Abstract
Protein kinase A-anchoring proteins (AKAPs) play important roles in the compartmentation of cAMP signaling, anchoring protein kinase A (PKA) to specific cellular organelles and serving as scaffolds that assemble localized signaling cascades. Although AKAPs have been recently shown to bind adenylyl cyclase (AC), the functional significance of this association has not been studied. In cardiac myocytes, the muscle protein kinase A-anchoring protein β (mAKAPβ) coordinates cAMP-dependent, calcium, and MAP kinase pathways and is important for cellular hypertrophy. We now show that mAKAPβ selectively binds type 5 AC in the heart and that mAKAPβ-associated AC activity is absent in AC5 knock-out hearts. Consistent with its known inhibition by PKA phosphorylation, AC5 is inhibited by association with mAKAPβ-PKA complexes. AC5 binds to a unique N-terminal site on mAKAP-(245–340), and expression of this peptide disrupts endogenous mAKAPβ-AC association. Accordingly, disruption of mAKAPβ-AC5 complexes in neonatal cardiac myocytes results in increased cAMP and hypertrophy in the absence of agonist stimulation. Taken together, these results show that the association of AC5 with the mAKAPβ complex is required for the regulation of cAMP second messenger controlling cardiac myocyte hypertrophy.
The formation of multimolecular protein complexes contributes to the specificity of intracellular signaling pathways, including those regulating cardiac myocyte hypertrophy. The cAMP-dependent protein kinase (PKA)3 is targeted to specific intracellular domains by protein kinase A-anchoring proteins (AKAPs) that often serve as scaffolding proteins for diverse signaling enzymes (1). In the heart, global disruption of PKA anchoring affects cardiac contractility, while the inhibited expression of individual AKAPs such as mAKAPβ or AKAP-Lbc attenuates adrenergic-induced hypertrophy of cultured neonatal myocytes (2–4). We have recently shown that specific AKAPs, namely AKAP79 and Yotiao, bind adenylyl cyclases (AC) (5, 6). However, the functional significance of AC-AKAP complexes has not been demonstrated.
mAKAPβ, expressed in striated myocytes, is one of two known splice variants encoded by the single mAKAP (AKAP6) gene (7). We previously published that mAKAPβ is primarily localized to the outer membrane of the nuclear envelope via direct binding to nesprin-1α (4, 8). In cardiac myocytes, mAKAPβ serves as the scaffold for a multimolecular signaling complex that in addition to PKA includes the ryanodine receptor (RyR2), the protein phosphatases PP2A and calcineurin, phosphodiesterase 4D3 (PDE4D3), exchange protein activated by cAMP (Epac1), ERK5, and MEK5 mitogen-activated protein kinases, molecules implicated in the regulation of cardiac hypertrophy (4, 7–13). mAKAPβ complexes facilitate cross-talk between MAP kinase, calcium, and cAMP signaling pathways, permitting feedback inhibition of cAMP levels and the dynamic regulation of PKA and ERK5 activity (4, 9–13). Accordingly, mAKAPβ RNAi attenuates adrenergic and cytokine-induced hypertrophy of cultured rat neonatal ventricular myocytes (4, 11).
Because mAKAPβ forms a complex with two cAMP effectors and a metabolizing enzyme for cAMP, we considered whether AC might also be an integral part of the mAKAPβ complex. We now demonstrate that type 5 adenylyl cyclase (AC5) binds directly a unique N-terminal site on mAKAPβ and is the predominant AC isoform associated with mAKAPβ in the heart. We show that AC5 bound to mAKAPβ is inhibited by PKA-dependent negative feedback. Importantly, inhibition of endogenous mAKAPβ-AC5 binding revealed the functional importance of these complexes for the regulation of cAMP-dependent myocyte hypertrophy.
MATERIALS AND METHODS
Reagents
Antibodies were obtained as described under supplemental methods. The mAKAPβ isoform is identical to residues 245–2314 of mAKAPα. Thus, all mutants are numbered according to mAKAPα. The mammalian expression plasmids for wild type and ΔPKA (deletion of residues 2053–2073) mAKAPβ are as previously described (4). Adenoviruses that express C-terminally Myc-tagged mAKAP 1–587, 245–340 (Myc-AC-BD), 245–587, 1286–1833, and 1835–2312 fragments were generated using the pTRE shuttle vector and the Adeno-X Tet-off System (Clontech) as previously described for Myc-mAKAP-(585–1286) (4). Encoded proteins are expressed in a doxycycline-inhibited manner when co-infected with adenovirus that constitutively express the tetracycline-controlled transactivator (tTA). All other expression vectors for AC isoforms and/or AKAP79 are described in supplemental methods.
