Abstract
This work examines the immobilization of myoglobin from horse skeletal muscle in hydrophilic polymer networks. Due to specific changes in the spectroscopic properties of hemoproteins during ligand binding, they could be employed in optical sensing devices. Two immobilization techniques were considered: imbibition and entrapment. Anionic hydrogels composed of methacrylic acid (MAA), cationic hydrogels composed of dimethylamino ethyl methacrylate (DMAEM), and neutral hydrogels composed of poly(ethylene glycol) monomethyl ether monomethacrylate (PEGMA; molecular weight = 200, 400, or 1000), all crosslinked with poly(ethylene glycol) dimethacrylate (PEGDMA) (molecular weight = 200, 600, or 1000), were synthesized by free-radical solution polymerization. By the imbibition method, MAA-based hydrogels incorporated the highest amount of myoglobin in comparison with PEGMA or DMAEM polymers. The evaluation of the correlation length of the networks revealed that MAA hydrogels had the highest correlation length in comparison with PEGMA-containing matrices or DMAEM hydrogels. Release experiments from MAA hydrogels at pHs 5.8 and 7.0 showed that the solute-transport mechanism was a combination of Fickian and chain relaxation diffusion. Myoglobin-loaded MAA hydrogels retained their heme reactivity after the immobilization process. The release of myoglobin incorporated by entrapment in MAA–PEGDMA hydrogels was highly influenced by the chain relaxation process. The diffusion coefficients of myoglobin incorporated by entrapment into anionic hydrogels were 2 orders of magnitude smaller (~10–13) than those for myoglobin incorporated by imbibition (10–11), both evaluated at pH 7.0. Substrate binding studies indicated that the protein biological activity was not compromised in those hydrogels loaded by the imbibition method, whereas prepolymeric solutions showed detrimental effects on protein stability.
Keywords: biological applications of polymers, diffusion, proteins, sensors
INTRODUCTION
Immobilized hemoproteins have been used in electrochemical and optical sensing devices. They are considered redox proteins; this refers to their ability to undergo electron transfer. Increasing interest has encouraged the study of protein electrochemistry to improve the understanding of the mechanisms of electron exchange between proteins and electrodes.1,2 Therefore, hemoproteins have been investigated for the development of highly selective and sensitive electrochemical biosensors.3 For electrochemical applications, direct electron transfer between hemoproteins and electrodes has been accomplished by the incorporation of proteins into films (surfactant films, polyion–surfactant or clay–surfactant composite films, and amphiphilic polymers),4 SP Sephadex,5 and gold nanoparticles.6–9
Hemoproteins are also well known for their optical properties, which change during the binding of the corresponding ligands.10 These optical characteristics have been investigated for the development of optical biosensors to detect dissolved oxygen (DO), nitric oxide (NO), and carbon monoxide (CO).11,12 In optical applications, sol–gels have been widely investigated as immobilization substrates.13 The entrapment of hemoproteins in sol–gel matrices has resulted in the retention of the spectroscopic properties and chemical functions of these proteins.10 However, a slight perturbation of the protein conformation has been observed. Raman studies on myoglobin have proved that entrapment in a sol–gel matrix hinders the conformational fluctuations necessary to open the heme pocket.10 Sol–gel immobilization techniques have demonstrated a high protein loading, control of the polarity and porosity of the matrix, and decreased protein release.9 Some disadvantages of the sol–gel methods are the difficulty of predicting the properties of the resultant glass, the aging of the material, and protein denaturation with time, which causes problems with sensor calibration.14
Hydrogels have emerged as alternatives to provide biomolecules with environments that could protect their biological function and allow their use in biosensor technology. They are transparent, making them appropriate to be used in optical sensing applications. Hydrogels have been extensively employed in the delivery of drugs, peptides, and proteins.15 These applications indicate that hydrogels could create suitable environments that offer protection from harsh external media and improve protein stability. 16,17 These materials can display variations in their swelling behavior, network structure, permeability, or mechanical strength as a result of different stimuli, such as changes in the pH, ionic strength of the surrounding media, temperature, electric or magnetic fields, and concentration.18,19
Hydrogels can be designed to possess precise features through the manipulation of specific properties. The degree of crosslinking, which determines the molecular weight between crosslinks, is a factor that can be used to modify mechanical strength, swelling ratio, and diffusion characteristics of hydrogels.20
The incorporation of drugs, peptides, or proteins inside hydrogels has been frequently performed by two methods. One method consists of entrapping the molecule inside the hydrogel during the polymerization. The other method is imbibition.21 Forming the hydrogel in the presence of the molecule can be an effective encapsulation approach, but polymerization conditions could provoke deleterious effects on molecule properties.22 Besides, removing the impurities and byproducts produced from the gel formation may produce leaching of some of the incorporated molecules. Moreover, side reactions between the hydrogel and the molecule may occur.23 In the case of protein incorporation by imbibition, the interactions between the protein and the hydrophilic polymer have the potential to produce irreversible adsorption.24 However, large proteins could be kept out from the hydrogel network by size exclusion.23
Hydrogels have been investigated for the development of long-term implantable biosensors.25 They are considered biocompatible and have been shown to act as effective antifouling sensor components.26 Glucose-responsive insulin-release devices couple two applications of hydrogels: sensing and drug delivery. These systems involve the immobilization of glucose oxidase in a pH-responsive hydrogel enclosed in a saturated insulin solution.17 For this application, hydrogels built from polyacrylamide, poly(2-hydroxyethyl methacrylate), poly(dimethylamino ethyl methacrylate), poly(diethylamino ethyl methacrylate), poly(ethylene glycol), and copolymers have been investigated.27
This project focused on the application of hydrophilic polymer networks as immobilization supports for horse skeletal myoglobin. Horse myoglobin was studied not only because it is commercially available but also because it shows an affinity toward interesting molecules such as oxygen, CO, cyanide, and NO.11,12 Three different polymer morphologies were investigated as protein supports and evaluated in their capacity for better immobilization by imbibition or entrapment techniques. The first was methacrylic acid (MAA) crosslinked with poly(ethylene glycol) dimethacrylate (PEGDMA). The second was poly (ethylene glycol) monomethyl ether monomethacrylate (PEGMA) crosslinked with PEGDMA. The third morphology was dimethylamino ethyl methacrylate (DMAEM) crosslinked with PEGDMA. By means of these polymer networks, it was possible to determine which kind of hydrogel—neutral, anionic, or cationic—could provide enhanced support for myoglobin without significantly affecting the biological activity.
The aforementioned morphologies were characterized by the evaluation of the correlation length and the partition coefficient. After the immobilization of myoglobin was accomplished by the two proposed techniques, release studies were performed. The capacity of the immobilized myoglobin to change its oxidation state and to bind CO was assessed during activity studies. Getting a better understanding of the relationships between the hydrogel structure and exhibited properties is critical for advances in the development and rational design of hydrogels with specific characteristics that allow permanent protein immobilization.28 The capacity of hydrogels to maintain the activity and to provide long-term stability for proteins will determine the feasibility of this material as biomolecule carriers in monitoring systems.
