Abstract
Poly (hydroxyalkanoates) (PHAs) have recently attracted a great deal of academic and industrial interest for their biodegradability and biocompatibility making them suitable for environmental and biomedical applications. Poly(3-hydroxybutyrate-) (PHB-) and Poly(DL-lactide-co-glycolide) (PLGA-) based nanoparticles were prepared using the dialysis method as yet unreported for the preparation of nanoparticles based on PHB. Processing conditions were varied in order to evaluate their influence on morphology, drug encapsulation, and size of nanoparticles. The relevant results obtained give a theoretical understanding of the phenomenon occurring during colloidal formation. The adopted procedure allows for a relatively small diameter and homogeneity in size distribution of the PHB nanoparticles to be obtained compared to other methods like the one based on solvent evaporation which leads to particles on microscale. The biocompatibility of PHB and relative nanoparticles was investigated and both exhibited very good cytocompatibility.
1. Introduction
Although a number of synthetic biodegradable polymers have been developed for biomedical applications, the use of natural biodegradable polymers remains attractive because of their abundance in nature, good biocompatibility, and ability to be readily modified [1].
The majority of drug delivery systems based on the use of natural polymers have been based on proteins and polysaccharides [2]. Poly(hydroxyalkanoates), synthesized by a wide variety of microorganisms, have recently attracted a great deal of interest and particular attention has been focused on the use of poly(3-hydroxybutyrate) (PHB) and related copolymers, mostly poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV), as carriers for drug delivery or scaffolds in tissue engineering. When compared with chemically produced polymers such as polyglycolate (PGA), polylactate (PLA), and poly(lactide-co-glycolide) (PLGA), which are mostly well known as biologically degradable drug carriers with good retarding characteristics, PHB and PHBV display advantages bound to their easier processibility, excellent biocompatibility, and propensity to biodegradating under different environmental conditions.
In addition, the controllable retarding properties of delivery systems based on PHB and PHBV can be modulated by variations in processing and molecular weight of the polymer and copolymer composition. Thus, it could be anticipated to be an ideal basic material for matrix composition [3].
Biosynthetic routes to the preparation of PHAs avoid the use of chemical catalysts, initiators, and solvents which may cause problems with acceptance of the final formulations if they are not completely eliminated in the workup of the polymer matrix. The involvement of smart microbial enzymes in the preparation of the PHAs polymer results in macromolecular matrices which are characterized by unique homogeneity in regioselectivity and chirality of the monomeric units. This guarantees a high confluence in the reproducibility of the physical-chemical features of the prepared matrices. Hydrolytic degradation of PHB in vitro proceeds to the monomer D-3-hydroxybutyricacid which is a normal constituent of blood and, in common with acetoacetate and acetone, constitutes one of the three ketone bodies produced endogenously by the ketogenesis process. It is, therefore, thought that PHB can be well tolerated in vivo [4].
Its use in drug release formulations has been reported in recent years and emulsion solvent evaporation has been the most frequently used microencapsulation method [1].
The production of relatively small nanoparticles, according to a general procedure applicable to different polymeric molecules, is not feasible on the grade of the results so far obtained [5].
Some disadvantages of the conventional methods include the difficulty and necessity of removing solvent and surfactant residues, low particle yields, numerous steps for preparation, and the necessity to use a high concentration of surfactant for the attainment of small spherical particles and polymeric micelles.
The dialysis method is a simple and effective method for the preparation of small, narrow-distributed nanoparticles mostly by using block graft copolymers and other amphiphilic materials [6]. Polymer, drug, and surfactants are dissolved in the same organic solvent and placed inside a dialysis tube with proper molecular weight cut-off. Dialysis is performed against a nonsolvent miscible with the former miscible. The displacement of the solvent inside the membrane is followed by the progressive aggregation of polymer, drug, and surfactants due to a loss of solubility and by the formation of homogeneous suspensions of micro-nanoparticles.
The mechanism of nanoparticles formation by dialysis method is not fully understood at present. It is thought that it may be based on a mechanism similar to that of nanoprecipitation proposed by the Fessi et al. [5].
The primary objective of the present work is the advancement of knowledge and theoretical understanding of the relationship among variables playing an important role in the development and optimization of the dialysis method for the preparation of polymeric nanoparticles suspensions. The work has been conducted with the practical end in mind of preparing nanodelivery structures for the delivery of hydrophobic bioactive agents in cancer therapy or tissue engineering applications. Retinoic acid (RA), a lipophyle drug with a polar head imparting amphiphilic character, was chosen as a model to be loaded into nanoparticles. Retinoic acid is the main biologically active derivative of Vitamin A or retinol [7], it is a potent regulator of gene transcriptions [8], it plays an important role in regulating cell growth, differentiation [7], embryonic development, apoptosis, and homeostasis [9], and it can reverse malignant cell growth in vitro and in vivo. All-transretinoic acid (ATRA) is being increasingly included in antitumour therapeutical schemes for the treatment of various diseases such as acute promyelocytic leukaemia, Kaposi's sarcoma, head and neck squamous cell carcinoma, ovarian carcinoma, bladder cancer, and neuroblastoma [10], and it has shown antiangiogenic effects in several systems [9]. Furthermore, it has been found to induce P19 cell differentiation into neurons [11]. However, due to its hydrophobicity [10] (solubility 0.21 μM in physiological solution, pH 7.4) [12] and short half-lives in blood [13], ATRA parenteral administration is very difficult and to date no commercial parenteral formulation is available. On the other hand, the oral formulations in clinical use are characterized by uncertain drug bioavailability due to the variable absorption in the gastrointestinal tract and the hepatic first pass effect, implying metabolism of ATRA to less active compounds by hepatocytes. Furthermore, its intestinal absorption is affected by the pH and fatty acid composition of intraluminal bile [14].
