Abstract
Background
Prospective studies have shown that C-reactive protein (CRP) is a predictor of hypertension. However, due to confounding variables a causal linkage between CRP and hypertension has not been clearly shown. We investigated whether high circulating human CRP concentrations can induce hypertension in rats.
Methods
A single intravenous injection of adeno-associated virus (AAV)-GFP or AAV-hCRP was administered and blood pressure was measured. Serum hCRP and endothelin-1 (ET-1) and urine cGMP were measured by ELISA. Serum nitric oxide (NO) was measured by the Griess method. Heart rate, maximum pressure, arterial elastance and mean aortic pressure, cardiac output, and dp/dt max were recorded.
Results
A single injection of AAV-hCRP resulted in efficient and sustained hCRP expression and led to increased blood pressure 2 months after gene transfer that persisted for another 2 months. This effect was associated with decreased NO production, as demonstrated by a decreased serum NO concentration and urinary cGMP excretion, and impairment of endothelial-dependent vascular relaxation. CRP transduction also increased expression of angiotensin type 1 receptor, ET-1 and endothelin type A receptor, decreased expression of angiotensin type 2 receptor and endothelial NO synthase in thoracic aortas, and increased arterial stiffness. Ex vivo studies indicated a similar detrimental effect of CRP that was reversed by the NO donor.
Conclusion
AAV vector-mediated CRP expression resulted in hypertension mediated through reduced NO production and subsequent alteration in ET-1 and renin-angiontensin system activation. Impaired arterial elasticity may also contribute to CRP-induced hypertension. These results support a causal role for CRP in the pathogenesis of hypertension.
Keywords: C-reactive protein, hypertension, endothelial cells, endothelin, angiotensin, inflammation
Hypertension is a major public health problem that affects more than 25% of the adult population worldwide. Increasing our understanding of the processes that lead to hypertension will allow for the implementation of valid measures for its prevention and treatment.
Chronic low-grade inflammation has been recently linked to hypertension 1, 2. C-reactive protein (CRP), one of multiple inflammation factors, has traditionally been viewed as a systemic marker of inflammation, but more recently, a flood of studies have demonstrated a powerful predictive relationship between increased CRP production and cardiovascular disease3.
In many case-control and cross-sectional studies, patients with essential hypertension have demonstrated increased concentrations of CRP 1, 2. More importantly, some prospective studies have explored whether CRP may be predictive of incident hypertension. For example, in the Women’s Health Study, a positive association between increasing concentrations of CRP and risk of developing hypertension was suggested 4. This effect was seen even in those women with low baseline blood pressure and in those without other conventional cardiovascular risk factors. Likewise, in a small study of incident hypertension, an 11-year follow-up revealed that men with CRP concentrations ≥3 mg/L were 3.6 times more likely to develop hypertension than men with concentrations ≤1 mg/L, even after adjustment for other known predictors 5.
Although observational studies such as these indicate that CRP is a predictor of hypertension, whether CRP is a cause or just a marker of hypertension has yet to be clarified. In this study, we used an adeno-associated virus (AAV) vector to obtain efficient and sustained CRP expression in rats with minimal associated inflammatory and immune responses. The effects of sustained CRP expression on blood pressure and potential mechanisms of CRP-induced hypertension were investigated using in vivo and ex vivo approaches.
METHODS
Adeno-associated virus (AAV)-mediated human CRP production
A 675-bp human CRP (hCRP) cDNA fragment was subcloned into double-stranded AAV vector plasmid (dsAAV) to create the plasmid dsAAV-hCRP. The AAV viral particles were prepared and purified according to a previously published protocol 6.
Animals and Gene delivery
Male Wistar rats weighing 180 to 200 g were randomized to one of three groups: the control group that received an injection of 1 ml saline, the GFP group that received an injection of AAV-GFP(5×1010 viron particles in saline solution), or the CRP group that received an injection of AAV-hCRP(5×1010 viron particles in saline solution). Rats were anaesthetized with pentobarbital. A single injection of AAV-GFP or AAV-hCRP was administered via the tail vein. After injection, the rats were kept warm under an infrared lamp until they returned to consciousness. All animals were sacrificed 4 months after injection, at which time liver, aorta, kidney were collected, frozen in liquid nitrogen and stored at −80°C prior to analysis.
