Abstract
Dihydropterin deaminase, which catalyzes the conversion of 7,8-dihydropterin to 7,8-dihydrolumazine, was purified 5850-fold to apparent homogeneity from Drosophila melanogaster. Its molecular mass was estimated to be 48 kDa by gel filtration and SDS-PAGE, indicating that it is a monomer under native conditions. The pI value, temperature, and optimal pH of the enzyme were 5.5, 40 °C, and 7.5, respectively. Interestingly the enzyme had much higher activity for guanine than for 7,8-dihydropterin. The specificity constant (kcat/Km) for guanine (8.6 × 106 m−1·s−1) was 860-fold higher than that for 7,8-dihydropterin (1.0 × 104 m−1·s−1). The structural gene of the enzyme was identified by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry analysis as CG18143, located at region 82A1 on chromosome 3R. The cloned and expressed CG18143 exhibited both 7,8-dihydropterin and guanine deaminase activities. Flies with mutations in CG18143, SUPor-P/Df(3R)A321R1 transheterozygotes, had severely decreased activities in both deaminases compared with the wild type. Among several red eye pigments, the level of aurodrosopterin was specifically decreased in the mutant, and the amount of xanthine and uric acid also decreased considerably to 76 and 59% of the amounts in the wild type, respectively. In conclusion, dihydropterin deaminase encoded by CG18143 plays a role in the biosynthesis of aurodrosopterin by providing one of its precursors, 7,8-dihydrolumazine, from 7,8-dihydropterin. Dihydropterin deaminase also functions as guanine deaminase, an important enzyme for purine metabolism.
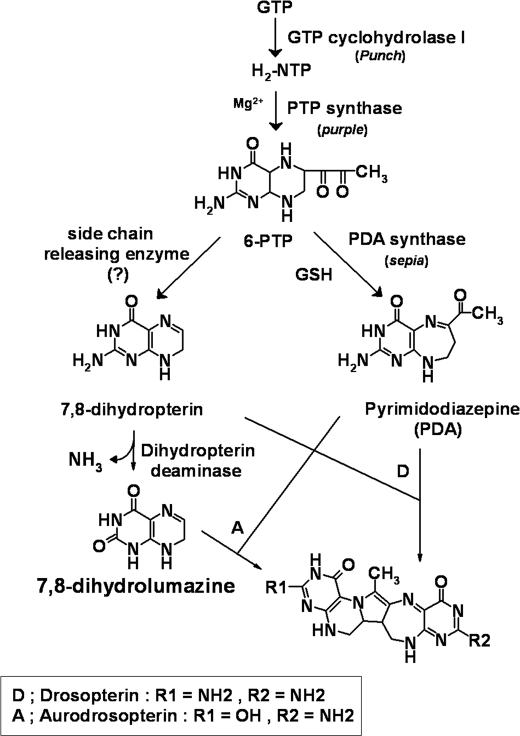
The complexity of the eye color phenotypes of the fruit fly Drosophila melanogaster has been the subject of numerous investigations for more than 90 years. Two classes of pigments contribute to the eye color of Drosophila: brown “ommochromes” and red “drosopterins.” Drosopterins, first reported by Lederer (1) and subsequently by Viscontini et al. (2), consist of at least five compounds, which have been referred to as drosopterin, isodrosopterin, neodrosopterin, aurodrosopterin, and “fraction e” (3). Among the red pigments, drosopterin and isodrosopterin are the major components, whereas aurodrosopterin and neodrosopterin are minor pigments in wild type flies.
The chemical structure of drosopterin was determined by Pfleiderer and co-worker (4). Drosopterin and its enantiomer, isodrosopterin, consist of a pentacyclic ring system containing a 5,6,7,8-tetrahydropterin (=2-amino-5,6,7,8-tetrahydropteridin-4(1H)-one), a 2-amino-3,7,8,9-tetrahydro-4H-pyrimido[4,5-b][1,4]diazepin-4-one, and a pyrrole ring (Scheme 1). Based on 1H NMR and UV/visible spectral analyses, the structure of aurodrosopterin was elucidated in 1993 by Yim et al. (5), who found that it is the same as that of drosopterin except that it has one less amino group in the pteridine portion. The presence or absence of an amino group in the pteridine moiety is the key characteristic that distinguishes drosopterin from aurodrosopterin (Scheme 1). They also reported the presence of isoaurodrosopterin based on thin layer chromatographic analyses of Drosophila head extracts using various solvent systems (5).
SCHEME 1.
Proposed pathway for the biosynthesis of drosopterins in D. melanogaster. The red eye pigments in Drosophila are collectively called drosopterins. Drosopterin (a major red eye pigment; labeled D) is produced nonenzymatically by the one-to-one condensation of 7,8-dihydropterin and PDA (10). Aurodrosopterin (a minor red eye pigment; labeled A), which has one less amino group in the pteridine portion of the structure, was shown to be produced nonenzymatically by the condensation of 7,8-dihydrolumazine and PDA (5).
The first step leading to the biosynthesis of drosopterins is the formation of 7,8-dihydroneopterin triphosphate from GTP by GTP cyclohydrolase I, which is encoded by Punch (6). 7,8-Dihydroneopterin triphosphate is then converted to 6-pyruvoyl tetrahydropterin (6-PTP)3 by PTP synthase, the product of the purple gene (7–9). Next 6-PTP is converted to pyrimidodiazepine (PDA) by PDA synthase, which is a member of the Omega class glutathione S-transferases and is encoded by the sepia gene (10, 11). Aurodrosopterin and its enantiomer, isoaurodrosopterin, are produced nonenzymatically by the one-to-one condensation of 7,8-dihydrolumazine and PDA under acidic conditions (5) in a manner similar to the production of drosopterin and isodrosopterin, which are produced by a similar nonenzymatic condensation of 7,8-dihydropterin and PDA (Scheme 1).
In the course of investigating the metabolic fate of tetrahydrobiopterin, Rembold and co-workers (12) found that tetrahydrobiopterin can be degraded to 6-hydroxylumazine by rat liver homogenates. They proposed that tetrahydrobiopterin was converted by nonenzymatic side chain release to 7,8-dihydropterin, which was converted to 7,8-dihydrolumazine, the deaminated counterpart of 7,8-dihydropterin, by a deaminase present in the crude extracts. 7,8-Dihydrolumazine is then converted to 7,8-dihydro-6-hydroxylumazine by xanthine oxidase and subsequently to 6-hydroxylumazine by autoxidation. This series of reactions was also observed in D. melanogaster. Takikawa et al. (13) demonstrated the conversion of 7,8-dihydropterin to 6-hydroxylumazine using partially purified fly extracts. However, the enzymatic properties of the deaminase and the identity of the gene encoding the protein have not yet been established.
Here we purified and characterized Drosophila dihydropterin deaminase and identified its structural gene, CG18143, by matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) analysis. We provide clear evidence that CG18143, previously annotated as the guanine deaminase gene, is directly involved in the biosynthesis of aurodrosopterin, a minor red eye pigment in Drosophila.
