Abstract
The discovery of new fluorescent proteins (FPs) that emit in the far-red part of the spectrum, where light absorption from tissue is significantly lower than in the visible, offers the possibility for non-invasive biological interrogation at the entire organ or small animal level in vivo. The performance of FPs in deep-tissue imaging depends not only on their optical characteristics, but also on the wavelength-dependent tissue absorption and the depth of the fluorescence activity. In order to determine the optimal choice of FP and illumination wavelength we compared the performance of five of the most promising FPs, i.e. tdTomato, mCherry, mRaspberry, mPlum, and Katushka. We experimentally measured the signal strength through mice and employed theoretical predictions to obtain an understanding on the performance of different illumination scenarios, especially as it pertains to tomographic imaging. It was found that the appropriate combination of red-shifted proteins and illumination wavelengths can improve detection sensitivity in small animals by at least 2 orders of magnitude compared to Green FP (GFP). It is also shown that the steep attenuation change of the hemoglobin spectrum around the 600nm range may significantly affect the detection sensitivity and necessitates the careful selection of illumination wavelengths for optimal imaging performance.
Keywords: Fluorescent proteins, optical tomography, whole body imaging
1. Introduction
The introduction of transgenes that express fluorescent proteins (FPs) into cells has revolutionized the ability to visualize many cellular and sub-cellular processes that are otherwise invisible with traditional means of optical imaging.1 FPs have therefore become an important tool in biomedical research and their utilization spans many application areas, including the assessment of cell bio-distribution and cell mobility, the study of protein activity and protein interactions in vivo or the dynamic visualization of transcription. Similarly, many application fields benefit from this technology, including cancer research,2,3 immunology,4 stem cell research,5 and others.
Ex vivo and intravital microscopy of FPs at the cellular level has led to unparalleled biological insights.6,7 Microscopic fluorescence imaging is generally performed on excised thin tissue slices, or in vivo at penetration depths typically of the order of 200–500 µm. For imaging deeper seated FP activity at the organ and whole animal level, macroscopic imaging has been considered2 to study for example cancer metastasis,8,9 angiogenesis,10 and monitoring cancer progression and treatment efficiency.11 In these studies, imaging is performed using a CCD camera in epi-illumination mode, i.e. by capturing fluorescence images from the animal side that is illuminated by the excitation light. Epi-illumination imaging can image deeper than microscopy, reaching depths of a few millimeters, but the images obtained are of significantly reduced resolution compared to that of microscopy due to tissue scattering. In addition, signals that come from deep-seated regions are highly attenuated compared to signals from more superficial activity and skin auto-fluorescence. This reduces the contrast achieved in epi-illumination mode, when imaging activity that seats deeper than a few millimeters, which makes whole body animal imaging challenging.
An alternative imaging geometry is trans-illumination, which illuminates the object of interest using one or multiple sources on one side and collects the emitted fluorescence from the opposite side. The signal attenuation is better balanced between deep and superficially seated fluorescence activity, since the combined excitation and emission light pathways are similar for all depths sampled. Trans-illumination data have been used in planar or three-dimensional tomographic imaging for imaging deep-seated fluorescence bio-distribution with increased sensitivity and resolution compared to epi-illumination imaging in phantoms and in vivo 12–14. Imaging of fluorescence proteins have been similarly showcased 13,15. However, conventional fluorescent proteins emit in the visible, where light absorption by tissue is strong. Therefore sensitive detection through entire animals has been challenging when imaging for example variants of the Green or Red fluorescent proteins at spectral windows in the 400–600 nm range.
The recent evolution of red-shifted FP’s16–18 that emit beyond the 600 nm barrier, where light absorption by tissue is considerably reduced, can enable sensitive in vivo detection of fluorescence proteins through entire animals using three-dimensional tomographic methods, in analogy to fluorescence tomography in the near infrared (NIR) 19,20. The performance of these proteins in whole body imaging, depends not only on the FP brightness, expression efficiency and toxicity but also on the particular attenuation of each protein by the wavelength-dependent tissue absorption. Since tissue absorption and scattering result in a non-linear attenuation of photon signal as a function of depth and optical properties, previous observations of FP expressing cells implanted at a shallow depth21 do not reflect the overall performance of each of the proteins when considering whole-body imaging.
In this paper we examine therefore the performance characteristics of promising new red-shifted FPs for whole-body imaging (i.e. tdTomato, mCherry, mRaspberry, mPlum, Katushka) and contrast these findings to imaging eGFP. These FP’s were selected to include fluorescent proteins of high brightness (i.e. tdTomato) or near-infrared-shifted emission spectra (i.e. mCherry, mRaspberry, mPlum, Katushka). Experimental measurements from cells expressing tdTomato and mCherry were translated with appropriate theoretical models to obtain performance predictions for whole body imaging. Overall red-shifted fluorescent proteins were found to improve detection sensitivity in whole body imaging applications by at least two orders of magnitude over GFP. In addition, the steep drop of the absorption coefficient of tissue at the ~590–630nm spectral region makes the performance sensitive to the selection of illumination parameters. Overall it is showcased that FPT of red-shifted FP’s can lead to an important biomedical imaging approach for the non-invasive assessment of cellular and sub-cellular function in vivo.
2. Methods
2.1 Cell lines, sample preparation, and surgical procedures
Lentivirus vectors bearing GFP (Clontech), mCherry or td-Tomato (from Dr. Roger Tsien, UCSD, CA) in front of the CMV promoter were constructed based on the lentivirus cloning vector CSCGW 22 thus resulting in LV-GFP, LV-mCherry and LV-tdTomato. Lentivirus vector preparation was previously described in Ref. 22. In brief, 293T human embryonic kidney fibroblasts cells were co-transfected with either the LV-GFP, the LV-mCherry, or the LV-td-tomato plasmids, with the lentivirus packaging genome CMVRΔ8.91 (from Dr. Didier Trono, Univ. Geneva, Switzerland) and with the envelope coding plasmid (pVSV-G; provided by Dr. Miguel Sena-Esteves, MGH). After 72 hrs, lentivirus vector supernatant was harvested, concentrated by ultracentrifugation and tittered as transducing units (t.u.)/ml on 293T cells in the presence of 10 µg/mL polybrene (Sigma) by counting the fluorescent positive cells 48 h post-infection. Human glioma cells transduced with the LV-GFP or LV-mCheery or LV-TdTomato were sorted by the FacsCalibur Cell sorter (BD Biosciences). The corresponding wild-type (WT) cells were also employed for control measurements.
