Abstract
In recent years, cell transplantation has drawn tremendous interest as a novel approach to preserving or even restoring contractile function to infarcted hearts. A typical human infarct involves the loss of approximately one billion cardiomyocytes, and so many investigators have sought to identify endogenous or exogenous stem cells with the capacity to differentiate into committed cardiomyocytes and repopulate lost myocardium. As a result of these efforts, dozens of stem cell types have been reported to have cardiac potential. These include pluripotent embryonic stem cells as well various adult stem cells resident in compartments including bone marrow, peripheral tissues, and the heart itself. Some of these cardiogenic progenitors have been reported to contribute replacement muscle through endogenous reparative processes or via cell transplantation in preclinical cardiac injury models. However, considerable disagreement exists regarding the efficiency and even the reality of cardiac differentiation by many of these stem cell types, making these issues a continuing source of controversy in the field. In this review, we consider approaches to cell fate mapping and establishing the cardiac phenotype, as well as the current state of the evidence for the cardiogenic and regenerative potential of the major candidate stem cell types.
Keywords: Stem cell, progenitor, myogenesis, cardiomyocyte, myocardial infarct
Introduction
In L. Frank Baum’s classic novel The Wonderful Wizard of Oz, the title character is only able to provide the Tin Woodman with a placebo heart made of velvet and filled with sawdust. One century later, the emerging field of regenerative medicine aspires to accomplish what Oz could not; that is, harness the potential of stem cells to build (or rebuild) heart tissue out of its component parts. Modern medical management has greatly improved the prospects for heart failure patients, but the only clinically available means of replacing lost myocardium remains whole-organ transplantation. The demand by patients with end-stage heart failure greatly exceeds the supply of suitable donor hearts, and so there has been considerable recent interest in cell-based therapies as an alternate means of “remuscularizing” injured hearts.
Valuable proof of principle for cell-based cardiac repair was provided by early preclinical studies in which terminally differentiated cardiomyocytes from fetal and neonatal sources were transplanted into infarcted rodent hearts, resulting in improved left ventricular function1–3. The transplanted cells retained their cardiac phenotype, including expression of sarcomeric proteins and formation of intercalated disc structures. Subsequent intravital imaging studies have confirmed the systolic activation of such grafts in both uninjured4 and infarcted4, 5 hearts, demonstrating that the nascent myocardium is capable of appropriate electromechanical integration. Thus, while recent work has taught us that cell therapy can also improve ventricular function by indirect (“paracrine”) mechanisms (for a review, see ref [11]), cardiogenic cell therapies offer the potential advantage of adding force-generating units to infarcted hearts that have lost muscle to scar tissue.
The clinical application of fetal and neonatal cardiomyocytes for cardiac repair is precluded by their limited supply and ethical concerns, and so the field has sought to identify other cardiogenic cell sources. We are pleased to report that there has been considerable recent progress, and cell-based cardiac repair has become a mainstream experimental concept with multiple clinical trials underway. At the same time, this field has witnessed a significant degree of controversy, particularly with regard to the capacity of certain unexpected stem cell types to differentiate into cardiomyocytes. In the present review, we begin by discussing techniques for demonstrating the cardiac potential of a candidate stem cell type and potential pitfalls in this phenotyping. We then consider a variety of reportedly cardiogenic stem cell types, both endogenous and exogenous, and evaluate the evidence in support of such claims and the prospects of each in cardiac repair.
Stem cells and the cardiac phenotype: definitions, methods, and potential artifacts
Stem cells are clonogenic cells capable of both self-renewal and differentiation into more specialized progeny. Traditionally, they have been divided into two broad categories: adult stem cells and embryonic stem cells (ESCs). Adult stem cells are derived from post-natal somatic tissues and are generally considered to be multipotent, meaning they can give rise to multiple differentiated cell types. ESCs, derived from the inner cell mass of blastocyst-stage embryos, are pluripotent, meaning they can in principle give rise to all the differentiated cell types of the post-natal organism. Differentiated somatic cell types can also be reprogrammed into a pluripotent state similar to ESCs via forced expression of stem cell related genes, the basis for the recently reported induced pluripotent stem cells (iPSCs)6.
Cardiac differentiation has been reported for a variety of stem cell types, including both expected and unexpected cell types. Since reports of differentiation into unexpected cell fates (i.e. “transdifferentiation” events) continue to be a source of controversy, it is worth considering the experimental techniques for tracing cell fate as well as associated potential pitfalls. In principle, tracing cell fate is straightforward and simply requires that one evaluate two parameters, the lineage (or “ancestry”) of any given cell and its phenotype at the time of evaluation. In practice, the challenges to determining each of these parameters and then rigorously demonstrating their co-localization within a single cell are often underestimated. Table 1 lists potential approaches to establishing the cardiac potential of a candidate progenitor cell type, as well as the major pros and cons of each approach. While not always possible, the use of multiple techniques is the most convincing way to demonstrate cardiac potential.
Table 1.
Approaches to determining the cardiac potential of progenitor cells.
| Advantages | Limitations | |
|---|---|---|
| Fate mapping using chemical label (e.g. BrdU, fluorescent dye, iron particles) to track progenitor cells |
|
|
| Fate mapping using in situ hybridization for species or gender-specific DNA sequence (e.g. Y-chromosome) to track progenitor cells |
|
|
| Fate mapping using genetic reporter (e.g. GFP) to track progenitor cells |
|
|
| Fate mapping using independent genetic reporters to track progenitor cells and neighboring host/co-cultured cells |
|
|
| Species-specific RT-PCR for cardiac transcripts |
|
|
| Direct assessment of in vivo graft function (e.g. intravital calcium imaging studies of GFP- tagged grafts) |
|
|
| Assessment of cardiac function (e.g. echocardiography) |
|
|
Tracing the lineage of putative cardiogenic progenitor cells is especially treacherous in experimental designs that involve the culture of admixed cells, co-culture with definitive cardiomyocytes, or in vivo transplantation studies. Two artifacts can confound such experiments: transfer of labels to neighboring cells7–10 and heterotypic cell fusion11–15. The first of these artifacts is best avoided by the use of indelible (e.g. genetic) cell lineage markers whenever possible. While even transgenes or transgene products have been reported to be exchanged between cultured cells under certain conditions16, 17, chemical labels (e.g. bromodeoxyuridine (BrdU), fluorescent cell tracker dyes, and fluorescent or iron-labeled particles) are known to be readily exchanged between co-cultured cells and between graft and host following transplantation8, 10. This is a particular concern in the context of myocardial infarction, which involves brisk infiltration by phagocytic cells that are capable of taking up both label and necrotic cardiomyocyte debris. As pointed out by our group and others7, 18, such transfer can be difficult or impossible to resolve at the light microscopic level.
Heterotypic cell fusion is a process whereby a donor and host cell merge, resulting in a fused cell with the genetic information of both cells, including any genetic marker (e.g. green fluorescent protein (GFP) expression) from the donor cells. Although cell fusion is generally considered to be a rare phenomenon, it can be augmented by inflammation, which predominates in the context of myocardial infarction or allotransplantation. The fusion event can result in a binucleated cell, a mononucleated cell with a single tetraploid syncharyon, or potentially even a karyotypically normal cell (if a fusion event is followed by reductive division in which an entire set of paired chromosomes is lost19.) Thus, to most convincingly exclude cell fusion in co-culture and transplantation experiments, investigators should supplement cytogenetic analysis with the use of separate genetic lineage markers to track donor and graft cells (e.g. transplantation of GFP+ cells into a β-galactosidase-expressing recipient or the Cre-lox recombination system, discussed in more detail below). Such precautions are not always possible, but they have been successfully employed by multiple groups13, 14, 20, 21 and should be regarded as the standard, particularly when asserting cardiac potential for unexpected stem cell types.
Demonstrating an unambiguous cardiac phenotype can also be a challenge. The most common approach is to show the expression of one or more cardiac-“specific” markers by immunocytochemistry or RT-PCR. In reality, there is no single absolutely specific cardiac marker, and so this approach requires the judicious selection of multiple markers and appropriate controls. (Table 2 lists commonly employed markers as well as the non-cardiac cell types in which they are also reportedly expressed.) Ideally, such phenotyping by cardiac markers is accompanied by functional assays, such as action potential recordings or fluorescent calcium imaging.
Table 2.
Commonly used cardiomyocyte markers and extracardiac tissues/cell types in which they can also be expressed.
| Marker | Extracardiac site(s) of expression |
|---|---|
| Nkx2.5 | Embryo: Pharyngeal endoderm, spleen, stomach, tongue110, 111 |
| GATA4 | Adult: Ovary, testis, lung, liver, and small intestine112 Embryo: Proximal anddistal gut, testis, ovary, liver, visceral endoderm, and parietalendoderm112, 113 |
| T-box 5 (Tbx5) | Embryo: Eye, forelimb, genital papilla, lung, mandible, trachea114 |
| Myocyte enhancer factor 2C (MEF2c) | Adult: Skeletal muscle, brain, lymphocytes115, 116 Embryo: Skeletal muscle, smooth muscle, brain117, 118 |
| Connexin-43 | Ovary, testis, smooth muscle, eye, brain, macrophages, fibroblasts119, 120 |
| Sarcomeric myosin heavy chain | Skeletal muscle121 |
| Sarcomeric actin | Skeletal muscle122 |
| Sarcomeric actinin | Skeletal muscle123 |
| Cardiac troponin I | Fetal skeletal muscle124 |
| Cardiac troponin T | Fetal skeletal muscle125 |
| Atrial natriuretic peptide | Brain126 |
| Smooth muscle α-actin | Smooth muscle, myofibroblasts127 |
| Desmin | Smooth muscle, skeletal muscle128 |
Evidence for cardiomyocyte repopulation in postnatal hearts
Until quite recently, it was accepted dogma that cardiogenesis was limited to the developing heart in mammalian organisms. This conventional view held that post-natal cardiac expansion under physiological or pathological conditions results from cardiomyocyte hypertrophy rather than via hyperplasia or recruitment of cardiac precursors. The pendulum has now swung the opposite direction, and a new paradigm has been proposed wherein there may be several resident and extracardiac progenitor cell populations that respond to cardiac injury by cell division and subsequent differentiation into functional cardiomyocytes.
