Abstract
Background
End-stage renal disease disproportionately affects black persons, but it is unknown when in the course of chronic kidney disease racial differences arise. Understanding the natural history of racial differences in kidney disease may help guide efforts to reduce disparities.
Methods
We compared white/black differences in the risk of end-stage renal disease and death by level of estimated glomerular filtration rate (eGFR) at baseline in a national sample of 2,015,891 veterans between 2001 to 2005.
Results
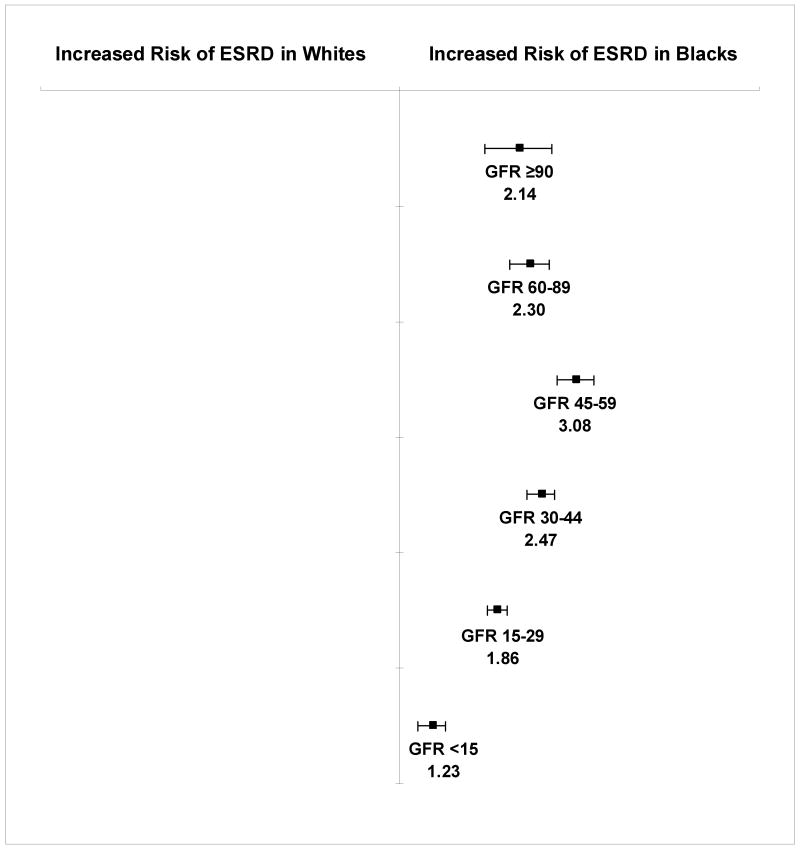
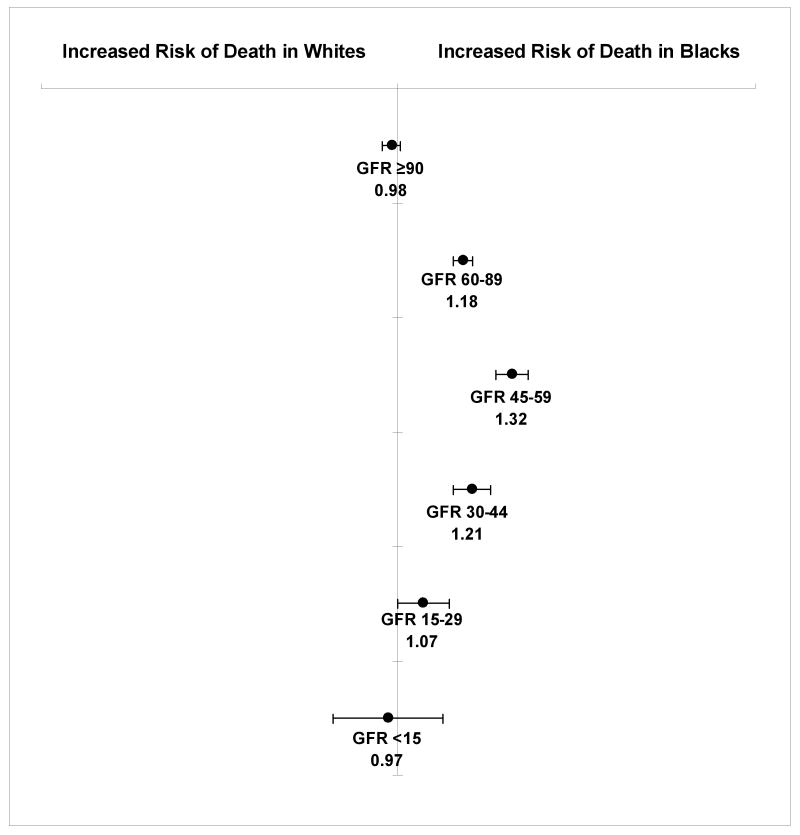
Rates of end-stage renal disease among black patients exceeded those among white patients at all levels of baseline eGFR. The adjusted hazard ratios (HR) for end-stage renal disease associated with black versus white race for patients with an eGFR ≥90, 60-89, 45-59, 30-44, 15-29, and <15 mL/min/1.73m2, respectively were 2.14 (95% confidence interval [CI], 1.72-2.65), 2.30 (95% CI, 2.02-2.61), 3.08 (95% CI, 2.74-3.46), 2.47 (95% CI, 2.26-2.70), 1.86 (95% CI, 1.75-1.98), and 1.23 (95% CI, 1.12- 1.34). We observed a similar pattern for mortality, with equal or higher rates of death among black persons at all levels of eGFR. The highest risk of mortality associated with black race was also observed among those with an eGFR 45-59 mL/min/1.73m2 (HR 1.32, 95% CI, 1.27-1.36).
