Abstract
Calcium oxalate dihydrate (COD) mineral and the urinary protein osteopontin/uropontin (OPN) are commonly found in kidney stones. To investigate the effects of OPN on COD growth, COD crystals were grown with phosphorylated OPN or a polyaspartic acid-rich peptide of OPN (DDLDDDDD, poly-Asp86–93). Crystals grown with OPN showed increased dimensions of the {110} prismatic faces attributable to selective inhibition at this crystallographic face. At high concentrations of OPN, elongated crystals with dominant {110} faces were produced, often with intergrown, interpenetrating twin crystals. Poly-Asp86–93 dose-dependently elongated crystal morphology along the {110} faces in a manner similar to OPN. In crystal growth studies using fluorescently tagged poly-Asp86–93 followed by imaging of crystal interiors using confocal microscopy, sectoral (compositional) zoning in COD was observed resulting from selective binding and incorporation (occlusion) of peptide exclusively into {110} crystal sectors. Computational modeling of poly-Asp86–93 adsorption to COD {110} and {101} surfaces also suggests increased stabilization of the COD {110} surface and negligible change to the natively stable {101} surface. Ultrastructural, colloidal-gold immunolocalization of OPN by transmission electron microscopy in human stones confirmed an intracrystalline distribution of OPN. In summary, OPN and its poly-Asp86–93 sequence similarly affect COD mineral growth; the {110} crystallographic faces become enhanced and dominant attributable to {110} face inhibition by the protein/peptide, and peptides can incorporate into the mineral phase. We, thus, conclude that the poly-Asp86–93 domain is central to the OPN ability to interact with the {110} faces of COD, where it binds to inhibit crystal growth with subsequent intracrystalline incorporation (occlusion).
Calcium oxalate is the major mineral phase of human renal calculi, constituting roughly 70% by weight of the stones (1). Two polymorphs of calcium oxalate, calcium oxalate monohydrate (COM)3 and calcium oxalate dihydrate (COD), are the most abundant mineral types, but others may exist in smaller amounts, including calcium phosphate minerals. It has been reported that the occurrence of COM, the more thermodynamically stable polymorph of calcium oxalate, is often at the core of most kidney stones and is approximately twice as frequent as COD (2), although both crystal types typically exist to some degree in most stones (3, 4). COM is commonly found in the urine of “stone formers,” but seldom is seen in healthy urine; on the other hand, COD crystals are typically found in the urine of both healthy people and stone formers and are routinely excreted during urination (5–7). Importantly, in patients with severe uremia and hypercalciuria, elongated, large rod-shaped COD crystals are not only often observed but are the sole mineral phase present in the kidneys in these pathologies (8, 9).
In comparing physiologic differences between calcium oxalate polymorphs, one study has shown that for a given amount of added crystals, ∼50% more COM than COD crystals bound to inner medullary collecting duct cells in vitro (10). Other studies have reported that COD crystals are less prone than COM crystals to adhere to cell surfaces, suggesting that COD might, thus, contribute to a lesser degree than COM to the retention of mineral in the renal collecting ducts leading to kidney stone formation (5, 11). This is supported by the fact that COM crystals are large cationic particulates, presenting more calcium ions than COD crystals at their surface that would have a stronger affinity for anionic molecules on renal epithelial cell membranes (10, 12). Further to this, COM and COD crystals bind to cultured renal cells with different face-selective affinities (13–16), and COM crystals are known to be more injurious to cell membranes than COD crystals (17). In this regard, Wesson et al. (1) proposed that the preferential formation of COD crystals in vivo protects against urolithiasis because they are less likely to adhere to renal tubular cells and are, thus, more readily excreted. This notion is supported by direct experimental measurements of the macromolecular adhesion force on specific crystal faces of COM or COD at the near-molecular level (13, 14). Given this, conversely, inhibition of the formation of COD crystals could lead to preferential COM deposition and the formation of kidney stones.
Although calcium and oxalate ionic concentrations are frequently supersaturated with respect to both COM and COD mineral polymorphs, normal human urine likely contains factors that can modulate calcium oxalate crystallization into COD (10). In this context the presence of urinary macromolecular inhibitors of crystal growth can cause preferential crystallization of COD, rather than COM, from a supersaturated solution of calcium chloride and sodium oxalate (10). Substantial elegant work has been performed on COM growth and the effect of citrate and peptides/proteins as crystal growth modifiers. Some macromolecules, including urinary osteopontin (OPN), contain polyanionic regions and net negative charges that have been shown to inhibit calcium oxalate crystallization (14, 18–21) and influence calcium oxalate growth in favor of COD (3, 10, 22, 23). Although there appears to be preferential inhibition of COM, several studies present evidence for higher affinity of OPN binding to COD, and here we investigate this further to show the effects of OPN (and a peptide of OPN) on COD crystal growth and provide information on peptide/protein occlusion that might facilitate crystal dissolution as originally proposed by Ryall et al. (see below) (23, 24).
OPN is a highly acidic, glycosylated phosphoprotein produced by many types of epithelial cells and can be found in normal plasma and in various body fluids such as bile, urine, and milk (25, 26). In normal kidneys, OPN is secreted by the thin and thick ascending limbs of the loop of Henle and distal nephrons (27–32). OPN contains a 15–20% aspartic acid residue content, and the mineral binding and inhibitory properties of OPN have often been partly attributed to an aspartic acid-rich sequence within this protein (10, 26, 33, 34). Likewise, post-translational phosphorylation of OPN has been shown to markedly enhance the mineral binding and inhibitory ability of this protein (34, 35). Furthermore, OPN also contains sialic acid (26, 27), which may play an indirect role in crystal binding by forming a bridge between transiently expressed crystal binding molecules and the cell surface (12). Several studies have shown that OPN has a higher affinity for COD than COM in normal urinal precipitates, with some evidence given for the incorporation of protein into calcium oxalate crystals (1, 23, 36).
OPN consistently localizes to kidney stones (37) and at physiologically relevant concentrations applied in vitro, acts as a potent inhibitor of the nucleation, growth, and aggregation of calcium oxalate crystals (27, 30, 34). In a rat model of urolithiasis, although increased OPN mRNA expression was associated with increased renal calculi formation, the urinary excretion level of OPN was less than in controls, discussed as being attributable to incorporation of OPN into stones (37, 38). In general, the inclusion of OPN plus other urinary macromolecules into renal calculi has been suggested to be part of a cellular defense mechanism designed to inhibit crystal growth and limit the growth of kidney stones, to interrupt inorganic crystal structure of the calcium oxalate minerals, and to provide an organic volume whose degradation by permeating proteases creates channels facilitating dissolution of the mineral phase (23, 24). Given these possibilities, our aim was to determine whether full-length phosphorylated OPN and a poly-Asp peptide of OPN affect COD crystal growth and morphology, and if so, we further aimed to identify the contribution of face-specific binding and intracrystalline incorporation (occlusion) of OPN peptide into COD. Combining experimental and computational approaches, we have identified preferential binding and unique occlusion of a peptide of OPN at a specific crystallographic face of COD. Furthermore, we present a possible adsorption mechanism in a model where multiple peptide carboxylate groups bind calcium atoms at the COD {110} surface. Taken together, our findings provide new information on the pathogenesis of renal calculi by describing specific actions of phosphorylated full-length OPN and a short peptide sequence of OPN (not having post-translational modifications) on specific COD crystal faces that modulate calcium oxalate growth and crystal morphologies.
EXPERIMENTAL PROCEDURES
Kidney Stones and Immunolocalization of OPN by Light and Electron Microscopy
Kidney stones from two female patients (age 66 and 69) obtained from the Kidney Stone Clinic of the Royal Victoria Hospital, McGill University Hospital Centre, were characterized as being calcium oxalate-containing stones by routine x-ray diffraction performed by a commercial service on a fragment of each stone. The bulk of the stones were washed briefly multiple times with tap water and fixed in 1% glutaraldehyde and 1% paraformaldehyde in 0.1 m sodium cacodylate buffer (pH 7.2) for 2 days. Stones were decalcified in a 4.13% EDTA solution containing 1% glutaraldehyde.