The degree to which such progenitors contribute to the renewal of adult myocardium is a source of considerable controversy. In our opinion, one of the most important advances in this area was reported recently by Hsieh et al., and we regard their study as a benchmark to evaluate findings by others in this evolving field of research22. In their elegant fate mapping study, these authors addressed two critical questions using mouse models of normal aging and cardiac injury. First, is there significant replacement of cardiomyocytes during normal aging? Second, do endogenous progenitor cells significantly contribute to the replacement of adult mammalian cardiomyocytes after injury?
Figure 1 illustrates the design and readout of the authors’ genetic pulse-chase labeling experiments. In brief, they generated a cardiac-restricted, inducible reporter mouse model by crossing two transgenic strains: the Z/EG reporter mouse in which constitutive β-galactosidase expression is replaced by the expression of GFP upon the removal of a loxP-flanked stop sequence by Cre recombinase, and a second transgenic mouse in which the cardiomyocyte-specific α-myosin heavy chain (MHC) promoter drives expression of a tamoxifen-activated form of Cre recombinase (MerCreMer). In theory, treatment of the resulting MerCreMer-ZEG mice with tamoxifen at a given time point (the ‘pulse’) should result in 100% of cardiomyocytes switching from expression of β-galactosidase to GFP (i.e. a “blue” to “green” switch). In reality, repeated dosing resulted in ~80% cardiomyocytes making the blue-to-green switch, while the remaining ~20% did not (i.e. an 80:20% ratio). The authors also performed appropriate control experiments to ensure the fidelity of their readout (e.g. confirming its reliable inducibility in cardiomyocytes and low leakiness in non-cardiomyocytes) and to exclude cell fusion.
Figure 1. Genetic fate mapping study indicating the replacement of adult mammalian cardiomyocytes by endogenous stem cells following injury.
MerCreMer mice (tamoxifen-dependent Cre recombinase expression from the α–MHC promoter) were crossed with the Z/EG reporter strain (ubiquitous lacZ expression, which is replaced by EGFP expression following Cre recombination), resulting in double heterozygous MerCreMer-ZEG mice. Pulsing the latter animals with tamoxifen induces a reporter switch from lacZ to EGFP expression in cardiomyocytes only. No reduction in the ratio of EGFP+ to lacZ+ cardiomyocytes was observed during normal aging, but a decrease in this ratio was observed following myocardial infarction, suggesting that new cardiomyocytes had been recruited from progenitor cells. (Summary of the experimental design used by Hsieh et al.22).
Their model assumes that cardiomyogenic precursors would not be labeled by the tamoxifen pulse because the αMHC-promoter should not be active in these presumably primitive cells. Thus, after a tamoxifen pulse and blue-to-green recombination in cardiomyocytes, any subsequent repopulation of cardiomyocytes from the unlabeled pool of progenitors would decrease the fraction of GFP-expressing (“green”) cardiomyocytes. In fact, during normal aging, the ratio of green/blue cardiomyocytes remained stable at 80:20%, indicating that no repopulation had occurred. By contrast, by three months following infarction, the mice showed a significant increase in the percentage of blue cardiomyocytes, resulting in a ~65:35% ratio of green-to-blue cardiomyocytes. This finding indicated that newly differentiated cardiomyocytes (blue because they have not undergone Cre-mediated recombination) had been recruited from the progenitor pool after the tamoxifen pulse. In important control experiments, the authors detected a similar frequency of cell death in β-galactosidase-expressing and GFP-expressing cardiomyocytes, ruling out the preferential death of GFP+ cardiomyocytes.
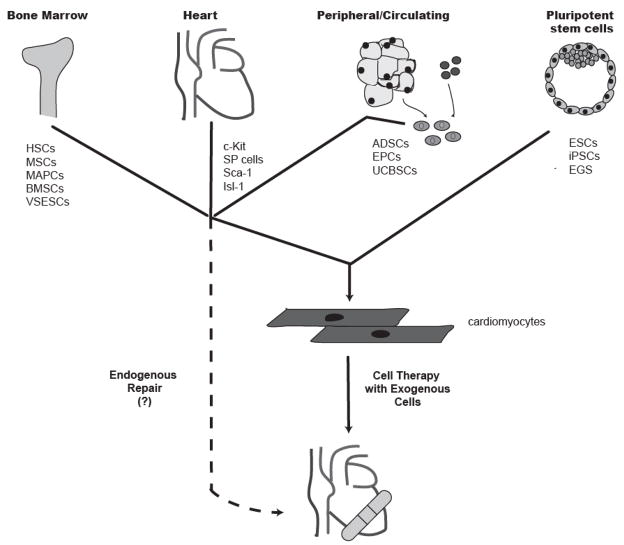
In sum, while Hsieh et al. did not find significant cardiac repopulation to occur during normal aging, their study is arguably the best evidence supporting cardiomyocyte repopulation, albeit modest, by endogenous progenitors following injury. At present, the phenotype and anatomic location of the cardiomyogenic progenitors implicated in their study remains undetermined. The authors observed that the cardiomyocyte repopulation following injury coincided with an increase in the number of intramyocardial cells expressing c-Kit (reported to be a marker of resident cardiac stem cells23, 24, as discussed below), but such data is only correlative. In the following sections, we consider some of the reportedly cardiogenic adult stem cell types that may underlie the endogenous cardiac repopulation demonstrated by Hsieh et al., as well as those that could contribute new muscle as an exogenous cell-based therapy. (Please see Figure 2). We have organized this discussion by the three compartments in which these candidate cell types reside: the bone marrow, the heart itself (so-called resident cardiac stem cells), and peripheral tissues (either circulating or in the stroma).
Figure 2. Sources of reported cardiogenic stem cells for potential use in cardiac repair.
Numerous stem cell types have been reported to be capable of differentiating into cardiomyocytes. These include multiple adult stem cell types within compartments including the bone marrow, the heart, and the circulation/peripheral tissues, as well pluripotent embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs). Efforts to “remuscularize” injured hearts are focused on either enhancing the participation of such progenitors in endogenous repair or delivering replacement cardiomyocytes derived from these cells. HSCs=hematopoietic stem cells, MSCs=mesenchymal stem cells, MAPCs=multipotent adult progenitor cells, BMSCs=bone marrow stem cells, VSESCs=very small embryonic stem cell-like stem cells, SP=side population, ADSCs=adipose stem cells, EPCs=endothelial progenitor cells, UCBSCs=umbilical cord blood stem cells, EGS=embryonic germ cells.
Bone marrow derived stem cells
Approximately one decade ago, a number of studies challenged the long-held view that adult stem cells could give rise to only a restricted set of differentiated cell types. These reports described “transdifferentiation” events, wherein adult stem cells differentiated into unexpected cell types, even across embryonic germ layer boundaries (e.g. neural stem cells giving rise to hematopoietic cell types). Although we now understand that many of these apparent transdifferentiation events actually resulted from technical artifacts and/or previously overlooked phenomenon, such as cell fusion and “ectopic” stem cells (e.g. hematopoietic stem cells resident in skeletal muscle)11, 12, the possibility that bone marrow derived stem cells might contribute to cardiac repair generated tremendous excitement. Importantly, the bone marrow is a very heterogenous compartment and contains multiple stem cell populations with putative cardiac potential (e.g. hematopoietic stem cells (HSCs)25, mesenchymal stem cells (MSCs)26–32, very small embryonic-like stem cells33, multipotent adult progenitor cells34, etc.) We here focus on those marrow-derived progenitors that have drawn the most attention by the field:
Hematopoietic stem cells (HSCs)
Interest in the cardiac potential of HSCs was stirred by animal studies in which donor-derived cells were identified in the hearts of bone marrow transplant recipients. In one such study, Jackson et al35 isolated an enriched population of HSCs (so-called side population or “SP” cells) from the bone marrow of transgenic mice that constitutively express β-galactosidase (LacZ+). They used these LacZ+ donor cells to repopulate the marrow of an irradiated wildtype recipient, which later underwent myocardial infarction by coronary ligation. Histological evaluation of the infarcted myocardium 4 weeks later revealed 0.02% of cardiomyocytes (and 3.3% of endothelial cells) to be LacZ+, indicating them to be donor-derived.
A subsequent study by Orlic et al reported a remarkable degree of regeneration following direct injection of HSCs in a mouse infarct model 25. These authors reported that the transplantation of lineage-null, c-Kit+ bone marrow stem cells from GFP-expressing transgenic mice into infarcted hearts resulted in the formation of nascent GFP+ myocardium that, on average, occupied over two-thirds of the infarct zone by 9 days post-transplantation. This impressive myocardial regeneration was accompanied by improved myocardial function and attenuated adverse ventricular remodeling.
A number of subsequent studies have failed to confirm the preceding authors’ findings. Murry et al. isolated HSCs using techniques similar to those of Orlic et al and used two transgenic mice strains as donors: mice constitutively expressing GFP and those in which the cardiac-specific α-MHC promoter drives expression of a nuclear targeted β-galactosidase (such that only cardiomyocytes show LacZ+ nuclei, thereby providing a convenient means of following both lineage and phenotype)36. After direct intracardiac injection into infarcted wildtype recipients, the latter authors were unable to find even a single donor-derived cardiomyocyte. In independent studies, both the Robbins37 and the Jacobsen13 labs were also unable to find bone marrow-derived cardiomyocytes following transplantation in murine infarct models. More recently, Rubart’s group used intravital imaging techniques to examine the functionality of bone marrow derived cells following transplantation into infarcted hearts. While these authors found the host cardiomyocytes exhibited cyclical calcium transients as expected, the graft cells did not, indicating they had not achieved a functional cardiac phenotype38.
Some (but not all) of these discrepancies regarding the cardiogenic potential of bone marrow-derived stem cells can perhaps be attributed to the previously described phenomenon of cell fusion. Using the Cre-lox recombination system, Alvarez-Dolado et al. showed that cell fusion accounted for the appearance of donor-derived parenchymal cells in a variety of tissues, including heart, following bone marrow transplantation12. Indeed, the frequency of fused cells observed by Alvarez-Dolado closely approximates the frequency of donor-derived cells found by Jackson et al35 and observed in studies by our group and others examining human recipients of gender-mismatched transplants (in which Y-chromosome in situ hybridization was used to determine cell origin)39, 40.
