Abstract
Mycobacterium tuberculosis modulates host immune responses through proteins and complex glycolipids. Here, we report that the glycosylphosphatidylinositol anchor phosphatidyl-myo-inositol hexamannosides PIM6 or PIM2 exert potent anti-inflammatory activities. PIM strongly inhibited the Toll-like receptor (TLR4) and myeloid differentiation protein 88 (MyD88)-mediated release of NO, cytokines, and chemokines, including tumor necrosis factor (TNF), interleukin 12 (IL-12) p40, IL-6, keratinocyte-derived chemokine, and also IL-10 by lipopolysaccharide (LPS)-activated macrophages. This effect was independent of the presence of TLR2. PIM also reduced the LPS-induced MyD88-independent, TIR domain-containing adaptor protein inducing interferon β (TRIF)-mediated expression of co-stimulatory receptors. PIM inhibited LPS/TLR4-induced NFκB translocation. Synthetic PIM1 and a PIM2 mimetic recapitulated these in vitro activities and inhibited endotoxin-induced airway inflammation, TNF and keratinocyte-derived chemokine secretion, and neutrophil recruitment in vivo. Mannosyl, two acyl chains, and phosphatidyl residues are essential for PIM anti-inflammatory activity, whereas the inosityl moiety is dispensable. Therefore, PIM exert potent antiinflammatory effects both in vitro and in vivo that may contribute to the strategy developed by mycobacteria for repressing the host innate immunity, and synthetic PIM analogs represent powerful anti-inflammatory leads.
Multifold interactions between Mycobacterium tuberculosis and host phagocytes determine immune responses to M. tuberculosis and tuberculosis pathogenesis (for review, see Refs. 1 and 2). Alveolar macrophages, the primary host cells for M. tuberculosis, and dendritic cells that carry mycobacterial antigens from the infection site to the draining lymph nodes to establish a T cell-mediated immune response contribute to modulate the innate immune response by secreting cytokines after recognition of microbial motives. Among them, TNF2 is an essential mediator for granuloma formation and containment of M. tuberculosis infection. Similarly, IL-12, interferon γ, but also IL-1, IL-18, IL-23, and nitric oxide are required for host defense (1–6). Phagocytes are also a source of immuno-modulatory cytokines, such as IL-10 and transforming growth factor-β, which dampen the immune response and inflammation. Mycobacteria-derived molecules down-modulating the immune system have been described, including the protein ESAT-6, mannose-capped lipoarabinomannan (ManLAM), and lipomannans (LM) (7–12). Here, we report that phosphatidyl-myo-inositol mannosides (PIM), the glycosylphosphatidylinositol (GPI) anchor structure of LAM and LM, exert strong anti-inflammatory activities.
Mycobacterial cell wall LAM, LM, and PIM are recognized by macrophages and dendritic cells through various pattern recognition receptors, including Toll-like receptors (TLRs) (13–16) and C-type lectins such as mannose receptor (MR/CD206) and dendritic cell-specific intercellular adhesion molecule-3 grabbing nonintegrin (DC-SIGN/CD209), central to M. tuberculosis binding and internalization by human dendritic cells (17–20). DC-SIGN and mannose receptor were proposed to mediate ManLAM inhibition of LPS-induced IL-12 production in dendritic cells, an activity ascribed to the mannosylated cap (8, 9). We showed recently that mycobacterial LM have a dual potential for pro-inflammatory and anti-inflammatory effects (11), tri- and tetra-acylated LM fractions exerting stimulatory effects through TLR2, TLR4, and MyD88 (21), whereas diacylated LM inhibit LPS-induced cytokine response independently of TLR2, SIGN-R1, and mannose receptor (12).
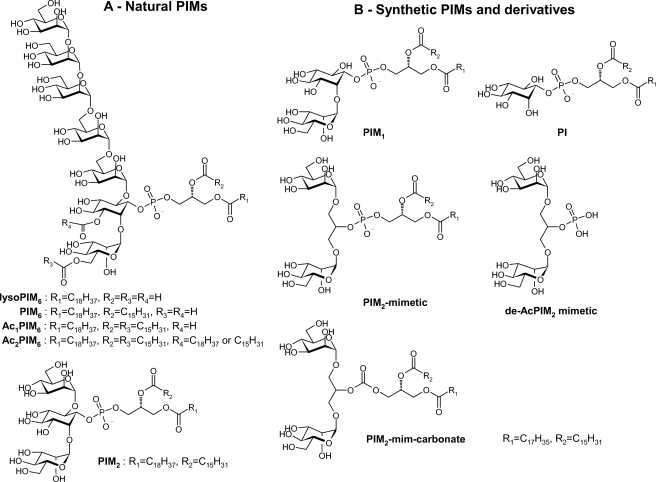
PIM are biosynthetic precursors of LM and LAM (22–25). Dimannoside (PIM2) and hexamannoside (PIM6) PIM are the two most abundant classes of PIM found in M. tuberculosis H37Rv and Mycobacterium bovis BCG (see Fig. 1). PIM purification and molecular chemical characterization revealed four major acyl forms, mono- to tetra-acylated (lyso-PIM for one acyl, PIM for two acyl, Ac1PIM for three acyl, and Ac2PIM for four acyl, respectively; see Fig. 1) for both PIM2 and PIM6 (26–29). Higher order PIM with mannose cap-like structures were found to preferentially associate with human MR and to contribute to phagosome-lysosome fusion (20). The degree of acylation influenced higher order PIM association with the MR, whereas PIM2 was recognized by DC-SIGN independently of its acylation degree. The complete synthesis of the different PIM has recently been reported (30–33).
FIGURE 1.
Natural PIM and synthetic PIM1 and PIM2 mimetics used in the study. Shown is a schematic representation of natural lyso-PIM6, PIM6, Ac1PIM6, Ac2PIM6, and PIM2 (A) and synthetic PIM1 (B) showing the C16 and C18 acyl groups on glycerol chain positions sn-2 and sn-1, the precursor PI, a synthetic mimetic of PIM2 (PIM2 mimetic) bearing C16 and C18 acyl chains, the de-acylated control molecule precursor of the PIM2 mimetic (de-AcPIM2 mimetic), and a PIM2 mimetic with replacement of the phosphodiester moiety by a carbonate.
Here, we analyzed isolated acyl forms of PIM and identified PIM2 and PIM6 but also synthetic PIM1 and a mimetic of PIM2 as strong inhibitors of endotoxin-induced proinflammatory responses in vitro and in vivo. Using macrophages from genetically modified mice, we characterized PIM inhibitory effects on MyD88, TRIF, and NFκB signaling pathways. Hence, not only complex mycobacterial lipoglycans like ManLAM and LM but also small molecular weight PIM analogues are potent inhibitors of host inflammatory responses.
EXPERIMENTAL PROCEDURES
Purification of LM Acyl Forms
The PIM-containing lipidic extract was obtained through purification of the M. bovis BCG phenolic glycolipids (34) as summarized in Gilleron et al. (26). M. bovis BCG PIM2 and PIM6 mono-, di-, tri-, and tetra-acylated forms were further fractionated using hydrophobic interaction chromatography as described by Gilleron et al. (21). The purity of the different acyl forms was assessed by 31P NMR and matrix-assisted laser desorption/ionization mass spectrometry.
