Although there are some exceptions1,2, numerous publications support the counter-regulatory roles of the angiotensin II (Ang II) type-2 receptor (AT2) against the AT1 receptor functions, such as inhibition of vascular contraction and hypertrophy3–5. However, it is still very uncertain as to how the AT2 receptor signals interfere with those of the AT1 receptor in the cardiovascular system5,6. Past findings suggest that the signal transduction of AT1 inhibition by the AT2 receptor may involve multiple distinct mechanisms. Some of these mechanisms appear to be indirect, such as production of nitric oxide through bradykinin opposing the vasoconstrictor actions of the AT1 receptor3. The direct inhibitory cross-talk of the two receptors occurs proximal to the receptor hetero-dimerization, as well as downstream from the receptors between AT1-activated protein kinases, epidermal growth factor (EGF) receptor kinase and extracellular signal-regulated kinase (ERK1/2)/p42/44 mitogen activated protein kinase (MAPK), etc, and AT2-activated protein phosphatases, protein phosphatese 2A (PP2A), SHP-1, and MAPK phosphatase-1 (MKP-1)7,8. The activation of the protein phosphatases by the AT2 receptor may or may not require hetero-trimeric G proteins (Gi or Gs) and/or the recently identified AT2 receptor C-terminal tail interacting proteins4–6.
Given that induction of hypertrophy of vascular smooth muscle cells (VSMCs) via the AT1 receptor appears to require a “triple-membrane-passing signal” involving a metalloprotease-dependent EGF receptor transactivation9,10, the article by Guilluy et al in this issue of Circ Res11 may not be so surprising, as it suggests the requirement of rather “twisty” three sequential phosphorylation/dephosphorylation events between a phosphatase, SHP-1, and two protein kinases for RhoA inhibition by the AT2 receptor (see Figure 7 in the article). Although negative regulation of RhoA through its Ser188 phosphorylation by the AT2 receptor has been demonstrated12,13, the two kinases—caseine kinase II (CK2) and Ste20-related kinase SLK—are novel downstream elements of the AT2 receptor.
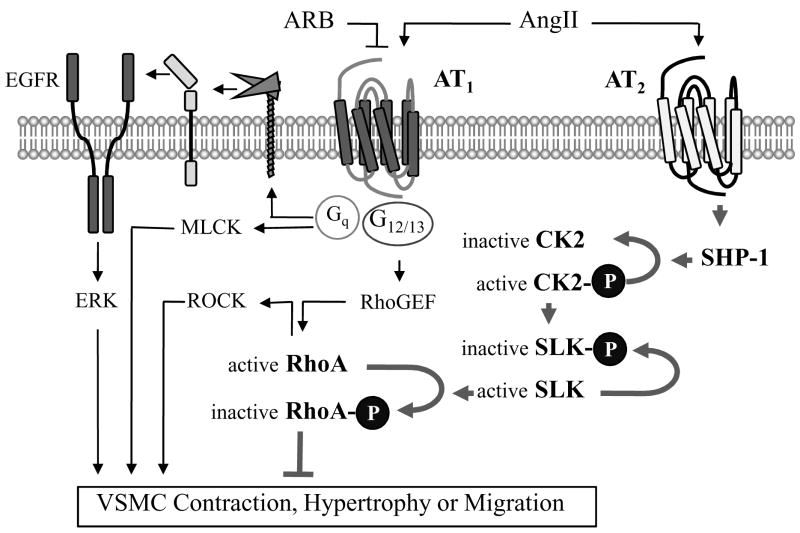
By using multiple distinct molecular approaches, a novel signal transduction cascade for inhibition of RhoA via the AT2 receptor, which is expected to counter-regulate RhoA activation by the AT1 receptor in VSMCs, becomes apparent11 (Figure). Rho-kinase (ROCK), the best-characterized effector of the small G protein RhoA, contributes to vascular contraction via Ca2+ sensitization. Moreover, the Rho/ROCK pathway has been implicated in a wide variety of cardiovascular pathogenic conditions including hypertension, atherosclerosis and cardiovascular hypertrophy14–17. It should be noted that both heterotrimeric G protein-dependent and -independent signal transductions have been proposed to mediate AT1 receptor function4,18,19. In addition to the production of reactive oxygen species4 and enhanced VSMC contraction, hypertrophy, as well as migration induced by the AT1 receptor, seem to require at least two parallel signal transduction cascades mediated through Gq and G12/13. The latter is primarily implicated in the Rho/ROCK cascade activation via RGS (regulator of G protein signaling)-domain containing Rho guanine nucleotide exchange factors (RhoGEFs)20–23. Inhibition of either cascade appears to block those pathogenic functions induced by the AT1 receptor20–23, and the study by Guilluy et al has further demonstrated that the RhoA inhibition mechanism via the AT2 receptor in VSMCs results in vasodilation11. The findings also indicate a strong support of this potential “triple twist” RhoA inhibition theory to explain the multiple tissue protective effects of AT1 receptor blockers beyond the expected AT1 inhibition, since the AT2 receptors could be strongly stimulated under these treatments.
