Abstract
Transforming growth factor-β1 (TGF-β1) is the most abundant TGF-β isoform detected in bone and is an important functional modulator of osteoclasts. TGF-β1 can induce osteoclast apoptosis; however, the apoptotic pathways involved in this process are not known. We show here that human osteoclasts express both type-I and type-II TGF-β receptors. In the absence of survival factors, TGF-β1 (1 ng/ml) induced osteoclast apoptosis. The expression of activated caspase-9, but not that of caspase-8, was increased by TGF-β1 stimulation, and the rate of TGF-β1-induced apoptosis was significantly lower in the presence of a caspase-9 inhibitor. To study further the mechanisms involved in TGF-β1-induced osteoclast apoptosis, we investigated TGF-β1 signaling, which primarily involves the Smad pathway, but also other pathways that may interfere with intracellular modulators of apoptosis, such as mitogen-activated protein (MAP) kinases and Bcl2 family members. We show here that early events consisted of a trend toward increased expression of extracellular signal-regulated kinase (ERK), and then TGF-β1 significantly induced the activation of p38 and Smad2 in a time-dependent manner. These signaling cascades may activate the intrinsic apoptosis pathway, which involves Bim, the expression of which was increased in the presence of TGF-β1. Furthermore, the rate of TGF-β1-induced osteoclast apoptosis was lower when Bim expression was suppressed, and inhibiting the Smad pathway abolished Bim up-regulation following TGF-β stimulation. This could correspond to a regulatory mechanism involved in the inhibition of osteoclast activity by TGF-β1.
Bone remodeling is a highly controlled physiological process that results from the balance between the formation and resorption of bone. Disruption of this equilibrium can give rise to bone disorders characterized by excessive bone resorption, such as osteoporosis (1). The bone resorbing cells, known as osteoclasts, are large, multinucleated cells (MNCs)2 of hematopoietic origin. Under the control of local and systemic factors, mainly macrophage-colony-stimulating factor (M-CSF) and receptor activator of nuclear factor-κB ligand (RANKL), monocyte/macrophage precursor cells undergo osteoclast differentiation, and this eventually leads to the formation of active bone-resorbing cells (2).
Most of the factors identified as affecting bone resorption enhance osteoclast formation or activity, but few factors have been shown to inhibit these processes. One important way to reduce osteoclast-resorbing activity is to induce osteoclast apoptosis (3). In recent years, there has been growing evidence to suggest that osteoclast apoptosis could be modulated by locally released signals. For instance, we and others have demonstrated the role of the TNF-related apoptosis-inducing ligand (TRAIL) pathway in osteoclast regulation (4–6). We have also shown that TRAIL is expressed by osteoclasts deprived of survival factors and that this is part of an autocrine loop that modulates osteoclast survival and thus bone remodeling (7).
Transforming growth factor-β1 (TGF-β1) is an important functional modulator of osteoclasts. TGF-β plays a major role in the proliferation, migration, differentiation, and survival of many cell types (8). The actions of TGF-β require first its binding to the TGF-β type-II receptor (TGF-β-RII) followed by the recruitment of TGF-β-RI. These ligand-receptor interactions lead to the phosphorylation of two cytoplasmic adaptors, Smad2 and Smad3, that in turn recruit Smad4 to form a trimeric complex of intracellular mediators that can enter the nucleus and initiate gene transcription (9). TGF-β is also able to activate the MAP kinases ERK, p38, and JNK by alternative pathways (10). TGF-β is known to influence bone metabolism in various ways. Firstly, TGF-β acts on bone formation by up-regulating the recruitment and proliferation of osteoblast precursors and by inhibiting their apoptosis (11). Secondly, TGF-β indirectly slows the formation and activation of osteoclasts by reducing RANKL expression and increasing osteoprotegerin production by osteoblasts (12). On the other hand, TGF-β has direct and contradictory actions on osteoclasts by limiting both the proliferation and the fusion of osteoclast precursors and enhancing RANKL-mediated differentiation (13, 14). In addition, TGF-β is a potent inducer of osteoclast apoptosis, although the mechanisms involved remain poorly understood (6, 15).
Our aim was to investigate the mechanisms of TGF-β-induced osteoclast apoptosis in human osteoclasts differentiated from cord blood monocytes (CBMs). We show here that TGF-β1 activates the intrinsic pathway of apoptosis in osteoclasts. We also show that the p38 and Smad pathways may be involved in mediating the actions of TGF-β and that Bim expression could be necessary for the execution of apoptosis.
EXPERIMENTAL PROCEDURES
Reagents
Recombinant human M-CSF, recombinant human GM-CSF, and caspase inhibitors were purchased from R&D systems (Minneapolis, MN); recombinant human TGF-β1 was obtained from Peprotech (Rocky Hill, NJ); staurosporine, monoclonal mouse antibodies directed against caspase-8, cleaved caspase-8, rabbit polyclonal antibodies against caspase-9, cleaved caspase-9, all anti-MAP kinases and anti-Smad antibodies, as well as the rabbit polyclonal antibody against actin came from Cell Signaling Technologies (Danvers, MA). The rabbit polyclonal antibody against TGF-β type-I and type-II receptors and the rabbit polyclonal antibody against Bcl-2 homologous were both purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Normal goat, rabbit, and mouse serum and LSAB2 staining kit were obtained from DAKO (Glostrup, Denmark). Soluble human RANKL was produced in our laboratory.