EXPERIMENTAL PROCEDURES
Materials
The samples of 7,8-dihydrolumazine and PDA were generously gifted to us by Professor Wolfgang Pfleiderer of Konstanz University in Konstanz, Germany. 7,8-Dihydroneopterin triphosphate was prepared enzymatically from GTP by GTP cyclohydrolase I purified from Escherichia coli according to a previously published procedure (14). Purified 7,8-dihydropterin was prepared from pterin by zinc-alkali reduction according to previously described methods (15). Drosopterin, isodrosopterin, aurodrosopterin, and neodrosopterin were isolated from Drosophila heads by methods published earlier (5). Other pteridines were purchased commercially from Schircks Laboratories (Jona, Switzerland).
Drosophila Strains
A wild type of D. melanogaster, Oregon-R, was used for the preparation of the enzymes. The breeding populations of the flies were maintained at 25 ± 1 °C in a culture cage on a standard yeast medium with propionic acid added as a mold inhibitor. For the purification of deaminase, adult flies (0–2 days old) were frozen at −70 °C until used. The fly strains used for the mutational analysis included the following. y1 w67c23;;P{y+mDint2 wBR.E.BR SUPor-P} KG04159 (16) was obtained from the Bloomington Stock Center (Department of Biology, Indiana University, Bloomington, IN), and ;;Df(3R)A321R1,ry (81F; 82A4-6) was a gift from S. A. Wasserman (University of California, San Diego, CA).
Deaminase Assays: Two Methods
The High Performance Liquid Chromatography (HPLC) Method
For the 7,8-dihydropterin deaminase assay, the standard reaction mixture contained 0.5 mm 7,8-dihydropterin, 100 mm potassium phosphate buffer (pH 7.5), and the enzyme in a final volume of 50 μl. After incubation at 40 °C for 10–30 min in the dark, the reaction was stopped by the addition of 5 μl of 30% trichloroacetic acid, and the mixture was centrifuged to remove the precipitated proteins. A 6.5-μl iodine solution (1% I2, 2% KI) was then added to the acidified reaction mixture to convert the reduced pteridines to their oxidized forms. After incubation for 1 h at room temperature, the excess iodine was reduced by the addition of 5 μl of 2% ascorbic acid. A portion of this mixture was subjected to HPLC on a reversed-phase C18 column (Inertsil ODS-3, 4.6 × 250 mm; GL Sciences) connected to a photodiode array detector (Waters 996) and a scanning fluorescence detector (Waters 474). The material was eluted isocratically with 85% (v/v) 50 mm ammonium acetate in 15% (v/v) methanol at a flow rate of 1 ml/min. The reaction products, lumazine and 6-hydroxylumazine, were monitored by their fluorescence intensities. For the guanine deaminase assay, the standard reaction mixture contained 0.1 mm guanine, 100 mm potassium phosphate buffer (pH 7.5), and the enzyme in a final volume of 100 μl and was incubated at 40 °C for 10–30 min in the dark. A portion of this mixture was subjected to HPLC with the same equipment as described above. The material was eluted isocratically with 50 mm sodium acetate buffer (pH 6.0) at a flow rate of 1 ml/min, and xanthine was monitored by the absorbance at 270 nm.
The Phenate Method
This assay was carried out spectrophotometrically as described previously (17) with some modifications. The reaction mixture contained a 0.25 mm concentration of each substrate, 100 mm potassium phosphate buffer (pH 7.5), and the enzyme in a final volume of 100 μl. After incubation at 40 °C for 30 min in the dark, the reaction was stopped by the addition of 350 μl of cold deionized water, and 2.5 μl of 3 mm MnSO4 solution was added to the sample. After 25 μl of hypochlorous acid reagent (10 ml of 5% NaOCl solution and 40 ml of water, pH 6.8) was added to the mixture and stirred, 30 μl of phenate reagent (2.5 g of NaOH and 10 g of phenol in 100 ml of water) was immediately added. After 10 min, the increase in absorption at 630 nm was measured relative to the absorption of the control solution. The same reaction mixture without the added enzyme was used as the control.
Purification of 7,8-Dihydropterin Deaminase
Dihydropterin deaminase was purified from head extracts of D. melanogaster by using conventional column chromatography. All of the operations were carried out at 4 °C.
Head Preparation
After the flies were frozen with liquid nitrogen, their heads were detached from their bodies by mechanical shock and separated from the other parts by sieving.
Crude Extracts
The fly heads (dry weight of 20 g) were homogenized in 80 ml of 0.1 m potassium phosphate buffer (pH 7.0) containing 2 mm phenylmethylsulfonyl fluoride. The homogenate was centrifuged at 25,000 × g for 1 h, and the resulting supernatant was used for the subsequent steps.
Ammonium Sulfate Fractionation
The proteins precipitated in 30–60% saturated ammonium sulfate were redissolved in 12 ml of 50 mm potassium phosphate buffer (pH 7.0). The solution was then dialyzed twice against 2 liters of the same buffer for 6 h.
Gel Permeation Chromatography
The dialyzed ammonium sulfate fraction was introduced into a Sephacryl HR S 300 column (2.5 × 78 cm), and the column was eluted with 50 mm potassium phosphate buffer (pH 7.0) at a flow rate of 30 ml/h.
Hydrophobic Interaction Chromatography
The fractions containing the active enzyme were combined (27.5 ml), and solid ammonium sulfate was added to yield a final concentration of 0.1 m. The sample was applied to a phenyl-Sepharose 6 fast flow column (1.5 × 11 cm) that had been equilibrated with 50 mm potassium phosphate (pH 6.1) containing 0.1 m ammonium sulfate. After the column was washed with the same buffer, elution was carried out with a linear gradient of ethylene glycol from 0 to 50% in 25 mm histidine HCl buffer (pH 6.1) at a flow rate of 30 ml/h.
Chromatofocusing
The active fractions were combined and concentrated to 5 ml with solid polyethylene glycol (molecular weight = 20,000). This material was dialyzed twice against 1 liter of 25 mm histidine HCl buffer (pH 6.1) for 6 h and loaded on a column of Polybuffer Exchanger 94 (1 × 37 cm) that had been equilibrated previously with 25 mm histidine HCl buffer (pH 6.1). The column was developed with 250 ml of elution buffer (10-fold diluted Polybuffer Exchanger 74, pH 5.0) at a flow rate of 15 ml/h. The active fractions were desalted with 20 mm phosphate buffer (pH 7.0) and concentrated using Amicon Centriplus Centrifugal Filter Devices (Millipore; molecular mass cutoff, 10,000 Da).
In Vitro Synthesis of Aurodrosopterin
The condensation reaction was performed as described previously (5) with some modifications. 7,8-Dihydropterin was incubated with the purified deaminase, and aliquots of the reaction mixture were taken out at various time points and acidified to pH 2.5 by the addition of 2 m HCl. Each acidified mixture was then incubated with PDA, which had been preincubated at pH 2.5 for 30 min, and the synthesized pigments were analyzed by thin layer chromatography (TLC).
Determination of Kinetic Parameters
The initial velocities of the catalytic reactions for 7,8-dihydropterin and guanine were determined under standard reaction conditions at various substrate concentrations. All of the experiments were performed three times. Ten different substrate concentrations were prepared within a range of 25–3000 μm for 7,8-dihydropterin and 10–300 μm for guanine. The initial velocities were measured within a 15–20% consumption range for each substrate.
