Abstract
Objective
To compare HIV transmission risk among patients randomized to episodic vs. continuous antiretroviral therapy.
Design
This was a substudy of the Strategies of Management of Antiretroviral Therapy (SMART) study, in which patients were randomized to continuous vs. CD4+ guided episodic antiretroviral therapy. Participants were surveyed about sexual activity and needle-sharing and had laboratory testing for gonorrhea, chlamydia, and syphilis.
Results
883 patients enrolled; the mean age was 45 years, 25% were women, and 78% were on antiretroviral therapy. At baseline, 136 participants (15.4%) had high-risk behavior (vaginal or anal sex without a condom, needle-sharing, or incident bacterial sexually-transmitted infection). Following randomization, the proportion of participants reporting high-risk behavior was stable and did not differ by randomized arm (p = 0.39). Among participants off therapy at baseline, high-risk behavior was less common 4 months after randomization among those who were randomized to start antiretroviral therapy (p = 0.03). HIV transmission risk (high-risk behavior while HIV-RNA level > 1500 copies/ml) with partners perceived to be HIV-uninfected was higher in the episodic therapy arm (p = 0.02).
Conclusions
Patients on episodic antiretroviral therapy did not decrease high-risk behavior, and because HIV-RNA levels were higher, this strategy may result in increased HIV transmission.
Keywords: antiretroviral therapy, HIV transmission risk, high-risk behavior, randomized trial
INTRODUCTION
Episodic antiretroviral therapy was suggested as a way of retaining the benefits of combination antiretroviral therapy while minimizing adverse effects of antiretroviral drugs 1, 2. However, interruption of antiretroviral therapy results in viral rebound, and plasma HIV-RNA levels are closely associated with the risk of perinatal and heterosexual transmission of HIV 3–5. Therefore, one of the concerns about episodic antiretroviral therapy is the possibility of increased risk of HIV transmission to sexual and needle-sharing partners 6.
The overall effect of the use of antiretroviral therapy on HIV transmission risk may include factors other than its effect on HIV-RNA level. Previous studies have suggested that the availability of antiretroviral therapy might result in increased risk behavior. Among gay men in the United States and western Europe, rates of unprotected anal sex increased in the years after introduction of potent combination antiretroviral therapy 7, 8. At the same time, rates of gonorrhea and syphilis increased among populations at risk for HIV infection 8. Furthermore, persons with previously diagnosed HIV infection were a prominent group in many of the recently reported outbreaks of syphilis, an infection that is both an indicator of risk behavior and a factor that increases the probability of HIV transmission 9, 10. Thus, based on these population and surveillance studies, it was hypothesized that availability of antiretroviral therapy led to increased sexual risk behavior among both HIV-infected and HIV-uninfected persons, perhaps because of a decreased perception of risk of HIV transmission while on treatment and/or a general improvement in health and well-being. If use of antiretroviral therapy is associated with an increase in risk behavior, this would partly negate the beneficial effect of antiretroviral therapy on genital viral load 11. A limitation of previous observational studies of the effect of antiretroviral therapy on HIV transmission risk behaviors is that the use of antiretroviral therapy was not randomized, making it difficult to adjust for possible confounding factors.
The Strategies for Management of Antiretroviral Therapy (SMART) study was a large, randomized clinical trial comparing episodic CD4+-guided antiretroviral therapy vs. continuous antiretroviral therapy. Enrollment in the SMART study and the episodic antiretroviral treatment were stopped early (January 11, 2006) because of an excess risk of death, opportunistic disease, and serious cardiovascular and metabolic events among participants on the episodic therapy arm 12. We report the results of a substudy comparing HIV transmission risk behavior among patients randomized to episodic therapy vs. continuous antiretroviral therapy in the SMART study. Among patients who entered the study on antiretroviral therapy, this randomized comparison allows the assessment of the effect of stopping versus continuing antiretroviral therapy on transmission risk behavior. On the other hand, among those who entered the study off antiretrovirals, the comparison is between (re-)starting versus deferring therapy.
METHODS
Study population
Between January 2002 and January 2006, 5,472 participants in 33 countries were enrolled in the SMART trial. A subset of geographically-diverse sites in the United States (New York City, Richmond, Washington DC, Denver, Portland, San Francisco, Detroit, Philadelphia, southern New Jersey) co-enrolled participants in this substudy on HIV transmission risk. Eligibility criteria for the SMART trial included a CD4+ lymphocyte count > 350 cells/mm3, and willingness to start or discontinue antiretroviral therapy according to randomization assignment. At enrollment participants could be antiretroviral-naïve, off antiretroviral therapy, but with a history of prior therapy, or on antiretroviral therapy 12. The study was reviewed by Institutional Review Boards at each site, and informed consent was obtained from each participant.
Study design
Study participants were randomized 1:1 to continuous antiretroviral therapy with the goal of maximal viral suppression (viral suppression [VS] strategy) vs. CD4+-guided episodic therapy with the threshold to stop therapy at CD4+ lymphocyte count > 350 cells/mm3 and the threshold to (re)start therapy at < 250 cells/mm3 (drug conservation [DC] strategy). The primary endpoint of the SMART study was the occurrence of a new opportunistic disease or death, and the initial results of the study have been previously reported 12. During the informed consent process patients were informed that HIV-RNA levels would be higher among patients randomized to the episodic therapy (either because of treatment interruption or deferral of therapy) and that this might increase the risk of HIV transmission to partners.