Synthetic PIM
PIM1 containing a C16 and a C18 chain in the glycerolipid unit was prepared following largely published procedures (30, 32, 35, 36) with some modifications (for details, see the supplemental information). The reference compound phosphatidylinositol (PI) was prepared by an analogous method. PIM2 mimetic was prepared by bisglycosylation of commercial 2-O-benzyl glycerol using tetra-O-methoxyacetyl-α-d-mannopyranosyl trichloro-acetimidate in the presence of trimethylsilyl triflate, debenzylation of the glycerol-2-O position, phosphorylation with the same phosphoramidite as used in the synthesis of PIM1, and deprotection by treatment with tert-butylamine (for the selective cleavage of the methoxyacetyl group) and final hydrogenolysis (for details, see the supplemental information).
Mice
6–12-Week-old mice deficient for TLR2 (37), MyD88 (38), TRIF (39), CD1 (40), ST2 (41), mannose receptor (42), or SIGNR1(43) and wild-type control C57Bl/6 (B6) mice were bred at the Transgenose Institute animal breeding facility (Orleans, France).
Primary Macrophage Cultures
Murine bone marrow cells were isolated from femurs and cultivated (106/ml) for 7 days in Dulbecco's minimal essential medium supplemented with 2 mm l-glutamine, 20% horse serum, and 30% L929 cell-conditioned medium as a source of macrophage colony-stimulating factor. After a further 3 days in fresh medium, the cell preparation contained a homogenous population of macrophages (97–98% CD11b+F4/80+). The bone marrow-derived macrophages (105 cells/well) in Dulbecco's minimal essential medium supplemented with 2 mm l-glutamine, 2 × 10−5 m β-mercaptoethanol, and 0.1% fetal calf serum were stimulated with 100 ng/ml LPS (Escherichia coli, serotype O111:B4; Sigma), 0.5 μg/ml synthetic bacterial lipopeptide Pam3CSK4 ((S)-2, 3-bis(palmitoyloxy)-(2-RS)-propyl]-N-palmitoyl-(R)-Cys- (S)-Ser-Lys4-OH) tri-hydrochloride, EMC Microcollections, Tuebingen, Germany), 30 ng/ml MALP2 (S-(2,3-bisacyloxypropyl)-cysteine-GNNDESNISFKEK (Alexis Biochemicals, Lausanne, Switzerland), 0.125 μm CpG ODN1826 (tccatgacgttcctgacgtt), or 3 μg/ml of poly(I·C) (poly(I)·poly(C) double strand, Amersham Biosciences). The PIM preparations or DMSO controls are added at the indicated concentrations 30 min before the stimuli. Lyophilized PIM preparations were solubilized in DMSO and added to the cultures at a non-cytotoxic 1% DMSO final concentration (3–7 μg/ml unless otherwise stated). The macrophages were activated with interferon-γ (500 units/ml) to study IL-12 release, and the supernatants were harvested after 24 h for further analysis. Alternatively, cells were collected at the indicated times for PCR gene expression analysis. Primary lung, bronchoalveolar or peritoneal resident macrophages, and spleen adherent cells were also activated with LPS in the presence of PIM. The absence of cytotoxicity of the stimuli was controlled using MTT incorporation.
Nuclear Translocation of NFκB
Bone marrow-derived macrophages stimulated with LPS plus PIM as above on microscopic slides for 1–4 h, washed with PBS, fixed in 4% paraformaldehyde, and permeabilized with Triton 100 × 0.5% were incubated with goat anti-murine NF-κBp65 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) for 1 h at room temperature, washed, and incubated with swine anti-goat IgG FITC Ab (Sigma). Cells presenting NFκB nuclear translocation were scored by confocal microscopy (Leica SP2).
Flow Cytometric Analysis (FACS)
Cells were saturated with mouse serum before staining for 20 min with fluorescence-conjugated antibodies CD11b-PerCP (clone M1/70), PE-F4/80 (clone BM8), fluorescein isothiocyanate anti-CD86 (clone GL1), PE-CD40 (clone 3/23), and isotype-matched control antibodies (all from Pharmingen except BM8, which was from eBioscience), and analyzed using a FACSCalibur flow cytometer and CellQuest Software (BD Biosciences).
Cytokine Enzyme-linked Immunosorbent Assay
Supernatants were harvested and assayed for cytokine content using commercially available enzyme-linked immunosorbent assay reagents for TNF, KC, IL-6, IL-10, and IL-12 p40 (Duoset R&D Systems, Abingdon, UK).
Nitrite Measurements
Nitrite concentrations in cell supernatants were determined using the Griess reaction (3% phosphoric acid, 1% p-aminobenzene sulfonamide, 1% N-1-napthyl ethylenediamide) as previously described (44).
Real-time Reverse Transcription-PCR
Expression of the indicated cytokine and chemokine genes was measured by real-time reverse transcription-PCR of bone marrow-derived macrophages 2 and 6 h after LPS stimulation (0.1 μg/ml) in the presence of synthetic PI, PIM1, PIM2 mimetic, deacylated Ac2PIM2 mimetic (all at 10 μg/ml). Expression of Hprt1, glyceraldehyde-3-phosphate dehydrogenase, 18 S, and β2-microglobulin housekeeping genes was used for normalization.
Airway Inflammation
LPS (1 μg) from E. coli (serotype O55:B5; Sigma) in saline containing PIM1 or PIM2 mimetic (50 μg) in DMSO (1.25% final) or saline plus DMSO alone was applied by nasal instillation in a volume of 40 μl under light ketamine-xylazine anesthesia. Airways resistance was evaluated by whole-body plethysmography (EMKA Technologies, Paris, France) over a period of 3 h (45). Enhanced respiratory pause as a measure of airway dysfunction (for details see Ref. 46) was registered and analyzed using Datanalyst Software (EMKA Technologies). At 24 h, myeloperoxidase activity was evaluated in lung, and bronchoalveolar lavage fluid was collected as described (46) for cytokine analysis and cell differential counts on 200 cells with Diff-Quik staining (Merz & Dade AG, Dudingen, Switzerland).
Statistical Analysis
Statistical significance was determined with Graph Pad Prism software (Version 4.0, San Diego, CA) by one way non-parametric analysis of variance followed by the Tukey post test. p values of <0.05 were considered statistically significant.