Blood pressure measurements
Systolic blood pressure was measured in conscious rats with a manometer–tachometer via the tail-cuff method.
Serum and urine analysis
Serum hCRP (RECI, Germany. The detection limit of the assay was 0.067 mg/L. Interassay and intra-assay precision of the kit were <5% and <10%, respectively.) and endothelin-1 (ET-1) (Rapidbio Lab, California. The detection limit of the assay was 0.5 pg/mL. Interassay and intra-assay precision of the kit were <10%.) and urine cGMP were measured by ELISA using commercial kits (Adlitteram Diagnostic laboratories Company, USA. The detection limit of the assay was 0.37 pmol/mL. Interassay and intra-assay precision of the kit were<10%.). Serum nitric oxide (NO) concentrations were measured by the Griess method.
Hemodynamic analysis
Under pentobarbital anaesthesia, a microtip P-V catheter (SPR-838, Millar Instruments, Houston, TX) was inserted into the right carotid artery and advanced into the left ventricle. Heart rate, maximum pressure, arterial elastance (Ea) and mean aortic pressure (MAP), cardiac output (CO), and dp/dt max were recorded and analyzed using a cardiac P-V analysis program (PVAN3.2, Millar Instruments).
Vasomotor function
Aorta rings were suspended in organ chambers, and response to norepinephrine (NE) and acetylcholine (Ach) was tested7.
Real-time PCR
Levels of mRNA transcripts were detected by real-time PCR following reverse transcription of total RNA. Primers for gene tested were shown in supplementary method. Results of real-time PCR were expressed as the ratio of target mRNA to that of GAPDH.
Western blotting
Protein samples (20 µg per lane) were loaded and transferred onto PVDF membranes. The primary antibodies were used against hCRP, endothelial NO synthase (eNOS), angiotensin type 1 receptor (AT1), angiotensin type 2 receptor (AT2), endothelin type A receptor (ETA) or β-actin. Protein bands were developed with enhanced chemiluminescence.
Ex vivo artery incubations
To determine whether CRP has direct effects on the vasculature, thoracic aortas were excised from naïve, 2 month old rats. The isolated aortas were cut into segments and treated with DMEM alone (control), DMEM with hCRP (50 mg/L), and DMEM plus CRP and S-nitroso-N-acetyl penicillamine (SNAP, 100 µM) for six hours. Tissues were homogenized as above and protein or mRNA expression for ET-1, ETA, AT1, AT2 and eNOS were determined.
Statistical analysis
All datas were expressed as mean ± SEM. The significance of differences in mean values was analyzed by one-way ANOVA followed by a post hoc least significant difference test. Statistical significance was defined as P < 0.05.
Results
AAV-mediated hCRP expression
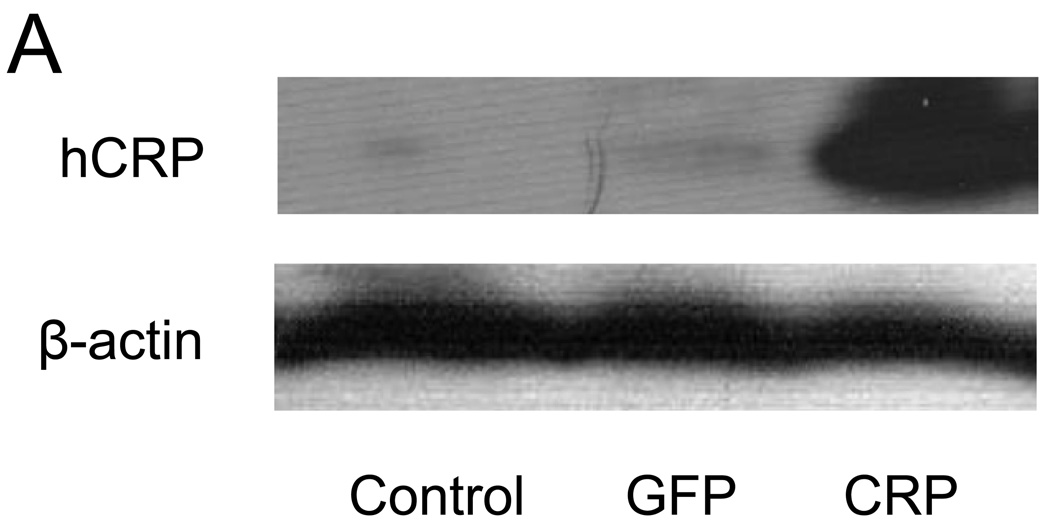
Liver protein concentrations of hCRP were assessed by western blotting 4 months following injection of saline or AAV. hCRP was not detectable in livers from rats injected with saline or AAV-GFP, while considerable hCRP expression was detected in livers from AAV-hCRP injected rats (Figure 1A). Similarly, assessment of serum samples 2 and 4 months following injection revealed that hCRP was undetectable in rats injected with saline or AAV-GFP, whereas rats injected with AAV-hCRP had readily detectable hCRP concentrations (Figure 1B). Real-time PCR also indicated expression of hCRP in kidneys and aortas (Figure 1C). Thus, AAV-hCRP injection induced long-term expression of hCRP and a corresponding increase in serum hCRP concentrations. These increases were not associated with any alteration in body weight or in serum cholesterol, triglycerides or glucose (Supplemental Data Table 1).
