Synopsis
As a result of the changes in utilization of imaging procedures that rely on ionizing radiation, the collective dose has increased by over 700% and the annual per-capita dose, by almost 600% over recent years. It is certainly possible that this growing use may have significant effects on public health. Although there are uncertainties related to the accuracy of calculated radiation exposure and the estimated biologic risk, there are measures that can be taken to reduce any potential risks while maintaining diagnostic accuracy. This article will review the existing data regarding biological hazards of radiation exposure associated to medical diagnostic testing, the methodology used to estimate radiation exposure and the measures that can be taken to effectively reduce it.
1.0 Introduction
In recent years, reports on radiation exposure resulting from medical imaging have unfailingly attracted intense attention by the media, whose reporting typically emphasizes the risks of such exposure. It is certainly possible that the growing use of imaging procedures that rely on ionizing radiation may have significant effects on public health. However, the potential health risks of ionizing radiation at the levels used in medical imaging are rarely portrayed in a balanced fashion that would highlight the patterns of use of medical imaging, the uncertainties about the magnitude of risk of cancer, or the undeniable benefits of medical imaging in specific scenarios. The purpose of this review is to provide an understanding of 1. the strengths and shortcomings of epidemiologic evaluations of radiation exposure to the general population, 2. the uncertainties related to radiation dosimetry and estimating the biologic risk (including carcinogenesis) resulting from exposure to ionizing radiation, and 3. the measures that can be taken to maximize our opportunities to perform the right study in the right patient with a radiation dose that is as low as reasonably achievable. In keeping with the main theme of the current issue of Cardiology Clinics, our review will focus on cardiac CT imaging in adults.
2.0 Radiation Exposure of the General Population
A preliminary report by the National Commission for Radiation Protection (NCRP) on the medical radiation exposures of the U.S. population in 20061 was mostly based on publicly available information from Medicare claims, Veterans Administration data, and information from the Agency for Healthcare Quality and Research. Among the nuclear medicine studies, which increased at a rate of 5% per year, cardiac imaging accounted for 57% of the procedures but 85% of the collective dose. CT imaging has received particular attention in recent reports of patient dose. In 2007, a review published in the New England Journal of Medicine2 reported an increase of CT images procedures from about 3 million in 1980 to an estimated 62 million in 2006. The preliminary NCRP report from 2008 suggested an increase of CT imaging studies by >10% per year. Among the CT studies performed in 2006, the estimated 800,000 coronary artery (CAC) scoring and “gated cardiac studies” (representing coronary CT angiography) accounted for 1.3% of the procedures and 1.5% of the collective E.
Taken together, the estimated 67 million CT (computed tomography) and 19 million nuclear medicine studies performed in 2006 accounted for 22% of imaging procedures that used ionizing radiation but 75% of the collective E (expressed in person-Sieverts). As a result of the changes in utilization of imaging procedures that rely on ionizing radiation, the collective dose has increased by over 700% and the annual per-capita dose, by almost 600% (from 0.53 mSv to 3 mSv), compared to previous reports on the exposure of the U.S. population to ionizing radiation in general3 and medical exposure in particular.4
The collective dose can be a useful index for quantifying dose in large populations but does not account for the fact that a large dose delivered to a small number of people is not the same as a small dose delivered to many people.5 Some medical physicists consider the collective dose a highly speculative and uncertain measure of risk that should, at levels of <100 mSv above background radiation, not be used for the purpose of estimating population health risks.5 The annualized per-capita doses were obtained by dividing the cumulative dose delivered to all patients by the size of the entire population, irrespective of age, occupation, location or health status. This methodology does, e.g., not account for the fact that 80% of the cumulative CT dose were delivered in the hospital setting, presumably in seriously ill in-patients.6 In comparison, the annual dose to individuals not receiving medical exposures has not or only minimally changed since 1987. Also, a disproportionately high number of CT imaging procedures is performed in the elderly. Put in perspective, it is quite conceivable that in the ill and the elderly, populations at increased risk of dying, medical imaging procedures that require ionizing radiation can allow management decisions that can improve quality of life or longevity, suggesting a favorable relationship between risks and benefits of radiation exposure in this patient group. Near the end of our review, we will discuss this concept in the context of cardiac imaging.
3.0 Biological Injury from Radiation
Most of what is known about the biological effects of ionizing radiation7 is derived from ex-vivo studies or from examining and following the survivors of the atomic bomb explosions in Hiroshima and Nagasaki. The effects of ionizing radiation depend on the dose and the rate at which the dose is delivered.
3.1 Markers of Radiation Injury
The acute effects of ionizing radiation are mostly mediated through impaired cell renewal (at the stage of mitosis) and triggering of apoptosis (which may not occur until after several cycles of cell division). The radiation sensitivity of cells and tissues is related to the rate of cellular proliferation, number of future divisions, and degree of differentiation. Among the most sensitive tissues are the precursor cells of hematopoiesis and the intestinal epithelium. As a result, absolute counts and relative changes of circulating lymphocytes, and the time to onset of emesis have emerged as useful semi-quantitative biological markers of absorbed radiation doses exceeding 6 Gy.8 Quantitative analysis of chromosomal aberrations of lymphocytes, mostly in the form of interchange between 2 separate chromosomes (dicentrics), in blood samples drawn >24 hours after exposure is the current reference standard for estimating the absorbed radiation dose in vivo.9
The dose-dependent effects of ionizing radiation fall into 2 categories. The severity of “deterministic” effects, which typically occur once tissue-specific thresholds are exceeded, varies with the dose. Examples for deterministic effects include the aforementioned influence on tissues with rapid turnover, but also hair loss and the development of skin erythema and cataracts. For “stochastic” effects, it is the probability, not severity, of occurrence that varies with dose, typically without an appreciable threshold and after a latency period that may last decades. The specific mechanisms that result in deterministic versus stochastic effects are not well known.
3.2 Risk of Carcinogenesis
The stochastic radiation effect of greatest concern is carcinogenesis. The dose-response relationship between ionizing radiation at the levels used in medical imaging and the development of malignancies is controversial.10 The data developed from survivors of atomic bomb explosions reflect uniform whole body irradiation at high levels and energy in a specific Japanese population. It is not universally accepted that these data can be extrapolated to the highly non-uniform partial body irradiation at much lower levels typical of medical imaging in other populations of various ethnicities.5
Several issues complicate the quantitative evaluation of carcinogenic risk related to ionizing radiation at effective doses <100 mSv (which reflects the definition of “low dose”). First, the effect appears to be small: neither in the Life Span Study of atomic bomb blast survivors11 nor in retrospective epidemiologic analyses in radiation workers,12 both studies with large sample sizes and long follow-up, was the risk of developing solid malignancies in the “low dose” range unequivocally different from no increased risk. It is important to realize that radiation-induced malignancies are indistinguishable from malignancies related to other carcinogens or biological processes. Therefore, the small potential risk of carcinogenesis would be superimposed on, and difficult to differentiate from, the substantial intrinsic average lifetime risks in the U.S. of developing (41%) or dying from (21%) a malignancy.13 This small potential would also vary greatly by sex, age, and health status of the individual. Finally, the general population is exposed to natural background radiation to geographically varying degrees that averages approximately 3.5 mSv per year. Given the random nature of the interaction of ionizing particles with critical cellular targets, it is conceivable that even the low levels of background radiation could contribute to the development of malignancies, which would be difficult to differentiate from the effects of a single exposure to medical radiation.
