studies of functional anatomy must be orchestrated in carrying out any real task. One approach to studying this connectivity uses fMRI to study correlations between active areas [33, 74]. An important finding has been that even at rest common brain areas appear to be active together (default state). Studies suggest that the connectivity between these areas change over the course of development. Figure 2 illustrates two sets of connections active at rest: a fronto-parietal network (related to orienting) and a cingulo-opercular network(related to executive attention). Children show many shorter connections and integration of the dorsal anterior cingulate in both networks. Adults show more segregation of the two networks and longer connections.
Figure 2.
The fronto-parietal network (orienting, online control) and the cingulo-opercular network (executive, set control) are active at rest and undergo a long developmental process shown as graphs for children C (age 9) and adults A [adapted from 32 ].
Abbreviations: aI/fO, anterior insula/frontal operculum; aPFC, anterior prefrontal cortex; dACC/msFC, dorsal anterior cingulate cortex/medial superior frontal cortex; dlPFC, dorsolateral prefrontal cortex; dF, dorsal frontal; IPL, inferior parietal lobule; IPS, intraparietal sulcus;
Some of the same brain areas found active during rest change when the person is given a task. For example, while the organization of anatomical areas in alerting and orienting is not fully known, some promising beginnings have taken place. In alerting, the source of the attentional influence on arousal appears in the locus coeruleus (lc). Cells in the lc have two modes of processing. One mode is sustained and may be related to the tonic level of alertness over long time intervals. This function is known to involve the right cerebral hemisphere more strongly than the left [17, 36]. Alertness is influenced by sensory events and by the diurnal rhythm. However, its voluntary maintenance during task performance may be orchestrated from the anterior cingulate [17, 36,62]. More phasic shifts of alerting can result from presenting any environmental signal. However, if the signal is likely to warn about an impending target, this shift results in a characteristic suppression of the intrinsic brain rhythms (e.g. alpha) within a few tens of milliseconds and a strong negative wave (contingent negative variation) recorded from surface electrodes that moves from a frontal generators toward sensory areas of the hemisphere opposite the expected target. Alerting has a surprisingly long time course of development before reaching adult levels of performance
INDIVIDUALITY
The finding that common brain networks are involved in self-regulation provides one important approach to evolution by looking at commonalities and differences with non-human organisms. Another approach of equal importance involves an examination of differences in the efficiency of this network between individuals. Such differences could rest in part upon genetic variation known to exist among individuals and in part upon differences in cultural or individual experience between people. The study of temperament examines individual differences in reactivity and self-regulation that are biologically based [81]. One of the most important of these individual differences has been called “effortful control.” It is a higher order factor consisting of a number of subscales measuring attentional and behavioral control.
Effortful control
Effortful control has been linked to the brain areas involved in self-regulation by imaging studies [106]. Whittle had 155 adolescents fill out a temperament scale and also measured the size of different brain structures and their activity. She found that the dorsal anterior cingulate size was positively related to effortful control and the ventral anterior cingulate activity was negatively related to effortful control. The reciprocal relation between the ventral and dorsal cingulate has also been reported in other imaging studies [28].
Attention Network Test
In our work we have used the Attention Network Test (ANT) to examine the efficiency of three brain networks underlying attention: alerting, orienting and executive attention [37]. The task requires the person to press one key if the central arrow points to the left and another if it points to the right. Conflict is introduced by having flankers surrounding the target point in either point the same (congruent) or opposite (incongruent) direction as the target. Cues presented prior to the target provide information on where or when the target will occur. Reaction times for the separate conditions are subtracted, providing three measures that represent the efficiency of the individual in alerting, orienting and executive networks. In a sample of 40 normal adults [37] we found each of these measure to be reliable over repeated presentations. In addition, we found no correlation among the measures. An analysis of reaction times found in this task shows large main effects for cue type and for the type of target. There were only two small interactions indicating some lack of independence among the cue conditions. One of these interactions when a cue directed orienting to the correct target location the influence of the surrounding flakers was reduced. In addition, omitting a cue, which produces relatively long reaction times, also reduced the size of the flanker interference. Presumably this is because some of conflict is resolved in parallel with alerting.
Subsequent work has confirmed the relative independence among networks, while showing that they can interact when conditions are made more difficult or otherwise changed. A study using fMRI [36] showed that the anatomy of these three networks was for the most part independent. In addition, each of the networks has a dominant neuromodulator arising from subcortical brain areas. The alerting network is modulated by norepinepherine arising in the locus coeruleus, the orienting network by acetylcholine from the basal forebrain and the executive network by dopamine from the ventral tegmental area [69].
Scores on the conflict network of the ANT have been shown to correlate with the temperament factor of effortful control (EC) at several ages during childhood. Gerardi-Caulton [45] carried out some of the first research linking EC to underlying brain networks of executive attention, using spatial conflict as a laboratory marker task. Similar findings linking parent reported EC to performance on laboratory attention tasks have been shown for 24-, 30-, and 36-month-olds [83], 3- and 5-year-olds [14] and 7-year-olds [47]. Some adult studies have also found a correlation between conflict resolution ability and effortful control [54] and in some disorders influence both executive [39] and effortful control.
Daily life
The correlation between conflict scores in this simple easily administered cognitive task and parental reports of effortful control form the basis for the association between self-regulation and executive attention. The anatomy found in the Whittle studies related to effortful control also closely corresponds to the anatomical areas activated by tasks like the ANT.
As discussed above, executive attention and ANT executive attention scores have been related to many aspects of child development. Effortful control is related to the empathy that children show toward others, their ability to delay an action and to avoid such behaviors as lying or cheating when given the opportunity [71, 84]. High levels of effortful control and ability to resolve conflict are related to fewer antisocial behaviors such as truancy in adolescents [31]. These findings have convinced us that self-regulation, a psychological function crucial for child socialization, can also be studied in terms of specific anatomical areas and their connections.
GENES AND EXPERIENCE BUILD NETWORKS
The common nature of brain networks such as those in Figure 1 argue strongly for the role of genes in their construction. This has led cognitive neuroscience to incorporate data from the growing field of human genetics [46, 73]. One method for doing this relates individual variations in genes (genetic alleles) to aspects of human behavior. Brain activity can serve as an intermediate level for relating genes to behavior as illustrated in Figure 3. As one example, the Attention Network Test (ANT) has been used to examine individual differences in the efficiency of executive attention. Strong heritability of the executive network [37] supported the search for genes related to individual differences in network efficiency.
Figure 3.