Data collection
This study assessed risk behavior known to result in HIV transmission and performed laboratory testing for bacterial sexually transmitted infections, as a second measure of risk behavior. Substudy-specific visits were at baseline, 4 months, 12 months, and annually thereafter. At each substudy visit, blood and urine specimens were collected, and patients completed a brief confidential survey of sexual and needle-sharing behavior, modeled after a set of questions suggested for HIV/STD behavioral surveillance by a workgroup for the Centers for Disease Control and Prevention 13. To minimize recall bias, the survey asked about sexual and needle-sharing behavior for the previous 2 months. Participants reported the number of sexual partners, but not the number of times they had sex with each partner. The type of sex (oral, anal, vaginal), condom use, and perceived HIV serostatus were reported for the last episode of sex with the main partner and, if applicable, occasional partner(s).
Laboratory testing for bacterial sexually-transmitted infections included urine DNA amplification testing (using any licensed test) for Neisseria gonorrhea and Chlamydia trachomatis, and serological testing for syphilis (screening rapid plasma reagin test [RPR] with testing for specific treponemal antibody [e.g., FTA-ABS test] for those with a positive RPR). Laboratory testing was performed at accredited local laboratories. In addition, patients also had CD4+ lymphocyte and HIV-RNA levels determined at each study visit. Genotypic resistance testing using the TRUGENE HIV-1 kit was performed at a central laboratory for baseline samples with an HIV-RNA level > 1000 copies/mL.
Outcome measures
The primary endpoint of this study was high-risk behavior, defined as any of the following: self-reported anal or vaginal sex without a condom, self-reported needle-sharing, or a new diagnosis of gonorrhea, chlamydia, or syphilis. Incident syphilis was defined as newly-positive non-treponemal test (RPR) confirmed by a treponemal test FTA-ABS) or a greater than 4-fold increase in RPR titer among patients with a positive RPR and FTA-ABS at baseline.
Participants were considered to have HIV transmission risk if they engaged in high-risk behavior as defined above while having an HIV-RNA level > 1500 copies/mL. This threshold for HIV-RNA levels was chosen because there were no cases of heterosexual transmission of HIV in the Rakai study of discordant couples when the infected partner had a plasma HIV-RNA level < 1500 copies/mL 4. Participants were considered to have transmission risk to HIV negative persons or those with unknown serostatus if they engaged in high-risk behavior with such partners while their HIV RNA level was > 1500 copies/mL. Participants were considered at risk of transmitting drug-resistant HIV if their baseline sample had at least one major resistance mutation 14 and they had high-risk behavior as defined above while their HIV RNA level was > 1500 copies/mL.
Statistical methods
Sample size calculation
We hypothesized that participants randomized to continuous antiretroviral therapy would more often have high-risk behavior than participants randomized to episodic antiretroviral therapy. Based on prior observational cohort studies 15–18 we hypothesized that approximately 20% of patients in the continuous therapy arm would have high-risk behavior. This study was powered to detect a two-fold difference in high-risk behavior (10% vs. 20%). When an interim analysis showed that the proportion of patients with high-risk behavior at baseline was lower than postulated (15%), the sample size was increased from 600 to 1010 participants.
Data analysis
Data were censored on 11 January 2006, the date when the CD4+-guided episodic ART strategy was modified. All comparisons between treatment arms were by intention-to-treat.
At baseline, the association between risk factors and high-risk behavior was assessed with Pearson’s χ2-test for independence, and logistic regression. We used generalized estimating equations (GEE) to compare the two randomized treatment arms longitudinally for proportions of patients with high-risk behavior (primary endpoint) and other endpoints. The treatment effect is presented as the ratio, between the two treatment groups, of the follow-up versus baseline odds ratios (comparing relative change from baseline). Subgroups of patients were compared for differential treatment effect by testing for interaction between treatment, follow-up, and subgroup indicators in the GEE models. Within treatment groups, changes in proportions over time were also assessed using GEE models.
When the survey and all laboratory tests were missing, the patient-visit was excluded from the analyses. Partially missing information was imputed as “no risk”, since prevalence of high-risk behavior was low. As sensitivity analysis, the primary endpoint analyses were repeated excluding all patient-visits where any information needed to ascertain the primary endpoint was missing. Analyses were performed with SAS version 9.1.
RESULTS
Participant characteristics
Of the 995 patients enrolling in the SMART study at participating sites, 883 (89%) co-enrolled in this substudy of transmission risk behavior between January 2002 and January 2006. Compared to participants who enrolled in the SMART study at participating sites but did not participate in this substudy, those who enrolled in the substudy were more often female (25% vs. 14%%, p = 0.01), more often reported heterosexual contact as their risk for HIV acquisition (47% vs. 31%, p = 0.002), and more often had an HIV-RNA level > 400 copies/ml at baseline (36% vs. 34%, p = 0.01). Of the 883 substudy participants, 440 were randomized to episodic therapy and 443 to continuous antiretroviral therapy. The randomized groups were well-balanced for demographic and clinical characteristics (Table 1).
Table 1.