Relationship between Low Dose Radiation and Risk of Lethal Malignancy
Given the small potential effect and the large number of confounding factors, a study setting out to establish the relationship between low levels of radiation and the development of malignancies would require hundreds of thousands of study subjects and decades of follow-up. Given the facts that 1. there is no definite evidence that suggests such a relationship exists but that 2. a relationship cannot be ruled out, several professional societies or committees have endorsed the conservative assumption that the risk of malignancies at low doses of radiation can be extrapolated from the risk of malignancies at high levels of radiation, and that there is no dose of radiation that cannot potentially cause malignancies.11, 14 This concept is commonly referred to as the “linear no threshold hypothesis” and is by no means universally accepted. A conceivable alternative “linear quadratic hypothesis” suggests that at low radiation doses the risk of malignancy is so low as to be unquantifiable in humans but that the risk rises exponentially at higher doses. Yet another concept termed “radiation hormesis,” even implies that exposure to low levels of radiation can convey health and survival benefits.15
The public summary of the Biological Effect of Ionizing Radiation (BEIR) VII report concedes that “statistical limitations make if difficult to evaluate cancer risk in humans” at doses of <100 mSv but overall the report endorses the linear no-threshold hypothesis.11 The report quotes a 5 in 100 age- and gender-average lifetime risk of dying from a malignancy attributable to radiation exposure among individuals of the general population who received an effective dose of 1 Sv. This risk would translate into 1 in 2000 patients (0.05%) who have received the 10 mSv dose that is typical of a coronary CT angiogram acquired using a retrospective gating protocol.
A recent study modeled age- and gender-specific whole body and organ lifetime attributable risks (LAR) of cancer associated the radiation exposure from a 64-slice coronary CTA.16 The computations were based on scanning protocols that used retrospective gating and generic Monte Carlo modeling of organ doses. The LARs were based on the linear no-threshold hypothesis. As expected, the risk varied by gender, age and scanning protocol (9.0 Tables Table 1), and ranged from 0.7% for 20 year-old women to 0.044% for 80 year-old men if no dose-sparing algorithm was used. The risks were 35% lower if dose sparing algorithms were used. At all ages, lung and breast cancer combined accounted for 80% to 85% of the LAR in women.
Table 1.
Estimated Risk of Cancer Incidence Attributable to a Single Coronary Computed Tomographic Angiogram Based on the Linear No-Threshold Hypothesis
| Gender | Age (years) | Risk |
|---|---|---|
| Female | 20 | 0.7% (1 in 143) |
| Female | 40 | 0.35% (1 in 286) |
| Female | 60 | 0.22% (1 in 546) |
| Female | 80 | 0.075% (1 in 1333) |
| Male | 20 | 0.15% (1 in 667) |
| Male | 40 | 0.099% (1 in 1010) |
| Male | 60 | 0.081% (1 in 1235) |
| Male | 80 | 0.044% (1 in 2273) |
Data reflect no dose-sparing algorithm used. Effective dose for women, 21 mSv; effective dose for men, 15 mSv. Risks were 35% lower if electrocardiographically tube current modulation was used as a dose-sparing scanning protocol. Data from Einstein AJ, Henzlova MJ, Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA 2007;298:317
4.0 Parameters to Quantify Radiation Dose
The objective of the design of scanner settings and scanning protocols is to obtain images of diagnostic quality. Image noise and motion artifacts are two key determinants of image quality in cardiac CT. The technical specifications that affect the temporal resolution of CT images and, hence, the probability of cardiac and coronary motion artifacts are addressed in Dr Sandra Halliburton's article elsewhere in this issue of Cardiology Clinics and have limited effect on patient dose. Conversely, the scanner settings that affect image noise also greatly affect x-ray tube output and patient dose.
4.1 Relationship of Scanner Settings, Tube Output and Image Quality
Image noise is inversely proportional to the number of photons received by the detector array. The product of tube current multiplied by exposure time (expressed in units of milliAmpere seconds, mAs), the tube voltage (expressed in units of peak kiloVolt, kVp), the reconstructed slice width and the degree of photon attenuation are the main determinants of the number of photons received by the detector array.17 The mAs mainly increases photon flux, and the kVp, photon energy. Photon attenuation increases with increases in body thickness. Therefore, adaptation of default scanner settings to a patient's body size can be needed to avoid excessive image noise that could interfere with confident image interpretation. For example, for every 4 - 5 cm of additional tissue to be traversed by photons the scanner output must be doubled for the level of image noise to remain constant.
Because patient dose is related to photon flux and energy, heavier patients may receive a higher dose of radiation, typically achieved by increasing mAs, to obtain images of diagnostic quality. Therefore, comparisons of doses between different CT scanning protocols are only meaningful at comparable levels of image noise. Because most of the additional dose is absorbed by the external fatty tissue, organ doses do not increase linearly with mAs.18 In this context, it is interesting to note that body weight is not an ideal predictor of tube output requirements in cardiac CT because increases in weight often reflect increased abdominal, not necessarily thoracic, thickness.19
4.2 Measurable Parameters in Radiation Dosimetry
The radiation output of CT scanners can be assessed in several ways. Parameters of radiation output that can be measured or calculated from measured values with standardized procedures lend themselves for the determination of diagnostic reference levels. Diagnostic reference levels are useful benchmarks for quality assurance efforts to identify practices that deliver, for specific radiological examinations, doses far above their peers. Diagnostic reference levels are typically set at the 75th to 80th percentile of clinical dose surveys.20, 21 Consistently exceeding diagnostic reference levels suggests the need for local review and practice adaptation. The use of diagnostic reference levels for benchmarking can reduce the median dose and dose variability as assessed in periodical surveys.22, 23
4.2.1 Computed Tomographic Dose Index
Most of the parameters used to measure radiation output were originally developed for imaging protocols that acquired single, parallel, transaxial slices, and can be applied to modern multidetector CT technology only with modifications. The computed tomography dose index (CTDI), a basic radiation dose parameter in CT, is defined as the integral under the radiation dose profile of a single rotation scan at a fixed table position divided by the nominal width of the radiation beam (Figure 1).24 The normalization to the nominal width of the radiation profile builds a “dose efficiency” factor into the value. Several variants of the CTDI exist that have been detailed elsewhere in terms relevant to clinicians.17, 25 In short, the CTDI100 represents a specific mode of measuring radiation exposure (in units of Coulomb/kilogram, C/kg) with an ionization chamber of 100 mm length. The weighted CTDI (CTDIw) is a calculated measure of the average absorbed dose in the scan plane (x and y axes) that is obtained from several CTDI100 measurements.