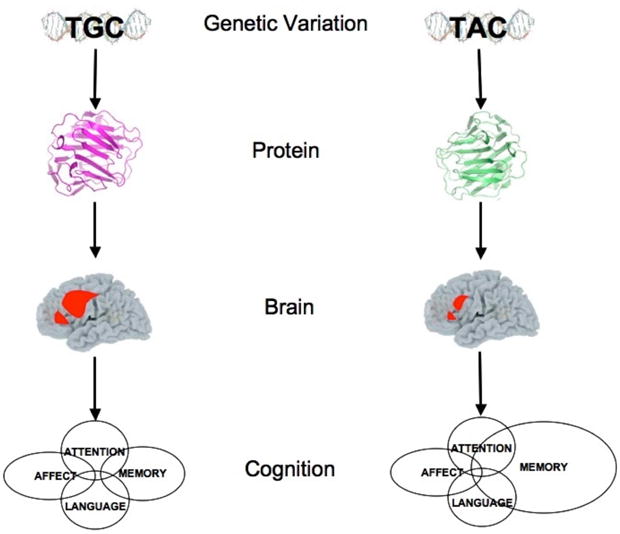
A strategy for relating brain networks to underlying molecular events [from 46]. The bottom of each columns are psychological functions, these are related to neural networks as shown in brain images and then to differences in protein configuration and to genetic variation at the to of each column
Neuromodulators
The association of the executive network with the neuromodulator dopamine provides a way of searching for candidate genes that might relate to the efficiency of the network [41, 69]. For example, several studies employing conflict related tasks have found alleles of the catechol-o-methyl transferase (COMT) gene were related to the ability to resolve conflict [23, 9, 73]. A number of other dopamine genes have also proved to be related to this form of attention, and research has suggested that genes related to serotonin transmission also influence executive attention [73 for a summary]. It has also been possible to show in brain imaging studies that some of these genetic differences are related to the degree to which the anterior cingulate is activated during task performance [35]. In the future, as suggested by Figure 3, it may be possible to relate genes to specific nodes within neural networks, allowing a much more detailed understanding of the origins of brain networks.
Logitudinal Study
Recently we have been conducting a longitudinal study to see if the genes we had shown to influence attention in adults would have specific roles in the development of self-regulation during infancy and childhood. Our study began when the infants were 7 months old. We retested and genotyped the children at age 2 years, will test them a final time at age 4 when they are able perform the ANT as a measure of executive attention. Because infants are not able to carry out voluntary attention tasks, we used a visual task in which a series of attractive stimuli are put on the screen in a repetitive sequence. Infants orient to them by moving their eyes (and head) to the location. On some trials infants show they anticipate what is coming by orienting prior to the stimulus. It had been shown previously that infants as young as 4 months of age have a significantly better than chance performance in their anticipations [15], and we argued that these anticipations must be based on an executive attention system. Anticipations then may serve as an early indicant of executive attention. In agreement with this idea we have shown [90] that infants who make them most anticipatory looks also exhibit a pattern of cautious reaching toward novel objects that predicts effortful control in older children [80, 83]. In addition, infants with more anticipatory looks showed more spontaneous attempts at self-regulation when presented with somewhat frightening objects. Along with the infants’ use of the anterior cingulate in detecting error [7, 108], these findings show that at 7 months they have a rudimentary executive attention system in place, even though parents are as yet unable to report it and infants do not carry instructed behaviors.
Rothbart & Derryberry [82] proposed a distinction between reactive and self-regulatory aspects of child temperament. They argued that early in life negative affect, particularly fear and orienting of attention served as regulatory mechanisms that were supplemented by parental regulation. Moreover, they [81] argued for developmental change in which effortful control only arose at about 3–4 years of age when parents could first report on their children’s self regulatory ability.
Our longitudinal study has confirmed but also revised and extended this analysis. We find a negative correlation at 7 months between parental reports of infant orienting of attention and negative affect. Orienting also correlates positively with reports of positive affect. By two years, orienting is no longer related to affect, but effortful control shows modest non-significant negative correlations with both positive and negative affect. There is substantial evidence that for children, adolescents and adults, effortful control shows a negative correlation with negative affect in western countries, although Ahadi, Rothbart, and Ye [1] found that in China effortful control was negatively correlated with positive affect in children. They argued that culture shaped the direction of the interaction of effortful control and emotion. We will be obtaining results from 4 year olds in our longitudinal study, and expect to find significant negative correlations between effortful control and negative affect.
Results of our longitudinal study suggest that early in life, orienting serves as a regulatory system and it both reduces negative affect [49] and increases positive affect. In this view both orienting and executive networks serve parallel regulatory functions during infancy. Later on, executive attention appears to dominate in regulating emotions and thoughts, but orienting still serves as a control system.
CHRNA4
The nicotinic cholinergic receptor modulates the release of dopamine in the mesolimbic system [67]. Polymorphisms in the CHRNA4 gene have been associated with nicotine dependence in humans and with cognitive performance [78]. The CHRNA4 C1545T polymorphism (rs1044396) has been associated with variation in performance of visuospatial attention [66] and in brain activity when performing visual attention tasks [107].
Since visuospatial attention requires orienting, we expected the CHRNA4 polymorphism to influence orienting in our subjects and thought it might also influence higher order attention. Our genetic findings provided support for the parallel model of regulation discussed above. At 7 months, CHRNA4 is related to aspects of anticipatory looking. We have regarded anticipatory, but not reactive looking to be a very early arising indicant of executive attention. However, this form of looking may also be related to the orienting network. At about two years the main influence of this gene appears to be on effortful control, which depends upon executive attention. In adults CHRNA4 seems to be related to tasks that clearly involve the orienting network [66], but these tasks may involve executive attention as well.
During infancy the T/T allele of CHRNA4 is related to better performance in anticipatory looking, but at Time 2 the C/C homozygotes have the highest scores on effortful control [103]. Since we regard anticipatory looking as an early measure of executive attention and effortful control also relates to executive attention this finding is puzzling. There is clearly cholinergic input to both the executive attention network and the orienting network. The surprising finding that alleles associated with higher attention during infancy (as measured by anticipation) have lower parent reported effortful control at age 2 may indicate that infants who control emotions through orienting are slower to transition to emotional control via the executive network. Since our measurement at 18–20 months occurs well before regulation by executive attention is complete, infants with strong orienting may not have made the transition. Our tests on the same children at age 4 years, when we will use both anticipatory looking and the attention network test [37], further explicate the relation of CHRNA4 to orienting and executive attention.
COMT
COMT plays an important role in dopamine metabolism by modulating extracellular levels of dopamine. The functional Val/Met polymorphism of COMT has a measurable effect on COMT enzyme activity, with the Val allele degrading extracellular dopamine more quickly than the less enzymatically active Met allele. The Met allele has been associated with anxiety and negative mood states [27], affective disorders [55], and decreased novelty seeking [77]. A finding from our current longitudinal study is that the COMT gene, which has consistently been shown to be related to executive attention in adults and older children, is also related to aspects of executive attention in toddlers [103]. We found that haplotypes of the COMT gene [24] influenced both anticipatory looking and nesting cup activity at 18–20 months, both tasks thought to be related to executive attention. At 7 months COMT was also related to positive affect as reported by parents. The finding of a relation of COMT to positive affect together with the influence of this gene on executive attention at 18–20 months could provide a genetic link between reactive emotion and emotional regulation during early development. However, it is also possible that COMT’s relation to positive affect in infancy is mediated by regulatory aspects of executive attention. It is not unlikely that the earliest form of executive attention is regulation of emotion, and this may occur in parallel with regulation by orienting. Evidence for these ideas is mixed in our current study in that positive affect in infancy was unrelated to later effortful control but other studies have shown such a connection [84].
