Abstract
Epidemiologic studies indicate that nearly 60% of schizophrenia (SZ) patients treated with conventional antipsychotic drugs develop extrapyramidal side effects (EPS) such as parkinsonism and tardive dyskinesia. Although the prevalence of EPS has decreased due to the newer antipsychotics, EPS continue to limit the effectiveness of these medicines. Ongoing monitoring of EPS is likely to improve treatment outcome or compliance and reduce the frequency of re-hospitalization. A quantitative analysis of handwriting kinematics was used to evaluate effects of antipsychotic medication type and dose in schizophrenia patients. Twenty-seven schizophrenia patients treated with risperidone, six schizophrenia patients who received no antipsychotic medication and 46 healthy comparison participants were enrolled. Participants performed a 20-minute handwriting task consisting of loops of various sizes and a sentence. Data were captured and analyzed using MovAlyzeR software. Results indicated that risperidone-treated participants exhibited significantly more dysfluent handwriting movements than either healthy or untreated SZ participants. Risperidone-treated participants exhibited lower movement velocities during production of simple loops compared to unmedicated patients. Handwriting dysfluency during sentence writing increased with dose. A 3-factor model consisting of kinematic variables derived from sentence writing accounted for 83% (r = .91) of the variability in medication dose. In contrast, we found no association between observer-based EPS severity ratings and medication dose. These findings support the importance of handwriting-based measures to monitor EPS in medicated schizophrenia patients.
1. Introduction
In schizophrenia the over-active fronto-striatal pathways in the brain cause the patient to indiscriminately associate thoughts with reality. Antipsychotic medications reduce the psychosis by inhibiting the transmission of dopamine throughout the fronto-striatal pathways in the brain. Conventional antipsychotics block the same dopamine receptors that produce motor disturbances such as parkinsonism. However, chronic dopamine receptor blockade can lead to permanent damage to these pathways resulting in tardive dyskinesia (TD). Epidemiologic studies indicate that nearly 60% of patients treated with conventional neuroleptic drugs develop EPS such as parkinsonism, while 20-25% of the patients develop irreversible TD (Caligiuri, Jeste, & Lacro, 2000). These serious conditions might have been prevented if EPS could have been detected early in the management of the patient's pharmacotherapy. Careful assessment of EPS is important as current strategies for managing EPS include prophylactic use of anticholinergic medication, dose reduction, or medication switching all of which extend costs to the patient and society (Lambert, 2007).
Observer-based rating scales continue to represent the state-of-the-art for assessing drug-induced EPS in the clinical setting. Rating the severity of EPS or parkinsonian signs requires a trained and experienced examiner. The examiner needs to engage the patient in several motor tasks involving the head, arms, and legs. However, suboptimal reliability, examiner bias, or nonlinearity of observer-based severity ratings limit their use in both clinical and research settings. Furthermore, the absence of reliable and precise EPS assessment methods is partly responsible for the inability to delineate the syndrome, determine its prevalence, or evaluate the efficacy of treatment. These problems have prompted researchers to develop reliable quantitative instrumental methods for objectively assessing EPS.
For objectively quantifying EPS researchers attempted various instrumental approaches based on load cells, strain gauges, accelerometers or electromyographs. Instrumental systems are able to measure movement abnormalities along a continuum of severity. Because of their high sensitivity, instrumental assessment can reveal subtle motor abnormalities that are below the threshold of detection by observer-based clinical evaluation, thus, allowing for the potential detection of subclinical motor phenomena. Instrumental measures are more objective as they are less influenced by examiner bias or experience. While these instruments found application in the research settings, they have not been adopted for routine clinical or bedside use because they require technical expertise to install and operate. Our goal is to develop simple techniques that provide quantitative objective measurement of EPS severity that can be widely used by neurologists, psychiatrists, or psychologists in the clinical setting.
The purpose of the present study was to evaluate our new approach to assessing EPS based on quantifying handwriting movements. The use of handwriting to assess EPS was first reported by Haase (1961; and later in 1978) and was adopted in more recent psychopharmacological studies (Gallucci, Phillips, Bradshaw, Vaddadi, & Pantelis, 1997; Gerken, Wetzel, & Benkert, 1991; Kuenstler, Juhnhold, Knapp, & Gertz, 1999). Gerken et al. (1991) expressed movement size as the area encompassed by handwriting in schizophrenic patients. Treatment with antipsychotics caused a 13% reduction in handwriting area in most of the samples in 75% of the treatment non-responders, while only in 33% of the treatment responders. The authors concluded that handwriting parameters could be better suited for evaluating neurological side effects of neuroleptic medication to predict treatment response than the conventional observer-based severity rating scales. Gallucci et al (1997) observed an association between handwriting duration consistency and medication type in their schizophrenia patients. Kuenstler et al (1999) used positron emission tomography (PET) in schizophrenic patients before and after treatment with drugs (haloperidol, clozapine, or risperidone). They found a relationship between reduction in handwriting size (expressed by area) and dopamine D2 receptor occupancy.