Cell Cultures
Umbilical cord blood from normal pregnant women was obtained at delivery after informed consent. Mononuclear leukocyte suspensions were isolated from the cord blood by density-gradient centrifugation and then washed and suspended in OPTI-MEM (Invitrogen, Burlington, Ontario, Canada) with antibiotics (1 IU/ml penicillin, 100 mg/ml streptomycin, 0.5 mg/ml amphotericin B) and 2% fetal bovine serum (Wisent, Montréal, PQ). They were plated at a density of 3 × 106 cells/ml. After incubating overnight, the cells were washed to remove non-adherent cells. The selected CBMs were cultured for another 3 weeks, in the same medium supplemented with GM-CSF (100 pg/ml), for the first 3 days and then with M-CSF (25 ng/ml) and RANKL (100 ng/ml). The medium was changed twice weekly. We have previously shown that fully differentiated osteoclasts form under these conditions (6, 16).
Immunocytochemistry
After 3 weeks of culture in eight-chamber Labtecks, mature cells were fixed with 1% paraformaldehyde in phosphate-buffered saline. Nonspecific binding sites were blocked with 5% skimmed milk, and the cells were then incubated overnight at 4 °C with the primary antibody directed against human TGF-β-RI (1:200), TGF-β-RII (1:200), cleaved caspase-8 (1:100), or cleaved caspase-9 (1:100) or with the same concentration of normal goat or mouse serum. After three washes, the specimens were exposed to 3% H2O2 in phosphate-buffered saline for 5 min to inactivate any endogenous peroxidase activity. Sequential incubations were then performed with biotinylated secondary antibody (45 min) and peroxidase-labeled streptavidin (45 min) from the biotin-streptavidin-peroxidase LSAB2 kit. Specific labeling was revealed with 3-amino-9-ethylcarbazole chromogen, which gave rise to a red precipitate where peroxidase had been bound. To identify multinucleated cells, the specimens were counterstained with hematoxylin.
Western Blot Analysis
To prevent cross-stimulation, osteoclast cultures were performed after removing M-CSF and RANKL, as well as reducing fetal bovine serum to 1% in the OPTI-MEM for 24 h prior to the experiments. TGF-β1 (1 ng/ml) or staurosporine (1 μm) was then added for various periods of time (3–24 h). The cells were then washed twice before being lysed for 20 min on ice in lysis buffer (150 mm NaCl, 50 mm Tris, 1% Nonidet P-40, 0.5% deoxycholate, 10 mm sodium pyrophosphate, 0.1% SDS, 5 mm EDTA). 50 μg of total proteins were separated by SDS-PAGE and transferred to a polyvinylidene difluoride membrane (Millipore, Billerica, MA). The membrane was blocked with 5% skimmed milk and incubated overnight at 4 °C with the primary antibody. For these experiments, we used primary antibodies against caspase-8 (1:250), caspase-9 (1:500), JNK (1:1000) and phospho-JNK (1:250), ERK and phospho-ERK (1:1000), p38 (1:1000) and phospho-p38 (1:500), Smad2 and phospho-Smad2 (1:1000), and Bcl2, Bid, Bax, and Bim (1:250). Anti-actin antibodies were used as a loading control. The membranes were then washed before being incubated for 1 h at room temperature with a horseradish peroxidase-conjugated anti-mouse or anti-rabbit antibody. Bound antibodies were visualized by an enhanced chemiluminescence (ECL) detection system (Amersham Biosciences). All bands were measured by densitometry and normalized for the optical density of the actin bands after stripping and reblotting the membranes (NIH ImageJ software, Bethesda, MD).
Study of Apoptosis
Osteoclast apoptosis was determined after removing M-CSF and RANKL, as well as reducing fetal bovine serum to 1% in the OPTI-MEM for 24 h prior to the experiments. The TACS Blue Label kit (R&D Systems) was then used to detect and quantify apoptosis. This terminal deoxynucleotidyltransferase-mediated dUTP nick end-labeling (TUNEL)-derived method provides in situ cytochemistry visualization of DNA fragmentation at the single cell level. The cells were fixed, washed, and permeabilized in an ethanol:acetic acid (2:1) solution for 10 min at −20 °C. They were then washed thoroughly with phosphate-buffered saline for rehydration. Biotinylated nucleotides were incorporated by terminal deoxynucleotidyl transferase for 2 h at 37 °C. Streptavidin-horseradish peroxidase conjugate was then added (45 min) followed by the substrate, TACS Blue Label (10 min). The resulting enzymatic reaction generated an insoluble, blue precipitate where DNA fragmentation had occurred. The specimens were then counterstained with Nuclear Fast Red for 10 min at room temperature to identify any multinucleated cells (cells with three or more nuclei) displaying nuclear fragmentation. The stained samples were examined using a light microscope, and the multinucleated cells were counted manually.
SiRNA-mediated Gene Knockdown
48 h before being stimulated, the differentiated cells were transfected with 30 nm Bim siRNA (SI026553559) or Smad2 siRNA (SI02757496) (Qiagen, Mississauga, Ontario, Canada) in a solution containing 0.03% HiperFect transfection reagent in serum-free OPTI-MEM. Cells were incubated at 37 °C for 1 h while stirring gently. The transfection efficiency was evaluated by fluorescence microscopy in one well of the culture containing a control green fluorescent protein siRNA, and the subsequent shutdown of Bim or Smad2 was assessed by Western blot. 24 h before stimulation, the transfection medium was removed and replaced with RANKL- and M-CSF-free OPTI-MEM 1% fetal bovine serum.
Statistical Analysis
Results are expressed as mean ± S.E., and the significance was determined by a paired Student's t test or one-way analysis of variance with Bonferroni post tests where applicable. Statistical significance was defined as p < 0.05.