Preparation of Heart Extracts
Frozen adult rat hearts (Pel-Freez Biologicals) or AC5+/+ and AC5−/− mouse hearts (generously provided by Kirk Hammond) were thawed in phosphate-buffered saline with protease inhibitors. The hearts were rinsed, diced, and homogenized with a polytron in lysis buffer lacking detergent. After addition of 1.0% Triton X-100, the extracts were further homogenized with a Dounce homogenizer, followed by centrifugation at 14,000 rpm to remove the insoluble fraction. For competition experiments, GST fusion proteins were added to the final homogenization step. The extracts were used immediately for experiments. For immunoprecipitations, extracts were incubated with 10 μl of serum and 20 μl of protein G-agarose for 3 h at 4 °C. The beads were washed twice with buffer containing 0.05% lubrol before measurement of AC activity as described in detail in the on-line supplement (6, 14).
Immunoprecipitation-AC Assay
Cardiac myocytes or transfected COS-7 cells were rinsed with phosphate-buffered saline and then lysed with lysis buffer (50 mm HEPES pH 7.4, 1 mm EDTA, 1 mm MgCl2, 150 mm NaCl, 0.5% C12E9, protease inhibitors). Cellular debris was removed by centrifugation, and an aliquot was removed to measure AC activity in the starting lysates. The clarified extracts were then subjected to immunoprecipitation followed by an AC assay upon addition of 50 nm Gαs-GTPγS and 100 μm forskolin, as detailed in the on-line supplement and previously described (6, 14).
GST Pull-down-AC Assays
GST fusion proteins (50 μg) were incubated with heart extract (∼1/3 heart per sample) for 30 min. Glutathione-agarose (30 μl of packed resin) was added for an additional 2 h. Samples were washed three times with wash buffer and assayed for AC activity directly on the resin as described in the on-line supplement (14).
Ventricular Myocyte Culture
Ventricular myocytes (over 90% free of fibroblasts) were prepared from 2–3-day-old Sprague-Dawley rats, as previously described (8). Adenoviral infection, immunocytochemistry, and [3H]leucine incorporation were performed as described in the on-line supplement. All data are expressed as means ± S.E. of the mean. Each n represents the results of individual experiments using separate primary cultures. p values were calculated using a two-tailed, paired Student's t test.
RESULTS
AC Activity Is Associated with mAKAPβ in Heart
To test whether AC associates with mAKAPβ, protein complexes were immunoprecipitated from adult rat heart extracts using multiple mAKAP antisera and were assayed for AC activity (IP-AC assay) (Fig. 1A). AC activity associated with each mAKAP sera was 3-fold greater than the respective preimmune control sera, representing 1.5 ± 0.8% of the total AC activity in heart extracts. IP-AC assays using rat neonatal ventricular myocytes confirmed the association of AC and mAKAPβ in the heart (Fig. 1B). mAKAP antiserum precipitated 10 ± 3% of the total AC activity in cardiac myocytes.
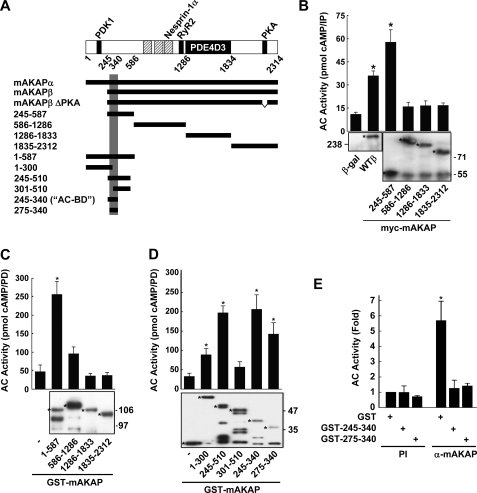
FIGURE 1.
AC5 associates with mAKAPβ in the heart. A, protein complexes were immunoprecipitated from adult rat heart extracts using mAKAP (VO54, VO56, or OR010), nesprin-1, or the corresponding preimmune (PI) sera. AC activity associated with the immunoprecipitates was measured in the presence of 50 nm Gαs + 100 μm forskolin (fsk). Significantly more AC activity was associated with the mAKAP and nesprin-1 antisera than the control PI sera. *, p < 0.05, n = 3. B, protein complexes were immunoprecipitated from rat neonatal ventricular myocyte extracts with VO56 mAKAP or PI sera. AC activity was assayed as in A. *, p < 0.04; n = 4. C, myocytes were infected with adenovirus expressing Myc-mAKAP-(585–1286), to inhibit endogenous mAKAPβ-nesprin-1α association, or β-gal control. AC activity in nesprin-1 or PI immunoprecipitates was measured by fsk-stimulated EIA. *, p < 0.05 compared with the other three conditions; representative of three experiments is shown. D, AC2, AC5, AC6, and/or Myc-tagged mAKAPβ were expressed in COS-7 cells. AC activity associated with Myc antibody immunoprecipitates was assayed as in A (left panel). The corresponding cell extracts were assayed for AC activity in the absence of phosphatase inhibitors (right panel). *, p < 0.05, n = 3. E, heart extracts were prepared from wild type (AC5+/+) or AC5 knock-out (AC5−/−) mice, and AC activity associated with mAKAP or PI immunoprecipitates was measured as in A. *, p < 0.05, n = 3.