EXPERIMENTAL
Hydrogel synthesis
Two ionic monomers were considered: anionic MAA and cationic DMAEM. The neutral polymers employed were PEGMAs with different tethered chain molecular weights: 200, 400, and 1000 g/mol. PEGDMA was employed as the crosslinker, and the following molecular weights were considered: 200, 600, and 1000 g/mol. The monomers and crosslinker agent were purchased from Polyscience, Inc. (Warrington, PA), and were used as received, with the exception of MAA, which was purified to remove the inhibitor agent hydroquinone monomethyl ether by ion exchange (Dihibit resin, Polyscience). The photoinitiator was 1-hydroxy cyclohexyl phenyl ketone at 99% (Sigma–Aldrich, Milwaukee, WI). A 1 : 1 (w/w) mixture of ethanol (EtOH; Fisher Scientific, Fair Lawn, NJ) and deionized (DI) water was employed to dilute the polymerization mixture. The hydrogels were prepared with the following monomer/cross-linker molar ratio: anionic, 97 : 3; cationic, 95 : 5; and neutral, 50 : 50. The molar ratio used to prepare the hydrogels depended on the desirable characteristics that were expected from the hydrogels, such as transparence and mechanical resistance. The dilution ratio for the anionic and neutral hydrogels was 1 : 1, and 3 : 2 for cationic hydrogels. Dilution was necessary to obtain mechanically stable films. The initiator percentage was 0.1% (w/w) of the polymer mixture.
The mixture of the monomer, crosslinker, solvent, and initiator was prepared in an amber bottle (Sigma–Aldrich) placed in an inert chamber and purged for 20 min with nitrogen to remove the excess of oxygen, which could interfere with the free-radical polymerization reaction. The polymerization solution was introduced by capillarity between two precleaned microscope slides (Fisher Scientific) separated by Teflon spacers of 0.030 in. (Small Parts, Inc., Miami Lakes, Fl) and located under ultraviolet light (UV; the light intensity fluctuated from 30 to 40 mW/cm2) for approximately 10 min. The resulting polymer was washed, depending on the ionic nature of the hydrogel. Anionic polymers were washed for 2 days with DI water followed by a wash with a 0.1M sodium phosphate buffer solution (pH 7.0) for 2 more days to promote swelling and completely eliminate any prepolymeric residue. Finally, the polymer was washed with DI water for 2 more days. The buffer and the DI water were changed every 24 h. For cationic hydrogels, the buffer was a 0.1M sodium phosphate (pH 5.8). Neutral hydrogels were washed with DI water. After washing, all hydrogels were cut into 1 in. diameter disks.
Protein immobilization by imbibition
Myoglobin was dissolved in DI water. The concentration of the resulting solution was estimated by measuring the UV absorbance at the characteristic peak in the Soret band (408 nm) that these proteins exhibit (Powerwave X-I microplate reader, Bio-Tek Instruments, Inc., Winooski, VT). The concentration of myoglobin ranged from approximately 0.25 to 0.5 mM. The extinction coefficient for myoglobin from horse at 408 nm is 188 mM−1 cm−1.29,30 The volume of the membrane was measured with a density kit coupled to an analytical balance (Voyager balance, Ohaus Corp., Pine Brook, NJ) before it was placed in the protein solution. A previously swollen polymer membrane was placed into a 120-mL amber bottle (Fisher Scientific) containing 10 mL of protein solution and kept at 5°C. After the equilibrium was achieved, the concentration and volume of the remaining solution were measured. The concentration of the remaining solution was assayed in triplicate. After the partition, the membranes were allowed to dry in a desiccator containing anhydrous calcium sulfate (Fisher Scientific). They were further dried in a vacuum oven at 35°C and 20 mmHg (VWR International, West Chester, PA). This drying process minimized membrane fracture. Through these experiments, it was possible to determine the partition coefficient of myoglobin from horse toward the various polymer morphologies.
Protein immobilization by entrapment
To perform the protein entrapment, the solvent fraction of the polymerization solution corresponding to DI water was replaced by aqueous myoglobin solution. Approximately 0.25 mg of myoglobin was added to the polymerization solution. After the addition of myoglobin to the polymerization solution, the hydrogel polymerization procedure was followed without any other variation.
Determination of the correlation length
Evaluation of the correlation length required the measurement of the membrane volume in the relaxed state after crosslinking, after drying, and in the swollen state. To determine these parameters, it was necessary to measure the hydrogel weight for each specific state (after crosslinking, drying, and swelling) in air and in a nonsolvent. The application of the Archimedes buoyancy principle allows the determination of the volume of a hydrogel by its immersion in a fluid with a known density. According to this principle, the difference between the weight in air and the weight in a nonsolvent over the density of the nonsolvent used is equal to the hydrogel volume. A density kit coupled to a balance was used to perform these measurements. Heptane was used as a nonsolvent to avoid swelling.
Release studies
A dried hydrogel with incorporated myoglobin was placed in a bottle containing 10 mL of a buffer solution. The buffer solution was continually agitated and maintained at 30°C. The buffer was changed at certain time intervals and replaced by fresh buffer. The myoglobin concentration in the buffer solutions was determined in triplicate from spectroscopic measurements at characteristic wavelengths. The test lasted until no more protein was released from the hydrogel. For the protein incorporated into MAA–PEGDMA hydrogels, the buffer was 0.1M sodium phosphate at pH 7.0 (PEGDMA200, PEGDMA600, and PEGDMA1000) and at pH 5.8 (PEGDMA600 and PEGDMA1000). With the desorption data obtained from the release experiments, it was possible to evaluate solute-transport mechanisms and diffusion coefficient (D). These experiments were performed for all the morphologies with at least three membranes.
Activity studies
Activity tests of hydrogels containing myoglobin consisted of changing the myoglobin oxidation state and evaluating its capacity to bind CO. The polymer network containing ferric myoglobin was placed in a quartz screw cap cell (Starna Cells, Inc., Atascadero CA), and an ultraviolet–visible (UV–vis) spectrum was taken (Powerwave X-I microplate reader, Bio-Tek Instruments). Then, a buffer solution with a small amount of sodium dithionate was added with a syringe through the septum cell. Sodium dithionate is known for its capacity to reduce hemoproteins from the metaquo state (FeIII; 408 nm) to the deoxy state (FeII; 435 nm).29 Spectra of the hydrogel in contact with sodium dithionate were collected over time to confirm the reduction of the protein to the ferrous state. Afterward, the buffer solution was removed from the cell with a syringe. Small quantities of CO (0.5–1.5 cm3) were transferred from a cylinder to the cell with a syringe. UV–vis spectra of the hydrogel after the addition of CO were taken over the course of time to verify the binding between myoglobin and CO.
RESULTS AND DISCUSSION
Protein immobilization by imbibition
The first method investigated for the immobilization of myoglobin was imbibition. Partition coefficients were determined to quantify the amount of protein incorporated inside the hydrogels after the imbibition process. The partition coefficient also allows us to determine the protein's affinity toward the polymer membrane or ability to remain in solution. The partition coefficient (Kd) was calculated with the following equation:31,32
| (1) |
where Cm and Cs are the concentrations of the solute in the membrane and in the solution, respectively; Ci is the initial concentration of solute in the solution; C0 is the concentration of solute in the solution after equilibrium; and Vs and Vm are the volumes of the solution and the membrane, respectively.