RESULTS
Human Osteoclasts Express TGF-β-RI and -RII Receptors
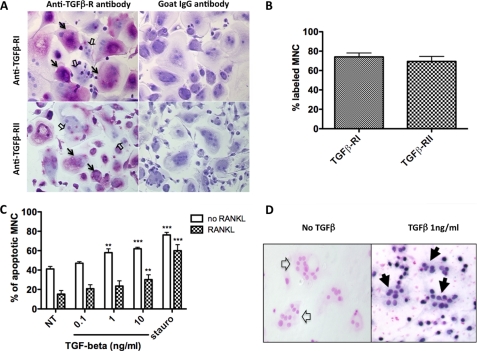
Immunocytochemistry was used to detect TGF-β-RI and -RII on the cell surface of unpermeabilized osteoclasts using polyclonal antibodies against TGF-β-RI and TGF-β-RII, and strong labeling was observed on MNCs, which contrasted with the nonspecific labeling on controls (Fig. 1A). The numbers of labeled cells showed that 74.0 ± 4.1% of MNCs expressed TGF-β-RI, whereas 69.4 ± 5.2% expressed TGF-β-RII (Fig. 1B). These findings show that both the TGF-β-RI and the TGF-β-RII receptors were expressed at the surface of human osteoclasts, suggesting that TGF-β could be bound to these receptors and potentially produce a direct action as a result.
FIGURE 1.
TGF-β receptor expression and TGF-β-induced apoptosis in human osteoclasts. A, at the end of the cultures, the expression of the TGF-β-RI and -RII receptors was evaluated by immunocytochemistry using specific antibodies. Polyclonal goat IgGs were used as negative controls. B, the percentage of labeled cells was determined for each TGF-β R type. Results are expressed as the percentage of labeled MNCs over total MNCs (mean ± S.E.), and three independent experiments were conducted. C, at the end of the CBM cultures, after the M-CSF and RANKL had been removed, different concentrations of TGF-β1 were added 24 h before the apoptosis was determined (0.1–10 ng/ml). The same experiments were performed in the presence of RANKL 100 ng/ml. Staurosporine (1 μm for 3 h) was used as a positive control of apoptosis induction. Cells with three or more nuclei were counted, and results of five independent experiments are expressed as the percentage of apoptotic MNCs over the total population of MNCs, mean ± S.E. **, p < 0.01; ***, p < 0.001, treated versus untreated. D, to quantify the number of apoptotic osteoclasts, TACS Blue labeling was used, resulting in blue staining of the nuclei of the apoptotic cells (positive multinucleated cells are delineated by black arrows, whereas non-apoptotic nuclei were stained pink/red (empty arrows) with a Nuclear Fast Red counterstain).
TGF-β Induces Osteoclast Apoptosis
At the end of the CBM cultures, after the M-CSF and RANKL had been removed, different concentrations of TGF-β1 were added 24 h before the apoptosis was determined (0.1–10 ng/ml). Results revealed that the percentage of apoptotic MNCs was 41 ± 2.5% in the absence of TGF-β1 and significantly increased in the presence of TGF-β1 at a concentration of 1 ng/ml (p < 0.01 versus NT) and 10 ng/ml (p < 0.001 versus NT). In experiments performed in the presence of RANKL, the percentages of apoptotic MNCs were lower (Fig. 1, C and D). Thus TGF-β1 induced osteoclast apoptosis, although less efficiently in RANKL-stimulated cells.
TGF-β Induces Caspase-9 Activation in Human Osteoclasts
To determine which apoptosis pathway was activated in response to TGF-β1 stimulation, we determined the levels of cleavage of caspase-8 and caspase-9, hallmarks of the activation of the extrinsic and intrinsic apoptotic pathways, respectively. In Western blot experiments, no detectable level of cleaved caspase-8 (43/45 kDa) was found after TGF-β1 stimulation. In contrast, increased cleavage of caspase-9 (35/37 kDa) occurred after exposure to 1 ng/ml TGF-β1. Staurosporine (1 μm) was used as a positive control of apoptosis induction and of the activation of caspase-8 and caspase-9 (Fig. 2A).
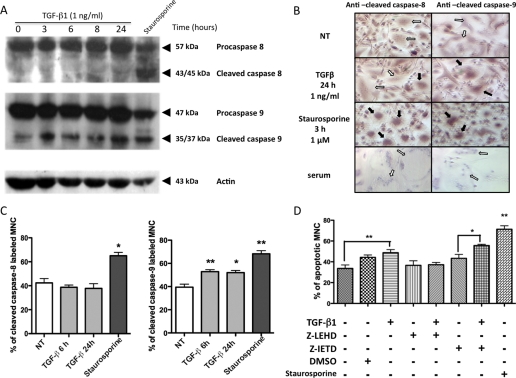
FIGURE 2.
Caspase activation in response to TGF-β stimulation. At the end of the CBM cultures, osteoclasts were cultured in a serum-reduced medium and deprived of RANKL and M-CSF for 24 h. They were then either treated or not with TGF-β1 (1 ng/ml) for 3, 6, 8, or 24 h or with staurosporine (1 μm) for 3 h as a positive control for apoptosis. A, Western blot analysis revealed the presence of the full-length caspase-8 (57 kDa) and of two fragments (43/45 kDa), corresponding to the cleaved caspase-8. The full-length caspase-9 appears at 47 kDa, and the two cleaved fragments appear at 35/37 kDa. B, immunocytochemistry was performed using antibodies specifically directed against the cleaved caspase-8 or the cleaved caspase-9 or normal serum as a negative control. Positive cells are indicated by black arrows, and negative cells are indicated by white arrows. NT, untreated. C, the expression of cleaved caspase-8 and caspase-9 was evaluated in these cultures in the presence of TGF-β1 (1 ng/ml) or staurosporine (1 μm). Results are expressed as the percentage of labeled MNCs from five different experiments (mean ± S.E.). D, caspase inhibitors (caspase-8 inhibitor Z-IETD, 10 μm, and caspase-9 inhibitor Z-LEHD 10 μm) were added 1 h before TGF-β1 stimulation, and osteoclast apoptosis was evaluated after 24 h using a terminal deoxynucleotidyltransferase-mediated dUTP nick end-labeling (TUNEL)-derived method. Staurosporine (1 μm) was used as the apoptotic inducer control. Results are expressed as the percentage of apoptotic MNCs over total MNCs (mean ± S.E.). Eight independent experiments were conducted. *, p < 0.05; **, p < 0.01, versus untreated. DMSO, dimethyl sulfoxide.