Nonetheless, the cardiac potential of HSCs remains controversial. The authors of the original study by Orlic et al. recently revisited this issue and again concluded that c-Kit+ bone marrow cells transdifferentiated following transplantation in a mouse infarct model and formed extensive replacement myocardium41. In this study, Rota et al. extended their original work to include the use of graft cells with both constitutive and cardiac-specific genetic reporters, Y-chromosome in situ hybridization (to exclude cell fusion, at least in the absence of reductive division), and intravital imaging studies. While this extensive study strengthens the case for HSC transdifferentiation, challenges to selected aspects of this work immediately followed38, indicating the debate is likely to continue.
Mesenchymal stem cells
MSCs are another well-characterized multipotent stem cell type that can be isolated from the bone marrow compartment. As typically prepared, MSCs are a fairly heterogenous cell population, but they generally express markers including Sca-1, CD29, CD44, CD81, CD106 in the mouse 42 and CD29, CD44, CD71, CD90, CD106, CD120a, and CD124 in humans43. MSCs differentiate into adipocyte, chondrocyte, and osteogenic phenotypes under specific media conditions43, and they can mediate immunomodulatory actions that help them avoid rejection after allotransplantation44.
The capacity of MSCs to differentiate into mesodermal lineages generated considerable interest in exploring the potential of these cells for cardiogenic differentiation and infarct repair. Makino et al.26 observed that the exposure of immortalized murine MSCs to 5-aza-deoxycytidine (5-aza-dC), an inhibitor of DNA methylation previously reported to promote the differentiation of pluripotent P19 embryonal carcinoma cells, resulted in the appearance of spontaneously beating foci. The cardiac phenotype of the treated cells was confirmed by a variety of techniques including RT-PCR (for markers including atrial natriuretic peptide (ANP), myosin light chain-2a and -2v, GATA4, and Nkx2.5), immunocytochemistry (for markers including sarcomeric MHC and α-actinin), electron microscopy, and electrophysiology (cardiac-type action potentials). Although similar findings with 5-aza-dC have been reported by others27, some have suggested that this cardiac induction may require immortalized MSCs45.
Safety concerns regarding 5-aza-dC led to interest in alternative, more targeted approaches to inducing cardiogenic differentiation by MSCs. Shim et al 28 isolated MSCs from the bone marrow of human patients undergoing coronary artery bypass surgery and treated the cells with insulin, dexamethasone, and ascorbic acid. The authors reported that the treated cells immunostained positively for α- and β-MHC and GATA4 but not for skeletal muscle markers such as skeletal MHC and MyoD. However, the efficiency of cardiogenesis by this approach appeared fairly low. The resultant “cardiomyocyte-like” cultures lacked appreciable spontaneous contractile activity, and only a small subset exhibited α-actinin-positive cross-striations. More recently, Shiota et al. have reported the cardiac induction of MSC-like progenitors derived via a complex culturing protocol involving the formation of spheres of marrow derived adherent cells46. After treatment with 5-aza-dC, the spheres showed spontaneous beating activity as well as immunoreactivity for cardiac markers including Nkx2.5 and MLC2v. The authors tested the capacity of these preparations to mediate cardiac repair in murine infarct model. They reported functional improvements following the transplantation of GFP-tagged, sphere-derived cells, but the degree of remuscularization was extremely low (~7 donor-derived cardiomyocytes per heart.)
The latter study is but one of many preclinical studies asserting beneficial effects on contractile function following the transplantation of MSCs in models of cardiac injury. Some27, 29, 32 but not all47 of these studies have concluded that MSCs transdifferentiate into cardiomyocytes in vivo. Reports favoring myocardial repopulation by MSC cells have generally shown only rare clusters of cells that lack a typical cardiomyocyte morphology but immunostained positively for one or more cardiac markers. Moreover, an important caveat with regard to the previously cited studies is that they used transferable chemical markers (such as BrdU), rather than a more indelible lineage marker to distinguish graft from host. Indeed, when Fazel et al.31 transplanted MSCs isolated from a constitutively β-galactosidase expressing mouse, they observed functional benefits but no LacZ+ cardiomyocytes.
Some insights into how MSCs might improve contractile function without directly repopulating the myocardium can be gleaned from an interesting series of studies by the Dzau group20, 48–50. Taken together, the latter work suggests that MSCs have limited cardiogenic potential but considerable promise in cell-based therapies via indirect (“paracrine”) effects on cardiac repair. These authors compared the consequences of transplanting MSCs overexpressing the pro-survival gene Akt1 to those obtained with control (i.e. β-galactosidase-expressing) MSCs in a rat infarct model. They found that the transplantation of Akt-expressing cells was far more efficacious, inhibiting adverse remodeling, inflammation, fibrosis and cardiomyocyte hypertrophy, while completely normalizing cardiac function48. Moreover, these effects did not appear mediated by cardiomyocyte repopulation, as studies using the Cre-lox recombination system revealed only rare MSC-derived cardiomyocytes, nearly all of which resulted from cell fusion20. Similar favorable effects were mediated by the injection of either medium conditioned by Akt-overexpressing MSCs49 or secreted frizzled-related protein 2 (SFRP-2), a soluble Wnt antagonist released by these cells51, implicating a paracrine mechanism of action.
Resident Cardiac Stem Cells
Side-population (SP) cells
Hierlihy et al. was the first to isolate SP cells from heart tissue using Hoechst 33342 dye exclusion, a technique previously developed to enrich resident progenitor cells from bone marrow and other organs52. Hoechst dye exclusion in primitive cells is conferred by the expression of various ATP-binding cassette (ABC) membrane transporters, such as those encoded by the multidrug resistance (MDR) genes. Hierlihy et al. found that cells with the SP phenotype comprised ~1% of all cells in the mouse heart, but they did not examine their cardiogenic potential. Martin et al.53 isolated cardiac SP cells from adult GFP+ mice and co-cultured the latter cells with cardiac main population cells from wildtype mice. After 14 days, an unspecified fraction of the GFP+ SP-derived cells immunostained for the striated muscle marker α-actinin; however, it should be noted that the antibody used does not distinguish between cardiac and skeletal forms of α-actinin.
In 2005, Pfister et al.21 described a subpopulation of murine cardiac SP cells that expresses the stem cell marker Sca-1 but is negative for the endothelial marker CD31. These cells comprised ~10% of the total SP cells (~500–1000 cells per adult mouse heart). By RT-PCR, it was shown that the CD31-/Sca1+ SP cells expressed Nkx2.5, GATA4, smooth muscle α-actin and desmin, but not α-actinin or α-MHC. After 2–3 weeks of co-culture with adult rat ventricular cardiomyocytes, it appeared that the SP cells had adopted a more mature cardiac phenotype, as evidenced by positive immunostaining for α-actinin, connexin43, and troponin I. These cells also exhibited contractions and calcium transients in response to field stimulation. Cell fusion was excluded by co-culture experiments using two separate genetic lineage markers and the Cre-lox recombination system.
Oyama et al. reported that they could induce the cardiac differentiation of SP cells from neonatal rat hearts without co-culture, by treating the cells with either oxytocin or the histone deacetylase inhibitor trichostatin A54. They also found that GFP-tagged SP cells homed to cryo-injured hearts following intravenous infusion. GFP+ cardiomyocytes were observed in the injured hearts (comprising 4.4% of all cells), but cell fusion was not excluded.
While we look forward to more definitive transplantation studies with cardiac SP cells, the in vivo response of endogenous SP cells to cardiac injury also remains a source of some uncertainty. On the one hand, Martin et al. reported that the number of cardiac SP cells increased following cryoinjury in the mouse heart55. By contrast, Mouquet et al observed an acute decrease in the number of cardiac SP cells post-infarction in a mouse model of permanent coronary ligation, followed by a gradual return to baseline levels over 7 days (via proliferation of resident cardiac SP cells and homing of bone marrow derived SP cells)56. At present, we can only speculate that the apparent discrepancies in SP cell dynamics between the two studies result from the differing modes of cardiac injury.
c-Kit+ cells
In 2003, Beltrami et al. reported a population of resident cardiac progenitors in adult rat heart that were identified by their expression of the surface antigen and stem cell marker, c-Kit23. Although rare overall (~1 c-Kit+ cell per 104 total myocardial cells), histological analysis revealed these cells to be located in small clusters within the interstitium of the ventricular and atrial myocardium with a higher density in the atria and the ventricular apex. Cardiac c-Kit+ cells were found to be self-renewing, clonogenic, and multipotent, differentiating into cardiomyocytes, smooth muscle and endothelial cells. While their differentiated progeny remained quite immature in vitro (i.e. lacking structural organization, identifiable sarcomeres, and contractile activity), this was in stark contrast to their in vivo capabilities. By twenty days following transplantation into the infarct border zone of syngeneic rats, BrdU-labeled c-Kit+ cells had colonized the infarcted area and appeared to have matured with expression of sarcomeric proteins (cardiac MHC), cross striations, and connexin43-positive intercalated discs. Morphometric analyses revealed that the injected cells had given rise to 13×106 new cardiomyocytes, corresponding to a 65-fold increase from the day of injection. This was accompanied by an increase in vascular density with incorporation of the graft cells. Similarly impressive cardiac regeneration and functional improvements by echocardiography were reported after the intracoronary infusion of c-Kit+ cardiac stem cells in both rat and canine infarct models57. More recently, these investigators have reported the isolation and expansion of c-Kit+ cells from human surgical specimens24.
The significance of these findings would be far reaching, but it remains to be seen whether this success can be reproduced in the target population of patients with ischemic heart disease. Other adult stem cell populations are known to be reduced with advanced age and/or disease; and, indeed, a recent study by Pouly et al. failed to demonstrate c-Kit+ resident stem cells in endomyocardial biopsies from typical human patients58.
Sca-1+ cells
Oh et al. reported the existence of resident cardiac progenitor cells in the adult mouse heart that express stem cell antigen-1 (Sca-1) but not c-Kit14. A small percentage of Sca-1+ cells expressed cardiac-specific genes like Nkx2.5 and sarcomeric proteins after treatment with the demethylating agent 5-aza-dC, but no spontaneous beating activity was observed. Following intravenous infusion in a mouse infarct model, Sca-1+ cells homed to injured myocardium, where they appeared to have differentiated into cardiomyocytes in the border zone with expression of sarcomeric α-actin, cardiac troponin I, and connexin 43. These authors used the Cre-lox recombination system to distinguish cell fusion from direct incorporation and concluded that each accounted for ~50% of the apparently donor-derived cells.