For light microscopy immunohistochemistry, the decalcified kidney stone samples were embedded in paraffin, and 5-μm sections were immunostained for OPN using rabbit anti-human OPN (LF-123) polyclonal antibodies (antibodies courtesy of Dr. Larry W. Fisher, National Institutes of Health, Bethesda, MD). Briefly, deparaffinized sections were treated with 1% bovine testicular hyaluronidase (Sigma) at 37 °C for 30 min followed by incubation with anti-OPN LF-123 antibody diluted 1:200 in 5% normal goat serum, 0.2% bovine serum albumin in Tris-buffered saline (TBS) with 0.01% Tween 20 (TBST) (50 mm Tris-HCl, 150 mm NaCl, 0.01% Tween 20 (pH 7.6)). Sections were washed with TBST, and secondary biotinylated goat anti-rabbit IgG (Caltag Laboratories, Invitrogen) antibody incubation was followed by TBST washing and application of the Vectastain ABC-AP kit (Vector Laboratories, Burlingame, CA) for an additional 45 min at room temperature. Color development was achieved by treatment with Fast Red TR/Naphthol AS-MX phosphate (Sigma) containing 1 mm levamisole to inhibit endogenous alkaline phosphatase activity. Sections were counterstained with methyl green (Vector Laboratories) and mounted with coverslips using Kaiser's glycerol jelly. Light micrographs were obtained using a DXC-950 3-CCD camera (Sony, Tokyo, Japan) mounted on an optical microscope (model Leitz DMRBE, Leica Wetzlar, Germany).
For ultrastructural immunolocalization of OPN in the kidney stones, aldehyde-fixed samples were fully decalcified with EDTA (also containing aldehyde), dehydrated, and then embedded in LR White acrylic resin (London Resin Co., Berkshire, UK) as previously described (39). Survey sections (0.5 μm) of embedded tissue were cut with a diamond knife on an ultramicrotome (Model Reichert Ultracut E, Leica), stained for light microscopy with toluidine blue, and coverslipped. For transmission electron microscopy, selected regions were trimmed, and ultrathin sections (80 nm) were placed on polyvinyl Formvar- and carbon-coated nickel grids. Grid-mounted tissue sections were processed for colloidal-gold immunocytochemistry by incubation of the sections with primary anti-OPN LF-123 antibody (1:10 dilution), after which immunolabeling patterns were visualized by incubation with protein A-colloidal gold complex (14 nm). Contrasting of the sections was performed using conventional uranyl acetate and lead citrate staining, and this was followed by their examination using a JEM 2000FXII transmission electron microscope (JEOL, Tokyo, Japan) operated at 80 kV.
Precipitation of Calcium Oxalate Crystals from Human Urine
The metastable limit of human urine with respect to oxalate, slightly below 4 mm sodium oxalate final concentration, was determined as described by Ryall et al. (40), and the protocol to precipitate calcium oxalate crystals from human urine was adapted from Doyle et al. (41). Briefly, fresh morning urine (200–500-ml volume) was collected without added anti-bacterial agents from 6 males between the ages of 23 and 45, with each having no history of kidney stones. The pH of each sample was taken, and urine analysis was performed to identify and discard abnormal samples using ChemStrip 5L (Roche Applied Science), which identifies the presence of leukocytes, glucose, ketones, or red blood cells in the samples. Urinary protein concentrations were quantified by the Micro BCA Protein Assay Reagent kit (Pierce, Thermo Fisher Scientific, Rockford, IL) as well as by the mini-Lowry method. A 200 mm solution of sodium oxalate was added to the urine samples to get a final concentration of 4 mm sodium oxalate, and the samples were left at room temperature for intervals of 1–3 h. Crystals precipitated in the samples were collected by centrifugation at 8200 rpm for 30 min followed by filtration harvesting through a hydrophilic polypropylene membrane (0.2–0.8-mm pore size, Gelman Sciences, Pall Corp., East Hills, NY) and washing with distilled and de-ionized water. To obtain/release crystal-bound proteins, 30 mg of crystals were demineralized with 7 ml of 0.25 m EDTA at pH 8.0 and 4 °C for a period of 3 days with gentle agitation (41). Dialysis was done against distilled, de-ionized water to remove EDTA using cellulose membranes with a 12-kDa molecular weight cutoff (Sigma) for more than 24 h at 4 °C. The extracts were collected in 15-ml conical tubes and concentrated 5-fold with the appropriate volume of distilled, de-ionized water.
Western Blotting for OPN from Urine-precipitated Crystal Extracts
Twenty μl of total protein extract from the precipitated crystals were separated by SDS-PAGE. The proteins were electrophoretically transferred to an Immobilon-P transfer membrane (Millipore). The membranes were blocked with 3% bovine serum albumin (Sigma) in phosphate-buffered saline and then probed with the following primary antibodies; 1) monoclonal mouse anti-human OPN Mb53 antibody (1:5,000 dilution, kindly provided by Dr. A. Chambers, University of Western Ontario, Canada), 2) polyclonal rabbit anti-human OPN LF-124 (anti-N terminus) or polyclonal rabbit anti-human LF-123 (anti-C-terminal) antibodies (LF-124 used at 1:2,000 dilution; LF-123 used at 1:10,000 dilution; kindly provided by Dr. Larry W. Fisher, National Institutes of Health, Bethesda, MD). This was followed by the appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies that included 1) goat anti-mouse-HRP/1:2000 (Dupont Biotechnology, Johnston, IA) for the monoclonal antibody and 2) goat anti-rabbit/1:2000 (Pierce, Thermo Fisher Scientific) for the polyclonal antibodies. The blots were visualized by chemiluminescence using ECL Plus (Amersham Biosciences).
OPN, Poly-Asp Peptide of OPN, and Calcium Oxalate Dihydrate (Weddelite) Crystal Growth in Vitro
To investigate the effects of full-length OPN and an acidic OPN peptide on COD growth, COD crystals were grown from aqueous CaCl2-Na2C2O4 solutions in the presence of OPN or the synthetic poly-Asp peptide. The OPN used for these experiments was full-length, phosphorylated bovine milk OPN as characterized (42) and supplied by Esben Sørensen (Department of Molecular and Structural Biology, University of Aarhus, Denmark) and Arla Foods (Denmark). Synthetic, linear polyaspartic acid-rich (poly-Asp) peptide with or without a fluorescein (FAM) tag was constructed according to the sequence of the aspartate-rich domain of bovine OPN (poly-Asp86–93, residues 86–93, DDLDDDDD) at the Sheldon Biotechnology Centre of McGill University, and peptide was purified by high performance liquid chromatography. Commercial polyaspartic acid (molecular mass range 5,000–15,000 daltons, range of 37–113 residues, Sigma) was also used to compare effects with the linear synthesized poly-Asp86–93. OPN, poly-Asp86–93, or commercial polyaspartic acid was added to the sodium oxalate solution before the crystal growth experiments, with various final concentrations ranging from 0.15 to 5 μm, which spans the physiologic range (43) and extends beyond.
The detailed procedure for the growth of COD crystals was essentially that described by Lepage and Tawashi (44). The growth experiments were performed in a free-drift system at 4 °C. In brief, 3 ml of 0.005 m sodium oxalate (Na2C2O4, Fisher, room temperature) with or without added protein or peptide was added to 5 ml of 1 m calcium chloride solution at 4 °C (CaCl2, Fisher) in 15-ml conical polypropylene tubes (Sarstedt). The Na2C2O4 solution was added to the center of the air-liquid interface of the CaCl2 solution using a micropipette. The mixture was left without agitation for 24 h at 4 °C. The COD crystals that formed were collected by centrifugation at 10,000 × g for 10 min at 4 °C. COD crystals are stable in air at 4 °C for 2 weeks; however, when these crystals are kept in normal saline at 37 °C, they are stable only for 24 h. This is attributable to the gradual transformation of the dihydrate form of calcium oxalate to the monohydrate form, which is more stable in normal saline at 37 °C (44). As COD is insoluble in alcohol (45), the crystals were washed briefly with distilled, de-ionized water (resistance >18 megaohms) and dehydrated sequentially in gradient 50, 70, and 100% ethyl alcohol solutions; the 100% ethyl alcohol was changed 3 times to ensure that the dehydration was complete. The crystals were kept at 4 °C and rehydrated sequentially as needed.
Morphological Imaging of COD Crystals by Scanning Electron Microscopy (SEM)
COD crystals grown in vitro were mounted with conductive carbon cement onto metallic SEM stubs. Samples were then sputter-coated with a 20–25-nm-thick gold-palladium thin film using a Hummer VI Sputter System (Anatech Ltd., Hayward, CA) and imaged using a Hitachi field-emission gun SEM operating at an accelerating voltage of 3–5 kV (model S-4700, Hitachi High Technologies America, Pleasanton, CA) and using working distances of <12 mm.