Baseline Characteristics by Treatment Group and Overall
| Episodic ART (DC Group) (N = 440) | Continuous ART (VS Group) (N = 443) | Total (N = 883) | |
|---|---|---|---|
| Demographics | |||
| Age (years; mean, SD) | 45.4 | 44.4 | 44.9 (8.8) |
| Gender (% female) | 25.7 | 24.8 | 25.3 |
| Race: | |||
| Hispanic (%) | 13.6 | 15.6 | 14.6 |
| Black (%) | 44.5 | 46.3 | 45.4 |
| White (%) | 40.5 | 34.8 | 37.6 |
| Other (%) | 1.4 | 3.4 | 2.4 |
| HIV acquisition risk factor (CDC classification) | |||
| MSM (%) | 49.1 | 45.1 | 47.1 |
| MSM/IDU (%) | 3.6 | 5.2 | 4.4 |
| IDU (%) | 12.7 | 9.9 | 11.3 |
| Heterosexual (%) | 32.3 | 35.2 | 33.7 |
| Other/unknown (%) | 2.3 | 4.5 | 3.4 |
| Education level | |||
| < high school (%) | 19.8 | 20.8 | 20.3 |
| High school or GED (%) | 29.3 | 28.2 | 28.8 |
| Some college (%) | 34.3 | 35.2 | 34.8 |
| Completed college (%) | 9.8 | 10.4 | 10.1 |
| Any post-graduate (%) | 6.8 | 5.4 | 6.1 |
| CD4+ (cells/mm3; median, IQR) | 567 | 549 | 555 (437, 733) |
| CD4+ nadir (cells/mm3; median, IQR) | 276 | 245 | 260 (150, 372) |
| HIV-RNA ≤ 400 copies/mL (%) | 53.2 | 53.7 | 53.5 |
| Prior recorded highest log HIV RNA (log copies/mL; median, IQR) | 4.7 | 4.8 | 4.8 (4.1, 5.3) |
| ART History | |||
| Antiretroviral naïve (%) | 6.4 | 8.1 | 7.2 |
| PI experienced (%) | 70.2 | 66.4 | 68.3 |
| NNRTI experienced (%) | 62.7 | 59.6 | 61.2 |
| On ART at baseline (%) | 77.7 | 77.7 | 77.7 |
| Time since first prescribed ART (years; median, IQR) | 6 | 6 | 6 (3, 9) |
| Prior AIDS-related illnesses (%) | 26.6 | 30.9 | 28.8 |
| Hepatitis B (%) | 3.4 | 1.8 | 2.6 |
| Hepatitis C (%) | 21.6 | 21.4 | 21.5 |
Abbreviations: DC, drug conservation; VS, viral suppression; IDU, injection drug use; MSM, male/male sex; SD, standard deviation; IQR, inter-quartile range; ART, antiretroviral therapy; PI, protease inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor.
Baseline risk behavior
Of the 875 participants who completed the baseline risk behavior survey, 20 (2%) reported having used injection drugs within the past 2 months, 2 of whom reported sharing needles (Table 2). 504 participants (58%) reported having sex within the past 2 months, with an average of two sexual partners. Sex with partners perceived to be HIV-uninfected or of unknown HIV serostatus was common. Of the entire study population, 18% reported anal or vaginal sex with a main partner who was perceived to be HIV-uninfected or of unknown status. Among those who reported being sexually active, 60% of episodes of anal or vaginal sex with main partners and 66% of episodes of anal or vaginal sex with occasional partners were with partners perceived to be HIV-uninfected or of unknown status. Condoms were used more frequently for sex with partners perceived to be HIV-uninfected or of unknown HIV status than for sex with HIV-infected partners (Table 2). One patient (0.1 %) had a positive urine test for gonorrhea at baseline, and 11 (1%) had a positive test for chlamydia.
Table 2.
Baseline sexual and needle-sharing behavior
| In population N (%)a | In subset N (%)b | |
|---|---|---|
| Self-reported risk behavior (last 2 months) | 875 | |
| Injected drugs | 20 (2.3) | |
| Shared needles | 2 (10.0) | |
| Had sex with another person (oral, anal or vaginal) | 504 (57.6) | |
| Number of sex partners | ||
| 1 | 314 (62.3) | |
| 2 | 82 (16.3) | |
| 3 or more | 95 (18.8) | |
| Not answered | 13 (2.6) | |
| Had sex with main partner | 346 (68.7) | |
| Not answered | 1 (0.2) | |
| Had sex with occasional partner | 199 (39.5) | |
| Not answered | 1 (0.2) | |
| Last episode of sex with the main partner was anal or vaginal | 263 (30.1) | |
| Perceived HIV status of main partner and condom use at the last episode (anal or vaginal sex) | ||
| HIV+, condom used | 47 (17.9) | |
| HIV+, condom not used | 57 (21.7) | |
| HIV-negative, condom used | 116 (44.1) | |
| HIV-negative, condom not used | 23 (8.7) | |
| Unknown HIV status, condom used | 9 (3.4) | |
| Unknown HIV status, condom not used | 10 (3.8) | |
| Not answered | 1 (0.4) | |
| Last episode of sex with an occasional partner was anal or vaginal | 131 (15.0) | |
| Perceived HIV status of the last occasional partner and condom use at the last episode (anal or vaginal sex) | ||
| HIV+, condom used | 21 (16.0) | |
| HIV+, condom not used | 20 (15.3) | |
| HIV-negative, condom used | 31 (23.7) | |
| HIV-negative, condom not used | 5 (3.8) | |
| Unknown HIV status, condom used | 31 (23.7) | |
| Unknown HIV status, condom not used | 19 (14.5) | |
| Not answered | 4 (3.1) | |
| High-risk behavior (self-reported anal or vaginal sex without condom or shared needles or laboratory evidence of STD) | 136 (15.4)c | |
| Self-reported high-risk behavior (anal or vaginal sex without condom or shared needles in past 2 months) | 126 (14.4) | |
| Self-reported high-risk sexual behavior with a partner who was HIV-negative or of unknown serostatus (anal or vaginal sex without condom) | 54 (6.2) | |
Percent relative to number of participants with surveys, N=875, unless otherwise noted
Percent relative to subsets defined by the bolded subheadings
Percent relative to number of participants with surveys or tests for STD, N=882. This include 36 participants with partialy missing information; of these, 7 have high-risk behavior, and for 29 the endpoint was imputed as “no risk behavior”. Survey and lab tests were missing for 1 participant (excluded).