DRD4
The 7 repeat allele of the DRD4 gene has been linked to ADHD and to the temperamental quality of risk taking. Adults and children with the 7 repeat allele have been shown to be higher in the temperamental quality of risk taking and at greater risk for attention deficit disorder than those with smaller numbers of repeats [3, 95]. In one series of studies [95] it was found that the orienting of 2 month old infants as rated by parents and observed during inspection of toys was related to the presence of the 7 repeat allele of the dopamine 4 receptor gene. This allele appears to interact with a gene related to serotonin transmission (5HTT) to influence orienting.
Evidence that environment can have a strong influence in the presence of the 7 repeat alleles has been reported by others [4, 101]. The same group [5] has also performed a parenting training intervention, finding that the training decreased externalizing behavior, but only for those children with the DRD4 7 repeat allele. This finding is important because assignment to the training group was random, insuring that the result was not due to something about the parents other than the training.
In our longitudinal study we used cheek swabs to extract DNA and determined genetic variation in a dozen of the genes that have been connected to attention in adult studies [91]. The children had been seen when at 7 months, but the genotyping took place when they returned to the laboratory at about 2 years of age. At this age, we added an observation of caregiver-child interaction in which the children played with toys in the presence of one of their parents. Raters observed the caregiver/child interaction and rated the parents on five dimensions of parental quality according to a schedule developed by NICHD [63]: ratings included support, autonomy, stimulation, lack of hostility and confidence in the child. Although all of the parents were likely concerned and caring, they did differ in their scores, and we divided the combined scores at the median into two groups. One of the groups were considered to show a higher quality of parenting, and the other a lower quality.
We were interested in whether parent reports of the child’s impulsivity and risk taking would be related to the child’s carrying the 7-repeat allele of the DRD4 gene, the parent’s scores on parenting quality, or an interaction of gene and parenting. We found a strong interaction between genes and parenting. For children without the 7-repeat polymorphism, variations in parenting within the range we examined were unrelated to the children’s scores on impulsivity and risk taking. For children carrying the 7-repeat gene variant, however, variations in parenting quality mattered. Children with this allele and high quality parenting showed normal levels of risk taking, but those with lower quality parenting showed very high values for risk taking.
It seems paradoxical that the 7 repeat allele associated with developmental psychopathology (attention deficit hyperactivity disorder) is also under positive selective pressure in recent human evolution [25]. Why should an allele related to ADHD be positively selected? We think that positive selection of the 7 repeat allele could well arise from its sensitivity to environmental influences. Parenting provides training for children in the values favored by the culture in which they live. For example, Rothbart and colleagues [1] found that in Western culture effortful control appears to regulate negative affect (sadness and anger), while in China (at least in the1980s) it was found to regulate positive affect (outgoingness and enthusiasm). In recent years the genetic part of the nature by nurture interaction has been given a lot of emphasis, but if genetic variations are selected according to the sensitivity they give children to cultural influences, this could support a greater balance between genes and environment. Theories of positive selection in the DRD4 gene have stressed the role of sensation seeking in human evolution [104]. Our new findings do not contradict this emphasis, but suggest a form of explanation that could have even wider significance. It remains to be seen whether the other 300 genes estimated to show positive selection would also increase an individual’s sensitivity to variations in rearing environments. We will be examining additional longitudinal data to test these ideas further.
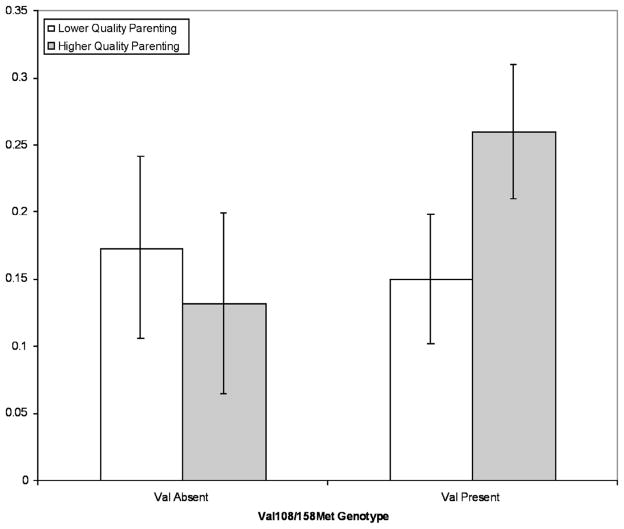
How could variation in genetic alleles lead to enhanced influence of cultural factors like parenting? The anterior cingulate receives input on both reward value and pain or punishment and this information is clearly important in regulating thoughts and feelings. Dopamine is the most important neuromodulator in these reward and punishment pathways. Thus changes in the availability of dopamine could enhance the influence of signals from parents related to reward and punishment. Another interaction has been reported between the serotonin transporter gene and parental social support on the temperamental dimension of behavioral inhibition or social fear [42]. To explain this interaction, Fox, Hane & Pine [43] argue that those children with a short form of the serotonin transporter gene who also have lower social support from parents show enhanced attention to threat and greater social fear. In our study of the DRD4, however, attention did not appear to be the mechanism by which the genetic variation influenced the child’s behavior. In our study there was no influence of the 7 repeat allele on executive attention, instead gene and environment interacted to influence the child’s behavior as observed by the caregiver. We also found that the COMT genotype showed an interaction with parenting quality and unlike the DRD4 it did operate through attention (see Figure 4). It is important to consider the multiple mechanisms by which genes may influence behavior. Clearly one important mechanism lies in the executive attention network we have been discussing in this chapter, but there must be other pathways that influence the same behavior.
Figure 4.
Haplotypes of the COMT gene interact with parenting to shape attention [from 103].
Parallel Control Systems
Overall these data support the important role of executive attention as a regulatory mechanism by 18–20 months of age as well as the role of orienting as a control mechanism at 6–7 months. It seems likely that the parallel activity of these two networks begins in infancy and continues to adulthood. The strong tendency for adults to look away as a self-regulatory strategy suggests a continued role for orienting even for adults. We hope our future longitudinal results and additional studies will provide information on the coordination between these two regulatory networks.
TRAINING ATTENTION AND SELF-REGULATION
We have shown that attention and self-regulation develop under the joint influence of genes and environment. This raises the issue of whether it would be possible to design experiences that would change attention and self-regulation. One possible way of doing this would be to find a way of improving the efficiency of the attention network through exercises [56,85,88]. We call this attention training. However, it may also be possible to develop a brain state which might be especially conducive to the ability to regulate other networks. We call this Attention State Training [98].
