Abstract
Poly(lactic-co-glycolic acid) (PLGA) is one of the more widely used polymers for biomedical applications. Nonetheless, PLGA lacks chemical moieties that facilitate cellular interactions and surface chemistries. Furthermore, incorporation of hydrophilic molecules is often problematic. The integration of polymer functionalities would afford the opportunity to alter device characteristics, thereby enabling control over drug interactions, conjugations, and cellular phenomena. In an effort to introduce amine functionalities and improve polymer versatility, we synthesized two block copolymers (PLGA-PLL 502H and PLGA-PLL 503H) comprised of PLGA and Poly(ε-carbobenzoxy-L-lysine) utilizing dicyclohexyl carbodiimide (DCC) coupling. PLGA-PLL micropsheres encapsulated approximately six-fold (502H) and three-fold (503H) more vascular endothelial growth factor (VEGF), and 41% (503H) more ciliary neurotrophic factor (CNTF) than their PLGA counterparts. While the amine functionalities were amenable to the delivery of large molecules and surface conjugations, they did not comprise polymer biocompatibility. With the versatile combination of properties, biocompatibility, and ease of synthesis, these block copolymers have the potential for diverse utility in the fields of drug delivery and tissue engineering.
Keywords: Block Copolymers, PLGA, Poly(amino acid), CNTF, VEGF
1. INTRODUCTION
The common polyester biomaterials, poly(lactic acid)(PLA), poly(glycolic acid)(PGA) and their copolymer poly(lactic-co-glycolic acid)(PLGA), lack chemical functionalities to elicit specific drug or cell interactions. Furthermore, their potential for the sustained delivery of hydrophilic molecules (i.e. proteins) is often limited [1]. This frequently results in poor encapsulation and a large “burst” release of the encapsulated drug within the first few hours or days [1–3]. This initial burst is due to desorption of surface associated hydrophilic molecules on devices comprised of hydrophobic polymers, not polymer degradation [1]. When introduced to an aqueous environment, these surface absorbed molecules are readily dissociated. These undesirable outcomes are often due to poor associations between the drug and polymer. In an effort to optimize these phase or electrostatic associations, many utilize alternative materials [4]. However, these alternatives present their own limitations (i.e. immunogenicity) [5]. To circumvent these limitations and establish therapeutic efficacy, large doses or site-specific administration are often required of devices comprised of the polyester biomaterials [6, 7].
Furthermore, these polymers lack functionalities to promote cellular phenomena such as adhesion and proliferation. Many have achieved cellular interactions or cell specific drug targeting through the addition of ligands to device surfaces [7–9]. However, a key shortcoming not enabling this addition to these polyesters, is the lack of functional groups on the aliphatic backbones, limiting the number of sites for potential incorporation and conjugation of biologically active molecules [7]. In an attempt to address these inadequacies, numerous groups have introduced functional groups to these degradable polyesters either through direct conjugation [10, 11], or with additives during device fabrication [7, 12].
Modifications of particular interest have included the addition of poly(amino acids). The incorporation of a poly(amino acid) allows one to tailor device characteristics such as hydrophilicity and surface charge while providing sites for conjugation and cellular interactions [13–15]. Much of this research has previously focused around PLA [10, 13, 14, 16]. Researchers have noted that devices comprised of a poly(L-lysine)(PLL)/PLA conjugate, have significantly improved protein encapsulation and facilitated surface conjugations over their PLA equivalents [16, 17]. However, with the hydrophobicity of PLA, its potential for drug delivery and tissue engineering applications is limited [18, 19]. In addition to a relatively complex conjugation scheme (ring-opening polymerization), a simpler technique was desired.
PLGA is a staple of drug delivery and tissue engineering [18, 20]. Not only is PLGA used in commercially available and FDA approved devices, but simple variations can alter the polymer’s rate of degradation and release of encapsulated agents [21, 22]. Because of these attributes, many have utilized PLGA in their efforts of controlled and site-specific drug delivery [23]. Lavik et al. found that by using dicyclohexyl carbodiimide (DCC) coupling, one is able to easily and efficiently conjugate PLL and PLGA,[24] thereby circumventing the aforementioned limitations of PLA.
However, a fundamental issue with incorporating PLL into biomaterials is the potential for cytotoxicity [25, 26]. It is well established that polycations, in general are toxic [25, 27]. This risk requires care to be taken when adding or modifying functional groups, as this can compromise the biocompatibility of a material or device [28]. Therefore, potential toxicity for any inclusion of PLL in a biomaterial must be thoroughly investigated.
Therefore, we looked to determine the potential of incorporating PLL and PLGA. We synthesized two block copolymers (PLGA-PLL 502H and PLGA-PLL 503H) comprised of PLGA (502H:Mn ~ 10 kDa or 503H:Mn ~ 25 kDa, respectively) and Poly(ε-carbobenzoxy-L-lysine)(Mw ~ 1000 Da). We hypothesized that the conjugation of PLL with PLGA would not only prove advantageous, as was the case with PLA, but the addition of amine functionalities would not sacrifice the biocompatibility and properties that PLGA is favored for.
Here we present a functionalized PLGA via synthesis of an amphipathic block copolymer comprised of PLGA and Poly(ε-carbobenzoxy-L-lysine). Conjugation of Poly(ε-carbobenzoxy-L-lysine) to different PLGA polymers introduced amine functionalities that significantly enhanced the incorporation of vascular endothelial growth factor A165 (VEGF) and ciliary neurotrophic factor (CNTF) in microspheres. These added functionalities enabled conjugations and their availability was explored via surface coupling of fluorescein isothiocyanate (FITC). By developing these block copolymers and establishing their biocompatibility both in vitro and in vivo, we were able to create a construct amendable for numerous biomedical applications while preserving the desired attributes of PLGA.
2. MATERIALS AND METHODS
2.1. Materials
Poly(lactic-co-glycolic acid) (PLGA) 502H (50:50 lactic to glycolic acid ratio and a Mn ~ 10 kDa) and PLGA 503H (50:50 lactic to glycolic acid ratio and a Mn ~ 25 kDa) were from Boehringer Ingelheim (Ingelheim, Germany). H signifies PLGA terminated with a carboxylic acid group. Poly(vinyl alcohol) (PVA) (88 mol% hydrolyzed) was from Polysciences (Warrington, PA, USA). Poly(ε-carbobenzoxy-L-lysine) (Mw ~1000 Da) was from Sigma (St. Louis, MO, USA). The Micro bicinchoninic (BCA) protein assay kit was from Pierce (Rockford, IL, USA). The enzyme-linked immunosorbent assays (ELISA DuoSet), vascular endothelial growth factor A165 (VEGF) and ciliary neurotrophic factor (CNTF) were from R&D Systems (Minneapolis, MN, USA). Deuterated dimethyl sulfoxide (D6 –DMSO) was from Camrbidge Isotope Laboratories, Inc. (Andover, MA, USA). All other chemicals were used as received from Sigma.
2.2. Synthesis and characterization of block copolymer
2.2.1. Conjugation
The block copolymers (PLGA-PLL 502H and PLGA-PLL 503H) were synthesized according to Lavik et al [24]. Briefly, PLGA 502H or PLGA 503H and Poly(ε-carbobenzoxy-L-lysine) (1:1 molar ratio) were dissolved in dimethyl formamide (DMF). Two molar equivalents (with respect to PLGA) of dicyclohexyl carbodiimide (DCC) and 0.1 molar equivalents of (dimethylamino-pyridine) DMAP were added and the reaction was allowed to run for 36 h under argon. Following conjugation of PLGA and Poly(ε-carbobenzoxy-L-lysine), the polymer solution was diluted with chloroform and filtered to remove N,N′-dicyclohexylurea (DCU), an insoluble by-product of the reaction. The presence of DCU was indicative of successful conjugation. The block copolymer was then precipitated in methanol, vacuum filtered and rinsed with ether to remove any unconjugated Poly(ε-carbobenzoxy-L-lysine), and lyophilized for at least 48 h.