RESULTS
Inhibition of LPS-induced Macrophage Stimulation by PIM6-purified Acyl Forms
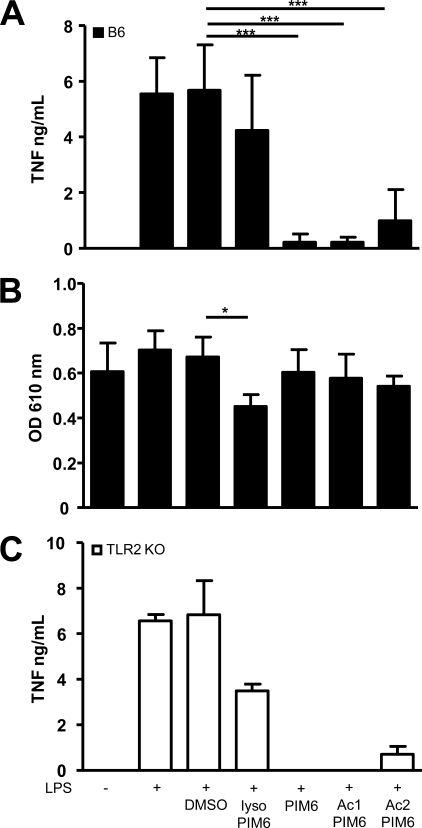
Unfractionated PIM stimulates TNF production by a monocytic cell line (47), and we showed previously that purified PIM6 are also slightly proinflammatory (27). However, because LM pro- and anti-inflammatory activities were separated according to the degree of acylation of the LM molecules, we asked whether certain PIM fractions could also inhibit macrophage activation. An enriched fraction of PIM6 was prepared from M. bovis BCG (26) and acyl forms bearing one to four fatty acids (see Fig. 1) were further purified as described (27). The inhibitory effect of lyso-PIM6, PIM6, Ac1PIM6, and Ac2PIM6 fractions on LPS-induced TNF secretion was first assessed using RAW 264.7 monocytic cell line and confirmed on primary bone marrow-derived macrophages (Fig. 2). The most effective inhibitors of TNF release were PIM6 and Ac1PIM6, whereas Ac2PIM6 was slightly less effective, and essentially no inhibition was seen with lyso-PIM6 (Fig. 2A). Similar results were obtained for NO release (data not shown). All PIM fractions were titrated and tested at a concentration at which only lyso-PIM6 exhibited a slight cytotoxicity (Fig. 2B). Thus, PIM-purified fractions inhibited TNF and NO secretion by primary macrophages depending on their level of acylation.
FIGURE 2.
PIM6-purified fractions inhibit TNF release by LPS-stimulated macrophages, independently of TLR2. Bone marrow-derived macrophages from wild-type (A and B) or TLR2-deficient (C) mice were incubated for 24 h with LPS in the presence of purified PIM6-acylated forms (lyso-PIM6, PIM6, Ac1PIM6, and Ac2PIM6; all at 6.7 μg/ml) or DMSO vehicle alone. TNF (A and C) was measured in the supernatants, and potential cytotoxicity (B) was verified on the cells by MTT bioassay (absorbance, 610 nm). Results are the mean ± S.D. from n = 6 mice per group from three independent experiments; *, p < 0.05; ***, p < 0.01. ko, knock out.
The Inhibition of LPS-induced TNF by PIM Is TLR2- independent
The unfractionated PIM preparation was shown to be a TLR2 agonist based on a reporter assay with cell lines transfected with the tlr2 gene (47), and we showed previously that the slight macrophage activation in response to PIM2 and PIM6 isolated fractions was dependent on the TLR2 pathway (27). We then examined whether TLR2 recognition was involved in the PIM anti-inflammatory activity using bone marrow-derived macrophages prepared from mice deficient for TLR2. The inhibitory effect of PIM fractions on LPS-induced TNF was independent of their recognition by TLR2, as cells deficient for TLR2 were efficiently inhibited by the PIM6 acyl fractions (Fig. 2C). The inhibition of LPS-induced TNF by PIM6, Ac1PIM6, or Ac2PIM6 was even accentuated in the absence of TLR2, which can be explained by their slight TLR2 agonist activity, whereas lyso-PIM6 remained poorly inhibitory. Thus, TLR2 is not required for mediating the inhibition of LPS-induced cytokine release by PIM fractions.
Specificity of PIM Fractions Inhibitory Effects for TLR4-mediated Stimulation
We next asked whether the inhibitory effects of the PIM fractions were specific for the TLR4 activation pathway. Specific TLR agonists, namely TLR4 agonist LPS, TLR2/TLR1 agonist Pam3CSK4 (bacterial lipopeptide), TLR2/TLR6 agonist Malp2, and TLR9 agonist CpG, were used to activate macrophages in the absence or in presence of PIM fractions. PIM6, Ac1PIM6, and Ac2PIM6 inhibited the production of NO and TNF (supplemental Fig. 1A and not shown) after stimulation by LPS but not after stimulation by bacterial lipopeptide, Malp2 or CpG. The inhibitory effect of PIM fractions on IL-12 p40 expression (supplemental Fig. 1B) after LPS stimulation was stronger and was also partially seen after Malp2 stimulation. Therefore, the inhibitory effects of the PIM fractions are preferentially targeted to the TLR4 signaling pathway, although the specificity does not seem absolute for IL-12 p40 release.
Separation of Anti- Versus Pro-inflammatory Activity in Synthetic PIM1
To confirm the inhibitory activity seen in mycobacterial-purified PIM fractions and to fully separate the anti-inflammatory activity from the slight TLR2-dependent proinflammatory activity seen with the natural PIM, we synthesized a monomannosylated PI (PIM1) bearing C16 and C18 chains (Fig. 3A). Synthetic PIM1 inhibited the LPS-induced secretion of IL-12 p40 (Fig. 3B), TNF, and NO (not shown) as efficiently as the natural fractions containing PIM6 or Ac1PIM6, whereas synthetic PI did not. Moreover, PIM1 was neither stimulatory for cytokine or NO production nor cytotoxic at the concentrations used (not shown). Thus, through synthesis we could definitely ascertain that the anti-inflammatory activities seen in the natural purified fractions was associated with the PIM structure, separate the PIM anti-inflammatory from the pro-inflammatory activity and demonstrate that a monomannoside PIM structure was sufficient for the anti-inflammatory activity.
FIGURE 3.
Anti-inflammatory activity of synthetic PIM1 and PIM2 mimetic. A, schematic representation of synthetic PIM1 showing the C16 and C18 acyl groups on glycerol chain positions sn-2 and sn-1 and the precursor PI. B, comparison of synthetic PIM1 to natural PIM6 or Ac1PIM6-purified fractions or to non-acylated PI (all at 10 μg/ml) for inhibiting LPS-induced release of IL-12 p40 by wild-type bone marrow-derived macrophages. C, schematic representation of a synthetic mimetic of PIM2 (PIM2 mimetic) and the de-acylated control molecule (de-AcPIM2 mimetic). Also shown are a comparison of synthetic PIM1, synthetic PIM2 mimetic, and non-acylated de-AcPIM2 mimetic for inhibiting LPS-induced release of TNF (D) and IL-12 p40 (E). Titration of natural PIM2 and synthetic PIM2 mimetic inhibitory activities as compared with PIM1 and non-acylated de-AcPIM2 mimetic for the inhibition of IL-12 p40 (F) and IL-10 (G) is shown. Results are the mean ± S.D. from n = 4–8 mice per group from 2–4 independent experiments; *, p < 0.05; ***, p < 0.01.