In cardiac myocytes, nesprin-1α anchors mAKAPβ at the nuclear envelope (15). AC was also immunoprecipitated from adult rat heart extract with a nesprin-1 antiserum (Fig. 1A). Detection of mAKAPβ and nesprin-1α in the mAKAP and nesprin-1 immunoprecipitates served as controls (supplemental Fig. S1). To determine whether AC association with nesprin-1α was direct or indirect through mAKAPβ, we expressed mAKAP-(585–1286) in cultured neonatal myocytes (Fig. 2A) to competitively inhibit endogenous mAKAPβ-nesprin-1α binding (15). AC activity associated with nesprin-1 co-immunoprecipitation was blocked by expression of mAKAP-(586–1286), but not by a β-galactosidase (β-gal) control (Fig. 1C). Thus, AC associates with mAKAPβ bound to nesprin-1α at the cardiac myocyte nuclear envelope.
FIGURE 2.
An N-terminal domain of mAKAPβ associates with AC in the heart. A, schematic of mAKAP domains and mutants used in this report. mAKAPβ is identical to mAKAPα-(245–2314) (7). Hatched bars indicate the three spectrin repeat domains required for nuclear envelope targeting (8). Binding sites are indicated for 3-phosphoinositide-dependent kinase-1 (PDK1) (7), nesprin-1 (15), ryanodine receptor (RyR2) (13), PDE4D3 (10), and PKA (8). The vertical gray bar marks the AC binding site. B, myocytes were infected with adenovirus expressing Myc-tagged full-length (WTβ), truncated mAKAPβ fragments, or β-gal control. AC binding to the mAKAP fragments was detected by Myc-antibody immunoprecipitation and measurement of associated AC activity in the presence of Gαs/fsk (*, p < 0.03; n = 3). Western blot (α-Myc) confirmed immunoprecipitation of mAKAP fragments (starred bands). C and D, GST-mAKAP fusion proteins were incubated with heart extracts prior to pull-down (PD) with glutathione resin and measurement of Gαs/fsk-stimulated AC assay. Western blots (α-GST) of GST proteins (starred bands) are shown below. The smallest GST-mAKAP protein that bound AC was 275–340. *, p < 0.05 compared with GST control; n = 3. E, GST-mAKAP fusion proteins or GST alone (5 μm) were incubated with heart extracts prior to immunoprecipitation of endogenous AC-mAKAPβ complexes with VO56 mAKAP-specific or PI antisera. Fold AC activity is shown normalized to the GST, PI samples. *, p < 0.05 compared with all other samples; n = 3.
mAKAP Associates with AC5 in Heart
AC5 and AC6 are the most abundant AC isoforms in cardiac myocytes (16, 17). Because of the lack of suitable, isoform-specific AC antibodies, we determined which AC isoforms bound mAKAPβ by co-expressing ACs and Myc-tagged mAKAPβ in COS-7 cells. AC5 and AC2 were significantly enriched in α-Myc immunoprecipitates (8-fold and 3-fold, respectively, over control, Fig. 1D, left panel). In contrast, AC6 and AC1 were not significantly associated with Myc-mAKAPβ (Fig. 1D, left panel and data not shown). The total AC activity in COS-7 cell extracts confirmed that each AC isoform was expressed 6–15-fold over background (Fig. 1D, right panel). IP-AC assay of mAKAPβ immunoprecipitates from wild type (AC5+/+) and AC5 knock-out (AC5−/−) mouse hearts demonstrated that AC5 is the major mAKAPβ-binding AC isoform in native tissue (Fig. 1E).
AC Binds the mAKAPβ N Terminus, Amino Acids 275–340
We mapped the mAKAPβ site that binds endogenous AC by expressing Myc-tagged mAKAP fragments in neonatal myocytes and performing an IP-AC assay (Fig. 2A). AC activity was only associated with wild-type mAKAPβ (WTβ) and mAKAP-(245–587) (Fig. 2B). Further mapping was accomplished by GST-pull-down assays using rat heart extracts and GST-mAKAP fusion proteins (Fig. 2, C and D). AC activity was consistently precipitated with mAKAP fragments that contained residues 275–340. The overlapping fusion protein GST-mAKAP-(1–300) also was able to precipitate AC activity to a lesser degree (Fig. 2D). To confirm that mAKAP-(275–340) was sufficient for binding AC, GST fusion proteins were used as competitive inhibitors in IP-AC assays using heart extracts. Both GST-mAKAP-(245–340) and -(275–340) fully inhibited endogenous AC-mAKAPβ binding, whereas GST alone had no effect (Fig. 2E).
