Abstract
The amyloid-β precursor protein (AβPP) is a ubiquitously expressed transmembrane protein whose cleavage product, the amyloid-β (Aβ) protein, is deposited in amyloid plaques in neurodegenerative conditions such as Alzheimer disease, Down syndrome, and head injury. We recently reported that this protein, normally associated with neurodegenerative conditions, is expressed by human embryonic stem cells (hESCs). We now report that the differential processing of AβPP via secretase enzymes regulates the proliferation and differentiation of hESCs. hESCs endogenously produce amyloid-β, which when added exogenously in soluble and fibrillar forms but not oligomeric forms markedly increased hESC proliferation. The inhibition of AβPP cleavage by β-secretase inhibitors significantly suppressed hESC proliferation and promoted nestin expression, an early marker of neural precursor cell (NPC) formation. The induction of NPC differentiation via the non-amyloidogenic pathway was confirmed by the addition of secreted AβPPα, which suppressed hESC proliferation and promoted the formation of NPCs. Together these data suggest that differential processing of AβPP is normally required for embryonic neurogenesis.
The amyloid-β precursor protein (AβPP)5 is a ubiquitously expressed transmembrane protein whose cleavage product, the amyloid-β (Aβ) protein, is deposited in amyloid plaques in the aged brain, following head injury, and in the neurodegenerative conditions of Alzheimer disease (AD) and Down syndrome (DS). AβPP has structural similarity to growth factors (1) and modulates several important neurotrophic functions, including neuritogenesis, synaptogenesis, and synaptic plasticity (2). The function of AβPP during early embryogenesis and neurogenesis has not been well described.
AβPP is processed by at least two pathways, the non-amyloidogenic and amyloidogenic pathways. Non-amyloidogenic processing of AβPP yields secreted AβPPα (sAβPPα), the secreted extracellular domain of AβPP that acts as a growth factor for many cell types and promotes neuritogenesis (3). Amyloidogenic processing of AβPP releases sAβPPβ, the AβPP intracellular domain, and Aβ proteins. The Aβ protein has both neurotoxic and neurotrophic properties (4) dependent on the differentiation state of the neuron; Aβ is neurotoxic to differentiating neurons via a mechanism involving differentiation-associated increases in the phosphorylation of the microtubule-associated protein tau (5) but neurotrophic to undifferentiated embryonic neurons. Evidence supporting a neurotrophic function for Aβ during development include its neurogenic activity toward rat neural stem cells (4–6). Consistent with these data, two studies have demonstrated increased hippocampal neurogenesis in young transgenic mice overexpressing human APPSw,Ind (7, 8).
Recently we reported that human embryonic stem cells (hESCs) express AβPP and that both the stemness of the cells and the pregnancy-associated hormone human chorionic gonadotropin alter AβPP expression (9). These results suggest a functional role for AβPP during early human embryogenesis. To further investigate the function of AβPP and its cleavage products during early embryonic neurogenesis, we examined the expression and processing of this protein and its role in proliferation and differentiation of hESCs into neural precursor cells (NPCs). We found that amyloidogenic processing of AβPP promotes hESC proliferation whereas non-amyloidogenic processing induces hESC differentiation into NPCs. These data reveal an important function for AβPP during early human embryonic neurogenesis. Our data imply that any dysregulation in AβPP processing that leads to altered sAβPPα/Aβ production could result in aberrant neurogenesis as reported in the AD and DS brains.
EXPERIMENTAL PROCEDURES
Propagation of Human Embryonic Stem Cells
Pluripotent H9 hESCs (passage 22–32; XX karyotype; also known as WA09, a National Institutes of Health registered line) were obtained from WiCell Research Institute (Madison, WI). Cells were plated onto irradiated mouse embryonic fibroblast (MEF) cells (1.875 × 105 cells/well; Biovintage, San Diego, CA) in 6-well plates (Fisher Scientific) coated with 1 ml of sterile 0.1% gelatin (Sigma-Aldrich) solution. Prior to addition of hESCs, MEF cells were grown in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum (Invitrogen) and 1% non-essential amino acids (Invitrogen). After 24 h of MEF plating, hESCs were plated on this MEF feeder layer and grown in the presence of DMEM-F-12 medium (Invitrogen) supplemented with 1% non-essential amino acids, 1 mm l-glutamine (Invitrogen), 0.1 mm 2-mercaptoethanol (Sigma-Aldrich), 4 ng/ml basic fibroblast growth factor (Invitrogen), and 20% Knock-outTM Serum Replacer (Invitrogen). Continual propagation of cells required colonies to be enzymatically lifted with 1 ml of a sterile solution of collagenase type IV (Invitrogen) (1 mg/ml of DMEM-F-12), dissected into multiple small pieces, and transferred onto a fresh MEF feeder layer every 4–5 days. hESCs also were grown on MatrigelTM (BD Biosciences), a basement membrane preparation extracted from a murine Englebreth-Holm-Swarm sarcoma, in the presence of mTeSR1 medium (StemCell Technologies, Inc., Vancouver, Canada), a defined culture medium developed by WiCell Research Institute (10). Matrigel (100 μg/ml in DMEM-F-12; 1 ml) was added to each well of a 6-well plate and left for 1 h at room temperature or at 4 °C overnight. hESCs were transferred onto these plates, cells were passaged by enzymatic lifting using a sterile solution of dispase (1 mg/ml in DMEM-F-12; Invitrogen), and the colonies were dissected into multiple small pieces, transferred onto new plates coated with Matrigel, and cultured in mTeSR1 medium. The culture medium (2.5 ml/well) was replaced every day in all the above culture conditions.
Differentiation of hESCs into Embryoid Bodies
This protocol mimics the formation of the blastocyst during human embryogenesis (11). Pluripotent hESCs (H9) grown on MEFs in a 6-well plate were rinsed twice with 1 ml of Dulbecco's phosphate-buffered saline (DPBS; without calcium or magnesium; Invitrogen)/well. Colonies were then incubated with 1 ml of dispase (0.5 mg/ml in DMEM-F-12) at 37 °C in 5% CO2 until the colonies detached intact while avoiding dispersing the colonies into single cells. T25 flasks (Fisher Scientific) were incubated with 5 ml of a 2% poly(2-hydroxyethyl methacrylate) (Sigma-Aldrich) solution for 5 min. The flask was placed in a horizontal position for 5 min with the cap on. This allowed optimal coating of the working surface area of the flask. This process was repeated for each side of the flask. The caps of coated flasks were opened, and the poly(2-hydroxyethyl methacrylate) solution was aspirated off. The open flasks were allowed to remain in the hood for 1 h to dry. After 1 h, the surface was washed twice with DPBS, and the detached hESC colonies were cultured in 15% characterized fetal bovine serum (Invitrogen) and 85% Iscove's modified Dulbecco's medium (Invitrogen) in these poly(2-hydroxyethyl methacrylate)-coated T25 flasks and incubated under standard conditions (37 °C in 5% CO2) on an orbital shaker (Boekel Orbitron, Feasterville, PA) with constant gentle rocking for 10–14 days to allow the hESCs to aggregate into cystic spheroidal structures. The poly(2-hydroxyethyl methacrylate) coat and the constant gentle rocking prevented the adherence of these spheroidal structures to the flask.
Differentiation of hESCs into Neural Precursor Cells
The protocol described below for the differentiation of hESCs into columnar NPCs mimics in vivo NPC development in terms of timing and morphology (12). In vitro, hESCs differentiate into columnar NPCs that organize into neural tubelike rosettes within 12–14 days. Considering that hESCs are equivalent to a 5–6-day embryo, development of the NPCs in vitro takes about 18–20 days, the time window when the neural tube forms in a human embryo (13, 14).