As Western blot results involve observing the whole culture, which contains cells other than osteoclasts, TGF-β-induced caspase activation in osteoclast cultures was also evaluated by immunocytochemistry using antibodies raised against the cleaved fragments of activated caspase-8 and caspase-9 (Fig. 2B). Cleaved caspase-8 expression was the same in untreated cells and in cells that had been treated with TGF-β1 (1 ng/ml) for 24 h, whereas it was significantly increased in the presence of staurosporine. In contrast, the percentage of MNC labeled with anti-cleaved caspase-9 antibodies was significantly increased by TGF-β1 stimulation when compared with untreated cells (Fig. 2C). These findings suggest that TGF-β1 induced apoptosis by activating caspase-9, and therefore the intrinsic pathway, in human osteoclasts.
To further confirm the implication of the intrinsic pathway in the TGF-β-induced apoptosis, MNC apoptosis levels were evaluated in the presence of caspase inhibitors. Cells were deprived of survival factors (M-CSF and RANKL) for 24 h and then preincubated with caspase inhibitors for 1 h before being exposed to TGF-β1 for 24 h (Fig. 2D). Apoptosis was detected in 33.6 ± 3.4% of the untreated MNCs; this corresponded to the basal level of apoptosis after survival factor deprivation. As expected, apoptosis was significantly increased in MNCs that had been exposed to TGF-β1 for 24 h. However, a preincubation with Z-LEHD, a specific caspase-9 inhibitor, totally prevented TGF-β1-induced apoptosis, and the level was similar to that of unstimulated cells. In contrast, the use of a caspase-8 inhibitor, Z-IETD, did not prevent apoptosis induction in TGF-β1-treated cells. These results confirm that the activation of caspase-9, but not that of caspase-8, is involved in TGF-β1-induced apoptosis in human osteoclasts.
Signal Transduction Induced by TGF-β in Human Osteoclasts
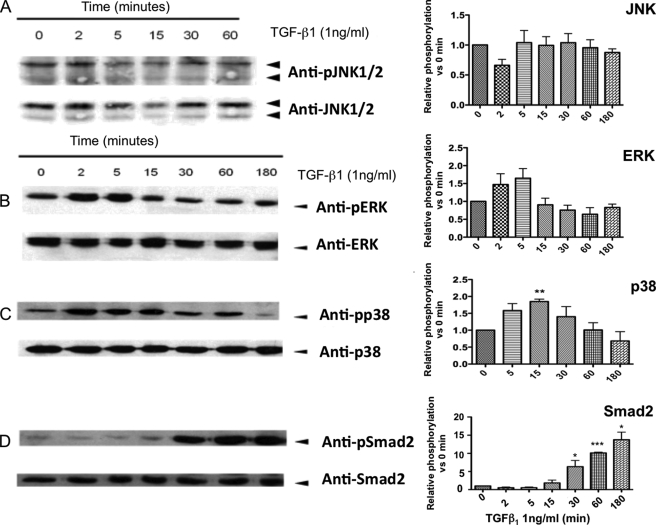
To further explain the activation of caspase-9 by TGF-β1, we investigated the signaling cascades activated in response to stimulation. The best known signaling cascades activated by TGF-β, including Smad and MAPKs (ERK, JNK, and p38) pathways, were investigated by Western blot (Fig. 3). TGF-β1 stimulation induced fast and transient phosphorylation of ERK after only 2 min, which peaked at 5 min (1.65-fold activation, p = 0.12), and then progressively decreased (Fig. 3B). TGF-β1 induced significant activation of p38 after 15 min, with a 1.9-fold increase in phosphorylation when compared with untreated cells (p < 0.01) (Fig. 3C). Finally, marked activation of Smad2 was detected from its phosphorylation 30 min after adding TGF-β1 (6.32-fold activation, p < 0.05), which indicated activation of the Smad pathway (Fig. 3D). Interestingly, we did not observe any significant modification of JNK phosphorylation within 3 h after TGF-β1 stimulation, suggesting that this MAPK may not be involved in downstream TGF-β stimulation in osteoclasts under these conditions (Fig. 3A).
FIGURE 3.
TGF-β1 activates signaling pathway components. At the end of the cultures, mature osteoclasts were treated with 1 ng/ml TGF-β1 for the times indicated. Then cells were rinsed on ice with cold phosphate-buffered saline, extracts were harvested, and 50 μg of protein were analyzed by Western blotting for the indicated phosphorylated (p) or total protein. We analyzed the band intensities using NIH ImageJ software, and the results are presented on each graph on the right of Western blot. Western blots of JNK (A), ERK (B), p38 (C), and Smad2 (D) are shown. The phosphorylation results are normalized for the total protein intensity and presented as relative phosphorylation versus the untreated cells (mean ± S.E.) for five different experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.005, treated versus untreated.