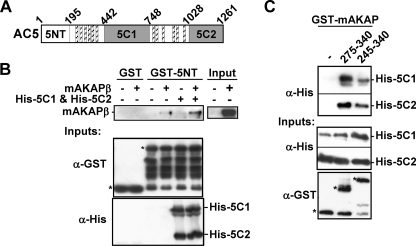
mAKAP Binds the Cytosolic Domains of AC5
AC5 contains three cytosolic domains that could interact with mAKAPβ, the divergent N-terminal (NT) and the conserved C1 and C2 domains (Fig. 3A) (18). GST fusions of the three cytosolic AC5 domains were used to pull-down mAKAPβ from cell lysates. Independently, each AC domain weakly pulled down mAKAPβ (data not shown). Therefore, we tested binding to mAKAP with a complex of all three AC5 domains. The presence of His6-tagged C1 and C2 domains increased mAKAPβ pull-down by GST-5NT (Fig. 3B). The AC5-mAKAPβ interaction appears to be direct, as GST-mAKAP-(275–340) and -(245–340) pulled down purified His6-tagged C1 and C2 domains in the presence of forskolin (Fig. 3C).
FIGURE 3.
mAKAPβ binds AC5 directly. A, AC5 has 12 transmembrane spans (hatched bars) and an N-terminal (5NT) and two catalytic (5C1 and 5C2) intracellular domains. B, cell lysates (500 μg) from COS-7 cells transfected with a mAKAPβ or control expression vector were incubated with 30 μg of GST or GST-5NT (1 μm) in the presence or absence of His6-tagged 5C1 and 5C2 (2 μm each) and 100 μm fsk. Complexes were precipitated with glutathione resin. Western blots for mAKAPβ and inputs are shown. 50% of the starting material is shown for mAKAPβ input. n = 3. C, His6-tagged 5C1 and 5C2 (1 μm) were incubated with 100 μm fsk and GST or GST-mAKAP fusion proteins and precipitated with glutathione resin. n = 3.
AKAP79, but not Yotiao, can also bind AC5 (5, 6). To determine whether mAKAPβ and AKAP79 contact common sites on AC5, we tested whether GST-mAKAP-(245–340) would competitively inhibit AKAP79-AC5 binding. Although GST-mAKAP-(245–340) competed with endogenous mAKAPβ binding to AC in heart extracts (Fig. 2E) and binds AC5 directly (Fig. 3C), it was unable to inhibit AKAP79-AC5 interactions (supplemental Fig. S2).
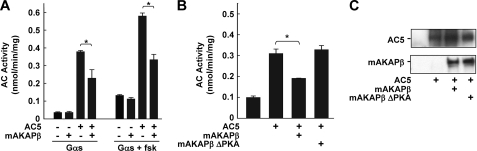
Inhibition of AC5 by mAKAPβ Complexes
To detect any effect of mAKAPβ association on AC5 activity, membranes isolated from COS-7 cells overexpressing AC5 and/or mAKAPβ were assayed for AC activity. AC5 was inhibited by 32 or 48% upon stimulation with Gαs or Gαs plus forskolin, respectively (Fig. 4A). The inhibition of AC5 by mAKAPβ did not appear to be a direct consequence of protein binding since addition of GST-tagged mAKAP-(275–340) or -(245–340) to AC5-containing membranes had no effect on AC5 activity and expression of Myc-AC-BD had no effect on AC activity in the absence of mAKAP (data not shown). Inhibition of AC5 by mAKAPβ was not due to altered AC5 protein levels (Fig. 4C). Instead, the inhibition of AC5 by mAKAPβ depended on PKA association with the complex. Expression of a mAKAPβ mutant unable to bind PKA (mAKAP ΔPKA (4), Fig. 2A) had no effect on AC5 activity in cell membranes (Fig. 4B), consistent with published evidence that AC5 is a PKA substrate and subject to phosphorylation-dependent feedback inhibition (19, 20). AC2 is not inhibited by PKA phosphorylation and was not inhibited by co-expression with mAKAPβ (data not shown).
FIGURE 4.
AC5 is inhibited by the mAKAPβ complex. A, AC activity associated with membranes prepared from COS-7 cells expressing AC5 and/or mAKAPβ was assayed in the presence of Gαs ± fsk. B, AC activity in membranes from COS-7 cells expressing the indicated proteins was assayed in the presence of Gαs and fsk. *, p < 0.05 and n = 3 for both A and B. C, representative Western blots of COS-7 membranes expressing the indicated proteins.