In this study nanoparticles based on PLGA, used as reference material, and PHB have been prepared and processing conditions were varied in order to evaluate their influence on morphology, size, and drug encapsulation capabilities.
In the perspective of a practical application in the pharmaceutical field, the biocompatibility of PHB and relative nanoparticles were submitted to in vitro cytotoxicity assays and the development of a final pharmaceutical form was investigated. Freeze-drying trials in the presence of cryoprotector agents were performed.
2. Materials and Methods
2.1. Materials
Poly(DL-lactide-co-glycolide) (PLGA) (lactide : glycolide 65 : 35 Mw 40–75 KDa), Poly(3-hydroxybutyrate) PHB from microbial fermentation (Mw 350 KDa), Pluronic-F127 and Retinoic Acid (RA) were purchased from Sigma. Regenerated cellulose dialysis membranes (MWCO 10 KDa) were purchased from Spectrum Labs. Before use, membranes were washed with deionized water as indicated by the manufacturer and conditioned in the desired solvent for 1 hour. All solvents were used as received: dimethyl sulfoxide (DMSO) from Baker, trifluoroethanol 99% (TFE), and ethanol from Aldrich. However, 3% mannitol solution was prepared by dissolving 3.0 g of mannitol (Baker) in 100 mL of deionized water.
2.2. PLGA-Based Nanoparticles Prepared by Dialysis Method
The nanoparticle suspensions were prepared by dialysis method according to a general procedure. Data relevant to individual experiments are summarized in Table 1 whereas typical examples are described in details in what follows.
Table 1.
PLGA-based nanoparticle formulations prepared by means of dialysis method.
| Run | Polymer | Volume(b) | Surfcant | RA | Solvent |
|---|---|---|---|---|---|
| (mg/mL) | (L) | (mg/mL) | (mg/mL) | (% (a)DMSO) | |
| LG-1 | 2.5 | 3 | — | — | 100 |
| LG-2 | 2.5 | 2 | — | — | 100 |
| LG-3 | 2.5 | 1 | — | — | 100 |
| LG-4 | 1.4 | 1 | — | — | 100 |
| LG-5 | 1.0 | 1 | — | — | 100 |
| LG-6 | 1.4 | 1 | — | — | 70 |
| LG-7 | 1.4 | 1 | — | 0.7 | 70 |
| LG-8 | 1.5 | 1 | 0.4 | — | 85 |
| LG-9 | 1.5 | 1 | 0.5 | — | 84.5 |
| LG-10 | 1.5 | 1 | 0.5 | 0.7 | 84.5 |
| LG-11 | 1.5 | 1 | 0.7 | 0.7 | 84.5 |
| LG-12 | 1.5 | 1 | 0.4 | 0.6 | 85 |
| LG-13 | 1.6 | 1 | 0.4 | 0.6 | 81 |
(a)DMSO/H2O (% vol/vol).
(b)Volume of water in the outer compartment.
2.2.1. Preparation of Unloaded Nanoparticles
An amount of 10 mL of solution 1 mg/mL of PLGA in DMSO were prepared. The solution was introduced into a dialysis tube and dialysed against 1 L × 3 of distilled water for 3 hours and with distilled water replacement at intervals of 3 ~ 4 hours during 24 hours.
2.2.2. Preparation of RA-Loaded Nanoparticles
An amount of 15 mg of PLGA were dissolved in 7.5 mL of DMSO and subsequently 700 μL of a solution of RA in DMSO (100 mg/mL), 470 μL of a solution of Pluronic F-127 in water (10 mg/mL), and 1 mL of water were added drop wise to the above solution. The resulting solution was stirred at room temperature for 30 minutes and was then introduced into a dialysis tube and dialysed against 1 L × 3 of distilled water for 3 hours and the distilled water exchange at intervals of 3 ~ 4 hours during 24 hours.
Concentrations of polymer, drug, and surfactant reported in Table 1 are related to the volume of the resulting solution.
2.3. PHB-Based Nanoparticles Prepared by Dialysis Method
The nanoparticle suspensions were prepared by dialysis method according to a general procedure. Data relevant to individual experiments are summarized in Table 2 whereas typical examples are described in detail in what follows.
Table 2.
PHB-based nanoparticles formulations prepared by means of dialysis method.
| Run | Polymer | Surfcant(a) | RA | Solvent |
|---|---|---|---|---|
| (mg/mL) | (mg/mL) | (mg/mL) | (TFE % Vol/Vol)(b) | |
| HB-1 | 1 | — | — | 90 |
| HB-2 | 1 | — | 0.3 | 90 |
| HB-3 | 1 | — | 0.5 | 90 |
| HB-4 | 1 | 2 | 0.3 | 96 |
(a)Pluronic F-127.
(b)TFE/DMSO % vol/vol.
2.3.1. Preparation of Unloaded Nanoparticles
An amount of 10 mg of PHB were dissolved in 9 mL of TFE and 1 mL of DMSO was added. The resulting solution was dialyzed against 1 L × 3 of distilled water for 3 hours and then distilled water replacement at intervals of 3-4 hours during 24 hours.
2.3.2. Preparation of RA-Loaded Nanoparticles
An amount of 10 mg of PHB were dissolved in 9.6 mL of TFE. Also, 200 μL of a solution of pluronic in TFE (10 mg/mL) and 300 μL of RA in DMSO (10 mg/mL) were added, and the resulting solution was stirred at room temperature for 20 minutes, then dialyzed against 1 L × 3 of distilled water for 3 hours and then distilled water exchange at intervals of 3-4 hours during 24 hours.