Increased Expression of Bax and Bim upon TGF-β Stimulation
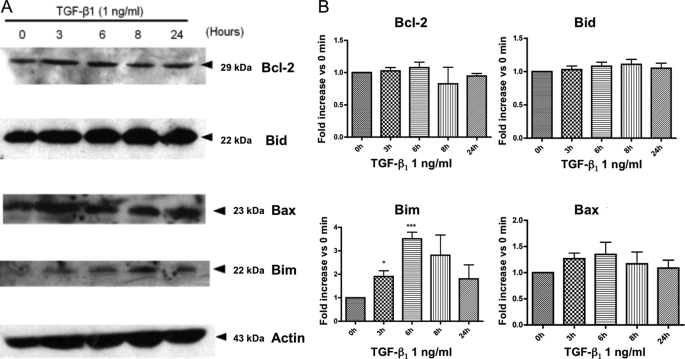
The complex of Smad proteins formed after TGF-β stimulation acts like a transcription factor. As the increased activity of ERK, p38, and Smad2 may modulate apoptosis-related gene transcription, we evaluated the expression of four common Bcl-2 homologs by Western blot after 3–24 h of TGF-β1 treatment (Fig. 4A). We did not observe any obvious change in the expression of the anti-apoptotic protein Bcl-2 or in the expression of the pro-apoptotic Bid. However, although this was not statistically significant, we did observe a consistent trend toward increased expression of the pro-apoptotic protein Bax (1.35-fold increase after 6 h when compared with untreated cells). Furthermore, a strong and significant increase of Bim expression was detected after 6 h of TGF-β1 stimulation (increased 3.52-fold when compared with untreated, p < 0.005) (Fig. 4B). This strongly suggests that increased expression of the pro-apoptotic protein Bim could be involved in TGF-β1-induced apoptosis in human osteoclasts.
FIGURE 4.
Expression of Bcl-2 homologs after TGF-β stimulation. At the end of the CBM cultures, mature osteoclasts were treated with TGF-β1 at 1 ng/ml for the time indicated. A, expression of four Bcl-2 homologs (Bcl-2, Bid, Bim, and Bax) was analyzed by Western blot. B, the intensity of the bands was analyzed using NIH ImageJ Software for four different experiments, protein expressions are normalized relative to the respective actin intensity, and the results are expressed as the expression relative to that of the untreated cells (0 h) (mean ± S.E.) (*, p < 0.05; ***, p < 0.005, treated versus untreated).
TGF-β Induced Caspase-9 Activation by Up-regulating Bim Expression
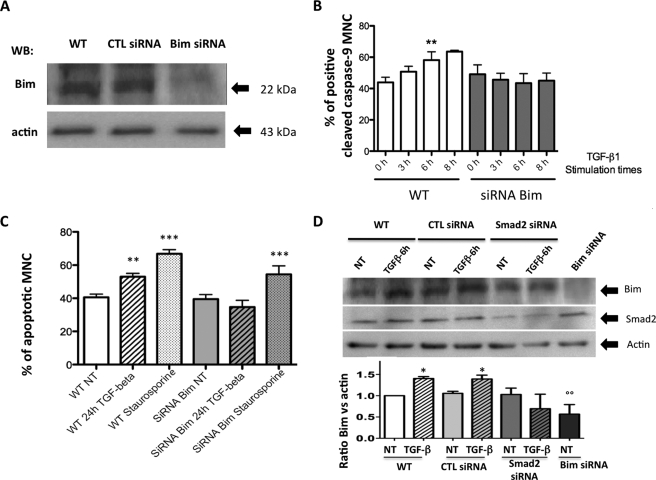
To further investigate whether Bim expression was required to mediate TGF-β1-induced apoptosis, we knocked down Bim expression by siRNA (Fig. 5A). Osteoclasts were then exposed to TGF-β1, and then caspase-9 cleavage and apoptosis were evaluated. Immunocytochemistry experiments revealed that caspase-9 cleavage occurred to the same extent in non-transfected cells and in cells that were transfected with the unsilencing control siRNA included in the kit. This ruled out a potential involvement of the transfection process in caspase-9 activation in our cultures. Following TGF-β1 stimulation, 58.2 ± 5.2% of the MNCs were labeled with anti-cleaved caspase-9 antibodies after 6 h, a significantly higher number than was observed in untreated cells (p < 0.01). However, this TGF-β-induced increase of caspase-9 cleavage was abolished when cells were transfected with Bim siRNA (Fig. 5B). Finally, TGF-β1-induced apoptosis was evaluated in cells that had or had not been transfected with Bim siRNA. In non-transfected cells, apoptosis was significantly increased from 40.58 ± 2.0% in non-treated cells to 53 ± 2.0% in MNCs that had been exposed to TGF-β1 (1 ng/ml) for 24 h (p < 0.01). However, siRNA Bim totally prevented TGF-β1-induced apoptosis (Fig. 5C and supplemental Fig. 5-1). To explore further whether the TGF-β1-stimulated Smad pathway was required to mediate the increase in Bim expression, we shut down Smad2 expression by siRNA. Expression of Bim was studied by Western blot in cells transfected by Smad2 siRNA. The increase in Bim expression after exposure to TGF-β1 (1 ng/ml) for 6 h was no longer observed in cells cultured in the presence of Smad2 siRNA (Fig. 5D and supplemental Fig. 5-2).
FIGURE 5.
