Abstract
Matrix metalloprotease (MMP)-2 plays a key role in many biological and pathological processes related to cell migration, invasion, and mitogenesis. MMP-2 is synthesized as a zymogen that is activated through either a conformational change or proteolysis of the propeptide. Several activating enzymes for pro-MMP-2 have been proposed, including metalloproteases and serine proteases. The mechanism of pro-MMP-2 activation by metalloproteases is well established, and the most studied activation mechanism involves cleavage of the propeptide by membrane type 1-MMP (MT1-MMP). In contrast, serine protease activation has not been thoroughly studied, although studies suggest that MT1-MMP may be involved in activation by thrombin and plasmin. Here, we demonstrate that factor Xa mediates MT1-MMP-independent processing of pro-MMP-2 in vascular smooth muscle cells and endothelial cells. Factor Xa and thrombin directly cleaved the propeptide on the carboxyl terminal sides of the Arg98 and Arg101 residues, whereas plasmin only cleaved the propeptide downstream of Arg101. Moreover, processed MMP-2 showed enzymatic activity that was enhanced by intermolecular autoproteolytic processing at the Asn109-Tyr peptide bond. In addition to its role in activation, factor Xa rapidly degraded MMP-2, thereby restricting excessive MMP-2 activity. Thrombin also degraded MMP-2, but the degradation was reduced greatly under cell-associated conditions, resulting in an increase in processed MMP-2. Overall, factor Xa and thrombin regulate MMP-2 enzymatic activity through its activation and degradation. Thus, the net enzymatic activity results from a balance between MMP-2 activation and degradation.
Matrix metalloprotease (MMP)3-2 is a member of the zinc-dependent endopeptidase family, which comprises 24 enzymes (1). MMP-2 plays a key role in many biological and pathological processes, including organ growth, endometrial cycling, wound healing, bone remodeling, tumor invasion, and metastasis (2). This enzyme functions through proteolysis of non-structural extracellular molecules and components of the basement membrane, including type IV collagen, fibronectin, elastin, laminin, aggrecan, and fibrillin (3).
Like most MMPs, MMP-2 is synthesized as a zymogen that is activated by conformational change (4) or proteolysis within the propeptide, which may involve membrane type MMPs (MT-MMPs) (5–9). The most studied activation mechanism for pro-MMP-2 is cleavage of the propeptide by MT1-MMP, which requires cooperative activity between MT1-MMP and tissue inhibitor of metalloprotease (TIMP)-2 (5, 10–12). Serine proteases, such as thrombin, factor Xa, activated protein C, and plasmin as well as the cysteine protease legumain are all known activators of pro-MMP-2 (13–17).
In addition to its role in coagulation, thrombin is involved in multiple cellular processes, including mitogenesis of fibroblasts (18), lymphocytes (19), mesenchymal cells (20), and smooth muscle cells (SMCs) (21, 22). Factor Xa acts as a potent mitogen for endothelial cells (23), fibroblasts (24), and vascular SMCs (25, 26). Both proteases can also elicit endothelial cell and SMC migration through pro-MMP-2 activation and subsequent extracellular matrix degradation (13, 27, 28). However, despite studies suggesting that MT1-MMP is involved in thrombin-mediated activation of pro-MMP-2, a detailed mechanism for MMP-2 activation has yet to be elucidated (15, 27).
In this study, we investigated the roles of factor Xa and thrombin in MMP-2 regulation. Data are presented to demonstrate that factor Xa mediates MT1-MMP-independent processing of pro-MMP-2 by cleavage of specific sites within the propeptide. Furthermore, factor Xa-processed MMP-2 showed enzymatic activity that was enhanced following intermolecular autoproteolytic cleavage. Thrombin also activated pro-MMP-2 through the same cleavage reaction. Interestingly, factor Xa and thrombin were also found to be involved in MMP-2 degradation. However, this activity was reduced greatly in thrombin-treated MMP-2 by the cell surface, which resulted in an increase in processed MMP-2.
EXPERIMENTAL PROCEDURES
Reagents
Human factor Xa, thrombin, and plasmin were purchased from Hematologic Technologies (Essex Junction, VT). Monoclonal antibodies specific to MMP-2 (sc-13595), TIMP-2 (sc-21735), and rabbit polyclonal MT1-MMP antibody (sc-30074) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Monoclonal anti-integrin αvβ3 antibody, polyclonal anti-integrin αv, monoclonal anti-glyceraldehyde-3-phosphate dehydrogenase, GM6001, and human TIMP-2 were purchased from Chemicon (Temecula, CA), and anti-Myc antibody (clone 9E10) was from Invitrogen. Type IV collagen, Pefabloc TH, and concanavalin A were purchased from Sigma, and the EnzChek gelatinase/collagenase assay kit was obtained from Molecular Probes, Inc. (Eugene, OR). siGENOME SMARTpool small interfering RNA against integrin αv was purchased from Dharmacon (Lafayette, CO). Recombinant tick anticoagulant protein (rTAP) was kindly provided by Dr. Yangsoo Jang.
Expression Plasmids and Site-directed Mutagenesis
BLAST programs from the National Center for Biotechnology Information were used to search for expressed sequence tags. Human testis cDNA (Marathon cDNA; Clontech, Palo Alto, CA) was used as a template to amplify the full-length cDNA for MMP-2 (GenBankTM accession number NM_004530). Oligonucleotide primers 5′-GCTACGATGGAGGCGCTAATGGCC-3′ (start codon underlined) and 5′-TCAGCAGCCTAGCCAGTCGGATTTG-3′ (stop codon underlined) were used for PCR with Advantage 2 polymerase (Clontech). The 2-kb PCR product was cloned into TOPO cloning vectors (Invitrogen) and sequenced completely. For the full-length MMP-2 expression plasmid, its open reading frame was digested with EcoRI and then recloned into the pcDNA3.1/Myc-His(−) B vector (Invitrogen) digested with EcoRI. All mutants used for the present study were generated by site-directed mutagenesis (Intron Biotechnology). The human MT1-MMP expression plasmid was kindly provided by Dr. Suneel Apte.
Cell Culture, Transfection, and Cell Treatments
Rat aortic SMCs (Bio-Bud), HEK293F, COS-1, MCF-7 (human breast adenocarcinoma) (ATCC number HTB-22), and human brain microvascular endothelial cell line (HBMEC) (29) were maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum. Human umbilical vein endothelial cells (HUVECs) were maintained on gelatin-coated plastic dishes in M199 culture medium containing 20% fetal bovine serum, 10 units/ml heparin, and 3 ng/ml basic fibroblast growth factor. The endothelial cells used in all experiments were from passages 3–5. Transfection with plasmids and small interfering RNA was performed using Lipofectamine 2000 according to the manufacturer's recommendations (Invitrogen). For secreted MMP-2, transfected COS-1 and HEK293F cells were cultured and then transferred to 293 SFM-II medium (Invitrogen).
Invasion Assay
Cell invasion assays were performed using Transwell inserts with 6.5-mm diameter polycarbonate 8-μm microporous membranes (Costar, Cambridge, MA). The filters were coated with Matrigel (BD Biosciences) at 7 μg/well. Trypsinized cells were pelleted by centrifugation at 1,500 rpm for 5 min and resuspended in DMEM containing 1% fetal bovine serum. A 100-μl cell suspension containing 1 × 104 cells in the presence and absence of 1 μm rTAP or 10 μm GM6001 was placed in the upper chamber, and factor Xa was subsequently added. Then 600 μl of DMEM containing 10% fetal bovine serum was added to the lower chamber. After 16 h, any cells remaining on the upper surface of the membrane were removed with a cotton swab. The lower side of the membrane was fixed in 4% formaldehyde and stained with 10% Giemsa. The cell number was counted using a light microscope.