Fluorescence Microscopy and Laser Confocal Microscopy of Crystals Grown with Poly-Asp86–93
COD crystals grown in the presence of fluorescein-tagged poly-Asp86–93 (as above) were suspended in absolute alcohol and deposited on microscope glass slides. Samples were viewed as whole mounts by immunofluorescence microscopy, and pictures were taken with Leica model DC300F camera mounted on a phase contrast/fluorescence microscope (Leica DM IL). For imaging of fluorescent peptide in the interior of the grown crystals using laser confocal microscopy, glass slides with COD containing fluorescein-tagged poly-Asp86–93 were coverslipped using Geltol mounting medium (Thermo Electron Corp., Pittsburgh, PA). Imaging at different focal planes was acquired using a Zeiss 510-META confocal microscope (Carl Zeiss GmbH, Jena, Germany), and fluorescein was visualized using 488-nm excitation (argon laser) and a 505–530-nm band-pass emission filter. All images were acquired at Nyquist resolution (70 nm/pixel), as recommended by the manufacturer, using a X63 numerical aperture (NA) 1.4 oil-immersion Plan-Apochromat objective lens (Zeiss). Line averaging (16×) was used to minimize noise. Optical sectioning was carried out using an interval of 0.5 μm, and stacks of image slices were analyzed using LSM Image Browser Release Version 4.2.
SHAPE Computer Modeling and Software Identification of Crystallographic Faces
The morphologies of COD crystals were identified and indexed by comparing an observed morphology and the angles of its polar-type corners of faces (i.e. crystal corners intersecting the c-axis of the COD crystal) to graphic renditions prepared by the computer software SHAPE (46), with reference to unit cell parameters, d-spacing, and x-ray powder diffraction file data for COD. SHAPE software renditions based on entered crystallographic parameters for COD matched exactly the observed SEM morphologies, validating differences in relative inhibition of specific faces. Two-dimensional schematic drawings of compositional sectoral zoning were prepared and calculated by re-orienting the three-dimensional morphological SHAPE drawings to match the orientations of selected crystals in light micrographs.
RosettaSurface Energy Calculations and Structure Predictions for Poly-Asp86–93
All simulations were performed using the RosettaSurface Monte Carlo plus-minimization structure-prediction program (47). Each execution of the program folds a peptide from a fully extended conformation and results in one energy-minimized candidate solution- and adsorbed-state structure. Large structural ensembles of 105 candidate solution- and adsorbed-state structures were generated from which the 100 lowest-energy structures from each state were chosen for further analysis. RosettaSurface accounts for solvent and solvent entropy via the Effective Energy Function-1 (EEF-1) implicit solvent model (48).
COD crystal coordinates were constructed for simulation using CrystalMakerTM (49) starting from the COD unit cell (50). Lattice plane terminations were chosen based on exposure of positively charged atoms (calcium) expected to interact with our negatively charged poly-Asp86–93 peptide and based on reasonable estimates of relative stability for individual crystallographic faces. Although multiple lattice plane terminations were run in the simulations, we present here the data for simulations of the {110} face during growth of an atomic plane terminating in high calcium density potentially available for extensive electrostatic peptide/protein binding which could in turn stabilize this termination. For the {101} face modeled here, we chose the normally stable termination leading to charge neutrality with the uppermost calcium-containing layer being partly buried under oxalate or lattice-incorporated H2O groups.
RESULTS
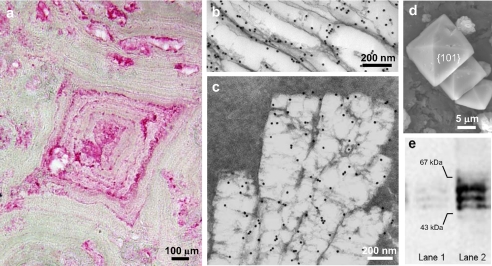
Immunohistochemical staining of human calcium oxalate kidney stones for osteopontin (Fig. 1a, a rhombic-shaped crystal at its center) often show layers/lamellae of concentrically deposited organic matrix surrounding apparent nidi of calcification; the layered appearance of the stones reflects the alternating cycles of crystal and matrix protein deposition at the growing stone surface (51). OPN is most abundantly localized within the lamellae as reported on previously using colloidal-gold immunolabeling (51). In addition to the strong immunolabeling of the lamellae, moderate OPN labeling was detected within the mineral compartment of the kidney stones (here intentionally decalcified during sample preparation to allow ultrathin sectioning and colloidal-gold immunolocalization of OPN by transmission electron microscopy; Fig. 1, b and c). In addition to immunogold labeling for OPN of the thin protein layer circumscribing individual crystals in decalcified samples of the stones (Fig. 1b), generally vacant, more central areas often had some residual matrix retained by the aldehyde (added to the decalcifying solution specifically for this purpose of retaining protein) that had clearly angular profiles reflecting the previously existing crystalline mineral phase (Fig. 1c).
FIGURE 1.
a, immunohistochemical localization of OPN in human kidney stone by light microscopy. Concentric layers/lamellae of organic matrix, rich in OPN, radiate from apparent nidi of calcification. b and c, at the ultrastructural level, colloidal-gold immunolabeling for OPN viewed by transmission electron microscopy shows OPN variably concentrated either at crystallite surfaces (b), here seen as voids after sample decalcification, or within the crystallites (within the void) and associated with a flocculent organic material evident after decalcification (c). d, scanning electron micrograph of COD crystals precipitated from normal male human urine by oxalate addition. Typical di-pyramidal COD crystals show prominent {101} crystallographic faces. e, Western blots for OPN of normal human urine (lane 1) and the protein extract of COD crystals precipitated from normal human urine (lane 2).
In the urine calcium oxalate precipitation experiments, crystals formed by increasing the calcium concentration and examined by SEM showed the typical di-pyramidal shapes characteristic of COD (Fig. 1d). Western blotting of urine was positive for OPN, and the total protein extract obtained from precipitated, washed, and subsequently decalcified crystals revealed particularly abundant OPN released from the COD crystals (Fig. 1e). Taken together, these results confirm previous reports describing that OPN is a major protein component of urine in healthy persons, that OPN is abundant in human kidney stones, and that OPN binds to COD crystals. More specifically, the intracrystalline immunogold localization data provide supporting evidence that a portion of the OPN likely resides within the mineral phase.
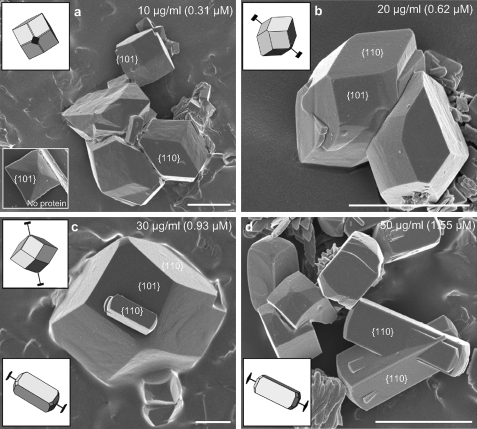
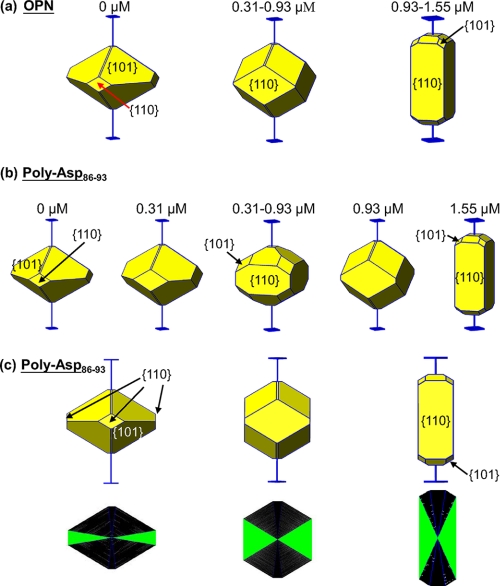
To elucidate how OPN might modify crystal chemistry and habit and whether OPN and OPN peptides might incorporate into COD crystals, COD precipitation experiments were carried out in aqueous CaCl2-Na2C2O4 solutions in the presence of full-length purified OPN or a synthesized 8-amino acid poly-Asp peptide of OPN. In these crystal growth experiments, more than 90% of the crystals precipitated as COD having characteristic tetragonal symmetry. Fig. 2 shows that upon the addition of OPN to the growth solution, the COD was more cuboidal in its crystal habit with the formation of extensive {110} prismatic faces (Fig. 2a), in contrast to having a {101} more di-pyramidal structure in the pure (control) system without protein/peptide (lower inset in Fig. 2a). The {110} prismatic faces of COD gained in morphologic importance (in their dimension) with increasing OPN, a result indicating that OPN interacts and modifies particularly these faces (Fig. 2, b–d). At an OPN concentration of 0.93 mm, elongated crystals started to appear attributable to {110} face-inhibition by OPN (Fig. 2c). At higher OPN concentrations, practically all COD crystals exhibited the elongated shape, often intergrown as penetration twins (Fig. 2d).