2.2.2. Deprotection
To expose primary amines, ~1.5 g of the block copolymer was dissolved in hydrogen bromide (HBr), 30 wt% in acetic acid (HBr/HOAc) and allowed to stir. After 1.5 h, ether was added to the solution and the precipitated polymer was removed. The polymer was washed with ether until an off-white brittle mass was obtained. The mass was then dissolved in chloroform, re-precipitated in ether and lyophilized for 48 h.
2.2.3. Characterization
Successful conjugation/deprotection was verified using ultraviolet-visible spectroscopy (UV-vis) (Cary 50 Bio UV-Vis Spectrophometer, Varian, Palo Alto, CA, USA). At 257 nm, the protecting carbobenzoxy (CBZ) group can be visualized. Presence of this CBZ group prior to deprotection verifies successful conjugation while its later absence is indicative of successful deprotection and thus amine exposure. 1H NMR was also utilized for determining successful conjugation/deprotection of PLGA-PLL. 1H NMR spectra were recorded at room temperature in D6 -DMSO on a 400 MHz Bruker (Germany) spectrometer and referenced to tetramethylsilane (TMS) peak (δ = 0.0 ppm). To ensure that conjugation was responsible for the presence of peaks associated with the CBZ group, and that unconjugated Poly(ε-carbobenzoxy-L-lysine) could successfully be removed, the reaction was carried out in the absence of DCC and DMAP. In this instance, no CBZ peaks were observed; indicating successful conjugation was necessary for the presence of CBZ peaks and ultimately amines.
2.3. Nanosphere fabrication
PLGA-PLL nanospheres were fabricated using a solvent evaporation method [23]. Two hundred milligrams of polymer (PLGA or PLGA-PLL) were dissolved in 2 ml of dichloromethane. This polymer solution was added dropwise to a 4 ml vortexing solution of 5% PVA (w/v). The solution was then emulsified via sonication (Tekmar Sonic Disruptor TM300, Mason, Ohio, USA) for 30 s at 38% amplitude. Following sonication, the emulsion was added to 50 ml of 5% PVA (w/v) and allowed to stir harden for 3 h. Nanospheres were then collected by centrifugation, washed three times with deionized water, and freeze dried for 72 h. Nanosphere size was determined using dynamic light scattering (DLS) (ZetaPals particle sizing software, Brookhaven Instruments Corp., Holtsville, NY, USA). We found the average diameters of the PLGA-PLL and PLGA 502H nanospheres to be 333 ± 4 nm and 290 ± 3 nm (mean ± SD), respectively.
2.4. Sponge fabrication
Salt-leached sponges were fabricated via methods described elsewhere [29]. Briefly, polymer (PLGA-PLL or PLGA) in chloroform (10% w/v) was added to Teflon vials with NaCl crystalline particles (size range 250–400 μm). Chloroform was allowed to evaporate and salt was removed by soaking sponges in deionized water. Sponges were then lyophilized for at least 48 h. Sponge and nanosphere morphology was observed via scanning electron microscopy (SEM) (Philips XL-30 environmental).
2.5. Quantification of Amine release
o-Phthaldialdehyde reagent solution (OPA) was used to detect primary amines via fluorescence (360 nm excitation/460 nm emission) on a Synergy HT Multi-detection microplate reader (BioTek Instruments, Winooski, VT, USA) [30].
2.5.1. Amine release from nanospheres and sponges
In 1.5 ml eppendorf tubes, 10 mg of PLGA-PLL (502H or 503H) or PLGA (502H or 503H) nanospheres/sponges were reconstituted with 0.5 mL of phosphate buffered saline (PBS). Mixtures were then incubated at 37°C on a rotating shaker. At specific time points (1 and 5 hours and 1, 3, 7 days, and once every 7 days thereafter until no material was present) the mixture was centrifuged and the supernatant was collected. An equal volume of PBS was then added to replace the withdrawn supernatant and the nanospheres/sponges were resuspended and returned to the shaker. Supernatant amine content for each of the sets of nanospheres/sponges was determined by OPA analysis.
2.5.2. Amine release from sponges and remaining mass
In eight, 1.5 ml eppendorf tubes, 10 mg of PLGA-PLL 502H or PLGA 502H sponge was suspended in 0.5 ml of PBS and incubated at 37°C. At eight predetermined time points (1, 3, 7, 14, 21, 28, 35 and 42 days) an eppendorf was removed, the supernatant extracted, and the remaining polymer was lyophilized for 24 h. The dry mass was later recorded and the supernatant amine content was determined via OPA analysis.
2.6. FITC conjugation to nanospheres
Fluorescein isothiocyanate (FITC) was initially dissolved in DMSO at 1 mg/ml and diluted to 0.5 mg/ml with 0.1M carbonate buffer (pH 9.7). Three milligrams of either PLGA-PLL 502H, PLGA-PLL 503H or PLGA 502H nanospheres were reconstituted in 1 ml of carbonate buffer in 1.5 ml eppendorf tubes and 0.1 ml of 0.5 mg/ml FITC solution was added. Samples were done in triplicate. Eppendorf tubes were covered and placed on an orbital shaker for 6 h at room temperature. Samples were then collected via centrifugation and washed with carbonate buffer three times. Samples were immediately reconstituted with 1 ml of carbonate buffer and were then analyzed for surface bound FITC (485 nm excitation/528 nm emission)(Initial Conjugation). The remaining volume was placed on an orbital shaker and allowed to degrade. After 24 h, samples were centrifuged and supernatants withdrawn. Nanospheres were reconstituted with an equal volume (as supernatant) of carbonate buffer. Supernatants and reconstituted nanospheres were then analyzed for FITC content (1 day Degradation and 1 day Supernatant). Values were then combined and compared to those from Initial Conjugation. PLGA 502H nanospheres were included for determining nanosphere autofluorescence and non-specifically adsorbed FITC.
2.7. Cell Viability
In vitro cytotoxicity of PLGA-PLL and PLGA 502H nanospheres was accessed using two primary cell types; rat endothelial cells (RECs) isolated from epididymal fat pad and human umbilical vein endothelial cells (HUVECs). RECs were maintained as described by Madri et al. and were not used after 12 passages [31]. RECs were cultured in medium containing Dulbecco’s Modified Eagle’s Medium (DMEM), 10% fetal bovine serum (FBS), 5% bovine aortic endothelial cell conditioned medium, 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 1mM sodium pyruvate, 1mM L-glutamine, and 1% penicillin/streptomycin. HUVECs were maintained in M199 medium containing 20% FBS, 2 mM L-glutamine, 100 units/mL penicillin, 100 μg/ml streptomycin, and 50 μg/ml endothelial cell growth supplement (ECGS) and were not used after five passages.