Matsuura and colleagues also reported the isolation of Sca-1 cells from adult murine hearts 59. Although 5-aza-dC failed to induce cardiac differentiation in their hands, they found that oxytocin resulted in a small fraction (~1%) of Sca-1+ cells exhibiting spontaneous beating activity and expression of sarcomeric α-actin, cardiac troponin I, Nkx2.5, and MHC. More recently, Wang et al. reported that Sca-1+ cells from the mouse heart could be induced to express cardiac markers by treatment with 5-aza-dC, FGF4, FGF8, and the Wnt antagonist Dickkopf-160. These authors also transplanted Sca-1+/CD31- cells into the acutely infarcted mouse heart and found that this intervention attenuated the progressive functional decline, while promoting angiogenesis and resulting in “modest” cardiomyocyte repopulation (i.e. LacZ+ cardiomyocytes that immunostained with troponin T, α-sarcomeric actin, and N-cadherin). Unfortunately, the authors did not determine the frequency of such cardiomyocytes or perform experiments to exclude cell fusion60.
Because humans do not have the Sca-1 gene61, it is unclear how an “orthologous” population of progenitors would be isolated from the human heart.
Cardiospheres
In 2004, Messina et al. described a novel isolation technique for resident cardiac progenitors from murine hearts as well as subcultures of human atrial or ventricular specimens62. Mild enzymatic digestion of the tissue specimens yielded small, round, phase-bright cells that clustered together in suspension. These sphere-generating cells were then allowed to adhere on poly-L-lysine coated plates and cultured in a media enriched with cytokines (epidermal growth factor, bFGF, cardiotrophin-1, thrombin). These “cardiosphere” (or CS)-derived cells were self-renewing, clonogenic, and expressed both endothelial (KDR in human, flk-1 in mouse cells, and CD31) and stem cell markers (CD34, c-Kit, Sca-1). Mouse CS-derived cells showed spontaneous contractile activity, while human CS cells did so only after 24 hours of co-culture with postnatal rat cardiomyocytes. CS-cells from both human and mouse demonstrated tri-lineage differentiation into cardiomyocytes, endothelial and smooth muscle cells; however, quantitative data as to the frequency of these events were not reported. CS-derived cardiomyocytes expressed cardiac markers including cardiac troponin I, ANP, and cardiac MHC. In vivo, CS-derived cells were reported to regenerate the infarcted mouse heart 62.
Subsequently, Smith et al. expanded on these findings by isolating CS-forming cells from human biopsy specimens63. The human cardiospheres, which were successfully isolated from 69 of 70 biopsies attempted, consistently expressed c-Kit but not the multidrug resistance gene, MDR1, indicating that these cells were phenotypically distinct from the resident cardiac progenitors previously identified in situ (c-Kit+, MDR1+)64, 65. Consistent with findings by Messina et al.62, human CS-derived cells did not spontaneously contract, but co-culture with neonatal rat cardiomyocytes evoked calcium transients in synchrony with neighboring cardiomyocytes, action potentials, and fast inward sodium current. Smith et al. also injected lentivirally-transduced LacZ+ human CS-derived cells into the border zone of infarcted SCID-beige mice63. Twenty days later, the CS-derived cells were found throughout border regions of the mouse heart, and occasional donor cells immunostained for α-sarcomeric actin and von Willebrand factor. Echocardiography showed improvements in global left ventricular function, but, given the apparently limited cardiomyocyte repopulation by LacZ+ cells, these functional effects were attributed to a combination of regeneration and paracrine effects.
Based on these studies, explant-derived cardiospheres appear to have cardiomyogenic potential and considerable promise for cardiac repair. However, a recent study by Shenje et al. gives some reason for caution7. These authors performed lineage tracing of cardiac explant-derived cells from a transgenic mouse with a highly sensitive cardiac reporter based on the Cre-lox recombination system (i.e. MLC2v-Cre/ZEG mice, in which expression of GFP indicates cardiac differentiation). They observed the emergence of small, round, phase-bright cells as described by others62, 63, but they did not place these explant-derived cells in suspension cultures so as to form cardiospheres. Notwithstanding this important technical difference, the explant-derived cells characterized by Shenje et al. had a perplexing phenotype in light of the preceding work. Their cells lacked expression of Sca-1, c-Kit, Nkx2.5, and ANP, and the sensitive MLC2v-Cre/ZEG genetic reporter was not activated. GFP+, sarcomeric actin-expressing explant-derived cells were observed, but these were convincingly shown by ultrastructural studies to result from phagocytosis of GFP-expressing myocytes cells, rather than activation of the reporter. (The latter finding underscores the challenge of co-localization studies at the light microscopic level when phagocytic cell types are present, a point that we have previously emphasized18.) Finally, the authors examined the functionality of these cells following transplantation with intravital imaging studies and found no evidence for calcium cycling by the graft.
Isl1+ cells
Islet-1 (Isl1), a LIM homeodomain transcription factor, is expressed by progenitor cells of the secondary heart field, a structure present during early development that gives rise to the formation of the outflow tract, the atria and the right ventricle. In 2005, Laugwitz et al. showed that a population of Isl1+ cells persists in neonatal mouse hearts and that these cells express the cardiac transcription factors Nkx2.5 and GATA4, but not Sca-1, CD31, or c-kit66. Isolated Isl1+ progenitor cells self-renew and maintain the ability to differentiate into functional cardiomyocytes in vitro and in vivo. Rare Isl1+ cells were identified in early postnatal rat, mouse, and human myocardium66, but they may not be present in adult human heart.
Stem cells in the periphery and/or circulation
Endothelial progenitor cells (EPCs)
EPCs are more properly viewed as both a circulating and a bone marrow stem cell type, as they are known to reside in both compartments. In 1997, Asahara and colleagues described the phenotype of EPCs, which proliferate in response to tissue ischemia, home to areas of injury, and either incorporate within or otherwise promote neovascularization67, 68. EPCs express markers including Flk-1, CD32, and CD133, and can differentiate into definitive endothelial cells67, 69–71. Initial interest in the application of EPCs to cardiac repair was naturally focused on their angiogenic properties.
The capacity of EPCs to transdifferentiate into cardiomyocytes was first reported by the Dimmeler group in 200372. In their study, CD34+ human EPCs were obtained from peripheral blood mononuclear cells of healthy adults or from patients with coronary artery disease. After co-culture with neonatal rat cardiomyocytes, EPCs were reported to transdifferentiate into cardiomyocytes based on morphology, α-sarcomeric actinin immunoreactivity by flow cytometry, and expression of other cardiac markers by immunostaining or RT-PCR with species-specific probes. Furthermore, the EPCs showed calcium transients that synchronized with adjacent rat cardiomyocytes suggesting communication with the host myocardium by gap junctions. Co-culture experiments with paraformaldehyde-fixed cardiomyocytes indicated that cell fusion was not required for EPCs to acquire the cardiac phenotype72–75. Still, the efficiency of cardiac induction by EPCs was very low: even after enhancement with inhibition of Notch signaling, ≪1% of EPCs expressed α-sarcomeric actinin75. Asahara’s group reported even lower rates of in vitro cardiac transdifferentiation following co-culture of EPCs with the rat heart-derived H9C2 cell line76. The latter authors also reported the in vivo cardiac differentiation of a related preparation of human circulating cells following transplantation into a rodent infarct model. This conclusion is complicated, however, by the definite demonstration of cell fusion between host myocytes and graft cells, using species-specific fluorescent in situ hybridization probes77.
Moreover, Gruh et al were unable to confirm the in vitro cardiac differentiation of EPCs following co-culture with primary myocytes78. These authors detected no expression of human cardiac transcripts (in contrast to the previously discussed studies73–75), and they concluded that the rare, ostensibly transdifferentiated EPCs observed by FACS or epifluorescence microscopy were artifacts due to overlying cells and/or autofluorescence. Thus, while the cardiac potential of EPCs remains a source of controversy, this report by Gruh et al. again underscores the challenges inherent to interpreting co-culture experiments.
Other post-natal stem cell types with reported cardiogenic potential
Several other post- (or peri-) natal stem cell types have been reported to have cardiogenic potential, among which adipose-derived stem cells (ADSCs) and umbilical cord-derived stem cells (UCBSCs) are just two more extensively studied examples. In 2004, Planat-Benard et al. reported the differentiation of spontaneously beating cells from ADSCs, albeit at a vanishingly low frequency (0.02–0.07%)79. Several cardiac markers were detected by RT-PCR and immunocytochemistry and the cells exhibited “pacemaker-like” action potentials, but these action potentials had a strikingly brief duration that would be unusual even for primitive cardiomyocytes. Recently, Yamada et al. reported a ten-fold higher yield of ADSCs from brown rather than white fat. Following transplantation in a mouse infarct model, brown fat ADSCs had dramatic beneficial functional effects and contributed to cardiac repopulation, but cell fusion was not excluded80.
The cardiogenic potential of UCBSCs remains controversial. On the one hand, three studies have described cardiomyogenic differentiation of UCBSCs in vivo 81 and in vitro following 5-aza-dc treatment 82 or co-culture with fetal mouse cardiomyocytes 83. On the other hand, two groups independently concluded that UCBSCs home to infarcted rodent hearts after intravenous infusion and exert functional benefits without differentiating into cardiomyocytes84,85.
Cell-based cardiac repair with exogenous pluripotent stem cells
In this section, we consider the cardiogenic potential of pluripotent stem cells—cell types that are uninvolved in endogenous reparative mechanisms but may nonetheless prove useful as an exogenous cell therapy. While the preclinical development of therapies with pluripotent stem cell types certainly lags that with adult stem cells, there has been a recent flurry of encouraging preclinical studies indicating functional efficacy with ESC-based cardiac repair86–91. It is no coincidence that these encouraging developments are happening now—they reflect a decade “on the learning curve” with human embryonic stem cells (hESCs) as well as the application of recent insights from developmental biology. Given the recent reprogramming of human somatic cells into ESC-like pluripotent cells, it is becoming increasingly plausible to envision a “second generation” of cell-based cardiac therapies that will exploit the tremendous expandability and unquestioned cardiac potential of pluripotent stem cells.