Pluripotent hESC (H9) colonies grown on MEFs in 6-well plates were rinsed twice with DPBS (1 ml/well) and then treated with dispase (1 ml of 1 mg/ml in DMEM-F-12) and incubated at 37 °C in 5% CO2 until the edges of the colonies began to curl up. The plate was then swirled to detach the colonies intact without dispersing the colonies into individual cells. The hESC colonies were grown in T25 flasks in a special embryonic stem cell growth medium (78.5% DMEM-F-12, 20% Knock-out Serum Replacer, 1% non-essential amino acids, 1 mm l-glutamine, and 0.1 mm 2-mercaptoethanol) for 4 days with daily replacement of medium to form embryonic stem cell aggregates. Cells were then adhered to the culture surface where they formed monolayer colonies in a chemically defined neural induction medium (32.6% F-12 (Invitrogen), 65.2% DMEM, 1% N2 supplement (Invitrogen), 1% non-essential amino acids, 0.2% 1 mg/ml heparin (Sigma-Aldrich), and 10 ng/ml basic fibroblast growth factor). Under this culture condition, columnar NPCs appear in the center of each colony and organize into neural tubelike rosettes after a total of 9–10 days of differentiation culture. The neural induction medium was replaced every other day. The NPCs in the rosettes were selectively isolated through differential enzymatic treatment using dispase (0.5 mg/ml in DMEM-F-12) and incubated for 2 h in neural induction medium to allow the non-neural cells to differentially attach to the T25 flasks. After this, the floating cells (mostly aggregates of NPCs) were transferred to new T25 flasks where they rolled up to form round clusters. Some of these clusters were collected and probed for nestin to confirm NPC differentiation. The remaining formed clusters were continuously passaged by manually splitting the clusters using a sterile scalpel.
Immunoblotting
Cells were collected, and immunoblot analyses were performed as described previously (9). Because of the dramatic changes that occur in protein expression during the dynamic developmental period under consideration, it was difficult to find an internal control to demonstrate equal protein loading (see Ref. 9). Given this variability, we chose to load samples according to total protein as described previously (5). The following antibodies (with dilution ratio) were used throughout this study: anti-human nestin monoclonal antibody, anti-human amyloid-β precursor protein, amino terminus (22C11) monoclonal antibody, anti-human amyloid-β precursor protein, carboxyl terminus polyclonal antibody (against YKFFEQMQN), anti-human anterior pharynx defective-1 (APH-1) polyclonal antibody, anti-human CD147 monoclonal antibody, and anti-human neuronal nuclei monoclonal antibody (Chemicon International, Temecula, CA); anti-human amyloid-β, amino acids 1–16 (6E10) monoclonal antibody, and anti-human amyloid-β amino acids 17–24 (4G8) monoclonal antibody (Signet Laboratories, Inc., Dedham, MA); anti-human PHF-1 monoclonal antibody (a kind gift from Dr. Peter Davies, Albert Einstein College of Medicine, Bronx, NY); anti-human BACE-1 polyclonal antibody (Abcam, Cambridge, MA); anti-human presenilin enhancer-2 (PEN-2) polyclonal antibody (Zymed Laboratories Inc., Inc., South San Francisco, CA); anti-human ADAM-10 polyclonal antibody (Calbiochem); anti-human glial fibrillary acidic protein (GFAP) polyclonal antibody (Dako North America, Inc., Carpinteria, CA); anti-human α-fetoprotein monoclonal antibody (R&D Systems, Inc., Minneapolis, MN); anti-human nicastrin (N-19) polyclonal antibody, anti-human presenilin 1 (B-6) monoclonal antibody, anti-human OCT-3/4 monoclonal antibody, anti-human CDK2 monoclonal antibody, anti-human caspase monoclonal antibody, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) goat polyclonal antibody (V-18), anti-human β-actin goat polyclonal antibody (C-11), and horseradish peroxidase-linked goat anti-mouse, goat anti-rabbit, and donkey anti-goat IgG (Santa Cruz Biotechnology, Inc., Santa Cruz, CA).
Quantitation of Secreted, Cell-soluble, and Cell-insoluble Aβ Fractions
hESC and NPC culture media were collected for Aβ enzyme-linked immunosorbent assay (ELISA). hESCs and NPCs were then washed twice with ice-cold DPBS and lysed with 50 mm Tris-HCl (pH 7.6) containing 1% Triton X-100 and 150 mm NaCl. The cell lysate was centrifuged at 60,000 × g for 20 min to obtain a supernatant and cell pellet. The supernatant (Triton-soluble fraction) was analyzed for Aβ by ELISA (see below). The cell pellet was washed once with 50 mm Tris-HCl (pH 7.6) containing 1% Triton X-100 and 150 mm NaCl to remove remaining Triton-soluble proteins. The pellet was sonicated in 50 mm Tris-HCl (pH 7.6) containing 6 m guanidine hydrochloride. Following centrifugation at 250,000 × g for 20 min, the supernatant was diluted 1:12 in doubly deionized water to give a final concentration of guanidine hydrochloride of 0.5 m (which has been shown not to affect protein-antibody binding (15)). This was the Triton-insoluble fraction. The samples were then subjected to a sandwich ELISA using Aβ-(1–40) and Aβ-(1–42) ELISA kits (Signet Laboratories, Inc.) according to the manufacturer's instructions. Briefly the plate was coated with antibody that captures the amino terminus of the Aβ peptide. Standards containing known amounts of Aβ peptide and samples were added to the wells and incubated overnight at 4 °C. The plate was washed to remove unbound peptide, and a primary antibody was added that binds to the carboxyl terminus of Aβ peptide. After incubation and washing, a secondary antibody conjugated to horseradish peroxidase was added. The plate was washed, o-phenylenediamine substrate was added, and bound Aβ peptide was visualized at 492 nm on a Molecular Devices SpectraMax Plus384 spectrophotometer. The level of Aβ was expressed as pg/mg of total cellular protein.
Preparation of Aβ Peptides
Aβ-(1–40) or Aβ-(1–42) peptides were synthesized, purified, and characterized by high pressure liquid chromatography analysis, amino acid analysis, and mass spectroscopy by the W. M. Keck Foundation Biotechnology Resource Laboratory (Yale University, New Haven, CT). Aβ peptides were reconstituted in sterile doubly deionized water under sterile conditions. The Aβ solution was sonicated indirectly three times for 45 s each (38 MHz) and then centrifuged at 10,000 × g for 10 min. The concentration of Aβ in the soluble supernatant was determined spectrophotometrically at 214 nm, and the supernatant was filtered (0.22-μm filter; Fisher Scientific) into a new microcentrifuge tube under sterile conditions and incubated for 3 days at 37 °C to form fibrillized Aβ (modified from Ref. 16). This Aβ was then diluted in mTeSR1 medium. Soluble, oligomeric, and fibrillar forms of Aβ also were prepared using the protocol described previously (17). Briefly Aβ was dissolved to 1 mm in 100% hexafluoroisopropanol (Sigma-Aldrich). Hexafluoroisopropanol was then removed under vacuum, and the peptide was stored at −20 °C. For the aggregation protocols, the peptide was first resuspended in dimethyl sulfoxide (DMSO; Fisher Scientific) to 5 mm. For oligomeric conditions, F-12 culture medium was added to bring the peptide to a final concentration of 100 μm, and the peptide was incubated at 4 °C for 24 h. For fibrillar conditions, 10 mm HCl was added to bring the peptide to a final concentration of 100 μm, and the peptide was incubated for 24 h at 37 °C. For unaggregated conditions, the 5 mm Aβ in DMSO was diluted directly into mTeSR1 medium.
Treatment of hESCs and Neural Precursor Cells with Aβ
hESCs were evenly plated in 6-well plates coated with Matrigel in 2.5 ml of mTeSR1 medium/well. Cells were treated each day with 0, 1, or 5 μm Aβ in mTeSR1 medium for a period of 5 days prior to cell counting using the trypan blue assay. hESCs differentiated into NPCs were treated with freshly prepared 0 or 5 μm fibrillar Aβ-(1–42) (fAβ-(1–42)) for 10 days in neural induction medium. The cells were then collected and stored at −80 °C for immunoanalyses of Aβ-induced changes in differentiation.
Treatment of hESCs and Neural Precursor Cells with Secretase Inhibitors and sAβPPα
hESCs were evenly plated in 6-well plates coated with Matrigel in 2.5 ml of mTeSR1 medium/well. After confirmation of even plating using the trypan blue assay, the cells were either treated with β-secretase inhibitor IV (100 nm), β-secretase inhibitor III (5.5 μm), or γ-secretase inhibitor IX (300 nm; all from Calbiochem) in mTeSR1 medium. Stock solutions of β-secretase inhibitor IV were prepared in methanol, and the β-secretase inhibitor III and γ-secretase inhibitor IX were prepared in DMSO. Corresponding concentrations of methanol or DMSO were added to control wells. Similarly hESCs were treated with sAβPPα (amino acids 19–612; Sigma-Aldrich) at 1 nm in mTeSR1 medium. After 5 days of treatment, the cells were trypsinized, and cell proliferation was assessed using the trypan blue assay. Cells also were collected in DPBS after 5 days of treatment and stored at −80 °C for immunoanalyses of the effect of the inhibitors or sAβPPα on hESC differentiation.