Conclusion
Racial differences in the risk of end-stage renal disease appear early in the course of kidney disease and are not explained by a survival advantage among blacks. Efforts to identify and slow progression of chronic kidney disease at earlier stages may be needed to reduce racial disparities.
Keywords: kidney disease, racial disparities, mortality
Introduction
Chronic kidney disease is now recognized as a major public health concern.1, 2 It is estimated that approximately 26.3 million adults in the US have non-dialysis dependent kidney disease and over 470,000 have end-stage renal disease, collectively representing over 13% of the US population.3-5 In the next 20 years, the burden of chronic kidney disease is expected to increase, with over 2 million individuals projected to be receiving renal replacement therapy (dialysis or kidney transplant) by 2030.2 In the US, black individuals shoulder a disproportionate burden of end-stage renal disease, comprising 32% of the end-stage renal disease population, but only 13% of the general population.5, 6 The increased risk for end-stage renal disease among blacks has been established by a large number of studies, 7-9 and the goal to eliminate this racial disparity has now found a place in the national health care agenda.10, 11
To date, research in this area has focused on the optimal delivery of care and treatment of complications associated with later stages of chronic kidney disease, such as dialysis and kidney transplantation, where race disparities have been well-documented.12-14 However, there is limited information on the presence and extent of racial differences in risk of adverse events at earlier stages of chronic kidney disease, where there may be the greatest potential to prevent or slow progression. The primary objective of this study was to compare white/black differences in end-stage renal disease and death through the course of kidney disease and to identify at what level of kidney function that racial disparities are greatest.
Method
Study Sample
The Department of Veterans Affairs (VA) health care system is the largest integrated health maintenance organization in the US.15 Geographically, the VA health care system is national in scope and offers comprehensive clinical services to US veterans. To be eligible for the study, we included all veterans of white or black race with one or more outpatient serum creatinine level recorded at a VA facility between October 2000 and September 2001. Patients entered the study at the time of their first creatinine measurement during this enrollment period. We excluded subjects who had already reached end-stage renal disease at the time of study entry, defined as receipt of chronic dialysis or kidney transplant.
Data Sources
We assembled the study cohort with multiple VA and non-VA data sources using previously described methods.16, 17 Briefly, creatinine measurements associated with outpatient visits were obtained from the VA Decision Support System Laboratory Results file.18 We used the VA National Patient Care Database, VA Fee Basis files, Medicare Denominator File, Immunology Case Registry, and inpatient and outpatient Medicare claims to ascertain demographic and comorbidity information.18, 19 We determined the date of death for cohort members using a validated VA death registry, the Beneficiary Identification and Records Locator Subsystem.20-22 Area-level socioeconomic data were obtained by matching an individual's residential zip code at the time of cohort entry to year 2000 United States Census zip code tabulation areas.6 Patients treated with chronic dialysis or kidney transplant during follow-up were identified by linking our cohort to the United States Renal Data System (USRDS), a comprehensive national end-stage renal disease registry.23
Patient Characteristics
The primary predictor variable for all analyses was white/black race. We preferentially designated race using the Medicare Denominator File based on its superior reliability to VA sources.24, 25 When Medicare race data were not available, we used race reported in VA data sources. Since the establishment of federal race reporting guidelines on October 1, 2002, the VA has achieved 95% agreement between observer reported data and self-reported race.24
We estimated glomerular filtration rate (eGFR) using the abbreviated Modification of Diet in Renal Disease (MDRD) formula based on age, sex, race, and serum creatinine level at the time of cohort entry.3 Patients were classified according to National Kidney Foundation guidelines by baseline eGFR level as follows: normal or increased (eGFR ≥90 ml/min/1.73m2), mildly decreased (eGFR 60-89 ml/min/1.73m2), moderately decreased (eGFR 45-59 and 30-44 ml/min/1.73m2), severely decreased (eGFR 15-29 ml/min/1.73m2) and kidney failure (eGFR <15 ml/min/1.73m2, not on dialysis). We identified co-existing illnesses using previously described methods, based on hospitalization discharge diagnoses, outpatient encounters, and procedure codes in VA and Medicare data sources between January 1999 and the time of cohort entry.16, 17, 26 We identified the following diagnosed conditions: coronary heart disease, heart failure, peripheral arterial disease, chronic obstructive lung disease, cerebrovascular disease, hypertension, diabetes mellitus, dementia, atrial fibrillation, hepatitis C virus (HCV) infection and human immunodeficiency virus (HIV) infection. Reliable information on individual-level socioeconomic status was not available in our data sources; we therefore assigned patients to categories of low, middle, or high socioeconomic status based on multiple residential zip code-level socioeconomic characteristics derived from the US Census using previously described techniques. 6, 17, 27
Outcomes
The primary outcomes were time from cohort entry to onset of end-stage renal disease (defined as initiation of chronic dialysis or kidney transplantation) and time from cohort entry to death, respectively. The observation period for end-stage renal disease extended through 2004, based on the availability of USRDS data, and through 2005 for death. Patients were censored at the time of death for the outcome of time to end-stage renal disease.