Embryonic stem cells
ESCs are pluripotent cells derived from the inner cell mass of preimplantation-stage blastocysts. Murine ESCs were first isolated in 198192, 93, and their human counterparts in 199894. ESCs from both species show similar features in culture: First, they can be propagated indefinitely as a stable, self-renewing population. Second, they have a pluripotent phenotype, meaning they can differentiate into cell types from all three primary germ layers both in vitro95 and in vivo92–94. The latter property represents both an advantage and a disadvantage to the use of these cells: the intra-cardiac transplantation of undifferentiated ESCs or preparations of insufficiently purified ESC-derived cardiomyocytes results in teratoma formation88, 90, 96. As this would obviously be a disastrous outcome, the development of cell therapies based on pluripotent stem cells will require tight control of their differentiation into useful cell types.
Human ESC-derived cardiomyocytes (hESC-CMs) have an unambiguous cardiac phenotype by transcriptional, immunocytochemical, ultrastructural, and functional endpoints. They express expected cardiac markers, including cardiac-specific transcription factors (e.g. Nkx2.5, GATA4, MEF2c, Tbx-5 and Tbx-20), sarcomeric proteins (e.g. cardiac troponins I and T, sarcomeric MHCs and actins), and chamber-specific proteins (e.g. ANP, MLC2V and MLC2A)97–99. They exhibit spontaneous beating activity, characteristic cardiac ionic currents, and nodal-, atrial-, and ventricular-like action potentials100, 101.
Historically, the most common method for deriving hESC-CMs in vitro has been via the formation of three-dimensional aggregates, so-called embryoid bodies, and treating the cells with high serum to induce differentiation97, 99. The efficiency of cardiogenesis is generally low in this approach, but the field has applied lessons from molecular and developmental biology to derive preparations of greater cardiac purity. Over a decade ago, the Field group demonstrated the power of genetic selection to purify cardiomyocytes from pluripotent stem cells by introducing a transgene in which the cardiac specific α-MHC promoter drives expression of neomyocin resistance102. After antibiotic selection of differentiated embryoid bodies, the resultant cultures contained 99.6% αMHC+ cardiomyocytes versus 0.6% in untreated cultures. Similar approaches have recently been shown to be successful with hESCs103, 104.
Our group and others have used insights from developmental biology to devise protocols to more efficiently guide the differentiation of hESCs into cardiomyocytes86, 87, 105. For example, we showed that the serial application of two TGF superfamily members, activin A and BMP4, followed by Percoll gradient purification, resulted in preparations of >80% β-MHC+ hESC-CMs86. Following injection into recently infarcted rat hearts, these cells formed stable grafts of human myocardium. Although we do not yet know whether hESC-CMs undergo systolic activation in vivo, echocardiographic and MRI studies indicated beneficial effects on global and regional left ventricular function. Interestingly, these effects appeared to require the presence of engrafted cardiomyocytes, as they were not observed with the transplantation of non-cardiac hESC derivatives. Multiple groups have now independently reported partial remuscularization and functional benefits following hESC-CM transplantation in rodent infarct models86, 88, 89.
Induced pluripotent stem cells
Three independent groups have reported the generation of ESC-like iPSCs by reprogramming human somatic cells with the viral delivery of four stem cell associated genes106–108. Ethical concerns are avoided because no embryos are harmed, and, in principle, the cells can be used in autologous cell therapies. There are risks associated with the integrating viruses currently used to generate iPSCs, but we anticipate that safer, non-viral approaches will be developed soon.
MacLellan’s group has demonstrated the cardiac phenotype of murine iPSC progeny in vitro and in vivo following blastocyst injection109. Murine iPSC-derived cardiomyocytes immunostain for sarcomeric MHC and troponin C, and they express transcripts including NKx2.5, GATA-4, MEF2c, ANP, MLC2v, and MLC2a. They exhibit calcium transients in response to field stimulation. Of note, Yamanaka’s group has reported that human iPSCs can be differentiated into cardiomyocytes using methods developed by our group for hESCs106. It is too soon to know whether iPSCs will be of value in cell-based cardiac repair, but they are an attractive new candidate.
Conclusions
Remuscularization of injured hearts is an ambitious goal, but recent studies indicating that adult mammalian hearts undergo limited cardiomyocyte repopulation in response to injury gives some reason for hope. In this review, we have considered only one aspect of this challenge—the identification of potential sources of replacement cardiomyocytes. Advances in stem cell and developmental biology have resulted in the identification of numerous candidate stem cell types with putative cardiogenic potential. Nonetheless, the ideal cell type remains uncertain, despite all claims to the contrary. In our opinion, the cardiogenic potential of bone marrow derived and circulating stem cells appears limited, but other candidates including certain resident cardiac stem cell populations and pluripotent stem cells are clearly capable of more efficient cardiogenesis. We are optimistic that research into cell-based cardiac repair will eventually yield effective myogenic therapies, but success will require rigorous attention to cardiac phenotyping, cell fate mapping, and preclinical and clinical testing.
Acknowledgments
The authors thank Drs. Kip Hauch and Charles Murry for critical scientific discussions and helpful comments. We apologize to colleagues in the field whose work could not be referenced due to space limitations.
Source of Funding
M.A. Laflamme is supported in part by NIH grants RO1-HL064387 and K08-HL80431.
Footnotes
Disclosures
M.A. Laflamme has a sponsored research agreement and is a consultant with Geron Corporation. Drs. Reinecke, Minami, and Zhu have no conflicts of interest to declare.
References
- 1.Leor J, Patterson M, Quinones MJ, Kedes LH, Kloner RA. Transplantation of fetal myocardial tissue into the infarcted myocardium of rat. A potential method for repair of infarcted myocardium? Circulation. 1996;94:II332–336. [PubMed] [Google Scholar]
- 2.Li RK, Jia ZQ, Weisel RD, Mickle DA, Zhang J, Mohabeer MK, Rao V, Ivanov J. Cardiomyocyte transplantation improves heart function. Ann Thorac Surg. 1996;62:654–660. doi: 10.1016/s0003-4975(96)00389-x. discussion 660–651. [DOI] [PubMed] [Google Scholar]
- 3.Scorsin M, Hagege AA, Marotte F, Mirochnik N, Copin H, Barnoux M, Sabri A, Samuel JL, Rappaport L, Menasche P. Does transplantation of cardiomyocytes improve function of infarcted myocardium? Circulation. 1997;96:II-188–193. [PubMed] [Google Scholar]
- 4.Rubart M, Pasumarthi KB, Nakajima H, Soonpaa MH, Nakajima HO, Field LJ. Physiological coupling of donor and host cardiomyocytes after cellular transplantation. Circ Res. 2003;92:1217–1224. doi: 10.1161/01.RES.0000075089.39335.8C. [DOI] [PubMed] [Google Scholar]
- 5.Zimmermann WH, Melnychenko I, Wasmeier G, Didie M, Naito H, Nixdorff U, Hess A, Budinsky L, Brune K, Michaelis B, Dhein S, Schwoerer A, Ehmke H, Eschenhagen T. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006;12:452–458. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 7.Shenje LT, Field LJ, Pritchard CA, Guerin CJ, Rubart M, Soonpaa MH, Ang KL, Galinanes M. Lineage tracing of cardiac explant derived cells. PLoS ONE. 2008;3:e1929. doi: 10.1371/journal.pone.0001929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terrovitis J, Stuber M, Youssef A, Preece S, Leppo M, Kizana E, Schar M, Gerstenblith G, Weiss RG, Marban E, Abraham MR. Magnetic resonance imaging overestimates ferumoxide-labeled stem cell survival after transplantation in the heart. Circulation. 2008;117:1555–1562. doi: 10.1161/CIRCULATIONAHA.107.732073. [DOI] [PubMed] [Google Scholar]
- 9.Coyne TM, Marcus AJ, Woodbury D, Black IB. Marrow stromal cells transplanted to the adult brain are rejected by an inflammatory response and transfer donor labels to host neurons and glia. Stem Cells. 2006;24:2483–2492. doi: 10.1634/stemcells.2006-0174. [DOI] [PubMed] [Google Scholar]
- 10.Burns TC, Ortiz-Gonzalez XR, Gutierrez-Perez M, Keene CD, Sharda R, Demorest ZL, Jiang Y, Nelson-Holte M, Soriano M, Nakagawa Y, Luquin MR, Garcia-Verdugo JM, Prosper F, Low WC, Verfaillie CM. Thymidine analogs are transferred from prelabeled donor to host cells in the central nervous system after transplantation: a word of caution. Stem Cells. 2006;24:1121–1127. doi: 10.1634/stemcells.2005-0463. [DOI] [PubMed] [Google Scholar]
- 11.Vieyra DS, Jackson KA, Goodell MA. Plasticity and tissue regenerative potential of bone marrow-derived cells. Stem Cell Rev. 2005;1:65–69. doi: 10.1385/SCR:1:1:065. [DOI] [PubMed] [Google Scholar]
- 12.Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez-Buylla A. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–73. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- 13.Nygren JM, Jovinge S, Breitbach M, Sawen P, Roll W, Hescheler J, Taneera J, Fleischmann BK, Jacobsen SE. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med. 2004;10:494–501. doi: 10.1038/nm1040. [DOI] [PubMed] [Google Scholar]