Effect of blocking Bim expression on TGF-β-induced caspase-9 activation and apoptosis in human osteoclasts. At the end of the culture, the cells were transfected with Bim siRNA 48 h before stimulation. 24 h before stimulation, the cells were placed in medium-reduced serum and deprived of RANKL and M-CSF. Cells were then stimulated with TGF-β1 at a concentration of 1 ng/ml for the times indicated. A, using Western blot (WB), the transfection of Bim siRNA was controlled by measuring Bim expression in wild type (WT) in unsilencing control (CTL) or Bim siRNA-transfected cells. B, the expression of cleaved caspase-9 was determined by immunocytochemistry in wild type and Bim siRNA-transfected cells after exposure to different TGF-β1 stimulation times (1 ng/ml). The results are expressed as the percentage of positive cleaved caspase-9 MNCs under each experimental condition for four different experiments. C, TGF-β1-induced apoptosis was evaluated in cells that had or had not been transfected with Bim siRNA and were then either treated or not with TGF-β1 (1 ng/ml) for 24 h or with staurosporine (1 μm) for 3 h. Results are expressed as the percentage of apoptotic MNCs over total MNCs (mean ± S.E.). Six independent experiments were conducted. **, p < 0.01; ***, p < 0.001; treated versus untreated (NT). D, expression of Bim was studied by Western blot in cells transfected by unsilencing control siRNA, Smad2, siRNA, or Bim siRNA in control experiments 48 h before stimulation with TGF-β1 (1 ng/ml) for 6 h as described above. Results are expressed as the expression relative to that of the untreated cells (three experiments). *, p < 0.05; treated versus untreated, °°, p < 0.01 versus NT wild type.
DISCUSSION
TGF-β has previously been described as inducing apoptosis in murine and human osteoclasts (6, 15), and in the present study, we investigated the sequence of events leading to such effects. We have shown that TGF-β receptors I and II were expressed at the surface of human osteoclasts and that TGF-β1 induced osteoclast apoptosis by activating the intrinsic pathway of apoptosis leading to caspase-9 activation. Furthermore, we have demonstrated that TGF-β1 induced a trend toward an early increase in ERK, the sequential phosphorylation of p38 and Smad2, and up-regulated Bim expression, which was related to the TGF-β1-induced osteoclast apoptosis.
TGF-β has a complex role in bone cells and bone remodeling. In human bone marrow cultures, TGF-β1 is known to be a potent inhibitor of osteoclast formation (17). TGF-β decreases RANKL expression and increases osteoprotegerin expression by osteoblasts in co-culture systems, thus indirectly decreasing osteoclast differentiation and activities by modulating the RANKL:osteoprotegerin ratio (12). TGF-β may also have a direct action on osteoclasts. TGF-β could increase osteoclast formation by stimulating RANK, αvβ3 integrin, calcitonin receptor, and NFATc1 expression when introduced at an early stage of the osteoclast differentiation (13, 14, 18). However, TGF-β is known to induce apoptosis in mature osteoclasts (6, 15). In vivo animal studies have indicated that bone mass increased when TGF-β pathways were inhibited, whether by inhibition of the type-I receptor or by knock-out of the latent TGF-β-binding protein 3 (19, 20), reflecting the net effect of TGF-β on bone as a result of its effects on bone cells, as well as on other intermediate cells. The presence of TGF-β receptors has been detected in osteoclasts from giant cell tumors of bone that are formed in a tumoral environment (21, 22). We confirm here that in other non-tumoral conditions, human osteoclasts express the TGF-β type-I and type-II receptors.
The intracellular pathways activated during TGF-β-induced apoptosis have been detailed in several studies of non-osteoclast cell types (23–27). The mechanisms used by TGF-β to induce apoptosis vary, depending on the cell type, and may involve death-associated protein kinases (DAPK), the MAP kinases p38 and JNK, or the Smad pathway (28–30).
We observed the sequential activation of p38 and Smad2 during TGF-β-induced osteoclast apoptosis activation. Although the p38 pathway is known to be involved in TGF-β-induced apoptosis, the activation of the p38 pathway has also been reported and associated with increased osteoclastogenesis (31, 32). This observation underlines the importance of cross-talk between pathways and the variability of the effects on different systems (33). The phosphorylation of Smad2 leads to the formation of a complex with pSmad3 and Smad4 that is translocated into the nucleus where it induces the transcription of target genes (11). The transcription of proapoptotic Bcl-2 homologs has often been implicated in Smad-dependent TGF-β-induced apoptosis (26). These two pathways involving p38 and Smads were therefore potential candidates for conducting TGF-β-induced osteoclast apoptosis as their activation is known to enhance the transcription of various apoptosis-related genes (25, 34, 35).
In murine bone marrow cultures, TGF-β1 has been shown to suppress osteoclast apoptosis in cultures performed in the presence of RANKL (36). RANKL, which is an osteoclast survival factor, may activate many signaling pathways that could interact with TGF-β signaling and so affect the final balance between osteoclast life or death (3). To clarify these potential types of cross-talk, we evaluated TGF-β-induced apoptosis in osteoclasts cultured in the presence of RANKL and did not observe any effect of TGF-β1 on osteoclast apoptosis studied under the same conditions (TGF-β1 1 ng/ml), although apoptosis was mildly induced with a higher dose of TGF-β1 (10 ng/ml). This could account for the apparent discrepancy between our results and those of Gingery et al. (36). This distinction may also help to clarify the proresorptive effect attributed to TGF-β in the development of lytic bone metastasis. Indeed, TGF-β is a major player in the vicious circle in which bone metastasis secrete factors that activate osteoclasts to resorb bone, leading to an increased TGF-β release from the bone matrix, which in turn stimulates tumor growth and parathyroid hormone-related protein production. Parathyroid hormone-related protein is a potent stimulator of RANKL production by osteoblasts, thus sustaining the vicious cycle (37). Under these conditions, TGF-β is secreted along with numerous cytokines and growth factors that stimulate osteoclast formation and activity.