MALDI-TOF Analysis and Data Base Search
The purified enzyme was subjected to SDS-PAGE, and the gel was silver-stained by a previously described method (18). The protein band was excised and in-gel digested with trypsin according to previously published procedures (19). MALDI-TOF mass spectra were acquired on a Voyager-DETM STR Biospectrometry Workstation. The peptide masses were measured as monoisotopic masses and analyzed against the NCBInr data base using the MS-Fit search algorithm (Protein Prospector, University of California San Francisco Mass Spectrometry Facility, San Francisco, CA). For the data base searches, no restrictions were placed on the species or organisms. Peptides were selected in the mass range of 1000–3000 Da.
Cloning and Expression of the Putative Gene
The coding sequence of CG18143 was amplified by reverse transcription-PCR. The total RNA was extracted from 0–2-day-old adult flies with TRIzol® reagent in accordance with the manufacturer's instructions (Invitrogen). PCR was carried out under the following conditions: one cycle of 94 °C for 4 min; 30 cycles of 94 °C for 1 min, 48 °C for 1 min, and 72 °C for 2 min; and one cycle of 72 °C for 7 min. For PCR, we used a gene-specific primer set, DPD1 (5′-GGGCCTTCCATATGGCAACCGTGTTTCTTGGAACT-3′) and DPD2 (5′-CGCGGATCCTCATTGGTATCCCTGTTTAATACGC-3′), designed to incorporate the NdeI and BamHI sites (underlined), respectively. The PCR products were subcloned into the multiple cloning site of the pET 15b vectors and transformed into BL21 cells. Transformants were cultured at 37 °C in 1000 ml of LB medium containing 50 μg/ml ampicillin, and protein expression was induced by the addition of 0.5 mm isopropyl d-thiogalactopyranoside at OD 0.5. After 3 h, the cells were collected by centrifugation at 5000 × g for 10 min and suspended in 40 ml of ice-cold Binding Buffer (pET system manual, Novagen). After sonication and centrifugation, the supernatant containing the soluble enzyme was bound to His·Bind Resin according to the manufacturer's protocol (Novagen).
Determination of the SUPor-P Insertion Site and Deficiency Region
For Southern blot analysis, genomic DNA extracted from ;;P{y+mDint2 wBR.E.BR SUPor-P} flies was cut by EcoRI or by a combination of EcoRI and EcoRV, separated on 1% agarose gels, and blotted onto Nytran® N membranes (Schleicher & Schuell). The blotted membranes were probed with a 32P-labeled DNA probe covering the 5′ end of the SUPor-P element using QuikHyb® Hybridization Solution (Stratagene) and autoradiographed. The inverse PCR method4 was used to obtain the genomic sequence flanking the insertion site of the P element in the ;;P{y+mDint2 wBR.E.BR SUPor-P} flies. A Plac1 (5′-CACCCAAGGCTCTGCTCCCACAAT-3′) and Plac4 (5′-ACTGTGCGTTAGGTCCTGTTCATTGTT-3′) primer set was used for the 5′ junction, and a Pry1 (5′-CCTTAGCATGTCCGTGGGGTTTGAAT-3′) and Pry4-added (5′-CAATCATATCGCTGTCTCACTCAGACT-3′) primer set was used for the 3′ junction. The amplified DNA fragments were directly sequenced using the Plac1 and Pry1 primers. The sequences were analyzed with basic local alignment search tool (BLAST) searches of the Drosophila Genome Database. After the insertion site of the SUPor-P element was identified, genomic DNA isolated from ;;SUPor-P/Df(3R)A321R1 flies was analyzed by PCR to determine whether the deficient region of ;;Df(3R)A321R1,ry (81F; 82A4-6) flies contains CG18143. A pair of primers that covers the CG18143 and P element insert region was used: Df-5′-primer (5′-TCGTCCACTGCCAGGAATCCTCCTTC-3′) and Df-3′-primer (5′-TATGTACAGGTGCGAGCAGTGCA-3′). The PCR conditions were as follows: one cycle of 94 °C for 4 min; 30 cycles of 94 °C for 1 min, 57 °C for 50 s, and 72 °C for 2 min; and one cycle of 72 °C for 7 min.
Genetics
A male of y1 w67c23 ;;P{y+mDint2 wBR.E.BR SUPor-P} was crossed to ;;TM3/TM6b females. One ;;SUPor-P/TM3 male resulting from this cross was crossed again to ;;TM3/TM6b females. From the cross between the males and ;;SUPor-P/TM3 females of the F2 generation, ;;SUPor-P homozygotes without the y1 mutation in the X chromosome were obtained. To delete the ry mutation from Df(3R)A321R1,ry flies, a recombination method was applied. First ;;Df(3R)A321R1,ry/TM3 males were crossed to Oregon-R virgins, and ;;Df(3R)A321R1,ry/+ virgins were then crossed to ;;TM3/TM6b males. Single male progeny carrying a TM6b balancer were mated in individual vials to ;;Df(3R)A321R1,ry/TM3 females to determine whether the males had a deficient third chromosome. Stocks were established by mating males and females carrying a TM3 balancer. Finally the males carrying a deficient third chromosome were crossed to ry506 females to determine whether the ry mutation on the third chromosome of the deficient fly was deleted. To get ;;Df(3R)A321R1/+, ;;SUPor-P/+, and ;;SUPor-P/Df(3R)A321R1 flies, Df(3R)A321R1/TM3 males were crossed with Oregon-R virgins, and ;;SUPor-P homozygote males were crossed with Oregon-R and ;;Df(3R)A321R1/TM3 virgins.
Quantification of Xanthine and Uric Acid
The xanthine and uric acid contents were determined by HPLC using a previously described method (20). Separations were performed on a 4.6 × 250-mm Inertsil ODS-3 reversed-phase column. Uracil, xanthine, and uric acid were monitored at 260, 273, and 292 nm, respectively.
Miscellaneous Methods
The protein concentration was determined by the Bradford method (21) with bovine serum albumin as the standard protein. The molecular mass of the native enzyme was estimated using a Superose S 12 column (1.0 × 40 cm) calibrated with standard molecular mass proteins and blue dextran (2,000,000 Da). SDS-PAGE was performed on 4–20% SDS-polyacrylamide gradient gels, and isoelectric focusing was carried out as described previously (22) on a polyacrylamide flat bed gel (0.1 × 14 × 15 cm). TLC was performed with microcrystalline cellulose plates (Eastman Kodak Co. Number 13255 without a fluorescence indicator). For one-dimensional TLC, a 3% ammonium chloride solution was used as a solvent. For two-dimensional TLC, isopropanol, 2% ammonium acetate (1:1, v/v) and 3% ammonium chloride solutions were used as the first and second developing solvents, respectively. Fluorescent spots were detected by a long wave UV lamp.