2.4. Granulometry in Suspension
Dimensional analyses were carried out by means of a Coulter LS230 Laser Diffraction Particle Size Analyzer equipped with small volume module plus. Nanoparticle suspensions were added into the cell until 30–50% obscuration of PIDS detector was reached. Deionized water was used as background and diameter distribution was processed using the Fraunhofer optical model. Three runs were performed on each sample.
2.5. Morphological Analysis
Nanoparticle morphology was investigated by means of scanning electron microscopy (SEM) using a JEOL LSM5600LV scanning electron microscope. The nanoparticle samples were purified by centrifugation and the resulting pellets were resuspended in deionized water and lyophilised. Gold sputtering was performed before SEM analysis.
2.6. Microscopy
For routine culturing and qualitative evaluation of morphology, cells were analyzed under an inverted microscope, Nikon Eclipse TE2000-U.
2.7. Evaluation of RA Incapsulation
Samples of purified and lyophilised PLGA and PHB nanoparticles were weighted properly and added into 6 mL of ethanol and stirred for 1 hour. After the removal of supernatant by centrifugation, the remaining nanoparticles were dissolved in 2 mL of DCM and this solution was extracted with 6 mL of ethanol. When the extraction was completed DCM was evaporated and the resulting solution was filtered through a membrane filter with a pore size of 0.45 μm. The alcoholic solutions of RA were joined and the final volume was adjusted to 25 mL. RA concentration was determined by UV.
Total drug content was calculated as the sum of RA amounts both on the surface and inside the nanoparticles.
Drug concentration was determined by using a standard curve obtained by plotting the average blank-corrected absorbance of each standard versus its RA concentration (1–9 mg/L).
2.8. In Vitro Biological Evaluation
2.8.1. Materials
Cell-line 3T3/BALB-c Clone A31 mouse embryo fibroblasts (CCL163) was purchased from American Type Culture Collection (ATCC) and propagated as indicated by the supplier. Dulbecco's Modified Eagles Medium (DMEM), 0.01 M pH 7.4 phosphate buffer saline without Ca2+ and Mg2+ (PBS), bovine calf serum (BCS), glutamine, and antibiotics (penicillin/streptomycin) were purchased from GIBCO Brl. Cell proliferation reagent WST-1 and Lactate dehydrogenase cytotoxicity detection kit (LDH) were purchased from Roche Diagnostic. In vitro toxicology assay kit Neutral Red based was purchased from Sigma Chemicals. Cell line. Cytotoxicity evaluations of polymers and relative NPs were carried out using the 3T3/BALB-C Clone A31 cell-line. Cells were grown in DMEM containing 10% Bovine Calf Serum BCS, 4 mM glutamine and 100 U/mL : 100 μg/mL penicillin : streptomycin (complete DMEM).
2.8.2. Subculturing
A 25 mL flask containing exponentially growing 3T3 cells was observed under an inverted microscope for cell confluence. The complete DMEM media was then removed and cells were rinsed for a few minutes with PBS. The buffer solution was removed and cells were incubated with 0.5 mL of trypsin/EDTA solution at 37°C in 5% CO2 incubator for 5 minutes or until the monolayer started to detach from the flask. Cells were suspended in an appropriate volume of DMEM at a split ratio of 1 : 6 or 1 : 10 in a 75 mL flask.
2.8.3. Determination of IC50 of Soluble Polymers and NPs
For the determination of the IC50 (50% inhibitory concentration (the material concentration at which 50% of cell death in respect to the control is observed) of bioeliminable polymers and relative NPs, a subconfluent monolayer of 3T3 fibroblast was trypsinized using a 0.25% trypsin, 1 mM EDTA solution, centrifuged at 200 × g for 5 minutes, resuspended in growth medium, and counted. Appropriate dilution was made in order to obtain 3 × 103 cells per 100 μL of medium, the final volume present in each well of a 96 well plate. Cells were incubated at 37°C, 5% CO2 for 24 hours until 60–70% confluence was reached. The medium from each well was then removed and replaced with medium containing a different concentration of polymeric materials (1–10 mg/mL) or nanoparticles (5–15 mg/mL). Control cells were incubated with fresh growth medium and wells containing only growth medium were used as blanks. After 24 hours of incubation with medium containing the polymeric sample cells were analyzed for viability with Cell Proliferation Reagent WST-1 and Neutral Red Uptake. Data are expressed as mean ± standard deviation (SD). The data were compared using Student's t-test and differences were considered significant at P < .05.
2.8.4. Cell Proliferation Assays
Quantitative proliferation of cells exposed to polymers and NPs was evaluated by means of two different assays.
2.8.5. WST-1 Cell Proliferation Assay
Cells were incubated with WST-1 reagent diluted 1 : 10 (as indicated by the manufacturer) for 4 hours at 37°C, 5% CO2. Plates were then analyzed with a Biorad Microplate Reader. Measurements of formazan dye absorbance were carried out at 450 nm, with the reference wavelength at 620 nm.
2.8.6. Neutral Red Uptake
Cells were incubated with Neutral Red diluted 1 : 10 (as indicated by the manufacturer) for 2 hours at 37°C 5% CO2. Medium containing the vital dye was then removed and cells were briefly washed with PBS. The incorporated dye was then liberated from the cells in an acidified ethanol solution. An increase or decrease in the number of cells or their physiological state results in a concomitant change in the amount of dye incorporated by the cells in the culture. Plates were then analyzed with a Biorad Microplate Reader. Measurements of Neutral Red absorbance were carried out at 540 nm with the reference wavelength at 620 nm.