The decision to initiate renal replacement therapy may not be dictated by uniform clinical criteria in practice. In order to support our assumption that rates of end-stage renal disease represent progression of disease, as a secondary outcome, we also calculated mean annual rates of change in eGFR based on serial creatinine measurements available during follow-up among those with moderate to severe kidney disease at baseline (eGFR 15-60 ml/min/1.73m2).
Statistical Analyses
We compared baseline characteristics between patients of white and black race using the t-test for continuous variables and the chi square test for categorical variables. Incidence rates for death and end-stage renal disease were obtained using a parametric survival-time model fitted to the exponential distribution and standardized to the median age of the overall cohort. We used Cox proportional hazard analysis to examine the association of white/black race with time to death and end-stage renal disease, respectively. Estimates were adjusted for age, sex, baseline comorbidities, and socioeconomic status. All analyses were stratified by level of eGFR at baseline. In order to account for potential differences in the delivery of health care and population served by each facility, estimates were adjusted for a fixed effect for VA center.
We estimated the rate of change in eGFR (ml/min/1.73m2 per year) using a mixed effects linear regression model which accounted for variations in the number and spacing of creatinine measurements and length of follow-up for each subject. For this analysis, we excluded patients who had already reached renal failure at the time of cohort entry (eGFR <15 mL/min/1.73m2) and individuals with an eGFR ≥60 mL/min/1.73m2, in whom estimates may be less precise. To avoid analyzing creatinine measurements which may not reflect chronic rates of progression, we excluded creatinine measurements that occurred 90 days before onset of end-stage renal disease or death.
All regression models were validated when checked by bootstrap methods or by comparing robust standard errors. For Cox models, the proportional hazards assumption was checked using the Schoenfeld test and by graphically comparing stratified log minus log(survivor function) versus time curves. Assumptions of the mixed effects model were tested and satisfied.
As a supplemental analysis, we analyzed end-stage renal disease and mortality among the subgroup of 86,649 women in the cohort. Analyses were conducted using Stata version 9.2 (Stata Corp, College Station, Tx). This study was approved by the Committee on Human Research at the San Francisco VA Medical Center.
Results
We identified 2,352,584 veterans aged 18-100 years with at least one outpatient serum creatinine measurement. There were 11,125 (0.5%) end-stage renal disease patients and 325,568 (13.9%) patients with non-white/black or unknown race who were excluded, leaving 2,015,891 patients for analysis. This group consisted of 1,704,101 (84.5%) white and 311,790 (15.5%) black patients (Table 1). Black individuals in our cohort were younger and included a greater proportion of women. White individuals had a higher prevalence of cardiovascular and lung disease, but a lower prevalence of diabetes, hypertension, HCV and HIV. A higher percentage of black (55.6%) compared with white (29.9%) patients were in the lowest tertile of socioeconomic status.
Table 1. Baseline Characteristics of 2,015,891 Outpatient Veterans, According to White/Black Race*.
| Characteristic | White Race | Black Race |
|---|---|---|
| Total (%) | 1,704,101 (84.5) | 311,790 (15.5) |
| Age (years) | 66.0 ± 12.4 | 59.1 ± 13.8 |
| Female Sex (%) | 4.0 | 6.1 |
| Estimated glomerular filtration rate, mL/min/1.73m2 (%) | ||
| ≥90 | 21.5 | 44.2 |
| 60-89 | 56.3 | 42.5 |
| 45-59 | 15.2 | 8.2 |
| 30-44 | 5.5 | 3.3 |
| 15-29 | 1.4 | 1.4 |
| <15 | 0.2 | 0.4 |
| Comorbidities (%) | ||
| Diabetes mellitus | 29.1 | 31.5 |
| Coronary heart disease | 39.4 | 25.7 |
| Stroke or transient ischemic attack | 15.6 | 12.3 |
| Heart failure | 15.8 | 12.0 |
| Peripheral arterial disease | 15.4 | 10.7 |
| Atrial fibrillation | 10.6 | 4.8 |
| Hypertension | 67.7 | 70.5 |
| Chronic obstructive lung disease | 30.0 | 23.3 |
| Dementia | 5.5 | 5.6 |
| Hepatitis C virus infection | 2.1 | 5.3 |
| Human immunodeficiency virus infection | 0.4 | 2.6 |
| Socioeconomic status, tertile (%) | ||
| Low | 29.9 | 55.6 |
| Middle | 35.0 | 24.6 |
| High | 35.2 | 19.8 |
p<0.001 for all characteristics; age reported as mean ± standard deviation.