To examine the effect of inhibitor treatment on NPCs, hESCs cultured on MEFs were treated with β-secretase inhibitor IV (100 nm) or γ-secretase inhibitor IX (300 nm) for 4–5 days and then cultured as described above for another 14 days to induce the formation of NPCs in the presence or absence of the above inhibitors in their respective media. Corresponding concentrations of methanol or DMSO were added to control wells and flasks. Fully formed NPC-containing rosettes were collected in DPBS and stored at −80 °C for immunoblot analyses. Similarly hESCs differentiated into NPCs were treated with freshly prepared sAβPPα (1 nm) for 10 days in neural induction medium. These cells were then collected and stored at −80 °C for immunoanalyses of sAβPPα-induced changes in differentiation.
Cell Viability and Proliferation Assays
hESCs were washed with DPBS and stained using the live/dead assay (4 μm ethidium homodimer and 2 μm calcein acetoxymethyl ester; Invitrogen). Cells were analyzed under a Zeiss Axiovert 200 fluorescence inverted microscope connected to a Fluo Arc light source and an Axio Cam MRC-5 camera. Images were visualized using Axio Vision 4.0. Calcein fluorescence was visualized using a fluorescein filter, and EthD-1 fluorescence was visualized using a rhodamine filter.
hESC proliferation was assessed using the Click-iTTM EdU Alexa Fluor® Assay according to the instructions of the manufacturer (Invitrogen). Briefly, cells plated on coverslips in 6-well plates were treated with fAβ1–42 in mTeSR1 medium for 36 h. Equal volumes of doubly deionized H2O were added to the controls. The wells were then incubated with 5-ethynyl-2′-deoxyuridine (EdUrd; 10 μm) in mTeSR1 medium for 2 h, the EdUrd was removed, and the cells were cultured for a further 36 h. Cells were then fixed with 3.7% formaldehyde in PBS and washed twice with 3% bovine serum albumin in PBS, and the cells were permeabilized with 0.5% Triton® X-100 in PBS for 20 min at room temperature. The cells were then washed twice with 3% bovine serum albumin in PBS prior to addition of the Click-iT reaction mixture for 30 min at room temperature in the dark. The cells were then washed with 3% bovine serum albumin in PBS prior to nuclear staining with Hoechst dye. Fluorescence was detected using a fluorescein isothiocyanate filter for EdUrd incorporation and a 4′,6-diamidino-2-phenylindole filter for nuclear staining.
Statistical Analysis
Statistical analysis was performed using the Student's t test and analyses of variance followed by pairwise comparisons with Fisher's protected least significant difference procedure to determine significant changes between treatment groups (Statview 5.0 and SuperAnova 3.0 programs, SAS Institute, Inc.).
RESULTS
AβPP and Secretase Expression in hESCs, Embryoid Bodies, and Neural Precursor Cells
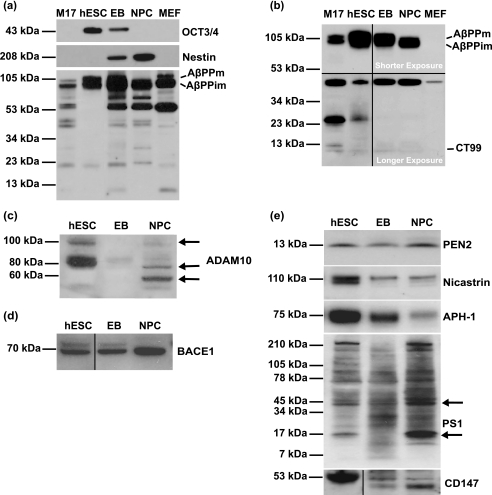
To understand the molecular functions of AβPP during early human embryogenesis, we examined whether the expression and/or proteolytic processing of AβPP was altered during hESC differentiation into NPCs. Pluripotent hESCs were differentiated into embryoid bodies (EBs) or into NPC-containing rosettes. EBs resemble early postimplantation embryos (blastocysts) in that they consist of endodermal, mesodermal, and ectodermal cells (18). Rosettes consist of more than 70% columnar NPCs and are the in vitro equivalent of a rudimentary neural tube (12). To ascertain the stage of hESC differentiation, we immunoprobed these cell lineages for octamer (OCT)-3/4, a 43-kDa POU transcription factor that is required to maintain stem cell self-renewal (i.e. pluripotency) and that is a master regulator of pluripotency that controls lineage commitment (19). This marker was absent in human M17 neuroblastoma cells and MEFs as expected for lineage-committed cells, was present in hESCs grown on Matrigel in mTeSR1 medium, was decreased in EBs, and was absent in NPCs (Fig. 1a, top panel). Concurrent with the loss of pluripotency was an increase in the expression of the NPC marker nestin in EBs and NPCs (Fig. 1a, middle panel).
FIGURE 1.
hESCs, EBs, and NPCs express AβPP and α-, β-, and γ-secretase. Undifferentiated hESCs, EBs, and NPCs and control M17 neuroblastoma cells and MEFs were cultured and collected, and the cell lysates were analyzed by immunoblot with monoclonal antibodies against OCT-3/4 (C-10; against amino acids 1–134 of human OCT-4) and nestin (clone 10C2) to confirm the differentiation of hESCs into NPCs. The immunoblot was then probed with 22C11, an AβPP amino terminus monoclonal antibody (a), and 6E10, a monoclonal antibody directed against amino acids 1–16 of Aβ (b). hESCs, EBs, and NPCs were analyzed by immunoblot with antibodies against α-secretase (ADAM-10; against amino acids 732–748 of human ADAM-10) (c), β-secretase (BACE-1; against amino acids 485–501 of human BACE) (d), and components of the γ-secretase complex (PEN-2, nicastrin, APH-1, PS-1, and CD147) (e). Molecular mass markers are given on the left-hand side. AβPPm, mature AβPP; AβPPim, immature AβPP.
We next probed this immunoblot with a series of antibodies specific for different regions of AβPP, including an antibody against amino acids 66–100 of the amino terminus of AβPP (22C11; Fig. 1a, lower panel), an antibody against the first 16 amino acids of Aβ (6E10; Fig. 1b), an antibody against the carboxyl-terminal 9 amino acids of AβPP (see supplemental Fig. 1a), and an antibody against amino acids 17–24 of Aβ (4G8; see supplemental Fig. 1b). Each antibody identified both the mature and immature forms of full-length AβPP that were variably expressed between cell lineages. The amino-terminal antibody identified a number of AβPP cleavage products including 72-, 58-, 47-, 42-, 29-, and 20-kDa variants. Antibodies directed against the Aβ sequence of AβPP recognized variants of different molecular mass compared with the amino-terminal antibody, including 47- and 25-kDa (both 6E10 and 4G8) and 78-, 67-, and 53-kDa (4G8) variants (Fig. 1b and supplemental Fig. 1b). A carboxyl-terminal antibody detected AβPP variants of 78, 67, and 41 kDa (supplemental Fig. 1a). The expression of both the amino- and carboxyl-terminal variants was generally increased with the differentiation of hESCs into EBs and NPCs. Antibodies directed against the carboxyl terminus of AβPP also identified the 99-amino acid carboxyl-terminal (∼10-kDa) fragment, a β-secretase cleavage product of AβPP (20), which was variably expressed during differentiation.
The presence of AβPP cleavage products in pluripotent hESCs, EBs, and NPCs suggests the presence of secretase enzymes in these cell lineages that modulate AβPP processing toward the amyloidogenic, non-amyloidogenic, and other pathways. To confirm the presence of secretase enzymes, we probed the immunoblot described above with antibodies against ADAM-10 (α-secretase) (21), BACE-1 (β-secretase) (20), and five known components of the γ-secretase complex: presenilin-1, nicastrin, APH-1, PEN-2, and CD147 (22). Immunoreactive bands representing the unprocessed proform of ADAM-10 (100 kDa) were detected in hESCs and NPCs, but the mature (active) form of ADAM-10 (60 kDa) was only detected in NPCs. An additional band (80 kDa) representing a partially processed form (23) also was apparent in hESCs, EBs, and NPCs (Fig. 1c). The expression of BACE-1 enzyme (70 kDa) was detected in hESCs and increased with differentiation toward NPCs (Fig. 1d). Although the expression of PEN-2 (13 kDa) did not change during hESC differentiation, the expression of nicastrin (110 kDa) and APH-1 (75 kDa) decreased (Fig. 1e). Expression of full-length PS-1 (47 kDa) increased with differentiation of hESCs into NPCs as did the carboxyl-terminal fragment of PS-1 (14 kDa; Fig. 1e) (24). The expression of the γ-secretase regulatory subunit CD147 decreased with differentiation of hESCs into NPCs (Fig. 1e). The differential expression of α-, β-, and γ-secretase enzymes and AβPP cleavage products observed between hESCs, EBs, and NPCs suggests that secretase enzymes are functionally active in generating amyloidogenic and non-amyloidogenic AβPP cleavage products during the differentiation of hESCs into germ line cells and NPCs.