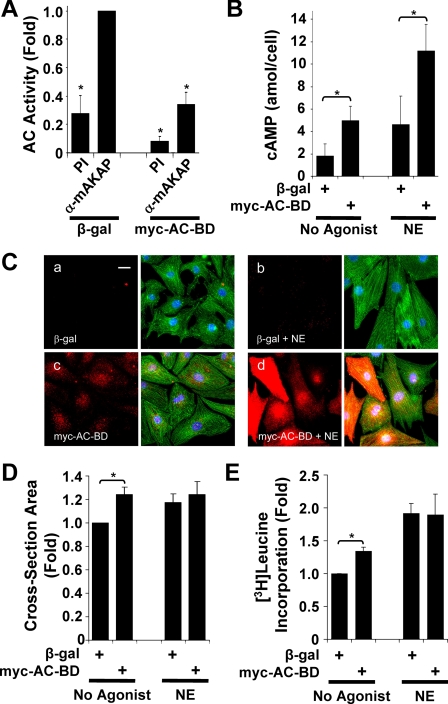
Expression of the AC Binding Domain in Myocytes
Analysis of AC5−/− mice revealed that AC5 contributes to the induction of pathologic cardiac remodeling in disease and with normal aging (21–23). Given that mAKAPβ inhibited AC5 activity (Fig. 4), we hypothesized that disruption of mAKAPβ-AC5 complexes in cardiac myocytes would enhance AC5 activity and affect myocyte hypertrophy. Therefore, we expressed Myc-AC-BD in cultured rat neonatal ventricular myocytes using a conditional adenovirus system (Fig. 5). Expression of Myc-AC-BD competitively inhibited endogenous mAKAPβ-AC binding in myocytes as measured by IP-AC assay (Fig. 5A), similar to the competition observed for GST-mAKAP-(245–340) in heart extracts (Fig. 2E). Interestingly, Myc-AC-BD not only displaced AC from endogenous mAKAPβ, but also increased total cAMP levels in myocytes (Fig. 5B). Unstimulated myocytes expressing Myc-AC-BD contained 2.7-fold greater cAMP than myocytes expressing β-gal (5.0 ± 1.3 versus 1.8 ± 1.0 attomol/cell) and 2.4-fold greater cAMP upon acute, maximal stimulation with the adrenergic agonist norepinephrine (NE, 10 μm) (11.2 ± 2.3 versus 4.6 ± 2.5 attomol/cell). Thus, mAKAPβ serves to constrain cAMP levels in myocytes.
FIGURE 5.
Expression of the mAKAPβ AC-binding domain (AC-BD) in myocytes increases total cellular cAMP and promotes cellular hypertrophy. Primary rat neonatal ventricular myocytes were infected with adenovirus that express Myc-AC-BD (Myc-tagged mAKAP 245–340) or β-gal. A, infected myocytes were cultured in minimal medium with 10 μm PE for 24 h. Protein complexes were immunoprecipitated with OR010 mAKAP-specific (α mAKAP) or preimmune (PI) serum. Associated AC activity was detected by EIA with fsk stimulation. Data are normalized to the AC activity precipitated from β-gal-expressing myocytes using α-mAKAP serum; *, p < 0.03 relative to the β-gal, α-mAKAP sample; n = 3. Equal amounts of mAKAPβ were precipitated in the absence or presence of Myc-AC-BD (data not shown). B, infected myocytes were maintained for 24 h in minimal medium and then incubated in fresh medium ± 10 μm NE for 30 min. Total cellular cAMP was measured by EIA. *, p ≤ 0.01; n = 4. C and D, infected myocytes were cultured for 2 days in minimal medium ± 10 μm NE. Myocytes expressing β-gal (C, panels a and b) or Myc-AC-BD (C, panels c and d) were stained with Myc (red) and α-actinin antibodies (green) and Hoechst 33258 DNA stain (blue). Separate red channels are shown to the left of the composite images. The scale bar in panel a represents 20 μm. The graph in D shows fold myocyte cross-section area normalized to the value for control cells expressing β-gal (849 ± 65 μm2). n = 5 independent experiments, each measuring >1000 cells per condition; *, p < 0.03. E, to measure the rate of protein synthesis, [3H]leucine was added for the last 18 h to the medium of myocytes treated as in C. Data are normalized as in D. n = 3; *, p < 0.03.
Elevated cAMP signaling is known to promote cardiomyocyte hypertrophy. Because disruption of the AC5-mAKAPβ complex leads to increased cAMP, we tested whether increased AC5 activity would affect myocyte morphology. Myocytes were infected with adenovirus expressing β-gal (Fig. 5C, panels a and b) or Myc-AC-BD (panels c and d) for 2 days in the absence (panels a and c) or presence (panels b and d) of NE. Consistent with the increase in cAMP levels (Fig. 5B), unstimulated cells expressing Myc-AC-BD were 24 ± 6% larger in cross-section area than cells expressing β-gal (Fig. 5D). Additionally, the larger cells exhibited greater myofibrillar organization, a characteristic of myocyte hypertrophy (Fig. 5C, panel C, green). As a separate control, addition of doxycycline, which inhibits protein expression in this conditional adenoviral system, abolished any difference between Myc-AC-BD and β-gal (data not shown). Similar results were obtained by expression of GFP-tagged mAKAP-(275–340) in transiently transfected myocytes (data not shown). Finally, total protein synthesis was measured as an additional marker for cardiac hypertrophy. [3H]leucine incorporation into newly synthesized protein was 34 ± 6% greater in unstimulated myocytes expressing myc-AC-BD compared with β-gal control (Fig. 5E). Although Myc-AC-BD expression increased total cAMP levels in myocytes, expression of the peptide did not result in further NE-induced myocyte hypertrophy, consistent with prior observations that NE stimulation of both α- and β-adrenergic receptors results in near maximal myocyte growth in vitro (24).