In our study, we examined whether measures of handwriting kinematics are sufficiently sensitive to the presence and severity of antipsychotic-induced EPS including parkinsonian bradykinesia and dyskinesia. We hypothesized that schizophrenia patients treated with an antipsychotic medication exhibits greater levels of impairment on measures of handwriting kinematics compared to unmedicated schizophrenia patients and healthy control participants. Furthermore, we hypothesized that severity of handwriting movement impairment is associated with dose of antipsychotic medication.
2. Methods
2.1 Participants
This study was a multi-site parallel group design. Seventy-four participants were recruited and tested at three sites: University of California San Diego, Minneapolis Veterans Administration Medical Center, and Indiana University School of Medicine in Indianapolis. Twenty-one risperidone-treated schizophrenia or schizoaffective disorder (hereafter referred to as schizophrenia or SZ patients), six unmedicated schizophrenia patients, and 47 normal healthy volunteers participated in this study. The medicated SZ group consisted of 11 males and 10 females with a mean (sd) age of 44.9 years (11.1) and mean illness duration of 19.0 (10.9) years. The unmedicated SZ group consisted of four males and two females with a mean age of 48.6 years (7.3) and mean illness duration of 16.2 (11.1) years. The normal healthy participant group consisted of 17 males and 30 females with a mean age of 42.0 years (10.2). Only patients treated with risperidone were included into the medicated SZ group. The six unmedicated patients served as a control group for the risperidone-medicated patients, while the normal healthy participant group served as a control for the schizophrenia patients.
2.2 Clinical Tests
Psychiatric diagnoses were based on DSM-IV criteria. We used the Positive and Negative Symptom Scale (PANSS: Kay, Fiszbein, & Opler, 1987) to evaluate the severity of psychosis symptoms and the Calgary Depression Scale (CDS: Addington, Addington, & Schissel, 1990) to evaluate depression symptoms. For both scales, higher scores indicate more severe psychopathology. Participants from each site received the same clinical evaluation and handwriting motor assessment, using the same procedures. Each participant was assessed during a single 1-hour session. They underwent an assessment of the severity and nature of psychosis. Standard observer-based rating scales were used to assess the severity of drug-induced EPS including parkinsonism (using the Simpson-Angus EPS scale, SAEPS) and TD (using the Abnormal Involuntary Movement Scale, AIMS). Table 1 shows the clinical characteristics of the two patient groups. Schizophrenia participants did not differ on measures of psychopathology, AIMS or BAS and there was a marginal trend for medicated patients to have more EPS based on SAEPS score than unmedicated patients (.05 < p < .10)
Table 1. Means (and standard deviations) for the clinical variables for the two schizophrenia groups.
| Clinical Variable | Risperidone-Treated Patients N = 21 |
Untreated Patients N = 6 |
|---|---|---|
| Age, Years | 44.9 (11.1) | 48.6 (7.3) |
| Illness duration, Years | 19.0 (10.9) | 16.2 (11.1) |
| AIMS -- Abnormal Involuntary Movement Scale, Total Score | 3.7 (3.5) | 1.8 (3.2) |
| SAEPS -- Simpson-Angus EPS Scale, Total Score | 4.3 (3.2) | 1.8 (1.9) |
| BAS -- Barnes Akathisia Scale, Global Score | 1.2 (1.1) | 1.8 (1.3) |
| PANNS -- Positive and Negative Symptom Scale, Total Score | 72.3 (15.5) | 69.3 (9.6) |
| CDS -- Calgary Depression Scale, Total Score | 14.4 (4.5) | 15.0 (2.9) |
2.3 Handwriting Tests
Handwriting movements were quantified using a non-inking pen with a Wacom UD 9×12 digitizing tablet (30 cm × 22.5 cm, sampling rate 100 Hz, RMS accuracy 0.01 cm) attached to a notebook computer running the MovAlyzeR software. A previous study demonstrated that schizophrenia patients with moderate levels of psychosis and with or without EPS are capable of performing the handwriting task without difficulty (Caligiuri et al., 2006). Participants sat at a table with the writing tablet positioned at an angle comfortable for writing. MovAlyzeR delivered visual cues to the examiner to instruct the participant on the next condition by showing a sample on paper. The handwriting procedures involved 15 task conditions: repetitive, overlaid circles, both with dominant and non-dominant hands, repetitive cursive l-loops with the dominant hand, continuous complex “lleellee” cursive pattern with sizes 1, 2, and 4 cm, respectively produced at comfortable speeds. Each pattern contained at last 16 up and down strokes. The overlaid circles at size 2 cm were also performed with a maximum-speed instruction with the dominant non-dominant hands. Finally, a standard sentence “Today is a nice day” was written using the dominant hand at normal speed within the 2 cm boundary. A template of two horizontal lines was used to guide the participant to write within one of the three lines with 1, 2, or 4 cm vertical size. Blocks of 3 trials per condition were performed in random order. Participants began the trial when prompted by the examiner and data collection began at the moment the pen came in contact with the tablet. Initiation delays, premature pen lifting lasting more than 3 seconds or non-consistent performance of the writing pattern caused the program to generate an error message and the trial was redone. The handwriting assessment lasted approximately 20 minutes.