Figure 1.
AAV-mediated expression of hCRP. (A) Representative western blot analysis of hCRP expression in liver 4 months after gene delivery. (B) Serum concentrations of hCRP 2 and 4 months after gene delivery. (C) Real-time PCR results of hCRP mRNA expression. Data represent mean ± SD of 3–6 rats/group. ND = not detected.
Arterial pressure responses to human CRP gene delivery
Blood pressure was measured at baseline and once per month following injections. AAV-hCRP injection resulted in a gradual rise in systolic blood pressure that became significantly increased 2 months after gene delivery. This hypertensive response became stable (21 mmHg higher than in controls; P =0.000) at 3 months after gene injection and remained so until the end of the study (Figure 2A). Conversely, systolic blood pressure in saline and AAV-GFP-treated rats did not change significantly throughout the study period (Figure 2A). Together, these results suggest that CRP gene transfer induced hypertension in rats.
Figure 2.
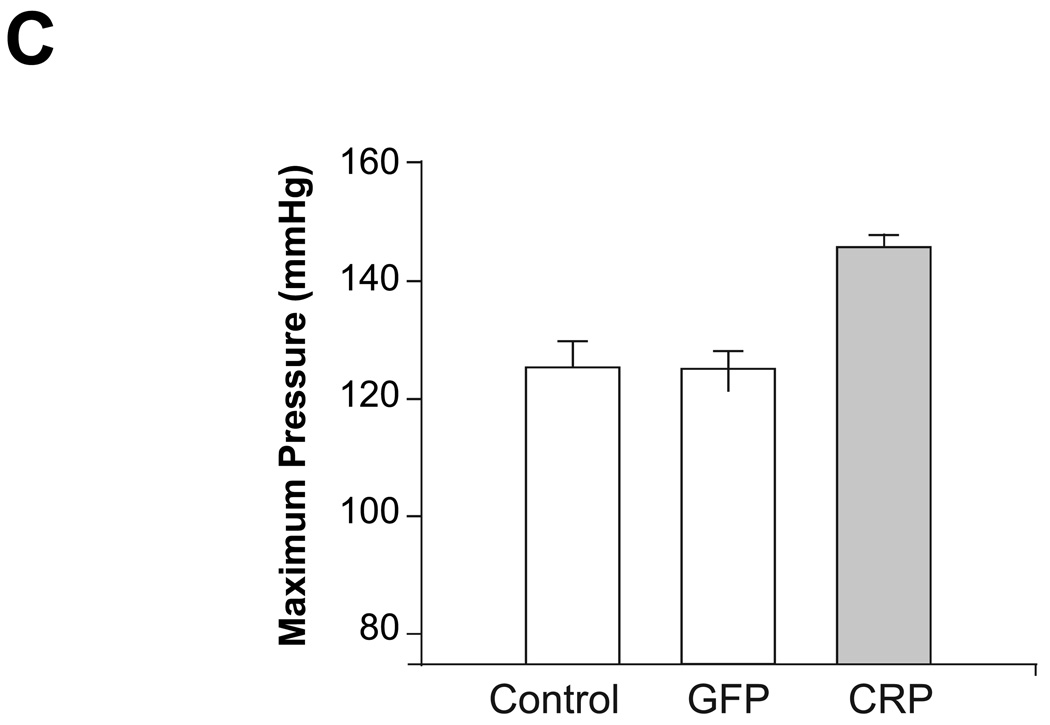
CRP caused hypertension in rats. (A) Systolic blood pressure was monitored from the beginning of the study using the tail-cuff method. Rats injected with AAV-hCRP had significantly increased blood pressure beginning 2 months after gene delivery (P =0.000). Hemodynamic parameters were increased by CRP gene transfer. (B) CRP gene delivery increased mean aortic pressure (P =0.001). (C) CRP gene delivery increased maximum left ventricular pressure (P =0.004). (D) CRP gene delivery increased arterial elastance (P =0.000). Data represent mean ± SEM of 6 to 8 rats/group. * P < 0.05 vs. control group.