Flow Cytometric Analysis
Cells were detached from 6-well plates using phosphate-buffered saline containing 5 mm EDTA and incubated with a monoclonal antibody against integrin αvβ3 at 4 °C for 2 h. The cells were washed and further incubated with fluorescein isothiocyanate-conjugated goat anti-mouse antibody (Chemicon) for 1 h. Flow cytometry was performed on a FACSCalibur flow cytometer (BD Biosciences), and data were analyzed using WinMDI software version 2.8 (Scripps Research Institute, La Jolla, CA).
Enzyme-linked Immunosorbent Assay, Zymography, Western Blotting, Protein Purification, and N-terminal Sequencing
MMP-2 present in the conditioned medium of transfected cells was quantitated using an MMP-2 enzyme-linked immunosorbent assay kit according to the manufacturer's recommendations (Calbiochem). The conditioned medium was analyzed for proteins with gelatinolytic activity by substrate lysis using 8% SDS-polyacrylamide gels containing 2 mg/ml gelatin. The gels were washed with 1% Triton X-100 for 1 h and incubated for 14–20 h at 37 °C in 50 mm Tris-HCl, pH 7.5, containing 20 mm CaCl2. Then the gels were stained with 0.2% Coomassie Brilliant Blue R-250 in 40% methanol and 10% acetic acid.
Cells were lysed in buffer containing 50 mm Tris-HCl, pH 7.5, 5 mm EDTA, 150 mm NaCl, 1% Nonidet P-40, and protease inhibitor mixture (Roche Applied Science) for 1 h at 4 °C and then centrifuged. The soluble portion of the lysate was used for Western blotting, which was performed by separation of reduced or nonreduced samples on SDS-PAGE, followed by electroblotting to polyvinylidine difluoride membrane and detection of bound antibody by enhanced chemiluminescence (Amersham Biosciences). MMP-2 was purified from the conditioned medium using gelatin-Sepharose according to the manufacturer's recommendations (Amersham Biosciences). The signal intensity of relevant bands from the zymogram and Western blot was quantitated using ImageJ software (National Institutes of Health, Bethesda, MD). To determine the N-terminal sequence of cleaved MMP-2, treated protein was blotted onto polyvinylidine difluoride membrane prior to Edman degradation (Tufts University Protein Chemistry Facility, Boston, MA).
Collagen IV and Fluorescein-conjugated Gelatin Digestion Assays
Substrates were incubated with gelatin-Sepharose-purified rat MMP-2 or conditioned medium containing MMP-2 (20 μg/ml per reaction) in 50 mm Tris-HCl, pH 7.5, plus 5 mm CaCl2 and 10 μg/ml leupeptin at 37 °C for 6 h with collagen IV as substrate (5 μg/reaction) or 1 h with fluorescein-conjugated gelatin, according to the manufacturer's recommendations. The digested substrates were analyzed by SDS-PAGE or a fluorescence microplate reader set for excitation at 495 nm and emission detection at 515 nm.
Statistical Analysis
Data are represented as the mean and S.D. of n experiments. Statistical analysis was performed using an unpaired Student's t test or analysis of variance. A p value less than 0.05 was considered statistically significant.
RESULTS
Factor Xa-mediated Propeptide Processing of MMP-2
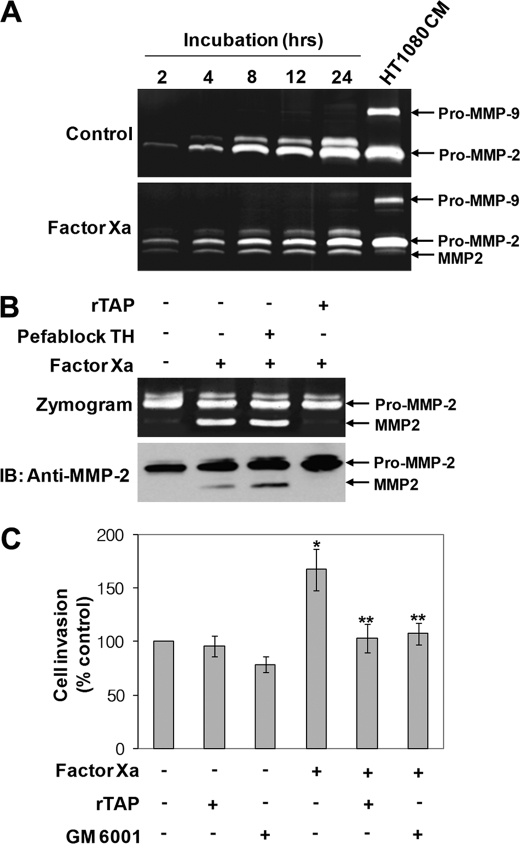
Factor Xa stimulates mitogenesis and extracellular matrix invasion of human vascular SMCs through activation of pro-MMP-2 (13). However, the exact mechanism of pro-MMP-2 activation in factor Xa-treated vascular SMC remains to be elucidated. Therefore, we investigated the molecular role of factor Xa-mediated activation of pro-MMP-2 in SMCs and endothelial cells. Zymography of conditioned medium isolated from factor Xa-treated rat aortic SMCs demonstrated that MMP-2 propeptide processing was induced by factor Xa over time (Fig. 1A). To investigate whether factor Xa catalytic activity or generation of active thrombin is involved, pro-MMP-2 processing was assessed in factor Xa-treated cells incubated with rTAP, a specific factor Xa inhibitor, or Pefablock TH, a thrombin inhibitor. Zymographic and Western blot analysis of the conditioned medium showed that pro-MMP-2 processing was inhibited significantly only in the presence of rTAP (Fig. 1B). These results indicate that enzymatic activity of factor Xa is essential for pro-MMP-2 processing, but the processing is thrombin-independent.
FIGURE 1.
Factor Xa-mediated propeptide processing of MMP-2 in rat aortic SMCs. A, serum-starved rat aortic SMCs were incubated in serum-free DMEM with or without 50 nm factor Xa for the indicated times, and the conditioned medium was analyzed by zymography. Conditioned medium from HT1080 cells was used as markers for MMP-2 and MMP-9 (n = 3). B, cells were pretreated with 1 μm rTAP or Pefablock TH for 30 min before incubation with factor Xa for 8 h. MMP-2 was concentrated from the conditioned medium using gelatin-Sepharose and analyzed by zymography and Western blotting (IB) with an anti-MMP-2 monoclonal antibody. The arrows indicate pro-MMP-2 and processed MMP-2 (n = 3). C, invasion assay for factor Xa-stimulated rat aortic SMCs. Note that non-stimulated cell invasion was slightly decreased in the presence of GM 6001. Data are represented as the mean and S.D. of n = 4 experiments. *, p < 0.05 factor Xa versus control (unstimulated cells); **, p < 0.05 factor Xa versus factor Xa plus inhibitors.
We then examined factor Xa-induced extracellular matrix invasion of rat aortic SMCs. In these invasion assays, cells were incubated with factor Xa in the presence and absence of rTAP or GM6001, an MMP inhibitor, for 16 h. As shown in Fig. 1C, factor Xa increased rat aortic SMC invasion significantly across the matrix gel relative to untreated cells. However, treatment with rTAP or GM6001 suppressed this invasion, suggesting that factor Xa-stimulated cell invasion is dependent on the proteolytic activation of pro-MMP-2. Similar to SMCs, pro-MMP-2 was also found to be processed in factor Xa-treated HUVECs, and factor Xa catalytic activity was essential for this processing independent of active thrombin (data not shown).