Abbreviations: STD, sexually transmitted disease
Factors associated with high-risk behavior at baseline
At baseline, 136 participants (15.4%) engaged in high-risk behavior. Factors associated with high-risk behavior included age less than 45 years, Black or White race (compared to Hispanics), male-male sex or male-male sex plus injection drug use as HIV acquisition risk factors, higher educational levels, and being off antiretroviral therapy (Table 3). At baseline, self-reported high-risk behavior was borderline associated with a diagnosis of gonorrhea or chlamydia (OR = 3.4, 95% CI = 1.0 to 11.7, p = 0.06).
Table 3.
Factors associated with high-risk behavior at baseline
| Factor | Number in subgroup | Number with high-risk behavior (%) | OR | 95% CI | p-valueb |
|---|---|---|---|---|---|
| Gender | 0.73 | ||||
| Male | 659 | 100 (15.2%) | 0.9 | 0.6–1.4 | |
| Female | 223 | 36 (16.1%) | 1.0 | ||
| Age | 0.03 | ||||
| < 45 years | 442 | 80 (18.1%) | 1.5 | 1.0–2.2 | |
| ≥ 45 years | 440 | 56 (12.7%) | 1.0 | ||
| Race/ethnicity | 0.04 | ||||
| Hispanic | 129 | 10 (7.8%) | 0.4 | 0.2–0.8 | |
| Black | 400 | 64 (16.0%) | 0.9 | 0.6–1.3 | |
| White | 332 | 60 (18.1%) | 1.0 | ||
| Other | 21 | 2 (9.5%) | 0.5 | 0.1–2.1 | |
| HIV acquisition risk factor a | 0.01 | ||||
| MSM | 415 | 80 (19.3%) | 1.0 | ||
| MSM/IDU | 39 | 8 (20.5%) | 1.1 | 0.5–2.4 | |
| IDU | 100 | 9 (9.0%) | 0.4 | 0.2–0.9 | |
| Heterosexual | 298 | 34 (11.4%) | 0.5 | 0.3–0.8 | |
| Other/unknown | 30 | 5 (16.7%) | 0.8 | 0.3–2.3 | |
| Educational level | 0.004 | ||||
| < high school | 179 | 12 (6.7%) | 1.0 | ||
| High school or GED | 253 | 38 (15.0%) | 2.5 | 1.2–4.9 | |
| Some college | 307 | 60 (19.5%) | 3.4 | 1.8–6.5 | |
| Completed college | 89 | 15 (16.9%) | 2.8 | 1.3–6.3 | |
| Any post-graduate | 54 | 11 (20.4%) | 3.6 | 1.5–8.6 | |
| Antiretroviral use at baseline | 0.05 | ||||
| On antiretroviral therapy | 686 | 97 (14.1%) | 0.7 | 0.4–1.0 | |
| Off antiretroviral therapy | 196 | 39 (19.9%) | 1.0 |
CDC classification system
Chi-square test for association between baseline high-risk behavior and subgroup factor
Abbreviations: MSM, men who have sex with men; IDU, injection drug use; CI, confidence interval; OR, odds ratio
Follow-up
The mean follow-up time was 25.5 months, and 54% of the participants were followed for at least 2 years. Sixteen participants (1.8%) were lost to follow-up. Behavioral surveys were available from 94.8% of the scheduled substudy visits, and full laboratory testing results were available from 90.4% of those visits; neither differed by randomized arm. In accordance with the SMART study design, participants in the episodic therapy arm received antiretroviral therapy for much less of the follow-up time than those in the continuous therapy arm (39% vs. 91% of follow-up time, respectively, p < 0.001), and the difference in antiretroviral therapy usage was greatest in the first year after randomization (24% vs. 94% of time). The median time to first (re)initiation of antiretroviral therapy in the episodic therapy arm was 16 months.
Changes in sexual activity and injection drug use
The proportion of participants reporting any sexual activity within the 2 months prior to study visits was similar in both treatment arms. Sexual activity appeared to decrease slightly in the first 4 months after study entry, and thereafter remained stable at approximately 55%. Similarly, there were no differences between the two randomized arms in the average number of sexual partners, perceived HIV status of partners, the proportion reporting having main or occasional partners, or in reported condom use with main or occasional partners (data not shown). Injection drug use was infrequently reported (approximately 2%) and did not differ by randomized arm.