Endogenously Produced Aβ Modulates hESC Proliferation
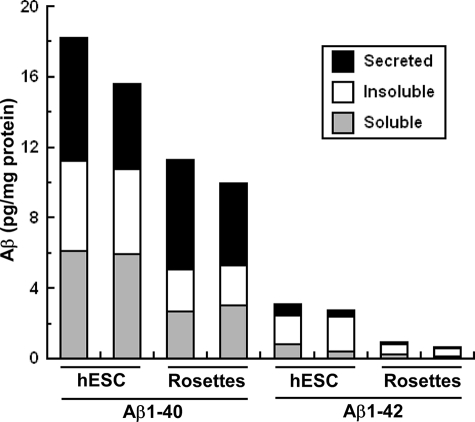
Given these data indicating the expression of secretases (Fig. 1e) and the AβPP carboxyl-terminal fragment in hESCs (Fig. 1b), we performed a sandwich ELISA for Aβ-(1–40) and Aβ-(1–42) in the soluble, insoluble, and secreted fractions of cultured hESCs (Fig. 2). We demonstrated for the first time that both Aβ species are present in hESCs and that Aβ-(1–40) is the dominant cellular and secreted species in hESCs and NPCs as reported in other cell systems. The expression of secreted, Triton-soluble cellular, and Triton-insoluble cellular fractions of Aβ-(1–40) and Aβ-(1–42) decreased 37 and 73%, respectively, during differentiation of hESCs into NPCs, suggesting a functional role for endogenous Aβ production prior to NPC formation.
FIGURE 2.
Endogenous production and secretion of Aβ-(1–40) and Aβ-(1–42) by hESCs decreases during differentiation into NPCs. hESCs and NPCs were cultured and collected for measurement of Triton-soluble and Triton-insoluble Aβ-(1–40) and Aβ-(1–42) by sandwich enzyme-linked immunosorbent assay. Medium was collected for measurement of secreted Aβ-(1–40) and Aβ-(1–42). Results represent two separate experiments.
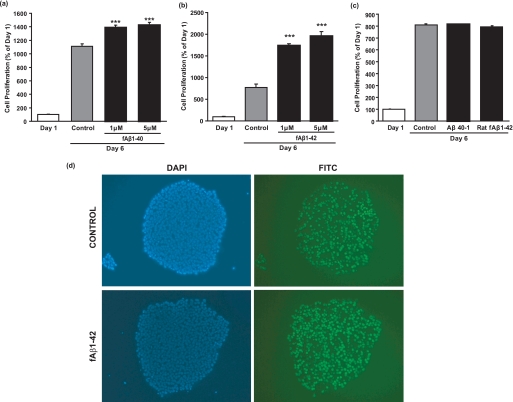
To investigate the function of Aβ in hESCs, we treated hESCs with fAβ for 5 days in mTeSR1 medium. fAβ-(1–40) treatment significantly increased hESC number by 25 and 28%, respectively, at 1 and 5 μm compared with control (Fig. 3a). Likewise in a separate experiment, fAβ-(1–42) treatment significantly increased hESC number by 126 and 154%, respectively, at 1 and 5 μm compared with 6-day control (Fig. 3b). hESC death as determined using the trypan blue staining technique was below 5% in treated cells (data not shown). To exclude the possibility that changes in hESC number were a result of nonspecific interaction with proteins, hESCs were treated with Aβ-(40–1) and rat fAβ-(1–42) for 5 days in mTeSR1 medium. No significant change in cell number was observed with treatment with these control peptides (Fig. 3c) indicating the specificity of the human Aβ1–40/42 proteins in mediating hESC proliferation. Incubation of hESCs with EdUrd, a nucleoside analog of thymidine that incorporates into DNA during cell division, confirmed that fAβ-(1–42) induced cell division (Fig. 3d).
FIGURE 3.
Aβ-(1–40)/Aβ-(1–42) induces hESC proliferation. Synthetic Aβ-(1–40) and Aβ-(1–42) were dissolved in sterile doubly deionized H2O followed by sonication and centrifugation. The supernatant was incubated at 37 °C for 3 days under sterile conditions to form fAβ-(1–40) and fAβ-(1–42). hESCs were then treated with 0, 1, and 5 μm fAβ-(1–40) (a) or fAβ-(1–42) (b) in mTeSR1 medium for 5 days prior to cell counting using the trypan blue assay. Results are expressed as mean ± S.E., n = 3 (***, p < 0.001 compared with 6-day control; representative of two experiments). c, Aβ-(40–1) (reverse) peptide was dissolved in DMSO and incubated at 37 °C in 10 mm HCl for 24 h. Synthetic rat Aβ-(1–42) was dissolved in sterile doubly deionized H2O followed by sonication and centrifugation. The supernatant was incubated at 37 °C for 3 days under sterile conditions to form rat fAβ-(1–42). hESCs were then treated with 0 or 1 μm Aβ-(40–1) or rat fAβ-(1–42) in mTeSR1 medium for 5 days prior to cell counting using the trypan blue assay. Results are expressed as mean ± S.E., n = 3. d, control and fAβ-(1–42)-treated hESCs were incubated with EdUrd in mTESR-1 medium for 2 h prior to fluorescence microscopy. Figures on the left show nuclear staining using a 4′,6-diamidino-2-phenylindole (DAPI) filter; figures on the right show EdUrd staining using a fluorescein isothiocyanate (FITC) filter.
We next tested which Aβ species were mitogenic by treating hESCs with soluble Aβ-(1–40), Aβ-derived diffusible ligands (ADDLs), or fAβ prepared according to the methods described previously (17). hESC number was moderately but significantly increased by soluble Aβ-(1–40) and fAβ-(1–40) at 1 μm (12 and 10%, respectively) but not at 5 μm concentration compared with 6-day control (Fig. 4a). Similarly fAβ-(1–42) significantly increased cell number at 5 μm (50%) compared with 6-day control (Fig. 4b). Conversely Aβ-(1–40) ADDLs significantly decreased cell number by 10 and 13% at 1 and 5 μm, respectively, compared with 6-day control. Aβ1–42 ADDLs significantly decreased cell number 35% at 5 μm compared with 6 d control. These results indicate differential effects of Aβ on hESC number dependent upon aggregation state. These results confirm the mitogenic effect of fAβ prepared in aqueous solution (Fig. 3, a and b), although the effects were more subtle possibly because of the presence of DMSO used in the latter protocol (17) that is a known differentiation agent (25–28). Treatment of hESCs with fAβ-(1–42) for 5 days did not induce nestin expression (Fig. 4c), indicating that Aβ peptides do not promote hESC differentiation into NPCs. To further confirm that Aβ peptides induce cell division, soluble, ADDL, and fibrillized forms of Aβ-(1–40) (5 μm) were added to hESCs, and the expression of a G1/S phase cell cycle marker (CDK2) was measured. All forms of Aβ-(1–40) induced a marginally significant increase in the expression of CDK2 (data not shown), suggestive of a role for Aβ-(1–40) in cell cycle signaling.
FIGURE 4.
Differential effects of Aβ-(1–40)/Aβ-(1–42) protein species on hESC proliferation. Synthetic Aβ-(1–40) and Aβ-(1–42), respectively, were dissolved to 1 mm in 100% hexafluoroisopropanol, which was then removed under vacuum, and the peptide was stored at −20 °C. The peptide was next resuspended in DMSO to 5 mm. For oligomeric Aβ (ADDL), F-12 culture medium was added to the peptide dissolved in DMSO to form a 100 μm solution and incubated at 4 °C for 24 h. For fibrillar Aβ, 10 mm HCl was added to bring the peptide to a final concentration of 100 μm, and the peptide was incubated at 37 °C for 24 h. For soluble Aβ (sAβ), Aβ-(1–40) or Aβ-(1–42) (5 mm) in DMSO were diluted directly into mTeSR1 medium. Cells were then treated each day with 0, 1, or 5 μm of soluble, oligomeric, or fibrillar Aβ-(1–40) (a) or Aβ-(1–42) (b) in mTeSR1 medium for a period of 5 days prior to cell counting. Results are expressed as mean ± S.E., n = 3 (significant increase: *, p < 0.05; **, p < 0.01; ***, p < 0.001 compared with 6-day control; significant decrease: #, p < 0.05; ##, p < 0.01; ###, p < 0.001 compared with 6-day control). c, hESCs treated with fAβ-(1–42) (1 μm; fibrillized in doubly deionized H2O) for 5 days were collected, and cell lysates were analyzed by immunoblot with an anti-nestin monoclonal antibody (clone 10C2), a polyclonal antibody against human β-actin, and a polyclonal antibody against human GAPDH. A control NPC sample expressing nestin is shown in the first lane. Molecular mass markers are shown on the left-hand side. Con, control.