Synthetic PIM2 Mimetic Recapitulates PIM Anti-inflammatory Activities
To avoid the long synthesis of natural PIM2, we prepared a structural analogue that conserves the main features of PIM2, namely the C16 and C18 acyl chains, two α-mannosyl residues, the diacylglycerol unit, and the phosphodiester linkage, all three groups being carried by a simple three-carbon glycerol scaffold instead of the complex myo-inositol (PIM2 mimetic; Fig. 3C). A related molecule was reported earlier to induce some interferon γ release by splenocytes in vitro (48). We assessed the inhibitory activity of the PIM2 mimetic on macrophages activated with LPS, in comparison to synthetic PIM1, and to a precursor of the PIM2 mimetic, dimannoside phosphate, devoid of acyl residues (de-AcPIM2 mimetic). PIM2 mimetic strongly inhibited the production of TNF and IL-12 p40, whereas the nonacylated precursor molecule was inactive (Fig. 3, D and E). Moreover, the compounds were neither stimulatory nor cytotoxic at concentrations up to 10 μg/ml.
PIM2 mimetic seemed more active than synthetic PIM1 (Fig. 3, D and E, and data not shown). To assess whether this was because of the presence of a second mannose moiety, PIM2 mimetic was further compared with the naturally purified PIM2. Both PIM2 mimetic and natural PIM2 strongly inhibited the production of IL-12 p40 (Fig. 3F) and TNF (data not shown) but also IL-10 (Fig. 3G) and NO (not shown) by LPS-activated macrophages, and they were more potent than PIM1, suggesting that indeed a second mannose moiety may increase the inhibitory effect. Replacement of the phosphodiester moiety by a carbonate (see Fig. 1) abrogated PIM2 mimetic inhibitory activity (supplemental Fig. 2), indicating that although the inosityl moiety is dispensable for the anti-inflammatory activity, the phosphodiester moiety is essential. Therefore, such PIM2 mimetics are much more readily accessible by total synthesis and recapitulate the anti-inflammatory activities seen in natural or synthetic PIM.
PIM Inhibition of MyD88 Versus TRIF-dependent Signals
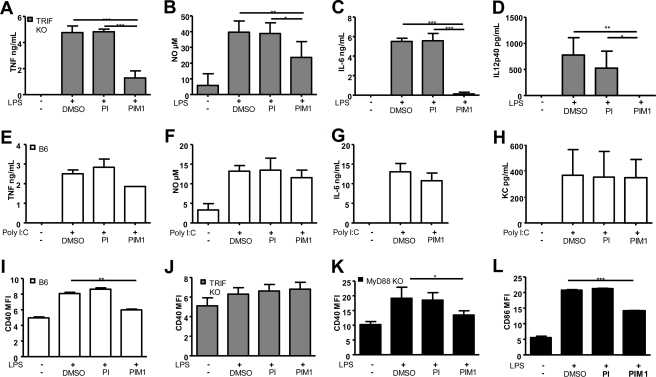
Because LPS/TLR4 can signal through two different pathways using, respectively, the adaptors MyD88/TIRAP or TRIF/TRAM, we next asked whether the inhibitory effects of the PIM fractions specifically target one of these pathways. PIM inhibitory activity was, thus, tested in MyD88- versus TRIF-deficient macrophages. Proinflammatory cytokine release upon LPS stimulation is strongly dependent on the MyD88 pathway (49) but largely independent of TRIF (Fig. 4, A–D). TNF, IL-12 p40, IL-6, and NO release by TRIF-deficient macrophages was strongly inhibited by synthetic PIM1 (Fig. 4, A–D), whereas PI was inactive, similar to what is seen in wild-type macrophages (data not shown and Fig. 3B). Conversely, TNF, IL-6, KC, or NO release after activation by TLR3 agonist poly(I·C), which stimulates a TRIF-dependent, MyD88-independent pathway, was poorly inhibited by PIM1 (Fig. 4, E–H). Both sets of results suggested that PIM inhibition of cytokine or NO release in response to LPS activation was independent of the TRIF pathway.
FIGURE 4.
Inhibition of MyD88 versus TRIF-dependent signals by synthetic PIM1. Wild-type, TRIF KO, or MyD88 KO bone marrow-derived macrophages were activated for 24 h with TLR4 agonist LPS or TLR3 agonist poly(I·C) in the presence of synthetic PIM1 (10 μg/ml), PI (10 μg/ml), or DMSO vehicle alone as indicated. PIM1 inhibited TNF (A), NO (B), IL-6 (C), and IL-12 p40 (D) by LPS-activated TRIF KO macrophages. Macrophage stimulation by poly(I·C), a TLR3 ligand using TRIF-dependent signaling, was not inhibited by PIM1 in terms of TNF (E), NO (F), IL-6 (G), or KC (H) release. LPS induction of CD40 was inhibited by PIM1 in wild-type macrophages (I). There was little CD40 induction in TRIF KO macrophages (J) as expected, and PIM1 inhibited CD40 and CD86 expression in MyD88 KO macrophages (K and L). Results are the mean ± S.D. from n = 4 mice per genotype and are from two experiments representative of three to five independent experiments; *, p < 0.05; ***, p < 0.01. MFI, mean fluorescence intensity.
We next asked whether the expression of the co-stimulatory molecules CD40 and CD86, which should be largely TRIF-dependent and MyD88-independent, was affected by PIM (Fig. 4, I–L). LPS-induced expression of co-stimulatory molecules such as CD40 was slightly reduced in the presence of synthetic PIM1 but not of PI in wild-type macrophages (Fig. 4I). As expected, LPS-induced CD40 and CD86 expression was essentially absent in TRIF-deficient macrophages (Fig. 4J and not shown). The expression of LPS-induced CD40 and CD86 was also strongly reduced in the presence of PIM1, but not of PI, in MyD88-deficient macrophages (Fig. 4, K and L). Therefore, PIM1 efficiently inhibited both signals emanating from LPS-TLR4 interaction, namely the MyD88-mediated TRIF-independent cytokine and NO release and the MyD88-independent, TRIF-mediated expression of co-stimulatory molecules CD40 and CD86.
Inhibition of NFκB Translocation by PIM
TLR4-dependent expression of IL12b, IL6, and Tnf genes is dependent upon NF-κB, and we next asked whether PIM activity affects NFκB translocation. NFκB staining is cytoplasmic in unstimulated macrophages and clearly nuclear 1 h after LPS stimulation. In the presence of PIM1 or PIM2 mimetic, cells exhibiting only NFκB nuclear staining after LPS stimulation were decreased by 25 and 90%, respectively (Fig. 5A). Similar results were obtained 4 h after LPS stimulation, indicating that NFκB translocation was not merely delayed in the presence of PIM (not shown). Furthermore, we verified that PIM2 mimetic inhibited the release of TNF and IL-12 p40 in primary macrophage populations, including lung and alveolar macrophages (Fig. 5B). Therefore, PIM1 and PIM2 mimetic reduced LPS-induced NFκB translocation, and the cytokine inhibitory effect was seen on different primary macrophage populations.
FIGURE 5.