Processing of Pro-MMP-2 by Factor Xa Is MT1-MMP- independent
Because MT1-MMP has been suggested to be a major activator of pro-MMP-2 (30–32), we hypothesized that this metalloprotease, as well as TIMP-2 and integrin αvβ3 (10–12, 33), mediated the activation of pro-MMP-2 in factor Xa-treated cells. Therefore, we examined the expression levels of MT1-MMP, TIMP-2, and integrin αvβ3 in these cells. Western blot analysis of cell lysates showed no difference in MT1-MMP expression between untreated and factor Xa-treated rat aortic SMCs and HUVECs (data not shown). Likewise, TIMP-2 expression was not changed by factor Xa treatment (data not shown). Flow cytometric analysis of integrin αvβ3 cell surface expression in HUVECs was also unaffected by factor Xa treatment (data not shown). Expression of integrin αvβ3 could not be examined in rat aortic SMCs, because an antibody to rat integrin αvβ3 was not commercially available. These data suggest that factor Xa processing of pro-MMP-2 is most likely MT1-MMP-independent, since expression of components of MT1-MMP-mediated pro-MMP-2 activation was not altered in factor Xa-treated cells.
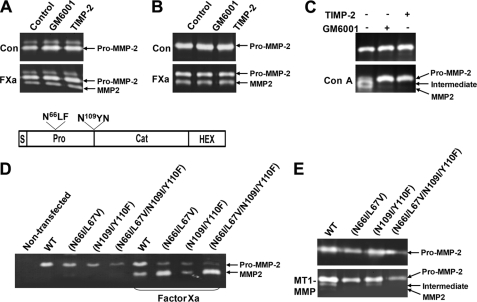
Examination of pro-MMP-2 processing in conditioned medium isolated from factor Xa-treated cells incubated with GM6001 and TIMP-2, metalloprotease inhibitors that abrogate MTI-MMP catalytic activity, further confirmed these results. Zymographic data revealed that pro-MMP-2 processing in rat aortic SMCs and HUVECs was unaffected by these inhibitors (Fig. 2, A and B). The conditioned medium of concanavalin A-treated HT-1080 cells was assessed as a control to demonstrate that MT1-MMP-mediated processing of pro-MMP-2 could be completely inhibited by these metalloprotease inhibitors (Fig. 2C).
FIGURE 2.
MT1-MMP-independent processing of pro-MMP-2 by factor Xa. A and B, effect of metalloprotease inhibitors on propeptide processing of MMP-2 in rat aortic SMCs (A) and HUVECs (B) with or without 50 nm factor Xa (FXa). After an 8-h incubation with 10 μm GM6001 or 2 μg/ml TIMP-2 in serum-free DMEM or M199 medium, conditioned medium was analyzed by zymography. The arrows indicate pro-MMP-2 and processed MMP-2. C, validation of the metalloprotease inhibitors to prevent MT1-MMP-mediated processing of pro-MMP-2. HT-1080 cells were treated with 50 μg/ml concanavalin A (Con A) in the presence and absence of the metalloprotease inhibitors for 16 h. D, zymograms of conditioned medium from COS-1 cells expressing pro-MMP-2 (WT), pro-MMP-2 N66I/L67V, pro-MMP-2 N109I/Y110F, and pro-MMP-2 N66I/L67V/N109I/Y110F treated with or without 50 nm factor Xa for 16 h. The drawing at the top shows the mutation sites. The various modules are represented as follows. S, signal peptide; Pro, propeptide; Cat, catalytic domain; HEX, hemopexin-like domain. E, zymograms of conditioned medium from COS-1 cells expressing pro-MMP-2 (WT), pro-MMP-2 N66I/L67V, pro-MMP-2 N109I/Y110F, and pro-MMP-2 N66I/L67V/N109I/Y110F with or without MT1-MMP (n = 3).
Activation of pro-MMP-2 by MT1-MMP involves cleavage of pro-MMP-2 at the Asn66-Leu peptide bond (5). Thus, to further investigate the role of MT1-MMP in factor Xa-mediated activation of pro-MMP-2, we generated pro-MMP-2 mutants incapable of cleavage by MT1-MMP and/or autolysis, namely N66I/L67V, N109I/Y110F, and N66I/L67V/N109I/Y110F. Transient transfection of these mutant plasmids into COS-1 cells did not affect cleavage, since processed MMP-2 was still detected in the conditioned medium of factor Xa-treated cells (Fig. 2D). To demonstrate the resistance of these mutants to cleavage, COS-1 cells were co-transfected with mutant pro-MMP-2 and MT1-MMP. In particular, N66I/L67V and N66I/L67V/N109I/Y110F were not cleaved by MT1-MMP, and N109I/Y110F did not undergo autoproteolytic processing following MT1-MMP-cleavage (Fig. 2E). Interestingly, fully activated MMP-2 was observed weakly in the conditioned medium of cells co-expressing wild type pro-MMP-2 with MT1-MMP (Fig. 2E). Taken together, these data suggest that the processing of pro-MMP-2 by factor Xa occurs in an MT1-MMP-independent manner.
MMP-2 Propeptide Is Proteolytically Processed Immediately Downstream of Arg98 and Arg101 by Factor Xa
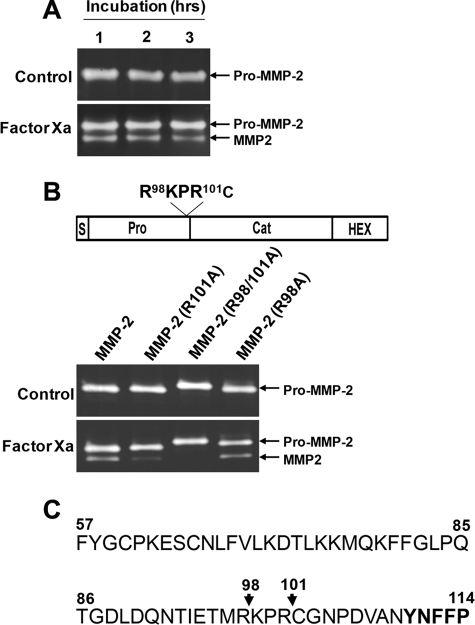
Based on the results described above, we postulated that factor Xa-mediated pro-MMP-2 processing may involve direct cleavage of the propeptide by factor Xa. To test this hypothesis, we incubated purified pro-MMP-2 with factor Xa under cell-free conditions. Zymographic analysis showed that factor Xa converted the purified pro-MMP-2 to its processed form (Fig. 3A), demonstrating direct cleavage of the propeptide by factor Xa.
FIGURE 3.
Factor Xa cleavage sites in the MMP-2 propeptide. A, direct cleavage of the propeptide by factor Xa. Purified pro-MMP-2 (100 ng/ml) from transfected COS-1 cells was incubated with 50 nm factor Xa for the indicated times, and zymographic analysis was performed. The arrows indicate pro-MMP-2 and processed MMP-2. B, zymograms of conditioned medium from COS-1 cells expressing pro-MMP-2, pro-MMP-2 R101A, pro-MMP-2 R98A/R101A, or pro-MMP-2 R98A treated with or without 50 nm factor Xa for 16 h. Note that the pro-MMP-2 R98A/R101A mutant migrates slower on the gel, possibly due to the replacement of two positively charged Arg residues with the uncharged Ala. The drawing at the top shows the mutation sites. C, partial sequence of the propeptide and start of the catalytic domain (boldface type). The arrows indicate factor Xa cleavage sites (n = 3).
Next, the factor Xa cleavage site within pro-MMP-2 was identified by N-terminal sequencing of the cleaved product. The N-terminal sequence of cleaved MMP-2, as determined by Edman degradation, was Cys102-Gly-Asn-Pro-Asp-Val, which lies within the conserved cysteine switch motif (1). Mutation of Arg101 to Ala reduced factor Xa-mediated processing of pro-MMP-2 dramatically but did not completely abolish this activity (Fig. 3B). Therefore, further site-directed mutagenesis was performed to substitute Arg98 and Arg98/Arg101 with Ala, generating the pro-MMP-2 R98A and pro-MMP-2 R98A/R101A mutants. Analysis of conditioned medium from COS-1 cell cultures expressing these mutants showed that the pro-MMP-2 R98A/R101A mutant was completely resistant to factor Xa-induced cleavage (Fig. 3B), whereas the pro-MMP-2 R98A mutant was normally processed. These results demonstrate that factor Xa cleaves pro-MMP-2 immediately downstream of Arg98 and Arg101 with a preference for Arg101 (Fig. 3, B and C).