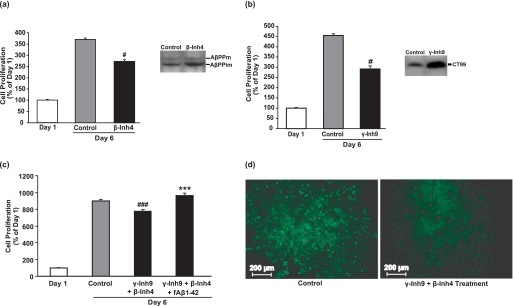
To confirm that endogenous Aβ synthesis and secretion by hESCs (Fig. 2) act in an autocrine manner to induce cell proliferation, we blocked Aβ production using inhibitors of β-secretase and γ-secretase. There was a significant 26, 23, and 36% decrease in hESC number with β-secretase inhibitor IV, β-secretase inhibitor III (data not shown), and γ-secretase inhibitor IX treatment, respectively, compared with 6-day controls (Fig. 5, a and b). Immunoblot analyses with an antibody against the carboxyl terminus of AβPP confirmed inhibition of β-secretase (increased full-length immature AβPP) and γ-secretase (increased the 99-amino acid carboxyl-terminal fragment). Inhibition of both β-secretase plus γ-secretase activity decreased cell number by 14% compared with untreated cells, a decrease that was reversed by the addition of 5 μm fAβ-(1–42) (Fig. 5c), confirming the direct autocrine effect of Aβ on hESCs. To exclude the possibility that the decrease in hESC number with secretase inhibitor treatment was due to a direct toxic effect of the inhibitors, treated cells were assayed using the live/dead cytotoxicity assay (Fig. 5d). The number of dead cells between control and inhibitor treatments showed no marked difference. Taken together, our results show that hESCs process AβPP to produce Aβ, which acts in an autocrine fashion to induce hESC proliferation but not differentiation.
FIGURE 5.
Secretase inhibitors decrease hESC proliferation. hESCs were treated with β-secretase inhibitor IV (β-Inh4; 100 nm) (a) or γ-secretase inhibitor IX (γ-Inh9; 300 nm) (b) in mTeSR1 medium for 5 days prior to cell counting. Stock solutions of β-secretase inhibitor IV and γ-secretase inhibitor IX were prepared in methanol and DMSO, respectively. Control wells contained corresponding concentrations of methanol or DMSO. Results are expressed as mean ± S.E., n = 3 (#, p < 0.05 compared with 6-day control). hESCs treated with β- and γ-inhibitors were lysed and analyzed by immunoblot to confirm the inhibitory action of these secretase inhibitors on AβPP processing using an antibody against the carboxyl terminus of AβPP. AβPPm, mature AβPP; AβPPim, immature AβPP; CT99, carboxyl-terminal fragment of AβPP. Molecular mass markers are given on the left-hand side. c, hESCs were treated with a combination of β-secretase inhibitor IV (100 nm) and γ-secretase inhibitor IX (300 nm) with or without aggregated Aβ-(1–42) (5 μm) in mTeSR1 medium for 5 days prior to cell counting by trypan blue assay. Results are expressed as mean ± S.E., n = 3 (significant increase: ***, p < 0.001; significant decrease: ###, p < 0.001 compared with 6-day control). d, cytotoxicity of β-secretase inhibitor IV (100 nm) plus γ-secretase inhibitor IX (300 nm) was tested using the live/dead assay. The images were captured using a Zeiss Axiovert 200 inverted fluorescence microscope. Magnification, 100×.
Non-amyloidogenic Processing of AβPP Regulates hESC Differentiation into Neural Precursor Cells
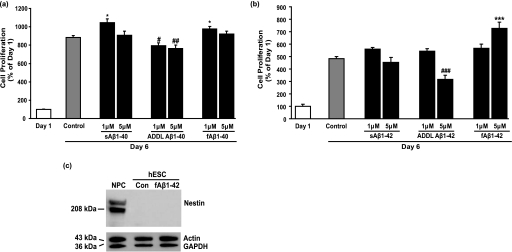
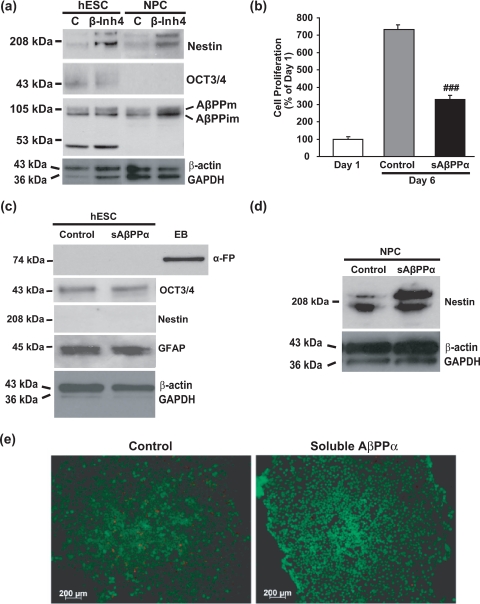
Mature ADAM-10 expression was absent in hESCs but was elevated in NPCs (Fig. 1c), suggesting that non-amyloidogenic processing of AβPP may be up-regulated during neural differentiation as reported previously for NPCs (29). To examine non-amyloidogenic processing of AβPP during differentiation, we treated hESCs with a β-secretase inhibitor. This resulted in an ∼3-fold increase in the expression of the early NPC marker nestin concurrent with a decrease in the expression of OCT-3/4 (Fig. 6a). This result suggests that products of the non-amyloidogenic pathway are important for neural differentiation, promoting hESC specification and loss of pluripotency. As in hESCs, continuous treatment of NPCs with β-secretase inhibitor IV increased nestin expression 2-fold compared with control and also increased immature AβPP expression 3-fold (Fig. 6a). To examine whether the non-amyloidogenic product of AβPP processing, the sAβPPα fragment, was required for hESC differentiation, we treated hESCs with sAβPPα. sAβPPα significantly decreased hESC proliferation by 55% compared with untreated cells over 5 days (Fig. 6b), explaining the absence of ADAM-10 expression in proliferating pluripotent hESCs. No significant difference in the number of dead cells was detected after sAβPPα treatment using the live/dead cytotoxicity assay, excluding the possibility of direct cytotoxicity of sAβPPα on hESCs (Fig. 6e). However, sAβPPα-treated cells showed no change in the expression of OCT-3/4 (a marker of pluripotency), nestin (a marker of NPCs), GFAP (a marker of astrocytes), or α-fetoprotein (an early marker of endoderm) (Fig. 6c), suggesting that although non-amyloidogenic processing of AβPP is necessary for hESC differentiation (Fig. 6a) sAβPPα is either not important or not sufficient at this early stage for neural differentiation. Because we observed the astrocytic marker GFAP in hESCs but never detected tau or α-tubulin (data not shown) expression and because tau and α-tubulin are expressed by astrocytes (e.g. Refs. 30 and 31), it would seem that hESCs, like adult neural stem cells (32, 33), normally express GFAP.
FIGURE 6.
β-secretase inhibition and sAβPPα promote neural differentiation. a, hESCs and NPCs were treated with β-secretase inhibitor IV (β-Inh4; 100 nm) and control (C) as described in Fig. 4 for 5 days prior to collection of cell lysates for immunoblot analyses for nestin, OCT-3/4, the carboxyl terminus of AβPP, β-actin, and GAPDH. b, hESCs were treated daily for 5 days with sAβPPα (1 nm) in mTeSR1 medium, and cell number was then determined using the trypan blue assay. Results are expressed as mean ± S.E., n = 3 (###, p < 0.001 compared with 6-day control). c, protein was extracted from collected hESCs and analyzed by immunoblot for α-fetoprotein (α-FP), OCT-3/4, nestin, GFAP, β-actin, and GAPDH. EBs were used as a positive control for α-fetoprotein. d, NPCs were treated with sAβPPα (1 nm) for 10 days, and cell lysates were analyzed by immunoblot for nestin, β-actin, and GAPDH. Molecular mass markers are given on the left-hand side. e, cytotoxicity of sAβPPα (1 nm) was tested using the live/dead assay. The images were captured using a Zeiss Axiovert 200 inverted fluorescence microscope. Magnification, 100×. AβPPm, mature AβPP; AβPPim, immature AβPP.