Synthetic PIM analogues inhibit NFκB nuclear translocation and act on different resident macrophage populations. A, NFκB translocation in LPS-stimulated macrophages; quantification of the cells exhibiting only NFκB nuclear staining 1 and 4 h after LPS stimulation in the absence or in the presence of PIM1 or PIM2 mimetic (n = 3 from two experiments). B, lung, bronchoalveolar (BAL), or peritoneal resident macrophages or spleen adherent cells were either untreated (medium) or activated with LPS in the presence of PIM2 mimetic (10 μg/ml) or DMSO vehicle alone and TNF or IL-12 p40 concentrations in the supernatants were measured at 24 h. Results are the mean ± S.D. from n = 6 mice per group from two independent experiments; *, p < 0.05; ***, p < 0.01.
In Vivo Inhibition of Endotoxin Induced Airway Inflammation by Synthetic PIM
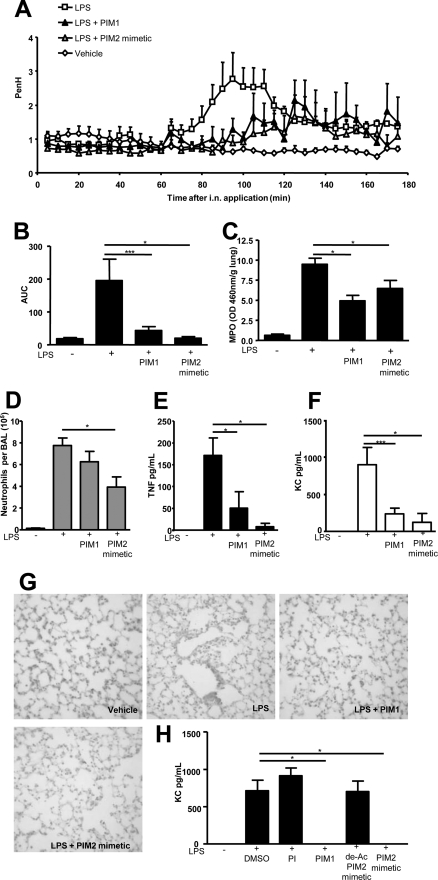
TNF is essential for acute LPS induced respiratory dysfunction as shown in TNF-deficient mice (46, 50). We then asked whether the inhibition of cytokine release by synthetic PIM1 or PIM2 mimetic was sufficient for reducing the airway response to endotoxin. Mice exposed to intranasal application of LPS developed an acute increase of enhanced respiratory pause, which was markedly decreased by co-administration of PIM1 or PIM2 mimetic (Fig. 6, A and B). The recruitment of inflammatory cells, mostly neutrophils, in the alveolar space seen after LPS treatment was decreased by co-administration of PIM1 or PIM2 mimetic (Fig. 6D) and so were neutrophils in the lung, assessed by lung tissue myeloperoxidase activity (Fig. 6C). In the bronchoalveolar fluid LPS-induced secretion of TNF, and KC, the neutrophil attracting chemokine, was reduced by PIM1 and PIM2 mimetic (Fig. 6, E and F). Microscopically, lung tissue sections showed strong inflammation and neutrophil infiltration after local LPS, which was partially reduced in the presence of PIM1 or PIM2 mimetic (Fig. 6G). Indeed, both compounds abrogated KC secretion by LPS-induced macrophages in vitro (Fig. 6H).
FIGURE 6.
In vivo inhibition of endotoxin-induced airway inflammation by synthetic PIM1 and PIM2 mimetic. Wild-type C57BL/6 mice were challenged intranasally (i.n.) with 1 μg of LPS in the absence or in the presence of synthetic PIM1 or PIM2 mimetic or vehicle (1.25% DMSO in saline). A representative experiment showing enhanced respiratory pause (Penh) recorded for 180 min using whole body plethysmography is shown (A). The bar graph in B represents the calculated area under the curve (AUC) from two independent experiments (shown in B–F). Neutrophil myeloperoxidase (MPO) activity in the lung was evaluated 24 h after challenge (C). The bronchoalveolar fluid (BAL) was analyzed for neutrophil counts (D) and concentration of TNF (E) and KC (F). PIM1 and PIM2 mimetic prevented the recruitment of neutrophils in the lung, as assessed by histological analysis. Representative hematoxylin and eosin staining of lung sections are shown (G, magnification, ×400). The inhibition of KC release by LPS-stimulated bone marrow-derived macrophages after incubation with PIM analogues (used as in Fig. 3) is shown in H. The values represent the mean ± S.D. of n = 6 mice per group from three independent experiments; *, p < 0.05.
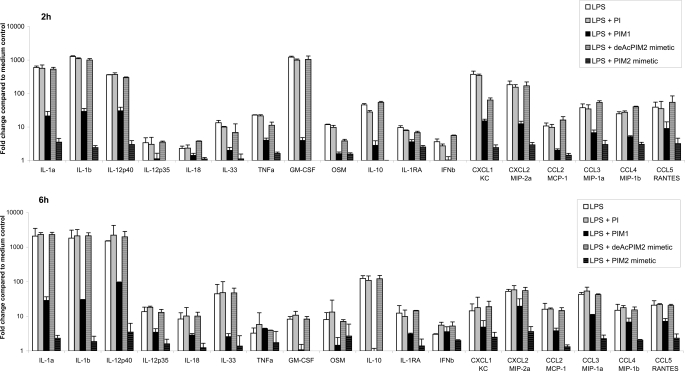
Furthermore, PIM1 and PIM2 mimetic potently inhibited the expression of a series of pro-inflammatory chemokines and cytokines, including CXCL1 (KC), CXCL2 (MIP-2α), CCL2 (MCP-1), CCL3 (MIP-1α), CCL4 (MIP-1β), CCL5 (RANTES), IL-1α, IL-1β, IL-12/IL-23 p40, IL-12 p35, IL-18, IL-33, TNF, granulocyte-macrophage colony-stimulating factor, oncostatin M, and interferon β but also of IL-10 as early as 2–6 h after LPS stimulation in vitro (Fig. 7). Therefore, the synthetic PIM1 or PIM2 mimetic effectively inhibited the airway inflammation in response to local LPS exposure in vivo, an activity likely reflecting their potent inhibition of a series of proinflammatory chemokines and cytokines.
FIGURE 7.
Inhibition of chemokine and cytokine gene expression by PIM analogues in LPS-stimulated macrophages. Real-time reverse transcription-PCR quantification of the expression of the indicated cytokine and chemokine genes 2 h (top) and 6 h (bottom) after LPS stimulation (0.1 μg/ml) of bone marrow-derived macrophages in the presence of synthetic PI, PIM1, PIM2 mimetic, and de-acylated Ac2PIM2 mimetic (all at 10 μg/ml) or DMSO vehicle alone. The data for the different genes are normalized versus the expression of Hprt1, glyceraldehyde-3-phosphate dehydrogenase, 18 S, β2-microglobulin housekeeping genes and presented as ratio of stimulated cells over unstimulated controls. Results are expressed as the mean ± S.D. of n = 2 mice and are from one experiment representative of two independent experiments. GM-CSF, granulocyte-macrophage colony-stimulating factor; IFNb, interferon β. OSM, oncostatin M.