DISCUSSION
In this report, we show that AC binds directly to mAKAPβ in cardiac myocytes, establishing mAKAPβ as the first identified AKAP to bind directly all of the components required for cAMP signaling, including enzymes for cAMP synthesis (AC), downstream signaling (PKA), and degradation (PDE4D3). (Epac1 binds mAKAPβ indirectly though PDE4D3 (11).) AC binding does not appear to compete with the other components of mAKAP complexes for which binding sites have been mapped, as AC (275–340), nesprin-1α (1065–1286) (4), PDE4D3 (1286–1831) (10), PKA (2055–2072) (8), and phosphatidyl-dependent kinase 1 (PDK1) in brain (227–232) (7) bind distinct mAKAP domains.
AC types 2 and 5 can bind mAKAPβ, although the predominant complex found in the heart is mAKAPβ-AC5 (Fig. 1). AC2 binding to mAKAP may be relevant in brain or skeletal muscle where mAKAPα or mAKAPβ are expressed, respectively. A very different pattern of specificity was detected for Yotiao, such that ACs 1, 2, 3, and 9, but not ACs 4, 5, and 6 bound this AKAP (5, 6). The N terminus of AC2 has previously been identified as the site of interaction with Yotiao (6); similarly, the N terminus of AC5 contributes to its increased binding affinity to mAKAPβ (Fig. 3B). Thus, interactions with non-conserved AC N termini may be a common theme for selective assembly of AKAP-AC complexes. Moreover, we show that multiple domains of AC5 (NT, C1, and C2) bind mAKAPβ, as observed for other AC regulators, including Gβγ, Gαs, and calmodulin (18).
mAKAPβ-AC5 Localization
mAKAPβ and nesprin-1α co-localize at the cardiac myocyte nuclear envelope, and significant AC activity was associated with mAKAPβ and indirectly with nesprin-1α (Fig. 1) (15). In the cardiac myocyte, the plasma membrane has many invaginations (the transverse tubular system) that bring it adjacent to the sarcoplasmic reticulum (SR) and presumably to the outer nuclear membrane with which the SR is continuous. AC5 has been detected on the cell surface and membranes of transverse tubules (25). There have also been reports of “functional” membrane-bound AC5 at the nuclear envelope (26, 27). Immunocytochemistry suggests that cAMP increases at numerous sites within cardiac myocytes including membranes associated with tubules, nuclear envelope, and sarcoplasmic reticulum (28). Furthermore, numerous G protein-coupled receptors are also targeted to the perinuclear region including β-adrenergic and prostaglandin receptors (reviewed in Ref. 29). Thus, the mAKAPβ scaffold may organize a novel, localized cAMP signaling complex at the nuclear envelope.
Role of AC in Heart
AC5, but not AC6, has been associated with pathologic remodeling. Disruption of the AC5 gene attenuated the normal inotropic response to sympathetic stimulation (30), and prevented the development of heart failure following thoracic aortic banding (23). Moreover, AC5-null mice are protected against age-induced cardiomyopathy, including cardiac hypertrophy, apoptosis, and fibrosis (21). The binding of AC5 to mAKAPβ is consistent with evidence that mAKAPβ complexes transduce hypertrophic cAMP signaling in cardiac myocytes. Studies using mAKAPβ RNAi showed a requirement for PKA-bound mAKAPβ expression in adrenergic-stimulated neonatal myocyte hypertrophy in culture (4), and the hypertrophic signaling enzymes calcineurin Aβ and ERK5 are both associated with mAKAPβ complexes (4, 11).
