Abstract
Cell-based therapy is a possible avenue for the treatment of Duchenne muscular dystrophy (DMD), an X-linked skeletal muscle-wasting disease. We have demonstrated that cultured myogenic progenitors derived from the adult skeletal muscle side population can engraft into dystrophic fibers of non-irradiated, non-chemically injured mouse models of DMD (mdx5cv) after intravenous and intra-arterial transplantation, with engraftment rates approaching 10%. In an effort to elucidate the cell surface markers that promote progenitor cell extravasation and engraftment after systemic transplantation, we show here that expression of the chemokine receptor CXCR4, whose ligand SDF-1 is overexpressed in dystrophic muscle, enhances the extravasation of these cultured progenitor cells into skeletal muscle after intra-arterial transplantation. One day post-transplantation, mice that received CXCR4-positive eGFP-positive cultured cells derived from the skeletal muscle side population displayed significantly higher amounts of eGFP-positive mononuclear cells in quadriceps and tibialis anterior than mice who received CXCR4-negative eGFP-positive cells derived from the same cultured population. At 30 days post-transplantation, significantly higher engraftment rates of donor cells were observed in mice that received CXCR4-positive cells versus those transplanted with CXCR4-negative fractions. Our data suggest the CXCR4 expression by muscle progenitor cells increases their extravasation into skeletal muscle shortly after transplantation. Furthermore, this enhanced extravasation likely promotes higher donor cell engraftment rates over time.
Keywords: Muscular Dystrophy, CXCR4, Muscle progenitor cells, Cell-based therapy, Cell therapy, Intra-arterial transplantation
INTRODUCTION
Duchenne Muscular Dystrophy (DMD) is an X-linked recessive disease characterized by mutations in the gene that encodes dystrophin, a membrane protein that links the actin cytoskeleton to extracellular matrix proteins 7,19,36. Lack of dystrophin expression leads to progressive muscle wasting and weakness due to degeneration of muscle fibers and infiltration of connective and adipose tissue. Restoring normal dystrophin expression in murine models of muscular dystrophy has been shown to reverse the mutant phenotype 9. Cell-based therapy for DMD has been proposed as a method for normal dystrophin delivery in which cells that can fuse into diseased muscle after transplantation are used as dystrophin gene shuttles.
Satellite cells are the predominant type of muscle precursors in skeletal muscle 6,33,50,51,54,58. Upon activation, satellite cells are able to divide and expand both in vitro and in vivo and give rise to muscle precursors or myoblasts. Rescue of dystrophin expression via fusion of culture-expanded wild type myoblasts to mdx myoblasts was successfully tested both in vitro and in vivo (myoblast transfer) by administering intramuscular cell injections in mdx mice 24,42. Following initial promising pre-clinical studies, wild-type myoblasts isolated from unaffected donors were injected intramuscularly in DMD patients in at least 6 different centers 16,25,35,39,40,57. Unlike the mouse studies, human clinical trials demonstrated that myoblast transfer was very inefficient, yet safe to those who received cells. Problems associated with the lack of efficiency included an estimated death of >90% of the injected cells within days from injection due to membrane attack by complement, lack of migration of introduced cells from the site of injection, lack of fusion of introduced cells to dystrophic myofibers, and failure by approximately 25% of donor cells fused to dystrophic myofibers to express dystrophin 5,17,46. These results revealed the need to return to mouse models to improve cell-based therapy approaches.
In late 1990s, a few groups described the existence of cells likely to be more primitive than satellite cells within muscle 18,22,45. These putative muscle `stem' cells have been purified from mouse skeletal muscle using different methods, including pre-plating 8,31,45,46, cell sorting 2,18,22,34,55 and fractionation based on specific antigens following pre-plating technique 56. Analogous to human hematopoetic stem cells isolated by Hoechst dye exclusion 15, mouse muscle side population (SP) cells have been isolated upon cell staining using the vital DNA dye Hoechst 33342, followed by FACS (fluorescence-activated cell sorting) 18,38. This method distinguishes SP cells from the main population of cells (MP cells), which exclude Hoechst 33342 significantly less efficiently. Muscle SP cells represent approximately 1% of the mononuclear cells in adult mouse skeletal muscle. Characterization of cell surface antigens via FACS analyses indicated that more than 95% of muscle SP cells are Sca-1+ CD45- CD43- c-kit-, and that 70% of muscle SP cells are CD34+, suggesting heterogeneity within this cell population 38. Analysis of Pax7-/- knockout mice, which lack satellite cells, revealed presence of normal amounts of muscle SP cells, indicating that muscle SP cells and satellite cells are likely to be distinct populations within skeletal muscle 51. Myogenic specification of mouse muscle cells within the side population occurs when these cells are co-cultured with primary myoblasts, suggesting that specific cell-cell interactions are necessary during this process.
Intravenous injection of unfractionated, uncultured muscle SP cells from wild-type male mice into lethally irradiated mdx female mice demonstrated that the muscle side population contains precursors with myogenic and hematopoetic activity 18. These progenitor cells, when transplanted via the tail vein of irradiated and non-irradiated mice, extravasate and engraft into mdx or mdx5cv muscle tissue 3,4,18,37. The more abundant MP cells, which include myoblasts, satellite cells, fibroblasts and endothelial cells, are significantly less efficient than SP cells at extravasating from the vasculature and engrafting into muscle following tail vein or femoral artery injection 3,4. Although intravenous injections of mouse muscle SP cells revealed the possibility of disseminating cells systemically, the percentage of engrafted cells did not reach levels that are considered to be therapeutically significant (~1% engraftment) 3,4,18. Studies from several groups that focused on intravenous donor cells delivery to dystrophic muscle have yielded comparable results using several donor cell types and mouse models 3,11,12,18,28,37.
In efforts to improve donor cell engraftment, a study by one group exploited the injection of donor cells into the iliac artery as an alternative delivery method. They demonstrated that injected cells can migrate from the circulation into all hind-limb muscles of the treated mice with an increased efficiency, especially after external muscle damage 56. The same group recently reported increased homing of human stem cells following intra-arterial injection in exercise-induced dystrophic muscles 14. Our group demonstrated that an injection of cultured cells derived from the side population significantly via the femoral artery increases engraftment into non-externally injured mdx5cv muscle, with 5-8% of muscle fibers expressing donor-derived transgenes 4. Another study demonstrated that intra-arterial injection of mouse meso-angioblasts, a type of vessel-associated fetal-derived stem cells, into mice with limb-girdle muscular dystrophy, leads to phenotypic and functional correction of the disease through widespread delivery of cells via the capillaries 48. The same study also reported that repeated intra-arterial injections can be performed, resulting in restoring protein expression in more than 50% of the total muscle fibers even four months after injection. The same group demonstrated that exposure of meso-angioblasts to several cytokines, including SDF-1 and TNF-a, significantly increased their homing into dystrophic muscle 13. These mouse studies have been apparently replicated in a dog model of muscular dystrophy with both dystrophin expression and partial functional improvement observed 49.