Figure.
Novel signal transduction cross-talk between AT1 and AT2 in VSMCs. The Rho/Rhokinase cascade inhibition by the AT2 receptor via the “triple-twist” theory involving SHP-1, CK2 and SLK not only inhibits AT1-induced vascular contraction, but also likely inhibits VSMC hypertrophy and migration. In addition, CK2 may be activated through the AT1 receptor, and AT2-activated SHP-1 has been shown to directly inactivate EGFR to inhibit AT1-activated hypertrophic signal transduction.
In addition, identification of the novel key components of the AT2 signal transduction will aid in exploring the molecular insight regarding the dynamic regulation of cardiovascular remodeling via the AT1 versus AT2, which likely involves far more additional cross-talk. Both cyclic AMP and cyclic GMP dependent kinases have been shown to phosphorylate RhoA at Ser18824, which in part explains the vasodilatory properties of these kinases in VSMCs. The study by Guilluy et al has identified SLK as a novel RhoA Ser188 kinase11. Interestingly, SLK has been shown to be able to activate apoptosis signal-regulated kinase-1 (ASK1) and p38 MAPK, leading to cell apoptosis25. This fits well with the past findings that AT2 mediates apoptosis in VSMCs via p38 MAPK activation26. Regarding CK2, activation of CK2 has been recently shown to mediate p27 degradation by Ang II likely through the AT1 receptor, which leads to cardiac hypertrophy27. The p27 degradation has also been implicated in vascular hyperplasia28. Therefore, inhibition of CK2 activity by the AT2 receptor may also antagonize the AT1 mediated detrimental effects through stabilization of p27 in addition to the RhoA inhibition.
Since the findings by Guilluy et al were mostly limited to VSMC culture and a ex vivo desendothelialized contraction assay using rat thoracic aorta rings, the relevance of this novel AT2 signal transduction in mediating the beneficial AT2 function in cardiovascular diseases remains unclear. Further expansion of the research in this field by using animal models of cardiovascular diseases has strong potential for future translation of the outcomes, which may lead to prevention of cardiovascular diseases linked to enhanced AT1 receptor signal transduction.
Acknowledgments
I thank Dr Gerald D. Frank for his critical reading of this comment.
Sources of Funding
Some of the work by the author referenced in this editorial was funded by National Institute of Health Grant HL076770, by American Heart Association Established Investigator Award 0740042N, and by W. W. Smith Charitable Trust Grant, H0605.
Footnotes
Disclosures
None.