2.4 Handwriting Data Analysis
Data in both x and y coordinates were low pass filtered at 8 Hz using a sinusoidal transition band of from 3.5 to 12.5 Hz (Teulings & Maarse, 1984). Subsequently, the first, second, and third time derivatives (i.e., velocity, acceleration, and jerk, respectively) were calculated. Movements were then segmented into successive up and down strokes. Per stroke, the following variables were extracted: (1) Vertical size (in cm), (2) Peak vertical velocity (in cm/s), (3) Normalized jerk averaged across a trial (ANJ) (Teulings, Contreras-Vidal, Stelmach, & Adler, 1997). Higher ANJ scores indicated more dysfluent pen movements. (4) Velocity-scaling (VS), defined as the slope of the linear regression across the 1, 2, and 4 cm conditions of the average peak velocity per stroke onto measured average vertical size per stroke (in cm/s/cm).
3. Results
3.1 Group Effects
Table 2 shows the means (and standard deviations) for the kinematic variables per group and condition. Because vertical velocity increases with vertical stroke height, mean velocities and stroke heights were calculated for the 1, 2, and 4-cm conditions separately. Velocity scaling was calculated using the three stoke height conditions for the overlaid circles and the two left-to-right loop patterns. We found no differences between dominant and nondominant hands on measures of vertical size, peak velocity, velocity scaling, and normalized jerk scores for 1, 2, and 4-cm loops in the SZ participants; therefore, data from both hands were combined for these analyses. Data were combined across three patterns (dominant and non-dominant hands, left-to-right loops) for analysis of vertical stroke size and peak velocity for each of the 1, 2, and 4-cm task conditions. For the analysis of normalized jerk for each of the 1, 2 and 4-cm task conditions, data were combined across four patterns (the previous 3 patterns plus the complex “lleellee” pattern).
Table 2. Means (and standard deviations) for the handwriting kinematic variables for the three schizophrenia groups.
| Kinematic Variable | Task Condition | Risperidone-treated Patients N = 21 |
Unmedicated Patients N = 6 |
Healthy Participants N = 47 |
Group Effect |
|---|---|---|---|---|---|
| Vertical Size (cm) | 1 cm | 1.08 (0.24) | 1.16 (0.24) | 0.93 (0.31) | p < .001 |
| 2 cm | 1.92 (0.24) | 2.02 (0.57) | 1.74 (0.32) | ns | |
| 4 cm | 3.72 (0.36) | 3.62 (0.93) | 3.40 (0.57) | p < .001 | |
| Sentence | 0.68 (0.16) | 0.65 (0.14) | 0.65 (0.16) | p < .05 | |
| Maximum Speed | 1.75 (0.31) | 1.63 (0.26) | 1.67 (0.40) | ns | |
| Peak Vertical Velocity (cm/s) | 1 cm | 6.49 (2.81) | 8.12 (3.55) | 6.27 (2.42) | p < .05 |
| 2 cm | 9.79 (3.98) | 12.14 (5.62) | 10.30 (4.05) | ns | |
| 4 cm | 15.57 (6.65) | 20.59 (9.55) | 15.95 (6.55) | p < .05 | |
| Sentence | 6.21 (2.04) | 6.09 (1.96) | 7.44 (1.99) | ns | |
| Maximum Speed | 17.22 (4.79) | 19.67 (5.35) | 17.77 (4.83) | ns | |
| Velocity Scaling (cm/s/cm) | 1-4 cm | 3.43 (1.79) | 5.10 (2.72) | 4.09 (2.31) | p < .05 |
| Average Normalized Jerk | 1 cm | 48.40 (82.43) | 18.74 (17.78) | 17.38(14.70) | p < .0001 |
| 2 cm | 58.35 (127.47) | 22.49 (19.12) | 21.08 (23.64) | ns | |
| 4 cm | 52.45 (82.47) | 18.88 (12.90) | 22.06 (31.91) | p < .001 | |
| Sentence | 43.59 (34.76) | 35.50 (22.29) | 20.16 (11.41) | p < .001 | |
| Maximum Speed | 24.70 (51.33) | 8.55 (0.92) | 9.81 (10.30) | ns | |
The complex interaction between handedness and group for some handwriting kinematic variables was not explored in the present study. However, exploratory analyses revealed that SZ patients exhibited less asymmetry than healthy participants. In healthy participants, the asymmetry led to faster movements for the dominant compared to nondominant hand across all conditions tested; whereas this hand dominance effect was not observed for SZ participants.