Current efforts are focused on not only maximizing engraftment of adult progenitor cells into non-externally injured muscle tissue, but also on identifying key molecules expressed by SP cells that mediate their extravasation and/or engraftment into dystrophic muscle. In this study, we show that CXCR4 expression by cultured cells derived from the side population enhances their ability to extravasate into dystrophic muscle after intra-arterial injection. CXCR4, also known as fusin, is an alpha-chemokine receptor that is specific for SDF-1, a powerful lymphocyte chemoattractant which is upregulated in murine and human dystrophic muscle and in other sites of tissue damage 1,23,26,27,29,30,43,44,47. Here we show that CXCR4 is upregulated in cultured SP-derived cells. Furthermore, delivery of CXCR4-positive SP-derived cells yields significantly higher levels of extravasation one day after transplantation versus CXCR4-negative SP-derived cells. Thirty days after transplantation, significantly higher muscle engraftment rates are observed in animals that received CXCR4-positive versus CXCR4-negative SP-derived cells. Our data suggest that CXCR4 expression plays an important role in promoting muscle progenitor cell extravasation to mdx5cv skeletal muscle after intra-arterial introduction. Enhanced extravasation likely facilitates increased levels of donor cell engraftment.
MATERIALS and METHODS
Mouse strains
C57BL/6Ros-5cv (mdx5cv) (X-linked muscular dystrophy) mice were obtained from the Jackson Laboratory, Bar Harbor, Maine. In the mdx5cv allele the dystrophin mRNA contains a 53 bp deletion of sequences from exon 10. Analysis of the genomic DNA uncovered a single A to T transversion in exon 10 that creates a new splice donor site that generates a frameshifting deletion in the processed mRNA. These animals display about 10 times fewer dystrophin positive revertant fibers than the mdx mouse allele 10,20,21,52.
Isolation of muscle SP cells
Mononuclear cells were isolated from skeletal muscle from 4-7 week-old mdx5cv donor tissue. Before Hoechst 33342 staining, red cells were lysed 3. Primary myoblasts were resuspended at 106 cells per ml and stained with 12.5 μg/ml Hoechst 33342 in PBS-0.5% BSA for 60 min at 37°C. In parallel, 106 cells were stained with Hoechst 33342 in the presence of 50 μM verapamil to set the gate for the isolation of SP cells by fluorescence-activated cell sorter (FACS, Becton Dickinson, Franklin Lakes, NJ). This gate was independently set to analyze the percentage of SP cells in each sort. To eliminate dead cells, 2 μg/ml propidium iodide was added to the cells before FACS analysis and sorting. FACS analysis and sorting was performed on a FACSVantage SE flow sorter (Becton Dickinson) 18.
Characterization of Freshly Isolated and Cultured Cells
For cell-lineage marker analysis, fresh and cultured cell samples were incubated for 30 minutes on ice with FITC-conjugated anti-mouse Sca1 (D7), PE-conjugated anti-mouse CD45 (30-F11), FITC-conjugated anti-mouse CD62L (MEL-14), FITC-conjugated anti-mouse CD184 (CXCR4), FITC-conjugated anti-mouse CD31 (MEC 13.3), PE-conjugated anti-mouse CD11a (2D7), PE-conjugated anti-mouse CD11b (M1/70), PE-conjugated anti-mouse CD49d (9C10), and biotin-conjugated anti-mouse CD62E (ELAM-1) and biotin-conjugated anti-mouse CD34 (clone RAM34) (BD Bioscience, San Jose, CA) followed by phycoerythrin-conjugated streptavidin mouse anti-rat secondary antibody (Invitrogen, Carlsbad, CA). Rat IgG-phycoerythrinconjugated and rat IgG-FITC-conjugated were used as negative controls. For immunohistochemical analysis of cultured cells, cells were stained with mouse anti-chicken Pax7 antibody (DSHB, University of Iowa, Iowa City, Iowa), mouse anti-human desmin antibody (clone D33, Dako, Denmark), anti-CD184 antibodies 12G5 and 2B11.
Cell Culture and Lentiviral Transduction
Following FACS sorting, cells were plated into collagen type I (Upstate Biotechnology, Lake Placid, NY) coated 384-well plates. Cells were transduced for 3-6 hours one day after sorting with 75 μl of inoculums of L-MSCV enhanced green fluorescent protein (eGFP) lentiviral particles (multiplicity of infection =10). Cells were washed twice with 1×PBS 15 hours after transduction in order to remove all viral particles. One hundred percent of cells and their progeny were transduced. Following transduction, cells were cultured for 21 days. While in culture, both floating and attached cells were transferred to larger wells (96, 48, 24, and 12 well plates) once they reached 70% confluence. All plate wells were coated with human fibronectin (Biocoat, Becton Dickinson), and proliferation medium was used: DMEM high glucose (CellGro, CellGenix, Freiburg, Germany) supplemented with 20% heat inactivated horse serum (Gibco, Carlsbad, CA), 2.5% chicken embryo extract (Accurate Chemicals, Westbury, NY), mouse stem cell factor (25 ng/ml, R & D Systems, Minneapolis, MN), epidermal growth factor (100 ng/ml, Sigma-Aldrich, St. Louis, MO), recombinant human fibroblast growth factor, basic (100 ng/ml, Promega, Madison, WI).
Intra-arterial transplantation
After FACS sorting, muscle-derived SP cells were prepared as described above. Before being injected into animals, cells were washed twice and resuspended in 50 μl of PBS; they were transplanted into the right iliac arteries of 4-7 week-old mdx5cv recipients. Each animal (n=20) received 50,000 cells in one injection. All injections were performed using the same protocol. Cells were injected via a custom-made catheter system, consisting of a 12-cm polyethylene 10 tube (PE-10, Becton Dickinson) that was slipped over a 30G needle and then connected to a Hamilton syringe. The catheter was constructed using PE-10 tubing that was stretched over an inserted 4-0 nylon suture. This reduced the outer diameter to about two-thirds of the initial size while maintaining the inner diameter (from 0.2 mm to 0.28 mm ID). Catheterization was performed using a Leica dissecting microscope (MZ16A). Mice were anesthetized with an intraperitoneal injection of ketamine PPPP and xylazine (10 mg/kg). An incision was made in the right groin, exposing the iliac artery. Two 6-0 silk ligatures were placed to secure the artery, and a microvessel clip was placed to occlude the vessel proximally. An incision was made in the iliac artery with a 30G needle. The tip of the catheter was inserted, advanced approximately 2 mm into the artery lumen, and the cells were injected slowly. After injection, the catheter was removed, and the vessel remained patent. The skin was closed with 5-0 cat gut suture. Following suture removal, the distal tip of the PE-10 was beveled to 45 degrees. All animal care was in accordance with institutional guidelines. Animals were injected with 0.075 mg/kg buprenex immediately before transplantation; their health condition was monitored every day.