FIGURE 2.
a–d, scanning electron micrographs of COD grown in solution in the presence of full-length, phosphorylated OPN at the indicated concentrations. With increasing amounts of OPN, the {110} prismatic crystallographic faces of COD grow in dimension and become predominant at the expense of the {101} faces, the latter being the predominant crystal face formed in the absence of added protein (lower inset in panel a). At the highest concentration of OPN used (d), most COD crystals are elongated and often intergrown as penetration twins. Schematic insets show SHAPE software-derived renditions of the crystals used for face identification and c axis determination (extension bars). Scale bars equal 5 μm.
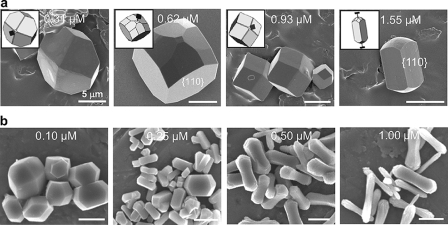
Given the high content of negatively charged aspartic acid residues in OPN and the presence of a contiguous stretch of these amino acids (residues 86–93) proposed to bind to calcium at the positively charged surface of COD, we performed COD precipitation experiments in which synthetic linear poly-Asp86–93 was used in the same crystal growth conditions to compare the effects of this peptide with full-length OPN. Increases in poly-Asp86–93 concentration, although less effective than full-length OPN (crystals showed delayed and less elongation), likewise enhanced the {110} prismatic face dimensions (Fig. 3a). Similar experiments using commercially purchased poly-Asp (Sigma), a large polymer of aspartic acid, exerted the most significant modification of COD morphology (Fig. 3b), not altogether surprising given the relatively high molecular mass (5–15 kDa) linear repeats of high unit charge.
FIGURE 3.
Scanning electron micrographs of COD grown in solution in the presence of synthetic, low (a) and high (b) molecular weight aspartic acid-rich peptides at the indicated concentrations. a, increases in linear poly-Asp86–93 concentration (sequence from OPN bovine sequence), although not as effective as full-length OPN, induced a similar elongating effect on COD crystal morphology, increasing the {110} faces at the expense of the {101} faces. b, high molecular weight and poly-Asp (Sigma) was more potent in similarly generating rod-shaped crystal morphologies, with some additional “rounding” effect at the poles of the elongated crystals. Schematic insets show SHAPE software-derived renditions of the crystals used for face identification and c-axis determination (extension bars). Scale bars equal 5 μm.
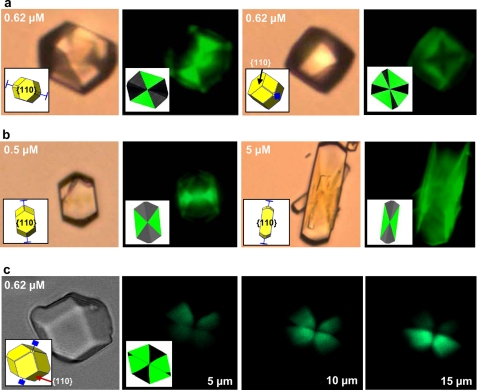
To examine possible incorporation of the poly-Asp86–93 linear peptide into the COD crystal interior, conventional and confocal fluorescence microscopy were used to examine the localization of fluorescently tagged poly-Asp86–93 (FAM-poly-Asp86–93). By conventional fluorescence light microscopy, COD crystals grown in the presence of FAM-poly-Asp86–93 displayed various polygonal fluorescence patterns (Fig. 4a), indicating incorporation (occlusion) of the peptide into COD crystals. The pattern of incorporation was that of sectoral compositional zoning crystallographically related to the elongated {110} face and, thus, could be indexed as {110} zoning (insets in Fig. 4a) using SHAPE crystal analysis software. The incorporation of FAM-poly-Asp86–93 at the {110} prismatic faces of COD crystals led not only to its inclusion but to the dose-dependent effect on the area and shape of these prismatic faces (Fig. 4b). The formation of these compositional zoning patterns in COD crystals was confirmed by laser scanning confocal microscopy (Fig. 4c), which enables “optical sectioning” to exclude out-of-focus flare and, thus, allows direct imaging of crystal interiors (20). These two-dimensional zoning patterns vary in appearance with the “depth” of the optical focus and allowing confirmation of the sectoral zoning through different focal planes (Fig. 4c). Fig. 5 summarizes how increasing OPN and poly-Asp86–93 concentrations inhibit the {110} faces of COD, leading to their increased relative prominence and resulting in elongated COD crystal morphology and specific {110} sectoral zoning within these crystals.
FIGURE 4.
Conventional fluorescence micrographs and laser confocal micrographs of COD crystals grown in the presence of fluorescently-tagged poly-Asp86–93 at the indicated concentrations. a and b, pyramidal, compositional (sectoral) zoning is observed in COD crystals reflecting selective incorporation during growth of fluorescently tagged poly-Asp86–93 specifically into {110} crystal sectors. Because of differences in orientation, the zoning pattern changes in appearance despite the crystals having similar morphology. Concentration of peptide affects the relative dimensions of the sectors as well as the area and shape of {110} prismatic faces (as in Fig. 3a). In this non-confocal image, fluorescence may originate from the interior of the crystal as well as from exterior faces. c, laser scanning confocal microscopy with focal plane imaging performed within crystal interiors (here shown for a crystal at a scanning depth of 5, 10, and 15 μm from the top of the crystal) confirms sectoral compositional zoning within COD crystals, revealing the boundaries of {110} crystal sectors. The overall morphology of the imaged crystal is shown by transmitted light in the left-most panel. Schematic insets show SHAPE software-derived renditions of the crystals used for face identification and c axis determination (blue extension bars).
FIGURE 5.
Summary of data using SHAPE software renditions of effects of full-length OPN and poly-Asp86–93 peptide on COD crystal growth morphology (a and b) and of occlusion of poly-Asp86–93 into COD in a sectoral compositional zoning pattern arising from {110} face binding of this peptide (c). Increases in OPN or poly-Asp86–93 concentrations in growth solution kinetically result in similar effects on COD crystal morphology; the same faces {110} are significantly inhibited and become predominant, and COD crystals elongate along their c-axis. {110} sectoral zones become dominant with increases in poly-Asp86–93 concentration, and the three schematics in the bottom row represent 2-dimensional planes taken from the center of COD crystals where poly-Asp86–93 incorporation (in increasing solution concentration from left to right) is shown by green triangles in two (of four) apposing pyramidal sectors. The {110} sectors (green) gain in proportion relative to other crystal-face sectors (black) as poly-Asp86–93 concentration increases.
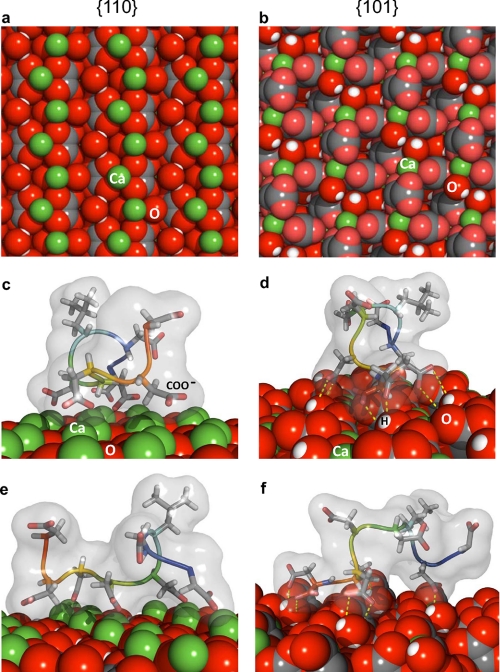
To investigate the mechanism underlying this face-specific interaction of poly-Asp86–93 with COD, we modeled poly-Asp86–93 adsorption to {110} and {101} crystal faces of COD (Fig. 6, a and b) using a Monte Carlo plus-minimization structure-prediction algorithm. In previous studies we have shown that this approach successfully predicted the binding of the salivary protein statherin to hydroxyapatite, in agreement with experimental solid-state NMR data (47, 52). Our calculations predict considerably stronger poly-Asp86–93 binding at the COD {110} surface relative to that of the COD {101} surface (Table 1). This prediction suggests stronger inhibition (higher relative stabilization) of the COD {110} surface compared with the {101} surface, a prediction consistent with our experimental observations demonstrating a gain in relative prominence of the {110} faces on COD crystals grown in the presence of protein/peptide (Fig. 3).