Thrombin Cleavage of Pro-MMP-2 on the C Terminus of Arg98 and Arg101
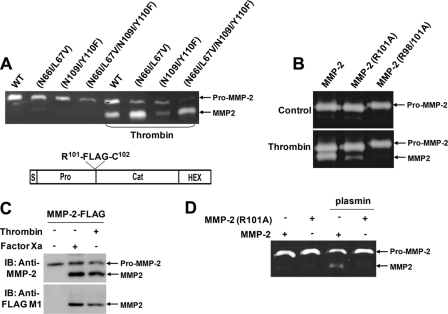
Since thrombin has been shown to activate pro-MMP-2 in endothelial and smooth muscle cells through MT1-MMP-dependent and -independent pathways (15, 27), we examined whether processing of pro-MMP-2 by thrombin in rat aortic SMCs and HUVECs involved MT1-MMP activity. GM6001 and TIMP-2 did not inhibit processing of the propeptide by thrombin (data not shown), suggesting that thrombin processing can occur independently of MT1-MMP activity. Furthermore, thrombin processing of pro-MMP-2 mutants incapable of MT1-MMP cleavage and/or autolysis was observed in COS-1 cells (Fig. 4A). Direct cleavage of purified pro-MMP-2 by thrombin occurred under cell-free conditions, albeit at a lower efficiency (data not shown).
FIGURE 4.
Thrombin cleaves the MMP-2 propeptide at the same sites as factor Xa. A, zymogram of conditioned medium from COS-1 cells expressing pro-MMP-2 (WT), pro-MMP-2 N66I/L67V, pro-MMP-2 N109I/Y110F, and pro-MMP-2 N66I/L67V/N109I/Y110F treated with or without 50 nm thrombin for 16 h. The arrows indicate pro- and processed MMP-2. B, zymograms of conditioned medium from COS-1 cells expressing pro-MMP-2, pro-MMP-2, R101A, or pro-MMP-2 R98A/R101A treated with or without thrombin for 16 h. C, conditioned medium of COS-1 cells expressing pro-MMP-2-RKPR101-FLAG (MMP-2-FLAG) was incubated with 50 nm factor Xa or thrombin for 16 h. Western blotting (IB) with anti-MMP-2 or anti-FLAG M1 antibody was performed to analyze the conditioned medium. The structure of this construct is shown above the gel. D, zymogram of conditioned medium from COS-1 cells expressing pro-MMP-2 or pro-MMP-2 R101A treated with or without plasmin for 16 h. (n = 3).
The MMP-2 propeptide contains one potential thrombin cleavage site (i.e. Xaa-Pro-Arg-Xaa-Xaa-Xaa, where Xaa is a non-acidic residue) (34), which corresponds to the factor Xa cleavage site (Arg98-Lys-Pro-Arg101-Cys). To investigate whether thrombin could cleave pro-MMP-2 within this motif, COS-1 cells expressing the pro-MMP-2 R101A mutant were treated with thrombin. Partial inhibition of thrombin-mediated pro-MMP-2 processing was observed (Fig. 4B). However, mutation of both Arg98 and Arg101 to Ala resulted in a complete loss of processing (Fig. 4B), suggesting that thrombin and factor Xa share MMP-2 cleavage sites.
To further verify that pro-MMP-2 is cleaved immediately downstream of Arg98-Lys-Pro-Arg101 by factor Xa and thrombin, a pro-MMP-2 mutant in which a FLAG epitope was inserted on the C-terminal side of Arg98-Lys-Pro-Arg101 was generated (Fig. 4C). In these experiments, anti-FLAG M1 antibody was used to detect processed MMP-2 by Western blotting, since this clone only recognizes proteins with a free N-terminal FLAG tag. The results show that processed MMP-2 was recognized specifically by the M1 antibody (Fig. 4C, bottom), whereas pro-MMP-2 and processed MMP-2 were detected by the anti-MMP-2 antibody (Fig. 4C, top). Since plasmin has also been shown to activate pro-MMP-2 (14, 35), we examined whether this enzyme could cleave the same bonds within the propeptide. As shown in Fig. 4D, plasmin processing of the propeptide did not occur in COS-1 cells expressing the pro-MMP-2 R101A mutant, indicating cleavage on the C-terminal side of Arg101.
Autoproteolytic Processing of Factor Xa-cleaved MMP-2 Is Required for Its Full Activation
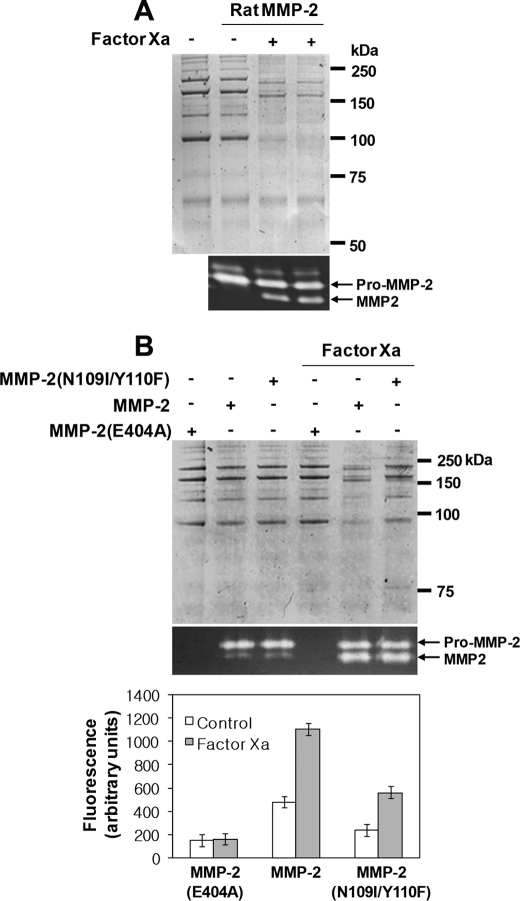
The proteolytic activity of MMP-2 purified from conditioned medium of rat aortic SMCs treated with or without factor Xa using collagen IV as a substrate was assessed. SDS-PAGE analysis showed that factor Xa-processed MMP-2 had greater proteolytic activity than MMP-2 from untreated rat aortic SMC, which exhibited low levels of enzymatic activity (Fig. 5A). Because factor Xa cleaves pro-MMP-2 on the N-terminal side of the Cys102 residue, this amino acid was retained after processing and may be capable of interacting with the catalytic domain zinc ion, keeping MMP-2 in a latent state. To test this, we investigated whether factor Xa-cleaved MMP-2 possessed any proteolytic activity or whether further autoproteolytic processing at the Asn109-Tyr peptide bond (36) was required. We compared proteolysis of collagen IV and fluorescein-conjugated gelatin in the conditioned medium of HEK293F cells expressing pro-MMP-2, the pro-MMP-2 E404A mutant, or the pro-MMP-2 N109I/Y110F mutant treated with or without factor Xa. A Glu404 → Ala mutation within the active site generates a proteolytically inactive enzyme (1). Our data showed that autoproteolytic processing was completely abolished in the conditioned medium of cells expressing the pro-MMP-2 N109I/Y110F mutant and MT1-MMP (Fig. 2E). These experiments were performed using conditioned medium from cells expressing the various constructs to avoid possible structural effects from the processed MMP-2 during purification. MMP-2 concentrations were measured by an enzyme-linked immunosorbent assay. All substrates were incubated with the same amount of MMP-2 and then analyzed by SDS-PAGE or fluorometry. Although MMP-2 was activated endogenously, its proteolytic activity increased significantly following cleavage by factor Xa (Fig. 5B). After factor Xa processing, the MMP-2 N109I/Y110F mutant exhibited lower enzymatic activity than the wild type protein, although its enzymatic activity increased after cleavage by factor Xa (Fig. 5B). Similar results were obtained in MCF-7 cells (data not shown). Collectively, these data demonstrated that factor Xa-processed MMP-2 was enzymatically active but required autoproteolytic processing at the Asn109-Tyr peptide bond for full activation.