Figure 1.
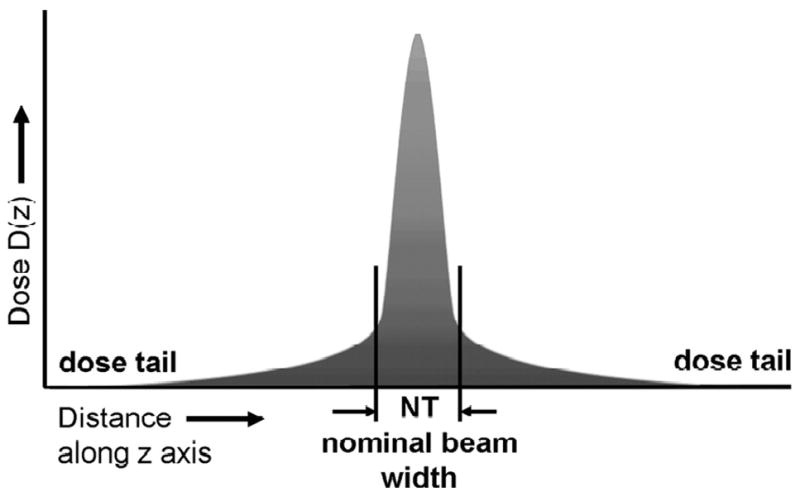
Distribution of radiation dose of a single axial CT can along the z-axis, perpendicular to the scan plane. The tails on either side of the dose profile are the result of x-ray beam divergence and internal radiation scatter. The Computed Tomographic Dose Index (CTDI) is defined as the integral under the area under the curve normalized to the nominal beam width (NT). From Bauhs JA, Vrieze TJ, Primak AN et al. CT dosimetry: comparison of measurement techniques and devices. Radiographics 2008:28;245.
The volume CTDI (CTDIvol; in units of milliGray, mGy) represents the average radiation within a specific volume and is now the preferred standardized measure of radiation output in CT dosimetry.17, 25, 26 The CTDIvol is convenient because it is derived from the easily-measured CTDI100, and it is useful for comparisons between different imaging protocols because it incorporates protocol-specific information on the spatial separation or overlap between successive scans. In helical CT, this spatial relationship between successive sans is dependent on the advance of the patient table during each gantry rotation (pitch). As a conceptual disadvantage, the CTDIvol does not reflect the total number of scans that make up a CT examination.
4.2.2 Dose Length Product
The dose-length product (DLP) best represents the integrated radiation output during a specific complete CT examination.17, 25, 26 The DLP (in units of mGy × cm) is defined as the CTDIvol multiplied by the scan length. The typical scan length for a coronary CTA is 12 cm. Because the numeric value of the DLP can vary with patient anatomy, the CTDIvol is more useful than the DLP for designing and comparing CT scanning protocols.
4.3 Estimates of Radiation Dose
The effective dose (E; in units of milliSievert, mSv) is a generic, calculated approximation of the biologic detriment (i.e. the stochastic risk) of a non-homogeneous body irradiation such as that which occurs during medical imaging of specific body regions. E represents the mean absorbed whole body dose that would result in the same total radiation detriment as the non-uniform partial-body irradiation in question. There is no measurable physical gold standard that represents E.10, 17, 25, 26
E is estimated in three steps.27 First, so-called Monte Carlo simulation is used to estimate the radiation doses (in units of mGy) received by individual organs. Monte Carlo simulation models the scattering of photons and the resulting absorption of radiation energy in standardized mathematical models of the human body, e.g. that of a man with a weight of 70 kg. The amount of ionization, and hence the probability of a relevant biological effect, along the track of an ionizing particle passing through the body varies between low (e.g. x-rays) and high (e.g. alpha particles) linear energy transfer sources. Therefore, in a second step in the estimation of E, the relative biological effectiveness of different types of ionizing radiation is represented by a radiation weighting factor. For x-rays, the radiation weighting factor is 1. As discussed in the section of “Biological Injury From Radiation” above, the radiation sensitivity of different tissues varies considerably. Therefore, the third step in the estimation of E applies tissue specific weighting factors that represent the radiation sensitivity of each organ or tissue. These tissue-specific weighting factors determine how much the individual organ doses contribute when E is calculated as the sum of the products obtained by multiplying organ doses with radiation and tissue-specific weighting factors.
The tissue-specific weighting factors are determined from population averages in the survivors of atomic bomb explosions. Because of the evolving knowledge of the biology and epidemiology of radiation injuries, several sets of tissue weighting factors exist. For example, the International Commission for Radiation Protection (ICRP) has published slightly differing sets in 1977,28 1991,29 and 2007.30 In addition, methodological differences in the calculation of E exist between the three 3 ICRP recommendations. These differences pertain e.g. to the incorporation of the so-called “remainder tissues” or tissues for which no organ coefficients exist, and to the best method of averaging dose over an organ. As a result, the numerical value of E can vary because of methodological differences alone even if the actual radiation exposure is identical. Table 2 gives an example of how the E of a coronary CT angiogram obtained with a typical single source 64-slice scanning protocol (CTDIvol, 59.9 mGy) might vary based on the various ICRP recommendations.
Table 2.
Differences in Effective Dose Estimates Based on the Recommendations in 3 Publications from the International Commission for Radiation Protection (ICRP).
| ICRP publication | 26 | 60 | 103 |
|---|---|---|---|
| Year | 1977 | 1990 | 2007 |
| Breast weighting coefficient | 0.15 | 0.05 | 0.12 |
| E (mSv) | 25 | 17 | 22 |
| Percent difference* | 151% | 100% | 134% |
Data are for a single source 64 detector row multislice coronary CT angiogram (volume Computed Tomographic Dose Index, 60 mGy). The differences in effective dose (E) are related to changing values of the tissue weighting coefficient of the breast, the handling of remainder tissues, and dose averaging.
The recommendations in ICRP publication 60, still widely used, were used as a reference to calculate relative differences in E between the three recommendations.
The European Working Group for Guidelines on Quality Criteria in CT has suggested a simplified method for the estimation of E. This estimate is obtained by multiplying the DLP with a conversion coefficient that varies dependent on which body region is scanned. The conversion coefficient for the thorax,31 which is relevant for cardiac CT, was recently revised from 0.017 to 0.014 mSv × mGy-1 × cm-1.
It is important to recognize that E is not an exact indicator of patient-specific absolute biological risk because is does not take into account variations of human anatomy (e.g. body weight at the upper and lower end of the spectrum) and because uncertainties about the radiation risk of human tissues remain.10 Because of the generic modeling and the many uncertainties relating to organ dose and organ risks, E should be reported as ranges rather than single numerical values with several significant figures.32 To obtain risk estimates that apply to individual patients, exact organ doses and organ-specific risk estimates related to age and gender are needed. This type of modeling is extraordinarily complex. Nonetheless, E is useful for comparisons of the biologic effect of different imaging protocols, different types of radiological examinations or even between imaging modalities that use different types of ionizing radiation. E can also help patients put the risk of a proposed radiological examination in perspective by comparison with the E received from natural background radiation (average, 3 mSv per year; range, 1 – 10 mSv). However, comparisons between E should keep the diagnostic objective in mind. Interpreting the E of a typical coronary CTA of 12 mSv as the equivalent of 600 chest x-rays or 1,200 panoramic dental x-rays may not be meaningful because neither chest x-rays nor dental x-rays are necessarily helpful in establishing or ruling out coronary artery disease as the cause of chest pain in a symptomatic patient.