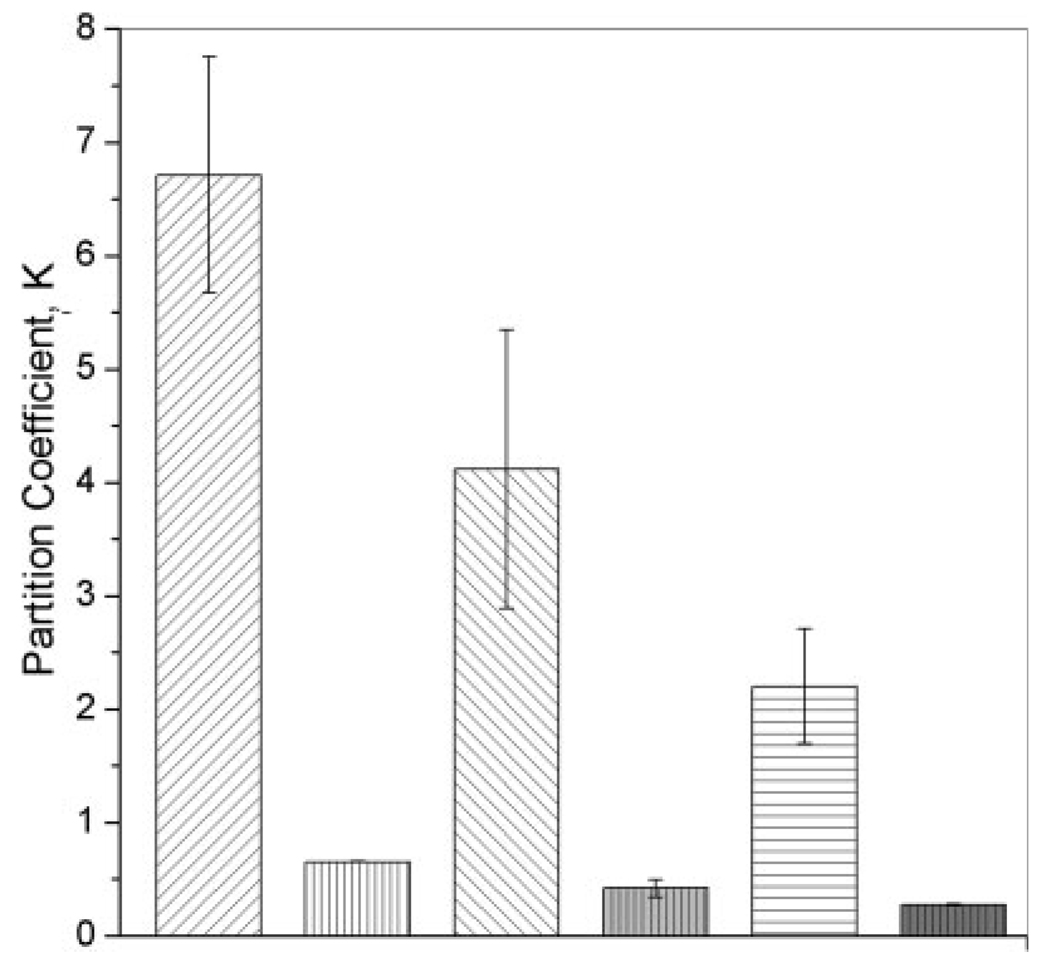
The values of the partition coefficient for MAA–PEGDMA and DMAEM–PEGDMA hydrogels are shown in Figure 1. An inverse relationship between the crosslinker length and amount of myoglobin incorporated was noticed for both polymer morphologies. Hydrogels synthesized with a larger cross-linker length incorporated a smaller protein amount than hydrogels with a shorter crosslinker length. The highest myoglobin incorporation degree through the imbibition immobilization technique was achieved in MAA-based hydrogels. Cationic hydrogels also allowed myoglobin incorporation by imbibition. However, the degree of incorporation of myoglobin in DMAEM-based hydrogels was approximately 10% of the one observed in MAA-based hydrogels. Neutral hydrogels did not adsorb any measurable amount of myoglobin.
Figure 1.
Plot of the partition coefficients for anionic and cationic hydrogels: ( ) MAA–PEGDMA200 (n = 6), (
) MAA–PEGDMA200 (n = 6), ( ) DMAEM–PEGDMA200 (n = 3), (
) DMAEM–PEGDMA200 (n = 3), ( ) MAA–PEGDMA600 (n = 6), (
) MAA–PEGDMA600 (n = 6), ( ) DMAEM–PEGDMA600 (n = 3), (
) DMAEM–PEGDMA600 (n = 3), ( ) MAA–PEGDMA1000 (n = 6), and (
) MAA–PEGDMA1000 (n = 6), and ( ) DMAEM–PEGDMA1000 (n = 3). The incorporation was performed in DI water at 5.0°C. Each bar represents an average plus or minus 1 standard deviation.
) DMAEM–PEGDMA1000 (n = 3). The incorporation was performed in DI water at 5.0°C. Each bar represents an average plus or minus 1 standard deviation.
To understand the effects and restrictions that the hydrogel structure imposed on the polymer behavior during protein loading, we investigated the structure of the networks used. A parameter known as the correlation length or mesh size was evaluated to characterize the structure of hydrogels. The correlation length is a measure of the space available for diffusion between two macromolecular chains. Because of the extent of randomization produced during the polymerization, this parameter can be evaluated only as an average value.18 The correlation length was evaluated with the equilibrium swelling theory applying the Peppas and Merril modification to the Flory–Rehner theory:33,34
| (2) |
where χ1 is the Flory–Huggins polymer–water inter-action parameter, V1 is the molar volume of water, ῡ is the specific volume of the polymer, υ2,s is the volume fraction of the swollen gel, υ2,r is the polymer volume fraction in the relaxed state, M̄C is the number-average molecular weight between the crosslinks, and M̄n is the molecular weight of the linear polymer chains prepared under the same conditions without crosslinking.
Polymer volume fractions of the hydrogels in both relaxed and swollen states, υ2,r, and υ2,s, can be evaluated from swelling studies with the following relationships: 35
| (3) |
| (4) |
where VP is the volume of the dry polymer, Vg,r is the volume of the hydrogel after crosslinking but before swelling, and Vg,s is the volume of the hydrogel after equilibrium swelling.
Evaluation of the networks correlation length revealed that MAA hydrogels possessed the highest correlation length [15.611–26.988 nm with a pH 7.4 phosphate-buffered solution (PBS)] in comparison with poly(ethylene glycol) (0.252–0.342 nm at pH 7.0 PBS) and DMAEM (1.461–0.645 nm at pH 5.8 PBS) hydrogels (see Fig. 2). The correlation length values of the neutral hydrogels did not show any statistical difference for the monomers or crosslinker lengths employed. The same trend was observed in the cationic hydrogels.
Figure 2.
Correlation length (nm) for various hydrogels morphologies: (□) PEGMA200–PEGDMA600 (n = 7), ( ) PEGMA200–PEGDMA1000 (n = 9), (
) PEGMA200–PEGDMA1000 (n = 9), ( ) PEGMA400– PEGDMA600 (n = 8), (■) PEGMA400–PEGDMA1000 (n = 8), (
) PEGMA400– PEGDMA600 (n = 8), (■) PEGMA400–PEGDMA1000 (n = 8), ( ) PEGMA1000–PEGDMA1000 (n = 9), (
) PEGMA1000–PEGDMA1000 (n = 9), ( ) MAA–PEGDMA200 (n = 7), (
) MAA–PEGDMA200 (n = 7), ( ) MAA–PEGDMA600 (n = 6), (
) MAA–PEGDMA600 (n = 6), ( ) MAA–PEGDMA1000 (n = 7), (
) MAA–PEGDMA1000 (n = 7), ( ) DMAEM–PEGDMA200 (n = 7), (
) DMAEM–PEGDMA200 (n = 7), ( ) DMAEM–PEGDMA600 (n = 6), and (
) DMAEM–PEGDMA600 (n = 6), and ( ) DMAEM–PEGDMA1000 (n = 6). Each bar represents an average plus or minus 1 standard deviation.
) DMAEM–PEGDMA1000 (n = 6). Each bar represents an average plus or minus 1 standard deviation.