Immunohistochemistry
Recipient animals were sacrificed, and skeletal muscles were fixed for 2 hours in 4% paraformaldehyde prior to being frozen in cold isopentane and stored at -80 °C. Whole muscle tissue was sectioned at -20°C into 10μm sections. The entire tibialis anterior and quadriceps muscles were sectioned from each host animal; 5 or 10 equidistant sections from throughout whole muscle were used for immunohistochemical analysis. Sections were fixed in 4% paraformaldehyde for 10 minutes on ice, transferred to ice-cold PBS for 5 minutes and blocked in PBS-10% FBS for 40 minutes before CXCR4 (ab1670-100, Abcam, Cambridge, MA) staining. Spectrin (NCL-SPEC2, Novacastra, Bannockburn, IL) staining was performed on some sections to identify cytoskeleton. Slides were incubated in primary antibodies for 16 hours at 4°C washed 3 times in PBS at room temperature, followed by 1 hour incubation with Texas Red-conjugated anti-mouse IgG and AMCA-conjugated anti-goat IgG (Jackson Immunoresearch, Westgrove, PA), respectively. eGFP expression was observed by fluorescent microscopy. The slides were mounted in Vectashield containing DAPI for nuclear counterstain (Vector Laboratories, Inc, Burlingame, CA). Immunohistochemistry was visualized using a Zeiss Axiophot microscope, and images were collected from the same microscopic field with separate filters for DAPI, FITC and Texas Red signals using a CCD camera (Diagnostic Instruments, Sterling Heights, MI). Signals were merged using a Macintosh computer and OpenLab software (Improvision, Waltham, MA). eGFP mononuclear cells were visualized and counted under fluorescence and light microscope.
Extravasation and Engraftment Quantitative and Statistical Analysis
To assess levels of progenitor cell extravasation and engraftment in host muscle, muscle sections from recipient animals were analyzed by fluorescence microscopy. Whole recipient tibialis anterior and quadriceps harvested one day after progenitor cell transplantation were sectioned longitudinally. Five equidistant tissue sections from throughout the entire recipient muscle were chosen. All mononuclear eGFP positive cells present in each of these sections were counted manually (Supplemental Table 1); this quantity represented the level of donor cell extravasation into host muscle. In addition, whole recipient tibialis anterior and quadriceps harvested thirty days after progenitor cell transplantation were also sectioned longitudinally. Ten equidistant tissue sections from throughout these whole recipient muscles were chosen for quantitative analysis. All eGFP positive and eGFP negative muscle fibers were counted manually in each of these sections. Percent engraftment was defined as number of eGFP positive myofibers per section divided by total number of myofibers per section (Supplemental Table 2).
To compare level of extravasation and percent engraftment among animals who received CXCR4-positive cells derived from the SP; CXCR4-negative cells derived from the SP; CXCR4-positive cells derived from the SP pretreated with CXCR4 blocking antibody; and freshly isolated SP cells, one-sided t-tests were performed. This analysis was performed for recipient tibialis anterior and quadriceps tissue sections from animals sacrificed 1 and 30 days after transplantation. The samples used for statistical analysis were comprised of sections listed in Supplemental Tables 1 and 2. A P-value of < 0.05 (95% confidence level) was used as the cut-off for statistical significance.
RESULTS
Cell surface marker analysis of cultured myogenic progenitor cells
To further understand the ability of certain muscle-derived progenitor cells to engraft into dystrophic muscle, we analyzed the expression of various cell-surface proteins potentially involved in extravasation and fusion to muscle fibers (Figure 1). We have previously demonstrated that cultured cells derived from the muscle side population extravasate from blood vessels and engraft into dystrophic muscles much more efficiently than freshly isolated SP cells or MP cells after intra-arterial delivery 4. We sought to identify the differences between cultured cells derived from the side population and freshly isolated SP cells that accounted for their differential abilities to extravasate and engraft. To assess surface protein expression, SP and MP cells were isolated from mdx5cv skeletal muscle transduced with eGFP lentiviral vectors and cultured for three weeks in proliferation media containing a cocktail of mouse stem cell factor, epidermal growth factor and recombinant human fibroblast growth factor as previously described 3. Cultured cells derived from the side population were consistently observed to adhere to culture dishes and change into various morphologies (data not shown) or remain unattached. In accordance with previously published data by our and other groups, neither MP nor SP cells differentiated into multinucleated myotubes in the growth media.
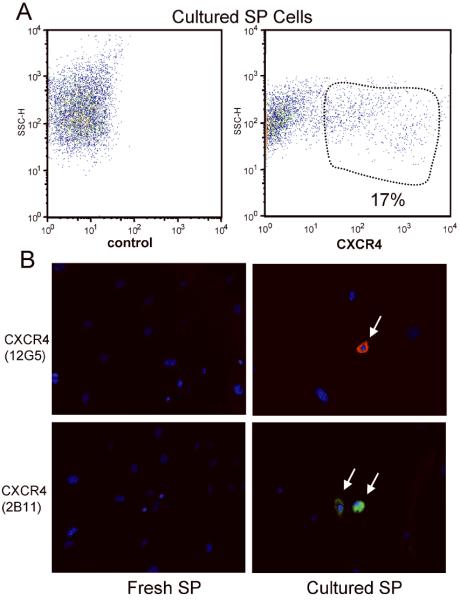
Figure 1. Expression of cell surface marker CXCR4 on cultured SP cells derived from mdx5cv skeletal muscles.
(a) FACS staining of cultured SP cells with control and CXCR4 antibodies. (b) Inmunohistochemistry using the 12G5 (Texas red) or 2B11 (FITC) CXCR4 clonal antibodies on freshly isolated and cultured SP cells. Nuclei were stained with DAPI (blue).
FACS analysis using antibodies to different surface markers and adhesion molecules was performed immediately after sorting for freshly isolated SP cells or after three weeks of culture (Table 1). Most cultured cells derived from the side population expressed Sca1 (96%), but only a few expressed CD31 (5%) and CD34 (4%), compared with 91% Sca1, 82% CD31 and 80% CD34 expression in freshly isolated SP cells. Only 60% of cultured MP cells expressed Sca-I, while 2% expressed CD31 and CD34 (not shown). Nearly 10% of cultured MP cells expressed CD45 (not shown); however, CD45 expression was found in only 2% of cultured SP-derived cells. Interestingly, approximately 17 % of SP-derived cultured cells expressed CXCR4, a chemokine receptor known to be involved in cell homing to bone marrow and spleen whose ligand, α-chemokine SDF-1, is overexpressed in dystrophic skeletal muscle (Figure 1a) 29,43. No CXCR4 expression was found in freshly isolated SP cells or cultured MP cells. In order to verify CXCR4 expression by cultured SP-derived cells, immunohistochemistry with two different clonal antibodies against CXCR4 was performed (Figure 1b). 18±3% of cultured SP-derived cells, after 5 independent experiments, were CXCR4-positive using both anti-CXCR4 antibodies (12G5-Cy5 in red and 2B11-FITC in green), while no expression was found in freshly isolated SP cells or cultured MP cells (not shown), confirming FACS analysis results. Expression of other surface molecules in cultured and freshly isolated SP cells was assessed using FACS analysis (Table 1).