5.0 Techniques to Reduce Radiation Dose
Given the oft-cited increase of cumulative and per-capita dose related to medical imaging in recent years, it is easily overlooked that, as a result of improvements in scanner technology and dose efficiency, the mean radiation dose per type of examination in general has decreased by a factor of 2 – 3 over the past 2 decades.33 Recently developed scanning protocols allow tremendous reduction of radiation dose in cardiovascular imaging on state-of-the-art multidetector CT scanners.
Certainly, considering on a per-patient basis whether the findings of a cardiac CT are likely to affect management meaningfully, and foregoing the study if it will not, must stand at the top of the hierarchy of dose-saving strategies. However, in this context it is equally important that the treating health care provider considers the risks of not performing a cardiac CT, i.e. the risk of missing an important diagnosis, if the examination is not performed because of concerns about radiation dose.10 As a second basic measure, to be addressed by the health care provider performing the imaging study, the scan length should be limited to include only the structures of interest, without routinely adding a “safety margin” above and below the heart.
Traditionally, cardiac scanning protocols with multidetector CT use continuous radiation output from the x-ray tube throughout the cardiac cycle.34 However, the images, typically reconstructed retrospectively within a short time window during the diastolic period of the cardiac cycle, represent only a small fraction of the radiation dose received. Given the relationships between x-ray tube settings and radiation dose detailed above, most dose reduction strategies in cardiac CT rely on reduction of tube voltage or tube current or both for a part or for all of the cardiac cycle. Some techniques of dose reduction that can be used for CT examinations of other body parts (e.g. automatic exposure control) are not available for, or not effective in, cardiac CT.18
5.1 Tube Current Modulation
As discussed above in the section on the relationship between scanner settings and image quality, photon attenuation is dependent upon the thickness and density of the body tissue to be traversed. The ovoid shape of the thorax, and the different densities along the length of the human body lend themselves to tube current modulation based on human anatomy. Angular (x,y) tube current modulation26 exploits the fact that the attenuation of the x-ray beam is higher when it traverses the body laterally than when it traverses the body from anterior or posterior locations. Therefore, during individual rotations of the gantry, the nominal tube current set by the operator is used only while scanning from laterally, and can be reduced by the scanner while scanning occurs in anteroposterior or posteroanterior direction. Similarly, attenuation of x-rays is higher near the bony structures of shoulder or hip regions than over the lungs or abdomen, and tube current can be adjusted by longitudinal (z) modulation26 accordingly throughout the table advance through the gantry. However, angular and longitudinal tube current modulation are currently not fully compatible with the approach of electrocardiographically controlled tube current modulation (ECTCM) discussed in the next paragraph.
5.1.1 Electrocardiographically Controlled Tube Current Modulation
ECTCM is a dose-sparing algorithm specific to cardiovascular CT. If high-quality reconstruction of the projection data into planar images is needed for only one time point of the cardiac cycle (such as for coronary CTA), the tube current can be reduced during the parts of the cardiac cycle that are unlikely to be used for reconstruction. With ECTCM nominal tube current is maintained during a time window of fixed length that can be determined by the operator but typically is set in late diastole, and the tube current is reduced to approximately 20% during the remainder of the cardiac cycle (Figure 2).18, 19 During the scan, the desired time window is identified prospectively based on the ECG. The length of the window of nominal tube current varies between different makes of scanners, and is programmable in some scanners. The effectiveness of ECTCM depends on the length of the window of nominal tube current, the level to which tube current is lowered for the remainder of the cardiac cycle, and the heart rate. ECTCM is more effective at low heart rates. In various studies, ECTCM has lowered the effective dose by between approximately 28% and 45%.35-38
Figure 2.
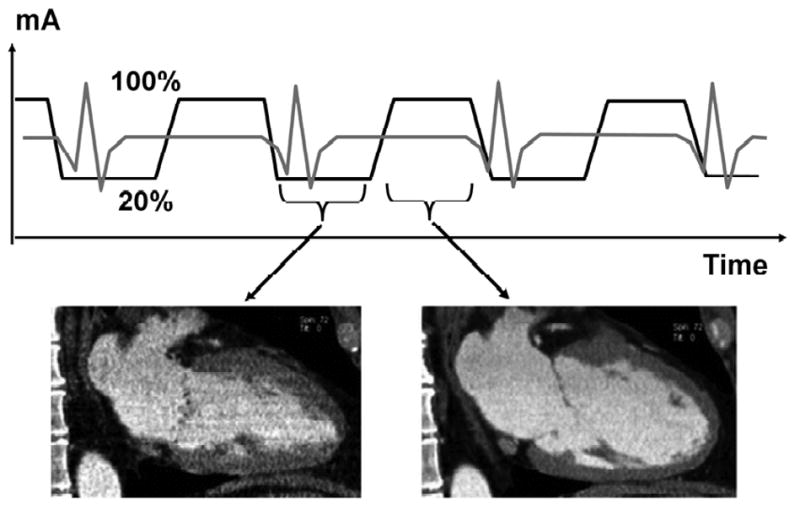
Principle of ECG-controlled tube current modulation. The tube current is a nominal value (100%) for a prospectively selected interval during diastole when images will likely be reconstructed (right). During the remainder of the cardiac cycle, tube current is reduced to 20%, which results in high image noise in images reconstructed during systole (left) but overall radiation dose to the patient is reduced. From Paul JF, Abada HT. Strategies for reduction of radiation dose in cardiac multislice CT. Eur Radiol 2007;17:2028
As a disadvantage, images reconstructed from projection data acquired during periods of reduced tube current will be noisy, and pathologic anatomic or functional findings that depend on incorporating these images into overall assessment of the examination may not be readily recognized. The low quality of images reconstructed during periods of low tube current may also be disadvantageous in patients with higher heart rates where optimal image quality with the least degree of motion artifact is often found during systole.39 In addition, ECTCM is not reliable in patients with arrhythmia or pronounced extrasystoli because the ECTCM algorithm may not identify the systolic and diastolic phase of the cardiac cycle correctly in this situation and may accidentally lower the tube current during the phase of the cardiac cycle that is desirable as a reconstruction window.