FIGURE 5.
Factor Xa-cleaved MMP-2 acquires full activation upon autoproteolytic processing at the Asn109-Tyr peptide bond. A, degradation of collagen IV by purified rat MMP-2. The digested substrates were analyzed by reducing SDS-PAGE. The zymogram shows that equal amounts of MMP-2 were used in each reaction (n = 2). The arrows indicate pro- and processed MMP-2. B, degradation of collagen IV and fluorescein-conjugated gelatin. Conditioned medium was obtained from HEK293F cells expressing pro-MMP-2, pro-MMP-2 E404A, and pro-MMP-2 N109I/Y110F treated with or without 50 nm factor Xa. The digested substrates were analyzed as described under “Experimental Procedures.” Data are represented as the mean and S.D. of n = 6. An analysis of variance test indicated statistically significant differences in factor Xa versus control and wild type versus the N109I/Y110F mutant, respectively (p < 0.05).
Factor Xa Negatively Regulates MMP-2 Activity through MMP-2 Degradation
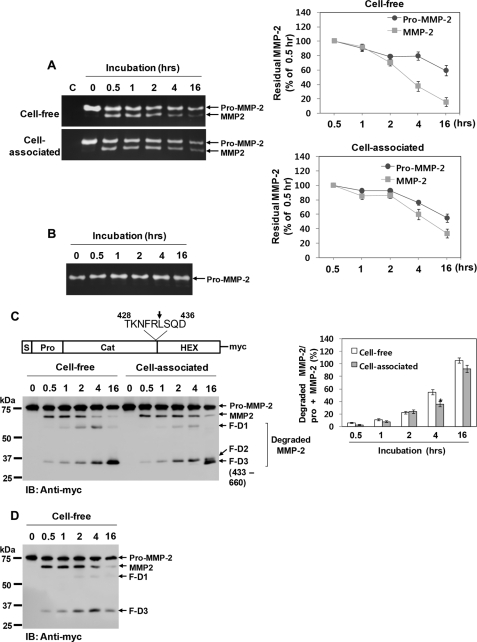
MMP-2 possesses several putative factor Xa cleavage sites within the catalytic and hemopexin-like domains, as well as the prodomain (34, 37). Therefore, whether factor Xa could negatively regulate MMP-2 activity through its degradation was examined. Purified pro-MMP-2 was incubated with factor Xa under cell-free or cell-associated conditions (HBMEC), and the reaction mixture was analyzed by zymography. The data revealed that the highest level of processing was attained after 30 min of incubation with factor Xa in both conditions (Fig. 6A). Further incubation did not increase the amount of processed MMP-2 but resulted in a gradual decrease in total MMP-2, where the level of the processed form declined more rapidly than pro-MMP-2 (Fig. 6A). Moreover, processed MMP-2 was eliminated more rapidly under cell-free conditions, whereas the decline in pro-MMP-2 was similar under both conditions (Fig. 6A). Pro-MMP-2 remained unaffected without factor Xa under cell-free (data not shown) and cell-associated conditions (Fig. 6B).
FIGURE 6.
Degradation of MMP-2 by factor Xa. A, zymograms of the reaction mixture from incubation of purified pro-MMP-2 (1 μg/ml) with 50 nm factor Xa in cell-free or cell-associated conditions (HBMEC) for the indicated times. The arrows indicate pro-MMP-2 and processed MMP-2. The line graphs show the ratio of MMP-2 at the indicated time points to MMP-2 at 0.5 h. Data are represented as the mean and S.D. of n = 3 experiments. B, zymogram of 1 μg/ml pro-MMP-2 incubated without factor Xa in HBMEC for the indicated times (n = 2). C, Western blotting (IB) using an anti-Myc antibody of 20 μg/ml pro-MMP-2-Myc/His incubated with 50 nm factor Xa under cell-free or cell-associated conditions for the indicated times. Cleavage products (F-D1, F-D2, and F-D3) are seen in factor Xa-treated MMP-2. The drawing at the top shows how cleavage at Arg433 generates the F-D3 fragment. The bar graph shows the ratio of the cleaved MMP-2 fragments (F-D1 + F-D2 + F-D3) to total MMP-2 (pro-MMP-2 + MMP-2). Data are represented as the mean and S.D. of n = 3 experiments. *, p < 0.05 cell-associated versus cell-free conditions. D, Western blotting using an anti-Myc antibody of 20 μg/ml pro-MMP-2-Myc/His E404A incubated with factor Xa under cell-free conditions for the indicated durations. Note that the degradation products (F-D1 and F-D3) are reduced relative to wild type MMP-2 (n = 2).
Next, experiments were performed using C-terminal Myc/His-tagged pro-MMP-2, followed by immunoblotting with an anti-Myc antibody. When purified pro-MMP-2-Myc/His was incubated with factor Xa under cell-free or cell-associated conditions, more degradation MMP-2 products were seen with a concomitant decrease in total MMP-2 (Fig. 6C). The decrease in pro-MMP-2 was similar in both conditions (Fig. 6C). However, the decrease in activated MMP-2 and degradation of total MMP-2 were reduced under cell-associated conditions, although the difference was not significant (Fig. 6C). These results indicate that processed MMP-2 may be more readily degraded by factor Xa under cell-free conditions and that its degradation may be reduced at the cell surface.
Through N-terminal sequencing of the cleaved product (F-D3, 35 kDa), we also identified an additional cleavage site on the C-terminal side of Arg433 near the hemopexin-like domain (Fig. 6C). However, a cleavage product with a slightly higher molecular weight (F-D2) seen at early time points was converted to the 35-kDa product under cell-associated conditions (Fig. 6C). The identity of the degradation products, F-D1 and F-D2, is presently unknown. These same degradation products were obtained when this experiment was performed using a proteolytically inactive MMP-2 mutant (pro-MMP-2-Myc/His E404A) (Fig. 6D), indicating that the anti-Myc-reactive fragments were not autoproteolytic in origin. Thus, we propose that factor Xa can rapidly inactivate activated MMP-2 through its degradation under cell-free conditions. However, this degradation may be impeded at the cell surface, leading to cell surface restriction of MMP-2 activity.
Interaction with the Cell Surface Increases Pro-MMP-2 Processing by Thrombin
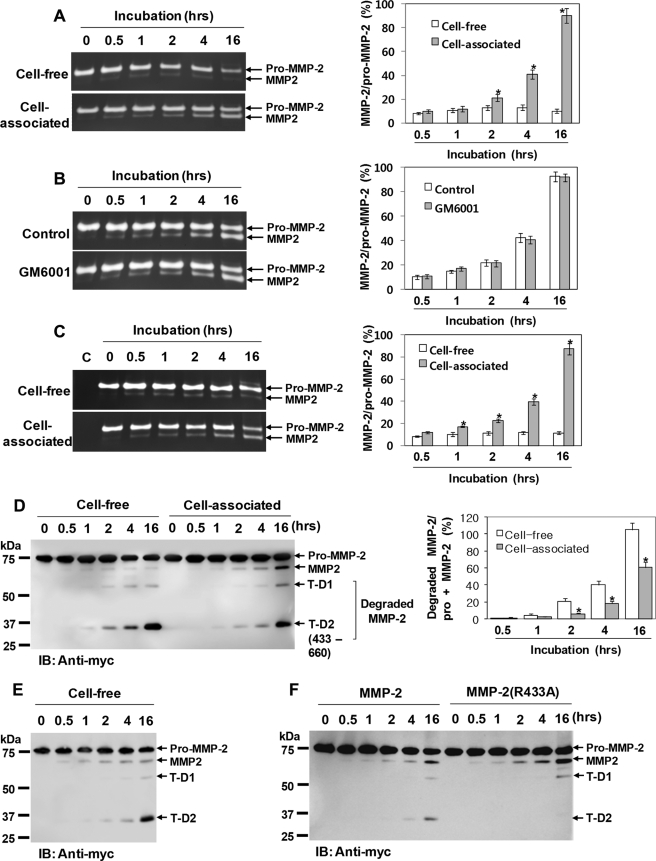
We further investigated whether thrombin could also regulate MMP-2 activity similarly to factor Xa. Thus, purified pro-MMP-2 was incubated with thrombin under cell-free or cell-associated conditions (HBMEC), and the reaction mixture was analyzed by zymography. As shown in Fig. 7A, little processed MMP-2 could be detected after 30 min of incubation with thrombin under both conditions. However, prolonged incubations did not increase this processing under cell-free conditions, unlike the cell-associated conditions (Fig. 7A).