3. Results and Discussion
3.1. PLGA-Based Nanoparticles Prepared by Dialysis Method
PLGA nanoparticles were prepared by dialysis technique and some processing conditions were changed to observe their effect on particle size, morphology, and drug loading. In addition, the concentration of polymer and the volume of water against which the polymer solution was dialyzed were varied. The possibility to use binary solvent systems to dissolve polymer and drug was investigated. A surfactant was added to the formulation in order to improve colloidal suspensions stability and drug encapsulation efficiency.
3.1.1. Influence of the Water Volume in the Receiving Compartment
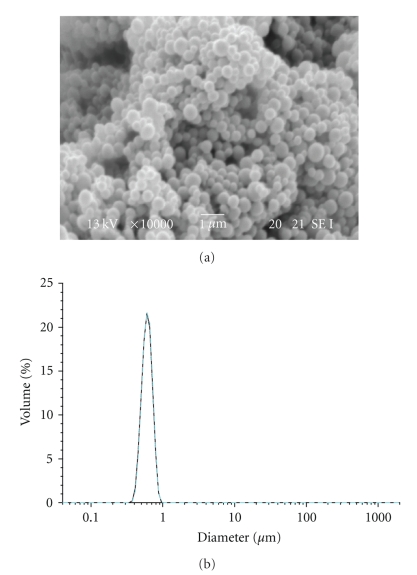
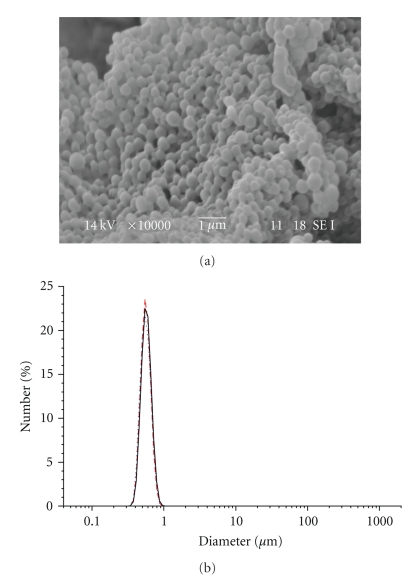
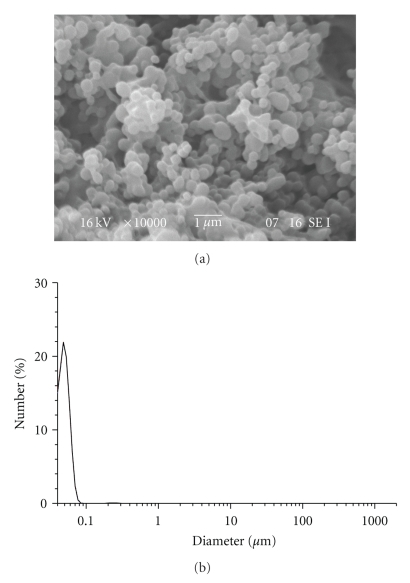
Dialysis procedure was performed for 24 hours in order to completely remove the organic solvent and to allow the formation of nanoparticles. The distilled water was exchanged at intervals of 3 ~ 4 hours. This procedure showed to be effective for the preparation of nanoparticles with spherical morphology and monomodal size distribution (Figure 1).
Figure 1.
SEM image and Particle size distribution of LG-1 NPs.
Water volume was varied from 3 L to 1 L in order to examine whether variation of osmotic pressure had any effect on nanoparticle size. The sizes of PLGA nanoparticles obtained by dialyzing the polymeric solution against 3, 2, and 1 L of distilled water are shown in Table 3.
Table 3.
Influence of water volume on particle size.
| Run | Polymer | Volume | Diameter size |
|---|---|---|---|
| (mg/mL) | (L) | (nm ± SD) | |
| LG-1 | 2.5 | 3 | 635 ± 102 |
| LG-2 | 2.5 | 2 | 634 ± 115 |
| LG-3 | 2.5 | 1 | 650 ± 105 |
Variation of the water volume did not affect particle diameter size and distribution. Based on these results, subsequent formulations were prepared by dialyzing the polymer organic solutions against 1 L of distilled water.
3.1.2. Effect of Polymer Concentration and Solvent Composition
The concentration of the polymer significantly affected the diameter size and distribution of the resulting nanoparticles. Nanoparticles were prepared from a solution of PLGA in DMSO with concentrations of 2.5, 1.4, and 1 mg/mL. A significant decrease in nanoparticle size was observed when reducing the polymer concentration (Table 4).
Table 4.
Influence of PLGA concentration and solvent composition on particle size.
| Run | Polymer | Volume(b) | Solvent | Diameter size | Diameter size |
|---|---|---|---|---|---|
| (mg/mL) | (L) | (% (a)DMSO) | (nm ± SD) | distribution | |
| LG-1 | 2.5 | 3 | 100 | 635 ± 102 | monomodal |
| LG-4 | 1.4 | 1 | 100 | 52 ± 13 | trimodal |
| LG-5 | 1.0 | 1 | 100 | 53 ± 11 | bimodal |
| LG-6 | 1.4 | 1 | 70 | 140 ± 20 | monomodal |
(a)% v/v DMSO/H2O.
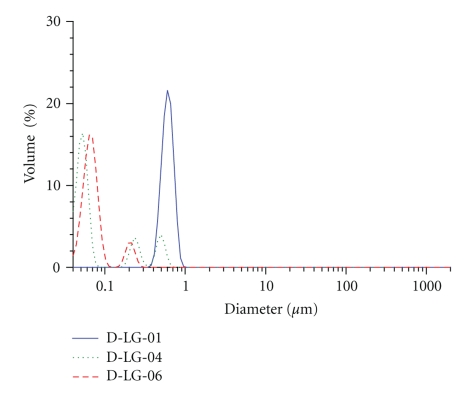
The homogeneity of the suspension was also affected. Bimodal and trimodal diameter size distribution was observed at lower polymer concentrations (Figure 2).