Table 1.
Percent Expression of Cell Surface Markers in Freshly Isolated SP Cells and Cultured Cells Derived from the Side Population
| Surface Marker | Freshly Isolated SP Cells | SP-derived Cultured Cells |
|---|---|---|
| Sca-1 | 91±8 | 96±2 |
| CD34 | 82±6 | 5±1 |
| CD31 | 80±7 | 4±0.5 |
| CD45 | 1±0.2 | 2±1 |
| CD11a | not assayed | 0 |
| CD65L | not assayed | 3±1 |
| CD62E | 0 | 0.5±0.5 |
| CD49d | 1±0.2 | 1±0.5 |
| CD11b | 1±0.4 | 0 |
| CXCR4 | 2±1 | 17±5 |
Percentages assayed by fluorescence-activated cell sorting. Data acquired from 5 independent experiments. CXCR4 expression increases nearly 9-fold after 21 days in culture, while CD34 and CD31 expression markedly decrease.
CXCR4 Expression Enhances Progenitor Cell Extravasation into Dystrophic Muscle
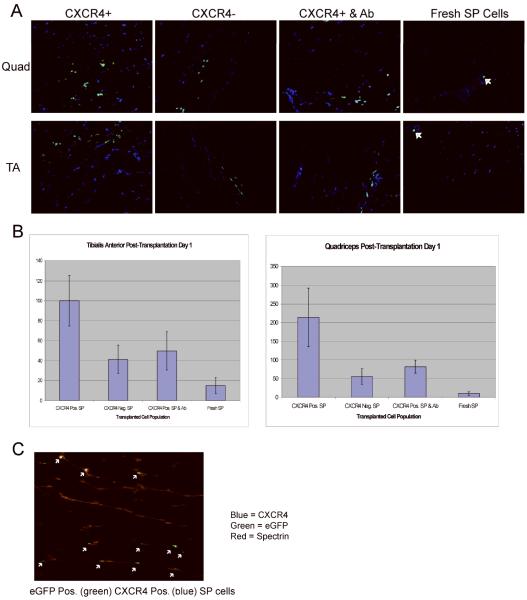
In order to investigate the potential contribution of the CXCR4 surface marker to the extravasation of SP-derived cultured cells into damaged muscle, eight mdx5cv 4-7 week-old mice were transplanted via iliac artery injection with 50,000 eGFP-positive lentiviral transduced mdx5cv SP cells. Cell were separated in four different groups and then transplanted: (i) CXCR4-positive cultured cells derived from the side population, (ii) CXCR4-negative cultured cells derived from the side population, (iii) CXCR4-positive SP-derived cultured cells previously incubated for one hour with a blocking CXCR4 antibody, and (iv) freshly isolated SP cells. Each group was introduced into two different animals. One day after transplantation, mice were sacrificed, and quadriceps and tibialis anterior of the right and left limbs were sectioned. eGFP mononuclear cell expression was analyzed through fluorescent microscopy in 5 muscle sections of each skeletal muscle obtained as described above (Figure 2a). eGFP-positive mononuclear donor cells were found in every right limb analyzed but not in the left limb, indicating rapid extravasation downstream from the site of injection. More importantly, greater numbers of donor eGFP-positive donor cells were observed in muscles transplanted with CXCR4-positive cells versus muscle from animals transplanted with CXCR4-negative cells (p<0.001), CXCR4-positive SP cells pretreated with blocking antibody (p<0.001), or freshly isolated SP cells (p<0.001) by one-tailed t-test (Figure 2b). There were no eGFP-positive cells in any of the left (contralateral) limbs analyzed, which is consistent with our previous studies 4. These results suggest that CXCR4 expression promotes rapid extravasation of myogenic precursor cells to dystrophic muscle downstream of the site of injection. Furthermore, the surface protein itself may play a role in the extravasation process, as treatment with a surface-side blocking antibody decreased extravasation rates.
Figure 2. Extravasation of donor SP cells into mdx5cv host muscles.
One day after intra-arterial delivery, eGFP-positive SP-derived donor mononuclear cells were found residing only in host muscle tissues downstream the site of injection. (a) CXCR4-positive cultured SP cells; CXCR4-negative cultured SP cells; CXCR4-positive cultured SP cells previously incubated with a CXCR4 blocking antibody; and freshly isolated SP cells were detected one day after injection in quadriceps and tibialis anterior. (b) Histograms show the average number of eGFP-positive mononuclear cells identified in sections of quadriceps or tibialis anterior. Each bar represents the average count in two mice; five sections were analyzed per mouse. Significantly more eGFP-positive mononuclear cells were identified in animals transplanted with CXCR4-positive eGFP-positive cells than those that received CXCR4-negative eGFP-positive cells. (c) Immunohistochemistry against CXCR4 (blue) shows CXCR4-positive eGFP mononuclear donor cells residing within the quadriceps of animals transplanted with CXCR4-positive eGFP-positive cultured SP cells. Spectrin (Texas red) staining performed to identify the cytoskeleton.
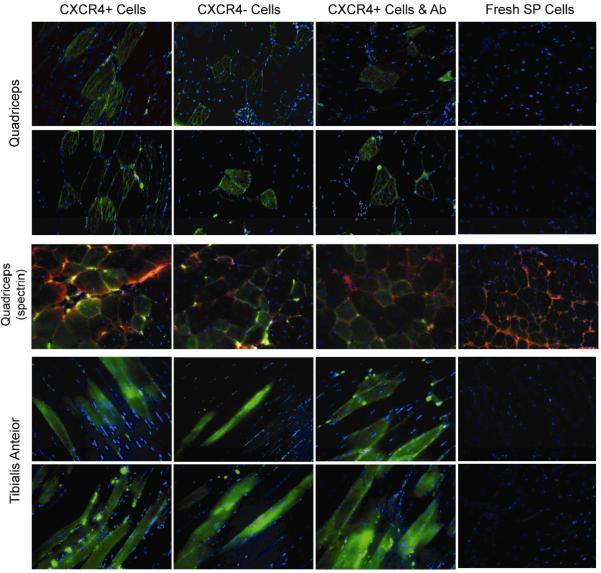
To investigate the role of CXCR4 expression in SP cell engraftment into dystrophic muscle, 12 mdx5cv mice were each transplanted with 50,000 eGFP-positive lentiviral transduced mdx5cv SP-derived cells that had been cultured as described for 21 days. Donor cells were sorted in four different groups and then transplanted using the same intra-arterial protocol: (i) CXCR4-positive cultured cells derived from the side population, (ii) CXCR4-negative cultured cells derived from the side population, (iii) CXCR4-positive SP-derived cultured cells previously incubated for one hour with a blocking CXCR4 antibody, and (iv) freshly isolated SP cells. Each group of cells was transplanted into three different mice. One month post transplantation animals were sacrificed, and quadriceps and tibialis anterior from both limbs were fixed and sectioned throughout the entire muscle as described above. eGFP-positive fibers, which indicated engraftment of eGFP-positive donor cells, were counted using fluorescence microscopy (Figure 3 and Supplemental Table 1). In the quadriceps and tibialis anterior muscles, the amount of CXCR4-positive donor cell engraftment was greater than engraftment of CXCR4-negative cultured SP cells (p=0.001 and p=0.04, respectively) by one-tailed t-test (Supplemental Table 1). As expected, CXCR4-positive cultured cells engrafted at a greater rate than freshly isolated SP cells in both quadriceps and tibialis anterior. No significant difference, however, was observed in engraftment rates of CXCR4-positive cells and CXCR4-positive cells treated with a blocking CXCR4 antibody 30 days after injection in the quadriceps (p=0.13) and the tibialis anterior (p=0.41). We believe that the blocking antibody may not have been effective in these long-term studies because of the length of time between injection and tissue analysis. Freshly isolated muscle SP cells engrafted at very low levels (at most 0.5%). As in previous studies 4, there were muscle sections from all injected mice with no engrafted fibers and some with up to 11% of donor engrafted green fibers in the quadriceps and up to 7% in the tibialis anterior (Figure 3 and Supplemental Table 2). There were also sections in which very little eGFP was detected. Engrafted fibers were found only in the right quadriceps and tibialis anterior and not in the contralateral limb.