The distribution of eGFR at baseline differed substantially between black and white individuals. The prevalence of an eGFR <60 mL/min/1.73m2 was much higher among white (22.2%) than black patients (13.3%). However, this primarily reflected a difference in the prevalence of moderate kidney disease (eGFR 30-59 mL/min/1.73m2), which was present in 20.7% of white but in only 11.5% of black persons. In contrast, the prevalence of severe kidney disease (eGFR 15-29 mL/min/1.73m2) was equal among white and black individuals (1.4%), while renal failure (eGFR <15 mL/min/1.73m2) was slightly more common in black (0.4%) compared with white patients (0.2%).
The median observation time was 3.7 (inter-quartile range [IQR] 3.4-4.0) years for end-stage renal disease and 4.8 (IQR 4.5-5.0) years for mortality. Death was far more common than incident end-stage renal disease: 14.3% (n=287,827) of patients died during 8,986,646 total person-years of observation, while only 0.8% (n=15,148) reached end-stage renal disease over 7,072,237 person-years. There were 4,379 cases of end-stage renal disease and 41,715 deaths among black members of the cohort and 10,769 cases of end-stage renal disease and 246,112 deaths among white individuals.
End-stage renal disease
The age-standardized incidence of end-stage renal disease per 1,000 person-years was consistently higher among black compared to white individuals at all levels of baseline eGFR (Table 2). In multivariate Cox proportional hazard analysis (Figure 1), the risk of end-stage renal disease was higher for black compared with white patients in all eGFR categories. However, the strength of this association varied by level of baseline eGFR and was of greatest magnitude for those with an eGFR 45-59 mL/min/1.73m2 and attenuated at eGFR levels above and below. While black patients with an eGFR 45-59 mL/min/1.73m2 had a greater than 3-fold increased risk of end-stage renal disease compared to their white counterparts, the excess risk was only 23% (95% CI, 12-34%) in the eGFR <15 mL/min/1.73m2 category.
Table 2. Age-Standardized Rates of End-Stage Renal Disease.
Rates are reported as end-stage renal disease events per 1,000 person-years with 95% confidence intervals in parentheses. Estimated glomerular filtration rate (GFR) reported in mL/min/1.73m2.
| Baseline Estimated GFR | Persons at Risk | Number of Events |
Observation Time (person-years) |
Age-Standardized Rate of End-Stage Renal Disease (per 1000 person-years) |
|---|---|---|---|---|
| White Race | ||||
| ≥90 | 365,484 | 182 | 1,296,354 | 0.2 (0.1, 0.3) |
| 60-89 | 959,619 | 929 | 3,419,386 | 0.1 (0.1, 0.2) |
| 45-59 | 258,144 | 1,116 | 892,314 | 1.7 (1.5, 1.8) |
| 30-44 | 93,850 | 2,288 | 300,680 | 12.5 (11.8, 13.1) |
| 15-29 | 23,674 | 4,318 | 62,649 | 94.3 (90.7, 97.9) |
| <15 | 3,330 | 1,936 | 4,540 | 472.3 (448.3, 496.4) |
| Black Race | ||||
| ≥90 | 137,806 | 164 | 493,031 | 0.4 (0.2, 0.6) |
| 60-89 | 132,648 | 367 | 474,320 | 0.4 (0.3, 0.4) |
| 45-59 | 25,520 | 495 | 86,128 | 6.3 (5.7, 7.0) |
| 30-44 | 10,242 | 871 | 31,497 | 32.8 (30.4, 35.2) |
| 15-29 | 4,305 | 1,596 | 9,907 | 179.5 (169.8, 189.3) |
| <15 | 1,269 | 886 | 1,432 | 615.6 (571.0, 660.3) |
Figure 1. Adjusted hazard ratios (HR) for end-stage renal disease (ESRD) in black versus white patients stratified by level of estimated glomerular filtration rate (GFR) at baseline.
Models adjusted for all variables listed in Table 1. Estimated GFR reported in mL/min/1.73m2. Error bars indicate 95% confidence intervals.