Results from the analyses of variance (ANOVA) for the 1-cm conditions indicated significant group differences for vertical size, F(2, 219) = 8.71, p < .001, peak vertical velocity, F(2, 219) = 3.89, p < .05, and average normalized jerk, F(2, 219) = 10.30, p < .0001. Post-hoc analyses indicated that medicated patients exhibited larger normalized jerk scores and lower peak velocities than either unmedicated patients or healthy participants (p < .05). In contrast, unmedicated patients exhibited larger peak velocities than healthy control participants (p < .05). This is related to the larger stroke heights in the unmedicated patients than in the healthy participants (p < .05).
Similarly, the 4-cm conditions show significant group differences for vertical size, F(2, 218) = 7.68, p < .001, peak vertical velocity, F(2, 218) = 4.02, p < .05, and average normalized jerk, F(2, 217) = 8.20, p < .001. Post-hoc analyses indicated that medicated patients exhibited again larger normalized jerk scores than either unmedicated or healthy participants. The unmedicated patients exhibited higher vertical velocities than medicated or healthy participants. Medicated patients exhibited larger vertical stroke heights than healthy participants, though. In contrast to the 1 and 4-cm conditions, no significant group differences were found for the 2-cm conditions.
There was a significant group effect for velocity scaling, F(2, 219) = 4.42, p < .05. Post-hoc analyses indicated that medicated patients exhibited significantly lower VS scores than either unmedicated or healthy participants.
For sentence writing, we found a significant group effect, but this effect was only for peak vertical velocity, F(2, 71) = 3.42, p < .05, and average normalized jerk, F(2, 71) = 9.06, p < .001. Post-hoc analyses indicated that either patient group exhibited lower movement velocities than the healthy participants. Medicated patients exhibited higher normalized jerk scores than unmedicated or healthy participants. No significant group effects were found for the maximum speed overlaid circles with dominant and non-dominant hands.
Figs. 1-4 show group means (with 95th confidence intervals) for each of the four kinematic variables for each handwriting conditions. Figs. 1 and 2 (vertical size and peak velocity) show that all three groups demonstrated some degree of velocity scaling (VS). Movement velocity increased in proportion to movement size. However, as shown in Fig. 3, the VS for medicated patients was significantly lower than the VS for the unmedicated and healthy participant groups. Fig. 4 shows that average normalized jerk was consistently elevated in the risperidone-treated patients compared to the other groups. As these patient groups had the same clinical severity, these results are likely attributed to antipsychotic treatment.
Fig. 1.
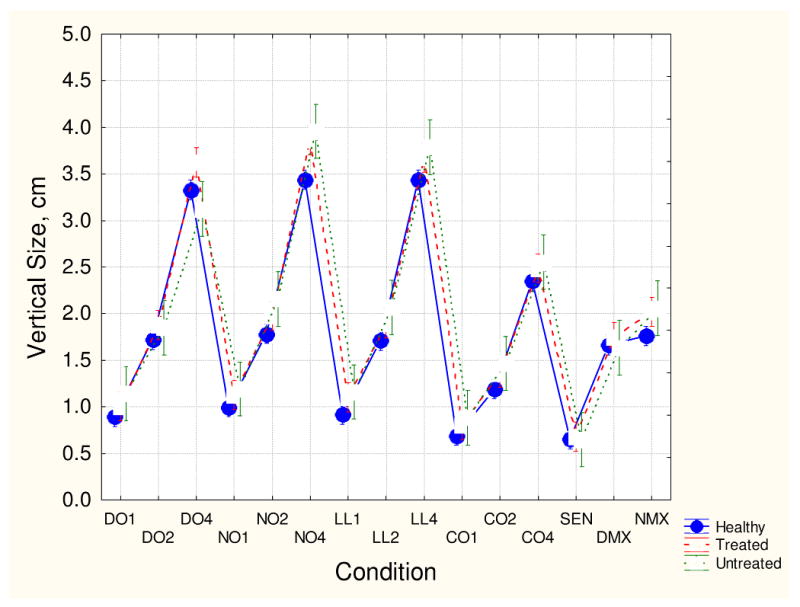
Mean vertical stroke size and 95th confidence intervals for the three participant groups for each handwriting conditions. The handwriting conditions are coded as D0 for dominant hand overlapping loops, N0 for non-dominant overlapping loops, LL for left-to-right loops, C0 for complex loops, SEN for sentence, and DMX and NMX for dominant and nondominant hand maximum speed overlaid circles. The numbers 1, 2, and 4 represent 1 cm, 2 cm, and 4 cm targets, respectively.