The (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (MTT) colorimetric assay of cell viability was utilized for determining cytotoxicity [32]. RECs (24 h: 12,500 cells/well; 72 h: 6,250 cells/well) or HUVECs (24 h: 10,000 cells/well; 72 h: 5,000 cells/well) were seeded in 24-well plates with 1 ml of respective media and allowed to adhere overnight. Plates used for RECs were coated first with 1.5% gelatin and rinsed with 1X PBS. Lower cell concentrations were used for 72 h incubation to eliminate the potential of overconfluency. For each plate a five point standard curve (100,000, 50,000, 10,000, 5,000, and 0 cells/well) was used for final determination of cell concentration. Following overnight adherence, transwell inserts were introduced with specified amounts of PLGA-PLL or PLGA 502H nanospheres in 0.5 ml of respective media. For controls and standards, transwells with media only were used. Plates were allowed to incubate for 24 or 72 h with transwell inserts before determining cell survival. All samples, excluding standards, were done in triplicate.
2.8. Subcutaneous implants
Eight 8- to 12-week-old male C57 black mice (C57BL/6) (Charles River Laboratories, Wilmington, MA, USA) were used in accordance with procedures approved by Animal Care and Use Committees of Yale University. Following induced anesthesia, an incision was made through the skin overlying the thoracic spine and two subcutaneous pockets were created bilaterally. Ten-millimeter sponges comprised of PLGA-PLL or PLGA 503H were cut with a cork borer. Sponges were then soaked in 70% ethanol for 30 min followed by a 6-point, 2-fold serial dilution rinse (ethanol:water). Two sponges from the same group were implanted in each mouse, one per pocket. The incision was closed with surgical clips. After 2 or 4 weeks, sponges were excised for histological analysis. Excised implants were fixed in 10% buffered formalin overnight, processed and paraffin embedded. Three of the four implanted sponges from each group were then sectioned (three 5-μm sections per sample) and stained with Masson’s trichrome. Collagen capsule thickness was measured at 15 different locations (five per sample) by a blinded observer. Images/measurements were taken on a Zeiss Axiovert 200 microscope (Zeiss, Thornwood, NY, USA).
2.9. VEGF and CNTF microspheres
Growth factors (GF), VEGF or CNTF, were encapsulated in PLGA-PLL or PLGA via similar methods described by Faranesh et al [33]. PLGA-PLL or PLGA was dissolved in dichloromethane at 100 mg/ml. Two hundred microliters of a 100 μg/ml GF:BSA (1:50) (20 μg GF:1000 μg BSA total) solution in PBS was added dropwise to 1ml of the polymer solution while vortexing. Five milliliters of a 1% (w/v) PVA with 5% (w/v) NaCl solution were subsequently added to the primary emulsion while vortexing. The resulting emulsion was poured into 40 ml 0.3% (w/v) PVA 5% (w/v) NaCl solution and stir hardened for 3 h. The particles were collected via centrifugation, washed three times with distilled water, and lyophilized. A GF:BSA mixture was used due to BSA’s stabilizing and protective properties during GF encapsulation [34–36]. Blank batches were prepared with 0.2 ml of PBS (without GF:BSA).
Microsphere protein loading was determined using a Micro BCA colorimetric assay [37]. Briefly, 10 mg of microspheres were added to 0.3 ml dichloromethane and allowed to dissolve for 30 min at 37° C. Following microsphere dissolution, 1 ml of PBS was added, vortexed, and allowed to phase separate at 37° C overnight. The aqueous phase was then extracted and protein content was determined using a Micro BCA assay [38]. Analysis of PLGA and PLGA-PLL blank microspheres were included for background/polymer absorbance.
VEGF and CNTF release from microspheres was determined in the following manner. In 1.5 ml eppendorf tubes, 10 mg of PLGA-PLL or PLGA 503H microspheres (GF:BSA or PBS alone) were reconstituted with 1 ml of PBS. Samples were prepared in triplicate. Mixtures were then incubated at 37°C on a rotating shaker. At specific time points (1 and 5 hours and 1, 3, 7 days, and once every 7 days there after until no pellets were present) the mixture was centrifuged and the supernatant was collected. An equal volume of PBS was then added to replace the withdrawn supernatant and the microspheres were resuspended and returned to the shaker. Immunoactive supernatant VEGF or CNTF concentrations were determined with an ELISA. Total VEGF/CNTF encapsulation was defined as the total amount of GF released, as determined by ELISA from release studies.
The volume-weighted mean diameter of VEGF and CNTF microspheres from each batch was determined using a Beckman Coulter Multisizer 3 (Fullerton, CA, USA) with a 200 μm diameter aperture based on a sample size of 2,000 microspheres. The mean diameter of PLGA-PLL and PLGA 503H VEGF microspheres were found to be 36 ± 22 μm and 50 ± 34 μm (mean ± SD), PLGA-PLL and PLGA 502H VEGF micropsheres 48 ± 20 μm and 57 ± 25μm, and PLGA-PLL and PLGA 503H CNTF microspheres 54 ± 23 μm and 60 ± 23 μm, respectively.
2.10. Statistics
All experiments were done in triplicate and data are presented as means ± standard error of the mean. Data were analyzed by using a one-way analysis of variance (ANOVA) followed by the Student-Newman-Keuls test for determining differences between groups. Differences were accepted as statistically significant with P < 0.05. Relations between measurements were analyzed by Pearson correlation coefficient (r).
3. RESULTS
3.1. Synthesis and characterization of block copolymer
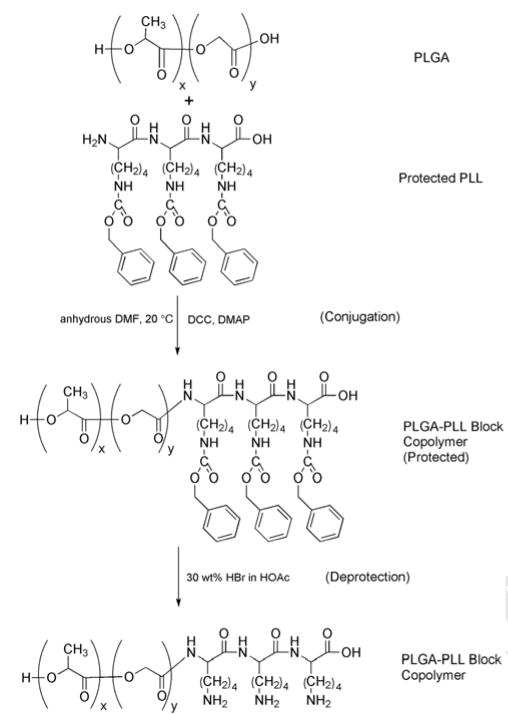
As seen in Scheme 1, Poly(ε-carbobenzoxy-L-lysine) was conjugated to PLGA via DCC coupling [39]. 1H NMR and UV-vis spectroscopy were utilized to demonstrate successful conjugation as well as deprotection of coupled PLGA-PLL. As seen in Figure 1, the benzene ring peak associated with the CBZ protecting groups (δ = 7.3 ppm) is clearly present in the protected PLGA-PLL while successfully removed in the deprotected form [24, 40].
Scheme 1.
Synthesis of PLGA-PLL block copolymer. DMAP: 4-dimethylaminopyridine; DMF: dimethylformamide.
Figure 1.
1H NMR spectra. Peak at δ = 7.3 ppm is associated with the benzene ring of the protecting CBZ group. The presence of this peak (PLGA-PLL (protected)) indicates successful conjugation of PLGA and Poly(ε-carbobenzoxy-L-lysine). Note the absence of this peak following deprotection (PLGA-PLL (deprotected)).