Figure 2.
Influence of PLGA concentration and solvent composition on particle size distribution.
Fessi et al. [15] reported that the origin of the mechanism of nanocapsule formation by nanoprecipitation could be explained in terms of interfacial turbulence or spontaneous agitation of the interface between two equilibrated liquid phases involving flow, diffusion, and surface processes, the so-called Maragoni effect. Although the mechanism is not fully understood at present, it is thought that the principle of PLGA nanoparticles formation by the dialysis procedure may be based on a mechanism similar to the Fessi et al. method, that is, by subsequent hydrophobic interactions among the polymer chains [6].
The precipitation of monodispersed colloids from homogeneous solution is a complex process. In a supersaturated solution nuclei rapidly generate by diffusive mechanism to form primary particles. These primary particles collide because of Brownian movements [16] and aggregate to form secondary particles [17]. The stability of the resulting particles depends on the balance of various attractive and repulsive forces acting between them. It is common to distinguish the two classes of colloids whose general behavior is entirely different. These classes are generally called lyophobic and lyophilic colloids; it is assumed that in the lyophilic colloids a strong affinity exists between the particles and the molecules of the dispersion medium, whereas in the lyophobic colloids the affinity is either weak or absent. The stability of lyophobic colloids is determined only by one factor (the electric charge of the particles), while two factors (charge and hydration) contribute to the stability of lyophilic colloids [16].
In the dialysis method, particles begin to form at the level of the inner surface of the dialysis membrane. A layer of supersaturated solution is originated by the flux of nonsolvent flowing into the dialysis tube which replaces the organic solvent. Reasonably at higher concentration of polymer, the concentration of primary particles that form at the level of this supersaturated layer is higher. Statistically, the number of collisions resulting in a permanent contact, which leads to the formation of secondary particles, is also higher and consequently secondary particles formed are characterized by larger diameters. Our results confirmed this hypothesis. Particles with bigger diameter were obtained at higher concentration of polymer but a polymodal distribution of diameters was observed when lower polymer concentrations were used.
PLGA was dissolved in the organic solvent and subsequently a percentage of nonsolvent (water) was added to the resulting solution. The resulting binary solvents system was dialyzed against water. The rationale behind this approach was that the presence of an amount of water in the organic solution would allow for better stabilization of the forming particles by hydration forces. Typically, the polar heads of the polymers chains are exposed onto the surface of the particles. This helps to avoid the formation of big secondary particles and aggregates, thus allowing nanoparticles with small diameter size to be obtained (Table 4, Figure 3).
Figure 3.
SEM image and particle size distribution of LG-6 NPs.
Nanoparticles with small size, monomodal distribution of diameter and fine spherical shape were obtained. They displayed a diameter size slightly higher than those prepared from a monosolvent solution of PLGA in DMSO at the same concentration. Such phenomena can be explained as the result of differences in solubility of the polymer, viscosity, or miscibility between the binary and monosolvent system [6].
3.1.3. Effect of Drug and Surfactant
Based on the previous results, the preparation of drug-loaded nanoparticles was carried out by adding RA dissolved in DMSO to a binary solvent solution of PLGA in DMSO and water.
The presence of the drug did not affect particle diameter size (Table 5) but large RA yellowish flakes formed during the dialysis process.
Table 5.
Effect of drug and surfactant on particle size and drug loading.
| Run | Polymer | Surfactant | RA | Diameter size | Drug loading |
|---|---|---|---|---|---|
| (mg/mL) | (mg/mL) | (mg/mL) | (nm ± SD) | (% w/w) | |
| LG-7 | 1.4 | — | 0.7 | 107 ± 39 | — |
| LG-8 | 1.5 | 0.4 | — | 531 ± 73 | — |
| LG-9 | 1.5 | 0.5 | — | — | — |
| LG-10 | 1.5 | 0.5 | 0.7 | — | — |
| LG-11 | 1.5 | 0.7 | 0.7 | — | — |
| LG-12 | 1.5 | 0.4 | 0.6 | 549 ± 170 | 1 |
| LG-13 | 1.6 | 0.4 | 0.6 | 508 ± 127 | 1.3 |
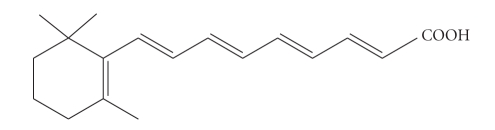
RA is a hydrophobic compound with one polar ionisable end group which confers amphiphilic character to the molecule (Figure 4).
Figure 4.
Structural formula of retinoic acid.
In nonpolar hydrocarbon solvents, RA self-assemble by forming tail-to-tail dimers which are stabilized by hydrogen bonding between the carboxyl groups of two RA molecules. On the contrary, in water RA self-assemble in micelle-like structures as driven by hydrophobic interactions among the rings of several molecules [18].
The loading process of hydrophobic drugs into the particles is thought to involve the hydrophobic interaction between the drug and the hydrophobic segment of the polymeric chains [6]. The micelles expose the polar heads of RA on the surface hampering the establishment of hydrophobic interactions with the polymer and reducing their encapsulation.