Fig. 4.
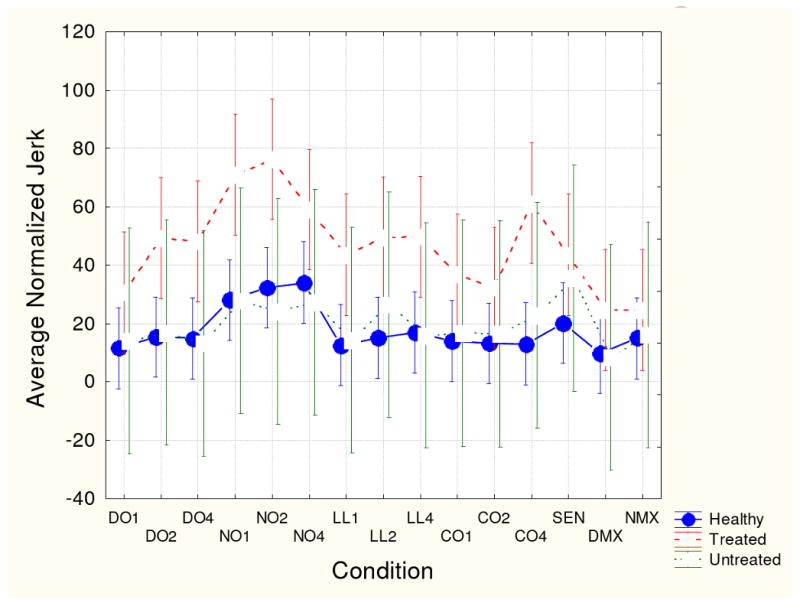
Mean average normalized jerk score and 95th confidence intervals for the three participant groups for each handwriting task. See Fig. 1 for key to conditions.
Fig. 2.
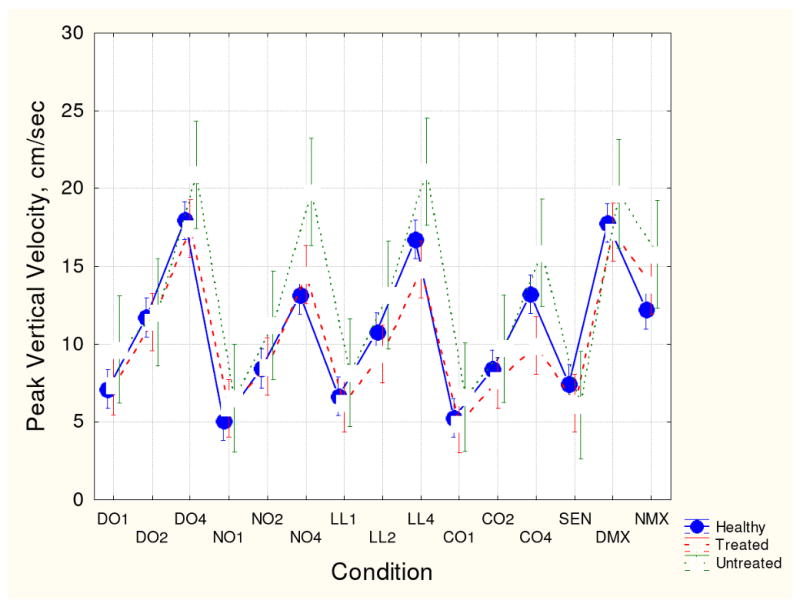
Mean peak vertical velocity and 95th confidence intervals for the three participant groups for each handwriting task. See Fig. 1 for key to conditions.
Fig. 3.