To examine further the role of training on attention networks we adopted a training method used by NASA to train monkeys for space travel [85, 89]. We developed and tested a five-day training intervention that uses computerized exercises. We tested the effect of training during the period of major development of executive attention, between 4 and 7 years of age [86,87,88]. We hoped to develop methods that could be used to observe improvements in conflict resolution following training. EEG data showed clear evidence of improvement in network efficiency in resolving conflict following training. The N2 component of the scalp recorded averaged electrical potential has been shown to arise in the anterior cingulate and is related to the resolution of conflict [20,102]. We found N2 differences between congruent and incongruent trials of the ANT in trained six-year-olds that resembled differences found in adults. In the four-year-olds, training seemed to influence more anterior electrodes that have been related to emotional control areas of the cingulate [12]. These data suggest that training altered the network for the resolution of conflict in the direction of being more like what is found in adults.
We also found a significantly greater improvement in intelligence in the trained group compared to the control children. This finding suggested that training effects had generalized to a measure of cognitive processing that is far removed from the training exercises. We did not observe changes in temperament over the course of the training, but this result was expected due to the short time elapsing between assessments.
A number of methods have been shown to improve attention in children [17, 22, 17, 86]. Many of these involve classroom training which may be easier to implement than individual exercises. We don’t have any expectation that our exercises are optimal or even better than other methods. The study of attention training as a whole suggests that networks can be shaped both in informal ways and by formal training. With the availability of imaging methods, it should be possible to design appropriate programs and activities for children of various ages and with various forms of difficulty. Our studies certainly support the importance of educational design in improving the lives of children. The practical application of brain research to the design of interventions for children has only recently been explored in some detail [99].
We have also used a training method called Integrated Body-Mind Training (IBMT) which is a form of meditation adapted from traditional Chinese Medicine [97]. This method seeks to develop an optimal state of balance between mind and body. We used 5 days of group practice, during which a coach answers questions and observes facial and body cues to identify those who are struggling with the method. The trainee concentrates on achieving a balanced state of mind while guided by the coach and the CD that teaches them to relax, adjust their breathing and use mental imagery. Because this approach is suitable for novices we hypothesized that a short period of training and practice might influence the efficiency of the executive attention network related to self-regulation [96, 97]. The control group was given a form of relaxation training very popular in the west
The two groups were given a battery of tests a week before training and immediately after the final training session. A standard computerized attention test measured orienting, alerting and the ability to resolve conflict (executive attention). The Attention Network Test (ANT). The Raven’s Standard Progressive Matrix, which is a standard culture fair intelligence test, an assay of mood state, the Profile of Mood States and a stress challenge of a mental arithmetic task followed by measures of cortisol and secretory immunoglobulin A (sig A). All of these are standard assays scored objectively by people blind to the experimental condition.
Our underlying theory was that IBMT training should improve functioning of the executive attention network. The experimental group showed significantly greater improvement than the control in the executive attention network, mood scales related to self-control and cortisol and immunoreactivity measures of stress to a mental arithmetic challenge [97]. More recently it has been found that the improvements involve a change of state. In that there is increased brain activity in areas related to the activation and control of the parasympathetic portion of the autonomic nervous systems [98].
The results that have been achieved with both attention training and attention state training argue that self-regulation may be modified at any age by the appropriate training method [98]. Important issues remain such as how long such improvement lasts and what are the underlying brain mechanisms changed by these various training methods.
AWARENESS
Although there here is a long tradition of the study of consciousness within Eastern and Western philosophy, cognitive neuroscience provides a somewhat new perspective on awareness and will both of which have been central to discussions of consciousness.
An important distinction in studies of awareness [53] is between general knowledge of our environment (ambient awareness) and detailed focal knowledge of a scene (focal awareness). As lay people we generally believe that we have full conscious awareness of our environment, even when our focal attention is upon our own internal thoughts. Experimental studies [76] show us how much this opinion is in error. In the study of “change blindness” when cues such as motion, that normally lead to a shift of attention, are suppressed, we have only a small focus for which we have full knowledge and even major semantic changes in the remainder of the environment are not reported.
Change blindness is closely related to the studies of visual search that have been prominent in the field of attention and involve an interaction between information in the ventral visual pathway processing object identity and information in the dorsal visual pathway that controls orienting to sensory information [29]. Visual search tasks have been important for examining what constraints attention provides to the nature of our awareness of a target event. There is clear evidence that attention to a visual event increases the brain activity associated with it. Most evidence arises from studies using event related electrical potentials with visual stimuli and these have clearly shown that early sensory components of the visual evoked potential P1 and N2 (80–150 millisec) are enlarged by the presence of attention [51].
In addition to changes in sensory systems, focal attention to the target of a visual search appears to involve the executive attention network (see Fig 1). Imaging studies suggest that whenever we bring to mind information, whether extracted from sensory input or from memory, we activate the executive attention network. In some studies a whole set of frontal areas become activated together, forming what is called a global workspace [19]. This global workspace becomes active about 300 millisec after a target event is presented. It provides the neural basis for the set of information on which a person is currently working in the process of problem solving.
The distinction between awareness and control (will) is traditional in studies of consciousness. However, one form of awareness, focal awareness, appears to involve the same underlying mechanism as involved in executive control. In this sense even though some forms of consciousness (e.g. ambient awareness) may have diverse sources within sensory specific cortex, there is also a degree of unity of the underlying mechanisms involved in focal awareness and conscious control. The distinction between focal and ambient factors in consciousness may help to clarify the sense of awareness present even when detailed accounts of the scene are not possible, as in change blindness.
GENERAL IMPLICATIONS
The goal of this paper was to examine the physical basis of the psychological concept of self-regulation. Self-regulation is clearly an important construct in developmental psychology which has significant implications for the successful socialization of children and their reproductive, scholarly and societal success. While self-regulation can be observed by parents and reliably measured it is difficult to see how one can determine the physical basis for such a concept.
The key to this problem rested in the ability to image the human brain when carrying our tasks that involve the conflict resolution that is a key computation of the executive attention network. Imaging has been able to discover brain networks involved in many tasks which humans carry out. Some of these are summarized in Figure 5.
Figure 5.

A list of areas of cognition and emotion for which neuroimaging studies have indicated neural networks involved. One selected study of the each network is referenced.
In the case of self-regulation, our effort to derive its physical basis depended upon the rather surprising relationship between a measure of executive attention derived from the Attention Network Test (ANT) and parental reports of effortful control. This allowed us to discover many nodes of the neural network that carries out resolution [34] of conflict and to discover the important role of anterior cingulate connectivity in influencing many brain areas involved in cognition and emotion. This discovery produced a putative brain circuitry underlying self-regulation. The anterior cingulate proved to have an important evolutionary history including the presence of special cells unique to areas involved in self-regulation and to humans and great apes. Specialization in this area could well be an important part of the unique human ability to delay gratification and to otherwise regulate their behavior in the service of long-term goals. This finding provides a renewed opportunity to explore differences between the human and other primate brains.