Figure 3. Tissue sections from mdx5cv mice transplanted with eGFP transduced SP cells.
eGFP was detected in fibers and mononuclear cells of transplanted animals injected with CXCR4-positive cultured SP cells; CXCR4-negative cultured SP cells; and CXCR4-positive cultured SP cells previously incubated with a CXCR4 blocking antibody, but not in animals transplanted with freshly isolated SP cells. Longitudinal (tibialis anterior) and transverse (quadriceps) muscle sections are presented. eGFP expression was detected in right quadriceps and right tibialis anterior one month after transplantation. Spectrin (Texas red) staining performed in some sections from right quadriceps to identify the cytoskeleton. Nuclei were stained with DAPI (blue). 20× magnification.
These results suggest that donor cell CXCR4 expression not only increases extravasation of donor cells but also results in increased cell engraftment into host tissue one month after transplantation. Given that CXCR4's ligand is a potent chemoattractant, CXCR4 expression likely promotes extravasation of donor cells into dystrophic tissue. Increased extravasation results in greater donor cell engraftment, possibly because more donor cells gain access to myogenic tissue and, thus, more cells can subsequently engraft. In summary, our data suggest that CXCR4 expression promotes extravasation into dystrophic muscle shortly after transplantation, and results in enhanced engraftment into dystrophic muscle over the long term.
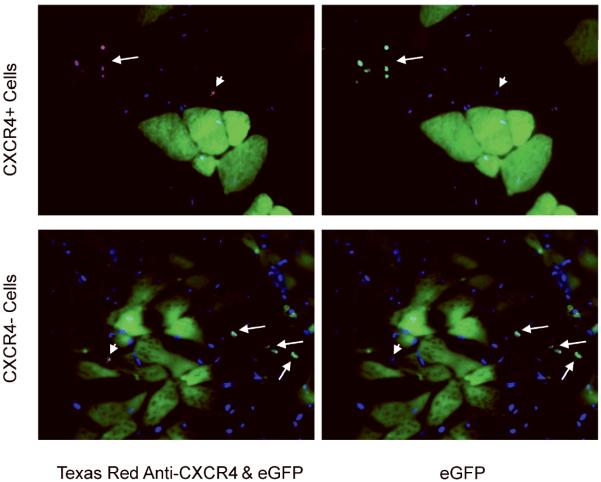
In addition, we evaluated the maintenance of long-term CXCR4 expression in mononuclear donor cells found in dystrophic muscle which did not fuse into host fibers after a month. CXCR4 (Texas red) and eGFP (green) co-expression was analyzed in the right quadriceps of animals injected with either CXCR4-positive or negative eGFP-positive cultured cells (Figure 4). Every eGFP-positive CXCR4-positive donor cell remained CXCR4-positive after a month residing in host tissue. No CXCR4-negative cell began to express this marker once in host tissue (Figure 4 arrows). Endogenous CXCR4 cells, probably inflammatory cells, were found in both cases (Figure 4 arrowheads).
Figure 4. CXCR4 expression in host tissue one month after transplantation.
CXCR4-positive mononuclear cells were detected in quadriceps from animals transplanted with CXCR4-positive cells and animals who received CXCR4-negative cells. Both eGFP-positive (donor) and eGFP negative (endogenous) CXCR4-positive cells (arrows and arrowheads, respectively) were found in tissues transplanted with CXCR4-positive cells. Only endogenous eGFP negative CXCR4-positive cells were found in tissues transplanted with eGFP-positive CXCR4-negative cells.
DISCUSSION
Mammals with muscular dystrophy are characterized by a progressive myofiber regenerative-degenerative process, which results in an inflammatory environment rich in cytokines and cell-signaling molecules. Mononuclear muscle precursor cells, named satellite cells, attempt to repair these dystrophic muscles by constantly replacing necrotic fibers. Another subpopulation of mononuclear cells isolated skeletal muscle, SP cells, can be introduced into the circulation via veins and arteries. These cells can then extravasate from vessels into skeletal muscle, engraft into muscle tissue and deliver their genetic material to host myofibers. The existence of muscle-resident adult progenitors with this ability makes skeletal muscle an attractive target tissue for cell-based therapy. Previous work has shown that this subpopulation of lineage uncommitted cells participate in the repair of dystrophic fibers when injected intra-arterially after several weeks in culture 4. The incorporation of only a few donor nuclei per muscle fiber may be sufficient to restore functional levels of an absent gene product to the entire fiber.
In order to elucidate the mechanisms by which muscle progenitor cells can extravasate from vessels and engraft in skeletal muscle, we compared cell surface markers of cultured cells derived from the side population and freshly isolated SP cells. Cultured cells express slightly different cell surface markers than freshly isolated SP cells. They lose CD34 and CD31 expression, and a subset of cells (~18%) begin to express CXCR4, a chemokine receptor for SDF-1 which is upregulated in dystrophic muscle and after tissue damage (Figure 1) 1,23,26,27,44,47. CXCR4/SDF-1 interaction may be important for the observed rapid extravasation of CXCR4-positive donor cells. This function would be consistent with CXCR4/SDF-1 interaction which drives extravasation of endogenously circulating CXCR4-positive cells (including CD4+ T lymphocytes), a regularly occurring process after injury and inflammation 1,23,26,27,44,47. Other cell surface markers and adhesion molecules are likely involved in this process as well, and we are currently working to identify other markers which participate in muscle progenitor cell extravasation.