In contrast to hESCs, sAβPPα treatment of NPCs induced a strong up-regulation of the expression of nestin (Fig. 6d) indicative of a role for sAβPPα in NPC formation. Together these results indicate that non-amyloidogenic products of AβPP regulate hESC differentiation toward NPCs.
Aβ Is Toxic to Neural Precursor Cells
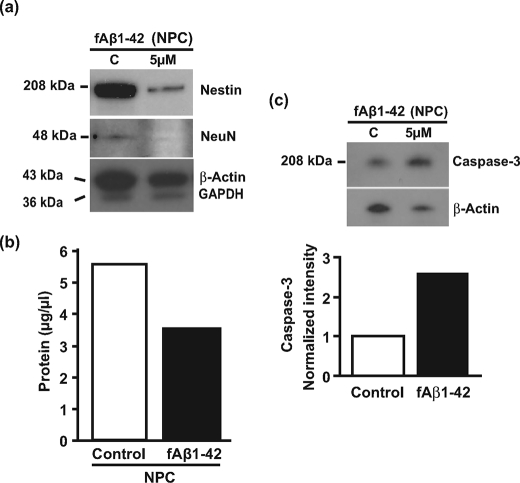
Because 1) Aβ production declines dramatically from hESCs to NPCs (Fig. 2), 2) Aβ toxicity in primary rat neurons is dependent upon differentiation-associated increases in tau and cyclin-dependent kinase 5 expression (5), and 3) undifferentiated hESCs are not susceptible to Aβ toxicity (Fig. 3), we examined whether the differentiation of hESCs into NPCs also increases susceptibility to Aβ toxicity by treating NPCs with fAβ-(1–42) for 10 days. fAβ-(1–42) induced a dramatic decrease in nestin (70%) and neuronal nuclei (95%) expression (Fig. 7a), indicating that Aβ was either toxic or suppressed nestin/neuronal nuclei expression. Because accurate counting of cells in rosettes is technically challenging, we used total protein concentration as a marker of cell number. fAβ-(1–42) treatment decreased protein concentration 37% compared with controls (Fig. 7b). To confirm that fAβ-(1–42) was toxic to NPCs, we measured the expression of caspase-3, a marker of apoptosis. Caspase-3 expression increased 2.6-fold (Fig. 7c), indicating that fAβ-(1–42) becomes toxic to a population of NPCs/neurons present in the rosettes.
FIGURE 7.
Aβ is toxic to NPC. a, NPCs were treated with synthetic human fAβ-(1–42) (5 μm) for 10 days in neural induction medium, and cell lysates were analyzed by immunoblot for the expression of nestin, NeuN, β-actin, and GAPDH. Molecular mass markers are given on the left-hand side. b, the protein concentration of these samples was measured using a bicinchoninic acid protein assay kit (Pierce). c, cell lysates from a were analyzed by immunoblot for human caspase-3 and β-actin. Quantitation of the blot is given below the blot. C, control.
DISCUSSION
Our data suggest that the differential processing of AβPP regulates hESC proliferation and differentiation (Figs. 1–6) of hESCs into NPCs. The up-regulation of the amyloidogenic pathway in hESCs was associated with cell proliferation as evidenced by the high expression of β-secretase and γ-secretase components but low expression of ADAM-10 in hESCs compared with EBs and NPCs (Fig. 1), the higher production of Aβ by hESCs compared with NPCs (Fig. 2), the mitogenic effects exerted by exogenous Aβ peptides on hESCs (Figs. 3 and 4), and the suppression of hESC proliferation upon inhibition of Aβ production (Fig. 5). The up-regulation of the non-amyloidogenic pathway was associated with hESC differentiation into NPCs as evidenced by an increase in functional ADAM-10 expression in NPCs (Fig. 1), β-secretase inhibitor-induced increases in nestin expression (Fig. 6a), and sAβPPα-induced inhibition of hESC proliferation (Fig. 6b) and increased nestin expression in NPCs (Fig. 6d).
The differential processing of AβPP in hESCs was clearly evidenced by the expression of different fragments of AβPP detected using carboxyl-terminal (78-, 67-, 53-, 47-, 41-, 25-, and 10-kDa) and amino-terminal (72-, 58-, 47-, 42-, 29-, and 20-kDa) antibodies. Although the functional significance of these AβPP cleavage products is unknown, their change in expression with differentiation suggests a crucial function during lineage specification and functional differentiation. Previously reported fragments include 22- and 29-kDa AβPP cleavage products in human platelets (34, 35) and PDAPP transgenic mice (36), respectively. Although the proteases involved in the generation of many of these fragments are unknown, the expression of α-secretase, β-secretase, and five components of the γ-secretase complex (Fig. 1) explain the presence of amyloidogenic and non-amyloidogenic processing of AβPP in hESCs.
Detection of both Aβ-(1–40) and Aβ-(1–42) in hESCs and NPCs confirms the processing of AβPP in the amyloidogenic pathway. Aβ-(1–40) was the predominant Aβ species generated by both hESCs and NPCs, accounting for ∼85–95% of total Aβ. Total Aβ-(1–40) and Aβ-(1–42) generation decreased 37 and 73%, respectively, between hESCs and NPCs, indicating that Aβ-(1–42) may play a more important role in hESC proliferation than in differentiation. The significant increase in the proliferation of hESCs with addition of soluble and fibrillar forms of Aβ-(1–40) and Aβ-(1–42) (Figs. 3, a and b, and 4, a and b) was not accompanied by changes in the expression of NPC markers (Fig. 4c), suggesting that Aβ promotes cell proliferation but not differentiation of hESCs into NPCs. Indeed β-secretase activity and Aβ production appear to be necessary for hESC proliferation and pluripotency (Fig. 5) because β-secretase inhibition induced hESC differentiation (Fig. 6). These finding are consistent with previous studies showing that Aβ is trophic toward Neuro-2A neuroblastoma and rodent neural stem cells (4–6, 17, 37, 38). Dose-dependent changes in proliferation were not always observed with soluble and fibrillar Aβ generated using DMSO, suggesting that DMSO might antagonize Aβ effects (25–28). However, hESC proliferation was significantly decreased in the presence of oligomeric Aβ, although it is not clear whether this is because of a decrease in the rate of cell proliferation or to an increase in toxicity as reported previously for neuroblastoma and neural stem cell cultures (17). Interestingly fAβ is toxic to NPCs (Fig. 7), indicating that although Aβ is required for pluripotency/proliferation of hESCs it becomes toxic to cells upon differentiation into a neuronal phenotype. Aβ toxicity may be mediated via the tau phosphorylation pathway (5) or via developmental up-regulation of apoptotic mediators such as caspase-2 (39) and caspase-3 (Fig. 7).
As hESCs differentiate down the NPC pathway, the non-amyloidogenic processing of AβPP increases sAβPPα production and decreases Aβ production, both of which are inhibitory to cell proliferation (Figs. 5 and 6). Inhibition of amyloidogenic processing of AβPP promotes nestin expression (Fig. 6), which is consistent with the differentiative effects associated with sAβPPα reported previously for neural stem cells (Ref. 29; for a review, see Ref. 40). Retinoic acid-induced neural differentiation of the embryonal carcinoma P19 cells markedly increases the abundance of AβPP mRNA (41), and sAβPP770 and to a lesser extent sAβPP695 promote the growth of rat embryonic day 13/14 neural stem cells (42, 43).
We have recently shown that NPC formation is induced by progesterone present in the “neural induction medium” (44, 45). Thus, the differentiation of hESCs into NPCs may be mediated by progesterone-induced modulation of AβPP processing toward the non-amyloidogenic pathway (Figs. 1 and 2). Future studies will confirm whether this early pregnancy hormone regulates AβPP metabolism for hESC differentiation.
There were a couple of interesting observations in our study of hESC differentiation. The first was that hESCs, like adult neural stem cells (32, 33), express GFAP, suggesting a pivotal function for this protein early in embryogenesis. These results bring into question its usefulness as an astrocytic marker alone. The second was that continued culturing of columnar NPC rosettes beyond 18–20 days led to the expression of a second nestin variant (∼220 kDa), suggesting the generation of a post-translationally modified version of nestin as neural precursor cells differentiate.