- 14.Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, Pocius J, Michael LH, Behringer RR, Garry DJ, Entman ML, Schneider MD. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci U S A. 2003;100:12313–12318. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reinecke H, Minami E, Poppa V, Murry CE. Evidence for fusion between cardiac and skeletal muscle cells. Circ Res. 2004;94:e56–60. doi: 10.1161/01.RES.0000125294.04612.81. [DOI] [PubMed] [Google Scholar]
- 16.Bergsmedh A, Ehnfors J, Spetz AL, Holmgren L. A Cre-loxP based system for studying horizontal gene transfer. FEBS Lett. 2007;581:2943–2946. doi: 10.1016/j.febslet.2007.05.045. [DOI] [PubMed] [Google Scholar]
- 17.Koyanagi M, Brandes RP, Haendeler J, Zeiher AM, Dimmeler S. Cell-to-cell connection of endothelial progenitor cells with cardiac myocytes by nanotubes: a novel mechanism for cell fate changes? Circ Res. 2005;96:1039–1041. doi: 10.1161/01.RES.0000168650.23479.0c. [DOI] [PubMed] [Google Scholar]
- 18.Laflamme MA, Murry CE. Regenerating the heart. Nat Biotechnol. 2005;23:845–856. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Olson S, Grompe M. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422:897–901. doi: 10.1038/nature01531. [DOI] [PubMed] [Google Scholar]
- 20.Noiseux N, Gnecchi M, Lopez-Ilasaca M, Zhang L, Solomon SD, Deb A, Dzau VJ, Pratt RE. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol Ther. 2006;14:840–850. doi: 10.1016/j.ymthe.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 21.Pfister O, Mouquet F, Jain M, Summer R, Helmes M, Fine A, Colucci WS, Liao R. CD31- but Not CD31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ Res. 2005;97:52–61. doi: 10.1161/01.RES.0000173297.53793.fa. [DOI] [PubMed] [Google Scholar]
- 22.Hsieh PC, Segers VF, Davis ME, MacGillivray C, Gannon J, Molkentin JD, Robbins J, Lee RT. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med. 2007;13:970–974. doi: 10.1038/nm1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 24.Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Lecapitaine N, Cascapera S, Beltrami AP, D’Alessandro DA, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, Leri A, Anversa P. Human cardiac stem cells. Proc Natl Acad Sci U S A. 2007;104:14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 26.Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H, Hata J, Umezawa A, Ogawa S. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103:697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tomita S, Li RK, Weisel RD, Mickle DA, Kim EJ, Sakai T, Jia ZQ. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation. 1999;100:II247–256. doi: 10.1161/01.cir.100.suppl_2.ii-247. [DOI] [PubMed] [Google Scholar]
- 28.Shim WS, Jiang S, Wong P, Tan J, Chua YL, Tan YS, Sin YK, Lim CH, Chua T, Teh M, Liu TC, Sim E. Ex vivo differentiation of human adult bone marrow stem cells into cardiomyocyte-like cells. Biochem Biophys Res Commun. 2004;324:481–488. doi: 10.1016/j.bbrc.2004.09.087. [DOI] [PubMed] [Google Scholar]
- 29.Piao H, Youn TJ, Kwon JS, Kim YH, Bae JW, Bora S, Kim DW, Cho MC, Lee MM, Park YB. Effects of bone marrow derived mesenchymal stem cells transplantation in acutely infarcting myocardium. Eur J Heart Fail. 2005;7:730–738. doi: 10.1016/j.ejheart.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 30.Nagaya N, Kangawa K, Itoh T, Iwase T, Murakami S, Miyahara Y, Fujii T, Uematsu M, Ohgushi H, Yamagishi M, Tokudome T, Mori H, Miyatake K, Kitamura S. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation. 2005;112:1128–1135. doi: 10.1161/CIRCULATIONAHA.104.500447. [DOI] [PubMed] [Google Scholar]
- 31.Fazel S, Chen L, Weisel RD, Angoulvant D, Seneviratne C, Fazel A, Cheung P, Lam J, Fedak PW, Yau TM, Li RK. Cell transplantation preserves cardiac function after infarction by infarct stabilization: augmentation by stem cell factor. J Thorac Cardiovasc Surg. 2005;130:1310. doi: 10.1016/j.jtcvs.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 32.Dai W, Hale SL, Martin BJ, Kuang JQ, Dow JS, Wold LE, Kloner RA. Allogeneic mesenchymal stem cell transplantation in postinfarcted rat myocardium: short- and long-term effects. Circulation. 2005;112:214–223. doi: 10.1161/CIRCULATIONAHA.104.527937. [DOI] [PubMed] [Google Scholar]
- 33.Dawn B, Tiwari S, Kucia MJ, Zuba-Surma EK, Guo Y, Sanganalmath SK, Abdel-Latif A, Hunt G, Vincent RJ, Taher H, Reed NJ, Ratajczak MZ, Bolli R. Transplantation of bone marrow-derived very small embryonic-like stem cells attenuates left ventricular dysfunction and remodeling after myocardial infarction. Stem Cells. 2008;26:1646–1655. doi: 10.1634/stemcells.2007-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeng L, Hu Q, Wang X, Mansoor A, Lee J, Feygin J, Zhang G, Suntharalingam P, Boozer S, Mhashilkar A, Panetta CJ, Swingen C, Deans R, From AH, Bache RJ, Verfaillie CM, Zhang J. Bioenergetic and functional consequences of bone marrow-derived multipotent progenitor cell transplantation in hearts with postinfarction left ventricular remodeling. Circulation. 2007;115:1866–1875. doi: 10.1161/CIRCULATIONAHA.106.659730. [DOI] [PubMed] [Google Scholar]
- 35.Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, Entman ML, Michael LH, Hirschi KK, Goodell MA. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001;107:1395–1402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA, Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 37.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 38.Scherschel JA, Soonpaa MH, Srour EF, Field LJ, Rubart M. Adult bone marrow-derived cells do not acquire functional attributes of cardiomyocytes when transplanted into peri-infarct myocardium. Mol Ther. 2008;16:1129–1137. doi: 10.1038/mt.2008.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laflamme MA, Myerson D, Saffitz JE, Murry CE. Evidence for cardiomyocyte repopulation by extracardiac progenitors in transplanted human hearts. Circ Res. 2002;90:634–640. doi: 10.1161/01.res.0000014822.62629.eb. [DOI] [PubMed] [Google Scholar]
- 40.Minami E, Laflamme MA, Saffitz JE, Murry CE. Extracardiac progenitor cells repopulate most major cell types in the transplanted human heart. Circulation. 2005;112:2951–2958. doi: 10.1161/CIRCULATIONAHA.105.576017. [DOI] [PubMed] [Google Scholar]
- 41.Rota M, Kajstura J, Hosoda T, Bearzi C, Vitale S, Esposito G, Iaffaldano G, Padin-Iruegas ME, Gonzalez A, Rizzi R, Small N, Muraski J, Alvarez R, Chen X, Urbanek K, Bolli R, Houser SR, Leri A, Sussman MA, Anversa P. Bone marrow cells adopt the cardiomyogenic fate in vivo. Proc Natl Acad Sci U S A. 2007;104:17783–17788. doi: 10.1073/pnas.0706406104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baddoo M, Hill K, Wilkinson R, Gaupp D, Hughes C, Kopen GC, Phinney DG. Characterization of mesenchymal stem cells isolated from murine bone marrow by negative selection. J Cell Biochem. 2003;89:1235–1249. doi: 10.1002/jcb.10594. [DOI] [PubMed] [Google Scholar]
- 43.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 44.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 45.Liu Y, Song J, Liu W, Wan Y, Chen X, Hu C. Growth and differentiation of rat bone marrow stromal cells: does 5-azacytidine trigger their cardiomyogenic differentiation? Cardiovasc Res. 2003;58:460–468. doi: 10.1016/s0008-6363(03)00265-7. [DOI] [PubMed] [Google Scholar]
- 46.Shiota M, Heike T, Haruyama M, Baba S, Tsuchiya A, Fujino H, Kobayashi H, Kato T, Umeda K, Yoshimoto M, Nakahata T. Isolation and characterization of bone marrow-derived mesenchymal progenitor cells with myogenic and neuronal properties. Exp Cell Res. 2007;313:1008–1023. doi: 10.1016/j.yexcr.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 47.Silva GV, Litovsky S, Assad JA, Sousa AL, Martin BJ, Vela D, Coulter SC, Lin J, Ober J, Vaughn WK, Branco RV, Oliveira EM, He R, Geng YJ, Willerson JT, Perin EC. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111:150–156. doi: 10.1161/01.CIR.0000151812.86142.45. [DOI] [PubMed] [Google Scholar]
- 48.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, Dzau VJ. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 49.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 50.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, Dzau VJ. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. Faseb J. 2006;20:661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 51.Mirotsou M, Zhang Z, Deb A, Zhang L, Gnecchi M, Noiseux N, Mu H, Pachori A, Dzau V. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci U S A. 2007;104:1643–1648. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hierlihy AM, Seale P, Lobe CG, Rudnicki MA, Megeney LA. The post-natal heart contains a myocardial stem cell population. FEBS Lett. 2002;530:239–243. doi: 10.1016/s0014-5793(02)03477-4. [DOI] [PubMed] [Google Scholar]
- 53.Martin CM, Meeson AP, Robertson SM, Hawke TJ, Richardson JA, Bates S, Goetsch SC, Gallardo TD, Garry DJ. Persistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac SP cells in the developing and adult heart. Dev Biol. 2004;265:262–275. doi: 10.1016/j.ydbio.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 54.Oyama T, Nagai T, Wada H, Naito AT, Matsuura K, Iwanaga K, Takahashi T, Goto M, Mikami Y, Yasuda N, Akazawa H, Uezumi A, Takeda S, Komuro I. Cardiac side population cells have a potential to migrate and differentiate into cardiomyocytes in vitro and in vivo. J Cell Biol. 2007;176:329–341. doi: 10.1083/jcb.200603014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martin CM, Ferdous A, Gallardo T, Humphries C, Sadek H, Caprioli A, Garcia JA, Szweda LI, Garry MG, Garry DJ. Hypoxia-inducible factor-2alpha transactivates Abcg2 and promotes cytoprotection in cardiac side population cells. Circ Res. 2008;102:1075–1081. doi: 10.1161/CIRCRESAHA.107.161729. [DOI] [PubMed] [Google Scholar]