RESULTS
Purification of Dihydropterin Deaminase
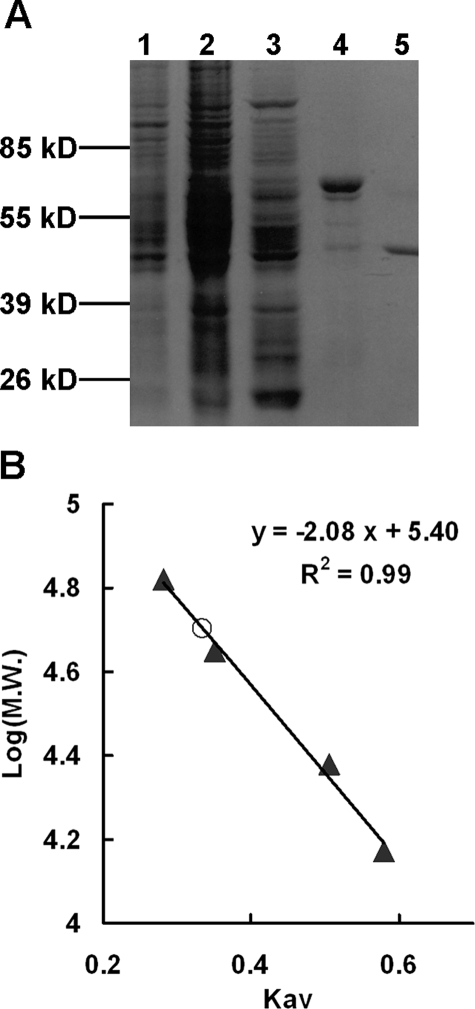
Dihydropterin deaminase from the heads of Drosophila was purified 5850-fold to apparent homogeneity. A summary of this purification is presented in Table 1. Throughout all of the column chromatographic steps, a single activity peak was observed, and the degree of purification was monitored by the increase in the specific activity and by SDS-PAGE (Fig. 1A). The enzyme was purified to a specific activity of 643 units/mg with an activity yield of 4.5%; more than 30 μg of pure protein was routinely obtained from 20 g (dry weight) of 0–2-day-old adult heads. The final preparation of the enzyme did not contain xanthine oxidase/dehydrogenase activity, which catalyzes the conversion of 7,8-dihydropterin to 7,8-dihydro-6-hydroxypterin. The molecular mass of the purified enzyme was estimated to be 48 kDa by 4–20% gradient SDS-PAGE (Fig. 1A) and by size exclusion chromatography on a calibrated Superose S 12 HPLC column (Fig. 1B), indicating that it is a monomer under native conditions.
TABLE 1.
Summary of purification of dihydropterin deaminase
One unit of activity is the amount of enzyme needed for the formation of 1 nmol of 7,8-dihydrolumazine from 7,8-dihydropterin/min at 50 °C.
| Enzyme preparation | Total protein | Specific activity | Activity yield | Relative specific activity |
|---|---|---|---|---|
| mg | units/mg | % | ||
| Crude extract | 4617 | 0.11 | 100 | 1.0 |
| Ammonium sulfate fraction | 2500 | 0.16 | 81.3 | 1.5 |
| Sephacryl HR S 300 eluate | 211 | 1.08 | 45.9 | 9.8 |
| Phenyl-Sepharose 6 Fast Flow eluate | 9.5 | 18.4 | 35.2 | 167 |
| Polybuffer Exchanger 94 eluate | 0.032 | 643.5 | 4.5 | 5850 |
FIGURE 1.
SDS-PAGE analysis and the molecular mass of the native enzyme. A, SDS-PAGE shows the progress of purification of dihydropterin deaminase. Lane 1, crude extract; lane 2, ammonium sulfate fraction; lane 3, Sephacryl HR S 300 column; lane 4, phenyl-Sepharose column; lane 5, Polybuffer Exchanger 94 column. Molecular masses of standard marker proteins are indicated. B, molecular mass of the purified dihydropterin deaminase (○) was estimated to be 48 kDa by HPLC on a calibrated Superose S 12 column (1.0 × 40 cm) as described under “Experimental Procedures.” Standard proteins (▴) used for calibration were bovine serum albumin (67,000 Da), ovalbumin (43,000 Da), chymotrypsinogen A (25,000 Da), and ribonuclease A (13,700 Da).
Identification of the Reaction Product
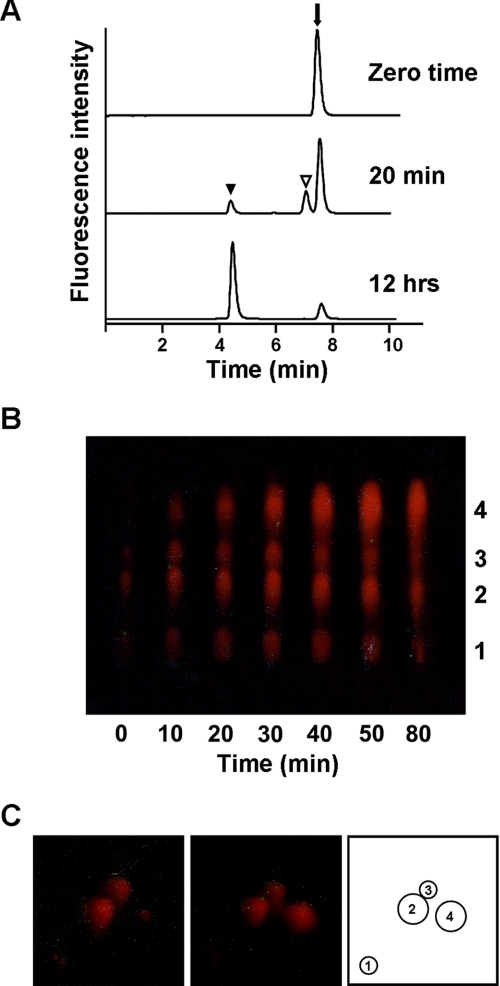
Forrest et al. (23) showed that the 5,6-double bonds of 7,8-dihydrolumazine are readily susceptible to nucleophilic attack, e.g. water addition. According to previous reports (12), 7,8-dihydrolumazine is converted to 7,8-dihydro-6-hydroxylumazine either in the presence or in the absence of xanthine oxidase, and 7,8-dihydro-6-hydroxylumazine is converted to 6-hydroxylumazine by auto-oxidation. We monitored the enzymatic deamination reaction of 7,8-dihydropterin at different time points (zero time, 20 min, and 12 h) by HPLC. After 20 min of the enzymatic reaction, three peaks were detected, the retention times of which were 4.4, 7.0, and 7.5 min, identical to those of standard 6-hydroxylumazine, lumazine, and pterin, respectively. The UV-visible spectra of these peaks were also identical to those of standard 6-hydroxylumazine, lumazine, and pterin, respectively (data not shown). When the enzymatic reaction was prolonged to 12 h, only 6-hydroxylumazine and pterin peaks were detected with the lumazine peak hardly detected (Fig. 2A, lower panel). These results indicate that 7,8-dihydrolumazine is formed from 7,8-dihydropterin by the enzyme and then nonenzymatically hydroxylated to 7,8-dihydro-6-hydroxylumazine, which is converted to 6-hydroxylumazine by iodine oxidation.
FIGURE 2.