Our findings clearly indicate that TGF-β1-induced apoptosis relies on the activation of the intrinsic pathway and caspase-9 cleavage. Caspase-8 cleavage, a characteristic of the extrinsic pathway, is not induced by TGF-β but still displays a relatively high basal level of activation. This is probably due to the presence and activity of endogenous TRAIL that is produced by osteoclasts when they are deprived of survival factors, as reported previously (7). It is, however, not surprising to find that TGF-β1 does not activate caspase-8 cleavage as its receptors do not bear a death receptor intracellular domain. The activation of caspase-9 cleavage, however, clearly relies on the expression of Bim, a member of the pro-apoptotic Bcl2 family. We demonstrate here that Bim expression is required to mediate TGF-β1-induced apoptosis, as has been reported in other cell types (25, 26, 28). In addition, Bim appears to be essential for the regulation of apoptosis in murine osteoclasts as this protein is rapidly induced in cytokine-deprived osteoclasts, and osteoclasts derived from Bim−/− mice are resistant to the withdrawal of cytokine-induced apoptosis (38). In our study, although the absence of survival factors could have induced increased Bim expression, we clearly show that in the absence of Bim expression, TGF-β1-induced apoptosis did not occur in osteoclasts. In addition, under the transcriptional control of Smad3/4 signaling, an up-regulation of Bim appeared to be crucial for TGF-β-induced apoptosis in Hep3B cells (39).
In the absence of survival factors such as RANKL, TGF-β1 induced apoptosis in our model of human osteoclast differentiation and also activated both the canonical Smad signaling pathways and the MAPK p38 pathway. An up-regulation of Bim expression was involved in triggering the intrinsic apoptotic pathway, leading to osteoclast apoptosis. Although Bim appeared to play an important role in the TGF-β-induced osteoclast apoptosis, further studies are necessary to relate this to the activation of the Smad pathway in our model, although this is strongly suggested by previously published data, and our results suggest that this could be driven by the TGF-β1-stimulated Smad2 pathway. In addition, we speculate that p38 may play a role as the p38 pathway mediates the induction of apoptosis through the activation of caspase-9 but not of caspase-8 (40); however, the precise mechanisms involved remain to be elucidated.
This pro-apoptotic effect of TGF-β1 on osteoclasts could be consistent with the hypothesis that TGF-β acts as a coupling agent in bone remodeling. After bone resorption has occurred, TGF-β is locally released from the bone matrix and then inhibits bone resorption partly by inducing osteoclast apoptosis and partly by stimulating osteoblastic bone formation.
Supplementary Material
This study was funded by grants from the Arthritis Society (to S. R.).
 The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 5-1 and 5-2.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 5-1 and 5-2.
- MNC
- multinucleated cell
- TGF-β1
- transforming growth factor-β1
- TGF-β-R
- transforming growth factor-β receptor
- TRAIL
- TNF-related apoptosis-inducing ligand
- RANK
- receptor activator of NF-κB
- RANKL
- receptor activator of NF-κB ligand
- MAP
- mitogen-activated protein
- MAPK
- MAP kinase
- ERK
- extracellular signal-regulated kinase
- JNK
- c-Jun NH2-terminal kinase
- M-CSF
- macrophage-colony-stimulating factor
- CBM
- cord blood monocyte
- Z
- benzyloxycarbonyl
- siRNA
- small interfering RNA.
REFERENCES
- 1.Riggs B. L., Parfitt A. M. (2005) J. Bone. Miner. Res. 20,177–184 [DOI] [PubMed] [Google Scholar]
- 2.Boyle W. J., Simonet W. S., Lacey D. L. (2003) Nature 423,337–342 [DOI] [PubMed] [Google Scholar]
- 3.Roux S., Chamoux E. (2007) Osteoclast apoptosis. in Cell Apoptotic Signaling Pathways ( Pickens C. O. ed.) pp. 163–184, Nova Science Publishers, Hauppauge, NY [Google Scholar]
- 4.Brunetti G., Oranger A., Mori G., Tamma R., Di Benedetto A., Pignataro P., Grassi F. R., Zallone A., Grano M., Colucci S. (2007) Ann. N.Y. Acad. Sci. 1116,316–322 [DOI] [PubMed] [Google Scholar]
- 5.Colucci S., Brunetti G., Cantatore F. P., Oranger A., Mori G., Pignataro P., Tamma R., Grassi F. R., Zallone A., Grano M. (2007) Apoptosis 12,1623–1632 [DOI] [PubMed] [Google Scholar]
- 6.Roux S., Lambert-Comeau P., Saint-Pierre C., Lépine M., Sawan B., Parent J. L. (2005) Biochem. Biophys. Res. Commun. 333,42–50 [DOI] [PubMed] [Google Scholar]
- 7.Chamoux E., Houde N., L'Eriger K., Roux S. (2008) J. Cell. Physiol. 216,536–542 [DOI] [PubMed] [Google Scholar]
- 8.Rahimi R. A., Leof E. B. (2007) J. Cell. Biochem. 102,593–608 [DOI] [PubMed] [Google Scholar]
- 9.Massagué J., Seoane J., Wotton D. (2005) Genes. Dev. 19,2783–2810 [DOI] [PubMed] [Google Scholar]