Changes in high-risk behavior
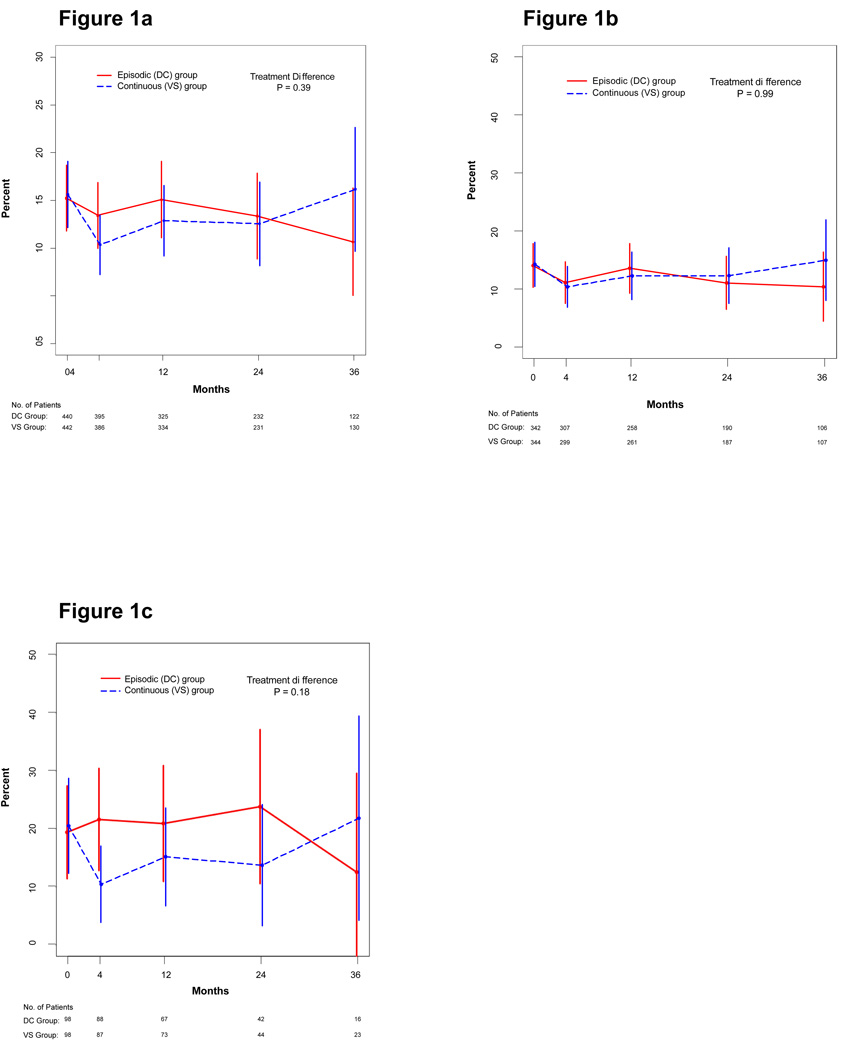
The proportion of participants reporting high-risk behavior (anal or vaginal sex without a condom, needle-sharing, or laboratory diagnosis of incident chlamydia, gonorrhea or syphilis) was similar in both study arms (Figure 1a; p = 0.39 for treatment difference through follow-up). In the continuous therapy group, high-risk behavior declined from 15.6% at baseline to 10.4% at month 4 (p = 0.005) and then returned closer to the baseline value. However, high-risk behavior appeared to decline somewhat in the episodic treatment group as well, and there was no evidence for a difference in high-risk behavior during the first 4 months in the episodic vs. continuous therapy arms (p = 0.15). As sensitivity analyses, we compared treatment groups for high-risk behavior after excluding all visits where information for assessing this endpoint was partially missing, and found similar results.
Figure 1.
Proportion of patients with high-risk behavior, by treatment group, for all patients (Figure 1a), among those who entered the study on antiretroviral therapy (Figure 1b), and among those who entered the study off antiretroviral therapy (Figure 1c). Error bars show ± 2 standard errors.
Incident bacterial sexually-transmitted infections were diagnosed at 37 follow-up visits, 1.8% of the 2024 visits at which testing was performed. There were no differences in the incidence of bacterial sexually-transmitted infections between the two treatment arms. Of the 38 incident infections diagnosed during follow-up, there were 13 cases of chlamydia, 5 cases of gonorrhea, and 20 cases of syphilis.
Subgroup analyses
We evaluated whether baseline factors influenced the difference in high-risk behavior between the two randomized arms. There was no treatment difference in high-risk behavior within subgroups of participants defined by gender, sexual preference, race, and age, and no evidence for differential treatment effects across these subgroups.
For participants on antiretroviral therapy at baseline, for whom the randomization was to interrupt vs. continue antiretroviral therapy, high-risk behavior remained stable over follow-up, and was very similar in the two arms (Figure 1b). However, among participants not on antiretroviral therapy at baseline, there was a significant decrease in transmission risk behavior among those randomized to start antiretroviral therapy (from 20% at baseline to 10% at 4 months, p= 0.05, Figure 1c). In contrast, among those randomized to defer therapy, the proportion with high-risk behavior remained stable. For participants not on antiretroviral therapy at baseline, the difference in high-risk behavior between the two treatment arms was significant at 4 months (p = 0.02), but not over the entire follow-up period (p = 0.11).