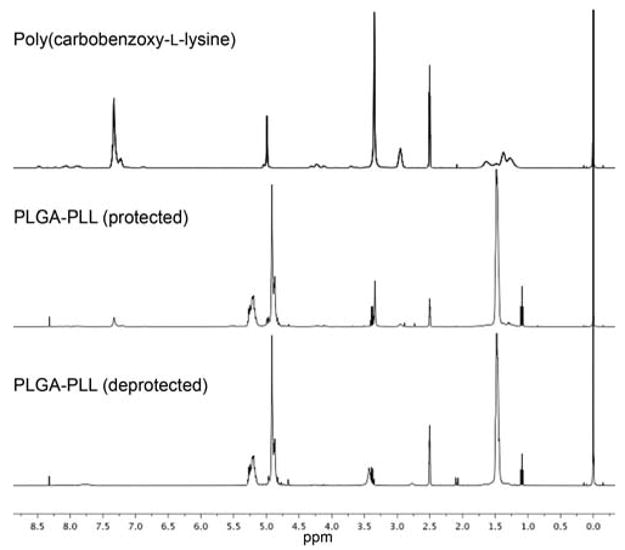
Furthermore, it is evident by the presence of the CBZ group peaks around 257 nm that PLGA and Poly(ε-carbobenzoxy-L-lysine) were successfully conjugated (Figure 2). Utilizing UV-vis analysis, the conjugation efficiency of PLGA 502H and Poly(ε-carbobenzoxy-L-lysine) was determined. Based on a standard curve of Poly(ε-carbobenzoxy-L-lysine) and assuming a 100% conjugation efficiency, the mass of polymer analyzed would have given an optical density of 1. However, as observed in the UV-vis spectrum (Figure 2), the CBZ peak absorbance was ~0.4, indicating ~40% conjugation efficiency. The increased efficiency, as compared to ~ 30% for PLGA-PLL 503H [24], may be attributed to the lower molecular weight of 502H. This results in a greater number of carboxyl groups for a given mass, thus increasing the probability of reaction with DCC.
Figure 2.
UV-vis spectra of the block copolymer PLGA-PLL before (solid line) and after deprotection (dashed line). At 257 nm, the protecting CBZ group can be visualized. Its presence indicates successful conjugation while its subsequent removal exposes amine functionalities.
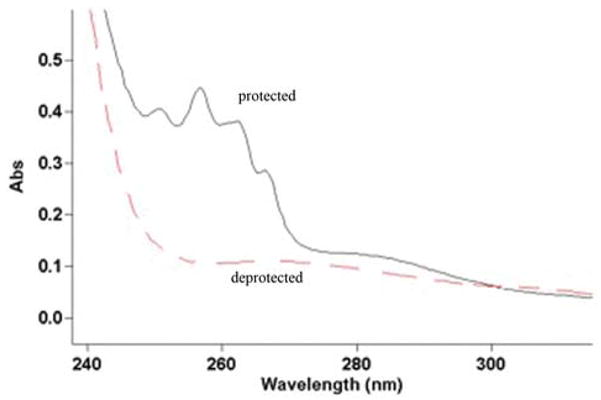
Fabricating nanospheres and salt-leached sponges tested the versatility of PLGA-PLL. As depicted in Figure 3, there were no discernable differences between these and PLGA devices. Efforts to fabricate devices (nano/microspheres and sponges) with a mixture (versus conjugation) of PLL and PLGA, under identical conditions, were aborted due to the insolubility of PLL with the various organic solvents used. This difficulty further illustrates the versatility of our modified PLGA.
Figure 3.
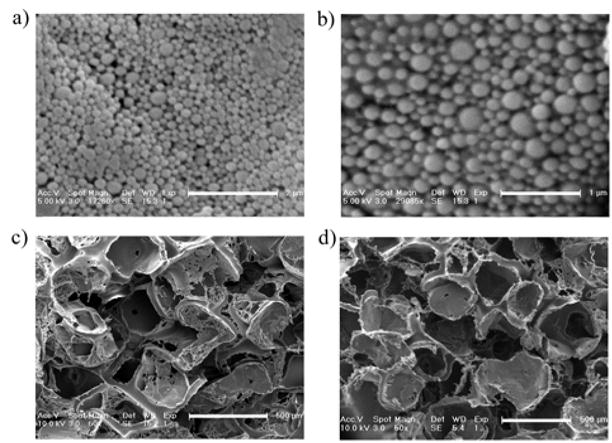
SEM micrographs of nanospheres a) PLGA-PLL 502H, b) PLGA 502H; and salt-leached sponges c) PLGA-PLL 503H, d) PLGA 503H. Scale bars a: 2 μm b: 1 μm, c/d: 500 μm.
3.2. Amine content and degradation of nanospheres and sponges
We further examined the amine content of both sponges and nanospheres comprised of PLGA-PLL using OPA (Figure 4A). There were more amines present in devices comprised of PLGA-PLL 502H versus 503H. This difference can be attributed to a greater conjugation efficiency and lower molecular weight of 502H. When degraded in PBS, a slower release of amines from PLGA-PLL 503H devices was observed in the supernatant, which can be attributed to 503H having a larger molecular weight and thus a slower rate of degradation [41]. Comparison of degradation rates of PLGA-PLL and PLGA 502H sponges in PBS (Figure 4B), and examination of its influence on amine release, revealed a strong correlation between PLGA-PLL degradation and amine release (r = 0.9421, P < 0.0001) (Figure 4C). As seen in Figure 4B, PLGA-PLL 502H had a slight delay in degradation at days 21 and 28. However, by day 35, degradation was equivalent to that of PLGA 502H.
Figure 4.
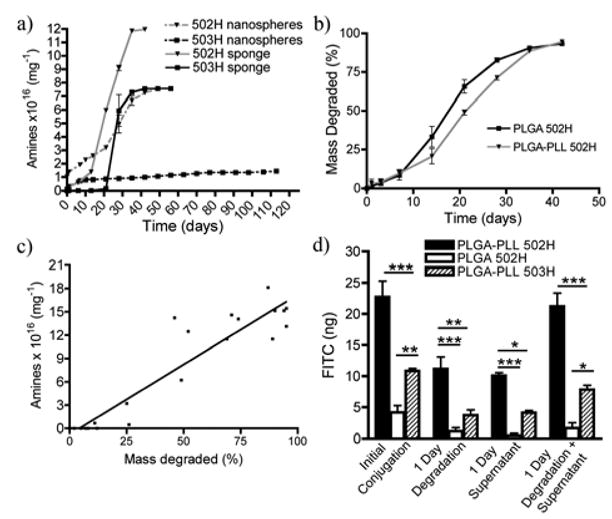
Cumulative release of NH2 from a) nanospheres (dashed lines) and salt-leached sponges (solid lines). b) Degradation of salt-leached sponges (represented as % of total mass degraded). c) Strong correlation (r = 0.9421, P < 0.0001) between amine release and degradation of PLGA-PLL 502H salt-leached sponge. d) Surface conjugation of FITC to PLGA-PLL 502H (black bars), PLGA 502H (white bars), and PLGA-PLL 503H (lined bars) nanospheres. Data (a,b, and d) represent mean ± SEM (n=3). * p < 0.05, ** p < 0.01, *** p < 0.001.
3.3. FITC conjugation to nanospheres
FITC was conjugated to the surface of PLGA-PLL nanospheres to help determine the reactivity and availability of the amine functionalities for further conjugations. There was significantly more FITC conjugated to the PLGA-PLL 502H nanospheres than PLGA-PLL 503H nanospheres (Figure 4D), which correlates with the greater degree of amines present in 502H than 503H (Figure 4A). Following 24-hour degradation in PBS, PLGA-PLL 502H still had significantly more FITC present. After 24 hours a decrease in surface bound FITC was observed, which can be attributed to the rapid loss of amines at early time points (Figure 4A). This loss was later accounted for in the supernatant (Figure 4D).