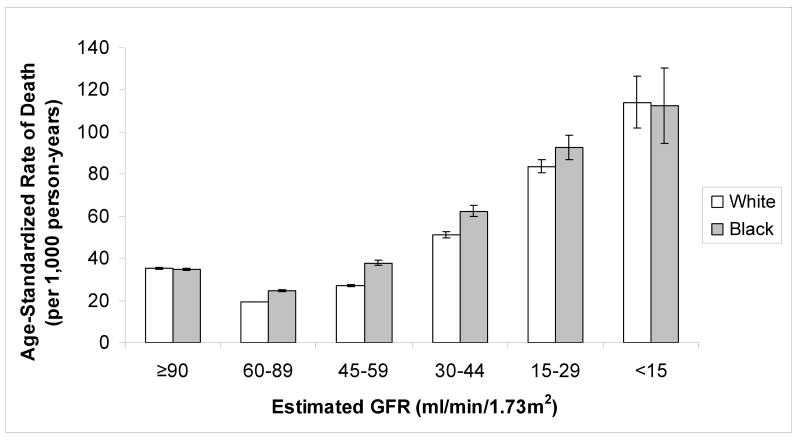
Death
Death rates were higher among black compared with white cohort members at all eGFR levels between 15 and 90 mL/min/1.73m2 (Figure 2). In adjusted analyses, the association of black race with mortality was strongest among patients with an eGFR 45-59 mL/min/1.73m2 (HR 1.32, 95% CI, 1.27-1.36) and was progressively attenuated at lower levels of eGFR (Figure 3). The adjusted risk of mortality associated with black race was two-fold greater among those with an eGFR 45-59 mL/min/1.73m2 (HR 1.32, 95% CI, 1.27-1.36) compared to those with an eGFR ≥60 mL/min/1.73m2 (HR 1.15, 95% CI, 1.11-1.18).
Figure 2. Age-standardized incidence of death by estimated glomerular filtration rate (GFR) at baseline.
Error bars indicate 95% confidence intervals.
Figure 3. Adjusted hazard ratios for death in black versus white patients stratified by level of estimated glomerular filtration rate (GFR) at baseline.
Models adjusted for all variables listed in Table 1. Estimated GFR reported in mL/min/1.73m2. Error bars indicate 95% confidence intervals.
Mean annual change in eGFR
For the outcome of mean annual change in eGFR, we included 420,334 patients with a baseline eGFR 15-60 mL/min/1.73m2 and analyzed 2,866,397 and 376,066 creatinine measurements among white and black individuals, respectively. Rates of estimated GFR decline were greater among black versus white patients for all eGFR categories studied. The mean annual change in eGFR (mL/min/1.73m2 per year) was -0.4 (95% CI, -0.4, -0.4), -0.4 (95% CI, -0.4, -0.3), and -0.5 (95% CI, -0.6, -0.4) for white patients and -0.5 (95% CI, -0.5, -0.4), -0.9 (95% CI, -1.0, -0.8), and -1.6 (95% CI, -1.9, -1.4) for black patients with a baseline eGFR 45-59, 30-44, and 15-29 mL/min/1.73m2.
Among the 86,649 women in this cohort, there were 4,485 deaths in 405,852 person-years, and 247 end-stage renal disease events in 316,358 person-years of observation. Rates of end-stage renal disease were greater among black versus white women for all categories of eGFR, but absolute rates were lower than among men. There was also a similar relationship between race, mortality and eGFR with comparable rates of death among black and white women in the eGFR >90 mL/min/1.73m2 category, but higher rates of death below this range. Fully adjusted models were consistent with these results and revealed that the association of race with time to end-stage renal disease and death reported for the primary analysis did not differ materially among women compared to men.
Discussion
In this large national cohort of patients receiving care in the Veterans Health Administration, black individuals were at higher risk for end-stage renal disease at all levels of kidney function when compared to whites, with the most pronounced differences observed in those with normal to moderately decreased eGFR. Differences in the risk of end-stage renal disease between blacks and whites were not explained by a lower competing risk of mortality. Instead, the risk of mortality associated with black race was increased in the setting of renal dysfunction, with the greatest relative increase in risk found among those with mild to moderate reductions of eGFR (30-89 mL/min/1.73m2). These findings demonstrate the presence of marked racial differences in end-stage renal disease and death early in the course of kidney disease and provide support for disparities research focused on prevention, targeted screening, and timely provision of therapies for chronic kidney disease among blacks.
Our findings highlight the importance of ongoing efforts in the renal community to improve awareness and recognition of chronic kidney disease.1, 3, 28 Current guidelines classify kidney disease primarily based on level of kidney function, to identify earlier stages of kidney disease and its antecedent risk factors, with the hope that adverse events can be prevented or delayed through targeted screening and early detection.1, 3 The classification of kidney disease by level of eGFR may also provide a platform for identifying groups vulnerable to poor renal outcomes. Our results demonstrate pronounced white/black differences in the risk of end-stage renal disease and death with mild reductions of eGFR, suggesting that these disparities will persist without interventions aimed at earlier stages of kidney disease.