References
- 1.Inagami T, Senbonmatsu T. Dual effects of angiotensin II type 2 receptor on cardiovascular hypertrophy. Trends Cardiovasc Med. 2001;11:324–328. doi: 10.1016/s1050-1738(01)00136-0. [DOI] [PubMed] [Google Scholar]
- 2.D’Amore A, Black MJ, Thomas WG. The angiotensin II type 2 receptor causes constitutive growth of cardiomyocytes and does not antagonize angiotensin II type 1 receptor-mediated hypertrophy. Hypertension. 2005;46:1347–1354. doi: 10.1161/01.HYP.0000193504.51489.cf. [DOI] [PubMed] [Google Scholar]
- 3.Carey RM. Cardiovascular and renal regulation by the angiotensin type 2 receptor: the AT2 receptor comes of age. Hypertension. 2005;45:840–844. doi: 10.1161/01.HYP.0000159192.93968.8f. [DOI] [PubMed] [Google Scholar]
- 4.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 5.Mogi M, Iwai M, Horiuchi M. Emerging concepts of regulation of angiotensin II receptors: new players and targets for traditional receptors. Arterioscler Thromb Vasc Biol. 2007;27:2532–2539. doi: 10.1161/ATVBAHA.107.144154. [DOI] [PubMed] [Google Scholar]
- 6.Berk BC. Angiotensin type 2 receptor (AT2R): a challenging twin. Sci STKE. 2003;2003:PE16. doi: 10.1126/stke.2003.181.pe16. [DOI] [PubMed] [Google Scholar]
- 7.AbdAlla S, Lother H, Abdel-tawab AM, Quitterer U. The angiotensin II AT2 receptor is an AT1 receptor antagonist. J Biol Chem. 2001;276:39721–39726. doi: 10.1074/jbc.M105253200. [DOI] [PubMed] [Google Scholar]
- 8.Shibasaki Y, Matsubara H, Nozawa Y, Mori Y, Masaki H, Kosaki A, Tsutsumi Y, Uchiyama Y, Fujiyama S, Nose A, Iba O, Tateishi E, Hasegawa T, Horiuchi M, Nahmias C, Iwasaka T. Angiotensin II type 2 receptor inhibits epidermal growth factor receptor transactivation by increasing association of SHP-1 tyrosine phosphatase. Hypertension. 2001;38:367–372. doi: 10.1161/01.hyp.38.3.367. [DOI] [PubMed] [Google Scholar]
- 9.Ohtsu H, Dempsey PJ, Frank GD, Brailoiu E, Higuchi S, Suzuki H, Nakashima H, Eguchi K, Eguchi S. ADAM17 mediates epidermal growth factor receptor transactivation and vascular smooth muscle cell hypertrophy induced by angiotensin II. Arterioscler Thromb Vasc Biol. 2006;26:e133–e137. doi: 10.1161/01.ATV.0000236203.90331.d0. [DOI] [PubMed] [Google Scholar]
- 10.Ohtsu H, Suzuki H, Nakashima H, Dhobale S, Frank GD, Motley ED, Eguchi S. Angiotensin II signal transduction through small GTP-binding proteins: mechanism and significance in vascular smooth muscle cells. Hypertension. 2006;48:534–540. doi: 10.1161/01.HYP.0000237975.90870.eb. [DOI] [PubMed] [Google Scholar]
- 11.Guilluy C, Rolli-Derinderen M, Loufrani L, Bourge A, Henrion D, Sabourin L, Loirand G, Pacaud P. Ste20-related kinase SLK phosphorylates Ser 188 of RhoA to nduce vasodilation in response to angiotensin II type 2 receptor activation. Circ Res. 2008;102:XXX–XXX. doi: 10.1161/CIRCRESAHA.107.164764. [DOI] [PubMed] [Google Scholar]
- 12.Savoia C, Tabet F, Yao G, Schiffrin EL, Touyz RM. Negative regulation of RhoA/Rho kinase by angiotensin II type 2 receptor in vascular smooth muscle cells: role in angiotensin II-induced vasodilation in stroke-prone spontaneously hypertensive rats. J Hypertens. 2005;23:1037–1045. doi: 10.1097/01.hjh.0000166845.49850.39. [DOI] [PubMed] [Google Scholar]
- 13.Andresen BT, Shome K, Jackson EK, Romero GG. AT2 receptors cross talk with AT1 receptors through a nitric oxide- and RhoA-dependent mechanism resulting in decreased phospholipase D activity. Am J Physiol Renal Physiol. 2005;288:F763–770. doi: 10.1152/ajprenal.00323.2004. [DOI] [PubMed] [Google Scholar]