The association of genetic variations with individual differences in the efficiency of the network provides a further method for discovery of those genes that serve to build the nodes and connections of the network during development. This link provides a molecular perspective to the physical basis of a complex psychological construct. The ability to find candidate genes related to the attention network rested on pharmacological findings linking different neuromodulators to the various networks. This opportunity is not present in a majority of the networks shown in Figure 5. However, other methods such as the use of full genome scans, the study of brain pathologies, and the use of hints from comparative studies of animals can be used to provide appropriate candidate genes for other networks.
What illumination will this molecular perspective provide? The conservation of genetic mechanisms along the phylogenetic scale provides a basis for relating human evolution to the evolution of our species. As the results of studies of the DRD4 gene suggest, human evolution may play a central role in behavior. Evidence for positive selection of alleles of this gene within recent human history, have led us to propose the possibility that alleles increasing the child’s influence by cultural factors like parenting can provide for improved reproductive success and thus be enhanced by positive selection. This connection between genetic variation and cultural influence shows that the molecular perspective can deepen our understanding of human nature in ways that may be unanticipated. While we remain very far from a deep understanding of the physical basis of many psychological concepts central to human nature the tools currently available appear adequate foster this effort.
Footnotes
The research reported in this paper was supported in part by National Institute of Human Development under grant HD38051. The authors are grateful to Yalchin Abdullaev, Charo Rueda, Brad Sheese,, Yiyuan Tang and other members of the Attention and Temperament Laboratory for their help with the research reported here.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahadi SA, Rothbart MK, Ye R. Children’s Temperament in the U.S. and China: Similarities and differences. European Journal of Personality. 1993;7:359–378. [Google Scholar]
- 2.Allman JJ, Watson KK, Tetreault NA, Hakeem AY. Intuition and autism: A possible role for Von Economo neurons. Trends in Cognitive Science. 2005;9:367–373. doi: 10.1016/j.tics.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 3.Auerbach J, Geller V, Letzer S, Shinwell E, Levine J, Belmaker RH, Ebstein RP. Dopamine D4 receptor (D4DR) and serotonin transporter promoter (5-HTTLPR) polymorphisms in the determination of temperament in two month old infants. Molecular Psychiatry. 1999;4:369–374. doi: 10.1038/sj.mp.4000531. [DOI] [PubMed] [Google Scholar]
- 4.Bakermans-Kranenburg MJ, van IJzendoorn MH. Gene-environment interaction of the dopamine D4 receptor (DRD4) and observed maternal insensitivity predicting externalizing behavior in preschoolers. Developmental Psychobiology. 2006;48:406–409. doi: 10.1002/dev.20152. [DOI] [PubMed] [Google Scholar]
- 5.Bakermans-Kranenburg MJ, van IJzendoorn MH, Pijlman FTA, Mesman J, Juffer F. Experimental evidenc for differential susceptibility: dopamine D4 Receptor Polymorphism (DRD4 VNTR) moderates intervention effects on toddlers externalizing behavior in a randomized controlled trial. Developmental Psychology. 2008;44:293–300. doi: 10.1037/0012-1649.44.1.293. [DOI] [PubMed] [Google Scholar]
- 6.Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. Journal of Neuroscience. 2001;21:RC165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger A, Tzur G, Posner MI. Infant babies detect arithmetic error. Proceedings of the National Academy of Science, USA. 2006;103:12649–12553. doi: 10.1073/pnas.0605350103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blair RJR, Morris JS, Frith CD, Perrett DI, Dolan RJ. Dissociable neural responses to facial expression of sadness and anger. Brain. 1999;1222:883–893. doi: 10.1093/brain/122.5.883. [DOI] [PubMed] [Google Scholar]
- 9.Blasi G, Mattay GS, Bertolino A, Elvevåg B, Callicott JH, Das S, Kolachana BS, Egan MF, Goldberg TE, Weinberger DR. Effect of cCatechol-O-Methyltransferase val158met genotype on attentional control. Journal of Neuroscience. 2005;25:5038–5045. doi: 10.1523/JNEUROSCI.0476-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Botvinick MM. Conflict monitoring and decision making: Reconciling two perspectives on anterior cingulate function. Journal of Cognitive, Affective and Behavioral Neuroscience. 2007;7:356–366. doi: 10.3758/cabn.7.4.356. [DOI] [PubMed] [Google Scholar]
- 11.Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 12.Bush G, Luu P, Posner MI. Cognitive and emotional influences in the anterior cingulate cortex. Trends in Cognitive Science. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 13.Carlson ST, Moses LJ. Individual differences in inhibitory control in children’s theory of mind. Child Development. 2001;72:1032–1053. doi: 10.1111/1467-8624.00333. [DOI] [PubMed] [Google Scholar]
- 14.Chang F, Burns BM. Attention in preschoolers: Associations with effortful control and motivation. Child Development. 2005;76:247–263. doi: 10.1111/j.1467-8624.2005.00842.x. [DOI] [PubMed] [Google Scholar]
- 15.Clohessy AB, Posner MI, Rothbart MK. Development of the functional visual field. Acta Psychologica. 2001;106:51–68. doi: 10.1016/s0001-6918(00)00026-3. [DOI] [PubMed] [Google Scholar]
- 16.Crottaz-Herbette S, Mennon V. Where and when the anterior cingulate cortex modulates attentional response: Combined fMRI and ERP evidence. Journal of Cognitive Neuroscience. 2006;18:766–780. doi: 10.1162/jocn.2006.18.5.766. [DOI] [PubMed] [Google Scholar]
- 17.Coull JT, Frith CD, Buchel C, Nobre AC. Orienting attention in time: behavioural and neuroanatomical distinction between exogenous and endogenous shifts. Neuropsychologia. 2000;38:808–819. doi: 10.1016/s0028-3932(99)00132-3. [DOI] [PubMed] [Google Scholar]
- 18.Dehaene S. The Number Sense. Oxford University Press; Oxford, UK: 1997. [Google Scholar]
- 19.Dehaene S. In: Attention and Performance XX: Functional Brain Imaging of Visual Cognition. Kanwisher N, Duncan J, editors. Oxford University Press; Oxford: pp. 205–224. [Google Scholar]
- 20.Dehaene S, Posner MI, Tucker DM. Localization of a neural system for error detection and compensation. Psychological Science. 1994;5:303–305. [Google Scholar]