Changes in HIV transmission risk
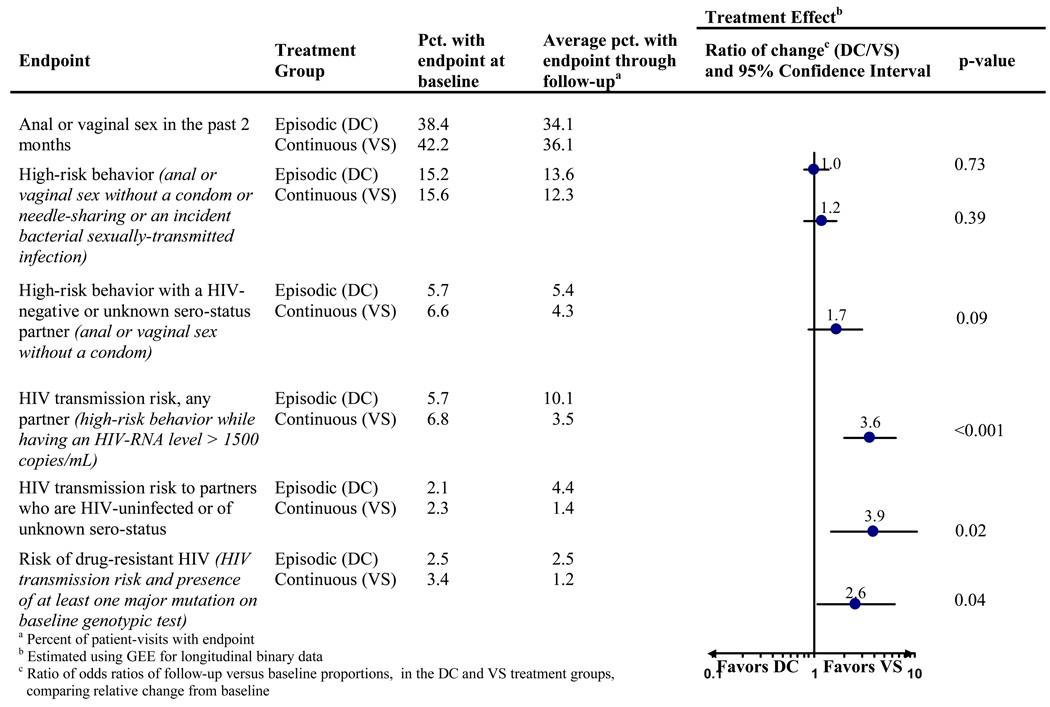
Because of the large difference in antiretroviral therapy usage by treatment arm during follow-up, participants in the episodic therapy arm had HIV-RNA levels greater than 1500 copies/mL much more often than participants in the continuous therapy arm (63% of follow-up time vs. 25%, respectively, p < 0.001). As a result of higher HIV-RNA levels and the lack of difference in high-risk behavior by treatment arm during follow-up, the proportion of participants with HIV transmission risk (high-risk behavior while having an HIV-RNA level > 1500 copies/ml) was higher in the episodic than in the continuous therapy arms (p < 0.001 for treatment difference; Figure 2). Similarly, transmission risk to a partner perceived to be HIV-uninfected or of unknown HIV serostatus was substantially higher in the episodic treatment arm (p = 0.02; Figure 2).
Figure 2.
Treatment effect (episodic [DC] vs. continuous [VS] antiretroviral therapy) on high-risk behavior and other outcomes, comparing change in proportions from baseline through follow-up between the two treatment groups
Finally, we evaluated the risk of transmitting drug-resistant HIV by treatment arm. At baseline, approximately 19% of participants had HIV-RNA level > 1000 copies/mL and an HIV strain with at least one major resistance mutation. Among those, the proportion with high-risk behavior remained stable, and was similar in both treatment arms. However, due to the higher HIV-RNA levels in the episodic treatment arm, the proportion of patients at risk of transmitting a resistant strain was also higher in this treatment arm (p = 0.04; Figure 2).
DISCUSSION
This is the first randomized study to evaluate the effect of antiretroviral therapy on transmission risk behavior. Episodic use of antiretroviral therapy did not decrease high-risk sexual or needle-sharing behavior that may result in HIV transmission, compared to continuous therapy use. Because HIV-RNA levels were higher among participants on episodic therapy while high-risk behavior remained similar to those in the continuous therapy arm, patients randomized to episodic therapy had a higher risk of transmitting HIV infection, including drug-resistant HIV. Additionally, among participants entering the study off antiretroviral therapy, high-risk behavior decreased initially among participants randomized to start antiretroviral therapy, compared to those randomized to defer therapy.
A number of cross-sectional studies have evaluated the association between antiretroviral therapy and sexual risk behavior. The results of individual studies differ, but a meta-analysis showed no association between antiretroviral therapy use and risk behavior 19. Longitudinal cohort studies have also had disparate results, with some showing an increase in risk behavior after starting combination antiretroviral therapy 20 and others showing stable or decreased risk behavior after starting 21, 22. Other studies suggested a more complex effect of starting antiretroviral therapy on risk behavior; women reported a decrease in number of sexual partners, but an increase in episodes of unprotected vaginal intercourse 23. Our study, the first to evaluate the effect of antiretroviral therapy in a randomized manner, found no evidence for a disinhibitory effect of being on antiretroviral therapy; as overall risk behavior was not affected by randomization to episodic versus continuous antiretroviral therapy.
We enrolled a population with demographic characteristics representative of those of persons in HIV care in the United States 24. A cross-sectional analysis of baseline risk behavior showed that participants who were on antiretroviral therapy reported less high-risk behavior. Starting antiretroviral therapy was followed by a significant decrease in high-risk behavior, to approximately the level observed among participants who were on antiretroviral therapy at enrollment. These findings have potential importance in the question of the optimal timing of initiation of antiretroviral therapy. If starting antiretroviral therapy is associated with decreases in both genital viral load and high-risk behavior as we have demonstrated, than initiation of antiretroviral therapy may prove to be an important strategy for the prevention of HIV transmission.
Our study has at least six limitations. First, sub-study participants had slightly different demographics from those who were eligible but did not co-enroll; however, the sub-study population closely resembles the SMART study population at participating clinical sites, since 89% of SMART participants at these sites co-enrolled in the sub-study‥ Second, the power of this substudy to detect differences in risk behavior by randomized arm was lower than planned because enrollment in the SMART study was stopped early, and the follow-up time was curtailed. However, this study was originally powered to detect a two-fold difference between treatment arms, and there was no trend suggesting any such difference. Third, we did not directly assess HIV transmission, but used the prevalence of high-risk behavior at elevated HIV-RNA levels as a surrogate for HIV transmission. Fourth, our survey was brief so that it would fit easily into study visits for a large clinical trial that assessed many other outcomes. As a result, the survey did not provide detailed information about the number and type of sexual encounters. Fifth, we may have underestimated the incidence of bacterial sexually-transmitted infections, both because of infrequent sampling and because we did not test for anal or pharyngeal gonorrhea and chlamydia 25. Finally, the risk of transmitting HIV resistant strains was likely underestimated, since genotypic testing was done only at baseline, and only for patients with HIV-RNA > 1000 copies/mL.