FIGURE 6.
Molecular modeling of poly-Asp86–93 peptide binding to {110} and {101} crystallographic faces of COD. Atomic configurations used for modeling at the surfaces of the {110} face (a) and {101} face (b) of COD. c–f, representative, top-scoring (low energy) models of poly-Asp86–93 adsorbed onto COD. c and e, two lateral views (∼90° to each other) of poly-Asp86–93 bound to a high calcium density plane in the {110} face of COD (with the N terminus of the peptide extending beyond the image plane in panel c, and with the N terminus of the peptide to the right in panel e. Some degree of lattice matching for five carboxylate (COO−) side chains aligned with calcium atoms occurs on the {110} face in this profile. d and f, two lateral views (∼90° to each other) of poly-Asp86–93 bound to the {101} face of COD (with the N terminus of the peptide extending beyond the image plane in panel d and with the N terminus of the peptide to the right in panel f. Hydrogen bonding is schematically illustrated by dashed yellow lines connecting donor-acceptor pairs. Green, calcium; gray, carbon; red, oxygen; white, hydrogen).
TABLE 1.
Poly-Asp86–93 peptide-COD adsorption energies in kcal/mol (estimated by RosettaSurface)
Binding energy (kcal/mol) is the difference between the mean energies of 100 lowest energy adsorbed- and solution-state candidate structures (error given is S.D.).
| COD crystal surface | {101} | {110} High calcium |
| Adsorption energy (kcal/mol) | −2.5 ± 5.7 | −23.4 ± 6.2 |
In the majority of the top-scoring configurations for poly-Asp86–93 adsorbed to the high calcium density {110} surface, the carboxylate residues of the peptide bind to calcium very strongly (Fig. 6, c and e). This strong, electrostatic binding of poly-Asp86–93 to the {110} face typically occurs through four or five aspartate carboxylate groups linked with crystal-surface calcium atoms such that a high degree of lattice matching can be observed between the peptide and {110} face (Fig. 6, c and e). The binding between carboxylate residues and calcium on the {110} face of COD yielded an average distance of 0.47 nm for the top-100 scoring decoys. In contrast, in the case of poly-Asp86–93 binding to the {101} face, the aspartate and leucine residues contribute only moderate hydrogen bonding with the {101} crystal surface upon peptide adsorption (Fig. 6, d and f; hydrogen bonds shown as yellow dashed lines represent degenerated, resonant donor-acceptor pairs) (53, 54). This significant binding of the peptide by hydrogen bonds to the COD {101} face is frequently balanced by desolvation penalties, thus rendering a binding energy near zero. Modeling also indicates that the poly-Asp86–93 peptide is mostly linear and unstructured in solution but becomes slightly more structured (less dispersed) upon adsorption to the {101} face and less structured (more dispersed) upon adsorption to the high calcium {110} face (data not shown). Coordinate files in Protein Data Bank format for the 10 lowest-scoring models of the OPN peptide bound to the {101} and {110} faces of COD are available as supplemental material.
DISCUSSION
Normal human urine is supersaturated with respect to calcium oxalate minerals, and it has been estimated that 1 × 107 calcium oxalate crystals can be produced and excreted daily in a healthy (non-stone forming) individual (55). In the face of these high ionic concentrations, inhibitory mechanisms must be in place to prevent pathologic calcification in the kidney and urine. In addition to small-molecule inhibitors like citrate, currently available in vitro and in vivo evidence suggest that urinary proteins such as OPN and trefoil factor 1 (TFF1) present in urine likewise serve as inhibitors of calcification (51, 56–59) and, moreover, may potentially decrease crystal retention and aggregation (55, 57). In addition, emerging data show that OPN may potentially direct the formation of COD and in the process incorporate (occlude) into the crystal structure (1, 23, 36). Interesting proposals for this occlusion include facilitating dissolution of the crystals from the inside-out by providing intracrystalline channels for fluid movement and facilitating phagocytosis of crystals by renal tubular cells for clearance of crystals from the urine (24, 36, 60, 61).
In the present study our objective was to examine how full-length OPN and one of its acidic peptides affected COD crystal growth and morphology and where small biologically relevant peptides might incorporate into COD. Such information would provide insight into when, where, and how biological molecules affect crystal growth and subsequent “maturation” or dissolution/clearance within the kidney and urine. Our data, deriving from several different experimental approaches, (i) confirm the presence and binding of OPN in kidney stones and in COD crystals precipitated from healthy human urine, (ii) show immunogold localization at the ultrastructural level consistent with the notion that OPN epitopes can be found at the surface of, and within, crystal interiors, (iii) demonstrate OPN peptide occlusion into COD in a sectoral compositional zoning pattern, and (iv) show that both full-length OPN and its poly-Asp86–93 peptide have significant and similar effects on COD crystal morphology, effects that ultimately might modify the fate of these crystalline particles in the context of this modification either reducing or promoting stone formation.
The concentric, lamellar immunohistochemical staining pattern for OPN commonly seen in kidney stones confirms that the protein is deposited in cyclical episodes that represent “growth rings” over time as the calcified mass grows within the urine/kidney. Both the concentric lamellae of matrix and perpendicularly arranged interlamellar organic structures (radial striations) all immunolabel intensely for OPN (29, 51). We extend these findings by showing that in addition to a surface coating of protein, a fine, flocculent organic material is frequently present in decalcified samples within the boundaries of profiles that clearly reflect crystalline structures present before the decalcification procedure (Fig. 1c). More specifically, we show that the crystal-coating layer and the internal flocculent material label strongly for OPN. Collectively, these localization studies at the light and electron microscopic levels suggest extensive incorporation of organics (and OPN) into all compartments of kidney stones.
A better temporal appreciation of organic inclusion into kidney stones can be obtained by intentionally and controllably inducing COD precipitation in urine which contains proteins that would likely be present and sequestered (more slowly) into kidney stones because of their mineral binding potential. The morphology of such precipitated COD shows {101} tetragonal di-pyramidal shapes typically seen in human urinary calculi (62–65) (Fig. 1d). The {101} tetragonal di-pyramid COD is the “equilibrium morphology” of COD generally equilibrated with growth medium, and this form often can be found in either pure growth solutions (as discussed below) or in impurity-loaded complex fluids where reactions have a propensity to reach an equilibrium state. Different growth conditions frequently used for the study of calcium oxalate commonly show at least some crystals of both the monohydrate and dihydrate forms. In an example of an equilibrated state, calcium oxalate crystals found in the major sources of commercially available fetal bovine serum routinely supplied as a growth- and differentiation-inducing supplement for cells in culture have been reported to sequester numerous proteins from the serum, and most of the COD crystals adopted the {101} tetragonal di-pyramidal shape (22). In this same study, Western blotting for OPN from protein extracts derived from the crystals separated from the fetal bovine serum confirmed likewise in this case the presence of abundant OPN bound by the crystals.
To assess the inhibitory potentials of OPN and its poly-Asp86–93 peptide on COD crystals, we performed COD growth experiments in the absence or presence of these organic molecules at physiologically relevant (or higher) concentrations (43). With increasing protein/peptide, the COD crystals become elongated along the c axis to form rod-shaped morphologies as the {110} tetragonal prismatic faces became dominant; these were capped with small {101} pyramids and other diminishing faces ({121} and {001}). This rod-shaped COD morphology, identified and referred to as the dodecahedral form of COD by Daudon et al. (9) based on in vivo observations at optical-microscopic resolution, rapidly became the predominant shape, with penetration twins of these crystals becoming frequent at relatively high concentrations of OPN, linear poly-Asp86–93, or commercial poly-Asp. The rod-shaped COD morphology is commonly seen in plant tissues where calcium oxalates are a common mineral phase. Interestingly, this form of COD has also been reported in patients having severe uremia (8) or severe hypercalciuria (9, 66). Calcium oxalate crystalluria particles experimentally induced in the urine of rats often showed elongated, dumbbell-shaped morphologies that were similarly reflected by the organic component of these particles after their decalcification (51). It is very likely that the elongated, rod-shaped COD represents a severe pathologic form of calcium oxalate commonly seen in both human stone disease and in a variety of animal models. To the best of our knowledge, this comprises the first in vitro report describing how a urinary protein or its peptide fragments facilitate the formation of rod-shaped COD crystals to resemble those observed in some severe renal stone diseases. Thus, our work here provides mechanistic insight into the lithogenic conditions and etiology that lead to the formation of various forms of COD in patients and, thus, may be useful in clinical diagnosis (9).