FIGURE 7.
Thrombin processing of pro-MMP-2 is increased significantly under cell-associated conditions. A, zymograms of 1 μg/ml pro-MMP-2 incubated with 50 nm thrombin under cell-free or cell-associated conditions (HBMEC) for the indicated times. The arrows indicate pro-MMP-2 and processed MMP-2. The bar graph shows the ratio of processed MMP-2 to pro-MMP-2. Data are represented as the mean and S.D. of n = 3 experiments. *, p < 0.05 cell-associated versus cell-free conditions. B, effect of 10 μm GM6001 on thrombin-mediated processing of pro-MMP-2 in HBMEC. Zymographic analysis was performed to analyze the incubation mixture. Data are represented as the mean and S.D. of n = 3 experiments. C, zymograms of 1 μg/ml pro-MMP-2 incubated with 50 nm thrombin under cell-free or cell-associated conditions (COS-1). The data shown represent the mean and S.D. of n = 3 experiments. *, p < 0.05 cell-associated versus cell-free conditions. D, Western blot analysis (IB) using an anti-Myc antibody of 20 μg/ml pro-MMP-2-Myc/His incubated with 50 nm thrombin under cell-free or cell-associated conditions (HBMEC) for the indicated times. Cleavage products (T-D1 and T-D2) are seen in thrombin-treated MMP-2. T-D2 was also generated by cleavage of MMP-2 at Arg433. The bar graph shows the ratio of the cleaved MMP-2 fragments (T-D1 + T-D2) to total MMP-2 (pro-MMP-2 + MMP-2). Data represent the mean and S.D. of n = 3 experiments. *, p < 0.05 cell-associated versus cell-free conditions. E, Western blot analysis using an anti-Myc antibody of 20 μg/ml pro-MMP-2-Myc/His E404A incubated with 50 nm thrombin under cell-free conditions for the indicated times. Note that cleavage products (T-D1 and T-D2) are also seen in thrombin-treated pro-MMP-2-Myc/His E404A (n = 2). F, Western blotting using an anti-Myc antibody of 20 μg/ml each of pro-MMP-2-Myc/His and pro-MMP-2-Myc/His R433A incubated with 50 nm thrombin under cell-associated conditions (HBMEC) for the indicated times (n = 3).
Because thrombin has been shown to activate pro-MMP-2 in an MT1-MMP-dependent manner (15, 27), we tested whether the increase in processing observed under cell-associated conditions was due to the proteolytic activity of MT1-MMP. However, zymography showed that the processing was unaffected by GM6001 (Fig. 7B). Likewise, when this experiment was performed in COS-1 cells that did not express MT1-MMP (38), the processing occurred normally as in HBMEC (Fig. 7C). Thus, these data suggest that thrombin processing of pro-MMP-2 can occur independently of the proteolytic activity of MT1-MMP.
For further confirmation, these experiments were also performed using pro-MMP-2-Myc/His. Analysis of the incubation mixture by Western blotting with anti-Myc antibody showed an increase in MMP-2 degradation and a corresponding decrease in activation under cell-free conditions (Fig. 7D). Thus, these results suggest that the cell surface may increase thrombin-mediated processing of pro-MMP-2 in an MT1-MMP-independent manner by decreasing MMP-2 degradation. Furthermore, we showed that a 35-kDa fragment (T-D2) was also generated by thrombin cleavage of MMP-2 on the C-terminal side of Arg433, which was determined by N-terminal sequencing of the cleaved product (Fig. 7D). The same degradation products were also seen in the reaction mixture containing the pro-MMP-2-Myc/His E404A mutant and thrombin, indicating that the cleavage was not autoproteolytic (Fig. 7E).
To ascertain whether reducing degradation led to the increase in thrombin-mediated processing of pro-MMP-2 at the cell surface, the pro-MMP-2 R433A mutant was generated. Analysis of the reaction mixture containing pro-MMP-2 or the mutant with thrombin under cell-associated conditions demonstrated that the cleavage product T-D2 was absent in the reaction mixture containing the mutant and thrombin with higher levels of processed MMP-2 (Fig. 7F), demonstrating that inhibition of MMP-2 degradation can increase its processing by thrombin.
DISCUSSION
A variety of proteases have been shown to be involved in the activation of pro-MMP-2, including metalloproteases (e.g. MMP-7 and MT-MMPs) (5–9, 39), serine proteases (e.g. thrombin, factor Xa, activated protein C, plasmin, trypsin-2, mast cell chymase, neutrophil elastase, cathepsin G, and proteinase-3) (13–16, 40–42), and a cysteine protease (e.g. legumain) (17). The mechanisms of pro-MMP-2 activation by the metalloproteases and the cysteine protease are well established. Pro-MMP-2 is activated by MT1-, MT2-, MT3-, and MT5-MMPs via cleavage of the propeptide at the Asn66-Leu peptide bond, followed by intermolecular autoproteolytic processing at the Asn109-Tyr peptide bond (5–8). Alternatively, this propeptide can be activated by MMP-7 and MT6-MMP by direct cleavage at the Asn109-Tyr peptide bond (9, 39). Legumain activates pro-MMP-2 by cleavage at the Asn109-Tyr and Asn111-Phe peptide bonds (17). Serine protease activation of pro-MMP-2, such as that mediated by thrombin and plasmin, has been well studied, with some reports indicating the involvement of MT1-MMP, which remains controversial (14, 15, 27, 35, 43, 44). However, the detailed molecular mechanism for pro-MMP-2 activation by serine proteases remains to be elucidated. Using metalloprotease inhibitors and pro-MMP-2 mutants incapable of cleavage by MT1-MMP, we demonstrated MT1-MMP-independent processing of pro-MMP-2 by factor Xa and thrombin. Factor Xa and thrombin cleaved pro-MMP-2 on the C-terminal side of Arg98 and Arg101, whereas plasmin processed the propeptide only on the C-terminal side of Arg101 and with low cleavage efficiency. The low efficiency of pro-MMP-2 cleavage by plasmin may reflect the lack of MT1-MMP expression in COS-1 cells (38), because MT1-MMP has been shown to be important for plasmin processing of pro-MMP-2 (14, 44).