In summary, episodic use of antiretroviral therapy did not affect high-risk sexual and needle-sharing behavior, compared to continuous therapy. Because episodic therapy resulted in higher HIV-RNA levels, the unchanged high-risk behavior increased the risk of HIV transmission, including the transmission of drug-resistant strains. Randomization to start antiretroviral therapy was associated with a trend towards decreased high-risk behavior, which may augment the effect of viral load reduction in decreasing HIV transmission.
Acknowledgements
We gratefully acknowledge the commitment of all the participants in the Transmission Risk Behavior substudy of the SMART study, and the many investigators and clinical staff. Birgit Grund had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Participating clinical sites (number of participants enrolled) and investigators are listed below.
Grant support provided by NIAD, NIH grants U01AI042170 and U01AI46362.
Richmond AIDS Consortium, Richmond, Virginia (n=175): Carol S. Clark, RN, Patricia W. Dodson, RN, BSN, CCRC, Luann Gahagan, RN, CCRC, Kathleen Genther, RN, CCRC, Martha L. Howe-Pittman, RN, CCRC, Johanna McKee, RN, CCRC, Vicky Watson, RN. Bronx AIDS Research Consortium, Bronx, New York (n=127): Richard B. Cindrich, MD, Eileen Dolce, RPA-C, Jonathan Shuter, MD, Luz M. Vasco. Wayne State University, Detroit, Michigan (n=97): Lawrence R. Crane, MD, Marti J. Farrough, BSN, RN, Joan M. Kopka, BA(Psych), RN, Jean C. Lee, PharmD, BCPS, Rodger D. MacArthur, MD. Community Consortium of San Francisco, San Francisco, California (n=96): Donald I. Abrams, MD, Jane Bailowitz, MD, Virgina Cafaro, MD, Milton Estes, MD, Malcolm John, MD, Harry Lampiris, MD, Steve O'Brien, MD, William Owen, MD, Simon Paul, MD, Robert Scott, MD, and colleagues. The Research and Education Group, Portland, Oregon (n=96): Diana Antoniskis, MD, Doug Beers, MD, Cliffton Ting Hong Bong, MD, Cindy Dietrich, RN, David Gilbert, MD, Joel Godbey, MD, Gordon Johnson, MD, Toni Kempner, RN, BSN, CCRC, Philip Todd Korthuis, MD, James Leggett, MD, Karen Loveless, RN, MS, CCRC, Michael S. MacVeigh, MD, Norma Martinez, RN, BSN, CCRC, Erin Merrifield, RN, BSN, Mary O'Hearn, MD, Robert K. Pelz, MD, James Sampson, MD. Southern New Jersey AIDS Clinical Trials, Camden, New Jersey (n=83): David Condoluci, DO, Kelly Freeman, CCRC, Deborah Goraj, RN, Christopher Lucasti, DO, Marlana Robinson, RN, P. Dawn Slowinski, MSN. Denver Community Programs for Clinical Research on AIDS, Denver, Colorado (n=73): Beverly Barber, RN, BSN, Mary Bessessen, MD, Joshua Blum, MD, Dale E. Britt, RN, ND, David L. Cohn, MD, Jeffrey Desjardin, MD, Rebecca Fernandez, NP, Leonor Melecio, Miguel Mogyoros, MD, Frances M. Moran, RN, BSN, Jack Rouff, MBA, Jennifer M. Saldanha, RN, MA, Judith C. Shlay, MD, Diane States, RN, BSN, Julia A. Weise, LCSW, Timothy Wright, BA. Temple University, Philadelphia, Pennsylvania (n=58): Shannon Frederick, PA, Kelly Lattanzi, CRNP, Ellen M. Tedaldi, MD, Mary van den Berg-Wolf, MD. Wide-Reaching AIDS Program, Washington DC (n=54): Shirley Cummins, Gordon Dickinson, MD, Christiane Jones, RN, Nancy Klimas, MD, Abeer Moanna, MD, David Rimland, MD. Philadelphia FIGHT, Philadelphia, Pennsylvania (n=19): New Jersey Medical School, Newark, New Jersey (n=1): Wanda Figueroa, MD, Jie Li, RN, BSN.
Footnotes
This study was presented in part at the 14th Conference on Retroviruses and Opportunistic Infections, February 2007, Los Angeles, CA.
None of the authors have financial conflicts of interest.