Our results suggest that mAKAPβ serves an additional function in the regulation of myocyte hypertrophy through the attenuation of AC5 activity by PKA feedback inhibition. Experiments using membranes containing AC5 and mAKAPβ performed in the presence of phosphatase and phosphodiesterase inhibitors revealed the importance of PKA associated with the complex to AC5 inhibition (Fig. 4). Accordingly, expression in myocytes of the mAKAPβ AC-binding domain disrupted endogenous AC-mAKAPβ complexes and increased total cAMP, resulting in increased cell size and protein synthesis in the absence of extracellular stimulation. Inhibition of AC5-mAKAPβ binding should also dissociate AC5 from PDE4D3 in the complex, which would otherwise oppose cAMP accumulation. In our model (Fig. 6), mAKAPβ is poised to balance cAMP production and degradation. Thus, assembly of the mAKAPβ complex may confer high fidelity, compartmentalized cAMP signaling to prevent cAMP overload, while facilitating selective activation of cAMP targets in response to receptor stimulation. In addition to revealing a novel aspect of cAMP regulation, our results also suggest a potential new strategy for the therapeutic manipulation of cAMP levels in vivo. While chronic stimulation of AC5 may be deleterious to the heart, acute elevation of AC5 activity may be, nevertheless, desirable in the maintenance of cardiac contractility during decompensation (31). The results obtained by mAKAPβ AC-BD overexpression suggest a potential mechanism by which to increase cAMP by targeting the interaction of ACs with AKAPs. In conclusion, we show for the first time that the anchoring of AC to an AKAP complex is required for the control of cAMP-dependent physiological events. This may represent a general paradigm for localized cAMP signaling and opens up a new chapter in cAMP signaling and regulation.
FIGURE 6.
Cyclic AMP regulation by the mAKAPβ complex. mAKAPβ serves as a scaffold for AC5, PKA, and PDE4D3. AC5 associated with the scaffold synthesizes cAMP, activating bound PKA. PKA can phosphorylate both AC5 and PDE4D3, inhibiting local cAMP production and increasing local cAMP degradation, respectively. In the absence of β-adrenergic stimulation, these negative feedback loops are important for maintenance of low basal cAMP levels. In the presence of β-adrenergic stimulation, these negative feedback loops may serve to modulate cAMP signaling. mAKAPβ is anchored in the cardiac myocyte to the outer nuclear membrane by nesprin-1α. mAKAPβ-bound AC5 is likely present on transverse tubules adjacent to the nucleus that would also contain β-adrenergic receptors and heterotrimeric G proteins. Other signaling enzymes that can bind mAKAPβ including Epac1, rap1, MEK5, and ERK5 that can also regulate PDE4D3 activity (11) are not shown in this model, but may also play important roles in the local regulation of cAMP levels.
Supplementary Material
Acknowledgments
We thank Kathryn Hassell for technical assistance and Drs. Tong Tang and Kirk Hammond for supplying AC5 wild type and KO hearts.
This work was supported, in whole or in part, by National Institutes of Health Grants GM60419 (to C. W. D.) and HL075398 (to M. S. K.).
 The on-line version of this article (available at http://www.jbc.org) contains supplemental methods and Figs. S1 and S2.
The on-line version of this article (available at http://www.jbc.org) contains supplemental methods and Figs. S1 and S2.
- PKA
- cAMP-dependent protein kinase
- AKAP
- protein kinase A-anchoring protein
- GST
- glutathione S-transferase
- AC
- adenylyl cyclase
- NE
- norepinephrine
- EIA
- enzyme immunoassay
- GTPγS
- guanosine 5′-3-O-(thio)triphosphate
- β-gal
- β-galactosidase.
REFERENCES
- 1.McConnachie G., Langeberg L. K., Scott J. D. (2006) Trends Mol. Med. 12,317–323 [DOI] [PubMed] [Google Scholar]
- 2.Fink M. A., Zakhary D. R., Mackey J. A., Desnoyer R. W., Apperson-Hansen C., Damron D. S., Bond M. (2001) Circ. Res. 88,291–297 [DOI] [PubMed] [Google Scholar]
- 3.Appert-Collin A., Cotecchia S., Nenniger-Tosato M., Pedrazzini T., Diviani D. (2007) Proc. Natl. Acad. Sci. U.S.A. 104,10140–10145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pare G. C., Bauman A. L., McHenry M., Michel J. J., Dodge-Kafka K. L., Kapiloff M. S. (2005) J. Cell Sci. 118,5637–5646 [DOI] [PubMed] [Google Scholar]