The development and preponderance of the {110} prismatic faces of COD observed in the present study reflect a kinetic response to increasing OPN/peptide concentration in solution, suggesting that these organic molecules inhibit growth through direct inhibitory binding of OPN/peptide to these crystallographic faces. Such face-specific binding inhibits crystal growth perpendicular to that face, as discussed for biologic calcification systems (67). The more OPN/peptide molecules present in a solution, the farther the COD morphology deviates from the equilibrium state, and more elongated crystals are formed (Fig. 5, a and b). Conserved among many mammalian and avian species, our selected poly-Asp86–93 peptide is a linear, contiguous stretch of amino acids rich in aspartic acid, as found in the primary sequence of OPN (68) and proposed to be one of the potent mineral binding motifs of this protein (34, 69). It has been shown that small, linear acidic polypeptides, some artificially constructed and others reflecting real protein sequence, can potently regulate the kinetics of biomineral growth (inhibition (34, 70, 71) or acceleration (72, 73)) and impurity-mineral interactions (74).
To provide information on a possible mechanism for binding, inhibition, and occlusion of poly-Asp86–93 related to COD crystal growth, we have used a modeling/simulation method (to our knowledge, the only one available) capable of accurately predicting the folding of a protein/peptide onto a solid-state (here mineral) surface (47, 52). This method allows for structural visualization and calculation of comparative adsorption energies of peptides to different mineral faces. The computational modeling predicts that the small, highly charged poly-Asp86–93 peptide is unstructured in solution, without specific orientation of functional side chains. Upon adsorption to COD crystals, the peptide may bind the crystal surface in one or more of many configurations. The binding energy of poly-Asp86–93 at the {101} face is approximately zero because the peptide-COD hydrogen bonding is frequently balanced by desolvation. Also, this face is stable and facilitates minimal electrostatic interaction. Therefore, poly-Asp86–93 adsorption at the COD {101} face is weak and minimally disruptive to the peptide intramolecular interactions (i.e. transient binding). Conversely, the strong binding predicted at the high calcium terminated {110} COD surface is disruptive to peptide intramolecular contacts. Binding of poly-Asp86–93 to the {110} surface is mostly electrostatic, with minimal hydrogen-bond contributions to the binding energy.
That poly-Asp86–93 peptide was less inhibitory than full-length OPN is not surprising given the large number of acidic amino acid stretches found in OPN and given that the protein is extensively post-translationally modified by phosphorylation. All these features of OPN, in its full-length form, would provide additional negative charges potentially clustered as groups and available for inhibitory crystal binding (35, 75, 76). In all scenarios, charge density may be a critical factor in determining the binding of OPN peptides. In addition to charge density, other characteristics of OPN and poly-Asp86–93 peptide in solution, such as hydrophilicity, may also account for abilities to influence growth of calcium oxalate minerals (73, 74).
Fluorescent molecules and fluorescently-tagged proteins have been previously and effectively used to study protein-mineral interactions (in COM studies (19, 20, 77–79)). Extending this approach to COD and using a poly-Asp86–93 peptide not previously examined by others, we have visualized by light and confocal microscopy the incorporation behavior of this peptide into the crystal structure of COD and revealed distinctive and informative three-dimensional sectoral (compositional) zoning patterns (77–80) that provide significant new insight into protein occlusion into biominerals. A promising scenario is that the selective, specific incorporation and segregation of fluorescent FAM-poly-Asp86–93 into pyramidal regions of a COD crystal, reflecting continuous binding to the {110} prismatic faces during COD growth, thus provides an example of creating an experimental visual record of the temporal and spatial occlusion of organics within a biomineral. The fluorescently labeled pyramidal growth sectors begin their formation at the center of the COD crystal and extend to the bases of the pyramids, reflecting continuous and gradual enlargement of the {110} faces attributable to peptide inhibition at these sites. Different pyramidal growth sectors within the same crystal meet centrally at their apices, and changes in fluorescence across adjacent growth sectors are abrupt; no fluorescence is seen in adjacent growth sectors that are not {110} sectors. This abrupt change in fluorescent peptide incorporation corresponds to sector boundaries reflecting interfacial compositional changes in COD crystal structure. Taken together, these results demonstrate that poly-Asp86–93 (and very likely other acidic peptides of OPN generated by proteolytic cleavage) binds to COD, inhibits growth normal to the {110} face, and exclusively occludes into its {110} growth sectors. These results are consistent with a variety of other in vitro studies suggesting intracrystalline OPN in urinary COD crystals (1, 23, 24, 36).
Of interest here, as deduced from simulation, is a possible scenario where the majority of poly-Asp86–93 carboxylate groups bind the {110} COD surface but leave remaining carboxylate groups available (or partially available) for binding of initially non-lattice calcium in solution. Strong peptide binding at the {110} crystal surface followed by the chelation of free calcium ions by solvent-exposed peptide carboxylate groups, might participate in the observed occlusion of peptide during further slow mineralization at that face.
The concept of protein inclusion (occlusion) into the crystal structure of biominerals has been commonly accepted as a strategy adapted by organisms to attain control over mineralization (81). In regulating biomineralization, intracrystalline proteins likely simultaneously (or uniquely) impart additional functional properties to the final structure of the biomineral, such as, among others, mechanical strength, optical characteristics, or varying textures (82, 83). In calcitic avian eggshell, for example, an extremely abundant organic matrix network occludes into the mineral phase, and OPN is a prominent protein of this matrix (84). Intracrystalline organics can also be components of biominerals that dislocate the mineral structure, distorting crystal lattice (85) and modifying their thermodynamic properties (86), thus rendering them more unstable and dissolvable. In this regard, recent studies have shown that urinary proteins situated within the bulk mineral phase of calcium oxalate raise lattice strains and reduce crystallite size because of intracrystalline defects and discontinuities, which in turn allow proteolytic invasion and crystal dissolution (36, 60, 87).
In our in vitro crystal growth experiments, the selective and nonequilibrated incorporation of peptide within COD crystals results in the formation of compositional zoning. The consequent sectoral intracrystalline heterogeneity suggests that the sectors containing occluded peptides may have different functional properties from neighboring sectors within the same crystal. In the context of the discussion above relating largely to the work done by Ryall et al. (23, 24, 36, 60, 87) and in linking this to physiologic defenses designed to decrease detrimental pathologic calcification as seen in uro/nephrolithiasis, one consequence of this may be that COD sectors with occluded peptide are susceptible to proteolytic, degradative “attack” by proteases, whose removal of occluded organics would increase the dissolution rate of the mineral phase or increase the urinary clearance of these particles. Moreover, in this scenario, these {110} sectors become dominant with increases in peptide concentration, and more intracrystalline peptides are sequestered within the COD, which in turn might ultimately promote their dissolution and/or clearance fate. Additional information about the surface and internal structure of the {110} faces and sectors is required to clarify whether this preferential interaction of peptides of OPN may favor the binding of COD crystals to renal epithelial cells and whether it is involved in the internalization of COD crystals via the phagocytic/endosomal pathway (24, 88).
CONCLUSIONS
The data reported here are consistent with an increasing number of reports describing protein/peptide occlusion in the “inorganic” phase of biomineralizing systems, and they provide an explanation for the presence and intracrystalline localization of abundant OPN and other proteins in kidney stones and in COD crystals artificially precipitated from urine. Intracrystalline proteins in biominerals can modify internal structure and physical properties of the mineral in which they reside. Proteins found in urine that incorporate into calcium oxalate crystals may perform a role in forming or inhibiting calcium oxalate urolithiasis. Our results show that with increasing OPN or poly-Asp86–93 concentrations in growth solutions, the {110} faces are greatly increased and eventually dominate the crystal morphology. The poly-Asp86–93 peptide of OPN may be central to the OPN effects on the {110} faces of COD, as its actions were similar on these faces. Finally, mineral-binding peptides of OPN may incorporate into {110} faces to develop {110} sectoral compositional zones in the crystals which may serve additional purposes related to crystal dissolution at later times.