- 21.Dennett D. Are we explaining consciousness yet? Cognition. 2001;79:221–237. doi: 10.1016/s0010-0277(00)00130-x. [DOI] [PubMed] [Google Scholar]
- 22.Diamond A, Barnett S, Thomas J, Munro S. Preschool improves cognitive control. Science. 2007;30:1387–1388. doi: 10.1126/science.1151148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diamond A, Briand L, Fossella J, Gehlbach L. Genetic and neurochemical modulation of prefrontal cognitive functions in children. American Journal of Psychiatry. 2005;161:125–132. doi: 10.1176/appi.ajp.161.1.125. [DOI] [PubMed] [Google Scholar]
- 24.Diatchenko L, Slade GD, Nackley AG, Bhalang K, Sigurdsson A, Belfer I, Goldman D, Xu K, Shabalina SA, Shagin D, Max MB, Makarov SS, Maixner W. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Human Molecular Genetics. 2005;14:135–143. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- 25.Ding YC, Chi HC, Grady DL, Morishima A, Kidd JR, Kidd KK, Flodman P, Spence MA, Schuck S, Swanson JM, Zhang YP, Moyzis RK. Evidence of positive selection acting at the human dopamine receptor D4 gene locus. Proceedings of the National Academy of Sciences of the USA. 2002;99:309–314. doi: 10.1073/pnas.012464099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dosenbach NUF, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RAT, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE. Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences of the USA. 2007;104:1073–1978. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drabant EM, Hariri AR, Meyer-Lindenberg A, Munoz KE, Mattay VS, Kolachana BS, et al. Catechol O-methyltransferase val158met genotype and neural mechanisms related to affective arousal and regulation. Archives of General Psychiatry. 2006;63:1396–1406. doi: 10.1001/archpsyc.63.12.1396. [DOI] [PubMed] [Google Scholar]
- 28.Drevets WC, Raichle ME. Reciprocal suppression of regional cerebral blood flow during emotional versus higher cognitive processes: Implications for interactions between emotion and cognition. Cognition and Emotion. 1998;12:353–385. [Google Scholar]
- 29.Driver J, Eimer M, Macaluso E. In: Attention and Performance XX: Functional Brain Imaging of Visual Cognition. Kanwisher N, Duncan J, editors. Oxford University Press; Oxford: pp. 267–300. [Google Scholar]
- 30.Duncan J, Seitz RJ, Kolodn J, Bor D, Herzog H, Ahmed A, Newell FN, Emslie H. A neural basis for general intelligence. Science. 2000;289:457–460. doi: 10.1126/science.289.5478.457. [DOI] [PubMed] [Google Scholar]
- 31.Ellis E, Rothbart MK, Posner MI. Individual differences in executive attention predict self-regulation and adolescent psychosocial behaviors. Anals of the New York Academy of Sciences. 2004;1031:337–340. doi: 10.1196/annals.1308.041. [DOI] [PubMed] [Google Scholar]
- 32.Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: A role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 33.Fair DA, Dosenbach NUF, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. Development of distinct control networks through segregation and integration. Proceedings of the National Academy of Sciences of the USA. 2007;104:13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan J, Flombaum JI, McCandliss BD, Thomas KM, Posner MI. Cognitive and brain consequences of conflict. Neuro Image. 2003;18:42–57. doi: 10.1006/nimg.2002.1319. [DOI] [PubMed] [Google Scholar]
- 35.Fan J, Fossella JA, Summer T, Wu Y, Posner MI. Mapping the genetic variation of executive attention onto brain activity. Proceedings of the National Academy of Science USA. 2003;100:7406–11. doi: 10.1073/pnas.0732088100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. Neuroimage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 37.Fan J, McCandliss BD, Sommer T, Raz M, Posner MI. Testing the efficiency and independence of attentional networks. Journal of Cognitive Neuroscience. 2002;3:340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- 38.Fan J, Wu Y, Fossella J, Posner MI. Assessing the heritability of attentional networks. BioMed Central Neuroscience. 2001;2:14. doi: 10.1186/1471-2202-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fernandez-Duque D, Black SE. Attentional networks in normal aging and Alzheimer’s disease. Neuropsychology. 2006;20:133–143. doi: 10.1037/0894-4105.20.2.133. [DOI] [PubMed] [Google Scholar]
- 40.Fonagy P, Target M. Early intervention and the development of self-regulation. Psychoanalytic Quarterly. 2002;22:307–335. [Google Scholar]
- 41.Fossella J, Sommer T, Fan J, Wu Y, Swanson JM, Pfaff DS, Posner MI. Assessing the molecular genetics of attention networks. BioMed Central Neuroscience. 2002;3:14. doi: 10.1186/1471-2202-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fox NA, Nichols KE, Henderson HA, Rubin KH, Schmidt LA, Hamer D, Ernst M, Pine DS. Evidence for a gene-environment interaction in predicting behavioral inhibition in middle childhood. Psychological Science. 2005;16:921–926. doi: 10.1111/j.1467-9280.2005.01637.x. [DOI] [PubMed] [Google Scholar]
- 43.Fox NA, Hane AA, Pine DS. Plasticity for affective neurocircuitry - How the environment affects gene expression. Current Directions in Psychological Science. 2007;16:1–5. [Google Scholar]
- 44.Fink GR, Markowitsch HJ, Reinkemeier Hl, Bruckbauer T, Kessler J, Heiss WD. Cerebral representation of one’s own past: Neural networks involved in autobiographical memory. Journal of Neurosceince. 1996;16:4275–4282. doi: 10.1523/JNEUROSCI.16-13-04275.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gerardi-Caulton G. Sensitivity to spatial conflict and the development of self-regulation in children 24–36 months of age. Developmental Science. 2000;3:397–404. [Google Scholar]
- 46.Green AE, Munafo MR, DeYoung CG, Fossella JA, Fan J, Gray JR. Using genetic data in cognitive neuroscience: From growing pains to genuine insights. Nature Neuroscience Review. 2008;9:710–720. doi: 10.1038/nrn2461. [DOI] [PubMed] [Google Scholar]
- 47.Gonzalez C, Fuentes LJ, Carranza JA, Estevez AF. Temperament and attention in the self-regulation of 7-year-old children. Personality and Individual Differences. 2001;30:931–946. [Google Scholar]
- 48.Grill-Spector K. In: Functional neuroimaging of visual cognition: Attention and Performance XX. Kanwisher N, Duncan J, editors. Oxford University Press; Oxford: 2004. pp. 169–193. [Google Scholar]
- 49.Harman C, Rothbart MK, Posner MI. Distress and attention interactions in early infancy. Motivation and Emotion. 1997;21:27–43. [Google Scholar]