The Cys102 residue of processed MMP-2 is thought to interact with the catalytic domain zinc ion and therefore may not possess enzymatic activity. However, MMP-2 cleaved by factor Xa was enzymatically active. Its enzymatic activity was enhanced after autoproteolytic processing at the Asn109-Tyr peptide bond, indicating that the cysteine-zinc ion pairing may be disrupted by cleavage at these sites. Consistent with previous data (14, 15), we showed that MMP-2 processed by thrombin and plasmin was also enzymatically active (data not shown). Cleavage of pro-MMP-2 at Arg101-Cys by furin and at Arg98-Lys by trypsin-2 has also been reported (40, 45). In contrast to our data, furin-cleaved MMP-2 did not exhibit enzymatic activity in COS-1 cells, and trypsin-2-cleavage of the propeptide resulted in only partial activation of pro-MMP-2 (40, 45). We also found that factor Xa- or thrombin-processed MMP-2 in the conditioned medium of COS-1 cells had substantially lower enzymatic activity than that obtained from HEK293F and MCF-7 cells (data not shown). This may reflect the high expression of TIMP-2 in COS-1 cells (data not shown), since this protein is a strong inhibitor of MMP-2 at high concentrations (46). High levels of TIMP-2 can inhibit the enzymatic activity of factor Xa-processed MMP-2 and further prevent autoproteolytic activation of processed MMP-2. Autoproteolytic activation of MT1-MMP-processed MMP-2 was detected marginally in COS-1 cells (Fig. 2E). Furthermore, trypsin-2-processed MMP-2 was obtained by incubating purified pro-MMP-2 with trypsin-2 under cell-free conditions (40). Therefore, autoproteolytic processing of trypsin-2-cleaved MMP-2 is not likely to occur, since cellular molecules, such as TIMP-2 and integrin αvβ3, may be required for the maturation of partially processed MMP-2 to its fully active form (10, 12, 33, 47). This may explain the partial activation of trypsin-2-processed MMP-2 seen under cell-free conditions. Reduced activity of factor Xa-processed MMP-2 was also found under cell-free conditions (data not shown).
Despite the importance of TIMP-2 and integrin αvβ3 in the autoproteolytic activation of MMP-2 following MT1-MMP-cleavage (10, 33, 47), these molecules are not likely to be involved in the complete activation of factor Xa-processed MMP-2. Full activation of MMP-2 was not affected in MCF-7 cells that express negligible amounts of TIMP-2 and HEK293F cells transfected with integrin αv small interfering RNA (data not shown). Other plausible candidates for involvement in the full activation are fibronectin and heparan sulfate proteoglycans, which display high binding affinity for MMP-2 (48, 49). Alternatively, unknown molecules may also play a role in this process. Thus, MMP-2 can be localized and concentrated at the cell surface via these molecules, which may promote autoproteolytic processing of factor Xa-processed MMP-2.
Because factor Xa generation occurs following intravascular injury for a long period of time (50, 51) and factor Xa-mediated MMP-2 activation is robust, it can exert deleterious effects on tissues unless it is tightly regulated. Regulation of MMP-2 activity occurs at multiple levels, including gene expression, compartmentalization, zymogen activation, and enzyme inactivation by extracellular inhibitors, such as TIMPs (3). Our data indicate that MMP-2 degradation might also be a mechanism of inactivation, since factor Xa appears to negatively regulate MMP-2 activity through its degradation. We also found that processing immediately followed factor Xa treatment, whereas further incubation with factor Xa resulted in a rapid decrease in processed MMP-2. One possible explanation for this event is that MMP-2 is more rapidly degraded by factor Xa upon activation compared with pro-MMP-2. In addition, pro-MMP-2 levels may continue to decrease, since pro-MMP-2 is also degraded and activated following factor Xa treatment, thereby leading to reduced pro-MMP-2 available for conversion to the activated form. Autodegradation is unlikely, because the inactive MMP-2 mutant was also degraded by factor Xa and thrombin, and MMP-2 remained intact in the absence of the enzymes. Previous studies showed MMP-2 degradation by plasmin and trypsin-2, further supporting the negative regulation of MMP-2 activity through degradation (35, 40). Thus, inactivation of MMP-2 activity through its degradation may be a general mechanism for the regulation of excessive enzymatic activity.
Here, we discovered that thrombin-mediated processing of pro-MMP-2 is greatly increased by the cell surface. We first speculated that MT1-MMP may be involved in this process because thrombin has been reported to activate pro-MMP-2 in an MT1-MMP-dependent manner (15, 27). Thus, we examined MT1-MMP dependence in thrombin-mediated processing of pro-MMP-2 using a metalloprotease inhibitor and MT1-MMP-deficient cells, all of which provided evidence for non-MT1-MMP components at the cell surface. Finally, we found that interaction with the cell surface greatly increases the processing by lowering the degradation of MMP-2. MMP-2 binding to the cell surface may either induce an unfavorable conformational change for thrombin cleavage or mask its cleavage sites, resulting in a decrease in degradation and a corresponding increase in activation. Although several studies have shown the interactions of MMP-2 with the cell surface via the MT1-MMP·TIMP-2 complex, fibronectin, heparan sulfate, and integrin αvβ3 (48, 49, 52, 53), further study will be necessary to elucidate the interacting molecule to induce molecular changes of MMP-2 that ultimately result in an increase in processed MMP-2.
This work was supported by a Korea Science and Engineering Foundation grant funded by the Korean government (MOST Grants ROA-2004-000-10297-0 and 2009-0081759).
- MMP
- matrix metalloprotease
- MT1-MMP
- membrane type 1 matrix metalloprotease
- SMC
- smooth muscle cell
- rTAP
- recombinant tick anticoagulant protein
- HBMEC
- human brain microvascular endothelial cell line
- DMEM
- Dulbecco's modified Eagle's medium
- HUVEC
- human umbilical vein endothelial cell
- TIMP
- tissue inhibitor of metalloprotease.
REFERENCES
- 1.Ra H. J., Parks W. C. (2007) Matrix Biol. 26,587–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woessner J. F., Jr. (1994) Ann. N.Y. Acad. Sci. 732,11–21 [DOI] [PubMed] [Google Scholar]
- 3.Somerville R. P., Oblander S. A., Apte S. S. (2003) Genome Biol. 4,216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bescond A., Augier T., Chareyre C., Garçon D., Hornebeck W., Charpiot P. (1999) Biochem. Biophys. Res. Commun. 263,498–503 [DOI] [PubMed] [Google Scholar]
- 5.Strongin A. Y., Collier I., Bannikov G., Marmer B. L., Grant G. A., Goldberg G. I. (1995) J. Biol. Chem. 270,5331–5338 [DOI] [PubMed] [Google Scholar]
- 6.Morrison C. J., Butler G. S., Bigg H. F., Roberts C. R., Soloway P. D., Overall C. M. (2001) J. Biol. Chem. 276,47402–47410 [DOI] [PubMed] [Google Scholar]