3.4. Cell viability
RECs and HUVECs were used to test potential polymer toxicity. Cells were exposed to PLGA-PLL 502H or PLGA 502H (1 or 10 mg of nanospheres/1.5 ml of media) for 24 or 72 hours. PLGA-PLL 502H nanospheres were analyzed due to their greater content of amines (Figure 4A). Cell viability was determined using the MTT assay [32]. As seen in Figures 5A-D, there were no statistical differences between cells exposed to no spheres (transwell alone) and to spheres (PLGA-PLL or PLGA) at either concentration or time point assessed.
Figure 5.
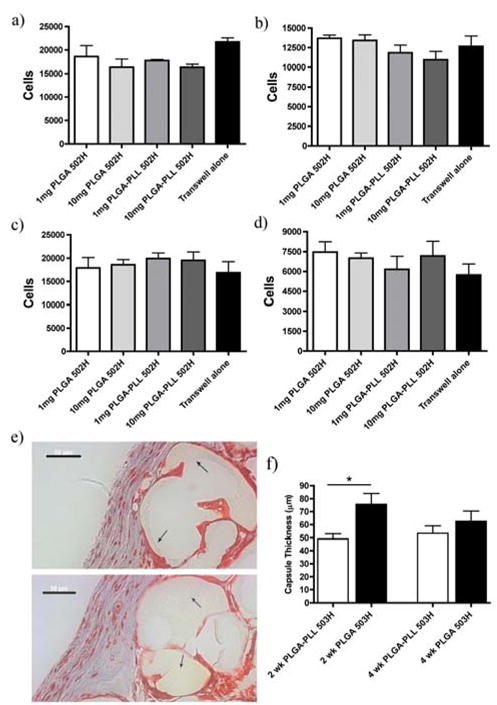
Cyto/Biocompatibility. Evaluation of PLGA or PLGA-PLL 502H nanosphere cell viability via MTT assay on Rat ECs a) 1 day, b) 3 day exposure and HUVECs c) 1, day d) 3 day exposure. e) Masson’s trichrome staining of sponges excised at 2 weeks post implantation. Top/Bottom: PLGA 503H/PLGA-PLL 503H. Arrows demarcate sponge and its location relative to the collagen capsule (blue). Scale bars: 50 μm. f) Quantification of collagen capsule thickness from subcutaneously implanted PLGA503H/PLGA-PLL 503H salt-leached sponges (n=15). All data represent mean ± SEM (n=3 unless stated otherwise). * p < 0.05.
3.5. Subcutaneous implants
Biocompatibility was examined in vivo via subcutaneous implantation of salt-leached sponges in C57/Bl6 mice. At 2 and 4 weeks post implantation, sponges were excised and collagen capsule formation was analyzed using Masson’s trichrome staining (Figure 5E). When implanted in vivo, PLGA elicits an inflammatory response resulting in fibrous capsule formation around the implant [42]. We therefore compared capsule thickness between the PLGA-PLL 503H and PLGA 503H at 2 and 4 weeks. These long-term time points enabled us to examine potential toxicity associated with material contact. As seen in Figure 5F, PLGA-PLL 503H capsule thickness at 2 weeks was less than that of PLGA 503H, and not significantly different at 4 weeks.
3.6. VEGF and CNTF microspheres
We encapsulated VEGF or CNTF in microspheres comprised of PLGA-PLL or PLGA 502H/503H. Shown in Figure 6A and 6C, PLGA-PLL microspheres released significantly more VEGF than their PLGA counterparts. While total VEGF release was approximately six times greater from PLGA-PLL 502H, and three times greater from PLGA-PLL 503H, total protein encapsulation (VEGF + bovine serum albumin (BSA)) was nearly equal (2.2 ± 0.8 and 2.4 ± 0.4 μg of protein/mg of PLGA 503H spheres and PLGA-PLL 503H spheres, respectively). Similar results were obtained when encapsulating CNTF (Figure 6E), with CNTF release from PLGA-PLL 503H micropsheres measuring approximately 41% greater than PLGA 503H. As was the case with VEGF microspheres, total protein encapsulation was nearly equal (0.44 ± 0.03 and 0.46 ± 0.02 ug/mg). While the block copolymers encapsulated/released more VEGF/CNTF than PLGA, the release kinetics were similar to those of PLGA (Figure 6B, D and F).
Figure 6.
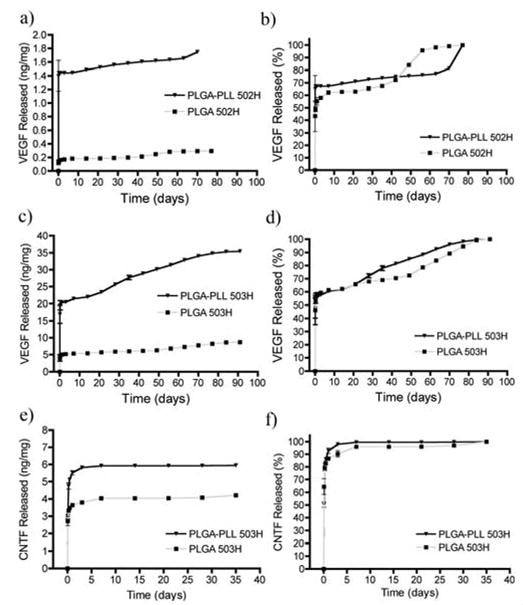
Cumulative release of VEGF/CNTF from microspheres. a) and c) VEGF released from 502H and 503H microspheres. b) and d) Data from a) and c) represented as % of total VEGF released. e) CNTF released from 503H microspheres. f) Data from e) represented as % of total CNTF released.
4. DISCUSSION
We investigated whether a simple modification to PLGA could augment drug delivery and provide moieties for further applications. While PLGA and its monomers, PLA and PGA, have numerous attractive attributes [23, 43], they are nonetheless limited by their chemical functionalities: -CH3, -OH and -COOH. By synthesizing an amphipathic block copolymer comprised of PLGA and Poly(ε-carbobenzoxy-L-lysine), we introduced amine functionalities. While earlier methods incorporated amine moieties by using a larger Poly(ε-carbobenzoxy-L-lysine)(Mw ~ 6300 Da) [24], functionality of our copolymer was realized using a smaller variant (Mw ~1000 Da).
Conjugation significantly improved encapsulation and delivery of large molecules (VEGF and CNTF) as compared to its PLGA counterparts. Furthermore, the block copolymer provided robust functionalities for coupling, while maintaining the properties PLGA is approved for. Of equivalent significance, the biocompatibility of PLGA was maintained. Therefore, using a simple chemistry to modify PLGA, we were able exploit the properties of a widely used polymer, while broadening its application.
Our block copolymer proved extremely useful for the encapsulation of the large molecule VEGF, an important angiogenic protein [44]. With VEGF’s ability to induce capillary formation, sustained delivery has proven to be a useful tool in regenerative medicine [20, 45]. Microspheres comprised of the block copolymer (502H and 503H) released more VEGF than their PLGA counterparts (Figure 6A and C). Additionally, the encapsulation and release of VEGF were found to be dependent on PLGA molecular weight (PLGA-PLL 502H versus PLGA-PLL 503H) [46]. However, while release was greater for PLGA-PLL microspheres, total protein loadings (VEGF + BSA) were comparable to that of PLGA. In conjunction, these results suggest that a preferential association between PLGA-PLL and VEGF exists.