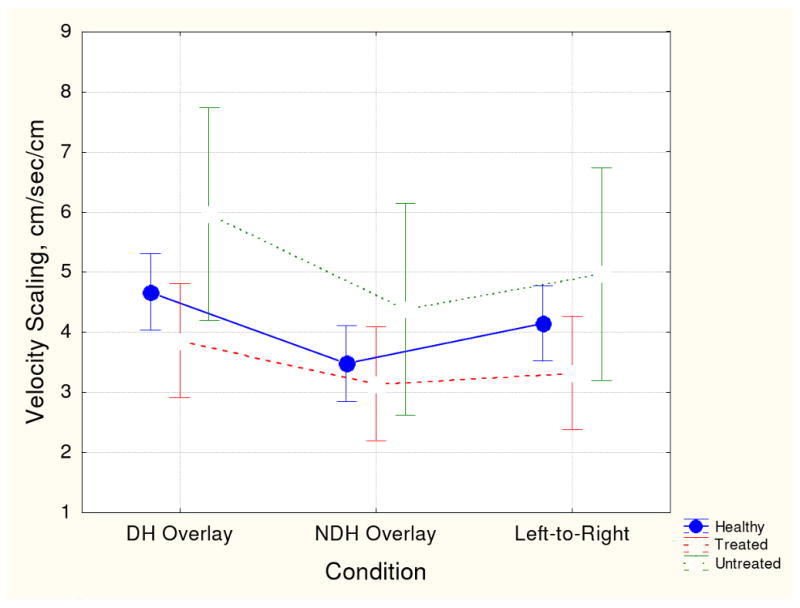
Mean velocity scaling score and 95th confidence intervals for the three participant groups for each handwriting task. See Fig. 1 for key to conditions.
3.2 Dose Effects
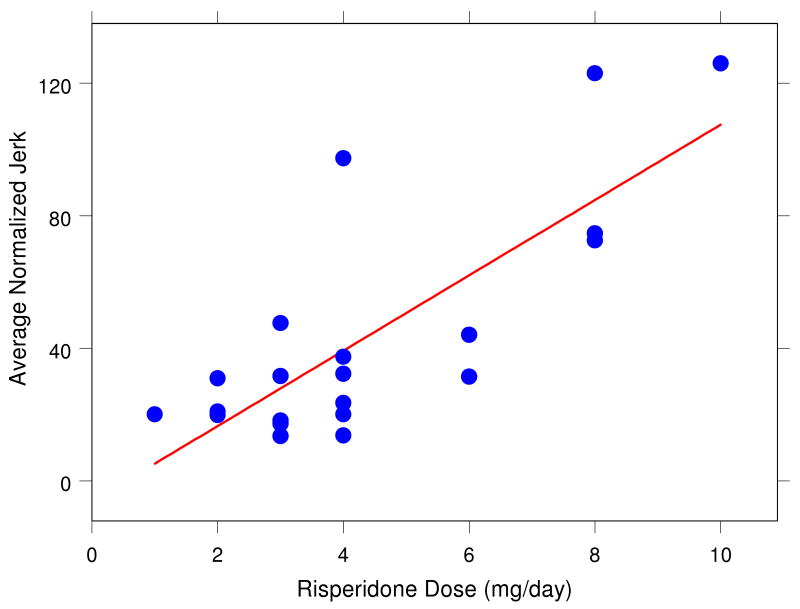
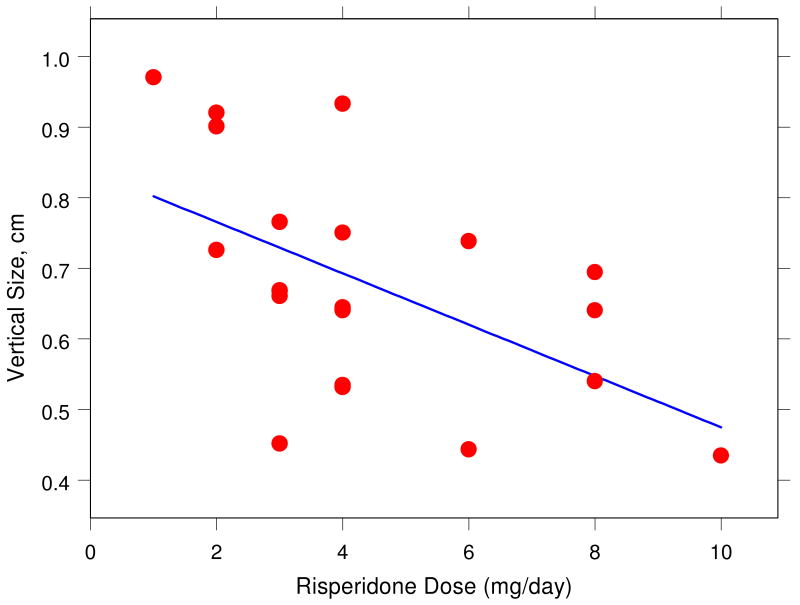
We examined whether the daily dose of risperidone results in changes in the observer-based EPS severity ratings and in handwriting kinematic variables. Figs. 5 and 6 illustrate that, for example, average normalized jerk and vertical size in the sentence-writing task systematically change with daily dose of risperidone. Careful analyses revealed that vertical size decreased with increasing dose in the 4 cm left-to-right loops (r = -.45, p < .05) and in the sentence (r = -.54, p < .01) conditions. Furthermore, peak vertical velocity decreased with increasing dose for the sentence condition (r = -.45, p < .05). Most clearly, average normalized jerk (ANJ) increased with dose in the sentence condition (r = .78, p < .0001). Each of these changes in kinematic variables is a sign of increased EPS. A multiple-regression analysis indicated that these three variables together accounted for as much as 83% (r = .91) of the variability in dose, F(3, 17) = 27.27, p < .00001.