To experimentally measure the relative intensity imparted by the different FPs examined, we prepared small volumes of the cells placed inside thin transparent glass capillary tubes of 0.9 mm inner diameter. The cells were suspended in PBS (phosphate buffer solution) and were slightly centrifuged to create a pellet of virtually constant cell density at the bottom of each tube. This procedure resulted in approximately 1 million of glioma-36 cells occupying 3 µL, and correspondingly 0.5 million U87 cells in 3 µL, the U87 cells being slightly bigger in size .
Human glioma cells expressing GFP and tdTomato were harvested by trypsinization and stereotactically implanted into the right frontal lobe of nude (nu/nu) athymic mice (500,000 cells/mouse, in 4µL PBS,) (from bregma, AP: −2 mm, ML: 1.5 mm V (from dura): 2 mm). The study was performed according to the procedures approved by the Massachusetts General Hospital.
2.2 Imaging system
The experimental setup used for epi-llumination and for trans-illumination imaging is similar to the one described in Refs. 12,14 The object (mouse) is placed in a water-tight chamber slightly compressed between two glass plates. The chamber is filled with a fluid that approximates the optical properties of the tissue for reducing stray light propagating through the sides of the mouse and simplifying the theoretical model required for quantitative measurements. Two continuous wave excitation sources were used, an Ar+ laser tuned at 488 and 514 nm (Melles Griot, Carlsbad, CA) and a frequency doubled Nd:YAG laser at 532 nm (B&W Tek. Newark, DE). For epi-llumination imaging, the excitation light is spatially expanded to uniformly illuminate the object or mouse imaged. In trans-illumination mode light is delivered through a galvanometer-controlled set of two-mirrors (Nutfield Technology, Inc., Windham, New Hampshire) that can x-y translate a 0.5 mm diameter spot on one side of the object imaged. Photons propagating though tissues are captured on the other side of the object using a Princeton Instruments Inc. (Trenton, NJ) VersArray CCD camera with 10242 pixel CCD array cooled to −70°C, through a 50 mm f/1.2 photographic lens (Nikon Corp. Japan). Multiple fluorescence bands were captured using 3-cavity bandpass interference filters (Andover Corp. Salem, NH and Omega Optical, Inc. Brattleboro, VT), placed in front of the lens, at 513±5 nm, 610±10nm, 630±10nm, and 670±30nm.
2.3 Spectrally dependent tissue attenuation
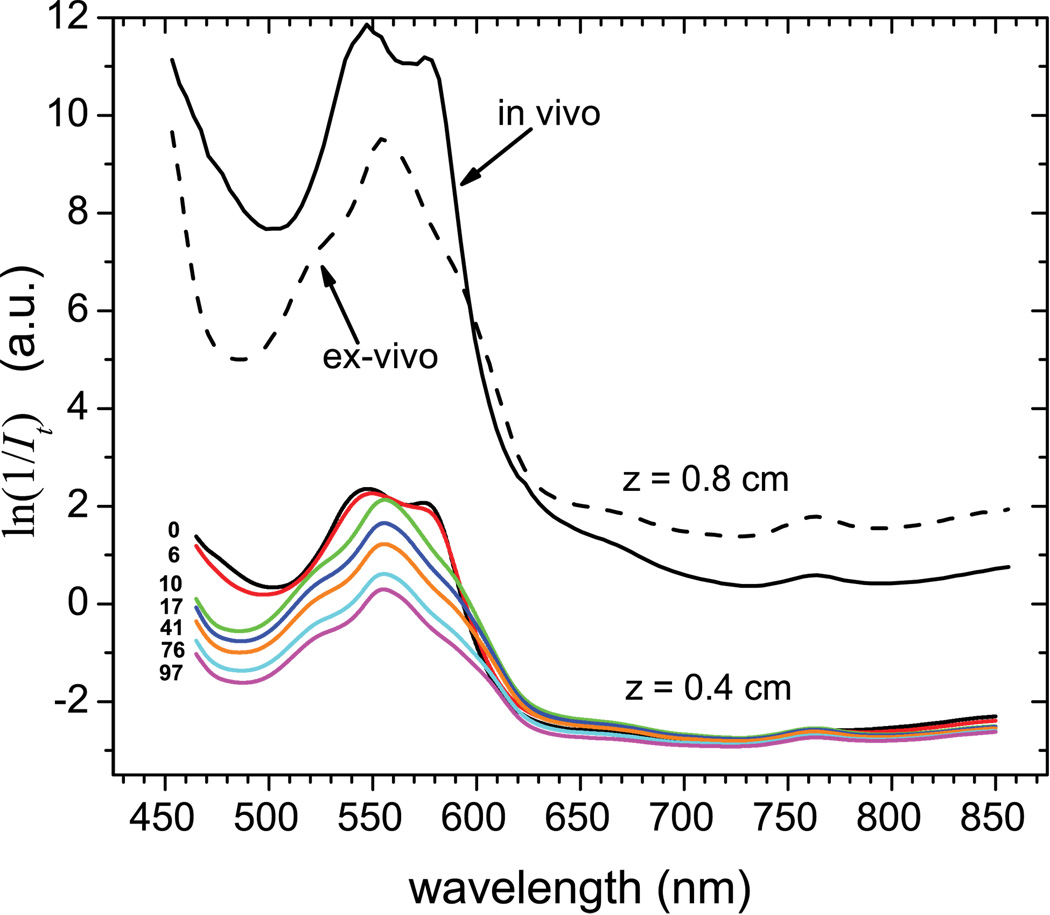
The fluorescence strength collected at different spectral bands depends on tissue attenuation characteristics for each of these bands. To determine this spectrally-dependent tissue attenuation, as it relates to the spectral bands selected for fluorescence measurements, we utilized a fiber-based spectrometer. The experimental setup consisted of a tungsten halogen lamp coupled to a multimode fiber to guide light onto the mouse tissue, acting as a point source. The light propagated inside the tissue and was collected by a second fiber, which was in contact with tissue on the opposite side of the source fiber, and delivered to a CCD spectrophotometer (Ocean Optics Inc. Dunedin, FL) to measure the intensity of the transmitted light It, which is proportional to the relative transmission T. The measured spectrum of the transmitted light was normalized by dividing with the reference spectrum of the illuminating source. The measurements were taken through the upper thoracic area along the dorsal-ventral direction both in vivo and post-mortem as a function of time after overdose injection with ketamine – xylazine. Fig. 1 depicts the relative spectral attenuation ln(1/It) as measured in vivo and post mortem for two different tissue thicknesses, i.e. 0.4 and 0.8 cm. It can be seen that the in vivo spectral curve is similar to the absorption curve of oxygenated hemoglobin, while the post mortem spectra are gradually becoming similar to the absorption spectrum of deoxygenated hemoglobin23.