Similarly, PLGA-PLL 503H microspheres released more CNTF, a neuroprotective protein,[47] than PLGA 503H (Figure 6E), also suggesting a preferential interaction between the growth factor and PLGA-PLL. CNTF has proven to be effective in protecting motor neurons in both injured and pathological conditions [48, 49]. However, systemic administration is limited by its short half-life and adverse side effects at high concentrations [50], suggesting that a controlled, local delivery is essential [51]. As with VEGF, total protein loading (CNTF + BSA) was equivalent. The marked decrease in CNTF and total protein loading, as compared to VEGF, may be attributed to factors that have proven to influence drug-polymer interactions, including charge density, isoelectric point and relative hydrophobicity [52–54]. These inherent properties may explain the differences observed between protein type and polymer (i.e. PLGA-PLL vs. PLGA). However, further experimentation would be required to fully elucidate the interaction between the encapsulated agent and polymer.
Of particular interest, PLGA-PLL release kinetics were similar to those of PLGA (Figures 6B, D, and F). This observation correlated with the noted lack of differences in morphology (Figure 3) and degradation rates between the PLGA-PLL and PLGA (Figure 4B). Furthermore, these release profiles were similar to those previously observed from PLGA microspheres [55–57]. Typically, the release of protein from PLGA microspheres often follows a tri-phasic release profile. In the initial burst phase (< 1 day), protein absorbed to the surface of the sphere is released. In the second phase, protein is released slowly, presumably by a diffusion process. The third phase is marked with an increase in protein release corresponding to massive bulk erosion of the polymer. The similarities in release kinetics emphasizes once again that our functionalization preserved the material properties of PLGA. By conserving these properties, one can infer from literature how variations such as molecular weight or copolymer ratio may influence drug release rates or encapsulation [21, 22]. This proves extremely advantageous, as compared to developing a new polymer system. With PLGA being one of the most widely used polymers in drug delivery and tissue engineering, being able to utilize its extensively studied properties only accentuates the potential our block copolymer has.
Conjugating Poly(ε-carbobenzoxy-L-lysine) directly to PLGA enabled us to introduce amine functionalities prior to device fabrication. This allowed for a greater enrichment of amines, which would have otherwise been limited by PLL solubility. However, a fundamental issue with incorporating PLL into biomaterials is the potential for cytotoxicity. Hence, establishing biocompatibility of our block copolymer was of particular importance. A key attribute of the block copolymer, is that while PLGA versatility was improved, biocompatibility was maintained. Others have found PLL concentration/molecular weight and cytotoxicity to be directly proportional [25, 26]. These findings coincide with our in vitro toxicity studies. At 24-hour time points, we reported similar findings, correlating low molecular weight PLL dosages, and cell viability [26]. These results proved promising, however, further toxicity analysis was undertaken in vivo. Groups investigating PLGA induced inflammation reported capsule thickness, over a similar time course, comparable to our measurements [42]. With PLGA-PLL 503H inducing a smaller or equivalent thickness (Figure 5f), this suggests that our conjugation of Poly(ε-carbobenzoxy-L-lysine) does not impair the biocompatibility of PLGA.
While amine functionalities were effectively introduced in both block copolymers, a slower release of amines from PLGA-PLL 503H devices was observed as compared to PLGA-PLL 502H. This correlated well with the prolonged longevity of 503H devices (Figures 4A and 6B). It is well established that PLGA molecular weight significantly influences its rate of degradation [41], suggesting that our block copolymer synthesis was effective in maintaining the characteristic properties of PLGA. The “burst” of amines, released from PLGA-PLL nanospheres at 1 and 5 h (Figure 4a), can be attributed to PLL and polymer degradation during nanosphere fabrication. Others have noted a similar release of copolymer constituents following nanosphere fabrication at early time points [58]. It is well documented that probe sonication is often associated with the denaturing of encapsulated agents during device fabrication [36]. It has been determined that sonication is responsible for polymer degradation as well [59].
Further device characterization found that more amines were present in devices comprised of PLGA-PLL 502H than 503H (Figure 4A). Successful conjugation of FITC validates this difference in amine content between PLGA-PLL 502H and 503H nanospheres (Figure 4D). FITC conjugation suggests that these amines are reactive, and can provide robust sites for surface modification and ligand conjugation [8], otherwise more difficult with PLGA alone. These surface conjugations would have the potential to facilitate site specific drug delivery and allow for restricted drug concentrations at the site of greatest efficacy [60]. Similar device modifications have proven useful in other systems, facilitating surface conjugations for cell specific delivery [7–9].
5. CONCLUSIONS
In summary, we functionalized PLGA in an effort to improve the material’s versatility. We found that the simple conjugation of Poly(ε-carbobenzoxy-L-lysine) to PLGA was advantageous in common biomaterial applications, particularly VEGF encapsulation. It was clearly demonstrated, that simple variations (i.e. polymer molecular weight) enabled the optimization for application specific demands. While synthesis of the block copolymer proved advantageous in numerous applications, the overall integrity and properties of PLGA were maintained. With biocompatibility uncompromised and established, we believe this block copolymer is an excellent platform for further biomedical applications.
Acknowledgments
This work was funded through the generous support of Richard and Gail Siegal, the Discovery Eye Foundation, and the Lincy Foundation. J.P.B. and R.R. would like to acknowledge NIH Neuroengineering Training Grant T90-DK070068. S.M.J. would like to acknowledge grant NIH HL085416. Authors would also like to thank Prof. W.M. Saltzman (Yale University) for contribution of VEGF, ELISA kits, and mice. RECs and HUVECs were generous gifts from Prof. J.A. Madri (Yale University) and Prof. J.S. Pober (Yale University).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thote AJ, Chappell JT, Gupta RB, Kumar R. Reduction in the initial-burst release by surface crosslinking of PLGA microparticles containing hydrophilic or hydrophobic drugs. Drug Dev Ind Pharm. 2005;31(1):43–57. doi: 10.1081/ddc-43985. [DOI] [PubMed] [Google Scholar]
- 2.Barichello JM, Morishita M, Takayama K, Nagai T. Encapsulation of hydrophilic and lipophilic drugs in PLGA nanoparticles by the nanoprecipitation method. Drug Dev Ind Pharm. 1999;25(4):471–6. doi: 10.1081/ddc-100102197. [DOI] [PubMed] [Google Scholar]