- 10.Javelaud D., Mauviel A. (2005) Oncogene 24,5742–5750 [DOI] [PubMed] [Google Scholar]
- 11.Janssens K., ten Dijke P., Janssens S., Van Hul W. (2005) Endocr. Rev. 26,743–774 [DOI] [PubMed] [Google Scholar]
- 12.Takai H., Kanematsu M., Yano K., Tsuda E., Higashio K., Ikeda K., Watanabe K., Yamada Y. (1998) J. Biol. Chem. 273,27091–27096 [DOI] [PubMed] [Google Scholar]
- 13.Fuller K., Lean J. M., Bayley K. E., Wani M. R., Chambers T. J. (2000) J. Cell. Sci. 113,2445–2453 [DOI] [PubMed] [Google Scholar]
- 14.Orcel P., Bielakoff J., De Vernejoul M. C. (1990) J. Cell. Physiol. 142,293–298 [DOI] [PubMed] [Google Scholar]
- 15.Hughes D. E., Dai A., Tiffee J. C., Li H. H., Mundy G. R., Boyce B. F. (1996) Nat. Med. 2,1132–1136 [DOI] [PubMed] [Google Scholar]
- 16.Roux S., Quinn J., Pichaud F., Orcel P., Chastre E., Jullienne A., De Vernejoul M. C. (1996) J. Cell. Physiol. 168,489–498 [DOI] [PubMed] [Google Scholar]
- 17.Chenu C., Pfeilschifter J., Mundy G. R., Roodman G. D. (1988) Proc. Natl. Acad. Sci. U.S.A. 85,5683–5687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fox S. W., Evans K. E., Lovibond A. C. (2008) Biochem. Biophys. Res. Commun. 366,123–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dabovic B., Levasseur R., Zambuto L., Chen Y., Karsenty G., Rifkin D. B. (2005) Bone 37,25–31 [DOI] [PubMed] [Google Scholar]
- 20.Mohammad K. S., Chen C. G., Balooch G., Stebbins E., McKenna C. R., Davis H., Niewolna M., Peng X. H., Nguyen D. H., Ionova-Martin S. S., Bracey J. W., Hogue W. R., Wong D. H., Ritchie R. O., Suva L. J., Derynck R., Guise T. A., Alliston T. (2009) PLoS ONE 4,e5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franchi A., Benvenuti S., Masi L., Malentacchi C., Arganini L., Brandi M. L., Santucci M. (2001) Appl. Immunohistochem. Mol. Morphol. 9,170–175 [DOI] [PubMed] [Google Scholar]
- 22.Zheng M. H., Fan Y., Wysocki S. J., Lau A. T., Robertson T., Beilharz M., Wood D. J., Papadimitriou J. M. (1994) Am. J. Pathol. 145,1095–1104 [PMC free article] [PubMed] [Google Scholar]
- 23.Birkey Reffey S., Wurthner J. U., Parks W. T., Roberts A. B., Duckett C. S. (2001) J. Biol. Chem. 276,26542–26549 [DOI] [PubMed] [Google Scholar]
- 24.Edlund S., Bu S., Schuster N., Aspenström P., Heuchel R., Heldin N. E., ten Dijke P., Heldin C. H., Landström M. (2003) Mol. Biol. Cell 14,529–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohgushi M., Kuroki S., Fukamachi H., O'Reilly L. A., Kuida K., Strasser A., Yonehara S. (2005) Mol. Cell. Biol. 25,10017–10028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramjaun A. R., Tomlinson S., Eddaoudi A., Downward J. (2007) Oncogene 26,970–981 [DOI] [PubMed] [Google Scholar]
- 27.Teramoto T., Kiss A., Thorgeirsson S. S. (1998) Biochem. Biophys. Res. Commun. 251,56–60 [DOI] [PubMed] [Google Scholar]
- 28.Ramesh S., Qi X. J., Wildey G. M., Robinson J., Molkentin J., Letterio J., Howe P. H. (2008) EMBO. Rep. 9,990–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoo J., Ghiassi M., Jirmanova L., Balliet A. G., Hoffman B., Fornace A. J., Jr., Liebermann D. A., Bottinger E. P., Roberts A. B. (2003) J. Biol. Chem. 278,43001–43007 [DOI] [PubMed] [Google Scholar]
- 30.Yu L., Hébert M. C., Zhang Y. E. (2002) EMBO. J. 21,3749–3759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karsdal M. A., Fjording M. S., Foged N. T., Delaissé J. M., Lochter A. (2001) J. Biol. Chem. 276,39350–39358 [DOI] [PubMed] [Google Scholar]
- 32.Karsdal M. A., Hjorth P., Henriksen K., Kirkegaard T., Nielsen K. L., Lou H., Delaissé J. M., Foged N. T. (2003) J. Biol. Chem. 278,44975–44987 [DOI] [PubMed] [Google Scholar]
- 33.Olson J. M., Hallahan A. R. (2004) Trends. Mol. Med. 10,125–129 [DOI] [PubMed] [Google Scholar]
- 34.Loesch M., Chen G. (2008) Front. Biosci. 13,3581–3593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wildey G. M., Patil S., Howe P. H. (2003) J. Biol. Chem. 278,18069–18077 [DOI] [PubMed] [Google Scholar]
- 36.Gingery A., Bradley E. W., Pederson L., Ruan M., Horwood N. J., Oursler M. J. (2008) Exp. Cell. Res. 314,2725–2738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kingsley L. A., Fournier P. G., Chirgwin J. M., Guise T. A. (2007) Mol. Cancer. Ther. 6,2609–2617 [DOI] [PubMed] [Google Scholar]
- 38.Akiyama T., Bouillet P., Miyazaki T., Kadono Y., Chikuda H., Chung U. I., Fukuda A., Hikita A., Seto H., Okada T., Inaba T., Sanjay A., Baron R., Kawaguchi H., Oda H., Nakamura K., Strasser A., Tanaka S. (2003) EMBO. J. 22,6653–6664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu J., Zhang L., Chen A., Xiang G., Wang Y., Wu J., Mitchelson K., Cheng J., Zhou Y. (2008) J. Cell. Physiol. 215,422–433 [DOI] [PubMed] [Google Scholar]
- 40.Sánchez-Capelo A. (2005) Cytokine Growth. Factor. Rev. 16,15–34 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.