DISCUSSION
Primary infection by M. tuberculosis concerns one-third of the global population, but it remains clinically silent in 9 of 10 infected individuals. The bacterium is still present and viable, ready to flare-up when the immune surveillance fails. To keep control of the immune system, there must be a fine balance between the mycobacteria and the host defense. Several mycobacterial products, including the protein ESAT-6, or the cell wall complex glycolipids LAM or LM down-modulate the host immune responses (7–12). Here we show that the small molecular weight PIM, previously shown to be weak TLR2 agonists, in fact also contain molecular moieties strongly inhibiting the host immune response.
In terms of structure/function relationships, at least one mannosyl and two fatty acids are required on the PIM molecule to inhibit the LPS-induced inflammatory response. Indeed, the most effective inhibitors of cytokine and NO release were PIM2, PIM6, and Ac1PIM6, whereas Ac2PIM6 was slightly less effective, and lyso-PIM6 was inactive. We reported previously that Ac2LM exhibited an inhibitory effect, whereas Ac1LM was inactive, clearly indicating that two fatty acids are required on the LM molecule to inhibit the LPS-induced TNF production, whereas three or four acyl chains are associated with LM pro-inflammatory activity (11, 12, 21). At least di-acylation of M. bovis BCG ManLAM was also necessary to inhibit LPS-induced IL-12 production by human dendritic cells (8). To further confirm that the PIM structure bearing two fatty acids was required and sufficient for anti-inflammatory activity, we synthesized PIM1. Indeed, we could show that a synthetic preparation of PIM1 was a potent inhibitor of LPS-induced release of cytokines and NO in vitro, whereas PI, which lacks the mannosyl moiety, was not inhibitory. Furthermore, using synthetic PIM derivatives, we could show that the inosityl moiety is dispensable for the anti-inflammatory activity, whereas the phosphodiester is essential.
ManLAM anti-inflammatory activity has been largely ascribed to the induction of the anti-inflammatory cytokine IL-10 (9, 10) through C-type lectins such as the mannose receptor and DC-SIGN. In contrast, PIM activity was not mediated by IL-10 overexpression, as PIM strongly inhibited not only pro-inflammatory cytokines like TNF, IL-12/IL-23 p40, and IL-6 but also IL-10 release. In fact, the inhibition of early transcriptional responses was suggestive of a direct effect as the expression of a large panel of LPS-induced cytokines and chemokines messages was drastically inhibited by PIM as early as 2 h after macrophage activation. Furthermore, the absence of C-type lectins mannose receptor and SIGN-R1 did not affect PIM inhibition of murine macrophage response (supplemental Fig. 3 and data not shown). Because PIM6 have a high affinity for human CD1b molecules (51) and PIM2 form complexes to mouse CD1d (52), we verified using CD1d-deficient macrophages that binding to CD1d was not required for PIM inhibition of proinflammatory cytokines (supplemental Fig. 3).
PIM inhibited TLR4 activation triggered not only by LPS but also by other TLR4 agonists such as M. tuberculosis H37Rv LM (12) or a synthetic lipid-A analog OM-197-MP-AC (53) (supplemental Fig. 4). Within the pathways triggered by TLR4, PIM inhibited clearly the MyD88-dependent, TRIF-independent secretion of pro-inflammatory cytokines but also the MyD88-independent, TRIF-dependent expression of co-stimulatory molecules. Central to cytokine transcription in response to TLR trigger is NFκB activation and translocation. Here, we showed a clear inhibitory effect of PIM on NFκB translocation induced by LPS/TLR4 activation.
PIM are GPI anchors of mycobacterial LAM and LM. LAM inserts into the plasma membrane of lymphomonocytic cells through their GPI anchors (54). PIM6 competitively inhibited LAM insertion, and the glycan moiety was important as PI was not as effective. LAM preferentially incorporated into specialized plasma membrane domains enriched in endogenous, host GPI-anchored molecules (54). On the other hand, protozoa GPI-anchor molecules have been shown to contribute to the regulation of host immune response by parasites such as Trypanosoma or Plasmodium. Although some GPI anchors have been reported to stimulate host inflammatory responses, GPI-anchored mucin from Trypanosoma cruzi membrane abrogated monocyte TNF and IL-12 expression (55), and treatment with GPI moiety of T. cruzi variant surface glycoproteins reduced macrophage TNF, IL-6, and IL-12 release while increasing IL-10 (56). Here we propose that GPI-anchor PIM are a potent additional weapon for mycobacteria to dampen and control the host immune responses. The GPI-anchor PIM activities are distinct from those of mycobacterial proteins such as ESAT-6, mediated through TLR2 and Akt, and of ManLAM, mediated through IL-10 overexpression and attributed to the mannosylated cap, absent in PIM structures. PIM activities are also distinct from those of T. cruzi GPI anchors as they are not mediated through IL-10 expression, and PIM do not trigger alternative macrophage activation (data not shown). Models of MD-2/TLR4 heterotetramer complex indicate multiple stabilizing contacts between TLR4 and MD-2 in the presence of a full agonist such as lipid A (57). PIM might interfere with the formation or the interactions of activated LPS·MD·2·TLR4 complex. However, we were able to reverse PIM inhibition of LPS-induced IL-12 p40 using inhibitors of kinases known to down-regulate IL-12 expression, such as phosphatidylinositol 3-kinase (data not shown), suggesting that the downstream signaling rather that the primary LPS·MD·2·TLR4 complex is affected by PIM.
We further asked whether PIM could contribute to the modulation of the innate immune response in vivo. In particular, because PIM traffic out of the mycobacterial phagosome and are released to the medium and bystander uninfected cells (58), it was important to determine whether PIM could dampen the innate immune responses in the lung, a target organ for mycobacterial infections. Indeed, we show that local administration of synthetic PIM1 or PIM2 mimetic inhibit endotoxin induced lung inflammation in terms of cytokine and chemokine secretion, inflammatory cell recruitment in the airways, and airway dysfunction. Readily available PIM2 structural analogs such as PIM2 mimetic that recapitulate or even improve the activity seen in natural or synthetic PIM are of considerable value as potential pharmacologically active leads.
In conclusion, we report the anti-inflammatory activities of PIM. Modulation of PIM release may represent an additional means of regulating the host innate immunity for mycobacteria. Indeed, GPI-anchor PIM could contribute to dampen the activation of infected macrophages and neighboring cells in the tuberculous granuloma and contribute to control the immune response in latent infection. PIM also represent a non-peptidic, small molecular weight, pathogen-derived immunomodulatory molecules with potential as immunotherapeutics.
Supplementary Material
Acknowledgments
We are grateful to Prof. M. C. Nussenzweig (The Rockefeller University, New York) for the kind gift of the mannose receptor-deficient mice, to Prof. S. Akira (Osaka University, Japan) for sharing the TLR-, MyD88-, and TRIF-deficient mice, to Profs. P. G. Fallon (Institute of Molecular Medicine, Trinity Centre for Health Sciences, Trinity College Dublin, Ireland) and A. N. McKenzie (Medical Research Council, Cambridge, UK) for giving us access to the SIGN-R1- and ST2-deficient mice, and to Dr. Jerome Nigou (Institut de Pharmacologie et de Biologie Structurale, Toulouse, France) for fruitful discussions.