Figure 1.
Spectral attenuation ln(1/It)of light transmitted through mouse thoracic area along the dorsal-ventral direction. Lower bundle of curves is a series of measurements for 0.4 cm tissue thickness as recorded in vivo (top line) and for consecutively in post-mortem period over a 0 – 97 min. Here, 0 min marks the administration of the ketamine – xylazine overdose. The higher set of two curves is for 0.8 cm thickness and for in vivo (solid curve) and ex vivo (dashed curve).
2.4 Epi-illumination imaging of FPs in tubes
The relative brightness Br of the fluorescent proteins examined, is defined as the peak fluorescence emission intensity of the FP expressing cells at optimum excitation wavelength, normalized to the peak emission of the reference fluorescent protein (here GFP) for the same excitation intensity. Although the values of the relative emission efficiency of the red shifted FPs are reported in the literature16,24, they were experimentally determined herein to account for possible experimental uncertainties, such as the variable cell density of the cells in the pellet or the level of expression.
A series of epi-illumination images of GFP, tdTomato, mCherry-expressing cells, and wild type cells in tubes excited with 488 and 532 nm laser lines were captured using the 610, 630, and 670 nm bandpass filters. The tubes were closely placed on a flat black absorbing surface and excited with an off-axis slightly-diverging p-polarized illuminating field. The fluorescent field was captured using a crossed polarizer to eliminate the reflections from the surface of the glass tubes. The intensity of the excitation field incident on the tubes was 50 and 70 µW/cm2 for the 488 and the 532 nm laser illumination respectively, and correspondingly the exposure times were set to 0.3s and 0.1s. The raw images were normalized for the exposure time and the interference filter bandwidth and were also corrected for the slight inhomogeneity of the illuminating field by dividing with the image of the illuminating field on a white flat diffusive surface in the absence of the tubes.
2.5 Trans-illumination imaging of FPs inside tissue
Two different experiments were performed to measure the intensity of fluorescence signals emanating from deep-tissue in trans-illumination mode, i.e. a) with cells in tubes inserted post-mortem into the esophagus of nude mice and b) using cells implanted in the brain to form a tumor. In both experiments mice were euthanized by carbon dioxide prior to the experiment to allow for flexibility in setting up the measurements without the need for prolonged anesthesia. Imaging was performed under consecutive illumination of the laser beam scanned at the 488 and 532 nm excitation wavelengths. All raw images were normalized for the exposure time, filter bandwidth and number of cells and signals are reported in counts/s/nm/106 cells.
In the implanted tube experiment, a mouse was euthanized and placed inside the chamber with the dorsal side towards the sources and the ventral towards the camera and is illuminated consecutively at two points on the dorsal side of its upper thorax. The four tubes were consecutively inserted and removed from the esophagus of the same mouse ensuring that the bottom of each tube was reaching the same location in the upper thoracic area, just above the heart. The tube in the esophagus was located 8 mm from the dorsal glass plate (source – tube distance rst = 0.8 cm) and 4 mm from the ventral glass plate (tube – detector distance rtd = 0.4 cm). Accurate and repeatable positioning of the tubes was critical to achieve identical geometry and imaging conditions and therefore allow for the direct comparison between the fluorescent protein signals. Fluorescence images were acquired at the 488 and 532 nm illumination laser lines and for 513 and 610 nm band pass filters, for GFP, tdTomato, and mCherry-expressing cell lines, as well as for the wild type cells that served as controls in order to independently record tissue and cell auto-fluorescence.
In the brain imaging experiments, two mice were implanted with GFP and tdTomato expressing cells to form tumors in the brain and were imaged 3 days after implantation. The brain was illuminated at 488, 514, and 532 nm with two 50 mW sources Illumination was incident on the ventral side and light was collected from the dorsal side. The other imaging parameters were kept the same as in the implanted tube experiments. Images of the head were captured at several excitation (488, 514, 532 nm) and fluorescence (610±10nm, 630±10nm, 670±30nm) wavelengths. The fluorescent images were post-processed by applying a threshold to reject background noise.12 A portion of the image recorded in the excitation wavelength was subtrtacted from the fluorescence image to correct for tissue autofluorescence.