- 5.Bauman A. L., Soughayer J., Nguyen B. T., Willoughby D., Carnegie G. K., Wong W., Hoshi N., Langeberg L. K., Cooper D. M., Dessauer C. W., Scott J. D. (2006) Mol. Cell 23,925–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piggott L. A., Bauman A. L., Scott J. D., Dessauer C. W. (2008) Proc. Natl. Acad. Sci. U.S.A. 105,13835–13840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michel J. J., Townley I. K., Dodge-Kafka K. L., Zhang F., Kapiloff M. S., Scott J. D. (2005) Mol. Cell 20,661–672 [DOI] [PubMed] [Google Scholar]
- 8.Kapiloff M. S., Schillace R. V., Westphal A. M., Scott J. D. (1999) J. Cell Sci. 112,2725–2736 [DOI] [PubMed] [Google Scholar]
- 9.Carlisle Michel J. J., Dodge K. L., Wong W., Mayer N. C., Langeberg L. K., Scott J. D. (2004) Biochem. J. 381,587–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dodge K. L., Khouangsathiene S., Kapiloff M. S., Mouton R., Hill E. V., Houslay M. D., Langeberg L. K., Scott J. D. (2001) EMBO J. 20,1921–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dodge-Kafka K. L., Soughayer J., Pare G. C., Carlisle Michel J. J., Langeberg L. K., Kapiloff M. S., Scott J. D. (2005) Nature 437,574–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kapiloff M. S., Jackson N., Airhart N. (2001) J. Cell Sci. 114,3167–3176 [DOI] [PubMed] [Google Scholar]
- 13.Marx S. O., Reiken S., Hisamatsu Y., Jayaraman T., Burkhoff D., Rosemblit N., Marks A. R. (2000) Cell 101,365–376 [DOI] [PubMed] [Google Scholar]
- 14.Dessauer C. W., Chen-Goodspeed M., Chen J. (2002) J. Biol. Chem. 277,28823–28829 [DOI] [PubMed] [Google Scholar]
- 15.Pare G. C., Easlick J. L., Mislow J. M., McNally E. M., Kapiloff M. S. (2005) Exp. Cell Res. 303,388–399 [DOI] [PubMed] [Google Scholar]
- 16.Katsushika S., Chen L., Kawabe J., Nilakantan R., Halnon N. J., Homcy C. J., Ishikawa Y. (1992) Proc. Natl. Acad. Sci. U.S.A. 89,8774–8778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishikawa Y., Katsushika S., Chen L., Halnon N. J., Kawabe J., Homcy C. J. (1992) J. Biol. Chem. 267,13553–13557 [PubMed] [Google Scholar]
- 18.Sadana R., Dessauer C. W. (2009) Neurosignals 17,5–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwami G., Kawabe J., Ebina T., Cannon P. J., Homcy C. J., Ishikawa Y. (1995) J. Biol. Chem. 270,12481–12484 [DOI] [PubMed] [Google Scholar]
- 20.Chen Y., Harry A., Li J., Smit M. J., Bai X., Magnusson R., Pieroni J. P., Weng G., Iyengar R. (1997) Proc. Natl. Acad. Sci. U.S.A. 94,14100–14104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan L., Vatner D. E., O'Connor J. P., Ivessa A., Ge H., Chen W., Hirotani S., Ishikawa Y., Sadoshima J., Vatner S. F. (2007) Cell 130,247–258 [DOI] [PubMed] [Google Scholar]
- 22.Okumura S., Vatner D. E., Kurotani R., Bai Y., Gao S., Yuan Z., Iwatsubo K., Ulucan C., Kawabe J., Ghosh K., Vatner S. F., Ishikawa Y. (2007) Circulation 116,1776–1783 [DOI] [PubMed] [Google Scholar]
- 23.Okumura S., Takagi G., Kawabe J., Yang G., Lee M. C., Hong C., Liu J., Vatner D. E., Sadoshima J., Vatner S. F., Ishikawa Y. (2003) Proc. Natl. Acad. Sci. U.S.A. 100,9986–9990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deng X. F., Rokosh D. G., Simpson P. C. (2000) Circ. Res. 87,781–788 [DOI] [PubMed] [Google Scholar]
- 25.Gao T., Puri T. S., Gerhardstein B. L., Chien A. J., Green R. D., Hosey M. M. (1997) J. Biol. Chem. 272,19401–19407 [DOI] [PubMed] [Google Scholar]
- 26.Boivin B., Lavoie C., Vaniotis G., Baragli A., Villeneuve L. R., Ethier N., Trieu P., Allen B. G., Hébert T. E. (2006) Cardiovasc. Res. 71,69–78 [DOI] [PubMed] [Google Scholar]
- 27.Belcheva M. M., Gucker S., Chuang D. M., Clark W. G., Jefcoat L. B., McHale R. J., Toth G., Borsodi A., Coscia C. J. (1995) J. Pharmacol. Exp. Ther. 274,1513–1523 [PubMed] [Google Scholar]
- 28.Yamamoto S., Kawamura K., James T. N. (1998) Microsc. Res. Tech. 40,479–487 [DOI] [PubMed] [Google Scholar]
- 29.Boivin B., Vaniotis G., Allen B. G., Hébert T. E. (2008) J. Recept. Signal Transduct. Res. 28,15–28 [DOI] [PubMed] [Google Scholar]
- 30.Okumura S., Kawabe J., Yatani A., Takagi G., Lee M. C., Hong C., Liu J., Takagi I., Sadoshima J., Vatner D. E., Vatner S. F., Ishikawa Y. (2003) Circ. Res. 93,364–371 [DOI] [PubMed] [Google Scholar]
- 31.Tepe N. M., Liggett S. B. (1999) FEBS Lett. 458,236–240 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.