Amyloidogenic pathways are normally associated with neurodegenerative diseases such as AD, DS, and frontotemporal dementia. Our data indicate parallels between neurogenesis and neurodegeneration, including AβPP and secretase expression, and processing of AβPP toward the amyloidogenic pathway (Figs. 1 and 2). The fetal brain has been reported to display a number of biochemical similarities to the AD brain, namely the presence of Aβ and AβPP (46, 47), hyperphosphorylated tau (48), and presenilin-1 expression (49). That amyloidogenic pathways are involved in neurogenesis has been reported recently by a number of workers (5, 6, 37, 38). In this context, an increase in neurogenesis has been reported in young transgenic mice overexpressing human mutant APP (7, 8). Moreover the overexpression of wild-type or familial AD mutant AβPP, which promotes Aβ generation (50), also has been shown to promote the re-entry of primary neurons into the cell cycle as demonstrated by the induction of DNA synthesis and cell cycle markers (51).
Developmental protein expression in the AD brain is intriguing in light of the fact that accumulating evidence suggests reactivation of the cell cycle in differentiated neurons of the AD brain (52–54). These data include 1) the ectopic expression of cell cycle proteins in those regions of the brain affected by AD (e.g. cyclin B1, CDC2, proliferating cell nuclear antigen, CDK4, Ki-67, and p16) but not in areas unaffected by AD pathology or in control brains, 2) chromosomal replication (endoreduplication) in differentiated AD neurons demonstrating completion of the S phase, 3) elevated cytoplasmic mitochondrial DNA and COX-1 expression suggestive of de novo mitochondrion synthesis, and 4) up-regulated growth factor signal transduction pathways.
Neural stem cells are generated continually from the subependymal zone of the lateral ventricles and from the hippocampal formation. These migrate and differentiate into granule cells of the olfactory bulb and dentate gyrus, respectively (55). The hippocampus shows constant neurogenesis even during adult life and is a prominent area affected in AD patients. In light of our results, the increase in ΑβPP processing in the AD brain may induce neural stem cell proliferation and/or differentiation as an attempt to repopulate neurons in and around the hippocampus. Our results and those of others strongly support this (4–6, 17, 37, 38). Because brain sAβPP levels are reduced in individuals with AD (56) and sAβPPα decreases hESC proliferation and promotes differentiation (Fig. 6), it is possible that the AD brain is promoting stem cell proliferation by suppressing sAβPPα production in favor of Aβ production. The reduced sAβPPα production might lead to decreased NPC formation to replace dying neurons. Likewise because brain sAβPPα levels are lower in individuals with DS compared with age-matched normal controls (57), stem cells in the DS brain may be incapable of differentiating correctly during neurogenesis to form functional neuronal connections with such changes in AβPP processing also leading to the higher incidence of AD in DS patients. Future studies are required to test these possibilities.
In conclusion, our data reveal that the early expression and differential processing of AβPP are normal processes important for neurogenesis during early embryogenesis. Subtle changes in the processing of AβPP by neuronal cells and/or resident neural stem cells in the adult during aging may underlie the cell cycle changes and apoptotic cell death observed in AD and DS. Our data indicate hESCs as a useful model for understanding both neurogenesis and neurodegeneration. Finally these results have important implications for current therapeutic strategies aimed at modulating Aβ production as well as stem cell replacement therapies for treating neurodegenerative diseases and head trauma.
Supplementary Material
Acknowledgments
We thank the WiCell Research Institute for providing hESC lines and technical support. We give special thanks to Katelin Shields for technical assistance with the ELISA.
This is Department of Veterans Affairs Geriatrics Research, Education and Clinical Center Manuscript 2009-07.
 The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
- AβPP
- amyloid-β precursor protein
- Aβ
- amyloid-β
- hESC
- human embryonic stem cell
- NPC
- neural precursor cell
- AD
- Alzheimer disease
- DS
- Down syndrome
- sAβPP
- soluble AβPP
- MEF
- mouse embryonic fibroblast
- DMEM
- Dulbecco's modified Eagle's medium
- DPBS
- Dulbecco's phosphate-buffered saline
- GFAP
- glial fibrillary acidic protein
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- ELISA
- enzyme-linked immunosorbent assay
- DMSO
- dimethyl sulfoxide
- fAβ
- fibrillar Aβ
- EdUrd
- 5-ethynyl-2′-deoxyuridine
- PBS
- phosphate-buffered saline
- EB
- embryoid body
- APH-1
- anterior pharynx defective-1
- PEN-2
- presenilin enhancer-2
- ADDL
- Aβ-derived diffusible ligand
- BACE
- β-site AβPP-cleaving enzyme
- OCT
- octamer.
REFERENCES
- 1.Rossjohn J., Cappai R., Feil S. C., Henry A., McKinstry W. J., Galatis D., Hesse L., Multhaup G., Beyreuther K., Masters C. L., Parker M. W. (1999) Nat. Struct. Biol. 6,327–331 [DOI] [PubMed] [Google Scholar]
- 2.Gralle M., Ferreira S. T. (2007) Prog. Neurobiol. 82,11–32 [DOI] [PubMed] [Google Scholar]
- 3.Milward E. A., Papadopoulos R., Fuller S. J., Moir R. D., Small D., Beyreuther K., Masters C. L. (1992) Neuron 9,129–137 [DOI] [PubMed] [Google Scholar]
- 4.Yankner B. A., Duffy L. K., Kirschner D. A. (1990) Science 250,279–282 [DOI] [PubMed] [Google Scholar]
- 5.Liu T., Perry G., Chan H. W., Verdile G., Martins R. N., Smith M. A., Atwood C. S. (2004) J. Neurochem. 88,554–563 [DOI] [PubMed] [Google Scholar]
- 6.López-Toledano M. A., Shelanski M. L. (2004) J. Neurosci. 24,5439–5444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin K., Galvan V., Xie L., Mao X. O., Gorostiza O. F., Bredesen D. E., Greenberg D. A. (2004) Proc. Natl. Acad. Sci. U.S.A. 101,13363–13367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.López-Toledano M. A., Shelanski M. L. (2007) J. Alzheimers Dis. 12,229–240 [DOI] [PubMed] [Google Scholar]
- 9.Porayette P., Gallego M. J., Kaltcheva M. M., Meethal S. V., Atwood C. S. (2007) Biochem. Biophys. Res. Commun. 364,522–527 [DOI] [PubMed] [Google Scholar]
- 10.Ludwig T. E., Bergendahl V., Levenstein M. E., Yu J., Probasco M. D., Thomson J. A. (2006) Nat. Methods 3,637–646 [DOI] [PubMed] [Google Scholar]
- 11.Itskovitz-Eldor J., Schuldiner M., Karsenti D., Eden A., Yanuka O., Amit M., Soreq H., Benvenisty N. (2000) Mol. Med. 6,88–95 [PMC free article] [PubMed] [Google Scholar]
- 12.Li X. J., Zhang S. C. (2006) Methods Mol. Biol. 331,169–177 [DOI] [PubMed] [Google Scholar]
- 13.Müller F., O'Rahilly R. (1985) Anat. Embryol. 172,157–169 [DOI] [PubMed] [Google Scholar]
- 14.Zhang S. C. (2003) J. Hematother. Stem Cell Res. 12,625–634 [DOI] [PubMed] [Google Scholar]
- 15.Morishima-Kawashima M., Ihara Y. (1998) Biochemistry 37,15247–15253 [DOI] [PubMed] [Google Scholar]