2.6 Calculation of light attenuation in tissue
Quantification of the relative intensity of fluorescent signals due to deep-seated FP activity was based on a diffusion-equation based model for photon propagation in tissue. The use of solutions of the diffusion equation for modeling photon propagation in tissues in the visible were shown valid when using a modified diffusion coefficient.25 To model the particular experimental geometry used herein, we assumed excitation light from a point source, with intensity Io, that is incident on the mouse surface and propagates for 0.8 cm inside tissue. At 0.8 cm, the excitation light reaches and excites the tubes of fluorescent protein with intensity Ix = T0.8Io, where T0.8 is the relative spectral transmittance for 0.8 cm as calculated from the tissue spectral measurements described in 2.3 (see also Fig. 1) obtained also for a point source. The relative emitted intensity then is , where em is the spectral emission of the FP is and is the measured relative brightness of the FP expressing cells. Finally, the emitted fluorescence propagates to the boundary of the tissue and the intensity that reaches the detector is Id ~ T0.4Ix, where T0.4 is the relative spectral transmittance of the remaining distance, or equivalently,
| (1) |
The accuracy of this simplified propagation model is initially tested by fitting the experimental data from the GFP-tdTomato-mCherry mouse-tube experiment (Section 2.5) and then applied to predict the intensity emission for new fluorescent proteins that attain good performance characterstics for in vivo applications.
3. Results
3.1 Relative brightness of the fluorescent proteins
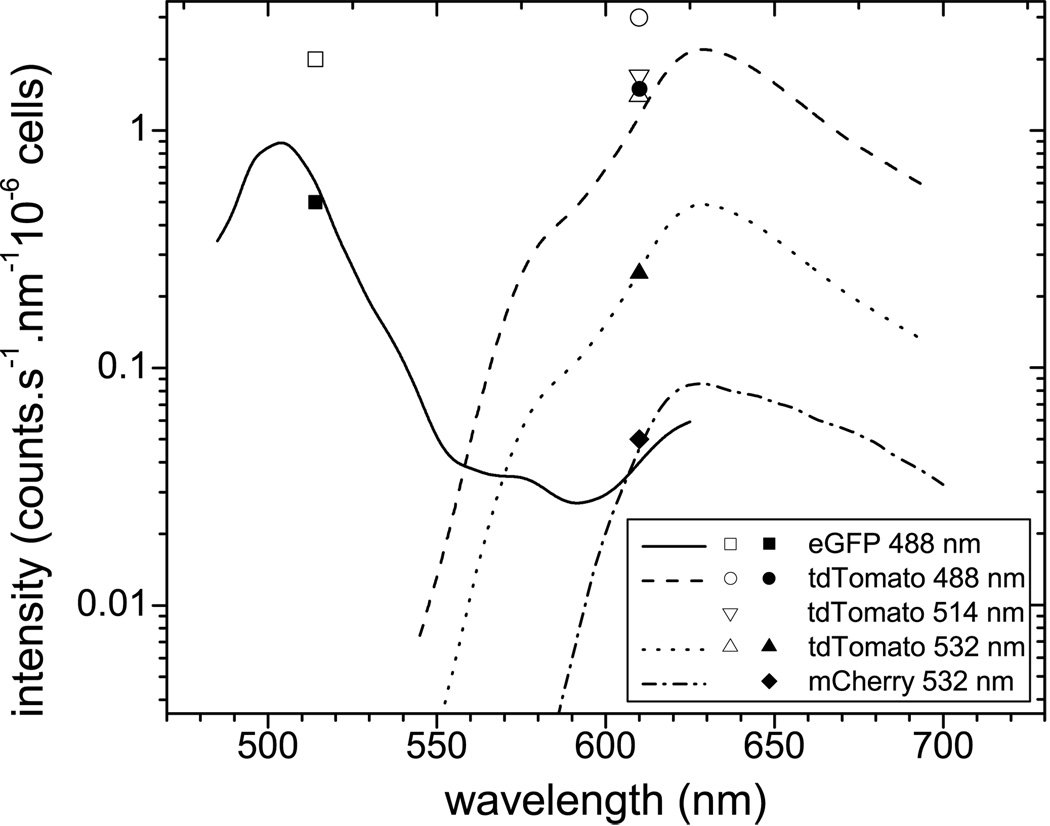
In the literature, the relative brightness of the fluorescent proteins is reported when excited at the optimum wavelength (see Table 1 for a summary of the optical properties of the most interesting FP’s). However, in practical applications the optimum excitation wavelength might not be available, and the actual relative brightness values can be different when exciting the proteins with the commercially available laser lines. Here we present the results of our experiments to determine the relative brightness for these wavelengths. In Fig.2 we present the normalized images of the fluorescent proteins in tubes acquired as described in Section 2.4. The intensity signal of the GFP was 4371 counts.s−1.nm−1 when excited at 488nm, for the tdTomato it was 5572 and 14488 counts.s−1.nm−1 when excited at 488 and 532 nm respectively, and for the mCherry it was 1447 counts.s−1.nm−1 when excited at 532nm. Taking into account the mismatch in the intensities of the excitation fields (50 and 70 µW/cm2 for the 488 and 532 nm respectively) the calculated relative brightness of the cells over GFP was 0.91 and 1.87 for tdTomato excited at 488 and 532 nm respectively, and 0.23 for mCherry excited at 532 nm. In Fig. 3 the data from the fluorescence epi-illumination images of Fig. 2 are plotted versus emission wavelength together with the relative emission spectra of the FPs as published in the literature 16,17,24. The results show that tdTomato is the brightest FP when excited at 532 nm, however its emission in 610 nm in only twice as bright compared to GFP fluorescence at 514 nm. The signal from the wild type cells marks the background/noise level.
Table 1.
Optical characteristics of the red-shifted FPs compared to GFP
| peak excitation |
mol. ext. coeff |
peak emission | qunt. yield |
relative brightness |
excitation | excitation mismatch coefficient |
reduced. rel. brightness |
ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|
|
(nm) |
ε (M−1cm−1) |
λem (nm) |
q | Br | λex (nm) |
|||||
| GFP | 489 | 53000 | 509 | 0.60 | 1 | 488 | 0.98 | 0.98 | 24 | |
| tdTomato | 554 | 138000 | 581 | 0.69 | 2.99 | 488/532 | 0.28/0.63 | 0.83/1.88 | 16 | |
| mCherry | 587 | 72000 | 610 | 0.22 | 0.49 | 532/594 | 0.08/0.37 | 0.18/0.40 | 16 | |
| mRaspb. | 598 | 86000 | 625 | 0.15 | 0.40 | 594 | 0.98 | 0.39 | 17 | |
| mRFP | 584 | 50000 | 607 | 0.25 | 0.39 | 594 | 0.94 | 0.37 | 16 | |
| mPlum | 590 | 41000 | 649 | 0.10 | 0.12 | 594 | 0.92 | 0.12 | 17 | |
| Katushka | 588 | 65000 | 635 | 0.34 | 0.67 | 594 | 0.92 | 0.62 | 18 |
Figure 2.