It is noteworthy that the higher incidence of end-stage renal disease among members of this cohort did not reflect a survival advantage among blacks with chronic kidney disease, as some have postulated.29-31 Consistent with a prior pooled analysis of four different cohorts and a recent analysis of data from the third National Health And Nutrition Examination Survey, we found that mortality rates were higher, not lower, in blacks with kidney disease.32, 33 Thus, it is likely that observed racial differences in the risk of end-stage renal disease underestimate true differences in rates of progression. Furthermore, the finding that the relative hazard of death and end-stage renal disease for blacks were both highest in patients with moderate reductions in eGFR (30-59 mL/min/1.73m2) suggests that the paradoxically lower prevalence of moderate kidney disease may be attributed to a relatively rapid exodus (due to death or progression) from this stage among blacks.31, 32, 34
While racial differences in risk of end-stage renal disease are widely recognized, explanatory mechanisms are poorly understood.7, 35, 36 Prior studies have found that black patients are more likely to have inadequately controlled diabetes, hypertension, and proteinuria than their white counterparts, despite a similar intensity of treatment, and are less likely to achieve quality of care goals.37-39 We observed significant differeces in the risk of end-stage renal disease despite uniform access to health care and extensive adjustment for sociodemographic variables and comorbid conditions. However, we did not adjust for blood pressure and proteinuria because these measures were not available for most patients. Thus, it is possible that the association of black race with end-stage renal disease and death observed here may be partly explained by racial differences in these characteristics which are known targets for intervention.
There are several other limitations to our study. Data were obtained during the course of regular outpatient clinical care; therefore, estimates of kidney function were not available for the entire source population. Although our results are consistent with other population based estimates of kidney disease, differences may exist in populations which include healthier patients who are not in clinical care.4, 34, 40 Findings may also not be generalizable to non-veterans, uninsured patients, women, or patients of other race/ethnicity groups. Finally, as mentioned above, we were unable to measure the contribution of several key predictors of progression including signs of kidney damage (such as proteinuria or hematuria), and blood pressure measurements, or individual-level socioeconomic factors because these were not available in our data sources.
Conclusions
Black patients have a higher risk of end-stage renal disease regardless of their baseline level of kidney function, that is most pronounced among those with moderate kidney disease. Similarly, racial differences in mortality arise and peak in the setting of mild to moderate reductions in eGFR. These findings support the substantial public health importance of efforts to prevent and slow progression of early kidney disease in blacks.
Acknowledgments
This study was supported by a NKF fellowship grant, NIH K23DK080645, K23AG028980, and R01AI069952, Kellogg Scholars in Health Disparities Program, Paso del Norte Health Foundation, and the VA Research Enhancement Award Program. These funding sources had no involvement in the design or execution of this study.
GTH has received research support from Catholic Healthcare West, Genentech, Biogen, Novartis, Roche, and NIDDK; he has received honoraria from NIEHS and Novartis. AMO receives royalties from UpToDate and research funding from the Centers for Disease Control. The other authors declare no potential financial conflicts of interest.
Funding Sources: This study was supported by a fellowship grant from the National Kidney Foundation, grants from the National Institutes of Health (K23DK080645-01A1, K23AG028980-03, R01AI069952-03), W.K. Kellogg Scholars in Health Disparities Program, Paso del Norte Health Foundation's Center for Border Health Research, and the San Francisco VA Research Enhancement Award Program to Improve Care for Older Veterans. These funding sources had no involvement in the design or execution of this study.
Footnotes
Potential Conflicts of Interest: GTH has received research support from the Catholic Healthcare West, Genentech, Biogen, Novartis, Roche and the National Institute of Diabetes and Digestive and Kidney Diseases; he has received honoraria from the National Institute of Environmental Health Sciences and Novartis. AMO receives royalties from UpToDate and research funding from the Centers for Disease Control. These funding sources played no role in the research presented here. The other authors declare no potential financial conflicts of interest.
Authorship: AIC, AMO, and DB had access to the data; all authors had a role in writing the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Levey AS, Atkins R, Coresh J, Cohen EP, Collins AJ, Eckardt KU, et al. Chronic kidney disease as a global public health problem: Approaches and initiatives - a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007 Jun 13; doi: 10.1038/sj.ki.5002343. [DOI] [PubMed] [Google Scholar]
- 2.Schoolwerth AC, Engelgau MM, Hostetter TH, Rufo KH, Chianchiano D, McClellan W, et al. Chronic kidney disease: a public health problem that needs a public health action plan. Prev Chronic Dis. 2006 [PMC free article] [PubMed] [Google Scholar]
- 3.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003 Jul 15;139(2):137–47. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 4.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, et al. Prevalence of chronic kidney disease in the United States. Jama. 2007 Nov 7;298(17):2038–47. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 5.United States Renal Data System 2006 Annual Data Report. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2006. [Google Scholar]
- 6.United States Census 2000. 2002 March 17, 2006 [cited July 3, 2006]; Available from: http://www.census.gov/main/www/cen2000.html.