In MAA–PEGDMA hydrogels, it was observed that the correlation length had an inverse relationship with the crosslinker length: the larger the crosslinker length, the smaller the correlation length. The calculation of the correlation length is based on the determination of the molecular weight between two adjacent crosslinkers. The results for this parameter (not shown) obtained for MAA-based hydrogels indicated that the molecular weight between cross-links increased when the crosslinker lengths decreased. Anionic hydrogels with shorter crosslinker lengths enclosed a higher number of monomer units between two adjacent crosslinkers. Therefore, the higher molecular weight between crosslinkers produced an increment in the mesh size. The increase in the crosslinker length was not able to counterbalance this effect. In addition, the reduction in the mesh size at larger crosslinker lengths may be related to the formation of loops and heterogeneous regions in the network.
The higher myoglobin incorporation reached in anionic hydrogels could be caused not only by the larger correlation length but also by the interactions between the protein and the carboxyl moieties in the MAA carrier. Globular proteins such as myoglobin are characterized by arranging their hydrophilic groups outside the molecule. Horse myoglobin has 11 residues of histidine, a hydrophilic group, in its bone chain. The pKa of histidine residues is 6.00, and they are positively charged over this value.36 The pKa of MAA is 4.66 at 20°C, and it becomes negatively charged over this value.37 As a result, it is possible that at a pH higher than 6.00, ionic interactions between MAA and histidine took place, increasing the affinity of myoglobin toward this specific polymer.
Moreover, anionic hydrogels are known to exhibit an increase in the swelling capacity at a pH higher than the pKa due to ionization.32 The former could have enhanced the incorporation of myoglobin in MAA-based hydrogels. In contrast, cationic hydrogels are un-ionized in a high pH environment and consequently do not swell to a large extent under these conditions.16 That could explain why DMAEM hydrogels adsorbed lower quantities of myoglobin than MAA hydrogels.
The difficulty in incorporating myoglobin inside a PEGMA-based hydrogel could be caused by the small correlation length displayed for these polymers. The hydrodynamic radius for denatured horse myoglobin has been reported to be 2.04 nm.38 The smaller correlation length of neutral hydrogels in comparison with the protein size could have provoked a size exclusion effect that hindered the incorporation of myoglobin inside PEGMA-based hydrogels. The preparation of PEGMA-based hydrogels has some restrictions related to the monomer/crosslinker ratio that can be used. To obtain stable membranes, the tethered chain length must be equal to or smaller than the crosslinker length, except for the monomer/crosslinker combination of PEGMA1000 and PEGDMA600 (unpublished data). The latter is still easily breakable. This constraint made it unfeasible to prepare structures with a considerably higher correlation length.
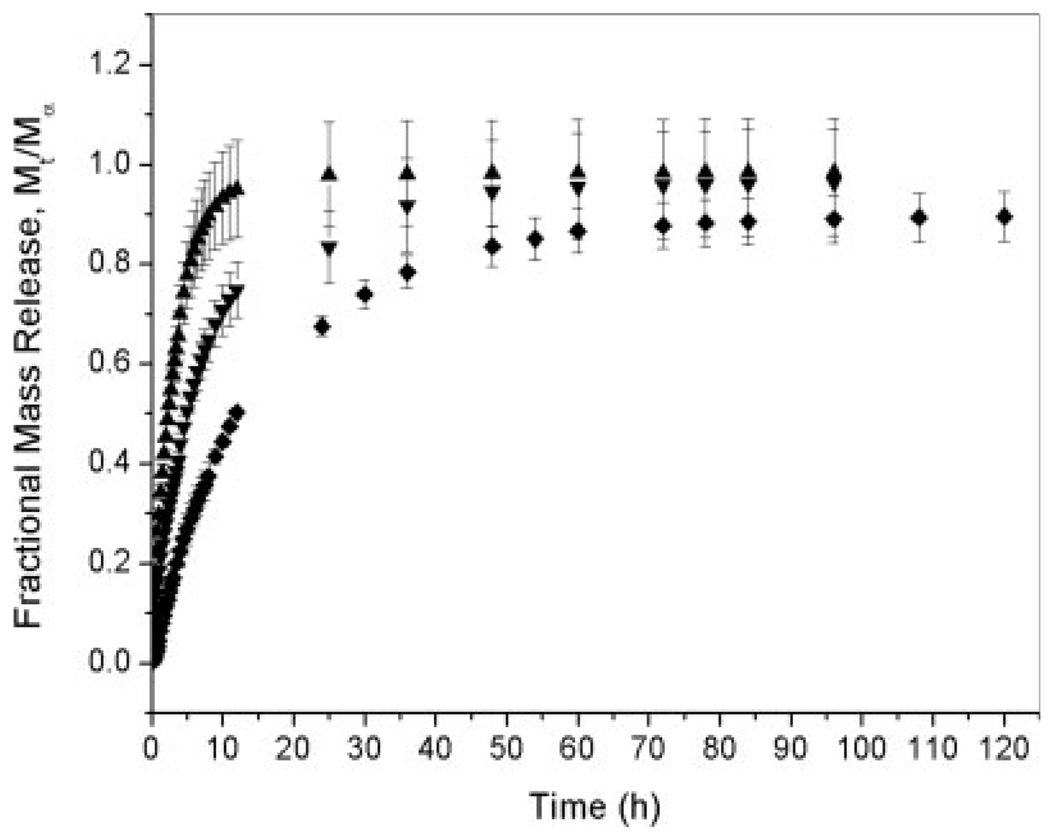
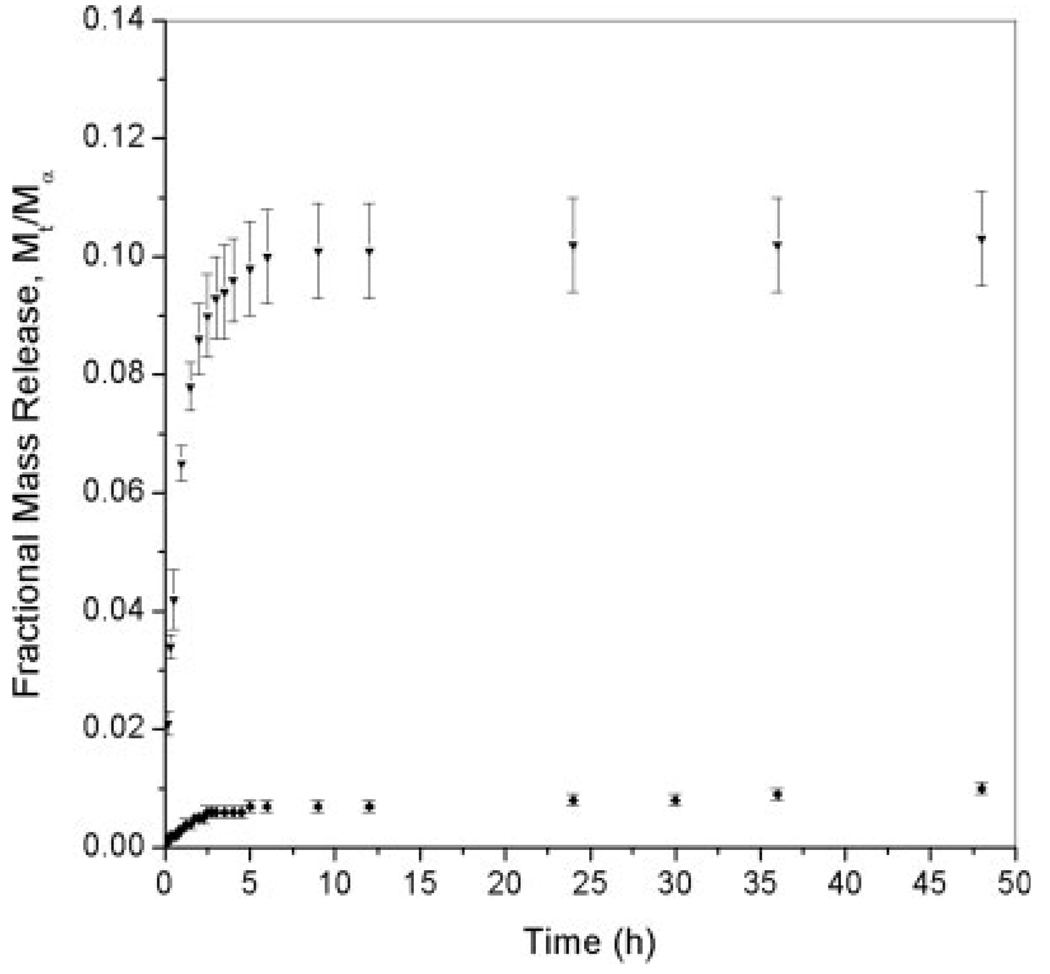
Considering that hydrogels have been extensively used as drug delivery systems, we expected that some of the protein incorporated would be released in the presence of a buffer solution. To evaluate the effect of the pH on the rate of myoglobin release from anionic hydrogels, release experiments were conducted in a sodium phosphate buffer (PBS) at pHs 7.0 and 5.8 (see Fig. 3 and Fig.4). The amount of protein released decreased significantly as a function of the pH. At pH 7.0, the total release fraction for MAA–PEGDMA200 and MAA–PEGDMA600 hydrogels was approximately 90%, whereas for MAA–PEGDMA1000 hydrogels, it was approximately 85%. In contrast, at pH 5.8, the amount of protein released was approximately 10% for MAA–PEGDMA600 and 1% for MAA–PEGDMA1000 polymer networks. At pH 5.8, the polymer was still collapsed because of the closeness of the pH buffer to the MAA pKa value, and it did not allow a substantial protonation of the polymer network. The collapsed polymer state hindered myoglobin diffusion from the anionic hydrogels. In contrast, MAA hydrogels at pH 7.0 were highly swollen. Highly swollen hydrogels allow greater solute transport because of the larger amounts of unbound water.32
Figure 3.