- 3.Uchida T, Yoshida K, Nakada Y, Nagareya N, Konishi Y, Nakai A, et al. Preparation and characterization of polylactic acid microspheres containing water-soluble anesthetics with small molecular weight. Chem Pharm Bull (Tokyo) 1997 Mar;45(3):513–7. doi: 10.1248/cpb.45.1539. [DOI] [PubMed] [Google Scholar]
- 4.Gu F, Amsden B, Neufeld R. Sustained delivery of vascular endothelial growth factor with alginate beads. J Controlled Release. 2004 May 18;96(3):463–72. doi: 10.1016/j.jconrel.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 5.Dusseault J, Tam SK, Ménard M, Polizu S, Jourdan G, Yahia L, et al. Evaluation of alginate purification methods: effect on polyphenol, endotoxin, and protein contamination. J Biomed Mater Res A. 2006 Feb 1;76(2):243–51. doi: 10.1002/jbm.a.30541. [DOI] [PubMed] [Google Scholar]
- 6.Murphy WL, Peters MC, Kohn DH, Mooney DJ. Sustained release of vascular endothelial growth factor from mineralized poly(lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials. 2000;21(24):2521–7. doi: 10.1016/s0142-9612(00)00120-4. [DOI] [PubMed] [Google Scholar]
- 7.Fahmy TM, Samstein RM, Harness CC, Saltzman WM. Surface modification of biodegradable polyesters with fatty acid conjugates for improved drug targeting. Biomaterials. 2005 Oct;26(28):5727–36. doi: 10.1016/j.biomaterials.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 8.Keegan ME, Royce SM, Fahmy T, Saltzman WM. In vitro evaluation of biodegradable microspheres with surface-bound ligands. J Controlled Release. 2006;110(3):574–80. doi: 10.1016/j.jconrel.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Gref R, Couvreur P, Barratt G, Mysiakine E. Surface-engineered nanoparticles for multiple ligand coupling. Biomaterials. 2003;24(24):4529–37. doi: 10.1016/s0142-9612(03)00348-x. [DOI] [PubMed] [Google Scholar]
- 10.Barrera DA, Zylstra E, Lansbury PT, Langer R. Copolymerization and degradation of poly(lactic acid co-lysine) Macromolecules. 1995 Jan;28(2):425–32. [Google Scholar]
- 11.Jo S, Engel PS, Mikos AG. Synthesis of poly(ethylene glycol)-tethered poly(propylene fumarate) and its modification with GRGD peptide. Polymer. 2000 Oct;41(21):7595–604. [Google Scholar]
- 12.Keegan ME, Falcone JL, Leung TC, Saltzman WM. Biodegradable microspheres with enhanced capacity for covalently bound surface ligands. Macromolecules. 2004 Dec;37(26):9779–84. [Google Scholar]
- 13.Caponetti G, Hrkach JS, Kriwet B, Poh M, Lotan N, Colombo P, et al. Microparticles of novel branched copolymers of lactic acid and amino acids: Preparation and characterization. J Pharm Sci. 1999 Jan;88(1):136–41. doi: 10.1021/js970457f. [DOI] [PubMed] [Google Scholar]
- 14.Cook AD, Hrkach JS, Gao NN, Johnson IM, Pajvani UB, Cannizzaro SM, et al. Characterization and development of RGD-peptide-modified poly(lactic acid-co-lysine) as an interactive, resorbable biomaterial. J Biomed Mater Res. 1997 Jun;35(4):513–23. doi: 10.1002/(sici)1097-4636(19970615)35:4<513::aid-jbm11>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 15.Yavin E, Yavin Z. Attachment and culture of dissociated cells from rat embryo cerebral hemispheres on polylysine-coated surface. J Cell Biol. 1974;62(2):540–6. doi: 10.1083/jcb.62.2.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edwards DA, Hanes J, Caponetti G, Hrkach J, Ben-Jebria A, Eskew ML, et al. Large porous particles for pulmonary drug delivery. Science. 1997 Jun 20;276(5320):1868–71. doi: 10.1126/science.276.5320.1868. [DOI] [PubMed] [Google Scholar]
- 17.Cook AD, Pajvani UB, Hrkach JS, Cannizzaro SM, Langer R. Colorimetric analysis of surface reactive amino groups on poly(lactic acid-co-lysine):poly(lactic acid) blends. Biomaterials. 1997 Nov 1;18(21):1417–24. doi: 10.1016/s0142-9612(97)00075-6. [DOI] [PubMed] [Google Scholar]
- 18.Jain RA. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials. 2000 Dec;21(23):2475–90. doi: 10.1016/s0142-9612(00)00115-0. [DOI] [PubMed] [Google Scholar]
- 19.Jalil R, Nixon JR. Biodegradable poly(lactic acid) and poly(lactide-co-glycolide) microcapsules: problems associated with preparative techniques and release properties. J Microencapsul. 1990 Jan 1;7(3):297–325. doi: 10.3109/02652049009021842. [DOI] [PubMed] [Google Scholar]
- 20.Elcin AE, Elcin YM. Localized angiogenesis induced by human vascular endothelial growth factor-activated PLGA sponge. Tissue Eng. 2006 Apr;12(4):959–68. doi: 10.1089/ten.2006.12.959. [DOI] [PubMed] [Google Scholar]
- 21.Park TG. Degradation of Poly(D,L-Lactic Acid) Microspheres - Effect of Molecular-Weight. J Controlled Release. 1994 May;30(2):161–73. [Google Scholar]
- 22.Park TG. Degradation of Poly(Lactic-Co-Glycolic Acid) Microspheres - Effect of Copolymer Composition. Biomaterials. 1995 Oct;16(15):1123–30. doi: 10.1016/0142-9612(95)93575-x. [DOI] [PubMed] [Google Scholar]
- 23.Hans ML, Lowman AM. Biodegradable nanoparticles for drug delivery and targeting. Current Opinion in Solid State & Materials Science. 2002 Aug;6(4):319–27. [Google Scholar]
- 24.Lavik EB, Hrkach JS, Lotan N, Nazarov R, Langer R. A simple synthetic route to the formation of a block copolymer of poly(lactic-co-glycolic acid) and polylysine for the fabrication of functionalized, degradable structures for biomedical applications. J Biomed Mater Res. 2001 May;58(3):291–4. doi: 10.1002/1097-4636(2001)58:3<291::aid-jbm1019>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 25.Fischer D, Li YX, Ahlemeyer B, Krieglstein J, Kissel T. In vitro cytotoxicity testing of polycations: influence of polymer structure on cell viability and hemolysis. Biomaterials. 2003 Mar;24(7):1121–31. doi: 10.1016/s0142-9612(02)00445-3. [DOI] [PubMed] [Google Scholar]
- 26.Strand BL, Ryan L, Veld PI, Kulseng B, Rokstad AM, Skjak-Braek G, et al. Poly-L-lysine induces fibrosis on alginate microcapsules via the induction of cytokines. Cell Transplant. 2001;10(3):263–75. doi: 10.3727/000000001783986800. [DOI] [PubMed] [Google Scholar]
- 27.Ferruti P, Knobloch S, Ranucci E, Duncan R, Gianasi E. A novel modification of poly(L-lysine) leading to a soluble cationic polymer with reduced toxicity and with potential as a transfection agent. Macromol Chem Phys. 1998;199(11):2565–75. [Google Scholar]
- 28.Wang YX, Robertson JL, Spillman WB, Claus RO. Effects of the chemical structure and the surface properties of polymeric biomaterials on their biocompatibility. Pharm Res. 2004 Aug;21(8):1362–73. doi: 10.1023/b:pham.0000036909.41843.18. [DOI] [PubMed] [Google Scholar]
- 29.Mikos AG, Thorsen AJ, Czerwonka LA, Bao Y, Langer R, Winslow DN, et al. Preparation and characterization of poly(L-lactic acid) foams. Polymer. 1994;35(5):1068–77. [Google Scholar]
- 30.Jones BN, Paabo S, Stein S. Amino-acid-analysis and enzymatic sequence determination of peptides by an improved ortho-phthaldialdehyde pre-column labelling procedure. J Liq Chromatogr. 1981;4(4):565–86. [Google Scholar]