5.2 Sequential scanning
Sequential scanning, sometimes referred to as “step-and-shoot,” is a technique new to multidetector CT scanning.18, 40 In this approach, the x-ray tube is “on,” prospectively triggered by the ECG, during only the part of the cardiac cycle to be used for image reconstruction, typically in late diastole. Given the fact that radiation is produced during only a part of the cardiac cycle, the dose reduction that can be achieved by this technique is very substantial, on the order of 77%-87%. As a disadvantage, no projection data are acquired or available for image reconstructions during other parts of the cardiac cycle. Therefore, this approach is not useful for CT examinations meant to assess functional aspects of the heart throughout the cardiac cycle. To maximize the probability of obtaining images of diagnostic quality, CT centers experienced in coronary CT angiography with use of sequential scanning recommend rigorous patient selection. Selection criteria include a heart rate of no more then 65-75 beats per minute and a heart rate variability of <10 beats per minute.41
5.3 Individual Adaptation of Tube Voltage and Current
Although “underexposure” of CT images is readily apparent in the form of image noise, “overexposure” in the form of areas that are too bright or too dark does not occur because of the normalization of CT data relative to water.18 Therefore, opportunities to reduce radiation dose by decreasing tube voltage or tube current, which will decrease radiation output, are currently not always realized. In theory, radiation dose is linearly related to tube current, and exponentially related to the square of the difference between tube voltage settings. Therefore, reducing tube voltage is a more effective means of reducing radiation dose. As an added benefit, the increased attenuation by iodine of photons at lower photon energy (such at it occurs with reduction of tube voltage) increases the contrast between the contrast-enhanced coronary artery lumen and the surrounding tissue. This advantage must be weighed against the increased image noise that results from higher attenuation of low-energy photons by the patient's body.
Several authors have suggested reducing tube voltage from the standard 120 kVp to to 100 kVp in patients with a body weight up to 75 kg or 85 kg. In 1 previous study the combined use of tube voltage reduction and ECTCM resulted in a reduction of the E received from coronary CT angiography performed with single-source 64-slice MDCT by 64% without appreciable reduction of subjectively perceived image quality.36
5.4 Barriers to the Consistent Use of Dose Saving Imaging Strategies
Among the strategies for radiation dose reduction, ECTCM was used in 73% of patients, sequential scanning (prospective triggering) in 6%, and reduction of tube voltage from typically 120 kVp to 100 kVp in 5% of examination in a recent international multicenter survey of radiation dose in cardiac CT.42 When interpreting this pattern of use of dose reduction strategies, it should be noted that at the time the survey was conducted in 2007, sequential scanning was not widely available. In addition, even at the time of this writing, the evidence base supporting the use of sequential scanning or reduced tube voltage is not as convincing as it is for ECTCM. Concerns that the use of these newer techniques might interfere with image quality to an extent that would reduce in a sizable number of examinations the confidence with which they can be interpreted may have contributed to the limited use of sequential scanning and tube voltage reduction. It should also be clearly understood that certain dose saving strategies cannot be used in certain patient or for examinations performed for functional imaging. Examples include patients with very fast or irregular heart rate, obese patients, or CT examinations performed to assess of left ventricular or valvular function exam. Studies are currently underway that examine the effect of sequential scanning or tube voltage reduction not only on image quality but also on diagnostic accuracy of coronary CTA.
6.0 Current Radiation Dose Values
Reliable data on dose estimates for various cardiac imaging procedures are not easily obtained. This is mainly because of 1. the uncertainties in the biological risk of ionizing radiation, 2. the generic and imprecise nature of radiation dose estimation that results in part from these uncertainties, and 3. the fact that the current requirements for radiation dose reporting in individual institutions by the U.S. Food and Drug Administration, American College of Radiology accreditation surveys, and the National Regulatory Commission do not translate into easily available public information.
A catalog of values and ranges of E in radiology and diagnostic nuclear medicine was compiled recently from a review of the literature between 1980 and 2007.43 A Science Advisory from the American Heart Association on ionizing radiation in cardiac imaging published in February 2009 modified, and added to, this information. Table 3 shows representative values and ranges of effective dose estimates from the literature for various radiological imaging studies.
Table 3.
Representative Values and Ranges of Effective Dose Estimates Reported in the Literature for Selected Radiological Studies
| Examination | Representative Effective Dose Value (mSv) | Range of Reported Effective Dose Values (mSv) | Administered Activity (MBq) |
|---|---|---|---|
| Chest x-ray PA and lateral | 0.1 | 0.05–0.24 | NA |
| CT chest | 7 | 4–18 | NA |
| CT abdominal | 8 | 4–25 | NA |
| CT pelvis | 6 | 3–10 | NA |
| Coronary calcium CT* | 3 | 1–12 | NA |
| Coronary CT angiogram† | 16 | 5–32 | NA |
| 64-Slice coronary CTA‡ | |||
| Without tube current modulation | 15 | 12–18 | NA |
| With tube current modulation21 | 9 | 8–18 | NA |
| Dual-source coronary CTA‡ | |||
| With tube current modulation | 13 | 6–17 | NA |
| Prospectively triggered coronary CTA‡22 | 3 | 2–4 | NA |
| Diagnostic invasive coronary angiogram | 7 | 2–16 | NA |
| Percutaneous coronary intervention or radiofrequency ablation | 15 | 7–57 | NA |
| Myocardial perfusion study | |||
| Sestamibi (1-day) stress/rest | 9 | — | 1100 |
| Thallium stress/rest | 41 | — | 185 |
| F-18 FDG | 14 | — | 740 |
| Rubidium-82 | 5 | — | 1480 |
PA indicates posteroanterior; CTA, CT angiography; and FDG, fluorodeoxyglucose; NA, not applicable.
Data combine prospectively triggered and retrospectively gated protocols. The representative effective dose is approximately 1 mSv for prospectively triggered coronary calcium CT scans and 3 mSv for retrospectively gated scans.
Includes data published between 1980 and 2007. Dose data may not reflect newest scanners and protocols.
64-Slice multidetector-row CT and dual-source CT studies published since 2005 only; data include a survey of the literature by Gerber et al.
Data from Mettler F, Jr., Huda W, Yoshizumi T, Mahesh M. A catalog of effective doses in radiology and nuclear medicine. Radiology 2008;248:254 and Gerber TC, Carr JJ, Arai AE, et al. Ionizing radiation in cardiac imaging: a science advisory from the American Heart Association Committee on Cardiac Imaging of the Council on Clinical Cardiology and Committee on Cardiovascular Imaging and Intervention of the Council on Cardiovascular Radiology and Intervention. Circulation 2009;119:1056.
The results of a first international survey of radiation dose in 1,965 cardiac CT angiography studies at 50 study sites were recently published.42 As a particular strength, this study reported not only effective dose estimates but also CTDIvol and DLP as measurable dosimetry parameters. Therefore, this article reports the first and only set of diagnostic reference levels for cardiac CT available to date. Based on their findings, the authors suggested a CTDI of 70 mGy and a DLP of 1200 mGy × cm as reference levels for coronary CTA for patients of average size.
An approximately sixfold variability of DLP (range, 331 – 2146 mGy × cm) between study sites was observed. There were no significant differences of DLP by geographic location (Figure 3). Multivariate regression analysis suggested the main determinants of the variability of radiation dose between sites were the user preferences for image noise, reconstruction algorithms (kernel determining in-pane sharpness, slice width (z-axis resolution), tube voltage (iodine contrast), and increased upper and lower limits of the scan range as a “safety zone,” as well as patient size. The predictors for DLP in multivariate regression analysis are detailed in Table 4.