Fractional release of myoglobin incorporated through imbibition in MAA–PEGDMA hydrogels for various crosslinker lengths in PBS at pH 5.8 and 30°C: (▲) MAA–PEGDMA200 (n = 4), (▼) MAA–PEGDMA600 (n = 5), and (◆) MAA–PEGDMA1000 (n = 3). Each point represents an average plus or minus 1 standard deviation.
Figure 4.
Fractional release of myoglobin incorporated through imbibition in MAA–PEGDMA hydrogels for various crosslinker lengths in PBS at pH 5.8 and 30°C: (▼) MAA–PEGDMA600 (n = 3) and (◆) MAA–PEGDMA1000 (n = 3). Each point represents an average plus or minus 1 standard deviation.
The solute-transport mechanism was evaluated through the application of the following equation:34
| (5) |
where Mt is the accumulative mass of solute released at time t, M∞ is the amount of solute released at infinite time, k is the characteristic constant of the polymer/solute system, and n is the diffusional exponent. The value of n allows the determination of the type of predominating diffusion mechanism in the system. Generally, for n = 0.5, transport follows Fickian behavior, and for n = 1, transport is relaxation-controlled. A value of n between 0.5 and 1 indicates anomalous transport. In this case, there are contributions from Fickian diffusion and relaxation processes.34,39,40
The values of n for MAA–PEGDMA hydrogels at pHs 5.8 and 7.0 were found to be in the range of 0.5–1.0 (see Table I). According to these results, it can be concluded that anomalous transport occurred during the release of myoglobin from anionic hydrogels. Therefore, the transport mechanism is a combination of Fickian diffusion and chain relaxation processes. The Fickian diffusion was promoted by the existent concentration gradient between the hydrogel containing protein and the buffer solution that was free of protein. The chain relaxation process was caused by the hydrogel swelling.
TABLE I.
Values of Parameters n, k, ϕF, ϕR, and D for Myoglobin Incorporated Through Imbibition into MAA–PEGDMA Hydrogels with Various Crosslinker Lengths
| MAA–PEGDMA200 (pH 7.0) |
MAA–PEGDMA600 | MAA–PEGDMA1000 | |||
|---|---|---|---|---|---|
| pH 7.0 | pH 5.8 | pH 7.0 | pH 5.8 | ||
| k | 0.29402 ± 0.00191 | 0.18087 ± 0.00224 | 0.06143 ± 0.00128 | 0.05904 ± 0.00277 | 0.00333 ± 0.00004 |
| n | 0.61677 ± 0.00736 | 0.62374 ± 0.01029 | 0.53438 ± 0.03207 | 0.91782 ± 0.02788 | 0.68215 ± 0.02134 |
| ϕF | 0.9319 ± 2.1963 × 10−4 | 0.9005 ± 1.820 × 10−3 | 0.1011 ± 1.8937 × 10−4 | 0.8555 ± 4.9936 × 10−3 | 0.0061 ± 2.7060 × 10−4 |
| ϕR | 0.0681 ± 2.1963 × 10−4 | 0.0995 ± 1.820 × 10−3 | 0.8988 ± 1.8937 × 10−4 | 0.1444 ± 4.9936 × 10−3 | 0.9939 ± 2.7060 × 10−4 |
| D (cm2/s) | 7.7647 × 10−9 ± 9.3886 × 10−11 | 3.4037 × 10−9 ± 5.4707 × 10−11 | 1.0995 × 10−9 ± 1.1575 × 10−10 | 1.6818 × 10−9 ± 1.2439 × 10−11 | 7.6023 × 10−11 ± 4.3082 × 10−12 |
The experiments were performed in PBS at pHs 7.0 and 5.8 at 30°C.
The values of D were determined with the Berens and Hopfenbers model. For this model, the sorption process is considered to be a linear superposition of independent contributions from Fickian diffusion and polymer relaxation.41 The sorption process for a disk of thickness (L) is described by the following equation:24,40,41
| (6) |
where ϕF is the fraction of sorption contributed by Fickian diffusion, ϕR is the fraction of sorption contributed by chain relaxation, and k is the first-order relaxation constant.
At later times, the sorption process is dominated by the relaxation of polymer chains, and the Fickian contribution can be neglected. For these conditions, a semilog plot of 1 − Mt/M∞ versus time can be used to evaluate k, ϕF, and ϕR. At short times, the relaxation process can be neglected. For 60% of the sorption process, a plot of ln(1 − Mt/M∞) versus time could be used to determine D.40
At pH 7.0, the results obtained for the Fickian (ϕF) and chain relaxation (ϕR) contributions indicate that the solute transport is the result of a coupling between the two contributions, and this confirms the previous analysis from the n values (see Table I). According to the results, Fickian diffusion contributed in a higher proportion to the solute transport in anionic morphologies. However, the chain relaxation contribution increased in hydrogels with larger crosslinker lengths. At pH 5.8, MAA–PEGDMA600 and MAA–PEGDMA1000 morphologies were markedly influenced by the chain relaxation mechanism. It could be observed that the chain relaxation contribution (ϕR) increased for larger crosslinker lengths. The value of the contribution parameter was 0.891 for MAA–PEGDMA600 and 0.994 for MAA–PEGDMA1000. These results indicate that for MAA–PEGDMA1000 hydrogels, the myoglobin transport followed mainly a chain relaxation mechanism.