Images of the protein pellets inside the capillary tubes. The images are normalized for the illuminating field inhomogeneity, the exposure time, and the emission bandwidth and are plotted versus excitation and emission wavelengths. Top left is a white light picture of the tubes. In all pictures the tubes from left to right contain glioma cells with: GFP, tdTomato, mCherry, and wild type. Excitation radiation: Nd:YAG laser 532nm, (first row) and Ar+ ion laser 488 nm (second row). Emission bandwidth filters centered at: 514 nm (first column), 610 nm (second column), 630 nm (third column), and 670 nm (fourth column).
Figure 3.
Fluorescent protein brightness. Discrete points are the compiled measurements from the normalized images in Fig. 2. Open (white) symbols: excitation with 488 nm, solid (black) symbols: excitation with 532 nm. Continuous lines are the emission spectra of the fluorescent proteins scaled to fit the experimental data.
The above results do not coincide with the values as reported in the literature because of the different excitation and emission wavelengths. Here, we introduce a model that accounts for the difference due to the wavelength mismatch that will be used to predict the reduced relative brightness values for every FP. The brightness B is proportional to the quantum yield q and the molar extinction coefficient
| (2) |
measured at the peak absorption/excitation wavelength and the relative brightness Br is normalized to the GFP value. According to Kasha – Vavilov rule the quantum yield is independent of the excitation wavelength, so the brightness is dependent only on the molar extinction coefficient at the wavelength of interest λex and is a fraction of the maximum brightness. The excitation mismatch factor is calculated by the spectral absorption data of the FPs as given in the literature and is given in Table 1 under the column labeled excitation mismatch coefficient. Assuming that the B ~ qεex relation holds true for adjacent wavelengths, we calculate the reduced relative brightness . The calculated values are 0.98 for GFP excited at 488 nm, 0.83/1.88 for tdTomato, excited at 488/532 nm and 0.18 for mCherry excited at 532 nm, which are consistent with our measurements as presented in Fig. 3. These values serve as the actual relative FP brightness and are used as a reference in the calculation of the relative fluorescence intensity through deep tissue.
3.2 Fluorescence intensity from activity located deep inside tissue
Here, we present the transillumination images of the fluorescence from the proteins located inside deep tissue from two experiments (Section 2.5): with the tubes inside the esophagus (Figs. 4 a–d) and with the brain tumors (Fig. e–f). The tubes with GFP, tdTomato, mCherry and control cells that were placed into the esophagus were excited with two illumination sources (the geometry is shown in Fig. 4 a). Representative images of the fluorescent signals of the tdTomato and WT-control cells excited at 488 nm and imaged with the 610 nm filter are illustrated in Figs. 4 b and c, respectively. Examination of the raw data shows that the fluorescence signal measured from the tdTomato cells (maximum: 2.2 counts/s/nm/106 cells) is comparable to the signal from the WT cells fluorescence image (maximum: 1.3 counts/s/nm/106 cells). This signal is actually attributed to the auto-fluorescence from the tissue, since the fluorescence from the wild type cells is negligible (Fig 3). Observation of the WT cell images for all wavelengths reveals that the auto-fluorescence signal intensity: a) was lower for the 488 nm excitation compared to the 532 nm, and b) was increasing for higher wavelengths (filters at 610, 630, and 670 nm). These observations seem to be in contradiction with the notion that auto-fluorescence is decreasing with increasing excitation and emission wavelength.26,27 However, light absorption by tissue is higher at 532 nm in comparison with 488 nm (Fig. 1); therefore more tissue molecules are excited at 532 nm to emit auto-fluorescence.
Figure 4.
Normalized images of the fluorescence emission of GFP and TdTomato. The two crosses indicate the position of the sources illuminating the mouse. a) – d) capillary tubes inserted in the esophagus Excited with Ar+ 488 nm radiation and imaged in the 600–620 nm spectral window. The capillary tube is physically located between the projections of the sources. a) plain reflectance image at excitation wavelength. b) raw image of the fluorescence emission of the inserted tdTomato tube. c) autofluorescence emission when the tdTomato tube is replaced with the wild-type – non fluorescing cells. d) Net tdTomato fluorescence as calculated when subtracting image c) from b). e) and f) are images of the fluorescence emission of 5.105 glioma-36 cells implanted in the brain expressing GFP and tdTomato respectively. All images are normalized to the same units (counts/s/nm/106 cells) for direct comparison.
To accurately calculate the actual fluorescent signal of each FP we subtracted the corresponding auto-fluorescence images (WT cells) from all FP fluorescence images. For example in Fig. 4 d we present the net fluorescence image of tdTomato cells excited at 488 nm, as calculated by subtraction of the corresponding autofluorescence image (Fig. 4c) from the original image (Fig. 4b); all images were similarly processed. The emanating intensity of GFP (ex.: 488nm, em.: 514 nm) was 0.49 counts/s/nm/106 cells, of tdTomato excitation (ex.: 488nm and 532nm, em.: 610 nm) was 1.53 and 0.25 counts/s/nm/106 cells respectively, while mCherry (ex.: 532nm, em.: 610 nm), is slightly above detection level with 0.025 counts/s/nm/106 cells. Although in epillumination mode tdTomato was excited more efficiently at 532 nm compared to 488 nm (see Fig.3), in deep tissue imaging it was found more sensitive for the 488nm excitation instead of 532 nm. This is due to the fact that the relative transmitted intensity for 0.8 cm thick ex vivo tissue is significantly higher at 488 nm, i.e. , as seen in Fig. 1. This is also an advantage for GFP excited at 488 nm and collected at 514 nm (which emits 0.49 counts/s/nm/106 cells).