- 7.Tarver-Carr ME, Powe NR, Eberhardt MS, LaVeist TA, Kington RS, Coresh J, et al. Excess risk of chronic kidney disease among African-American versus white subjects in the United States: a population-based study of potential explanatory factors. J Am Soc Nephrol. 2002 Sep;13(9):2363–70. doi: 10.1097/01.asn.0000026493.18542.6a. [DOI] [PubMed] [Google Scholar]
- 8.Xue JL, Eggers PW, Agodoa LY, Foley RN, Collins AJ. Longitudinal study of racial and ethnic differences in developing end-stage renal disease among aged medicare beneficiaries. J Am Soc Nephrol. 2007 Apr;18(4):1299–306. doi: 10.1681/ASN.2006050524. [DOI] [PubMed] [Google Scholar]
- 9.Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Stamler J. End-stage renal disease in African-American and white men. 16-year MRFIT findings. Jama. 1997 Apr 23-30;277(16):1293–8. [PubMed] [Google Scholar]
- 10.Healthy People 2010. 2nd. Washington, DC: Department of Health and Human Services; 2000. [Google Scholar]
- 11.Powe NR. Let's get serious about racial and ethnic disparities. J Am Soc Nephrol. 2008 Jul;19(7):1271–5. doi: 10.1681/ASN.2008040358. [DOI] [PubMed] [Google Scholar]
- 12.Ayanian JZ, Cleary PD, Weissman JS, Epstein AM. The effect of patients' preferences on racial differences in access to renal transplantation. N Engl J Med. 1999 Nov 25;341(22):1661–9. doi: 10.1056/NEJM199911253412206. [DOI] [PubMed] [Google Scholar]
- 13.Kinchen KS, Sadler J, Fink N, Brookmeyer R, Klag MJ, Levey AS, et al. The timing of specialist evaluation in chronic kidney disease and mortality. Ann Intern Med. 2002 Sep 17;137(6):479–86. doi: 10.7326/0003-4819-137-6-200209170-00007. [DOI] [PubMed] [Google Scholar]
- 14.Powe NR. Let's Get Serious About Racial and Ethnic Disparities. J Am Soc Nephrol. 2008 Jun 4; doi: 10.1681/ASN.2008040358. [DOI] [PubMed] [Google Scholar]
- 15.Ashton CM, Souchek J, Petersen NJ, Menke TJ, Collins TC, Kizer KW, et al. Hospital use and survival among Veterans Affairs beneficiaries. N Engl J Med. 2003 Oct 23;349(17):1637–46. doi: 10.1056/NEJMsa003299. [DOI] [PubMed] [Google Scholar]
- 16.O' Hare AM, Bertenthal D, Covinsky KE, Landefeld CS, Sen S, Mehta K, et al. Mortality risk stratification in chronic kidney disease: one size for all ages? J Am Soc Nephrol. 2006 Mar;17(3):846–53. doi: 10.1681/ASN.2005090986. [DOI] [PubMed] [Google Scholar]
- 17.Choi AI, Rodriguez RA, Bacchetti P, Bertenthal D, Volberding PA, O' Hare AM. Racial Differences in End-Stage Renal Disease Rates in HIV Infection versus Diabetes. J Am Soc Nephrol. 2007 Oct 17; doi: 10.1681/ASN.2007040402. [DOI] [PubMed] [Google Scholar]
- 18.Maynard C, Chapko MK. Data resources in the Department of Veterans Affairs. Diabetes Care. 2004 May;27 2:B22–6. doi: 10.2337/diacare.27.suppl_2.b22. [DOI] [PubMed] [Google Scholar]
- 19.Backus L, Mole L, Chang S, Deyton L. The Immunology Case Registry. J Clin Epidemiol. 2001 Dec;54 1:S12–5. doi: 10.1016/s0895-4356(01)00442-5. [DOI] [PubMed] [Google Scholar]
- 20.Cowper DC, Kubal JD, Maynard C, Hynes DM. A primer and comparative review of major US mortality databases. Ann Epidemiol. 2002 Oct;12(7):462–8. doi: 10.1016/s1047-2797(01)00285-x. [DOI] [PubMed] [Google Scholar]
- 21.Fisher SG, Weber L, Goldberg J, Davis F. Mortality ascertainment in the veteran population: alternatives to the National Death Index. Am J Epidemiol. 1995 Feb 1;141(3):242–50. doi: 10.1093/oxfordjournals.aje.a117426. [DOI] [PubMed] [Google Scholar]
- 22.Page WF, Mahan CM, Kang HK. Vital status ascertainment through the files of the Department of Veterans Affairs and the Social Security Administration. Ann Epidemiol. 1996 Mar;6(2):102–9. doi: 10.1016/1047-2797(95)00126-3. [DOI] [PubMed] [Google Scholar]
- 23.United States Renal Data System. 2006 [cited July 28, 2006]; Available from: http://www.usrds.org.