The values obtained for D confirmed the behavior observed for myoglobin release from anionic hydrogels (see Table I). The myoglobin D values for MAA-based hydrogels at a PBS buffer with pH 7.0 were 7.7647 × 10−9 cm/s for MAA–PEGDMA200, 3.4037 × 10−9 cm/s for MAA–PEGDMA600, and 1.6818 × 10−9 cm/s for MAA–PEGDMA1000. D increased for hydrogels with shorter crosslinker lengths. These results are in agreement with release experiments in which the amount of myoglobin released was higher for MAA-based hydrogels having shorter crosslinker lengths. Two reasons could explain the results obtained through the application of the Berens and Hopfenbers model: the correlation length and the amount of incorporated myoglobin were higher at shorter crosslinker lengths. A larger correlation length could provide a larger space for diffusion, facilitating the myoglobin transport from the hydrogel to the buffer solution. As the amount of protein incorporated inside the polymer networks became higher, the concentration gradient increased, and this could induce Fickian diffusion.
The D values of myoglobin released at pH 5.8 were smaller than the values obtained for myoglobin released at pH 7.0. At the lower pH, the MAA–PEGDMA600 value was 1.0995 × 10−9 cm/s, and for MAA–PEGDMA1000, it was 7.06023 × 10−11 cm/s. The difference between D at pH 7.0 and D at pH 5.8 could lie in the degree of swelling of the networks at different pHs. In anionic structures such as MAA-based hydrogels, the swelling response is improved at higher pHs. Larger solute transport is usually observed in highly swollen hydrogels.32 In addition, the mechanical strength of anionic hydrogels decreased substantially at pH 5.8, and this prevented the performance of release experiments for MAA–PEGDMA200 hydrogels at this pH.
After release experiments were completed, the biological activity of myoglobin permanently encapsulated inside the hydrogel was assessed. The peaks displayed in the Soret band were used to monitor the changes in the spectral properties for myoglobin-loaded anionic hydrogels at pH 7.0. Usually, peaks at the Soret band for myoglobin in metaquo and deoxy states are located at 408 and 435 nm, respectively. The carboxy–myoglobin complex shows a peak in the Soret band at 425 nm.29,30 The presence or development of these peaks was used to corroborate the occurrence of the reactions.
The effect of sodium dithionite and CO on the spectroscopic properties of myoglobin incorporated into MAA–PEGDMA200 hydrogels after release is shown in Figure 5. The spectra taken after the end of the release experiment showed a peak at 409 nm. The interaction with sodium dithionate produced the disappearance of the peak at 409.2 nm and the appearance of a new peak near to 431 nm. The observed change in the spectroscopic properties indicated that permanently immobilized protein inside MAA hydrogels changed its oxidation state from metaquo to deoxy. Hydrogels containing reduced myoglobin were placed in contact with CO, and the formation of a new peak at approximately 423 nm was observed. This peak indicated the binding of myoglobin and CO. The capacity of the remaining myoglobin after release experiments to be reduced by sodium dithionate and to bind CO is a clear indication of the activity retention.
Figure 5.
Effect of sodium dithionite and CO on the spectroscopic properties of myoglobin incorporated into MAA–PEGDMA200 hydrogels after their release. The solid, black line shows the spectrum before dithionate and CO; the spectra after the addition of sodium dithionate are shown by the dashed, black line (t = 0 min) and the solid, gray line (t = 5 min); and the spectra after the addition of CO are shown by the dashed, gray line (0.5 cm3 CO, t = 0 min) and the black, dashed line (0.5 cm3 CO, t = 0 min).
Myoglobin spectroscopic properties did not change in the presence of sodium dithionite during the activity test for myoglobin remaining inside anionic hydrogels at pH 5.8 after release experiments. From this result, it could not be categorically concluded that the protein contained inside the hydrogels was not biologically active. It is probable that MAA-based hydrogels at pH 5.8 were still collapsed, hindering the diffusion of sodium dithionite inside the hydrogel and preventing the protein reduction.
Protein immobilization by entrapment