Finally, we imaged the two mice with brain tumors expressing GFP and tdTomato in transillumination mode. The GFP tumor was excited at 488 and fluorescence was collected at 513 nm and the tdTomato tumor was excited at 488, 514 and 532 nm and was collected at 610 nm. For example, in Figs. 4 e) and f) we present the fluorescence trans-illumination fluorescence images of GFP and tdTomato tumors (excitation 488 nm, emission 610 nm) overlaid on the corresponding reflectance images of the heads. The peak fluorescence signals for GFP and tdTomato is 2 and 3 counts/s/nm/106 cells, respectively, and are both located at the right hemisphere of the brain, which is congruent with the implantation position. It is evident that the intensity of the FPs from the tumors is higher compared to the signal coming from the tubes and this can be probably attributed to the lower light absorption of the tissue in the head area, to the different tissue and tumor “geometry”, and/or to the fast growth of the tumor over the 3 days between implantation and imaging. In Fig. 5 all the above results from the tube and tumor imaging experiments are summarized in a plot of intensity versus emission wavelength (closed black symbols – inserted tubes, open white symbols – brain tumors).
Figure 5.
Normalized fluorescence intensity of GFP and tdTomato emitted from the FP’s cells inside tubes inserted into the esophagus and cells implanted in the brain as compiled from the images like in Fig 4. FP’s are excited with 488, 514, and 532 nm. Symbols: solid – fluorescence from cells in tubes, open – fluorescence from brain tumors, lines: theoretical fitting for the intensity of the fluorescent proteins in inserted tubes. Solid line –GFP excited at 488 nm, dashed lines, dashed lines – tdTomato excited at 488 and 532 nm, and dotted line – mCherry excited at 532 nm. All experimental data are normalized to the same units (counts/s/nm/106 cells) for direct comparison.
3.3 Theoretical calculation of the transmitted fluorescent signal
To evaluate the theoretical model in Eq. (1) we examined the ability to predict the experimental measurements presented in Section 3.2. Spectrally dependent intensity profiles of the fluorescence from deep tissue were calculated using together a) the spectral emission curves em reported in the literature,16,17,24 b) the previously measured relative brightness from Sect. 3.1, and c) the ex vivo transmission T0.8,0.4 from Fig. 1. The resulting profile (shown in Fig. 5 - lines) demonstrates good match between the experimental data and the theoretical approximation in the 514 and 610 nm centered bandpass emission windows.
Using this model we then predicted the performance of the new red-shifted fluorescent proteins for in vivo applications, in particular mRFP, mPlum, mRaspberry and Katushka. For this calculation we used the in vivo relative attenuation curves from Fig. 1 and the calculated reduced relative brightness from Table 1. While a wide range of illumination wavelengths can be studied, we opted for examining the performance of laser lines that are generally available in a cost-efficient form, i.e. the well established laser lines at 488 and 532 nm using Argon-ion or doubled-frequency Nd-Yag lasers and the newly developed 594 nm continuous wave diode-pumped solid state (CW DPSS) laser line.
Figure 6 depicts a comparative plot of the 4mm deep-tissue fluorescent signal from the different FP’s placed 8mm away from the sources (see location details in Section 2.6). The maximum signal achieved for each FP is tabulated in Table 2. Except for GFP that emits in the green area of the spectrum, the detected fluorescence from the other FPs is above 600 nm because it is “shaped” by the spectral profile of the tissue absorption. For the particular depth where the tubes containing the fluorescence protein expressing cells were placed, the maximum signal from Katushka excited at 594 nm was calculated to be 46 times stronger than GFP. Respectively, mRaspberry, mCherry and mRFP was found to be 24, 18 and 17 times brighter than GFP. The mPlum maximum signal was 8.5 times brighter than GFP, but its spectral profile is smoother and shifted further in the red spectral region, with its peak at 650 nm, similar to Katushka. Finally, although tdTomato is the brightest protein in epi-illumination mode, it is by-far outperformed in deep tissue imaging applications by the other proteins.
Figure 6.
Relative fluorescence intensity of various FPs located inside the body of a mouse 8 mm from the sources and 4 mm from the detector position. The FP’s are excited with the optimal laser choices, all of them were assumed to have the same light output power. GFP maximum intensity is normalized to 1.
Table 2.
Maximum intensity of the red-shifted FPs compared to GFP inside tissue.
| excitation | peak emission | maximum signal |
max. sign. | max. sign. | max. sign. | ||
|---|---|---|---|---|---|---|---|
| λex (nm) |
λem(nm) | − 2 nm | + 2 nm | “reverse” geometry |
|||
| GFP | 488 | 505 | 1 | 1 | 1 | 1 | |
| tdTomato | 488/532 | 630 | 6.36/1.82 | 6.02/2.64 | 6.68/1.24 | 129/105 | |
| mCherry | 532 | 625 | 18.2 | 9.98 | 31.3 | 207 | |
| mRaspb. | 594 | 630 | 24.3 | 13.7 | 40.9 | 318 | |
| mRFP | 594 | 625 | 17.9 | 9.81 | 30.6 | 206 | |
| mPlum | 594 | 650 | 8.49 | 4.85 | 14.0 | 141 | |
| Katushka | 594 | 645 | 45.7 | 26.03 | 75.61 | 732 |
The signal strength from the FP’s shown in Fig. 6 is sensitive on the wavelength used for illumination, since the optical properties change rapidly in the area around 600nm, as also shown in Fig.1. Therefore the calculations herein, but also the overall performance achieved is highly dependent on the exact excitation wavelength employed. To demonstrate this, the relative fluorescence intensities are calculated for two additional cases: when the 593 nm exctitation wavelength is shifted by −2 nm (towards the UV) and by +2 nm (towards IR). The results are presented in Table 2 and deviations between 50 – 200% may be present, for example, the Katushka fluorescent signal can be 26 to 75 times higher than GFP.