Supplementary Material
Acknowledgments
We thank Dr. Denise Arsenault for input during the course of this study and Dr. Esben Sørensen and Arla Foods for the generous supply of osteopontin protein.
This work was funded by Canadian Institutes of Health Research Grant MT11360 (to M. D. M.) and by the Arnold and Mabel Beckman Foundation.
 The on-line version of this article (available at http://www.jbc.org) contains supplemental Protein Data Bank files.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Protein Data Bank files.
- COM
- calcium oxalate monohydrate
- COD
- calcium oxalate dihydrate
- OPN
- osteopontin
- FAM
- 5-carboxyfluorescein
- SEM
- scanning electron microscopy.
REFERENCES
- 1.Wesson J. A., Worcester E. M., Wiessner J. H., Mandel N. S., Kleinman J. G. (1998) Kidney Int. 53,952–957 [DOI] [PubMed] [Google Scholar]
- 2.Mandel N. S., Mandel G. S. (1989) J. Urol. 142,1516–1521 [DOI] [PubMed] [Google Scholar]
- 3.Durrbaum D., Rodgers A. L., Sturrock E. D. (2001) Urol. Res. 29,83–88 [DOI] [PubMed] [Google Scholar]
- 4.Mandel N. S., Mandel G. S., Hasegawa A. T. (1987) J. Urol. 138,557–562 [DOI] [PubMed] [Google Scholar]
- 5.Cerini C., Geider S., Dussol B., Hennequin C., Daudon M., Veesler S., Nitsche S., Boistelle R., Berthézène P., Dupuy P., Vazi A., Berland Y., Dagorn J. C., Verdier J. M. (1999) Kidney Int. 55,1776–1786 [DOI] [PubMed] [Google Scholar]
- 6.Elliot J. S., Rabinowitz I. N. (1980) J. Urol. 123,324–327 [DOI] [PubMed] [Google Scholar]
- 7.Dyer R., Nordin B. E. (1967) Nature 215,751–752 [DOI] [PubMed] [Google Scholar]
- 8.Lewis R. D., Lowenstam H. A., Rossman G. R. (1974) Arch. Pathol. 98,149–155 [PubMed] [Google Scholar]
- 9.Daudon M., Jungers P., Bazin D. (2008) in Second International Urolithiasis Research Symposium ( Evan A. P., Lingeman J. E., McAteer J. A., Williams J. C. eds) American Institute of Physics, Indianapolis, IN [Google Scholar]
- 10.Wesson J. A., Worcester E. M. (1996) Scanning Microsc. 10,415–424 [PubMed] [Google Scholar]
- 11.Mandel N. S. (1994) J. Am. Soc. Nephrol. 5,S37–45 [DOI] [PubMed] [Google Scholar]
- 12.Verkoelen C. F., van der Boom B. G., Kok D. J., Romijn J. C. (2000) Kidney Int. 57,1072–1082 [DOI] [PubMed] [Google Scholar]
- 13.Wesson J. A., Ward M. D. (2006) Curr. Opin. Nephrol. Hypertens. 15,386–393 [DOI] [PubMed] [Google Scholar]
- 14.Sheng X., Jung T., Wesson J. A., Ward M. D. (2005) Proc. Natl. Acad. Sci. U.S.A. 102,267–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lieske J. C., Norris R., Swift H., Toback F. G. (1997) Kidney Int. 52,1291–1301 [DOI] [PubMed] [Google Scholar]
- 16.Lieske J. C., Toback F. G., Deganello S. (1996) Calcif. Tissue Int. 58,195–200 [DOI] [PubMed] [Google Scholar]
- 17.Wiessner J. H., Mandel G. S., Mandel N. S. (1986) J. Urol. 135,835–839 [DOI] [PubMed] [Google Scholar]
- 18.Qiu S. R., Wierzbicki A., Orme C. A., Cody A. M., Hoyer J. R., Nancollas G. H., Zepeda S., De Yoreo J. J. (2004) Proc. Natl. Acad. Sci. U.S.A. 101,1811–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taller A., Grohe B., Rogers K. A., Goldberg H. A., Hunter G. K. (2007) Biophys. J. 93,1768–1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grohe B., O'Young J., Ionescu D. A., Lajoie G., Rogers K. A., Karttunen M., Goldberg H. A., Hunter G. K. (2007) J. Am. Chem. Soc. 129,14946–14951 [DOI] [PubMed] [Google Scholar]
- 21.Langdon A., Wignall G. R., Rogers K., Sørensen E. S., Denstedt J., Grohe B., Goldberg H. A., Hunter G. K. (2009) Calcif. Tissue Int. 84,240–248 [DOI] [PubMed] [Google Scholar]
- 22.Pedraza C. E., Chien Y. C., McKee M. D. (2008) J. Cell. Biochem. 103,1379–1393 [DOI] [PubMed] [Google Scholar]
- 23.Ryall R. L., Chauvet M. C., Grover P. K. (2005) BJU Int. 96,654–663 [DOI] [PubMed] [Google Scholar]
- 24.Grover P. K., Thurgood L. A., Fleming D. E., van Bronswijk W., Wang T., Ryall R. L. (2008) Am. J. Physiol. Renal Physiol. 294,F355–F361 [DOI] [PubMed] [Google Scholar]
- 25.Brown L. F., Berse B., Van de Water L., Papadopoulos-Sergiou A., Perruzzi C. A., Manseau E. J., Dvorak H. F., Senger D. R. (1992) Mol. Biol. Cell 3,1169–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rittling S. R., Denhardt D. T. (1999) Exp. Nephrol. 7,103–113 [DOI] [PubMed] [Google Scholar]
- 27.Shiraga H., Min W., VanDusen W. J., Clayman M. D., Miner D., Terrell C. H., Sherbotie J. R., Foreman J. W., Przysiecki C., Neilson E. G., Hoyer J. R. (1992) Proc. Natl. Acad. Sci. U.S.A. 89,426–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez C. A., Hoyer J. R., Wilson P. D., Waterhouse P., Denhardt D. T. (1993) Lab. Invest. 69,355–363 [PubMed] [Google Scholar]
- 29.Khan S. R. (1997) World J. Urol. 15,236–243 [DOI] [PubMed] [Google Scholar]
- 30.Asplin J. R., Arsenault D., Parks J. H., Coe F. L., Hoyer J. R. (1998) Kidney Int. 53,194–199 [DOI] [PubMed] [Google Scholar]
- 31.Tawada T., Fujita K., Sakakura T., Shibutani T., Nagata T., Iguchi M., Kohri K. (1999) Urol. Res. 27,238–242 [DOI] [PubMed] [Google Scholar]
- 32.Xie Y., Sakatsume M., Nishi S., Narita I., Arakawa M., Gejyo F. (2001) Kidney Int. 60,1645–1657 [DOI] [PubMed] [Google Scholar]
- 33.Fisher L. W., Torchia D. A., Fohr B., Young M. F., Fedarko N. S. (2001) Biochem. Biophys. Res. Commun. 280,460–465 [DOI] [PubMed] [Google Scholar]
- 34.Hoyer J. R., Asplin J. R., Otvos L. (2001) Kidney Int. 60,77–82 [DOI] [PubMed] [Google Scholar]
- 35.Kazanecki C. C., Uzwiak D. J., Denhardt D. T. (2007) J. Cell. Biochem. 102,912–924 [DOI] [PubMed] [Google Scholar]
- 36.Lyons Ryall R., Fleming D. E., Doyle I. R., Evans N. A., Dean C. J., Marshall V. R. (2001) J. Struct. Biol. 134,5–14 [DOI] [PubMed] [Google Scholar]
- 37.Kohri K., Nomura S., Kitamura Y., Nagata T., Yoshioka K., Iguchi M., Yamate T., Umekawa T., Suzuki Y., Sinohara H., Kurita T. (1993) J. Biol. Chem. 268,15180–15184 [PubMed] [Google Scholar]
- 38.Yasui T., Sato M., Fujita K., Tozawa K., Nomura S., Kohri K. (2001) Urol. Res. 29,50–56 [DOI] [PubMed] [Google Scholar]
- 39.McKee M. D., Nanci A. (1995) Microsc. Res. Tech. 31,44–62 [DOI] [PubMed] [Google Scholar]
- 40.Ryall R. L., Hibberd C. M., Marshall V. R. (1985) Urol. Res. 13,285–289 [DOI] [PubMed] [Google Scholar]