Figure 3.
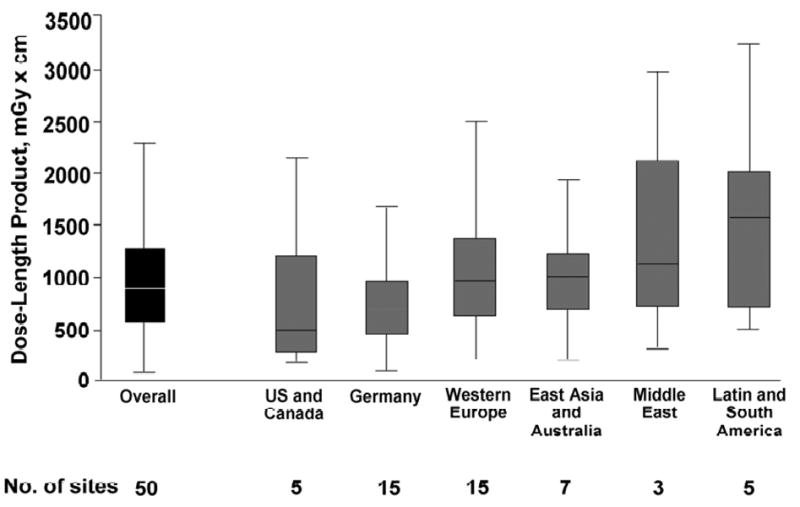
Box-and-whiskers plot of dose length product overall and by geographic location of study sites. Middle horizontal line, median; upper and lower edges of boxes, 25th and 75th percentiles of the interquartile range (IQR), respectively; lower whiskers, 25th percentile minus 1.5 times IQR; upper whiskers, 75th percentile plus 1.5 times IQR. From Hausleiter J, Meyer T, Hermann F, et al. Estimated radiation dose associated with cardiac CT angiography. JAMA 2009;301:500
Table 4.
Predictors for Dose Length Product in Multivariate Linear Regression Analysis.
| Predictors | Effects (%) (95% CI) |
p |
|---|---|---|
| Patient wt, 10-kg increase | 5 (4 to 6) | <0.001 |
| Indication, noncoronary vs coronary | -1 (-5 to 4) | 0.31 |
| Heart rhythm, nonsinus vs sinus | 10 (2 to 19) | 0.01 |
| Heart rate, 10-bpm increase | 1 (-1 to 1) | 0.98 |
| Scan length, 1-cm increase | 5 (4 to 6) | <0.001 |
| Automated exposure control | 0 (-3 to 3) | 0.97 |
| ECTCMb | -25 (-23 to -28) | <0.001 |
| Tube voltage 100 kV vs ≥120 kV or greater | -46 (-42 to -51) | <0.001 |
| Sequential vs spiral scanning | -78 (-77 to -79) | <0.001 |
| Site experience in CCTA, 12-mo increase | -1 (-1 to 0) | 0.03 |
| Performed CCTAs/mo, 10-CCTA increase | 0 (0 to 1) | 0.03 |
| 64-slice CT system vs Siemens single-source 64c | ||
| GE 64 | 97 (88 to 106) | <0.001 |
| Philips 64 | 11 (5 to 19) | <0.001 |
| Siemens dual-source 64 | 23 (17 – 30) | <0.001 |
| Toshiba 64 | 59 (47 to 71) | <0.001 |
CI, confidence interval; CT, computed tomography; CCTA, cardiac CT angiography; DLP, dose length product.
Predictors for radiation dose are presented as % change in DLP (mGy × cm)
Electrocardiographically controlled tube current modulation
The Siemens single-source 64-slice CT system with the lowest median DLP in this study was used as a reference. The association with DLP is shown for the remaining four 64-slice systems within the linear regression analysis.
From Hausleiter J, Meyer T, Hermann F, et al. Estimated radiation dose associated with cardiac CT angiography. JAMA 2009;301:500
7.0 Summary and Practical Implications
The risk of causing a malignancy at the radiation dose levels used in cardiac imaging is hypothetical, not proven, and estimates of radiation dose have a wide margin of error. However, in the absence of certainty, the consensus opinions of influential expert panels advocate adopting a conservative estimate of radiation risks. The median effective dose estimate for coronary CTA in a recent international survey of radiation doses in cardiac CT angiography (12 mSv) is in the same range as of a 1-day myocardial scintigraphy stress test using Tc-99m sestamibi (9 mSv).10 Information in that survey reflected the status quo of CT scanner technology in 2007. In the meantime, sophisticated CT imaging protocols that use prospective triggering of radiation dose output have become widely available that can reduce the effective dose estimates to a fraction (2 – 4 mSv) of that of retrospectively gated protocols.
Radiation protection for patients involves justification, optimization and limitation of exposure. How can we use all this complex information in making medical decisions that have the best interest of the patient at heart? A recent Science Advisory from the American Heart Association10 put forth a basic conceptual framework. Ultimately, the goal is to choose the right test for the right patient.
Coronary CTA is a highly accurate technique for defining the presence of coronary artery disease. Given the potential consequences of failing to identify coronary artery disease in symptomatic patients, one could consider the small hypothetical risk justified. In addition, the patient cohort of symptomatic patients with intermediate probability of CAD is predominantly older than 50 years of age, and many of these patients may well die from other (including cardiac) causes before such a malignancy can become clinically apparent. Conversely, coronary CT angiography may not be a proven or recommended screening tool for asymptomatic, low-risk patients given the fact that, at this time, no data support the concept of using imaging evidence for “subclinical” atherosclerosis as a basis for management decisions. In the asymptomatic patient group, the small hypothetical risk may outweigh the unproven, potential benefit.
For now, cardiac CT should be ordered consistent with established appropriateness criteria and expert consensus.44-46 Clearly, an individualized consideration of risks and benefits of cardiac CT in each patient is needed, and ideally risks and benefits should be discussed with the patient whenever practical. Can the clinical question at hand be addressed by means that do not use ionizing radiation? Has the patient perhaps recently at another institution undergone an imaging procedure that involved ionizing radiation that could help address the clinical question? Which among the appropriate imaging modalities will expose the patient to the least amount of radiation? These considerations should also include the risk of missing an important, potentially life-threatening diagnosis if imaging is not performed because of concerns about radiation. If a cardiac CT is deemed necessary, the available scanning options to reduce radiation dose that apply to the type of examination and the individual patient should be utilized unless justified reasons exist not to do so. Foregoing one-size-fits-all approaches to cardiac CT in favor of individualizing scanning protocols represents an all-important opportunity to keep patient dose as low as reasonably achievable.