4. Discussion and conclusions
The ability to select optimal red-shifted fluorescent proteins for whole body applications becomes an involved task due to the availability of a large number of potent fluorescent proteins and the steep change in the absorption spectrum of tissue around the 600nm wavelength. In addition the limited availability of economically available laser lines to appropriately excite these proteins in tomographic mode, (i.e using point illumination) further complicates the FP selection. This work therefore studied the relative performance of red-shifted FP’s assuming whole body imaging and realistic experimental considerations that relate to tomographic setups.
Two different experimental approaches were presented to measure the intensity of the FP signals emerging from deep areas, i.e using tubes containing FP expressing cells implanted into mice post-mortem and direct implantation of cells in mouse brains. Although the latter is directly related to realistic biomedical applications, the implanted tube approach offered many advantages, i.e.
Since a single mouse cadaver was employed, the body size, positioning and “geometry” remained the same in all the measurements so that accurate comparisons could be performed between the different FP’s through tissue. In contrast tumor cell implantation in different mice may result in differences in implantation site (depth) and overall geometry which presents particular challenges in accurately accounting for accurate one-to-one comparison, due to the strong non-linear dependence of optical signal intensity to depth.
The concentration (number per volume) of the cells inside the tubes is measured in advance; on the other hand tumor growth rate is not the same between different mice.
The implantable tube approach offers accurate control measurements when the wild type cells were employed, enabling the direct measurement of tissue autofluorescence, in the same geometry as the one used for the FP signal measurement. This measurement can be directly subtracted from the other measurements to offer accurate measurements of FP related fluorescence only.
The obvious disadvantage is that these measurements do not directly relate to in vivo measurements because in vivo and post-mortem tissue spectra have significant differences, as shown in Fig.1. However, the use of the spectra in Fig.1 allow for calculations of in vivo related values from the ones achieved post-mortem by taking into account the relative attenuation differences in each spectral region examined.
As demonstrated in Section 3.3, a small deviation in the excitation wavelength may yield a significant deviation in sensitivity performance, therefore further red-shifting the excitation wavelength may yield significant sensitivity gains. In addition, the relative fluorescence intensity gain of the red-shifted FPs compared to GFP depends on the position of the FPs inside the body. Herein, the geometry examined assumed that the source – protein and the protein – detector distances are 0.8 and 0.4 cm, respectively. This was selected as a typical worst case scenario approach, where the highly attenuated excitation wavelength (<600nm) propagates for a longer distance compared to the emission light. Other combinations where the fluorescence activity is deeper in the tissue (closer to the source plane) will yield higher signal intensity and sensitivity. For example, we have calculated the intensities in the “reverse geometry” were the source-protein and the protein-detectror distances were 0.4 cm and 0.8 cm respectively, with the detected fluorescence from Katushka being more than 700 times higher than GFP (see “reverse geometry” column in Table 2). Finally, more superficial activity (closer to the CCD camera plane) can be detected also in epi-illumination mode.
As a conclusion, the new FPs emitting in the red-near infrared part of the spectrum are expected to boost whole-body imaging due to the high intensity fluorescent signals when properly excited. The maximum signals coming from red-shifted fluorescent proteins located deep inside tissue are stronger than GFP in every case examined experimentally and theoretically. The improvement in signal would be even higher if appropriate light sources at 600–610 nm were available. From our calculation the highest emission intensity signal is expected from Katushka when excited with 594 nm laser radiation, although these values may be modified by certain biological parameters such as expression levels and expression sustainability, the pH of the microenvironment and the detection system utilized. However Katushka, and mRaspberry and mCherry appear to be the most suitable choice for whole body fluorescent protein imaging applications.
Acknowledgements
The authors would like to thank Jason Gaglia for useful discussions. This research was supported in part by National Institutes of Health grants 1R01EB00438201 and 1R21-CA110167.
References
- 1.Hoffman RM. The multiple uses of fluorescent proteins to visualize cancer in vivo. Nat. Rev. Cancer. 2005;5(10):796–806. doi: 10.1038/nrc1717. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman RM. Green fluorescent protein imaging of tumour growth, metastasis, and angiogenesis in mouse models. Lancet Oncol. 2002;3(9):546–556. doi: 10.1016/s1470-2045(02)00848-3. [DOI] [PubMed] [Google Scholar]
- 3.Shah K, Jacobs A, Breakefield XO, Weissleder R. Molecular imaging of gene therapy for cancer. Gene Ther. 2004;11(15):1175–1187. doi: 10.1038/sj.gt.3302278. [DOI] [PubMed] [Google Scholar]