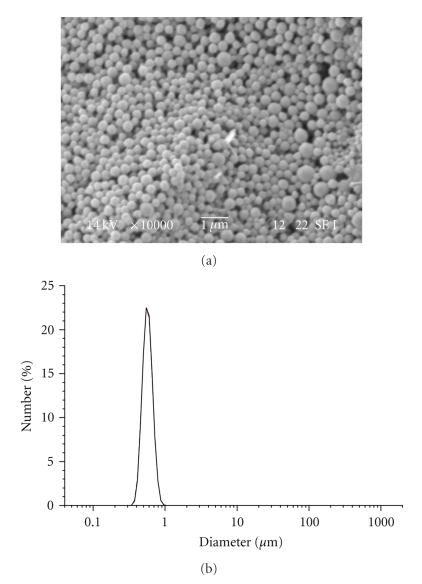
In order to reduce the formation of flakes and to increase the loading efficiency, a surfactant (Pluronic F-127) was added to the formulation. Pluronic: due to its amphiphilic character, could mediate the interaction between the polymer and the drug avoiding the formation of flakes and precipitates. The presence of the surfactant influenced the average diameter size of the nanoparticles but had only a slight effect on the formation of flakes which was only partially reduced. Particle size increased resulting in the range of 500–700 nm (Figure 5).
Figure 5.
SEM image and particle size distribution of LG-10 NPs.
PLGA, RA, and pluronic concentrations were varied in order to optimize the formulation and increase the loading efficiency. Particles with an average diameter size of 530 nm and a drug loading of 1.3% were obtained (Figure 6).
Figure 6.
SEM image and particle size distribution of LG-13 NPs.
Nanoparticles drug content resulted lower than expected. Attempts to increase the loading were carried out by dialysing the polymer, drug, and surfactant solution against ethanol instead of water during the first three hours of dialysis (LG-14). Ethanol acts as a nonsolvent for PLGA, allowing for the formation of nanoparticles and as a solvent for RA, avoiding the formation of flakes and precipitates. Nanoparticles with an average diameter size of 630 nm, with a very good spherical shape, and with a drug content of 2.2% were obtained (Figure 7).
Figure 7.
SEM image and particle size distribution of LG-14 NPs.
As expected, flakes and precipitates of RA did not form during the dialysis and the drug loading reached higher values, even though it was still low. In this case, the low drug loading could be due to the leakage of RA out of the dialysis tube during the dialysis step. Being soluble in ethanol and having a molecular weight much lower than that of the cut-off of the dialysis membrane (MWRA: 300.4 Da; MWCOmembrane: 10000 Da), it could have easily passed through the membrane's pores during the process. These experiments seem to confirm that the loading efficacy is the major drawback of the dialysis procedure [6].
3.2. PHB-Based Nanoparticles Prepared by Dialysis Method
PHB nanoparticles were prepared by dialysis method and some processing conditions were varied in observing their effect on nanoparticle characteristics such as particle size, morphology, and drug loading. More precisely, the influence of the concentration of drug and the addition of a surfactant in the formulation mixture was evaluated.
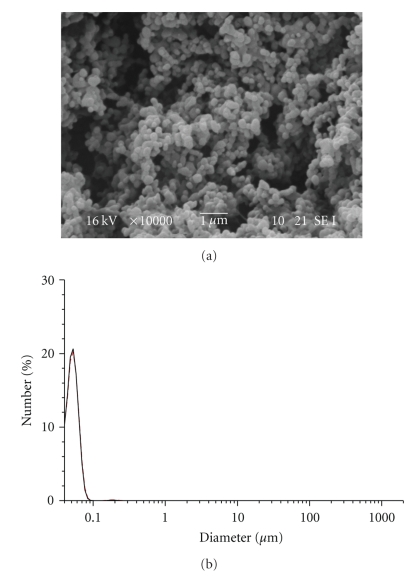
In the dialysis process, solvents to make nanoparticles are limited to water-miscible solvents. These solvents can dissolve both the polymer and the drug as water-immiscible solvents such as dichloromethane cannot diffuse out or evaporate from the dialysis membrane to the outer aqueous environment [6]. Chlorinated solvents such as dichloromethane are commonly used to dissolve poly(hydroxyalkanoates) but water miscible-solvents such as DMSO, acetone, THF, and DMF were found to be ineffective in solubilizing PHB. However, PHB showed good solubility in TFE at 50°C and it was, therefore, chosen as a solvent. On the contrary, RA displays solubility in polar solvents such as DMSO and acetone but it is poorly soluble in TFE. Based on the results obtained from the preparation of PLGA nanoparticles, it was decided to use a binary solvent system in the nanoparticle formulations. TFE and DMSO mixture was chosen which was able to dissolve both the polymer and the drug without compromising the basic concept of the dialysis process. PHB-unloaded nanoparticles with an average diameter size of 55 nm were successfully obtained (Table 6).
Table 6.
PHB nanoparticles prepared by dialysis method.
| Run | Surfactant | RA | Diameter size | Drug loading |
|---|---|---|---|---|
| (mg/mL) | (mg/mL) | (nm ± SD) | (% w/w) | |
| HB-1 | — | — | 53 ± 13 | — |
| HB-2 | — | 0.5 | 53 ± 16 | 0.94 |
| HB-3 | — | 0.3 | 50 ± 20 | — |
| HB-4 | 2 | 0.3 | 53 ± 17 | 1.04 |
b% v/v TFE/DMSO.
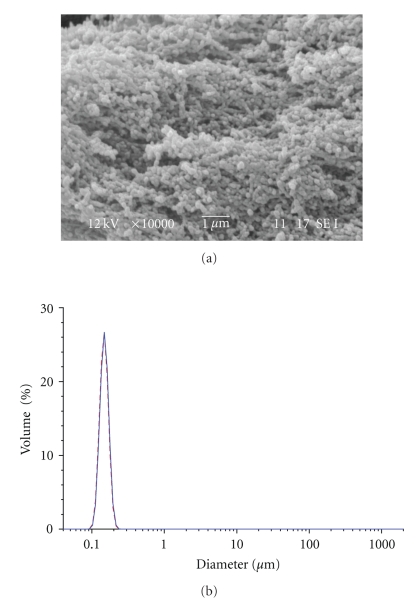
Nanoparticles showed a good spherical shape and very narrow size distribution of diameter (Figure 8).