It is up to the cardiac imaging community to develop the information needed to make rational determinations of the potential risk and benefits in individual patients. Emphasizing the importance and use of measurable x-ray tube output metrics in cardiac CT and routinely including such information in the imaging record to facilitate the determination of diagnostic reference levels and trends will help us understand to what levels of radiation dose patients are exposed with each permutation of scanner technology. In the clinical realm, studies of the value of detecting subclinical atherosclerosis in the form of noncalcified plaque for improving longevity are difficult to conceive and conduct but are pivotal if the use of cardiac CT in asymptomatic patients with risk factors is to be justified. These efforts are needed understand in which circumstances cardiac CT conveys benefit to patients by facilitating management decisions that will help them live better and longer.
Acknowledgments
Supported in part by NIH grant 1R01EB007986-02 (“Non-Invasive Localization of Vulnerable Plaques”) awarded to Birgit Kantor, M.D. and Cynthia H. McCollough, PhD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mettler F, Jr, Thomadsen B, Bhargavan M, et al. Medical Radiation Exposure in the U.S.2006: Preliminary Results. Health Physics. 2008;95(5):502–507. doi: 10.1097/01.HP.0000326333.42287.a2. [DOI] [PubMed] [Google Scholar]
- 2.Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007 Nov 29;357(22):2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 3.National Council on Radiation Protection and Measurements (NCRP) Ionizing radiation exposure of the population of the United States: recommendations of the National Council on Radiation Protection and Measurements. Bethesda, MD: National Council on Radiation Protection and Measurements (NCRP); 1987. p. 93. [Google Scholar]
- 4.National Council on Radiation Protection and Measurements (NCRP) Exposure of the U.S. population from diagnostic medical radiation: recommendation of the National Council on Radiation Protection and Measurements. Bethesda, MD: National Council on Radiation Protection and Measurements (NCRP); 1989. p. 100. [Google Scholar]
- 5.Health Physics Society (HPS) Radiation Risk in Perspective. [March 12, 2009]; http://hps.org/documents/risk_ps010-1.pdf (accessed March 12, 2009)
- 6.National Council on Radiation Protection and Measurements (NCRP) Ionizing radiation exposure of the population of the United States (prepublication copy) Bethesda, MD: National Council on Radiation Protection and Measurements (NCRP); 2009. p. 160. [Google Scholar]
- 7.Hall EJ, Giaccia AJ. Radiation biology for the radiologist. Philadelphia, PA (USA): Lippincott, Williams and Wilkins; 2006. [Google Scholar]
- 8.Goans RE. Clinical care of the radiation accident patient: patient presentation, assessment and initial diagnosis. In: Ricks RC, Berger ME, O'Hare FM, editors. The medical basis for radiation accident preparedness: The clinical care of victims. Boca Raton, FL (USA): Parthenon Publishing Group; 2002. [Google Scholar]
- 9.Lloyd DC, Edwards AA, Moquet JE, Guerrero-Carbajal YC. The role of cytogenetics in early triage of radiation casualties. Appl Radiat Isot. 2000 May;52(5):1107–1112. doi: 10.1016/s0969-8043(00)00054-3. [DOI] [PubMed] [Google Scholar]
- 10.Gerber TC, Carr JJ, Arai AE, et al. Ionizing radiation in cardiac imaging: a science advisory from the American Heart Association Committee on Cardiac Imaging of the Council on Clinical Cardiology and Committee on Cardiovascular Imaging and Intervention of the Council on Cardiovascular Radiology and Intervention. Circulation. 2009 Feb 24;119(7):1056–1065. doi: 10.1161/CIRCULATIONAHA.108.191650. [DOI] [PubMed] [Google Scholar]
- 11.Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation Board on Radiation Effects Research Division on Earth and Life Studies National Research Council of the National Academies. Health risks from exposure to low levels of ionizing radiation: BEIR VII-Phase 2. Washington, DC: National Academies Press; 2006. [PubMed] [Google Scholar]
- 12.Cardis E, Vrijheid M, Blettner M, et al. The 15-Country Collaborative Study of Cancer Risk among Radiation Workers in the Nuclear Industry: estimates of radiation-related cancer risks. Radiat Res. 2007;167:396–416. doi: 10.1667/RR0553.1. [DOI] [PubMed] [Google Scholar]
- 13.Ries LAG, Melbert DK, Mariotto A, et al. Lifetime risk (percent) of being diagnosed with cancer and lifetime risk (percent) of dying from cancer, by site and race/ethnicity. [March 13, 2009];SEER Cancer Statistic Review. 1975-2004; based on November 2006 SEER data submission, posted to the SEER Web site, 2007. Tables I-14 and I-17 [ http://seer.cancer.gov/csr/1975_2005/results_merged/topic_lifetime_risk.pdf.
- 14.National Council on Radiation Protection and Measurements (NCRP) Risk estimates for radiation protection. Bethesda, MD: National Council on Radiation Protection and Measurements (NCRP); 1993. p. 115. [Google Scholar]
- 15.Wikipedia - the free encyclopedia. Radiation hormesis. [March 12, 2009]; http://en.wikipedia.org/wiki/Radiation_hormesis.
- 16.Einstein AJ, Henzlova MJ, Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA. 2007 Jul 18;298(3):317–323. doi: 10.1001/jama.298.3.317. [DOI] [PubMed] [Google Scholar]
- 17.Gerber TC, Kuzo RS, Morin RL. Techniques and parameters for estimating radiation exposure and dose in cardiac computed tomography. Int J Cardiovasc Imaging. 2005 Feb;21(1):165–176. doi: 10.1007/s10554-004-5338-6. [DOI] [PubMed] [Google Scholar]
- 18.McCollough CH, Primak AN, Braun N, Kofler J, Yu L, Christner J. Strategies for Reducing Radiation Dose in CT. Radiol Clin North Am. 2009 Jan;47(1):27–40. doi: 10.1016/j.rcl.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paul JF, Abada HT. Strategies for reduction of radiation dose in cardiac multislice CT. Eur Radiol. 2007 Aug;17(8):2028–2037. doi: 10.1007/s00330-007-0584-3. [DOI] [PubMed] [Google Scholar]
- 20.Anonymous. ACR practice guideline for diagnostic reference levels in medical x-ray imaging. [March 12, 2009];2006 http://www.acr.org/SecondaryMainMenuCategories/quality_safety/RadSafety/RadiationSafety/guideline-diagnostic-reference.aspx.