- 50.Haxby JV. In: Functional neuroimaging of visual cognition: Attention and Performance XX. Kanwisher N, Duncan J, editors. Oxford University Press; Oxford: 2004. pp. 83–97. [Google Scholar]
- 51.Hillyard SA, Di Russo F, Martinez A. In: Functional neuroimaging of visual cognition: Attention and Performance XX. Kanwisher N, Duncan J, editors. Oxford University Press; Oxford: 2004. pp. 381–390. [Google Scholar]
- 52.Johnson SC, Schmitz TW, Kawahara-Baccus TN, Rowley HA, Alexander AL, Lee JH, Davidson RJ. The cerebral response during subjective choice with and without self-reference. Journal of Cognitive Neuroscience. 2005;17:1897–1906. doi: 10.1162/089892905775008607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iwasaki S. Spatial attention and two modes of visual consciousness. Cognition. 1993;49:211–233. doi: 10.1016/0010-0277(93)90005-g. [DOI] [PubMed] [Google Scholar]
- 54.Kanske P. Exploring executive attention in emotion. Dresden; Sachsisches Digitaldruckzentrum: 2008. [Google Scholar]
- 55.Karayiorgou M, Altemus M, Galke BL, Goldman D, Murphy DL, Ott J, Gogos JA. Genotype determining low catechol-O-methyltransferase activity as a risk factor for obsessive-compulsive disorder. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:4572–4575. doi: 10.1073/pnas.94.9.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klingberg T, Forssberg H, Westerberg H. Training of working memory in children with ADHD. Journal of Clinical and Experimental Neuropsychology. 2002;24:781–791. doi: 10.1076/jcen.24.6.781.8395. [DOI] [PubMed] [Google Scholar]
- 57.Knutson B, Fong GW, Bennett SM, Adams CM, Homme D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: Characterization with rapid event-related fMRI. Neuroimage. 2003;18:263–272. doi: 10.1016/s1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- 58.Koch C, Tsuchiya N. Attention and consciousness: Two distinct brain processes. Trends in Cognitive Science. 2007;11:16–22. doi: 10.1016/j.tics.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 59.Kochanska G. Children’s temperament, mothers’ discipline, and security of attachment: Multiple pathways to emerging internalization. Child Development. 1995;66:597–615. [Google Scholar]
- 60.Kochanska G, Murray K, Jacques TY, Koenig AL, Vandegeest KA. Inhibitory control in young children and its role in emerging internationalization. Child Development. 1996;67:490–507. [PubMed] [Google Scholar]
- 61.Levitin D. This is your brain on music. Penguin Press; London: 2006. [Google Scholar]
- 62.Mottaghy FM, Willmes K, Horwitz B, Muller HW, Krause BJ, Sturm W. Systems level modeling of a neuronal network subserving intrinsic Alertness. Neuroimage. 2006;29:225. doi: 10.1016/j.neuroimage.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 63.NICHD Early Child Care Research Network. The NICHD Study of Early Child Care: A comprehensive longitudinal study of young children’s lives. 1993. ERIC Document Reproduction Service No. ED3530870. [Google Scholar]
- 64.Ochsner KN, Ludlow DH, Knierim K, Hanelin J, Ramachandran T, Glover GC, Mackey SC. Neural correlates of individual differences in pain-related fear and anxiety. Pain. 2006;129:69–77. doi: 10.1016/j.pain.2005.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ochsner KN, Kossyln SM, Cosgrove GR, Cassem EH, Price BH, Nierenberg AA, Rauch SL. Deficits in visual cognition and attention following bilateral anterior cingulotomy. Neuropsychologia. 2001;39:219–230. doi: 10.1016/s0028-3932(00)00114-7. [DOI] [PubMed] [Google Scholar]
- 66.Parasuraman R, Greenwood PM, Kumar R, Fossella J. Beyond heritability – Neurotransmitter genes differentially modulate visuospatial attention and working memory. Psychological Science. 2005;16:200–207. doi: 10.1111/j.0956-7976.2005.00804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pidoplichko VI, DeBiasi M, Williams JT, Dani JA. Nicotine activates and desensitizes midbrain dopamine neurons. Nature. 1997;390:401–404. doi: 10.1038/37120. [DOI] [PubMed] [Google Scholar]
- 68.Posner MI. Attention: The mechanism of consciousness. Proceedings of the National Academy of Sciences, USA. 1994;91:7398–7402. doi: 10.1073/pnas.91.16.7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Posner MI, Fan J. In: Topics in Integrative Neuroscience. Pomerantz JR, editor. Cambridge University Press; New York New York: 2008. pp. 31–61. [Google Scholar]
- 70.Posner MI, Raichle ME. Images of Mind. Scientific American Library; New York: 1996. [Google Scholar]
- 71.Posner MI, Rothbart MK. Educating the Human Brain. APA Books; Washington DC: 2007. [Google Scholar]
- 72.Posner MI, Rothbart MK, Sheese BE, Tang Y. The anterior cingulate gyrus and the mechanisms of self regulation. Journal of Cognitive, Affective and Social Neuroscience. 2007;7:391–395. doi: 10.3758/cabn.7.4.391. [DOI] [PubMed] [Google Scholar]
- 73.Posner MI, Rothbart MK, Sheese BE. Attention genes. Developmental Science. 2007;10:24–29. doi: 10.1111/j.1467-7687.2007.00559.x. [DOI] [PubMed] [Google Scholar]
- 74.Posner MI, Sheese BE, Odludas Y, Tang Y. Analyzing and shaping neural networks of attention. Neural Networks. 2006;19:1422–1429. doi: 10.1016/j.neunet.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 75.Raz A, Buhle J. Typologies of attentional networks. Nature, Neuroscience Reviews. 2006;7:367–379. doi: 10.1038/nrn1903. [DOI] [PubMed] [Google Scholar]
- 76.Rensink RA, O’Regan JK, Clark JJ. To see or not to see: The need for attention to perceive changes in scenes. Psychological Science. 1997;8:368–373. [Google Scholar]
- 77.Reuter M, Hennig J. Association of the functional catechol-O-methyltransferase Val158 Met polymorphism with the personality trait of extraversion. NeuroReport. 2005;16:1135–1138. doi: 10.1097/00001756-200507130-00020. [DOI] [PubMed] [Google Scholar]
- 78.Rigbi A, Kanyas K, Yakir A, Greenbaum L, Pollak Y, Ben-Asher E, Lancet D, et al. Why do young women smoke? V. Role of direct and interactive effects of nicotinic cholinergic receptor gene variation on neurocognitive function. Genes, Brain, and Behavior. 2008;7:164–172. doi: 10.1111/j.1601-183X.2007.00329.x. [DOI] [PubMed] [Google Scholar]
- 79.Rothbart MK, Ahadi SA, Hershey KL. Temperament and social behavior in childhood. Merrill-Palmer Quarterly. 1994;40:21–39. [Google Scholar]
- 80.Rothbart MK, Ahadi SA, Hershey K, Fisher P. Investigations of temperament at three to seven years: The Children’s Behavior Questionnaire. Child Development. 2001;72:1394–1408. doi: 10.1111/1467-8624.00355. [DOI] [PubMed] [Google Scholar]