- 56.Mouquet F, Pfister O, Jain M, Oikonomopoulos A, Ngoy S, Summer R, Fine A, Liao R. Restoration of cardiac progenitor cells after myocardial infarction by self-proliferation and selective homing of bone marrow-derived stem cells. Circ Res. 2005;97:1090–1092. doi: 10.1161/01.RES.0000194330.66545.f5. [DOI] [PubMed] [Google Scholar]
- 57.Dawn B, Stein AB, Urbanek K, Rota M, Whang B, Rastaldo R, Torella D, Tang XL, Rezazadeh A, Kajstura J, Leri A, Hunt G, Varma J, Prabhu SD, Anversa P, Bolli R. Cardiac stem cells delivered intravascularly traverse the vessel barrier, regenerate infarcted myocardium, and improve cardiac function. Proc Natl Acad Sci U S A. 2005;102:3766–3771. doi: 10.1073/pnas.0405957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pouly J, Bruneval P, Mandet C, Proksch S, Peyrard S, Amrein C, Bousseaux V, Guillemain R, Deloche A, Fabiani JN, Menasche P. Cardiac stem cells in the real world. J Thorac Cardiovasc Surg. 2008;135:673–678. doi: 10.1016/j.jtcvs.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 59.Matsuura K, Wada H, Nagai T, Iijima Y, Minamino T, Sano M, Akazawa H, Molkentin JD, Kasanuki H, Komuro I. Cardiomyocytes fuse with surrounding noncardiomyocytes and reenter the cell cycle. J Cell Biol. 2004;167:351–363. doi: 10.1083/jcb.200312111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang X, Hu Q, Nakamura Y, Lee J, Zhang G, From AH, Zhang J. The role of the sca-1+/CD31- cardiac progenitor cell population in postinfarction left ventricular remodeling. Stem Cells. 2006;24:1779–1788. doi: 10.1634/stemcells.2005-0386. [DOI] [PubMed] [Google Scholar]
- 61.Holmes C, Stanford WL. Concise review: stem cell antigen-1: expression, function, and enigma. Stem Cells. 2007;25:1339–1347. doi: 10.1634/stemcells.2006-0644. [DOI] [PubMed] [Google Scholar]
- 62.Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F, Salio M, Battaglia M, Latronico MV, Coletta M, Vivarelli E, Frati L, Cossu G, Giacomello A. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–921. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 63.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marban E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 64.Quaini F, Urbanek K, Beltrami AP, Finato N, Beltrami CA, Nadal-Ginard B, Kajstura J, Leri A, Anversa P. Chimerism of the transplanted heart. N Engl J Med. 2002;346:5–15. doi: 10.1056/NEJMoa012081. [DOI] [PubMed] [Google Scholar]
- 65.Urbanek K, Quaini F, Tasca G, Torella D, Castaldo C, Nadal-Ginard B, Leri A, Kajstura J, Quaini E, Anversa P. Intense myocyte formation from cardiac stem cells in human cardiac hypertrophy. Proc Natl Acad Sci U S A. 2003;100:10440–10445. doi: 10.1073/pnas.1832855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, Lin LZ, Cai CL, Lu MM, Reth M, Platoshyn O, Yuan JX, Evans S, Chien KR. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–653. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 68.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 69.Shi Q, Rafii S, Wu MH, Wijelath ES, Yu C, Ishida A, Fujita Y, Kothari S, Mohle R, Sauvage LR, Moore MA, Storb RF, Hammond WP. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998;92:362–367. [PubMed] [Google Scholar]
- 70.Murohara T, Ikeda H, Duan J, Shintani S, Sasaki K, Eguchi H, Onitsuka I, Matsui K, Imaizumi T. Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. J Clin Invest. 2000;105:1527–1536. doi: 10.1172/JCI8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rafii S. Circulating endothelial precursors: mystery, reality, and promise. J Clin Invest. 2000;105:17–19. doi: 10.1172/JCI8774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Badorff C, Brandes RP, Popp R, Rupp S, Urbich C, Aicher A, Fleming I, Busse R, Zeiher AM, Dimmeler S. Transdifferentiation of blood-derived human adult endothelial progenitor cells into functionally active cardiomyocytes. Circulation. 2003;107:1024–1032. doi: 10.1161/01.cir.0000051460.85800.bb. [DOI] [PubMed] [Google Scholar]
- 73.Koyanagi M, Urbich C, Chavakis E, Hoffmann J, Rupp S, Badorff C, Zeiher AM, Starzinski-Powitz A, Haendeler J, Dimmeler S. Differentiation of circulating endothelial progenitor cells to a cardiomyogenic phenotype depends on E-cadherin. FEBS Lett. 2005;579:6060–6066. doi: 10.1016/j.febslet.2005.09.071. [DOI] [PubMed] [Google Scholar]
- 74.Rupp S, Koyanagi M, Iwasaki M, Diehl F, Bushoven P, Schranz D, Zeiher AM, Dimmeler S. Genetic proof-of-concept for cardiac gene expression in human circulating blood-derived progenitor cells. J Am Coll Cardiol. 2008;51:2289–2290. doi: 10.1016/j.jacc.2008.01.062. [DOI] [PubMed] [Google Scholar]
- 75.Koyanagi M, Bushoven P, Iwasaki M, Urbich C, Zeiher AM, Dimmeler S. Notch signaling contributes to the expression of cardiac markers in human circulating progenitor cells. Circ Res. 2007;101:1139–1145. doi: 10.1161/CIRCRESAHA.107.151381. [DOI] [PubMed] [Google Scholar]
- 76.Murasawa S, Kawamoto A, Horii M, Nakamori S, Asahara T. Niche-dependent translineage commitment of endothelial progenitor cells, not cell fusion in general, into myocardial lineage cells. Arterioscler Thromb Vasc Biol. 2005;25:1388–1394. doi: 10.1161/01.ATV.0000168409.69960.e9. [DOI] [PubMed] [Google Scholar]
- 77.Iwasaki H, Kawamoto A, Ishikawa M, Oyamada A, Nakamori S, Nishimura H, Sadamoto K, Horii M, Matsumoto T, Murasawa S, Shibata T, Suehiro S, Asahara T. Dose-dependent contribution of CD34-positive cell transplantation to concurrent vasculogenesis and cardiomyogenesis for functional regenerative recovery after myocardial infarction. Circulation. 2006;113:1311–1325. doi: 10.1161/CIRCULATIONAHA.105.541268. [DOI] [PubMed] [Google Scholar]
- 78.Gruh I, Beilner J, Blomer U, Schmiedl A, Schmidt-Richter I, Kruse ML, Haverich A, Martin U. No evidence of transdifferentiation of human endothelial progenitor cells into cardiomyocytes after coculture with neonatal rat cardiomyocytes. Circulation. 2006;113:1326–1334. doi: 10.1161/CIRCULATIONAHA.105.559005. [DOI] [PubMed] [Google Scholar]
- 79.Planat-Benard V, Menard C, Andre M, Puceat M, Perez A, Garcia-Verdugo JM, Penicaud L, Casteilla L. Spontaneous cardiomyocyte differentiation from adipose tissue stroma cells. Circ Res. 2004;94:223–229. doi: 10.1161/01.RES.0000109792.43271.47. [DOI] [PubMed] [Google Scholar]
- 80.Yamada Y, Wang XD, Yokoyama S, Fukuda N, Takakura N. Cardiac progenitor cells in brown adipose tissue repaired damaged myocardium. Biochem Biophys Res Commun. 2006;342:662–670. doi: 10.1016/j.bbrc.2006.01.181. [DOI] [PubMed] [Google Scholar]
- 81.Kogler G, Sensken S, Airey JA, Trapp T, Muschen M, Feldhahn N, Liedtke S, Sorg RV, Fischer J, Rosenbaum C, Greschat S, Knipper A, Bender J, Degistirici O, Gao J, Caplan AI, Colletti EJ, Almeida-Porada G, Muller HW, Zanjani E, Wernet P. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med. 2004;200:123–135. doi: 10.1084/jem.20040440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bonanno G, Mariotti A, Procoli A, Corallo M, Rutella S, Pessina G, Scambia G, Mancuso S, Pierelli L. Human cord blood CD133+ cells immunoselected by a clinical-grade apparatus differentiate in vitro into endothelial- and cardiomyocyte-like cells. Transfusion. 2007;47:280–289. doi: 10.1111/j.1537-2995.2007.01104.x. [DOI] [PubMed] [Google Scholar]
- 83.Nishiyama N, Miyoshi S, Hida N, Uyama T, Okamoto K, Ikegami Y, Miyado K, Segawa K, Terai M, Sakamoto M, Ogawa S, Umezawa A. The significant cardiomyogenic potential of human umbilical cord blood-derived mesenchymal stem cells in vitro. Stem Cells. 2007;25:2017–2024. doi: 10.1634/stemcells.2006-0662. [DOI] [PubMed] [Google Scholar]
- 84.Ma N, Stamm C, Kaminski A, Li W, Kleine HD, Muller-Hilke B, Zhang L, Ladilov Y, Egger D, Steinhoff G. Human cord blood cells induce angiogenesis following myocardial infarction in NOD/scid-mice. Cardiovasc Res. 2005;66:45–54. doi: 10.1016/j.cardiores.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 85.Leor J, Guetta E, Feinberg MS, Galski H, Bar I, Holbova R, Miller L, Zarin P, Castel D, Barbash IM, Nagler A. Human umbilical cord blood-derived CD133+ cells enhance function and repair of the infarcted myocardium. Stem Cells. 2006;24:772–780. doi: 10.1634/stemcells.2005-0212. [DOI] [PubMed] [Google Scholar]
- 86.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O’Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 87.Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, Henckaerts E, Bonham K, Abbott GW, Linden RM, Field LJ, Keller GM. Human cardiovascular progenitor cells develop from a KDR(+) embryonic-stem-cell-derived population. Nature. 2008;453:524–8. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 88.Caspi O, Huber I, Kehat I, Habib M, Arbel G, Gepstein A, Yankelson L, Aronson D, Beyar R, Gepstein L. Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. J Am Coll Cardiol. 2007;50:1884–1893. doi: 10.1016/j.jacc.2007.07.054. [DOI] [PubMed] [Google Scholar]