Fig. 5.
Relationship between average normalized jerk score during sentence writing and daily dose of risperidone (in mg) for 21 medicated patients (r = .78, p < .0001).
Fig. 6.
Relationship between vertical stroke size during sentence writing and daily dose of risperidone (in mg) for 21 medicated patients (r = -.54, p < .01).
In contrast, there were no significant correlations (p > .05) between the observer-based severity ratings and risperidone dose. This supports the notion that objective handwriting measures are better suited to detect dose-related EPS than the conventional clinical severity ratings.
4. Discussion
This study produced several important findings. First, that risperidone-treated schizophrenia patients exhibit significant motor impairment and that these impairments can be readily quantified using a naturalistic measure of handwriting movements recorded by a digitizing tablet. Second, our results show that in a sentence-writing task, the severity of motor impairment as measured by three handwriting kinematic variables is significantly related to the average daily dose of risperidone. Specifically, decreased vertical stroke height, decreased peak vertical velocity and increased average normalized jerk (all signs of EPS) account for 83% of the variability in daily risperidone dose. In contrast, conventional, observer-based EPS assessments appeared not able to detect differences between risperidone-medicated and unmedicated groups or a relationship with risperidone dose. These findings suggest that handwriting quantification could play a crucial role in the clinical setting when managing troublesome EPS and can offer valuable support for the clinician's decision to switch antipsychotics or alter dosage (cf. Lambert, 2007).
Schizophrenia (SZ) patients differed from healthy participants on each of the four kinematic variables in this study (vertical size, peak vertical velocity, velocity scaling, and average normalized jerk). Specifically, SZ patients exhibited increased vertical size and peak velocity in the 1 and 4-cm loop tasks, decreased vertical velocity on the sentence task, decreased velocity scaling, and increased average normalized jerk on the 1 and 4-cm loop tasks and sentences. These findings confirm that SZ patients exhibit fine motor abnormalities as measured by handwriting kinematics.
To be useful for the assessment of medication effects, handwriting measures need to distinguish medicated from unmedicated patients. Two of our variables met this criterion. Medicated patients exhibited lower velocity scaling scores and lower movement velocities (for the 1 and 4-cm loops) than unmedicated patients. Because the medicated and unmedicated patient groups were comparable in terms of severity of psychosis and depression these differences in handwriting kinematics cannot be attributed to differences in psychopathology. Further support for the sensitivity of our handwriting procedures to detect movement abnormalities associated with antipsychotic medication comes from the strong association between handwriting kinematics during sentence production and daily dose of risperidone. In contrast, the observer-based EPS assessments appeared not sensitive to the medication status of our SZ patients, as they did not show a relationship with daily dose of risperidone.
We observed differences between unmedicated SZ patients and healthy participants on some handwriting kinematic variables (e.g. ANJ). While unmedicated SZ patients exhibited smoother handwriting movements compared to medicated patients for sentence production, their handwriting movements were more dysfluent than healthy participants confirming a mild hyperkinetic movement disorder. These findings are in agreement with the well-established reports of mild EPS (particularly of the hyperkinetic form) among unmedicated and never-medicated SZ patients (Cortese, et al. 2005) and the non-linear scale of the observer ratings. Previous studies have shown that 10-15% of SZ patients exhibit some form of EPS prior to exposure to antipsychotic medication while, the prevalence seems to be even higher when using the more sensitive instrumental measures (Caligiuri, Lohr, & Jeste, 1993; Cortese et al., 2005).