Myoglobin incorporation through entrapment was not possible in PEGMA–PEGDMA and DMAEM–PEGDMA hydrogels under the studied conditions. The contact between myoglobin and polymerization solutions of neutral and cationic polymers produced protein precipitation. The formation of protein aggregates was more notorious when the polymerization solutions contained EtOH in comparison with the effect produced by monomer and crosslinker mixtures. The protein precipitation could be caused by the influence of poly(ethylene glycol) and EtOH.42–44
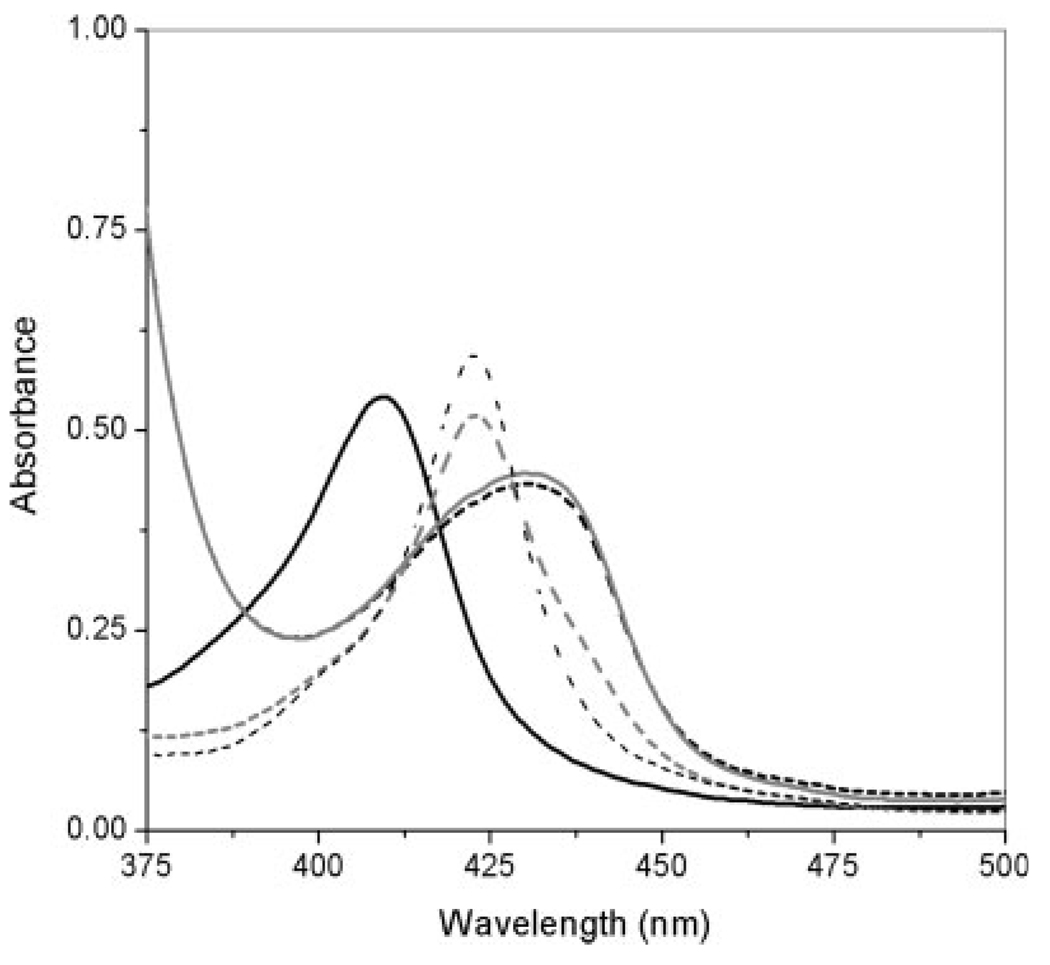
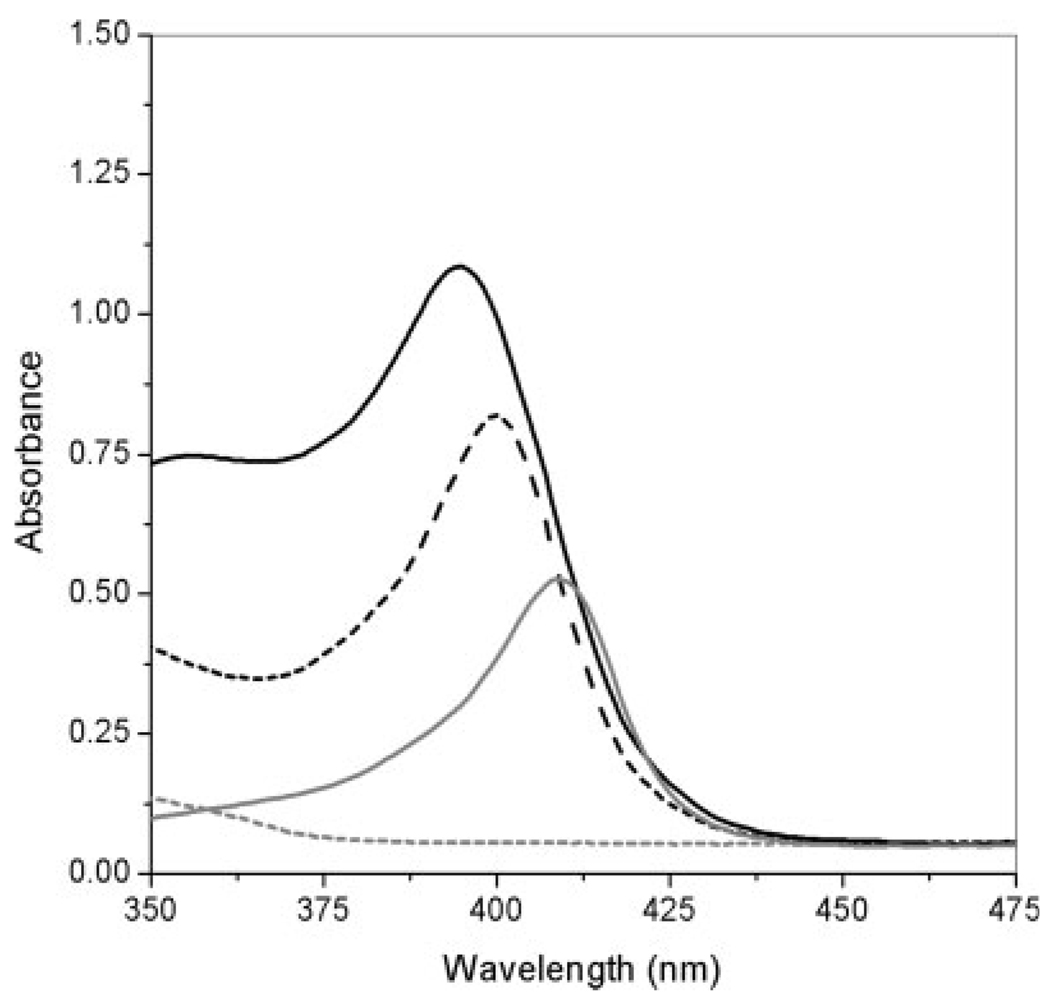
The contact of myoglobin with the polymerization solutions of MAA-based hydrogels did not produce protein precipitation. However, a displacement in the myoglobin characteristic peak at the Soret band from 408 to 400 nm was observed immediately after the protein was in contact with the polymerization solution. After the polymerization of the network, the peak was observed to appear at 395 nm (see Fig. 6). The low pH of the anionic polymerization solutions could be adversely affecting the protein structure. However, changes in the monomer, crosslinker, and solvent ratios have demonstrated an improvement in the proteins’ biological activity (unpublished data).
Figure 6.
Effect of the MAA–PEGDMA200 polymerization solution on the Soret characteristic peak of horse myoglobin. The dashed, gray line shows the spectrum of the MAA–PEGDMA200 polymerization solution without myoglobin; the solid, gray line shows the spectrum of free myoglobin in the metaquo state; the dashed, black line shows the spectrum of the MAA–PEGDMA200 polymerization solution immediately after the addition of myoglobin; and the black, solid line shows the spectrum of MAA–PEGDMA200 containing myoglobin after polymerization
The three anionic morphologies employed behaved in a similar manner during myoglobin release. No statistical differences were observed. The evaluation of n revealed that the release of myoglobin incorporated by entrapment in MAA–PEGDMA hydrogels was highly influenced by the chain relaxation process (see Table II). The D values of myoglobin incorporated by entrapment were of the order of magnitude of 10−11 cm2/s. A reduction of 2 orders of magnitude was observed in comparison with the D values of myoglobin incorporated by imbibition in the same hydrogel morphologies, both evaluated in PBS at pH 7.0. The incorporation through entrapment could hinder the diffusion of the protein from the polymer structure because the network was built around the protein during the polymerization process.
TABLE II.
Values of Parameters n, k, ϕF, ϕR, and D for Myoglobin Incorporated Through Entrapment in MAA–PEGDMA Hydrogels with Various Crosslinker Lengths
| MAA–PEGDMA200 | MAA–PEGDMA600 | MAA–PEGDMA1000 | |
|---|---|---|---|
| k | 0.00155 ± 0.00071 | 0.00137 ± 0.00049 | 0.0020 ± 0.00086 |
| n | 0.94164 ± 0.09514 | 0.99578 ± 0.078 | 0.94153 ± 0.09495 |
| ϕF | 0.2010 ± 4.2789 × 10−3 | 0.1736 ± 5.9042 × 10−3 | 0.1861 ± 3.8082 × 10−3 |
| ϕR | 0.7990 ± 4.2789 × 10−3 | 0.8264 ± 5.9042 × 10−3 | 0.8139 ± 3.8082 × 10−3 |
| D (cm2/s) | 2.2245 × 10−11 ± 1.5101 × 10−12 | 3.4343 × 10−11 ± 9.3681 × 10−13 | 3.2871 × 10−11 ± 6.3542 × 10−13 |
The experiments were performed in PBS at pH 7.0 at 30°C.
CONCLUSIONS
Two different techniques were studied to immobilize myoglobin from horse skeletal muscle in hydrophilic polymer networks: imbibition and entrapment. The best results for myoglobin immobilization were achieved by the imbibition technique inside MAA–PEGDMA hydrogels. Imbibition allowed the incorporation of greater amounts of myoglobin in anionic morphologies. Release experiments performed with a PBS buffer at pH 7.0 showed that these hydrogels allowed the desorption of approximately 90% of the protein previously loaded. Nonetheless, the myoglobin remaining inside the hydrogels maintained the heme reactivity. These results indicated that myoglobin incorporated by imbibition inside MAA–PEGDMA hydrogels was able to successfully overcome the adsorption process into the polymer, the polymer drying, and the release process without undergoing significant unfavorable effects on its heme reactivity. On the basis of the results, the use of hydrogels as matrices in which proteins such as myoglobin can be permanently contained without the alteration of their biological properties seems promising. These networks could find applications in the development of optical biosensors with hemo-proteins as recognition elements.