- 89.van Laake LW, Passier R, Monshouwer-Kloots J, Verkleij AJ, Lips DJ, Freund C, den Ouden K, Ward-van Oostwaard D, Korving J, Tertoolen LG, van Echteld CJ, Doevendans PA, Mummery CL. Human embryonic stem cell-derived cardiomyocytes survive and mature in the mouse heart and transiently improve function after myocardial infarction. Stem Cell Research. 2007;1:9–24. doi: 10.1016/j.scr.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 90.Leor J, Gerecht S, Cohen S, Miller L, Holbova R, Ziskind A, Shachar M, Feinberg MS, Guetta E, Itskovitz-Eldor J. Human embryonic stem cell transplantation to repair the infarcted myocardium. Heart. 2007;93:1278–1284. doi: 10.1136/hrt.2006.093161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tomescot A, Leschik J, Bellamy V, Dubois G, Messas E, Bruneval P, Desnos M, Hagege AA, Amit M, Itskovitz J, Menasche P, Puceat M. Differentiation in vivo of cardiac committed human embryonic stem cells in postmyocardial infarcted rats. Stem Cells. 2007;25:2200–2205. doi: 10.1634/stemcells.2007-0133. [DOI] [PubMed] [Google Scholar]
- 92.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 94.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 95.Trounson A. The production and directed differentiation of human embryonic stem cells. Endocr Rev. 2006;27:208–219. doi: 10.1210/er.2005-0016. [DOI] [PubMed] [Google Scholar]
- 96.Nussbaum J, Minami E, Laflamme MA, Virag JA, Ware CB, Masino A, Muskheli V, Pabon L, Reinecke H, Murry CE. Transplantation of undifferentiated murine embryonic stem cells in the heart: teratoma formation and immune response. Faseb J. 2007;21:1345–1357. doi: 10.1096/fj.06-6769com. [DOI] [PubMed] [Google Scholar]
- 97.Kehat I, Kenyagin-Karsenti D, Snir M, Segev H, Amit M, Gepstein A, Livne E, Binah O, Itskovitz-Eldor J, Gepstein L. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest. 2001;108:407–414. doi: 10.1172/JCI12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mummery C, Ward D, van den Brink CE, Bird SD, Doevendans PA, Opthof T, Brutel de la Riviere A, Tertoolen L, van der Heyden M, Pera M. Cardiomyocyte differentiation of mouse and human embryonic stem cells. J Anat. 2002;200:233–242. doi: 10.1046/j.1469-7580.2002.00031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xu C, Police S, Rao N, Carpenter MK. Characterization and enrichment of cardiomyocytes derived from human embryonic stem cells. Circ Res. 2002;91:501–508. doi: 10.1161/01.res.0000035254.80718.91. [DOI] [PubMed] [Google Scholar]
- 100.He JQ, Ma Y, Lee Y, Thomson JA, Kamp TJ. Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization. Circ Res. 2003;93:32–39. doi: 10.1161/01.RES.0000080317.92718.99. [DOI] [PubMed] [Google Scholar]
- 101.Sartiani L, Bettiol E, Stillitano F, Mugelli A, Cerbai E, Jaconi ME. Developmental changes in cardiomyocytes differentiated from human embryonic stem cells: a molecular and electrophysiological approach. Stem Cells. 2007;25:1136–1144. doi: 10.1634/stemcells.2006-0466. [DOI] [PubMed] [Google Scholar]
- 102.Klug MG, Soonpaa MH, Koh GY, Field LJ. Genetically selected cardiomyocytes from differentiating embronic stem cells form stable intracardiac grafts. J Clin Invest. 1996;98:216–224. doi: 10.1172/JCI118769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Anderson D, Self T, Mellor IR, Goh G, Hill SJ, Denning C. Transgenic enrichment of cardiomyocytes from human embryonic stem cells. Mol Ther. 2007;15:2027–2036. doi: 10.1038/sj.mt.6300303. [DOI] [PubMed] [Google Scholar]
- 104.Huber I, Itzhaki I, Caspi O, Arbel G, Tzukerman M, Gepstein A, Habib M, Yankelson L, Kehat I, Gepstein L. Identification and selection of cardiomyocytes during human embryonic stem cell differentiation. Faseb J. 2007;21:2551–2563. doi: 10.1096/fj.05-5711com. [DOI] [PubMed] [Google Scholar]
- 105.Mummery C, Ward-van Oostwaard D, Doevendans P, Spijker R, van den Brink S, Hassink R, van der Heyden M, Opthof T, Pera M, de la Riviere AB, Passier R, Tertoolen L. Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm-like cells. Circulation. 2003;107:2733–2740. doi: 10.1161/01.CIR.0000068356.38592.68. [DOI] [PubMed] [Google Scholar]
- 106.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 107.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 108.Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–6. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 109.Schenke-Layland K, Rhodes KE, Angelis E, Butylkova Y, Heydarkhan-Hagvall S, Gekas C, Zhang R, Goldhaber JI, Mikkola HK, Plath K, Maclellan WR. Reprogrammed Mouse Fibroblasts Differentiate into Cells of the Cardiovascular and Hematopoietic Lineages. Stem Cells. 2008;26:1537–46. doi: 10.1634/stemcells.2008-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lints TJ, Parsons LM, Hartley L, Lyons I, Harvey RP. Nkx-2.5: a novel murine homeobox gene expressed in early heart progenitor cells and their myogenic descendants. Development. 1993;119:969. doi: 10.1242/dev.119.3.969. [DOI] [PubMed] [Google Scholar]
- 111.Kasahara H, Bartunkova S, Schinke M, Tanaka M, Izumo S. Cardiac and extracardiac expression of Csx/Nkx2.5 homeodomain protein. Circ Res. 1998;82:936–946. doi: 10.1161/01.res.82.9.936. [DOI] [PubMed] [Google Scholar]
- 112.Arceci RJ, King AA, Simon MC, Orkin SH, Wilson DB. Mouse GATA-4: a retinoic acid-inducible GATA-binding transcription factor expressed in endodermally derived tissues and heart. Mol Cell Biol. 1993;13:2235–2246. doi: 10.1128/mcb.13.4.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Morrisey EE, Ip HS, Lu MM, Parmacek MS. GATA-6: a zinc finger transcription factor that is expressed in multiple cell lineages derived from lateral mesoderm. Dev Biol. 1996;177:309–322. doi: 10.1006/dbio.1996.0165. [DOI] [PubMed] [Google Scholar]
- 114.Chapman DL, Garvey N, Hancock S, Alexiou M, Agulnik SI, Gibson-Brown JJ, Cebra-Thomas J, Bollag RJ, Silver LM, Papaioannou VE. Expression of the T-box family genes, Tbx1-Tbx5, during early mouse development. Dev Dyn. 1996;206:379–390. doi: 10.1002/(SICI)1097-0177(199608)206:4<379::AID-AJA4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 115.Swanson BJ, Jack HM, Lyons GE. Characterization of myocyte enhancer factor 2 (MEF2) expression in B and T cells: MEF2C is a B cell-restricted transcription factor in lymphocytes. Mol Immunol. 1998;35:445–458. doi: 10.1016/s0161-5890(98)00058-3. [DOI] [PubMed] [Google Scholar]
- 116.Martin JF, Schwarz JJ, Olson EN. Myocyte enhancer factor (MEF) 2C: a tissue-restricted member of the MEF-2 family of transcription factors. Proc Natl Acad Sci U S A. 1993;90:5282–5286. doi: 10.1073/pnas.90.11.5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Edmondson DG, Lyons GE, Martin JF, Olson EN. Mef2 gene expression marks the cardiac and skeletal muscle lineages during mouse embryogenesis. Development. 1994;120:1251–1263. doi: 10.1242/dev.120.5.1251. [DOI] [PubMed] [Google Scholar]
- 118.Leifer D, Krainc D, Yu YT, McDermott J, Breitbart RE, Heng J, Neve RL, Kosofsky B, Nadal-Ginard B, Lipton SA. MEF2C, a MADS/MEF2-family transcription factor expressed in a laminar distribution in cerebral cortex. Proc Natl Acad Sci U S A. 1993;90:1546–1550. doi: 10.1073/pnas.90.4.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Eugenin EA, Branes MC, Berman JW, Saez JC. TNF-alpha plus IFN-gamma induce connexin43 expression and formation of gap junctions between human monocytes/macrophages that enhance physiological responses. J Immunol. 2003;170:1320–1328. doi: 10.4049/jimmunol.170.3.1320. [DOI] [PubMed] [Google Scholar]
- 120.Beyer EC, Kistler J, Paul DL, Goodenough DA. Antisera directed against connexin43 peptides react with a 43-kD protein localized to gap junctions in myocardium and other tissues. J Cell Biol. 1989;108:595–605. doi: 10.1083/jcb.108.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pette D, Staron RS. Myosin isoforms, muscle fiber types, and transitions. Microsc Res Tech. 2000;50:500–509. doi: 10.1002/1097-0029(20000915)50:6<500::AID-JEMT7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 122.Boheler KR, Carrier L, de la Bastie D, Allen PD, Komajda M, Mercadier JJ, Schwartz K. Skeletal actin mRNA increases in the human heart during ontogenic development and is the major isoform of control and failing adult hearts. J Clin Invest. 1991;88:323–330. doi: 10.1172/JCI115295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fridlianskaia II, Goncharova EI, Borisov AB, Krylova TA, Pinaev GP. [Monoclonal antibodies to the muscle isoform of alpha-actinin--a marker for the study of the differentiation of skeletal and cardiac muscles] Tsitologiia. 1989;31:1234–1237. [PubMed] [Google Scholar]
- 124.Sasse S, Brand NJ, Kyprianou P, Dhoot GK, Wade R, Arai M, Periasamy M, Yacoub MH, Barton PJ. Troponin I gene expression during human cardiac development and in end-stage heart failure. Circ Res. 1993;72:932–938. doi: 10.1161/01.res.72.5.932. [DOI] [PubMed] [Google Scholar]
- 125.Anderson PA, Malouf NN, Oakeley AE, Pagani ED, Allen PD. Troponin T isoform expression in humans. A comparison among normal and failing adult heart, fetal heart, and adult and fetal skeletal muscle. Circ Res. 1991;69:1226–1233. doi: 10.1161/01.res.69.5.1226. [DOI] [PubMed] [Google Scholar]
- 126.Gardner DG, Deschepper CF, Ganong WF, Hane S, Fiddes J, Baxter JD, Lewicki J. Extra-atrial expression of the gene for atrial natriuretic factor. Proc Natl Acad Sci U S A. 1986;83:6697–6701. doi: 10.1073/pnas.83.18.6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ruzicka DL, Schwartz RJ. Sequential activation of alpha-actin genes during avian cardiogenesis: vascular smooth muscle alpha-actin gene transcripts mark the onset of cardiomyocyte differentiation. J Cell Biol. 1988;107:2575–2586. doi: 10.1083/jcb.107.6.2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Paulin D, Li Z. Desmin: a major intermediate filament protein essential for the structural integrity and function of muscle. Exp Cell Res. 2004;301:1–7. doi: 10.1016/j.yexcr.2004.08.004. [DOI] [PubMed] [Google Scholar]