- 41.Doyle I. R., Ryall R. L., Marshall V. R. (1991) Clin. Chem. 37,1589–1594 [PubMed] [Google Scholar]
- 42.Sørensen E. S., Petersen T. E. (1993) J. Dairy Res. 60,189–197 [DOI] [PubMed] [Google Scholar]
- 43.Thurgood L. A., Grover P. K., Ryall R. L. (2008) Urol. Res. 36,103–110 [DOI] [PubMed] [Google Scholar]
- 44.Lepage L., Tawashi R. (1982) J. Pharm. Sci. 71,1059–1062 [DOI] [PubMed] [Google Scholar]
- 45.Bartholomew R. S., Rebello P. F. (1979) Am. J. Ophthalmol. 88,1026–1028 [DOI] [PubMed] [Google Scholar]
- 46.Dowty E. (2002) SHAPE Windows Professional 6.0 Ed., Kingsport, TN [Google Scholar]
- 47.Masica D. L., Gray J. J. (2009) Biophys. J. 96,3082–3091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lazaridis T., Karplus M. (1999) Proteins Struct. Funct. Genet. 35,133–152 [DOI] [PubMed] [Google Scholar]
- 49.Palmer D. (2005) Crystal Maker, 7.1 Ed., Yarnton, Oxfordshire, UK [Google Scholar]
- 50.Tazzoli V., Domeneghetti C. (1980) Am. Mineral 65,327–334 [Google Scholar]
- 51.McKee M. D., Nanci A., Khan S. R. (1995) J. Bone Miner. Res. 10,1913–1929 [DOI] [PubMed] [Google Scholar]
- 52.Makrodimitris K., Masica D. L., Kim E. T., Gray J. J. (2007) J. Am. Chem. Soc. 129,13713–13722 [DOI] [PubMed] [Google Scholar]
- 53.Morozov A. V., Kortemme T., Tsemekhman K., Baker D. (2004) Proc. Natl. Acad. Sci. U.S.A. 101,6946–6951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kortemme T., Morozov A. V., Baker D. (2003) J. Mol. Biol. 326,1239–1259 [DOI] [PubMed] [Google Scholar]
- 55.Koul H. K., Koul S., Fu S., Santosham V., Seikhon A., Menon M. (1999) J. Am. Soc. Nephrol. 10, Suppl. 14, S417–421 [PubMed] [Google Scholar]
- 56.Worcester E. M. (1996) Semin. Nephrol. 16,474–486 [PubMed] [Google Scholar]
- 57.Wesson J. A., Johnson R. J., Mazzali M., Beshensky A. M., Stietz S., Giachelli C., Liaw L., Alpers C. E., Couser W. G., Kleinman J. G., Hughes J. (2003) J. Am. Soc. Nephrol. 14,139–147 [DOI] [PubMed] [Google Scholar]
- 58.Kleinman J. G., Wesson J. A., Hughes J. (2004) Nephron Physiol. 98,p43–47 [DOI] [PubMed] [Google Scholar]
- 59.Chutipongtanate S., Nakagawa Y., Sritippayawan S., Pittayamateekul J., Parichatikanond P., Westley B. R., May F. E., Malasit P., Thongboonkerd V. (2005) J. Clin. Invest. 115,3613–3622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fleming D. E., Van Riessen A., Chauvet M. C., Grover P. K., Hunter B., van Bronswijk W., Ryall R. L. (2003) J. Bone Miner. Res. 18,1282–1291 [DOI] [PubMed] [Google Scholar]
- 61.Chauvet M. C., Ryall R. L. (2005) J. Struct. Biol. 151,12–17 [DOI] [PubMed] [Google Scholar]
- 62.Gibson R. I. (1974) Am. Mineral 59,1177–1182 [Google Scholar]
- 63.Berg W., Schnapp J. D., Schneider H. J., Hesse A., Hienzsch E. (1976) Eur. Urol. 2,92–97 [DOI] [PubMed] [Google Scholar]
- 64.Berg W., Lange P., Bothor C., Rössler D. (1979) Eur. Urol. 5,136–143 [DOI] [PubMed] [Google Scholar]
- 65.Werness P. G., Bergert J. H., Smith L. H. (1981) J. Cryst. Growth 53,166–181 [Google Scholar]
- 66.Daudon M., Barbey F., Jungers P. (2004) Rev. Med. Suisse Romande 124,455–459 [PubMed] [Google Scholar]
- 67.Addadi L., Weiner S. (1985) Proc. Natl. Acad. Sci. U.S.A. 82,4110–4114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oldberg A., Franzén A., Heinegård D. (1986) Proc. Natl. Acad. Sci. U.S.A. 83,8819–8823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sodek J., Ganss B., McKee M. D. (2000) Crit. Rev. Oral Biol. Med. 11,279–303 [DOI] [PubMed] [Google Scholar]
- 70.Wang L., Qiu S. R., Zachowicz W., Guan X., Deyoreo J. J., Nancollas G. H., Hoyer J. R. (2006) Langmuir 22,7279–7285 [DOI] [PubMed] [Google Scholar]
- 71.Elhadj S., Salter E. A., Wierzbicki A., De Yoreo J. J., Han N., Dove P. M. (2006) Cryst. Growth Des. 6,197–201 [Google Scholar]
- 72.Kim I. W., Darragh M. R., Orme C., Evans J. S. (2006) Cryst. Growth Des. 6,5–10 [Google Scholar]
- 73.Elhadj S., De Yoreo J. J., Hoyer J. R., Dove P. M. (2006) Proc. Natl. Acad. Sci. U.S.A. 103,19237–19242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stephenson A. E., DeYoreo J. J., Wu L., Wu K. J., Hoyer J., Dove P. M. (2008) Science 322,724–727 [DOI] [PubMed] [Google Scholar]
- 75.Gericke A., Qin C., Spevak L., Fujimoto Y., Butler W. T., Sørensen E. S., Boskey A. L. (2005) Calcif. Tissue Int. 77,45–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pampena D. A., Robertson K. A., Litvinova O., Lajoie G., Goldberg H. A., Hunter G. K. (2004) Biochem. J. 378,1083–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hunter G. K., Grohe B., Jeffrey S., O'Young J., Sørensen E. S., Goldberg H. A. (2009) Cells Tissues Organs 189,44–50 [DOI] [PubMed] [Google Scholar]
- 78.O'Young J., Chirico S., Al Tarhuni N., Grohe B., Karttunen M., Goldberg H. A., Hunter G. K. (2009) Cells Tissues Organs 189,51–55 [DOI] [PubMed] [Google Scholar]
- 79.Touryan L. A., Clark R. H., Gurney R. W., Stayton P. S., Kahr B., Vogel V. (2001) J. Cryst. Growth 233,380–388 [Google Scholar]
- 80.Reeder R. J., Grams J. C. (1987) Geochim. Cosmochim. Acta 51,187–194 [Google Scholar]
- 81.Albeck S., Addadi I., Weiner S. (1996) Connect. Tissue Res. 35,365–370 [DOI] [PubMed] [Google Scholar]
- 82.Aizenberg J., Hanson J., Ilan M., Leiserowitz L., Koetzle T. F., Addadi L., Weiner S. (1995) FASEB J. 9,262–268 [DOI] [PubMed] [Google Scholar]
- 83.Berman A., Addadi L., Weiner S. (1988) Nature 331,546–548 [Google Scholar]
- 84.Chien Y. C., Hincke M. T., Vali H., McKee M. D. (2008) J. Struct. Biol. 163,84–99 [DOI] [PubMed] [Google Scholar]
- 85.Pokroy B., Fitch A. N., Marin F., Kapon M., Adir N., Zolotoyabko E. (2006) J. Struct. Biol. 155,96–103 [DOI] [PubMed] [Google Scholar]
- 86.De Yoreo J. J. V., P. G. (2003) in Biomineralization ( Dove P. M., De Yoreo J.J., Weiner S. ed) 1st ed., pp. 57–93, The Mineralogical Society of America, Washington, D. C [Google Scholar]
- 87.Ryall R. L., Grover P. K., Thurgood L. A., Chauvet M. C., Fleming D. E., van Bronswijk W. (2007) Urol. Res. 35,1–14 [DOI] [PubMed] [Google Scholar]
- 88.Lieske J. C., Deganello S. (1999) J. Am. Soc. Nephrol. 10, Suppl. 14, S422–429 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.