CXCR4 expression on SP-derived cells also results in increased donor cell engraftment at one month post transplantation. Increased engraftment in the quadriceps and tibialis anterior was observed at one month post single transplantation in mice that received CXCR4-positive cells versus those that were injected with CXCR4-negative cells. This likely occurred because CXCR4/SDF-1 interactions promoted increased extravasation shortly after cell delivery, which lead to a greater number of donor cells in dystrophic tissue that subsequently engrafted into myofibers. Given that CXCR4's ligand is a chemoattractant, it is unlikely that CXCR4 itself plays a direct role in donor cell fusion with host myofibers. Rather, CXCR4 may serve as a localization signal that targets SP cells in the circulation to dystrophic muscle. Future work aims to elucidate surface molecules on muscle progenitor cells which drive their fusion with host myofibers. Increasing the expression of these molecules may further increase cell engraftment after intra-arterial delivery.
Our results also suggest the possibility that SP-derived CXCR4-negative cells express markers that allow them to engraft into host muscle once these cells are present in the dystrophic myogenic environment. In short term studies (1 day post-transplantation), CXCR4-positive cells showed greater extravasation potential than CXCR4-negative cells. However, in long term studies (30 days post-transplantation), CXCR4-negative cell engraftment, while significantly less than CXCR4-positive cell engraftment, was higher than may have been expected given their poorer relative extravasation potential. This suggests that the CXCR4-negative transplanted population, while relatively poor at extravasation (nearly a fifth of the number of cells than CXCR4-positive 1 day post-transplantation), may express alternative or additional surface markers that enhance their ability to engraft into dystrophic muscle. At thirty days post transplantation, CXCR4-negative cells attained engraftment levels just half of those observed after CXCR4-positive cell transplantation. Gene expression profiling on these different cell types could yield insights into the molecules and pathways involved. Future goals include identifying markers differentially expressed in muscle SP-derived CXCR4-negative and CXCR4-positive populations that directly contribute to engraftment into dystrophic muscle. Future delivery experiments will focus on transplanting a cell population that expresses both CXCR4 and these markers.
To assess donor cell extravasation, sections from the entire quadriceps and the tibialis anterior were analyzed. Extravasation was not uniformly distributed in host muscle; some sections did not show any engraftment, while higher efficiency (up to 11%) of eGFP expression was observed in others. This suggests that the delivered cells were more likely to extravasate from certain post-capillary venules versus others. This may have been determined by vessel morphology and/or differential expression of surface markers on vessel endothelial cells. Areas of skeletal muscle with more damage could release chemoattractants that recruit donor cells more actively. Consistent with previous studies, extravasation of donor cells into skeletal muscle was observed only in the limbs downstream of the injection sites. This suggests that extravasation may occur shortly after intra-arterial delivery and before these cells enter the venous circulation 4. It is possible that donor cells quickly lose their extravasation potential once they are introduced into the circulation and therefore are not observed in the contralateral limb. Both humeral and cellular immunity may be involved in limiting donor cell extravasation. Future work will aim to determine the factors that contribute to the observed engraftment patterns.
Intra-arterial transplantation is a promising approach for donor cell delivery in clinical settings, since artery catheterization is a safe and widely-implemented clinical technique performed by interventional cardiologists and radiologists 32,41,53. This technique has been widely implemented clinically for over a decade and can be repeated in the same patient without complications. Our successful delivery of progenitor cells to dystrophin-deficient muscle reinforces the utility of this approach for cell-based clinical therapies of primary myopathies. Furthermore, this study identifies a key cell surface molecule, CXCR4, whose expression may contribute to the delivery of cells to dystrophic tissue and, therefore, enhance progenitor cell engraftment after systemic transplantation.
Supplementary Material
Acknowledgements
We are grateful to E. Gussoni, J. Guyon and J. Schienda for fruitful discussion and critical reading of the manuscript. This study was supported by funds from the National Institutes of Health (5P50NS040828; 5P30HD018655; RO1 AR047721), the Bernard and Alva B. Gimbel Foundation and the Howard Hughes Medical Institute. L.M.K. is an Investigator of the Howard Hughes Medical Institute.
ABBREVIATIONS
- DMD
Duchenne muscular dystrophy
- SP
Side population
- FACS
Fluorescence-activated cell sorting
- MP
Main population
REFERENCES
- 1.Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med. 1997;185:111–120. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asakura A, Seale P, Girgis-Gabardo A, Rudnicki MA. Myogenic specification of side population cells in skeletal muscle. J Cell Biol. 2002;159:123–134. doi: 10.1083/jcb.200202092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachrach E, Li S, Perez AL, Schienda J, Liadaki K, Volinski J, Flint A, Chamberlain J, Kunkel LM. Systemic delivery of human microdystrophin to regenerating mouse dystrophic muscle by muscle progenitor cells. Proc Natl Acad Sci U S A. 2004;101:3581–3586. doi: 10.1073/pnas.0400373101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachrach E, Perez AL, Choi YH, Illigens BM, Jun SJ, del Nido P, McGowan FX, Li S, Flint A, Chamberlain J, Kunkel LM. Muscle engraftment of myogenic progenitor cells following intraarterial transplantation. Muscle Nerve. 2006;34:44–52. doi: 10.1002/mus.20560. [DOI] [PubMed] [Google Scholar]
- 5.Beauchamp JR, Morgan JE, Pagel CN, Partridge TA. Dynamics of myoblast transplantation reveal a discrete minority of precursors with stem cell-like properties as the myogenic source. J Cell Biol. 1999;144:1113–1122. doi: 10.1083/jcb.144.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bischoff R. The satellite cell and muscle regeneration. In: Engel AG, Franzini-Armstrong C, editors. Myology. 2nd ed. Volume 1. Mc Graw Hill; New York: 1994. pp. 97–119. [Google Scholar]