- 31.Madri JA, Williams SK. Capillary endothelial cell-cultures- phenotypic modulation by matrix components. J Cell Biol. 1983;97(1):153–65. doi: 10.1083/jcb.97.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Denizot F, Lang R. Rapid colorimetric assay for cell-growth and survival- modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986;89(2):271–7. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 33.Faranesh AZ, Nastley MT, de la Cruz CP, Haller MF, Laquerriere P, Leong KW, et al. In vitro release of vascular endothelial growth factor from gadolinium-doped biodegradable microspheres. Magn Reson Med. 2004 Jun;51(6):1265–71. doi: 10.1002/mrm.20092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perez C, Castellanos IJ, Costantino HR, Al-Azzam W, Griebenow K. Recent trends in stabilizing protein structure upon encapsulation and release from bioerodible polymers. J Pharm Pharmacol. 2002 Mar;54(3):301–13. doi: 10.1211/0022357021778448. [DOI] [PubMed] [Google Scholar]
- 35.Bilati U, Allemann E, Doelker E. Strategic approaches for overcoming peptide and protein instability within biodegradable nano- and microparticles. Eur J Pharm Biopharm. 2005 Apr;59(3):375–88. doi: 10.1016/j.ejpb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 36.van de Weert M, Hennink WE, Jiskoot W. Protein instability in poly(lactic-co-glycolic acid) microparticles. Pharm Res. 2000 Oct 1;17(10):1159–67. doi: 10.1023/a:1026498209874. [DOI] [PubMed] [Google Scholar]
- 37.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 38.Cohen S, Yoshioka T, Lucarelli M, Hwang LH, Langer R. Controlled delivery systems for proteins based on poly(lactic glycolic acid) microspheres. Pharm Res. 1991 Jun;8(6):713–20. doi: 10.1023/a:1015841715384. [DOI] [PubMed] [Google Scholar]
- 39.Hermanson GT. Bioconjugate Techniques. San Diego: Academic Press; 1996. [Google Scholar]
- 40.Deng C, Chen X, Yu H, Sun J, Lu T, Jing X. A biodegradable triblock copolymer poly(ethylene glycol)-b-poly(l-lactide)-b-poly(l-lysine): Synthesis, self-assembly, and RGD peptide modification. Polymer. 2007 Jan 5;48(1):139–49. [Google Scholar]
- 41.Wang N, Qiu JS, Wu XS. Tailored Polymeric Materials for Controlled Delivery Systems. In: Mc Culloch I, Shalaby SW, editors. ACS Symposium Series 709. Washington DC: ACS; 1998. pp. 242–54. [Google Scholar]
- 42.Wang YD, Ameer GA, Sheppard BJ, Langer RNm. A tough biodegradable elastomer. Nat Biotechnol. 2002 Jun;20(6):602–6. doi: 10.1038/nbt0602-602. [DOI] [PubMed] [Google Scholar]
- 43.Mooney DJ, Organ G, Vacanti JP, Langer R. Design and fabricationo f biodegradable polymer devices to engineer tubular tissues. Cell Transplant. 1994;3(2):203–10. doi: 10.1177/096368979400300209. [DOI] [PubMed] [Google Scholar]
- 44.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13(1):9–22. [PubMed] [Google Scholar]
- 45.Jay SM, Shepherd BR, Bertram JP, Pober JS, Saltzman WM. Engineering of multifunctional gels integrating highly efficient growth factor delivery with endothelial cell transplantation. FASEB J. 2008 May 1; doi: 10.1096/fj.08-108803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niwa T, Takeuchi H, Hino T, Kunou N, Kawashima Y. Preparations of biodegradable nanospheres of water-soluble and insoluble drugs wtih D,L-lactide glycolide copolymer by a novel spontaneous emulsification solvent diffusion method, and the drug release behavior. J Controlled Release. 1993;25(1–2):89–98. [Google Scholar]
- 47.Clatterbuck RE, Price DL, Koliatsos VE. Ciliary neurotrophic factor prevents retrograde neuronal death in the adult central-nervous-system. Proc Natl Acad Sci U S A. 1993;90(6):2222–6. doi: 10.1073/pnas.90.6.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clatterbuck RE, Price DL, Koliatsos VE. Ciliary neurotrophic factor prevents retrograde neuronal death in the adult central nervous system. Proc Natl Acad Sci USA. 1993 Mar 15;90(6):2222–6. doi: 10.1073/pnas.90.6.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Emerich DF, Winn SR, Hantraye PM, Peschanski M, Chen EY, Chu Y, et al. Protective effect of encapsulated cells producing neurotrophic factor CNTF in a monkey model of Huntington’s disease. Nature. 1997 Mar 27;386(6623):395–9. doi: 10.1038/386395a0. [DOI] [PubMed] [Google Scholar]
- 50.Dittrich F, Thoenen H, Sendtner M. Ciliary neurotrophic factor: pharmacokinetics and acute-phase response in rat. Ann Neurol. 1994 Feb 1;35(2):151–63. doi: 10.1002/ana.410350206. [DOI] [PubMed] [Google Scholar]
- 51.Nkansah MK, Tzeng SY, Holdt AM, Lavik EB. Poly(lactic-co-glycolic acid) nanospheres and microspheres for short- and long-term delivery of bioactive ciliary neurotrophic factor. Biotechnol Bioeng. 2008 Aug 1;100(5):1010–9. doi: 10.1002/bit.21822. [DOI] [PubMed] [Google Scholar]
- 52.Alexis F. Factors affecting the degradation and drug-release mechanism of poly(lactic acid) and poly[(lactic acid)-co-(glycolic acid)] Polym Int. 2005;54(1):36–46. [Google Scholar]
- 53.Peters MC, Isenberg BC, Rowley JA, Mooney DJ. Release from alginate enhances the biological activity of vascular endothelial growth factor. Journal of Biomaterials Science-Polymer Edition. 1998;9(12):1267–78. doi: 10.1163/156856298x00389. [DOI] [PubMed] [Google Scholar]
- 54.Siegel SJ, Kahn JB, Metzger K, Winey KI, Werner K, Dan N. Effect of drug type on the degradation rate of PLGA matrices. Eur J Pharm Biopharm. 2006;64(3):287–93. doi: 10.1016/j.ejpb.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 55.Diwan M, Park TG. Pegylation enhances protein stability during encapsulation in PLGA microspheres. J Controlled Release. 2001 Jun;73(2–3):233–44. doi: 10.1016/s0168-3659(01)00292-9. [DOI] [PubMed] [Google Scholar]
- 56.Viswanathan NB, Patil SS, Pandit JK, Lele AK, Kulkarni MG, Mashelkar RA. Morphological changes in degrading PLGA and P(DL)LA microspheres: implications for the design of controlled release systems. J Microencapsul. 2001 Nov-Dec;18(6):783–800. doi: 10.1080/02652040110065440. [DOI] [PubMed] [Google Scholar]
- 57.Bouillot P, Ubrich N, Sommer F, Duc TM, Loeffler JP, Dellacherie E, et al. Protein encapsulation in biodegradable amphiphilic microspheres. Int J Pharm. 1999 Apr;181(2):159–72. doi: 10.1016/s0378-5173(99)00023-x. [DOI] [PubMed] [Google Scholar]
- 58.Avgoustakis K, Beletsi A, Panagi Z, Klepetsanis P, Karydas AG, Ithakissios DS. PLGA-mPEG nanoparticles of cisplatin: in vitro nanoparticle degradation, in vitro drug release and in vivo drug residence in blood properties. Journal of controlled release: official journal of the Controlled Release Society. 2002 Feb 19;79(1–3):123–35. doi: 10.1016/s0168-3659(01)00530-2. [DOI] [PubMed] [Google Scholar]
- 59.Reich G. Ultrasound-induced degradation of PLA and PLGA during microsphere processing: influence of formulation variables. European journal of pharmaceutics and biopharmaceutics: official journal of Arbeitsgemeinschaft für Pharmazeutische Verfahrenstechnik eV. 1998 Mar 1;45(2):165–71. doi: 10.1016/s0939-6411(97)00152-5. [DOI] [PubMed] [Google Scholar]
- 60.Eldridge JH, Hammond CJ, Meulbroek JA, Staas JK, Gilley RM, Tice TR. Controlled vaccine release in the gut-associated lymphoid-tissues. 1. Orally-administered biodegradable microspheres target the peyers patches. J Controlled Release. 1990;11(1–3):205–14. [Google Scholar]