The present study included 15 handwriting tasks, designed to inform us about potential effects of task complexity, handedness, magnitude of movement, and meaningfulness on performance. For the purpose of assessing medication effect, this set of 15 can be reduced to the 1, 2, and 4-cm loops and a sentence, each replicated 3 times. We obtained our results from the standard sentence “Today is a nice day”. We have no reasons to assume that different sentences would yield other results. These four tasks yield 4 key kinematic variables: vertical size, peak vertical velocity, velocity scaling and average normalized jerk demonstrated to characterize increased EPS as a function of diagnosis and antipsychotic medication. On the other hand, the complex loops, overlaid circles drawn with the non-dominant hand, and overlaid circles drawn at maximum speed appeared not useful for the quantification of EPS.
The present study forms a first step towards identifying key variables that distinguish SZ patients from healthy participants, medicated from unmedicated patients, and different dosages of risperidone. Follow-up studies will be needed to further establish that handwriting movement measures can help identify patients at risk of developing EPS. Longitudinal studies that follow patients throughout changes in medication are ongoing and will help address the question of whether handwriting quantification can detect change in medication-induced EPS.
Acknowledgments
This research was supported by NIH grant R44 MH073192.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophrenia Research. 1990;3:247–251. doi: 10.1016/0920-9964(90)90005-r. [DOI] [PubMed] [Google Scholar]
- Caligiuri MP, Lohr JB, Jeste DV. Parkinsonism in neuroleptic-naive schizophrenic patients. American Journal of Psychiatry. 1993;150:1343–1348. doi: 10.1176/ajp.150.9.1343. [DOI] [PubMed] [Google Scholar]
- Caligiuri MP, Jeste DV, Lacro JP. Antipsychotic-induced movement disorders in the elderly: epidemiology and treatment recommendations. Drugs and Aging. 2000;17:363–384. doi: 10.2165/00002512-200017050-00004. [DOI] [PubMed] [Google Scholar]
- Caligiuri MP, Teulings HL, Filoteo JV, Song D, Lohr JB. Quantitative measurement of handwriting in the assessment of drug-induced parkinsonism. Human Movement Science. 2006;25:510–522. doi: 10.1016/j.humov.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Cortese L, Caligiuri MP, Malla AK, Manchanda R, Takhar J, Haricharan R. Relationship of neuromotor disturbances to psychosis symptoms in first-episode neuroleptic-naive schizophrenia patients. Schizophrenia Research. 2005;75:65–75. doi: 10.1016/j.schres.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Gallucci RM, Phillips JG, Bradshaw JL, Vaddadi KS, Pantelis C. Kinematic analysis of handwriting movements in schizophrenic patients. Biological Psychiatry. 1997;41:830–833. doi: 10.1016/S0006-3223(96)00544-6. [DOI] [PubMed] [Google Scholar]
- Gerken A, Wetzel H, Benkert O. Extrapyramidal symptoms and their relationship to clinical efficacy under perphenazine treatment. A controlled prospective handwriting-test study in 22 acutely ill schizophrenic patients. Pharmacopsychiatry. 1991;24:132–137. doi: 10.1055/s-2007-1014456. [DOI] [PubMed] [Google Scholar]
- Haase HJ. Extrapyramidal modification of fine movements: a “conditio sine qua non” of the fundamental therapeutic action of neuroleptic drugs. Review of Canadian Biology. 1961;20:425–449. [PubMed] [Google Scholar]
- Haase HJ. The purely neuroleptic effects and its relation to the “neuroleptic threshold”. Acta Psychiatrica Belgica. 1978;78:19–36. [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kuenstler U, Juhnhold U, Knapp WH, Gertz HH. Positive correlation between reduction in handwriting area and D2 dopamine receptor occupancy during treatment with neuroleptic drugs. Psychiatry Research. 1999;90:31–39. doi: 10.1016/s0925-4927(98)00054-7. [DOI] [PubMed] [Google Scholar]
- Lambert TJ. Switching antipsychotic therapy: what to expect and clinical strategies for improving therapeutic outcomes. Journal of Clinical Psychiatry. 2007;68 6:10–13. [PubMed] [Google Scholar]
- Teulings HL, Maarse FJ. Digital recording and processing of handwriting movements. Human Movement Science. 1984;3:193–217. [Google Scholar]
- Teulings HL, Contreras-Vidal JL, Stelmach GE, Adler CH. Coordination of fingers, wrist, and arm in Parkinsonian handwriting. Experimental Neurology. 1997;146:159–170. doi: 10.1006/exnr.1997.6507. [DOI] [PubMed] [Google Scholar]