- 7.Campbell KP, Kahl SD. Association of dystrophin and an integral membrane glycoprotein. Nature. 1989;338:259–262. doi: 10.1038/338259a0. [DOI] [PubMed] [Google Scholar]
- 8.Cao B, Zheng B, Jankowski RJ, Kimura S, Ikezawa M, Deasy B, Cummins J, Epperly M, Qu-Petersen Z, Huard J. Muscle stem cells differentiate into haematopoietic lineages but retain myogenic potential. Nat Cell Biol. 2003;5:640–646. doi: 10.1038/ncb1008. [DOI] [PubMed] [Google Scholar]
- 9.Cox GA, Cole NM, Matsumura K, Phelps SF, Hauschka SD, Campbell KP, Faulkner JA, Chamberlain JS. Overexpression of dystrophin in transgenic mdx mice eliminates dystrophic symptoms without toxicity. Nature. 1993;364:725–729. doi: 10.1038/364725a0. [DOI] [PubMed] [Google Scholar]
- 10.Danko I, Chapman V, Wolff JA. The frequency of revertants in mdx mouse genetic models for Duchenne muscular dystrophy. Pediatr Res. 1992;32:128–131. doi: 10.1203/00006450-199207000-00025. [DOI] [PubMed] [Google Scholar]
- 11.Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- 12.Ferrari G, Stornaiuolo A, Mavilio F. Failure to correct murine muscular dystrophy. Nature. 2001;411:1014–1015. doi: 10.1038/35082631. [DOI] [PubMed] [Google Scholar]
- 13.Galvez BG, Sampaolesi M, Brunelli S, Covarello D, Gavina M, Rossi B, Constantin G, Torrente Y, Cossu G. Complete repair of dystrophic skeletal muscle by mesoangioblasts with enhanced migration ability. J Cell Biol. 2006;174:231–243. doi: 10.1083/jcb.200512085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gavina M, Belicchi M, Rossi B, Ottoboni L, Colombo F, Meregalli M, Battistelli M, Forzenigo L, Biondetti P, Pisati F, Parolini D, Farini A, Issekutz AC, Bresolin N, Rustichelli F, Constantin G, Torrente Y. VCAM-1 expression on dystrophic muscle vessels has a critical role in the recruitment of human blood-derived CD133+ stem cells after intra-arterial transplantation. Blood. 2006;108:2857–2866. doi: 10.1182/blood-2006-04-018564. [DOI] [PubMed] [Google Scholar]
- 15.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gussoni E, Pavlath GK, Lanctot AM, Sharma KR, Miller RG, Steinman L, Blau HM. Normal dystrophin transcripts detected in Duchenne muscular dystrophy patients after myoblast transplantation. Nature. 1992;356:435–438. doi: 10.1038/356435a0. [DOI] [PubMed] [Google Scholar]
- 17.Gussoni E, Blau HM, Kunkel LM. The fate of individual myoblasts after transplantation into muscles of DMD patients. Nat Med. 1997;3:970–977. doi: 10.1038/nm0997-970. [DOI] [PubMed] [Google Scholar]
- 18.Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, Kunkel LM, Mulligan RC. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999;401:390–394. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- 19.Hoffman EP, Monaco AP, Feener CC, Kunkel LM. Conservation of the Duchenne muscular dystrophy gene in mice and humans. Science. 1987;238:347–350. doi: 10.1126/science.3659917. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman EP, Morgan JE, Watkins SC, Partridge TA. Somatic reversion/suppression of the mouse mdx phenotype in vivo. J Neurol Sci. 1990;99:9–25. doi: 10.1016/0022-510x(90)90195-s. [DOI] [PubMed] [Google Scholar]
- 21.Im WB, Phelps SF, Copen EH, Adams EG, Slightom JL, Chamberlain JS. Differential expression of dystrophin isoforms in strains of mdx mice with different mutations. Hum Mol Genet. 1996;5:1149–1153. doi: 10.1093/hmg/5.8.1149. [DOI] [PubMed] [Google Scholar]
- 22.Jackson KA, Mi T, Goodell MA. Hematopoietic potential of stem cells isolated from murine skeletal muscle. Proc Natl Acad Sci U S A. 1999;96:14482–14486. doi: 10.1073/pnas.96.25.14482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jankowski K, Kucia M, Wysoczynski M, Reca R, Zhao D, Trzyna E, Trent J, Peiper S, Zembala M, Ratajczak J, Houghton P, Janowska-Wieczorek A, Ratajczak MZ. Both hepatocyte growth factor (HGF) and stromal-derived factor-1 regulate the metastatic behavior of human rhabdomyosarcoma cells, but only HGF enhances their resistance to radiochemotherapy. Cancer Res. 2003;63:7926–7935. [PubMed] [Google Scholar]
- 24.Karpati G, Pouilot Y, Zubrzycka-Gaarn E, Carpenter S, Ray PN, Worton RG, Holland P. Dystrophin is expressed in mdx skeletal muscle fibers after normal myoblast implantation. Am J Pathol. 1989;135:27–32. [PMC free article] [PubMed] [Google Scholar]
- 25.Karpati G, Ajdukovic D, Arnold D, Gledhill RB, Guttmann R, Holland P, Koch PA, Shoubridge E, Spence D, Vanasse M, et al. Myoblast transfer in Duchenne muscular dystrophy. Ann Neurol. 1993;34:8–17. doi: 10.1002/ana.410340105. [DOI] [PubMed] [Google Scholar]
- 26.Kollet O, Shivtiel S, Chen YQ, Suriawinata J, Thung SN, Dabeva MD, Kahn J, Spiegel A, Dar A, Samira S, Goichberg P, Kalinkovich A, Arenzana-Seisdedos F, Nagler A, Hardan I, Revel M, Shafritz DA, Lapidot T. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. J Clin Invest. 2003;112:160–169. doi: 10.1172/JCI17902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kucia M, Ratajczak J, Reca R, Janowska-Wieczorek A, Ratajczak MZ. Tissue-specific muscle, neural and liver stem/progenitor cells reside in the bone marrow, respond to an SDF-1 gradient and are mobilized into peripheral blood during stress and tissue injury. Blood Cells Mol Dis. 2004;32:52–57. doi: 10.1016/j.bcmd.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 28.LaBarge MA, Blau HM. Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell. 2002;111:589–601. doi: 10.1016/s0092-8674(02)01078-4. [DOI] [PubMed] [Google Scholar]
- 29.Lapidot T, Kollet O. The essential roles of the chemokine SDF-1 and its receptor CXCR4 in human stem cell homing and repopulation of transplanted immune-deficient NOD/SCID and NOD/SCID/B2m(null) mice. Leukemia. 2002;16:1992–2003. doi: 10.1038/sj.leu.2402684. [DOI] [PubMed] [Google Scholar]
- 30.Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106:1901–1910. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- 31.Lee JY, Qu-Petersen Z, Cao B, Kimura S, Jankowski R, Cummins J, Usas A, Gates C, Robbins P, Wernig A, Huard J. Clonal isolation of muscle-derived cells capable of enhancing muscle regeneration and bone healing. J Cell Biol. 2000;150:1085–1100. doi: 10.1083/jcb.150.5.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levi DS, Alejos JC, Moore JW. Future of interventional cardiology in pediatrics. Curr Opin Cardiol. 2003;18:79–90. doi: 10.1097/00001573-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Mauro A. Satellite cells of skeletal muscle. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKinney-Freeman SL, Jackson KA, Camargo FD, Ferrari G, Mavilio F, Goodell MA. Muscle-derived hematopoietic stem cells are hematopoietic in origin. Proc Natl Acad Sci U S A. 2002;99:1341–1346. doi: 10.1073/pnas.032438799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mendell JR, Kissel JT, Amato AA, King W, Signore L, Prior TW, Sahenk Z, Benson S, McAndrew PE, Rice R, et al. Myoblast transfer in the treatment of Duchenne's muscular dystrophy. N Engl J Med. 1995;333:832–838. doi: 10.1056/NEJM199509283331303. [DOI] [PubMed] [Google Scholar]