Figure 8.
SEM image and particle size distribution of HB-1 NPs.
The small diameter of the PHB nanoparticles obtained is remarkable in comparison to those of PHB microspheres prepared by other techniques such as solvent evaporation.
3.2.1. Effect of Drug and Sufactant
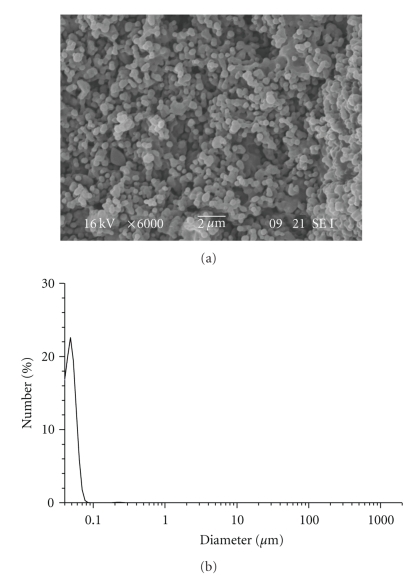
RA-loaded PHB nanoparticles were prepared by adding to the PHB solution in TFE the solution of retinoic acid in DMSO at various concentrations. The average diameter size of retinoic acid-loaded nanoparticles was 55 nm with a drug content of 0.94% (Figure 9).
Figure 9.
SEM image and particle size distribution of HB-2 NPs.
The addition of RA did not affect the diameter of the resulting nanoparticles. Flakes of retinoic acid formed during the dialysis process as it occurred during the preparation of PLGA nanoparticles.
In order to reduce the formation of flakes and precipitates and to increase the drug content a surfactant, pluronic was added to the formulation. Particles with an average size diameter of 55 nm were obtained with a drug content of 1.05% (HB-4). In this case, the surfactant did not affect the diameter size of the nanoparticles and did not improve the RA encapsulation (Table 6, Figure 10).
Figure 10.
SEM image and particle size distribution of HB-4 NPs.
3.3. In Vitro Citotoxicity Studies
In order to evaluate the biocompatibility of polymers and their resulting nanoparticles, in vitro experiments were carried out by using the balb 3T3 mouse embryo fibroblasts clone A31 cell line, following the procedures of ISO 10993—Part 5: “Test for cytotoxicity—in vitro methods.”
Cytotoxicity, defined as the “in vitro evaluation of toxicological risks using cell culture” is a rapid, standardized, sensitive, and inexpensive way to assess the in vitro biocompatibility of materials to be used in biomedical applications. Assays deal with the assessment of various aspects of cellular function such as cell viability and proliferation, loss of membrane integrity, reduced cell adhesion, biosynthetic activity, and altered cell morphology. Moreover, the information gained from these types of investigations may be used in the design of further in vivo experiments [19].
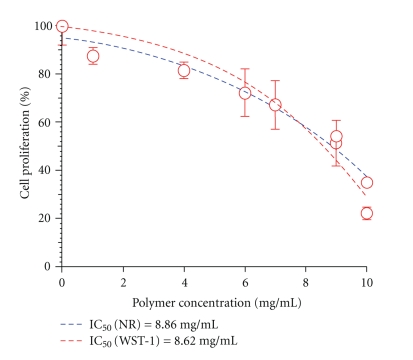
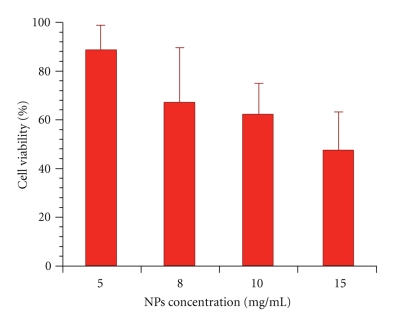
PHB and relative nanoparticles were suspended in Dulbecco's Modified Eagles Medium since they are not water soluble at different concentrations ranging between 1 and 10 mg/mL. Cells were incubated for 24 hours with polymer and nanoparticle suspensions and then tested for cell viability and proliferation using WST-1 tetrazolium salt and Neutral Red Uptake. The obtained results predominantly indicate a high level of cytocompatibility of the investigated materials. In vitro cytotoxicity of PHB resulted fairly low as highlighted by the high IC50 values obtained both with tetrazolium salts and neutral red assays (Figure 11). PHB-based nanoparticles prepared by dialysis method also exhibited very good cytocompatibility and can be considered fully biocompatible (Figure 12).
Figure 11.
In Vitro PHB Citotoxicity.
Figure 12.
In Vitro PHB Nanoparticles Citotoxicity.
4. Conclusions
Dialysis methods were investigated for the preparation of PLGA and PHB nanoparticles encapsulating retinoic acid. Some processing conditions were varied in order to observe their effect on nanoparticle characteristics. The utilization of binary solvent solution composed by a mix of polymer and drug solvent and nonsolvent proved to be effective for preparing nanoparticles with small size and a narrow distribution of diameters. The small diameter of PHB nanoparticles prepared by this method was quite exceptional when compared with those of PHB microsphere prepared by other techniques commonly used such as solvent evaporation, even though they were characterized by low drug loading efficiency.
The acquired results and relevant discussion promote a better understanding of the suitability and versatility of the method applied for the preparation nanodrug delivery systems.
A careful in vitro investigation of the cytotoxicity of PHB and the resulting nanoparticles show their high cytocompatibilities, thus indicating the suitability of the prepared nanosystems as carriers for controlled release of drugs and eventually active agents.