- 21.Gray JE, Archer BR, Butler PF, et al. Reference values for diagnostic radiology: application and impact. Radiology. 2005 May;235(2):354–358. doi: 10.1148/radiol.2352020016. [DOI] [PubMed] [Google Scholar]
- 22.Suleiman OH, Conway BJ, Quinn P, et al. Nationwide survey of fluoroscopy: radiation dose and image quality. Radiology. 1997 May;203(2):471–476. doi: 10.1148/radiology.203.2.9114107. [DOI] [PubMed] [Google Scholar]
- 23.Van Unnik JG, Broerse JJ, Geleijns J, Jansen JT, Zoetelief J, Zweers D. Survey of CT techniques and absorbed dose in various Dutch hospitals. Br J Radiol. 1997 Apr;70(832):367–371. doi: 10.1259/bjr.70.832.9166072. [DOI] [PubMed] [Google Scholar]
- 24.Bauhs JA, Vrieze TJ, Primak AN, Bruesewitz MR, McCollough CH. CT dosimetry: comparison of measurement techniques and devices. Radiographics. 2008 Jan-Feb;28(1):245–253. doi: 10.1148/rg.281075024. [DOI] [PubMed] [Google Scholar]
- 25.Morin RL, Gerber TC, McCollough CH. Radiation dose in computed tomography of the heart. Circulation. 2003 Feb 18;107(6):917–922. doi: 10.1161/01.cir.0000048965.56529.c2. [DOI] [PubMed] [Google Scholar]
- 26.McCollough CH, Bruesewitz MR, Kofler JM., Jr CT dose reduction and dose management tools: overview of available options. Radiographics. 2006 Mar-Apr;26(2):503–512. doi: 10.1148/rg.262055138. [DOI] [PubMed] [Google Scholar]
- 27.McCollough CH, Schueler BA. Calculation of effective dose. Medical Physics. 2000;27(5):828–837. doi: 10.1118/1.598948. [DOI] [PubMed] [Google Scholar]
- 28.International Commission on Radiological Protection (ICRP) 1977 recommendations of the International Commission on Radiological Protection (ICRP Publication 26) Ann ICRP. 1977;1 doi: 10.1016/j.icrp.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 29.International Commission on Radiological Protection (ICRP) 1990 recommendations of the International Commission on Radiological Protection (ICRP Publication 60) Ann ICRP. 1991;21:1–201. [PubMed] [Google Scholar]
- 30.International Commission on Radiological Protection (ICRP) 2007 recommendations of the International Commission on Radiological Protection (ICRP Publication 103) Ann ICRP. 2007;37:1–332. doi: 10.1016/j.icrp.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Shrimpton PC, Wall BF, Yoshizumi TT, Hurwitz LM, Goodman PC. Effective dose and dose-length product in CT. Radiology. 2009 Feb;250(2):604–605. doi: 10.1148/radiol.2502081340. [DOI] [PubMed] [Google Scholar]
- 32.Martin CJ. Effective dose: how should it be applied to medical exposures? Br J Radiol. 2007 Aug;80(956):639–647. doi: 10.1259/bjr/25922439. [DOI] [PubMed] [Google Scholar]
- 33.McCollough CH. Dose in computed tomography: how to quantitate, how to reduce. Paper presented at: NCRP 43rd Annual Meeting: Advances in Radiation Protection in Medicine; Arlington, VA. 2007. [Google Scholar]
- 34.Gerber TC, Kuzo RS, Karstaedt N, et al. Current results and new developments of coronary angiography with use of contrast-enhanced computed tomography of the heart. Mayo Clinic Proceedings. 2002;77(1):55–71. doi: 10.4065/77.1.55. [DOI] [PubMed] [Google Scholar]
- 35.Gerber TC, Stratmann BP, Kuzo RS, Kantor B, Morin RL. Effect of acquisition technique on radiation dose and image quality in multidetector row computed tomography coronary angiography with submillimeter collimation. Invest Radiol. 2005 Aug;40(8):556–563. doi: 10.1097/01.rli.0000170628.69792.cb. [DOI] [PubMed] [Google Scholar]
- 36.Hausleiter J, Meyer T, Hadamitzky M, et al. Radiation dose estimates from cardiac multislice computed tomography in daily practice: impact of different scanning protocols on effective dose estimates. Circulation. 2006 Mar 14;113(10):1305–1310. doi: 10.1161/CIRCULATIONAHA.105.602490. [DOI] [PubMed] [Google Scholar]
- 37.Jakobs TF, Becker CR, Ohnesorge B, et al. Multislice helical CT of the heart with retrospective ECG gating: reduction of radiation exposure by ECG-controlled tube current modulation. Eur Radiol. 2002 May;12(5):1081–1086. doi: 10.1007/s00330-001-1278-x. [DOI] [PubMed] [Google Scholar]
- 38.Trabold T, Buchgeister M, Kuttner A, et al. Estimation of radiation exposure in 16-detector row computed tomography of the heart with retrospective ECG-gating. ROFO-Fortschritte auf dem Gebiet der Rontgenstrahlen und der Bildgebenden V. 2003;175(8):1051–1055. doi: 10.1055/s-2003-40926. [DOI] [PubMed] [Google Scholar]
- 39.Sanz J, Rius T, Kuschnir P, et al. The importance of end-systole for optimal reconstruction protocol of coronary angiography with 16-slice multidetector computed tomography. Invest Radiol. 2005 Mar;40(3):155–163. doi: 10.1097/01.rli.0000153930.34439.e4. [DOI] [PubMed] [Google Scholar]
- 40.Earls JP, Berman EL, Urban BA, et al. Prospectively gated transverse coronary CT angiography versus retrospectively gated helical technique: improved image quality and reduced radiation dose. Radiology. 2008 Mar;246(3):742–753. doi: 10.1148/radiol.2463070989. [DOI] [PubMed] [Google Scholar]
- 41.Earls JP. How to use a prospective gated technique for cardiac CT. J Cardiovasc Comput Tomogr. 2009 Jan-Feb;3(1):45–51. doi: 10.1016/j.jcct.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 42.Hausleiter J, Meyer T, Hermann F, et al. Estimated radiation dose associated with cardiac CT angiography. JAMA. 2009 Feb 4;301(5):500–507. doi: 10.1001/jama.2009.54. [DOI] [PubMed] [Google Scholar]
- 43.Mettler F, Jr, Huda W, Yoshizumi T, Mahesh M. A catalog of effective doses in radiology and nuclear medicine. Radiology. 2008;248:254–263. doi: 10.1148/radiol.2481071451. [DOI] [PubMed] [Google Scholar]
- 44.Bluemke DA, Achenbach S, Budoff M, et al. Noninvasive coronary artery imaging: magnetic resonance angiography and multidetector computed tomography angiography: a scientific statement from the american heart association committee on cardiovascular imaging and intervention of the council on cardiovascular radiology and intervention, and the councils on clinical cardiology and cardiovascular disease in the young. Circulation. 2008 Jul 29;118(5):586–606. doi: 10.1161/CIRCULATIONAHA.108.189695. [DOI] [PubMed] [Google Scholar]
- 45.Budoff MJ, Achenbach S, Blumenthal RS, et al. Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation. 2006 Oct 17;114(16):1761–1791. doi: 10.1161/CIRCULATIONAHA.106.178458. [DOI] [PubMed] [Google Scholar]
- 46.Hendel RC, Patel MR, Kramer CM, et al. ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American College of Radiology, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, American Society of Nuclear Cardiology, North American Society for Cardiac Imaging, Society for Cardiovascular Angiography and Interventions, and Society of Interventional Radiology. J Am Coll Cardiol. 2006 Oct 3;48(7):1475–1497. doi: 10.1016/j.jacc.2006.07.003. [DOI] [PubMed] [Google Scholar]