- 36.Monaco AP, Bertelson CJ, Colletti-Feener C. Kunkel LM. Localization and cloning of Xp21 deletion breakpoints involved in muscular dystrophy. Hum Genet. 1987;75:221–227. doi: 10.1007/BF00281063. [DOI] [PubMed] [Google Scholar]
- 37.Montanaro F, Liadaki K, Volinski J, Flint A, Kunkel LM. Skeletal muscle engraftment potential of adult mouse skin side population cells. Proc Natl Acad Sci U S A. 2003;100:9336–9341. doi: 10.1073/pnas.1133179100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Montanaro F, Liadaki K, Schienda J, Flint A, Gussoni E, Kunkel LM. Demystifying SP cell purification: viability, yield, and phenotype are defined by isolation parameters. Exp Cell Res. 2004;298:144–154. doi: 10.1016/j.yexcr.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 39.Morandi L, Bernasconi P, Gebbia M, Mora M, Crosti F, Mantegazza R, Cornelio F. Lack of mRNA and dystrophin expression in DMD patients three months after myoblast transfer. Neuromuscul Disord. 1995;5:291–295. doi: 10.1016/0960-8966(94)00070-p. [DOI] [PubMed] [Google Scholar]
- 40.Neumeyer AM, Cros D, McKenna-Yasek D, Zawadzka A, Hoffman EP, Pegoraro E, Hunter RG, Munsat TL, Brown RH., Jr. Pilot study of myoblast transfer in the treatment of Becker muscular dystrophy. Neurology. 1998;51:589–592. doi: 10.1212/wnl.51.2.589. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen T, Hung PM, Tuan NQ, Hermiller J, Douglas JS, Jr., Grines C. Balloon angioplasty. J Interv Cardiol. 2001;14:563–569. doi: 10.1111/j.1540-8183.2001.tb00373.x. [DOI] [PubMed] [Google Scholar]
- 42.Partridge TA, Morgan JE, Coulton GR, Hoffman EP, Kunkel LM. Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal myoblasts. Nature. 1989;337:176–179. doi: 10.1038/337176a0. [DOI] [PubMed] [Google Scholar]
- 43.Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, Nagler A, Ben-Hur H, Many A, Shultz L, Lider O, Alon R, Zipori D, Lapidot T. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 44.Ponomaryov T, Peled A, Petit I, Taichman RS, Habler L, Sandbank J, Arenzana-Seisdedos F, Magerus A, Caruz A, Fujii N, Nagler A, Lahav M, Szyper-Kravitz M, Zipori D, Lapidot T. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J Clin Invest. 2000;106:1331–1339. doi: 10.1172/JCI10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qu-Petersen Z, Deasy B, Jankowski R, Ikezawa M, Cummins J, Pruchnic R, Mytinger J, Cao B, Gates C, Wernig A, Huard J. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002;157:851–864. doi: 10.1083/jcb.200108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qu Z, Balkir L, van Deutekom JC, Robbins PD, Pruchnic R, Huard J. Development of approaches to improve cell survival in myoblast transfer therapy. J Cell Biol. 1998;142:1257–1267. doi: 10.1083/jcb.142.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ratajczak MZ, Kucia M, Reca R, Majka M, Janowska-Wieczorek A, Ratajczak J. Stem cell plasticity revisited: CXCR4-positive cells expressing mRNA for early muscle, liver and neural cells `hide out' in the bone marrow. Leukemia. 2004;18:29–40. doi: 10.1038/sj.leu.2403184. [DOI] [PubMed] [Google Scholar]
- 48.Sampaolesi M, Torrente Y, Innocenzi A, Tonlorenzi R, D'Antona G, Pellegrino MA, Barresi R, Bresolin N, De Angelis MG, Campbell KP, Bottinelli R, Cossu G. Cell therapy of alpha-sarcoglycan null dystrophic mice through intra-arterial delivery of mesoangioblasts. Science. 2003;301:487–492. doi: 10.1126/science.1082254. [DOI] [PubMed] [Google Scholar]
- 49.Sampaolesi M, Blot S, D'Antona G, Granger N, Tonlorenzi R, Innocenzi A, Mognol P, Thibaud JL, Galvez BG, Barthelemy I, Perani L, Mantero S, Guttinger M, Pansarasa O, Rinaldi C, Cusella De Angelis MG, Torrente Y, Bordignon C, Bottinelli R, Cossu G. Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature. 2006;444:574–579. doi: 10.1038/nature05282. [DOI] [PubMed] [Google Scholar]
- 50.Schultz E, Jaryszack DL, Valliere CR. Response of satellite cells to focal skeletal muscle injury. Muscle Nerve. 1985;8:217–222. doi: 10.1002/mus.880080307. [DOI] [PubMed] [Google Scholar]
- 51.Seale P, Rudnicki MA. A new look at the origin, function, and “stem-cell” status of muscle satellite cells. Dev Biol. 2000;218:115–124. doi: 10.1006/dbio.1999.9565. [DOI] [PubMed] [Google Scholar]
- 52.Sicinski P, Geng Y, Ryder-Cook AS, Barnard EA, Darlison MG, Barnard PJ. The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science. 1989;244:1578–1580. doi: 10.1126/science.2662404. [DOI] [PubMed] [Google Scholar]
- 53.Silva MB, Jr., Bohannon WT, Santana D. Technical note on renal angioplasty and stenting: new developments that facilitate its performance. Vascular. 2004;12:42–50. doi: 10.1258/rsmvasc.12.1.42. [DOI] [PubMed] [Google Scholar]
- 54.Stockdale FE, Hager EJ, Fernyak SE, DiMario JX. Myoblasts, satellite cells, and myoblast transfer. Adv Exp Med Biol. 1990;280:7–11. doi: 10.1007/978-1-4684-5865-7_2. [DOI] [PubMed] [Google Scholar]
- 55.Tamaki T, Akatsuka A, Ando K, Nakamura Y, Matsuzawa H, Hotta T, Roy RR, Edgerton VR. Identification of myogenic-endothelial progenitor cells in the interstitial spaces of skeletal muscle. J Cell Biol. 2002;157:571–577. doi: 10.1083/jcb.200112106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Torrente Y, Tremblay JP, Pisati F, Belicchi M, Rossi B, Sironi M, Fortunato F, El Fahime M, D'Angelo MG, Caron NJ, Constantin G, Paulin D, Scarlato G, Bresolin N. Intraarterial injection of muscle-derived CD34(+)Sca-1(+) stem cells restores dystrophin in mdx mice. J Cell Biol. 2001;152:335–348. doi: 10.1083/jcb.152.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tremblay JP, Malouin F, Roy R, Huard J, Bouchard JP, Satoh A, Richards CL. Results of a triple blind clinical study of myoblast transplantations without immunosuppressive treatment in young boys with Duchenne muscular dystrophy. Cell Transplant. 1993;2:99–112. doi: 10.1177/096368979300200203. [DOI] [PubMed] [Google Scholar]
- 58.Yablonka-Reuveni Z, Rivera AJ. Temporal expression of regulatory and structural muscle proteins during myogenesis of satellite cells on isolated adult rat fibers. Dev Biol. 1994;164:588–603. doi: 10.1006/dbio.1994.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.