Identification of dihydropterin deaminase reaction product. A, HPLC chromatogram of the enzyme reaction product. 7,8-Dihydropterin was incubated with the purified enzyme (0.6 μg) under standard reaction conditions. After iodine oxidation, the materials were analyzed on a C18 column at various times. The enzyme converted 7,8-dihydropterin to 7,8-dihydrolumazine, which was converted nonenzymatically to 7,8-dihydro-6-hydroxylumazine. Positions of the elution of 6-hydroxylumazine (black arrowhead), lumazine (white arrowhead), and pterin (arrow) are indicated. B, in vitro synthesis of aurodrosopterins from PDA and the enzyme reaction product. 7,8-Dihydropterin was incubated with the purified enzyme, and aliquots of the reaction mixture at the indicated time points were incubated with PDA as described under “Experimental Procedures.” The synthesized pigments were identified by one-dimensional TLC. The identity of each spot is as follows: 1, neodrosopterin; 2, drosopterin; 3, isodrosopterin; and 4, aurodrosopterin. C, analysis of the synthesized pigments by two-dimensional TLC. The red pigments extracted from the heads of Oregon-R (left panel) were combined and analyzed with synthesized pigment (middle panel) by two-dimensional TLC. The synthesized pigments appeared at the same location as natural aurodrosopterin extracted from wild type heads. Numbering is as follows: 1, neodrosopterin; 2, drosopterin; 3, isodrosopterin; and 4, aurodrosopterin (right panel).
To identify the deaminase reaction product, we also tried to synthesize aurodrosopterin, which is produced nonenzymatically by the one-to-one condensation of 7,8-dihydrolumazine and PDA under acidic conditions (5). For this purpose, aliquots of the deaminase reaction mixture taken out at various time points were incubated with PDA as described under “Experimental Procedures.” The synthesized pigments were analyzed by one-dimensional TLC (Fig. 2B). At the early stage of the deamination reaction, only drosopterin and isodrosopterin were detected; as the deamination reaction proceeded, the synthesis of aurodrosopterins gradually increased (Fig. 2B). After the substrate had been fully exhausted, the synthesized pigments were detected at the same position as that of natural aurodrosopterin on a two-dimensional TLC plate (Fig. 2C). Consequently the enzymatic reaction product is 7,8-dihydrolumazine, which is the precursor of aurodrosopterin in nonenzymatic synthesis.
Substrate Specificity
Various pteridine compounds were tested for substrate specificity. When the deaminase activity for 7,8-dihydropterin was 100%, the activities for tetrahydropterin, tetrahydrobiopterin, and tetrahydroneopterin were determined to be 71, 62, and 63%, respectively. The enzyme had no deaminase activities for other dihydropteridines such as 7,8-dihydrobiopterin, 7,8-dihydroneopterin, and sepiapterin; it also did not show deaminase activity with fully oxidized pteridines such as pterin, biopterin, neopterin, monapterin, xanthopterin, isoxanthopterin, aminopterin, 6-formylpterin, 6-carboxypterin, and 6-hydroxymethylpterin. Moreover the enzyme did not use folic acid, PDA, drosopterin, or isodrosopterin as a substrate.
To determine whether the high reactivities with tetrahydropteridines are due to the nonenzymatic conversion of tetrahydropteridines to 7,8-dihydropterin, the spectral changes in the reaction mixture were examined during the deaminase reaction. When tetrahydropterin was incubated under the standard reaction condition in the absence of enzyme, the UV-visible spectrum of the reaction mixture increased gradually to that of 7,8-dihydropterin up until 20 min. The highest deaminase activity was obtained when the aliquot was taken out at 20 min and used as the source of the substrate. Moreover when the deaminase assay with tetrahydropterin was coupled with 6,7-dihydropteridine reductase (EC 1.5.1.34), which specifically converts quinonoid dihydropteridines to their tetrahydro forms (24), no deaminase activity was detected. This result strongly suggests that the reason for the high deaminase activities for tetrahydropteridines is that tetrahydropteridines are converted to 7,8-dihydropterin by nonenzymatic side chain release at position 6 via quinonoid dihydropteridines.
Interestingly the enzyme exhibited 40-fold higher activities for guanine than for 7,8-dihydropterin (Table 2) along with 60% activity for 8-azaguanine and 5% activity for guanosine relative to 7,8-dihydropterin. No activity was found for adenine and adenosine (data not shown).
TABLE 2.
Kinetic parameters of the purified enzyme for 7,8-dihydropterin and guanine
The purified enzyme for 7,8-dihydropterin and guanine exhibited Michaelis-Menten kinetics. The Km and Vmax were calculated using the Lineweaver-Burk plotting method. The turnover number, kcat, was calculated from the specific activity and the molecular mass of the enzyme (48 kDa). All values are means ± S.D. of three determinations.
| Substrate |
||
|---|---|---|
| 7,8-Dihydropterin | Guanine | |
| Vmax (nmol·min−1·μg−1) | 8.9 ± 0.2 | 23.6 ± 2.1 |
| Km (μm) | 1621.0 ± 73.4 | 75.7 ± 3.3 |
| kcat (s−1) | 16.3 ± 0.4 | 649.0 ± 58.2 |
| kcat/Km (m−1·s−1) | 1.0 × 104 ± 4.9 × 102 | 8.6 × 106 ± 4.1 × 105 |
Other Biochemical Characteristics
The enzyme exhibited optimal catalytic activities at pH 7.5 and 40 °C against both 7,8-dihydropterin and guanine. The overall pH and temperature profiles of the catalytic activity for guanine were almost the same as those for 7,8-dihydropterin within the error ranges. However, in 0.1 m glycine, NaOH buffers of pH 9.0 and 9.5, the catalytic activities for 7,8-dihydropterin were significantly lower than those for guanine. The pI value of the enzyme, as determined by analytical isoelectric focusing on a nondenaturing gel, was 5.7.
Because the deaminase efficiently uses both 7,8-dihydropterin and guanine as substrates, the kinetic parameters of the enzyme were compared (Table 2). The Km values for 7,8-dihydropterin and guanine were determined to be 1620 and 75.7 μm, respectively, and the Vmax values for 7,8-dihydropterin and guanine were 8.9 and 23.6 nmol/min/μg of protein, respectively. The specificity constant (kcat/Km) for guanine (8.6 × 106 m−1·s−1) was 860-fold higher than that for 7,8-dihydropterin (1.0 × 104 m−1·s−1).
Various compounds were tested for their potential inhibitory effect on deaminase activities (Table 3). The deaminase activities for both 7,8-dihydropterin and guanine were completely inhibited in the presence of 10−4 m p-chloromercuribenzoate. At concentrations of 10−3 and 10−2 m, potassium fluoride, allopurinol, lumazine, guanosine, adenine, and adenosine showed similar weak inhibitory effects. However, KCN and pterin showed different inhibitory effects on deaminase activities for the two substrates. KCN inhibited dihydropterin deaminase activity strongly (98.6% inhibition at 10−2 m), whereas it had almost no inhibitory effect on guanine deaminase activity at the same concentration. On the other hand, pterin strongly inhibited guanine deaminase activity (57.8% inhibition at 10−3 m), whereas it had a weak effect on dihydropterin deaminase activity.
TABLE 3.