This work was supported by CNRS, ANR-05-MIIM-038-02 Grant TB-SIGN, and European Union FEDER Grant 1649-32264.
 The on-line version of this article (available at http://www.jbc.org) contains supplemental Schemes 1–3 and Figs. 1–4.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Schemes 1–3 and Figs. 1–4.
- TNF
- tumor necrosis factor
- IL
- interleukin
- LPS
- lipopolysaccharide
- BCG
- bacillus Calmette Guérin
- DC-SIGN
- dendritic cell-specific intracellular adhesion molecule-3 grabbing nonintegrin
- KC
- keratinocyte-derived chemokine
- MR
- mannose receptor
- LAM
- lipoarabinomannan
- LM
- lipomannans
- Man
- mannosyl unit
- ManLAM
- mannose-capped LAM
- MyD88
- myeloid differentiation protein 88
- PI
- phosphatidyl-myo-inositol
- PIM
- PI mannosides
- TLR
- Toll-like receptor
- MTT
- 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide
- GPI
- glycosylphosphatidylinositol
- TRIF
- TIR domain-containing adaptor protein inducing interferon β
- KO
- knock out.
REFERENCES
- 1.Flynn J. L., Chan J. (2001) Annu. Rev. Immunol. 19,93–129 [DOI] [PubMed] [Google Scholar]
- 2.Flynn J. L. (2006) Microbes Infect. 8,1179–1188 [DOI] [PubMed] [Google Scholar]
- 3.Cooper A. M., Kipnis A., Turner J., Magram J., Ferrante J., Orme I. M. (2002) J. Immunol. 168,1322–1327 [DOI] [PubMed] [Google Scholar]
- 4.Jouanguy E., Lamhamedi-Cherradi S., Altare F., Fondanèche M. C., Tuerlinckx D., Blanche S., Emile J. F., Gaillard J. L., Schreiber R., Levin M., Fischer A., Hivroz C., Casanova J. L. (1997) J. Clin. Invest. 100,2658–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altare F., Durandy A., Lammas D., Emile J. F., Lamhamedi S., Le Deist F., Drysdale P., Jouanguy E., Döffinger R., Bernaudin F., Jeppsson O., Gollob J. A., Meinl E., Segal A. W., Fischer A., Kumararatne D., Casanova J. L. (1998) Science 280,1432–1435 [DOI] [PubMed] [Google Scholar]
- 6.Fremond C. M., Togbe D., Doz E., Rose S., Vasseur V., Maillet I., Jacobs M., Ryffel B., Quesniaux V. F. (2007) J. Immunol. 179,1178–1189 [DOI] [PubMed] [Google Scholar]
- 7.Pathak S. K., Basu S., Basu K. K., Banerjee A., Pathak S., Bhattacharyya A., Kaisho T., Kundu M., Basu J. (2007) Nat. Immunol. 8,610–618 [DOI] [PubMed] [Google Scholar]
- 8.Nigou J., Zelle-Rieser C., Gilleron M., Thurnher M., Puzo G. (2001) J. Immunol. 166,7477–7485 [DOI] [PubMed] [Google Scholar]
- 9.Geijtenbeek T. B., Van Vliet S. J., Koppel E. A., Sanchez-Hernandez M., Vandenbroucke-Grauls C. M., Appelmelk B., Van Kooyk Y. (2003) J. Exp. Med. 197,7–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gringhuis S. I., den Dunnen J., Litjens M., van Het Hof B., van Kooyk Y., Geijtenbeek T. B. (2007) Immunity 26,605–616 [DOI] [PubMed] [Google Scholar]
- 11.Quesniaux V. J., Nicolle D. M., Torres D., Kremer L., Guérardel Y., Nigou J., Puzo G., Erard F., Ryffel B. (2004) J. Immunol. 172,4425–4434 [DOI] [PubMed] [Google Scholar]
- 12.Doz E., Rose S., Nigou J., Gilleron M., Puzo G., Erard F., Ryffel B., Quesniaux V. F. (2007) J. Biol. Chem. 282,26014–26025 [DOI] [PubMed] [Google Scholar]
- 13.Brightbill H. D., Libraty D. H., Krutzik S. R., Yang R. B., Belisle J. T., Bleharski J. R., Maitland M., Norgard M. V., Plevy S. E., Smale S. T., Brennan P. J., Bloom B. R., Godowski P. J., Modlin R. L. (1999) Science 285,732–736 [DOI] [PubMed] [Google Scholar]
- 14.Heldwein K. A., Fenton M. J. (2002) Microbes Infect. 4,937–944 [DOI] [PubMed] [Google Scholar]
- 15.Stenger S., Modlin R. L. (2002) Curr. Opin. Immunol. 14,452–457 [DOI] [PubMed] [Google Scholar]
- 16.Takeda K., Kaisho T., Akira S. (2003) Annu. Rev. Immunol. 21,335–376 [DOI] [PubMed] [Google Scholar]
- 17.Tailleux L., Schwartz O., Herrmann J. L., Pivert E., Jackson M., Amara A., Legres L., Dreher D., Nicod L. P., Gluckman J. C., Lagrange P. H., Gicquel B., Neyrolles O. (2003) J. Exp. Med. 197,121–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maeda N., Nigou J., Herrmann J. L., Jackson M., Amara A., Lagrange P. H., Puzo G., Gicquel B., Neyrolles O. (2003) J. Biol. Chem. 278,5513–5516 [DOI] [PubMed] [Google Scholar]
- 19.Pitarque S., Herrmann J. L., Duteyrat J. L., Jackson M., Stewart G. R., Lecointe F., Payre B., Schwartz O., Young D. B., Marchal G., Lagrange P. H., Puzo G., Gicquel B., Nigou J., Neyrolles O. (2005) Biochem. J. 392,615–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torrelles J. B., Azad A. K., Schlesinger L. S. (2006) J. Immunol. 177,1805–1816 [DOI] [PubMed] [Google Scholar]
- 21.Gilleron M., Nigou J., Nicolle D., Quesniaux V., Puzo G. (2006) Chem. Biol. 13,39–47 [DOI] [PubMed] [Google Scholar]
- 22.Khoo K. H., Dell A., Morris H. R., Brennan P. J., Chatterjee D. (1995) Glycobiology 5,117–127 [DOI] [PubMed] [Google Scholar]
- 23.Besra G. S., Morehouse C. B., Rittner C. M., Waechter C. J., Brennan P. J. (1997) J. Biol. Chem. 272,18460–18466 [DOI] [PubMed] [Google Scholar]
- 24.Gilleron M., Nigou J., Cahuzac B., Puzo G. (1999) J. Mol. Biol. 285,2147–2160 [DOI] [PubMed] [Google Scholar]
- 25.Korduláková J., Gilleron M., Puzo G., Brennan P. J., Gicquel B., Mikusová K., Jackson M. (2003) J. Biol. Chem. 278,36285–36295 [DOI] [PubMed] [Google Scholar]