Acknowledgments
Contract grant sponsor: National Center for Research Resources (NCRR), a Component of the National Institutes of Health (NIH); contract grant number: P20RR016470-04. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NCRR or NIH.
References
- 1.Nassar AEF, Willis WS, Rusling JF. Anal Chem. 1995;67:2386. doi: 10.1021/ac00110a010. [DOI] [PubMed] [Google Scholar]
- 2.Ma HY, Hu NF, Rusling JF. Langmuir. 2000;16:4969. [Google Scholar]
- 3.Lu HY, Li Z, Hu NF. Biophys Chem. 2003;104:623. doi: 10.1016/s0301-4622(03)00121-2. [DOI] [PubMed] [Google Scholar]
- 4.Hu NF. Pure Appl Chem. 2001;73:1979. [Google Scholar]
- 5.Fan CH, Wang HY, Sun S, Zhu DX, Wagner G, Li GX. Anal Chem. 2001;73:2850. doi: 10.1021/ac001397s. [DOI] [PubMed] [Google Scholar]
- 6.Gu HY, Yu AM, Chen HY. Electroanal Chem. 2001;516:119. [Google Scholar]
- 7.Liu SQ, Dai ZH, Chen HY, Ju HX. Biosens Bioelectron. 2004;19:963. doi: 10.1016/j.bios.2003.08.025. [DOI] [PubMed] [Google Scholar]
- 8.Gu HY, Yu AM, Yuan SS, Chen HY. Anal Lett. 2002;35:647. [Google Scholar]
- 9.Xu XX, Liu SQ, Li B, Ju HX. Anal Lett. 2003;36:2427. [Google Scholar]
- 10.Ferrer ML, Del Monte F, Mateo CR, Gomez J, Levy D. J Sol-Gel Sci Technol. 2003;26:1169. [Google Scholar]
- 11.Chung KE, Lan EH, Davidson MS, Dunn BS, Valentine JS, Zink JI. Anal Chem. 1995;67:1505. [Google Scholar]
- 12.Blyth DJ, Aylott JW, Richardson DJ, Russell DA. Analyst. 1995;120:2725. doi: 10.1039/a806921b. [DOI] [PubMed] [Google Scholar]
- 13.McCool BA, Cashon R, Karles G, DeSisto WJ. J Non-Cryst Solids. 2004;333:143. [Google Scholar]
- 14.Jin W, Brennan JD. Anal Chim Acta. 2002;461:1. [Google Scholar]
- 15.Chen LY, Tian ZG, Du YM. Biomaterials. 2004;25:3725. doi: 10.1016/j.biomaterials.2003.09.100. [DOI] [PubMed] [Google Scholar]
- 16.Qiu Y, Park K. Adv Drug Delivery Rev. 2001;53:321. doi: 10.1016/s0169-409x(01)00203-4. [DOI] [PubMed] [Google Scholar]
- 17.Peppas NA. Curr Opin Colloid Interface Sci. 1997;2:531. [Google Scholar]
- 18.Peppas NA, Bures P, Leobandung W, Ichikawa H. Eur J Pharm Biopharm. 2000;50:27. doi: 10.1016/s0939-6411(00)00090-4. [DOI] [PubMed] [Google Scholar]
- 19.Gupta P, Vermani K, Garg S. Drug Discovery Today. 2002;7:569. doi: 10.1016/s1359-6446(02)02255-9. [DOI] [PubMed] [Google Scholar]
- 20.Elliott JE, Macdonald M, Nie J, Bowman CN. Polymer. 2004;45:1503. [Google Scholar]
- 21.van de Wetering P, Metters AT, Schoenmakers RG, Hubbell JA. J Controlled Release. 2005;102:619. doi: 10.1016/j.jconrel.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 22.Kim SW, Bae YH, Okano T. Pharm Res. 1992;9:283. doi: 10.1023/a:1015887213431. [DOI] [PubMed] [Google Scholar]
- 23.Gehrke SH, Uhden LH, McBride JF. J Controlled Release. 1998;55:21. doi: 10.1016/s0168-3659(98)00019-4. [DOI] [PubMed] [Google Scholar]
- 24.am Ende MT, Mikos AG. In: Protein Delivery: Physical Systems. Sanders LM, Hendren RW, editors. New York: Plenum; 1997. p. 139. [Google Scholar]
- 25.Herber S, Olthuis W, Bergveld P. Sens Actuators B. 2003;91:378. [Google Scholar]
- 26.Brahim S, Narinesingh D, Guiseppi-Elie A. Biosens Bioelectron. 2002;17:973. doi: 10.1016/s0956-5663(02)00089-1. [DOI] [PubMed] [Google Scholar]
- 27.Podual K, Doyle FJ, Peppas NA. J Controlled Release. 2000;67:9. doi: 10.1016/s0168-3659(00)00195-4. [DOI] [PubMed] [Google Scholar]
- 28.Jeong B, Gutowska A. Trends Biotechnol. 2002;20:305. doi: 10.1016/s0167-7799(02)01962-5. [DOI] [PubMed] [Google Scholar]
- 29.Antonini E, Brunori M. Hemoglobin and Myoglobin in Their Interactions with Ligands. North Holland: Amsterdam; 1971. [Google Scholar]
- 30.Imai K. In: Metalloproteins, Chemical Properties, Biological Effects: Bioactive Molecules. Otsuka CI, Yamanaka T, editors. New York: Elsevier; 1988. p. 115. [Google Scholar]
- 31.Bell CL, Peppas NA. Biomaterials. 1996;17:1203. doi: 10.1016/0142-9612(96)84941-6. [DOI] [PubMed] [Google Scholar]
- 32.am Ende MT, Hariharan D, Peppas NA. React Polym. 1995;25:127. [Google Scholar]
- 33.Peppas NA, Merrill EW. J Appl Polym Sci. 1977;21:1763. [Google Scholar]
- 34.Merrill EW, Dennison KA, Sung C. Biomaterials. 1993;14:1117. doi: 10.1016/0142-9612(93)90154-t. [DOI] [PubMed] [Google Scholar]
- 35.Peppas NA. Hydrogels in Medicine, Pharmacy Applications. Boca Raton, FL: CRC; 1987. [Google Scholar]
- 36.Nelson DL, Cox MM. Lehninger: Principles of Biochemistry. New York: Worth; 2000. [Google Scholar]
- 37.Institute for Health and Consumer Protection. Dortmund, Germany: European Chemicals Bureau, Methacrylic Acid, Summary Risk Assessment Report; CAS 79-41-4. 2002
- 38.Zhou HX. J Phys Chem B. 2002;106:5769. [Google Scholar]
- 39.Thimmegowda MC, Sathyanarayana PM, Shariff G, Ashalatha MB, Ramani R, Ranganathaiah C. J Biomater Sci Polym Ed. 2002;13:1295. doi: 10.1163/15685620260449705. [DOI] [PubMed] [Google Scholar]
- 40.Torres-Lugo M, Peppas NA. Macromolecules. 1999;32:6646. [Google Scholar]
- 41.Berens AR, Hopfenberg HB. Polymer. 1978;19:489. [Google Scholar]
- 42.Guo MN, Narsimhan G. Biotechnol Prog. 1991;7:54. [Google Scholar]
- 43.Guo R, Narsimham G. Presented at the Institute of Food Technologists Annual Meeting; June 25–29, 1994; Atlanta, GA. [Google Scholar]
- 44.Harris JM, Zalipsky S. Chemistry and Biological Applications of Polyethylene Glycol. Washington, DC: American Chemical Society; 1997. [Google Scholar]