Effect of various compounds on deaminase activity
Reaction mixtures containing 1 unit of the purified enzyme and the indicated amount of selected compounds were incubated under the standard reaction conditions, and the amounts of liberated ammonia were determined by the phenate method. All values are the means of three determinations. PCMB, p-chloromercuribenzoate.
| Compound concentration |
Inhibition |
|
|---|---|---|
| Dihydropterin deaminase | Guanine deaminase | |
| m | % | |
| PCMB | ||
| 10−4 | 100 | 100 |
| KF | ||
| 10−2 | 3.5 | 14.3 |
| 10−3 | 1.2 | 8.1 |
| 10−4 | 0.0 | 3.6 |
| KCN | ||
| 10−2 | 98.6 | 2.8 |
| 10−3 | 44.7 | 0.0 |
| 10−4 | 3.7 | 0.0 |
| Allopurinol | ||
| 10−3 | 10.1 | 11.0 |
| 10−4 | 6.2 | 5.2 |
| Lumazine | ||
| 10−3 | 10.0 | 17.8 |
| 10−4 | 3.2 | 4.5 |
| Pterin | ||
| 10−3 | −8.2 | 57.8 |
| 10−4 | −2.1 | 18.9 |
| Guanosine | ||
| 10−3 | 5.1 | 12.5 |
| 10−4 | 3.8 | 1.8 |
| Adenine | ||
| 10−3 | 14.1 | 24.0 |
| 10−4 | 10.3 | 7.1 |
| Adenosine | ||
| 10−3 | 16.7 | 22.7 |
| 10−4 | 11.5 | 5.6 |
Cloning and Expression of the Structural Gene of Dihydropterin Deaminase
To identify the structural gene, the purified enzyme was analyzed by MALDI-TOF mass spectrometry. Thirty-seven peptides (range, 1000–3000 Da) were analyzed by a data base search that identified the enzyme as the product of the Drosophila CG18143 gene, located at the 82A1 region of the cytological map of chromosome 3R (GenBankTM accession number AE003607; NCBI RefSeq accession number NP_ 649439). It encodes 448 amino acids as deduced from the open reading frame sequence of CG18143; the theoretical molecular mass and pI value are predicted to be 48,884 Da and 5.97, respectively, which are in close agreement with the values determined with the purified enzyme. Fig. 3 shows that the product of CG18143 is highly homologous to guanine deaminases from other sources, such as human (NCBI RefSeq accession number NP_004284), mouse (NCBI RefSeq accession number NP_034396), and yeast (NCBI RefSeq accession number NP_010043). We cloned the open reading frame of CG18143 into the pET 15b vector, expressed the proteins in E. coli, and purified them with a Ni2+ affinity column. The purified recombinant protein also showed deaminase activities for both 7,8-dihydropterin and guanine. Consequently it is clear that Drosophila CG18143 is the structural gene of dihydropterin deaminase, which also has guanine deaminase activity.
FIGURE 3.
Multiple sequence alignment. Sequence alignment of Drosophila CG18143 was performed with guanine deaminase from human, mouse, and yeast using the ClustalW program. Identical and semiconserved amino acids are highlighted in black and gray, respectively. CG18143 (NCBI RefSeq accession number NP_649439) is highly homologous to guanine deaminases of human (NCBI RefSeq accession number NP_004284), mouse (NCBI RefSeq accession number NP_034396), and yeast (NCBI RefSeq accession number NP_010043): 41% identity and 71% similarity, 43% identity and 71% similarity, and 34% identity and 66% similarity, respectively. The putative Zn2+ binding motif is indicated by a bracket.
The in Vivo Role of CG18143 in Aurodrosopterin Biosynthesis
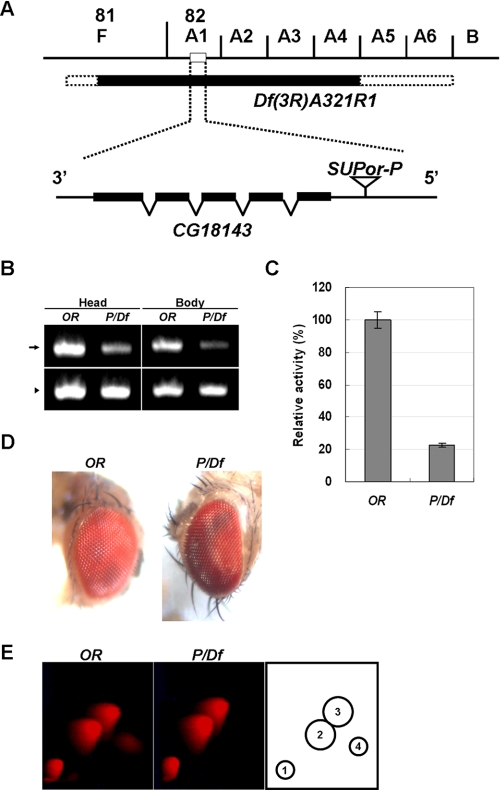
Dihydropterin deaminase converts 7,8-dihydropterin to 7,8-dihydrolumazine, which acts as an immediate precursor of aurodrosopterin (6). We used mutational analysis to examine whether dihydropterin deaminase actually participates in the biosynthesis of aurodrosopterin. To establish the mutant flies, two kinds of flies were used: one carried a single SUPor-P element near the transcription initiation site of CG18143, and the other was a deficiency line, Df(3R)A321R1, described under “Experimental Procedures” (Fig. 4A). The SUPor-P element is a modified P element that carries the suppressor of Hairy-wing (su(Hw)) binding regions, and its gene expression can be altered by the su(Hw) protein as a result of interfering with the interaction between enhancer/silencer elements and their promoter (16). In comparison with wild type flies, the mRNA level of CG18143 was severely decreased in the head and body of the SUPor-P/Df(3R)A321R1 transheterozygotes (Fig. 4B), and dihydropterin deaminase activity in the head also decreased to about 22% of the activity in the wild type flies (Fig. 4C). The mutant flies exhibited slightly more dark reddish eye color than the wild type flies (Fig. 4D). Moreover the mutant transheterozygotes had dramatically decreased amounts of aurodrosopterin in their heads, whereas the levels of the other red eye pigments were normal (Fig. 4E). All these results support the conclusion that the dihydropterin deaminase encoded by CG18143 plays a direct role in the biosynthesis of aurodrosopterin by providing its precursor, 7,8-dihydrolumazine.
FIGURE 4.
Mutational analysis of the function of CG18143 in aurodrosopterin biosynthesis. A, cytological map of chromosome 3R showing CG18143 and its environs. The solid bar represents the deficiency region, including CG18143, in the Df(3R)A321R1 fly. Deficiency breakpoint limits are depicted by stippled bars. The SUPor-P element of the ;;P{y+mDint2 wBR.E.BR SUPor-P} fly is indicated 98 bp upstream from the first exon of CG18143. B, comparison of CG18143 mRNA level (arrow) between Oregon-R (OR) and SUPor-P/Df(3R)A321R1 (P/Df) transheterozygotes. Total RNA was extracted and reverse transcribed followed by PCR with specific primers as described under “Experimental Procedures.” The levels of glyceraldehyde-3-phosphate dehydrogenase mRNA (arrowhead) were used as internal controls. C, relative activities of dihydropterin deaminase in wild type flies (OR) and mutant flies (P/Df). The ammonium sulfate fraction (30–60%) from 2-day-old adult head extracts was used as the source of the enzyme. Vertical bars represent mean ± S.E. D, eye color phenotype of 2-day-old wild type and P/Df transheterozygote mutants. E, analysis of red pigments in the heads of Oregon-R (left panel) and P/Df transheterozygote (middle panel) by two-dimensional TLC. The fluorescent spots were detected by a long wave UV lamp. In P/Df transheterozygotes, aurodrosopterin was specifically decreased compared with the wild type flies. The identity of each spot is as follows: 1, neodrosopterin; 2, drosopterin; 3, isodrosopterin; and 4, aurodrosopterin (right panel).