Drug release studies of retinoic acid, carried out by an original method that might better reflect bioavailability data, will constitute the subject of a forthcoming paper.
Acknowledgments
The present work was performed within the framework of PRIN 2006 project, Bioactive Polymeric Materials for Applications in Tissue Engineering & Regenerative Medicine, Prot. 2006038548, cofunded by MIUR, is gratefully acknowledged.
References
- 1.Maia JL, Santana MHA, Ré MI. The effect of some processing conditions on the characteristics of biodegradable microspheres obtained by an emulsion solvent evaporation process. Brazilian Journal of Chemical Engineering. 2004;21(1):1–12. [Google Scholar]
- 2.Park J, Ye M, Park K. Biodegradable polymers for microencapsulation of drugs. Molecules. 2005;10(1):146–161. doi: 10.3390/10010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim GJ, Bang KH, Kim YB, Rhee YH. Preparation and characterization of native poly(3-hydroxybutyrate) microspheres from Ralstonia eutropha. Biotechnology Letters. 2000;22(18):1487–1492. [Google Scholar]
- 4.Pouton CW, Akhtar S. Biosynthetic polyhydroxyalkanoates and their potential in drug delivery. Advanced Drug Delivery Reviews. 1996;18(2):133–162. [Google Scholar]
- 5.Jeon H-J, Jeong Y-I, Jang M-K, Park Y-H, Nah J-W. Effect of solvent on the preparation of surfactant-free poly(DL-lactide-co-glycolide) nanoparticles and norfloxacin release characteristics. International Journal of Pharmaceutics. 2000;207(1-2):99–108. doi: 10.1016/s0378-5173(00)00537-8. [DOI] [PubMed] [Google Scholar]
- 6.Jeong Y-I, Cho C-S, Kim S-H, et al. Preparation of poly(DL-lactide-co-glycolide) nanoparticles without surfactant. Journal of Applied Polymer Science. 2001;80(12):2228–2236. [Google Scholar]
- 7.Armstrong JL, Redfern CPF, Veal GJ. 13-cis retinoic acid and isomerisation in paediatric oncology—is changing shape the key to success? Biochemical Pharmacology. 2005;69(9):1299–1306. doi: 10.1016/j.bcp.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Noy N. Retinoid-binding proteins: mediators of retinoid action. Biochemical Journal. 2000;348(3):481–495. [PMC free article] [PubMed] [Google Scholar]
- 9.Maiti TK, Ghosh KS, Debnath J, Dasgupta S. Binding of all-trans retinoic acid to human serum albumin: fluorescence, FT-IR and circular dichroism studies. International Journal of Biological Macromolecules. 2006;38(3–5):197–202. doi: 10.1016/j.ijbiomac.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 10.Zuccari G, Carosio R, Fini A, Montaldo PG, Orienti I. Modified polyvinylalcohol for encapsulation of all-trans-retinoic acid in polymeric micelles. Journal of Controlled Release. 2005;103(2):369–380. doi: 10.1016/j.jconrel.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 11.Newman KD, McBurney MW. Poly(D,L lactic-co-glycolic acid) microspheres as biodegradable microcarriers for pluripotent stem cells. Biomaterials. 2004;25(26):5763–5771. doi: 10.1016/j.biomaterials.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 12.Szuts EZ, Harosi FI. Solubility of retinoids in water. Archives of Biochemistry and Biophysics. 1991;287(2):297–304. doi: 10.1016/0003-9861(91)90482-x. [DOI] [PubMed] [Google Scholar]
- 13.Jeong Y-I, Kang M-K, Sun H-S, et al. All-trans-retinoic acid release from core-shell type nanoparticles of poly(ε-caprolactone)/poly(ethylene glycol) diblock copolymer. International Journal of Pharmaceutics. 2004;273(1-2):95–107. doi: 10.1016/j.ijpharm.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 14.Ozpolat B, Lopez-Berestein G, Adamson P, Fu CHJ, Williams AT. Pharmacokinetics of intravenously administered liposomal all-trans-retinoic acid (ATRA) and orally administered ATRA in healthy volunteers. Journal of Pharmacy & Pharmaceutical Sciences. 2003;6(2):292–301. [PubMed] [Google Scholar]
- 15.Bilati U, Allémann E, Doelker E. Development of a nanoprecipitation method intended for the entrapment of hydrophilic drugs into nanoparticles. European Journal of Pharmaceutical Sciences. 2005;24(1):67–75. doi: 10.1016/j.ejps.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 16.Molina-Bolívar JA, Ortega-Vinuesa JL. How proteins stabilize colloidal particles by means of hydration forces. Langmuir. 1999;15(8):2644–2653. [Google Scholar]
- 17.Privman V, Goia DV, Park J, Matijević E. Mechanism of formation of monodispersed colloids by aggregation of nanosize precursors. Journal of Colloid and Interface Science. 1999;213(1):36–45. doi: 10.1006/jcis.1999.6106. [DOI] [PubMed] [Google Scholar]
- 18.Noy N. The ionization behavior of retinoic acid in aqueous environments and bound to serum albumin. Biochimica et Biophysica Acta. 1992;1106(1):151–158. doi: 10.1016/0005-2736(92)90233-c. [DOI] [PubMed] [Google Scholar]
- 19.Chiellini F, Bartoli C, Dinucci D, Piras AM, Anderson R, Croucher T. Bioeliminable polymeric nanoparticles for proteic drug delivery. International Journal of Pharmaceutics. 2007;343(1-2):90–97. doi: 10.1016/j.ijpharm.2007.05.012. [DOI] [PubMed] [Google Scholar]