- 4.Griekspoor A, Zwart W, Neefjes J. Presenting antigen presentation in living cells using biophysical techniques. Curr. Opin. Microbiol. 2005;8(3):338–343. doi: 10.1016/j.mib.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Wobus AM, Boheler KR. Embryonic stem cells: Prospects for developmental biology and cell therapy. Physiol. Rev. 2005;85(2):635–678. doi: 10.1152/physrev.00054.2003. [DOI] [PubMed] [Google Scholar]
- 6.Helmchen F, Denk W. Deep tissue two-photon microscopy (vol 2, pg 932, 2005) Nat. Methods. 2006;3(3):235–235. doi: 10.1038/nmeth818. [DOI] [PubMed] [Google Scholar]
- 7.Jain RK, Munn LL, Fukumura D. Dissecting tumour pathophysiology using intravital microscopy. Nat. Rev. Cancer. 2002;2(4):266–276. doi: 10.1038/nrc778. [DOI] [PubMed] [Google Scholar]
- 8.Yang M, Baranov E, Jiang P, Sun FX, Li XM, Li LN, Hasegawa S, Bouvet M, Al-Tuwaijri M, Chishima T, Shimada H, Moossa AR, Penman S, Hoffman RM. Whole-body optical imaging of green fluorescent protein-expressing tumors and metastases. Proc. Natl. Acad. Sci. U. S. A. 2000;97(3):1206–1211. doi: 10.1073/pnas.97.3.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kishimoto H, Kojima T, Watanabe Y, Kagawa S, Fujiwara T, Uno F, Teraishi F, Kyo S, Mizuguchi H, Hashimoto Y, Urata Y, Tanaka N, Fujiwara T. In vivo imaging of lymph node metastasis with telomerase-specific replication-selective adenovirus. Nat. Med. 2006;12(10):1213–1219. doi: 10.1038/nm1404. [DOI] [PubMed] [Google Scholar]
- 10.Yang M, Baranov E, Li XM, Wang JW, Jiang P, Li L, Moossa AR, Penman S, Hoffman RM. Whole-body and intravital optical imaging of angiogenesis in orthotopically implanted tumors. Proc. Natl. Acad. Sci. U. S. A. 2001;98(5):2616–2621. doi: 10.1073/pnas.051626698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katz MH, Takimoto S, Spivack D, Moossa AR, Hoffman RM, Bouvet M. A novel red fluorescent protein orthotopic pancreatic cancer model for the preclinical evaluation of chemotherapeutics. Journal of Surgical Research. 2003;113(1):151–160. doi: 10.1016/s0022-4804(03)00234-8. [DOI] [PubMed] [Google Scholar]
- 12.Ntziachristos V, Turner G, Dunham J, Windsor S, Soubret A, Ripoll J, Shih HA. Planar fluorescence imaging using normalized data. J. Biomed. Opt. 2005;10(6) doi: 10.1117/1.2136148. [DOI] [PubMed] [Google Scholar]
- 13.Zacharakis G, Kambara H, Shih H, Ripoll J, Grimm J, Saeki Y, Weissleder R, Ntziachristos V. Volumetric tomography of fluorescent proteins through small animals in vivo. Proc. Natl. Acad. Sci. U. S. A. 2005;102(51):18252–18257. doi: 10.1073/pnas.0504628102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zacharakis G, Shih H, Ripoll J, Weissleder R, Ntziachristos V. Normalized transillumination of fluorescent proteins in small animals. Mol. Imaging. 2006;5(3):153–159. [PubMed] [Google Scholar]
- 15.Turchin IV, Plehanov VI, Orlova AG, Kamenskiy VA, Kleshnin MS, Shirmanova MV, Shakhova NM, Balalaeva IV, Savitskiy AP. Fluorescence diffuse tomography of small animals with DsRed2 fluorescent protein. Laser Phys. 2006;16(5):741–746. [Google Scholar]
- 16.Shaner NC, Campbell RE, Steinbach PA, Giepmans BNG, Palmer AE, Tsien RY. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp red fluorescent protein. Nat. Biotechnol. 2004;22(12):1567–1572. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Jackson WC, Steinbach PA, Tsien RY. Evolution of new nonantibody proteins via iterative somatic hypermutation. Proc. Natl. Acad. Sci. U. S. A. 2004;101(48):16745–16749. doi: 10.1073/pnas.0407752101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shcherbo D, Merzlyak EM, Chepurnykh TV, Fradkov AF, Ermakova GV, Solovieva EA, Lukyanov KA, Bogdanova EA, Zaraisky AG, Lukyanov S, Chudakov DM. Bright far-red fluorescent protein for whole-body imaging. Nat. Methods. 2007;4(9):741–746. doi: 10.1038/nmeth1083. [DOI] [PubMed] [Google Scholar]
- 19.Weissleder R, Tung CH, Mahmood U, Bogdanov A. In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat. Biotechnol. 1999;17(4):375–378. doi: 10.1038/7933. [DOI] [PubMed] [Google Scholar]
- 20.Gurfinkel M, Ke S, Wen XX, Li C, Sevick-Muraca EM. Near-infrared fluorescence optical imaging and tomography. Dis. Markers. 2003–2004;19(2–3):107–121. doi: 10.1155/2004/474818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winnard PT, Kluth JB, Raman V. Noninvasive optical tracking of red fluorescent protein-expressing cancer cells in a model of metastatic breast cancer. Neoplasia. 2006;8(10):796–U712. doi: 10.1593/neo.06304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sena-Esteves M, Tebbets JC, Steffens S, Crombleholme T, Flake AW. Optimized large-scale production of high titer lentivirus vector pseudotypes. J. Virol. Methods. 2004;122(2):131–139. doi: 10.1016/j.jviromet.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 23.Zijlstra WG, Buursma A, Meeuwsenvanderroest WP. Absorption-Spectra of Human Fetal and Adult Oxyhemoglobin, De-Oxyhemoglobin, Carboxyhemoglobin, and Methemoglobin. Clin. Chem. 1991;37(9):1633–1638. [PubMed] [Google Scholar]
- 24.Patterson GH, Knobel SM, Sharif WD, Kain SR, Piston DW. Use of the green fluorescent protein and its mutants in quantitative fluorescence microscopy. Biophys. J. 1997;73(5):2782–2790. doi: 10.1016/S0006-3495(97)78307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ripoll J, Ntziachristos V. From finite to infinite volumes: Removal of boundaries in diffuse wave imaging. Phys. Rev. Lett. 2006;96(17) doi: 10.1103/PhysRevLett.96.173903. [DOI] [PubMed] [Google Scholar]
- 26.Muller MG, Georgakoudi I, Zhang QG, Wu J, Feld MS. Intrinsic fluorescence spectroscopy in turbid media: disentangling effects of scattering and absorption. Appl. Optics. 2001;40(25):4633–4646. doi: 10.1364/ao.40.004633. [DOI] [PubMed] [Google Scholar]
- 27.Weissleder R, Ntziachristos V. Shedding light onto live molecular targets. Nat. Med. 2003;9(1):123–128. doi: 10.1038/nm0103-123. [DOI] [PubMed] [Google Scholar]