References
- 1.Lori F, Lisziewicz J. Structured treatment interruptions for the management of HIV infection. JAMA. 2001 Dec 19;286(23):2981–2987. doi: 10.1001/jama.286.23.2981. [DOI] [PubMed] [Google Scholar]
- 2.Hogg RS, Havlir D, Miller V, Montaner JS. To stop or not to stop: that is the question, but what is the answer? Aids. 2002 Mar 29;16(5):787–789. doi: 10.1097/00002030-200203290-00015. [DOI] [PubMed] [Google Scholar]
- 3.Mofenson LM, Lambert JS, Stiehm ER, et al. Risk factors for perinatal transmission of human immunodeficiency virus type 1 in women treated with zidovudine. Pediatric AIDS Clinical Trials Group Study 185 Team. N Engl J Med. 1999 Aug 5;341(6):385–393. doi: 10.1056/NEJM199908053410601. [DOI] [PubMed] [Google Scholar]
- 4.Quinn TC, Wawer MH, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 5.Tovanabutra S, Robison V, Wongtrakul J, et al. Male viral load and heterosexual transmission of HIV-1 subtype E in northern Thailand. J Acquir Immune Defic Syndr. 2002 Mar 1;29(3):275–283. doi: 10.1097/00126334-200203010-00008. [DOI] [PubMed] [Google Scholar]
- 6.Teicher E, Casagrande T, Vittecoq D. Enhanced risk of HIV sexual transmission during structured treatment interruption. Sex Transm Infect. 2003 Feb;79(1):74. doi: 10.1136/sti.79.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen SY, Gibson S, Katz MH, et al. Continuing increases in sexual risk behavior and sexually transmitted diseases among men who have sex with men: San Francisco, Calif, 1999–2001, USA. Am J Public Health. 2002 Sep;92(9):1387–1388. [PMC free article] [PubMed] [Google Scholar]
- 8.Dodds JP, Mercey DE, Parry JV, Johnson AM. Increasing risk behaviour and high levels of undiagnosed HIV infection in a community sample of homosexual men. Sex Transm Infect. 2004 Jun;80(3):236–240. doi: 10.1136/sti.2003.007286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicoll A, Hamers FF. Are trends in HIV, gonorrhoea, and syphilis worsening in western Europe? Bmj. 2002 Jun 1;324(7349):1324–1327. doi: 10.1136/bmj.324.7349.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heffelfinger JD, Swint EB, Berman SM, Weinstock HS. Trends in primary and secondary syphilis among men who have sex with men in the United States. Am J Public Health. 2007 Jun;97(6):1076–1083. doi: 10.2105/AJPH.2005.070417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katz MH, Schwarcz SK, Kellogg TA, et al. Impact of highly active antiretroviral treatment on HIV seroincidence among men who have sex with men: San Francisco. Am J Public Health. 2002 Mar;92(3):388–394. doi: 10.2105/ajph.92.3.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The SMART Study Group. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006 Nov 30;355(22):2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 13.Rietmeijer CA, Lansky A, Anderson JE, Fichtner RR. Developing standards in behavioral surveillance for HIV/STD prevention. AIDS Educ Prev. 2001 Jun;13(3):268–278. doi: 10.1521/aeap.13.3.268.19740. [DOI] [PubMed] [Google Scholar]
- 14.Johnson VA, Brun-Vezinet F, Clotet B, et al. Update of the drug resistance mutations in HIV-1: Fall 2006. Top HIV Med. 2006 Aug–Sep;14(3):125–130. [PubMed] [Google Scholar]
- 15.Flaks RC, Burman WJ, Gourley PJ, Rietmeijer CA, Cohn DL. HIV transmission risk behavior and its relation to antiretroviral treatment adherence. Sex Transm Dis. 2003 May;30(5):399–404. doi: 10.1097/00007435-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Wenger NS, Kusseling FS, Beck K, Shapiro MF. Sexual behavior of individuals infected with the human immunodeficiency virus. The need for intervention. Arch Intern Med. 1994;154(16):1849–1854. [PubMed] [Google Scholar]
- 17.Erbelding EJ, Stanton D, Quinn TC, Rompalo A. Behavioral and biologic evidence of persistent high-risk behavior in an HIV primary care population. AIDS. 2000;14:297–301. doi: 10.1097/00002030-200002180-00012. [DOI] [PubMed] [Google Scholar]
- 18.Kozal MJ, Amico KR, Chiarella J, et al. Antiretroviral resistance and high-risk transmission behavior among HIV-positive patients in clinical care. AIDS. 2004 Nov 5;18(16):2185–2189. doi: 10.1097/00002030-200411050-00011. [DOI] [PubMed] [Google Scholar]
- 19.Crepaz N, Hart TA, Marks G. Highly active antiretroviral therapy and sexual risk behavior: a meta-analytic review. JAMA. 2004 Jul 14;292(2):224–236. doi: 10.1001/jama.292.2.224. [DOI] [PubMed] [Google Scholar]
- 20.Desquilbet L, Deveau C, Goujard C, Hubert JB, Derouineau J, Meyer L. Increase in at-risk sexual behaviour among HIV-1-infected patients followed in the French PRIMO cohort. Aids. 2002 Nov 22;16(17):2329–2333. doi: 10.1097/00002030-200211220-00014. [DOI] [PubMed] [Google Scholar]
- 21.Glass TR, Young J, Vernazza PL, et al. Is unsafe sexual behaviour increasing among HIV-infected individuals? Aids. 2004 Aug 20;18(12):1707–1714. doi: 10.1097/01.aids.0000131396.21963.81. [DOI] [PubMed] [Google Scholar]
- 22.Bouhnik AD, Moatti JP, Vlahov D, Gallais H, Dellamonica P, Obadia Y. Highly active antiretroviral treatment does not increase sexual risk behaviour among French HIV infected injecting drug users. J Epidemiol Community Health. 2002 May;56(5):349–353. doi: 10.1136/jech.56.5.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson TE, Gore ME, Greenblatt R, et al. Changes in sexual behavior among HIV-infected women after initiation of HAART. Am J Public Health. 2004 Jul;94(7):1141–1146. doi: 10.2105/ajph.94.7.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. HIV/AIDS surveillance report, 2004. 2005. pp. 1–46. [Google Scholar]
- 25.Kent CK, Chaw JK, Wong W, et al. Prevalence of rectal, urethral, and pharyngeal chlamydia and gonorrhea detected in 2 clinical settings among men who have sex with men: San Francisco, California, 2003. Clin Infect Dis. 2005 Jul 1;41(1):67–74. doi: 10.1086/430704. [DOI] [PubMed] [Google Scholar]