- 24.Sohn MW, Zhang H, Arnold N, Stroupe K, Taylor BC, Wilt TJ, et al. Transition to the new race/ethnicity data collection standards in the Department of Veterans Affairs. Popul Health Metr. 2006 Jul 6;4(1):7. doi: 10.1186/1478-7954-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arday SL, Arday DR, Monroe S, Zhang J. HCFA's racial and ethnic data: current accuracy and recent improvements. Health Care Financ Rev. 2000 Summer;21(4):107–16. [PMC free article] [PubMed] [Google Scholar]
- 26.Borzecki AM, Wong AT, Hickey EC, Ash AS, Berlowitz DR. Identifying hypertension-related comorbidities from administrative data: what's the optimal approach? Am J Med Qual. 2004 Sep-Oct;19(5):201–6. doi: 10.1177/106286060401900504. [DOI] [PubMed] [Google Scholar]
- 27.Diez Roux AV, Merkin SS, Arnett D, Chambless L, Massing M, Nieto FJ, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001 Jul 12;345(2):99–106. doi: 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- 28.Brown WW, Peters RM, Ohmit SE, Keane WF, Collins A, Chen SC, et al. Early detection of kidney disease in community settings: the Kidney Early Evaluation Program (KEEP) Am J Kidney Dis. 2003 Jul;42(1):22–35. doi: 10.1016/s0272-6386(03)00405-0. [DOI] [PubMed] [Google Scholar]
- 29.Newsome BB, McClellan WM, Coffey CS, Allison JJ, Kiefe CI, Warnock DG. Survival advantage of black patients with kidney disease after acute myocardial infarction. Clin J Am Soc Nephrol. 2006 Sep;1(5):993–9. doi: 10.2215/CJN.01251005. [DOI] [PubMed] [Google Scholar]
- 30.Hunsicker LG, Adler S, Caggiula A, England BK, Greene T, Kusek JW, et al. Predictors of the progression of renal disease in the Modification of Diet in Renal Disease Study. Kidney Int. 1997 Jun;51(6):1908–19. doi: 10.1038/ki.1997.260. [DOI] [PubMed] [Google Scholar]
- 31.Hsu CY, Lin F, Vittinghoff E, Shlipak MG. Racial differences in the progression from chronic renal insufficiency to end-stage renal disease in the United States. J Am Soc Nephrol. 2003 Nov;14(11):2902–7. doi: 10.1097/01.asn.0000091586.46532.b4. [DOI] [PubMed] [Google Scholar]
- 32.Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol. 2004 May;15(5):1307–15. doi: 10.1097/01.asn.0000123691.46138.e2. [DOI] [PubMed] [Google Scholar]
- 33.Mehrotra R, Kermah D, Fried L, Adler S, Norris K. Racial Differences in Mortality Among Those with kidney disease. J Am Soc Nephrol. 2008 Apr 2; doi: 10.1681/ASN.2007070747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McClellan W, Warnock DG, McClure L, Campbell RC, Newsome BB, Howard V, et al. Racial differences in the prevalence of chronic kidney disease among participants in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Cohort Study. J Am Soc Nephrol. 2006 Jun;17(6):1710–5. doi: 10.1681/ASN.2005111200. [DOI] [PubMed] [Google Scholar]
- 35.Ishani A, Grandits GA, Grimm RH, Svendsen KH, Collins AJ, Prineas RJ, et al. Association of single measurements of dipstick proteinuria, estimated glomerular filtration rate, and hematocrit with 25-year incidence of end-stage renal disease in the multiple risk factor intervention trial. J Am Soc Nephrol. 2006 May;17(5):1444–52. doi: 10.1681/ASN.2005091012. [DOI] [PubMed] [Google Scholar]
- 36.Rostand SG, Kirk KA, Rutsky EA, Pate BA. Racial differences in the incidence of treatment for end-stage renal disease. N Engl J Med. 1982 May 27;306(21):1276–9. doi: 10.1056/NEJM198205273062106. [DOI] [PubMed] [Google Scholar]
- 37.Heisler M, Smith DM, Hayward RA, Krein SL, Kerr EA. Racial disparities in diabetes care processes, outcomes, and treatment intensity. Med Care. 2003 Nov;41(11):1221–32. doi: 10.1097/01.MLR.0000093421.64618.9C. [DOI] [PubMed] [Google Scholar]
- 38.Rehman SU, Hutchison FN, Hendrix K, Okonofua EC, Egan BM. Ethnic differences in blood pressure control among men at Veterans Affairs clinics and other health care sites. Arch Intern Med. 2005 May 9;165(9):1041–7. doi: 10.1001/archinte.165.9.1041. [DOI] [PubMed] [Google Scholar]
- 39.Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988-2000. Jama. 2003 Jul 9;290(2):199–206. doi: 10.1001/jama.290.2.199. [DOI] [PubMed] [Google Scholar]
- 40.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004 Sep 23;351(13):1296–305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]