- 7.Nakada M., Yamada A., Takino T., Miyamori H., Takahashi T., Yamashita J., Sato H. (2001) Cancer Res. 61,8896–8902 [PubMed] [Google Scholar]
- 8.Pei D. (1999) J. Biol. Chem. 274,8925–8932 [DOI] [PubMed] [Google Scholar]
- 9.Nie J., Pei D. (2003) Cancer Res. 63,6758–6762 [PubMed] [Google Scholar]
- 10.Caterina J. J., Yamada S., Caterina N. C., Longenecker G., Holmbäck K., Shi J., Yermovsky A. E., Engler J. A., Birkedal-Hansen H. (2000) J. Biol. Chem. 275,26416–26422 [DOI] [PubMed] [Google Scholar]
- 11.Hernandez-Barrantes S., Toth M., Bernardo M. M., Yurkova M., Gervasi D. C., Raz Y., Sang Q. A., Fridman R. (2000) J. Biol. Chem. 275,12080–12089 [DOI] [PubMed] [Google Scholar]
- 12.Wang Z., Juttermann R., Soloway P. D. (2000) J. Biol. Chem. 275,26411–26415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rauch B. H., Bretschneider E., Braun M., Schrör K. (2002) Circ. Res. 90,1122–1127 [DOI] [PubMed] [Google Scholar]
- 14.Baramova E. N., Bajou K., Remacle A., L'Hoir C., Krell H. W., Weidle U. H., Noel A., Foidart J. M. (1997) FEBS Lett. 405,157–162 [DOI] [PubMed] [Google Scholar]
- 15.Lafleur M. A., Hollenberg M. D., Atkinson S. J., Knäuper V., Murphy G., Edwards D. R. (2001) Biochem. J. 357,107–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson M. T., Smith M. M., Smith S. M., Jackson C. J., Xue M., Little C. B. (2009) Arthritis Rheum. 60,780–791 [DOI] [PubMed] [Google Scholar]
- 17.Chen J. M., Fortunato M., Stevens R. A., Barrett A. J. (2001) Biol. Chem. 382,777–783 [DOI] [PubMed] [Google Scholar]
- 18.Kahan C., Seuwen K., Meloche S., Pouysségur J. (1992) J. Biol. Chem. 267,13369–13375 [PubMed] [Google Scholar]
- 19.Chen D., Carpenter A., Abrahams J., Chambers R. C., Lechler R. I., McVey J. H., Dorling A. (2008) J. Exp. Med. 205,1739–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ozaki Y., Nishimura M., Sekiya K., Suehiro F., Kanawa M., Nikawa H., Hamada T., Kato Y. (2007) Stem Cells Dev. 16,119–129 [DOI] [PubMed] [Google Scholar]
- 21.Wang Z., Kong L., Kang J., Morgan J. H., 3rd, Shillcutt S. D., Robinson J. S., Jr., Nakayama D. K. (2009) Neurosci. Lett. 451,199–203 [DOI] [PubMed] [Google Scholar]
- 22.McNamara C. A., Sarembock I. J., Gimple L. W., Fenton J. W., 2nd, Coughlin S. R., Owens G. K. (1993) J. Clin. Invest. 91,94–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bono F., Herault J. P., Avril C., Schaeffer P., Lormeau J. C., Herbert J. M. (1997) J. Cell. Physiol. 172,36–43 [DOI] [PubMed] [Google Scholar]
- 24.Blanc-Brude O. P., Chambers R. C., Leoni P., Dik W. A., Laurent G. J. (2001) Am. J. Physiol. Cell Physiol. 281,C681–C689 [DOI] [PubMed] [Google Scholar]
- 25.Koo B. H., Kim D. S. (2003) J. Biol. Chem. 278,52578–52586 [DOI] [PubMed] [Google Scholar]
- 26.Herbert J., Bono F., Herault J., Avril C., Dol F., Mares A., Schaeffer P. (1998) J. Clin. Invest. 101,993–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henderson N., Markwick L. J., Elshaw S. R., Freyer A. M., Knox A. J., Johnson S. R. (2007) Am. J. Physiol. Lung Cell Mol. Physiol. 292,L1030–L1038 [DOI] [PubMed] [Google Scholar]
- 28.Galis Z. S., Kranzhöfer R., Fenton J. W., 2nd, Libby P. (1997) Arterioscler. Thromb. Vasc. Biol. 17,483–489 [DOI] [PubMed] [Google Scholar]
- 29.Callahan M. K., Williams K. A., Kivisäkk P., Pearce D., Stins M. F., Ransohoff R. M. (2004) J. Neuroimmunol. 153,150–157 [DOI] [PubMed] [Google Scholar]
- 30.Hotary K., Allen E., Punturieri A., Yana I., Weiss S. J. (2000) J. Cell Biol. 149,1309–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilles C., Polette M., Piette J., Munaut C., Thompson E. W., Birembaut P., Foidart J. M. (1996) Int. J. Cancer 65,209–213 [DOI] [PubMed] [Google Scholar]
- 32.Ellerbroek S. M., Fishman D. A., Kearns A. S., Bafetti L. M., Stack M. S. (1999) Cancer Res. 59,1635–1641 [PubMed] [Google Scholar]
- 33.Deryugina E. I., Ratnikov B., Monosov E., Postnova T. I., DiScipio R., Smith J. W., Strongin A. Y. (2001) Exp. Cell Res. 263,209–223 [DOI] [PubMed] [Google Scholar]
- 34.Jenny R. J., Mann K. G., Lundblad R. L. (2003) Protein Expr. Purif. 31,1–11 [DOI] [PubMed] [Google Scholar]
- 35.Mazzieri R., Masiero L., Zanetta L., Monea S., Onisto M., Garbisa S., Mignatti P. (1997) EMBO J. 16,2319–2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strongin A. Y., Marmer B. L., Grant G. A., Goldberg G. I. (1993) J. Biol. Chem. 268,14033–14039 [PubMed] [Google Scholar]
- 37.Lonsdale-Eccles J. D., Hogg D. H., Elmore D. T. (1980) Biochim. Biophys. Acta 612,401–409 [DOI] [PubMed] [Google Scholar]
- 38.Cao J., Drews M., Lee H. M., Conner C., Bahou W. F., Zucker S. (1998) J. Biol. Chem. 273,34745–34752 [DOI] [PubMed] [Google Scholar]
- 39.Crabbe T., Smith B., O'Connell J., Docherty A. (1994) FEBS Lett. 345,14–16 [DOI] [PubMed] [Google Scholar]
- 40.Sorsa T., Salo T., Koivunen E., Tyynelä J., Konttinen Y. T., Bergmann U., Tuuttila A., Niemi E., Teronen O., Heikkilä P., Tschesche H., Leinonen J., Osman S., Stenman U. H. (1997) J. Biol. Chem. 272,21067–21074 [DOI] [PubMed] [Google Scholar]
- 41.Tchougounova E., Lundequist A., Fajardo I., Winberg J. O., Abrink M., Pejler G. (2005) J. Biol. Chem. 280,9291–9296 [DOI] [PubMed] [Google Scholar]
- 42.Shamamian P., Schwartz J. D., Pocock B. J., Monea S., Whiting D., Marcus S. G., Mignatti P. (2001) J. Cell. Physiol. 189,197–206 [DOI] [PubMed] [Google Scholar]
- 43.Nguyen M., Arkell J., Jackson C. J. (1999) Lab. Invest. 79,467–475 [PubMed] [Google Scholar]
- 44.Monea S., Lehti K., Keski-Oja J., Mignatti P. (2002) J. Cell. Physiol. 192,160–170 [DOI] [PubMed] [Google Scholar]
- 45.Cao J., Rehemtulla A., Pavlaki M., Kozarekar P., Chiarelli C. (2005) J. Biol. Chem. 280,10974–10980 [DOI] [PubMed] [Google Scholar]
- 46.Cao J., Sato H., Takino T., Seiki M. (1995) J. Biol. Chem. 270,801–805 [DOI] [PubMed] [Google Scholar]
- 47.English J. L., Kassiri Z., Koskivirta I., Atkinson S. J., Di Grappa M., Soloway P. D., Nagase H., Vuorio E., Murphy G., Khokha R. (2006) J. Biol. Chem. 281,10337–10346 [DOI] [PubMed] [Google Scholar]
- 48.Wallon U. M., Overall C. M. (1997) J. Biol. Chem. 272,7473–7481 [DOI] [PubMed] [Google Scholar]
- 49.Yu W. H., Woessner J. F., Jr. (2000) J. Biol. Chem. 275,4183–4191 [DOI] [PubMed] [Google Scholar]
- 50.Ragosta M., Gimple L. W., Gertz S. D., Dunwiddie C. T., Vlasuk G. P., Haber H. L., Powers E. R., Roberts W. C., Sarembock I. J. (1994) Circulation 89,1262–1271 [DOI] [PubMed] [Google Scholar]
- 51.Marmur J. D., Rossikhina M., Guha A., Fyfe B., Friedrich V., Mendlowitz M., Nemerson Y., Taubman M. B. (1993) J. Clin. Invest. 91,2253–2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Butler G. S., Butler M. J., Atkinson S. J., Will H., Tamura T., Schade van Westrum S., Crabbe T., Clements J., d'Ortho M. P., Murphy G. (1998) J. Biol. Chem. 273,871–880 [DOI] [PubMed] [Google Scholar]
- 53.Brooks P. C., Strömblad S., Sanders L. C., von Schalscha T. L., Aimes R. T., Stetler-Stevenson W. G., Quigley J. P., Cheresh D. A. (1996) Cell 85,683–693 [DOI] [PubMed] [Google Scholar]