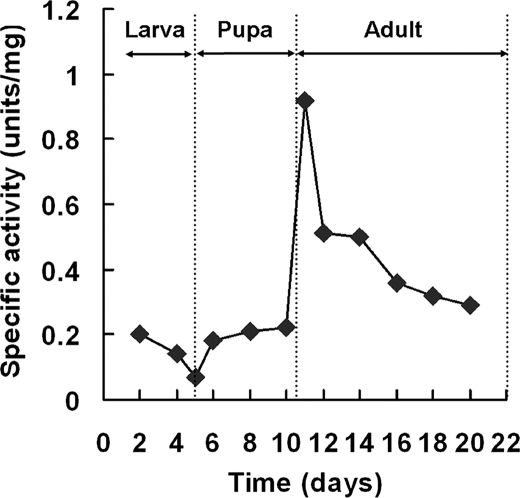
Developmental Profile of Dihydropterin Deaminase Activity
We compared the specific activity of dihydropterin deaminase at different developmental stages of the wild type flies. As shown in Fig. 5, the deaminase activity increased considerably just before and after eclosion and remained relatively high for a few days in adult flies. The enzymes involved in the biosynthesis of the pteridine pigments, such as GTP cyclohydrolase I (25) and 6-PTP synthase (26), also showed their highest activity in the late pupa and in the newly emerged adult. The developmental profile of dihydropterin deaminase agreed well with the fact that the formation of red eye pigments starts during the late pupal stage and continues over several days in the eclosed fly (27).
FIGURE 5.
Developmental profile of dihydropterin deaminase activity. An ammonium sulfate fraction (30–60%) of crude extracts was prepared from whole bodies synchronized at the indicated stages. The standard reaction mixtures, containing 35 μg of protein from each developmental stage, were assayed by the HPLC method. All values are the means of three determinations.
Function of CG18143 as Guanine Deaminase
The purified enzyme showed high guanine deaminase activity and a high kcat/Km value for guanine. Therefore, we also examined the guanine deaminase activity and the levels of related purine metabolites in the CG18143 mutant. According to a previous report (28), the amount of free uric acid in male D. melanogaster is the highest in 1-day-old adults and declines sharply with age to about 50% of the initial level in 5-day-old flies. Free xanthine concentrations are low in newly emerged adult flies but increase sharply until 5 days of adult age after which they decline. The fly homogenates from 2-day-old adult flies were chosen to compare the amounts of guanine deaminase activity, free xanthine, and uric acid in wild type and mutant flies. In the mutant CG18143 flies, the guanine deaminase activity was severely decreased to about 27% of the activity in the wild type flies, and the amounts of free xanthine and uric acid were also considerably decreased to about 76 and 59%,respectively, of the levels in wild type flies. These results support the conclusion that the product of CG18143 also functions as guanine deaminase, a member of the purine metabolic pathway.
DISCUSSION
Based on structural considerations, the deamination of a pteridine compound is required for the production of dihydrolumazine and for the synthesis of aurodrosopterin eye pigment. However, the molecular properties of the enzyme catalyzing this step and the identity of the gene encoding the deaminase have not been established. In the present study, we purified and characterized Drosophila dihydropterin deaminase and identified its structural gene. The enzyme has dual functions: as dihydropterin deaminase, it directly facilitates the biosynthesis of aurodrosopterin eye pigment by providing its precursor, 7,8-dihydrolumazine, and as guanine deaminase, it is involved in purine metabolism in D. melanogaster.
The purified dihydropterin deaminase showed high activities toward tetrahydropteridines such as tetrahydropterin, tetrahydrobiopterin, and tetrahydroneopterin. Its true substrate is 7,8-dihydropterin, which is derived from the tetrahydro form of pteridines. The enzyme had no deaminase activities for other dihydropteridines or for fully oxidized pteridines. The enzyme also exhibited high deaminase activity for guanine. The Km value for guanine was 21-fold lower than that for 7,8-dihydropterin, and the turnover number (kcat) and specificity constant (kcat/Km) for guanine were 40- and 860-fold higher, respectively, than those for 7,8-dihydropterin. This means that the enzyme has a much higher catalytic efficiency for guanine than for 7,8-dihydropterin in vivo. The enzyme also showed relatively high activity for 8-azaguanine, a synthetic analog of guanine.
Because of the similarities between the chemical structures of purine and pteridine, it might be possible for guanine deaminase to deaminate pteridine compounds as well as guanine. Nevertheless every pteridine deaminase examined so far uses purine as a substrate (13, 29), and conversely, guanine deaminases from bacterial, fungal, and mammalian sources use no pteridines as substrates (30, 31). In the present study, we report that pteridine deaminase purified from Drosophila also functions as guanine deaminase. The molecular mass of the purified enzyme, 48 kDa, is in good agreement with the masses of guanine deaminase subunits from various sources; the Drosophila enzyme is a monomer under native conditions, whereas other guanine deaminases have generally been found to be dimeric except for rabbit liver guanine deaminase, which is a monomer (30, 31). It should be noted that Drosophila dihydropterin deaminase shares a 9-residue N-terminal motif with other aminohydrolases and amidohydrolases (PGX(V/I)DXH(T/V/I)H) (Fig. 3). In particular, the invariant His-X-His portion of the motif has been implicated in the association of a bound zinc ion, which plays key roles in stabilizing the attacking hydroxide ion during catalysis (32).
The purified enzyme shares pH and temperature effects similar to its two enzymatic activities. Both the dihydropterin and guanine deaminase activities of the enzyme were highly sensitive to mercurial p-chloromercuribenzoate, indicating an essential cysteine –SH at the active site. However, the characteristics of the catalytic activity for 7,8-dihydropterin differed from those for guanine in some aspects; dihydropterin deaminase activities were much lower at pH 9.0 and 9.5 than those of guanine deaminase (data not shown), and KCN and pterin showed different inhibitory effects toward dihydropterin and guanine deaminase activity (Table 3). The differential effects of pH, KCN, and pterin might originate from the local environment of the active site; i.e. specific residues or the zinc ions in the Drosophila dihydropterin deaminase participate differentially in recognizing 7,8-dihydropterin and guanine, causing these differential effects of pH and inhibitors on catalytic activities for the two substrates.
Aurodrosopterin and isoaurodrosopterin are produced nonenzymatically by the one-to-one condensation of 7,8-dihydrolumazine and PDA (5) in a manner similar to drosopterin and isodrosopterin, which are produced from 7,8-dihydropterin and PDA (10). PDA is synthesized from 6-PTP by PDA synthase (10, 11), but the origins of the other precursors, 7,8-dihydropterin and 7,8-dihydrolumazine, are not fully understood. Yim et al. (33) reported the presence of an enzyme in D. melanogaster that releases the three-carbon side chain from 7,8-dihydroneopterin triphosphate (or 6-PTP) to 7,8-dihydropterin, and the dihydropterin deaminase, responsible for the synthesis of 7,8-dihydrolumazine, was purified to a homogeneous form in this study. The MALDI-TOF analysis of the pure protein revealed that Drosophila CG18143 is the structural gene for the deaminase. The biochemical and enzymological properties (molecular size, substrate specificity, pI value, and optimal pH and temperature) of the recombinant protein expressed from the CG18143 open reading frame were almost identical to those of the native enzyme purified from the fly head extract.