- 16.Wei W., Wang X., Kusiak J. W. (2002) J. Biol. Chem. 277,17649–17656 [DOI] [PubMed] [Google Scholar]
- 17.Dahlgren K. N., Manelli A. M., Stine W. B., Jr., Baker L. K., Krafft G. A., LaDu M. J. (2002) J. Biol. Chem. 277,32046–32053 [DOI] [PubMed] [Google Scholar]
- 18.O'Shea K. S. (1999) Anat. Rec. 257,32–41 [DOI] [PubMed] [Google Scholar]
- 19.Niwa H., Miyazaki J., Smith A. G. (2000) Nat. Genet. 24,372–376 [DOI] [PubMed] [Google Scholar]
- 20.Vassar R., Bennett B. D., Babu-Khan S., Kahn S., Mendiaz E. A., Denis P., Teplow D. B., Ross S., Amarante P., Loeloff R., Luo Y., Fisher S., Fuller J., Edenson S., Lile J., Jarosinski M. A., Biere A. L., Curran E., Burgess T., Louis J. C., Collins F., Treanor J., Rogers G., Citron M. (1999) Science 286,735–741 [DOI] [PubMed] [Google Scholar]
- 21.Lammich S., Kojro E., Postina R., Gilbert S., Pfeiffer R., Jasionowski M., Haass C., Fahrenholz F. (1999) Proc. Natl. Acad. Sci. U.S.A. 96,3922–3927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaether C., Haass C., Steiner H. (2006) Neurodegener. Dis. 3,275–283 [DOI] [PubMed] [Google Scholar]
- 23.McCulloch D. R., Akl P., Samaratunga H., Herington A. C., Odorico D. M. (2004) Clin. Cancer Res. 10,314–323 [DOI] [PubMed] [Google Scholar]
- 24.Kim T. W., Pettingell W. H., Jung Y. K., Kovacs D. M., Tanzi R. E. (1997) Science 277,373–376 [DOI] [PubMed] [Google Scholar]
- 25.Wen J., Xia Q., Lu C., Yin L., Hu J., Gong Y., Yin B., Monzen K., Yuan J., Qiang B., Zhang X., Peng X. (2007) J. Cell. Biochem. 102,149–160 [DOI] [PubMed] [Google Scholar]
- 26.Hay D. C., Zhao D., Fletcher J., Hewitt Z. A., McLean D., Urruticoechea-Uriguen A., Black J. R., Elcombe C., Ross J. A., Wolf R., Cui W. (2008) Stem Cells 26,894–902 [DOI] [PubMed] [Google Scholar]
- 27.Hay D. C., Zhao D., Ross A., Mandalam R., Lebkowski J., Cui W. (2007) Cloning Stem Cells 9,51–62 [DOI] [PubMed] [Google Scholar]
- 28.McBurney M. W., Jones-Villeneuve E. M., Edwards M. K., Anderson P. J. (1982) Nature 299,165–167 [DOI] [PubMed] [Google Scholar]
- 29.Kwak Y. D., Brannen C. L., Qu T., Kim H. M., Dong X., Soba P., Majumdar A., Kaplan A., Beyreuther K., Sugaya K. (2006) Stem Cells Dev. 15,381–389 [DOI] [PubMed] [Google Scholar]
- 30.Miller F. D., Naus C. C., Durand M., Bloom F. E., Milner R. J. (1987) J. Cell Biol. 105,3065–3073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyazono M., Iwaki T., Kitamoto T., Shin R. W., Fukui M., Tateishi J. (1993) Acta Neuropathol. 86,236–241 [DOI] [PubMed] [Google Scholar]
- 32.Doetsch F., García-Verdugo J. M., Alvarez-Buylla A. (1997) J. Neurosci. 17,5046–5061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackson E. L., Garcia-Verdugo J. M., Gil-Perotin S., Roy M., Quinones-Hinojosa A., VandenBerg S., Alvarez-Buylla A. (2006) Neuron 51,187–199 [DOI] [PubMed] [Google Scholar]
- 34.Tang K., Hynan L. S., Baskin F., Rosenberg R. N. (2006) J. Neurol. Sci. 240,53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Q. X., Evin G., Small D. H., Multhaup G., Beyreuther K., Masters C. L. (1995) J. Biol. Chem. 270,14140–14147 [DOI] [PubMed] [Google Scholar]
- 36.Esh C., Patton L., Kalback W., Kokjohn T. A., Lopez J., Brune D., Newell A. J., Beach T., Schenk D., Games D., Paul S., Bales K., Ghetti B., Castaño E. M., Roher A. E. (2005) Biochemistry 44,13807–13819 [DOI] [PubMed] [Google Scholar]
- 37.Calafiore M., Battaglia G., Zappalà A., Trovato-Salinaro E., Caraci F., Caruso M., Vancheri C., Sortino M. A., Nicoletti F., Copani A. (2006) Neurobiol. Aging 27,606–613 [DOI] [PubMed] [Google Scholar]
- 38.Heo C., Chang K. A., Choi H. S., Kim H. S., Kim S., Liew H., Kim J. A., Yu E., Ma J., Suh Y. H. (2007) J. Neurochem. 102,493–500 [DOI] [PubMed] [Google Scholar]
- 39.Troy C. M., Rabacchi S. A., Friedman W. J., Frappier T. F., Brown K., Shelanski M. L. (2000) J. Neurosci. 20,1386–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Atwood C. S., Huang X., Moir R. D., Smith M. A., Tanzi R. E., Roher A. E., Bush A. I., Perry G. (2001) in Alzheimer's Disease: Advances in Etiology, Pathogenesis and Therapeutics ( Iqbal K. ed) pp. 341–361, John Wiley & Sons, Ltd., London [Google Scholar]
- 41.Yoshikawa K., Aizawa T., Maruyama K. (1990) Biochem. Biophys. Res. Commun. 171,204–209 [DOI] [PubMed] [Google Scholar]
- 42.Hayashi Y., Kashiwagi K., Ohta J., Nakajima M., Kawashima T., Yoshikawa K. (1994) Biochem. Biophys. Res. Commun. 205,936–943 [DOI] [PubMed] [Google Scholar]
- 43.Ohsawa I., Takamura C., Morimoto T., Ishiguro M., Kohsaka S. (1999) Eur. J. Neurosci. 11,1907–1913 [DOI] [PubMed] [Google Scholar]
- 44.Gallego M. J., Porayette P., Kaltcheva M., Bowen R. L., Vadakkadath Meethal S., Atwood C. S. (2008) Nat. Precedings http://hdl.handle.net/10101/npre.2008.2671.1 [Google Scholar]
- 45.Gallego M. J., Porayette P., Kaltcheva M. M., Meethal S. V., Atwood C. S. (2009) Stem Cells Dev. 18,737–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takashima S., Kuruta H., Mito T., Nishizawa M., Kunishita T., Tabira T. (1990) Brain Dev. 12,367–371 [DOI] [PubMed] [Google Scholar]
- 47.Arai Y., Suzuki A., Mizuguchi M., Takashima S. (1997) Brain Dev. 19,290–294 [DOI] [PubMed] [Google Scholar]
- 48.Goedert M., Jakes R., Crowther R. A., Six J., Lübke U., Vandermeeren M., Cras P., Trojanowski J. Q., Lee V. M. (1993) Proc. Natl. Acad. Sci. U.S.A. 90,5066–5070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berezovska O., Xia M. Q., Page K., Wasco W., Tanzi R. E., Hyman B. T. (1997) J. Neuropathol. Exp. Neurol. 56,40–44 [DOI] [PubMed] [Google Scholar]
- 50.Citron M., Westaway D., Xia W., Carlson G., Diehl T., Levesque G., Johnson-Wood K., Lee M., Seubert P., Davis A., Kholodenko D., Motter R., Sherrington R., Perry B., Yao H., Strome R., Lieberburg I., Rommens J., Kim S., Schenk D., Fraser P., St George Hyslop P., Selkoe D. J. (1997) Nat. Med. 3,67–72 [DOI] [PubMed] [Google Scholar]
- 51.McPhie D. L., Coopersmith R., Hines-Peralta A., Chen Y., Ivins K. J., Manly S. P., Kozlowski M. R., Neve K. A., Neve R. L. (2003) J. Neurosci. 23,6914–6927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raina A. K., Zhu X., Rottkamp C. A., Monteiro M., Takeda A., Smith M. A. (2000) J. Neurosci. Res. 61,128–133 [DOI] [PubMed] [Google Scholar]
- 53.Hirai K., Aliev G., Nunomura A., Fujioka H., Russell R. L., Atwood C. S., Johnson A. B., Kress Y., Vinters H. V., Tabaton M., Shimohama S., Cash A. D., Siedlak S. L., Harris P. L., Jones P. K., Petersen R. B., Perry G., Smith M. A. (2001) J. Neurosci. 21,3017–3023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herrup K., Yang Y. (2007) Nat. Rev. Neurosci. 8,368–378 [DOI] [PubMed] [Google Scholar]
- 55.Kuhn H. G., Svendsen C. N. (1999) BioEssays 21,625–630 [DOI] [PubMed] [Google Scholar]
- 56.Palmert M. R., Usiak M., Mayeux R., Raskind M., Tourtellotte W. W., Younkin S. G. (1990) Neurology 40,1028–1034 [DOI] [PubMed] [Google Scholar]
- 57.Busciglio J., Pelsman A., Wong C., Pigino G., Yuan M., Mori H., Yankner B. A. (2002) Neuron 33,677–688 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.