- 26.Gilleron M., Ronet C., Mempel M., Monsarrat B., Gachelin G., Puzo G. (2001) J. Biol. Chem. 276,34896–34904 [DOI] [PubMed] [Google Scholar]
- 27.Gilleron M., Quesniaux V. F., Puzo G. (2003) J. Biol. Chem. 278,29880–29889 [DOI] [PubMed] [Google Scholar]
- 28.Hsu F. F., Turk J., Owens R. M., Rhoades E. R., Russell D. G. (2007) J. Am. Soc. Mass Spectrom. 18,479–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsu F. F., Turk J., Owens R. M., Rhoades E. R., Russell D. G. (2007) J. Am. Soc. Mass Spectrom. 18,466–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stadelmaier A., Schmidt R. R. (2003) Carbohydr. Res. 338,2557–2569 [DOI] [PubMed] [Google Scholar]
- 31.Liu X., Stocker B. L., Seeberger P. H. (2006) J. Am. Chem. Soc. 128,3638–3648 [DOI] [PubMed] [Google Scholar]
- 32.Dyer B. S., Jones J. D., Ainge G. D., Denis M., Larsen D. S., Painter G. F. (2007) J. Org. Chem. 72,3282–3288 [DOI] [PubMed] [Google Scholar]
- 33.Boonyarattanakalin S., Liu X., Michieletti M., Lepenies B., Seeberger P. H. (2008) J. Am. Chem. Soc. 130,16791–16799 [DOI] [PubMed] [Google Scholar]
- 34.Vercellone A., Puzo G. (1989) J. Biol. Chem. 264,7447–7454 [PubMed] [Google Scholar]
- 35.Ainge G. D., Parlane N. A., Denis M., Hayman C. M., Larsen D. S., Painter G. F. (2006) Bioorg. Med. Chem. 14,7615–7624 [DOI] [PubMed] [Google Scholar]
- 36.Ainge G. D., Hudson J., Larsen D. S., Painter G. F., Gill G. S., Harper J. L. (2006) Bioorg. Med. Chem. 14,5632–5642 [DOI] [PubMed] [Google Scholar]
- 37.Michelsen K. S., Aicher A., Mohaupt M., Hartung T., Dimmeler S., Kirschning C. J., Schumann R. R. (2001) J. Biol. Chem. 276,25680–25686 [DOI] [PubMed] [Google Scholar]
- 38.Kawai T., Adachi O., Ogawa T., Takeda K., Akira S. (1999) Immunity 11,115–122 [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto M., Sato S., Hemmi H., Hoshino K., Kaisho T., Sanjo H., Takeuchi O., Sugiyama M., Okabe M., Takeda K., Akira S. (2003) Science 301,640–643 [DOI] [PubMed] [Google Scholar]
- 40.Mendiratta S. K., Martin W. D., Hong S., Boesteanu A., Joyce S., Van Kaer L. (1997) Immunity 6,469–477 [DOI] [PubMed] [Google Scholar]
- 41.Townsend M. J., Fallon P. G., Matthews D. J., Jolin H. E., McKenzie A. N. (2000) J. Exp. Med. 191,1069–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee S. J., Evers S., Roeder D., Parlow A. F., Risteli J., Risteli L., Lee Y. C., Feizi T., Langen H., Nussenzweig M. C. (2002) Science 295,1898–1901 [DOI] [PubMed] [Google Scholar]
- 43.Lanoue A., Clatworthy M. R., Smith P., Green S., Townsend M. J., Jolin H. E., Smith K. G., Fallon P. G., McKenzie A. N. (2004) J. Exp. Med. 200,1383–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tannenbaum S. R. (1982) Anal. Biochem. 126,131–138 [DOI] [PubMed] [Google Scholar]
- 45.Lefort J., Singer M., Leduc D., Renesto P., Nahori M. A., Huerre M., Créminon C., Chignard M., Vargaftig B. B. (1998) J. Immunol. 161,474–480 [PubMed] [Google Scholar]
- 46.Schnyder-Candrian S., Quesniaux V. F., Di Padova F., Maillet I., Noulin N., Couillin I., Moser R., Erard F., Vargaftig B. B., Ryffel B., Schnyder B. (2005) J. Immunol. 175,262–269 [DOI] [PubMed] [Google Scholar]
- 47.Jones B. W., Means T. K., Heldwein K. A., Keen M. A., Hill P. J., Belisle J. T., Fenton M. J. (2001) J. Leukocyte Biol. 69,1036–1044 [PubMed] [Google Scholar]
- 48.Singh-Gill G., Larsen D. S., Jones J. D., Severn W. B., Harper J. L. ( June2, 2005) European Patent Number WO 2005049631 [Google Scholar]
- 49.Fremond C. M., Yeremeev V., Nicolle D. M., Jacobs M., Quesniaux V. F., Ryffel B. (2004) J. Clin. Invest. 114,1790–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Togbe D., Grivennikov S. I., Noulin N., Couillin I., Maillet I., Jacobs M., Maret M., Fick L., Nedospasov S. A., Quesniaux V. F., Schnyder B., Schnyder-Candrian S. (2007) Eur. J. Immunol. 37,768–779 [DOI] [PubMed] [Google Scholar]
- 51.Ernst W. A., Maher J., Cho S., Niazi K. R., Chatterjee D., Moody D. B., Besra G. S., Watanabe Y., Jensen P. E., Porcelli S. A., Kronenberg M., Modlin R. L. (1998) Immunity 8,331–340 [DOI] [PubMed] [Google Scholar]
- 52.Zajonc D. M., Ainge G. D., Painter G. F., Severn W. B., Wilson I. A. (2006) J. Immunol. 177,4577–4583 [DOI] [PubMed] [Google Scholar]
- 53.Savoy F., Nicolle D. M., Rivier D., Chiavaroli C., Ryffel B., Quesniaux V. F. (2006) Immunobiology 211,767–777 [DOI] [PubMed] [Google Scholar]
- 54.Ilangumaran S., Arni S., Poincelet M., Theler J. M., Brennan P. J., Nasir-ud-Din, Hoessli D. C. (1995) J. Immunol. 155,1334–1342 [PubMed] [Google Scholar]
- 55.de Diego J., Punzón C., Duarte M., Fresno M. (1997) J. Immunol. 159,4983–4989 [PubMed] [Google Scholar]
- 56.Stijlemans B., Baral T. N., Guilliams M., Brys L., Korf J., Drennan M., Van Den Abbeele J., De Baetselier P., Magez S. (2007) J. Immunol. 179,4003–4014 [DOI] [PubMed] [Google Scholar]
- 57.Walsh C., Gangloff M., Monie T., Smyth T., Wei B., McKinley T. J., Maskell D., Gay N., Bryant C. (2008) J. Immunol. 181,1245–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beatty W. L., Rhoades E. R., Ullrich H. J., Chatterjee D., Heuser J. E., Russell D. G. (2000) Traffic 1,235–247 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.