- 81.Rothbart MK, Bates JE. Temperament in children’s development. In: Damon W, Lerner R, Eisenberg N, editors. Handbook of Child Psychology: Vol. 3, Social, Emotional, and Personality Development. New York: Wiley; 2006. pp. 99–166. [Google Scholar]
- 82.Rothbart MK, Derryberry D. In: Advances in developmental psychology. Lamb ME, Brown AL, editors. Erlbaum; Hillsdale, NJ: 1981. pp. 37–86. [Google Scholar]
- 83.Rothbart MK, Ellis LK, Rueda MR, Posner MI. Developing mechanisms of effortful control. Journal of Personality. 2003;71:1113–1143. doi: 10.1111/1467-6494.7106009. [DOI] [PubMed] [Google Scholar]
- 84.Rothbart MK, Rueda MR. In: Developing Individuality in the Human Brain: A Festschrift Honoring Michael I. Posner. Mayr U, Awh E, Keele SW, editors. American Psychological Association; Washington, DC: 2003. [Google Scholar]
- 85.Rueda MR, Checa P, Santonja M. Training executive attention in preschoolers: Lasting effects and transfer to affective self-regulation. Paper presented at the 2008 Annual Meeting of the Cognitive Neuroscience Society; San Francisco, CA. [Google Scholar]
- 86.Rueda MR, Fan J, Halparin J, Gruber D, Lercari LP, McCandliss BD, Posner MI. Development of attention during childhood. Neuropsychologia. 2004;42:1029–1040. doi: 10.1016/j.neuropsychologia.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 87.Rueda MR, Posner MI, Rothbart MK. In: Handbook of Self-regulation: Research, Theory, and Applications, New York. Baumeister RF, Vohs KD, editors. Guilford Press; 2004. pp. 283–300. [Google Scholar]
- 88.Rueda MR, Rothbart MK, McCandliss BD, Saccamanno L, Posner MI. Training, maturation and genetic influences on the development of executive attention. Proceedings of the National Academy of Sciences of the USA. 2005;102:14931–14936. doi: 10.1073/pnas.0506897102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rumbaugh DM, Washburn DA. Intelligence of apes and other rational beings. Yale University Press; New Haven: 2003. [Google Scholar]
- 90.Sheese BE, Rothbart MK, Posner MI, White LK, Fraundorf SH. Executive attention and self regulation in infancy. Infant Behavior and Development. 2008;31:501–510. doi: 10.1016/j.infbeh.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 91.Sheese BE, Voelker PM, Rothbart MK, Posner MI. Parenting quality interacts with genetic variation in Dopamine Receptor DRD4 to influence temperament in early childhood. Development and Psychopathology. 2007;19:1039–1046. doi: 10.1017/S0954579407000521. [DOI] [PubMed] [Google Scholar]
- 92.Shelton AL, Gabrieli JDE. Neural correlates of encoding space from route and survey perspectives. Journal of Neuroscience. 2002;22:2711–2717. doi: 10.1523/JNEUROSCI.22-07-02711.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Smith EE, Jonides J, Marshuetz G, Koeppe RA. Components of verbal working memory. Proceedings of the National Academy of Sciences of the USA. 1998;95:876–882. doi: 10.1073/pnas.95.3.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sridharan R, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of the National Academy of Sciences of the USA. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Swanson J, Oosterlaan J, Murias M, Schuck S, Flodman P, Spence MA, Wasdell M, Ding Y, Chi H, Smith M, Mann M, Carlson C, Kennedy MJ, Sergeant J, Leung P, Zhang Y, Sadeh A, Chen C, Moyzis R, Posner MI. Attention deficit/hyperactivity disorder children with a 7-repeat allele of the dopamine receptor D4 gene have extreme behavior but normal performance on critical neuropsychological tests of attention. Proceedings of National Academy of Sciences. 2000;97:4754–4759. doi: 10.1073/pnas.080070897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tang Y-Y, Hu B, Lu Q, Li J, Fan Y, Wang Y, Tan L, Cui Y, Posner MI. IBMT long-term effects on brain default model. in process. [Google Scholar]
- 97.Tang YY, Ma Y, Wang J, Fan Y, Feng S, Lu Q, Yu Q, Sui D, Rothbart MK, Fan M, Posner MI. Short term meditation training improves attention and self-regulation. Proceedings of the National Academy of Sciences of the USA. 2007;104:17152–17156. doi: 10.1073/pnas.0707678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tang YY, Posner MI. Attention training and attention state training, Trends in Cognitive Science. doi: 10.1016/j.tics.2009.01.009. in press. [DOI] [PubMed] [Google Scholar]
- 99.Tough P. Whatever it takes. Houghton Mifflin; Boston: 2008. [Google Scholar]
- 100.Ungerleider LG, Courtney SM, Haxby HV. A neural system for human visual working memory. Proceedings of the National Academy of Sciences of the USA. 1998;95:883–890. doi: 10.1073/pnas.95.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.van IJzendoorn MH, Bakermans-Kranenburg MJ. DRD4 7-repeat polymorphism moderates the association between maternal unresolved loss or trauma and infant disorganization. Attachment and Human Development. 2006;8:291–307. doi: 10.1080/14616730601048159. [DOI] [PubMed] [Google Scholar]
- 102.van Veen V, Carter CS. The timing of action-monitoring processes in the anterior cingulate cortex. Journal of Cognitive Neuroscience. 2002;14:593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- 103.Voelker P, Sheese BE, Rothbart MK, Posner MI. Variations in COMT gene interact with parenting to influence attention in early development. Neuroscience. doi: 10.1016/j.neuroscience.2009.05.059. in process. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang ET, Kodama G, Baldi P, Moyzis RK. Global landscape of recent inferred Darwinan selection for Homo sapiens. Proceedings of the National Academy of Science. 2006;103:135–140. doi: 10.1073/pnas.0509691102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Washburn DA. Stroop-like effects for monkeys and humans: Processing speed or strength of association. Psychological Science. 1994;5:375–379. doi: 10.1111/j.1467-9280.1994.tb00288.x. [DOI] [PubMed] [Google Scholar]
- 106.Whittle SL. Unpublished doctoral dissertation. University of Melbourne; Australia: 2007. The neurobiological correlates of temperament in early adolescents. [Google Scholar]
- 107.Winterer G, Musso F, Konrad A, Vucurevuc G, Stoeter P, Sander T, Gallinat J. Association of attentional network function with exon 5 variations of the CHRNA4 gene. Human Molecular Genetics. 2007;16:2165–2174. doi: 10.1093/hmg/ddm168. [DOI] [PubMed] [Google Scholar]
- 108.Wynn K. Addition and subtraction by human infants. Nature. 1992;358:749–750. doi: 10.1038/358749a0. [DOI] [PubMed] [Google Scholar]
- 109.Zilles K. In: From Monkey Brain to Human Brain. Dehaene S, Duhamel J-R, Hauser MD, Rizzolatti G, editors. MIT Press; Cambridge: 2005. pp. 41–56. [Google Scholar]