- 14.Noma K, Oyama N, Liao JK. Physiological role of ROCKs in the cardiovascular system. Am J Physiol Cell Physiol. 2006;290:C661–668. doi: 10.1152/ajpcell.00459.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loirand G, Guerin P, Pacaud P. Rho kinases in cardiovascular physiology and pathophysiology. Circ Res. 2006;98:322–334. doi: 10.1161/01.RES.0000201960.04223.3c. [DOI] [PubMed] [Google Scholar]
- 16.Shirai H, Autieri M, Eguchi S. Small GTP-binding proteins and mitogen-activated protein kinases as promising therapeutic targets of vascular remodeling. Curr Opin Nephrol Hypertens. 2007;16:111–115. doi: 10.1097/MNH.0b013e3280148e4f. [DOI] [PubMed] [Google Scholar]
- 17.Shimokawa H, Rashid M. Development of Rho-kinase inhibitors for cardiovascular medicine. Trends Pharmacol Sci. 2007;28:296–302. doi: 10.1016/j.tips.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Hunyady L, Catt KJ. Pleiotropic AT1 receptor signaling pathways mediating physiological and pathogenic actions of angiotensin II. Mol Endocrinol. 2006;20:953–970. doi: 10.1210/me.2004-0536. [DOI] [PubMed] [Google Scholar]
- 19.Oro C, Qian H, Thomas WG. Type 1 angiotensin receptor pharmacology: signaling beyond G proteins. Pharmacol Ther. 2007;113:210–226. doi: 10.1016/j.pharmthera.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohtsu H, Mifune M, Frank GD, Saito S, Inagami T, Kim-Mitsuyama S, Takuwa Y, Sasaki T, Rothstein JD, Suzuki H, Nakashima H, Woolfolk EA, Motley ED, Eguchi S. Signal-crosstalk between Rho/ROCK and c-Jun NH2-terminal kinase mediates migration of vascular smooth muscle cells stimulated by angiotensin II. Arterioscler Thromb Vasc Biol. 2005;25:1831–1836. doi: 10.1161/01.ATV.0000175749.41799.9b. [DOI] [PubMed] [Google Scholar]
- 21.Harris DM, Cohn HI, Pesant S, Zhou RH, Eckhart AD. Vascular smooth muscle G(q) signaling is involved in high blood pressure in both induced renal and genetic vascular smooth muscle-derived models of hypertension. Am J Physiol Heart Circ Physiol. 2007;293:H3072–3079. doi: 10.1152/ajpheart.00880.2007. [DOI] [PubMed] [Google Scholar]
- 22.Wirth A, Benyo Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, Orsy P, Horvath B, Maser-Gluth C, Greiner E, Lemmer B, Schutz G, Gutkind JS, Offermanns S. G12-G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med. 2008;14:64–68. doi: 10.1038/nm1666. [DOI] [PubMed] [Google Scholar]
- 23.Ohtsu H, Higuchi S, Shirai H, Eguchi K, Suzuki H, Hinoki A, Brailoiu E, Eckhart AD, Frank GD, Eguchi S. Central role of Gq in the hypertrophic signal transduction of angiotensin II in vascular smooth muscle cells. Endocrinology. 2008 doi: 10.1210/en.2007-1694. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ellerbroek SM, Wennerberg K, Burridge K. Serine phosphorylation negatively regulates RhoA in vivo. J Biol Chem. 2003;278:19023–19031. doi: 10.1074/jbc.M213066200. [DOI] [PubMed] [Google Scholar]
- 25.Hao W, Takano T, Guillemette J, Papillon J, Ren G, Cybulsky AV. Induction of apoptosis by the Ste20-like kinase SLK, a germinal center kinase that activates apoptosis signal-regulating kinase and p38. J Biol Chem. 2006;281:3075–3084. doi: 10.1074/jbc.M511744200. [DOI] [PubMed] [Google Scholar]
- 26.Miura S, Karnik SS. Ligand-independent signals from angiotensin II type 2 receptor induce apoptosis. EMBO J. 2000;19:4026–4035. doi: 10.1093/emboj/19.15.4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hauck L, Harms C, Rohne J, Gertz K, Dietz R, Endres M, von Harsdorf R. Protein kinase CK2 links extracellular growth factor signaling with the control of p27(Kip1) stability in the heart. Nat Med. 2008;14:315–324. doi: 10.1038/nm1729. [DOI] [PubMed] [Google Scholar]
- 28.Diez-Juan A, Castro C, Edo MD, Andres V. Role of the growth suppressor p27Kip1 during vascular remodeling. Curr Vasc Pharmacol. 2003;1:99–106. doi: 10.2174/1570161033386709. [DOI] [PubMed] [Google Scholar]