The evidence that guanine deaminase plays a significant role in urate production via the xanthine pathway in mammals (34) agrees well with our data that CG18143 mutants had decreased levels of xanthine and urate. Guanine deaminase catalyzes the hydrolytic deamination of guanine to xanthine, which leads to irreversible elimination of the guanine base from further use as guanylate nucleotides. The diagnostic usefulness of guanine deaminase has also been indicated in humans; for example, the activity of the enzyme in serum is among the most sensitive indicators of liver diseases because of its near absence in normal serum, erythrocytes, and lymphoid cells (35). Moreover various guanine deaminase activities have been found in cancerous breast and kidney tissues (36). In Drosophila, the role of guanine deaminase still remains to be determined.
This work was supported by Korea Research Foundation Grant KRF-2008-313-E00068 funded by the Korean Government (Ministry of Education and Human Resources Development) and by the second stage of the Brain Korea 21 project in 2009.
E. J. Rehm, unpublished.
- 6-PTP
- 6-pyruvoyl tetrahydropterin
- pterin
- 2- amino-4-hydroxypteridine
- PDA
- pyrimidodiazepine
- HPLC
- high performance liquid chromatography
- TLC
- thin layer chromatography
- MALDI-TOF
- matrix-assisted laser desorption/ionization time-of-flight.
REFERENCES
- 1.Lederer E. (1940) Biol. Rev. Camb. Philos. Soc. 15,273–306 [Google Scholar]
- 2.Viscontini M., Hadorn E., Karrer P. (1957) Helv. Chim. Acta 40,579–585 [Google Scholar]
- 3.Schwinck I. (1971) Genetics 68,s59–s60 [Google Scholar]
- 4.Theobald N., Pfleiderer W. (1978) Chem. Ber. 111,3385–3402 [Google Scholar]
- 5.Yim J., Kim S. J., Walcher G., Pfleiderer W. (1993) Helv. Chim. Acta 76,1970–1979 [Google Scholar]
- 6.Mackay W. J., O'Donnell J. M. (1983) Genetics 105,35–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yim J. J., Grell E. H., Jacobson K. B. (1977) Science 198,1168–1170 [DOI] [PubMed] [Google Scholar]
- 8.Krivi G. G., Brown G. M. (1979) Biochem. Genet. 17,371–390 [DOI] [PubMed] [Google Scholar]
- 9.Park Y. S., Kim J. H., Jacobson K. B., Yim J. J. (1990) Biochim. Biophys. Acta 1038,186–194 [DOI] [PubMed] [Google Scholar]
- 10.Wiederrecht G. J., Paton D. R., Brown G. M. (1984) J. Biol. Chem. 259,2195–2200 [PubMed] [Google Scholar]
- 11.Kim J., Suh H., Kim S., Kim K., Ahn C., Yim J. (2006) Biochem. J. 398,451–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rembold H., Metzger H., Gutensohn W. (1971) Biochim. Biophys. Acta 230,117–126 [DOI] [PubMed] [Google Scholar]
- 13.Takikawa S., Tsusue M., Gyure W. L. (1983) Insect Biochem. 13,361–368 [Google Scholar]
- 14.Yim J. J., Brown G. M. (1976) J. Biol. Chem. 251,5087–5094 [PubMed] [Google Scholar]
- 15.Kaufman S. (1967) J. Biol. Chem. 242,3934–3943 [PubMed] [Google Scholar]
- 16.Roseman R. R., Johnson E. A., Rodesch C. K., Bjerke M., Nagoshi R. N., Geyer P. K. (1995) Genetics 141,1061–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taras M. J., Arnold E. F., Hoak R. D., Rand M. C. (1981) in Standard Methods for the Examination of Water and Wastewater ( Greenberg A. E. ed) pp. 350–352, American Public Health Association, Washington, D. C [Google Scholar]
- 18.Gharahdaghi F., Weinberg C. R., Meagher D. A., Imai B. S., Mische S. M. (1999) Electrophoresis 20,601–605 [DOI] [PubMed] [Google Scholar]
- 19.Arnott D., O'Connell K. L., King K. L., Stults J. T. (1998) Anal. Biochem. 258,1–18 [DOI] [PubMed] [Google Scholar]
- 20.Hilliker A. J., Duyf B., Evans D., Phillips J. P. (1992) Proc. Natl. Acad. Sci. U.S.A. 89,4343–4347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bradford M. M. (1976) Anal. Biochem. 72,248–254 [DOI] [PubMed] [Google Scholar]
- 22.Awdeh Z. L., Williamson A. R., Askonas B. A. (1968) Nature 219,66–67 [DOI] [PubMed] [Google Scholar]
- 23.Forrest H. S., van Baalen C., Viscontini M., Piraux M. (1960) Helv. Chim. Acta 43,1005–1010 [Google Scholar]
- 24.Park D., Park S., Yim J. (2000) Biochim. Biophys. Acta 1492,247–251 [DOI] [PubMed] [Google Scholar]
- 25.Fan C. L., Hall L. M., Skrinska A. J., Brown G. M. (1976) Biochem. Genet. 14,271–280 [DOI] [PubMed] [Google Scholar]
- 26.Kim N. S., Kim J. S., Park D. K., Rosen C., Dorsett D., Yim J. (1996) Genetics 42,1157–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwinck I., Mancini M. (1973) Arch. Genet. 46,41–52 [PubMed] [Google Scholar]
- 28.Massie H. R., Shumway M. E., Whitney S. J. (1991) Exp. Gerontol. 26,609–614 [DOI] [PubMed] [Google Scholar]
- 29.Rembold H., Gyure W. L. (1972) Angew. Chem. Int. Ed. Engl. 11,1061–1072 [DOI] [PubMed] [Google Scholar]
- 30.Rossi C. A., Hakim G., Solaini G. (1978) Biochim. Biophys. Acta 526,235–246 [DOI] [PubMed] [Google Scholar]
- 31.Gupta N. K., Glantz M. D. (1985) Arch. Biochem. Biophys. 236,266–276 [DOI] [PubMed] [Google Scholar]
- 32.Liaw S. H., Chang Y. J., Lai C. T., Chang H. C., Chang G. G. (2004) J. Biol. Chem. 279,35479–35485 [DOI] [PubMed] [Google Scholar]
- 33.Yim J., Jacobson K. B., Crummett D. C. (1981) Insect Biochem. 11,363–370 [Google Scholar]
- 34.Farkas W. R., Stanawitz T., Schneider M. (1978) Science 199,786–787 [DOI] [PubMed] [Google Scholar]
- 35.Kuzmits R., Seyfried H., Wolf A., Müller M. M. (1980) Enzyme 25,148–152 [DOI] [PubMed] [Google Scholar]
- 36.Canbolat O., Durak I., Cetin R., Kavutcu M., Demirci S., Oztürk S. (1996) Breast Cancer Res. Treat. 37,189–193 [DOI] [PubMed] [Google Scholar]