CRP overexpression impairs endothelial-dependent vessel relaxation
Ring tension studies were conducted to determine if CRP overexpression affected relaxation responses in isolated aortic rings. In all groups, acetylcholine (1 nM to 100 µM) produced dose-dependent relaxation in aortic rings precontracted with 1 µM NE. However, the maximum relaxation observed in rings isolated from AAV-hCRP-treated animals was significantly attenuated compared to that in saline-treated controls (43.3 ± 6.1% vs. 69.0 ± 4.6%, respectively; P =0.011) (Figure 3A). In contrast, AAV-GFP treatment did not alter maximum aortic ring relaxation (73.7 ± 8.9%). Furthermore, there was no significant difference between the groups in aortic ring contractile responses to NE (data not shown), suggesting that CRP impairs endothelial-dependent relaxation independent of effects on the contractile response to NE.
Figure 3.
NO biosynthesis was decreased by CRP gene delivery. (A) Endothelial-dependent relaxation to acetylcholine in aortic segments was impaired by CRP gene transfer. (B) CRP gene delivery decreased serum NO concentration. (C) CRP gene delivery decreased urine cGMP excretion. Data are presented as the mean ± SEM of 6 to 8 rats/group. * P < 0.05 vs. control group.
Hemodynamic analysis
Prior to sacrifice at the end of the study (i.e. 4 months following injections), mean intra-aortic and maximum left ventricular pressures were measured by invasive hemodynamic monitoring. These data showed similar differences between the groups through non-invasive systolic blood pressure measurements by the tail-cuff method. Specifically, mean aortic pressure (136.7 ± 2.7 vs. 119.9 ± 4.7 mmHg; P =0.001) and maximum left ventricular pressure (145.7 ± 2.0 vs. 125.1 ± 4.7 mmHg; P =0.004) were significantly higher in the AAV-hCRP group than in the saline control group, but not altered in the AAV-GFP group (Figures 2B and 2C). Conversely, heart rates or dP/dt max did not differ significantly among the treatment groups. CO in the CRP group tended to be decreased compared to the control group, but the difference was not significant (Supplemental Data Table 2). Ea provides a reliable estimate of aortic impedance and was found to be significantly increased in the AAV-hCRP group compared to the saline control group (1.03 ± 0.07 vs. 0.53 ± 0.07 mmHg/µl, respectively; P =0.000) but not altered in the AAV-GFP group (Figure 2D), suggesting that inflammation-mediated increases in CRP may enhance arterial stiffening.
Nitric oxide production
Plasma NO is a potent endothelium-derived vasodilator and signalling molecule that is involved in the control of vascular tone and endothelial function. To evaluate whether AAV-hCRP-induced hypertension was associated with altered NO biosynthesis, we measured serum NO and urine cGMP concentrations as well aortic eNOS expression (see below) 4 months after gene delivery. CRP gene delivery induced a significant reduction in serum NO concentrations (32.8 ± 3.7 vs. 45.4 ± 2.4 µmol/L; P =0.04) and urine cGMP excretion (0.44 ± 0.12 vs. 1.49 ± 0.32 nmol/mmol creatinine; P =0.019) compared to values in saline control animals (Figure 3B, 3C) whereas AAV-GFP injection had no effect. These observations suggest that CRP gene delivery decreased NO production.
Aortic expression of eNOS, ET-1, ETA, AT1 and AT2
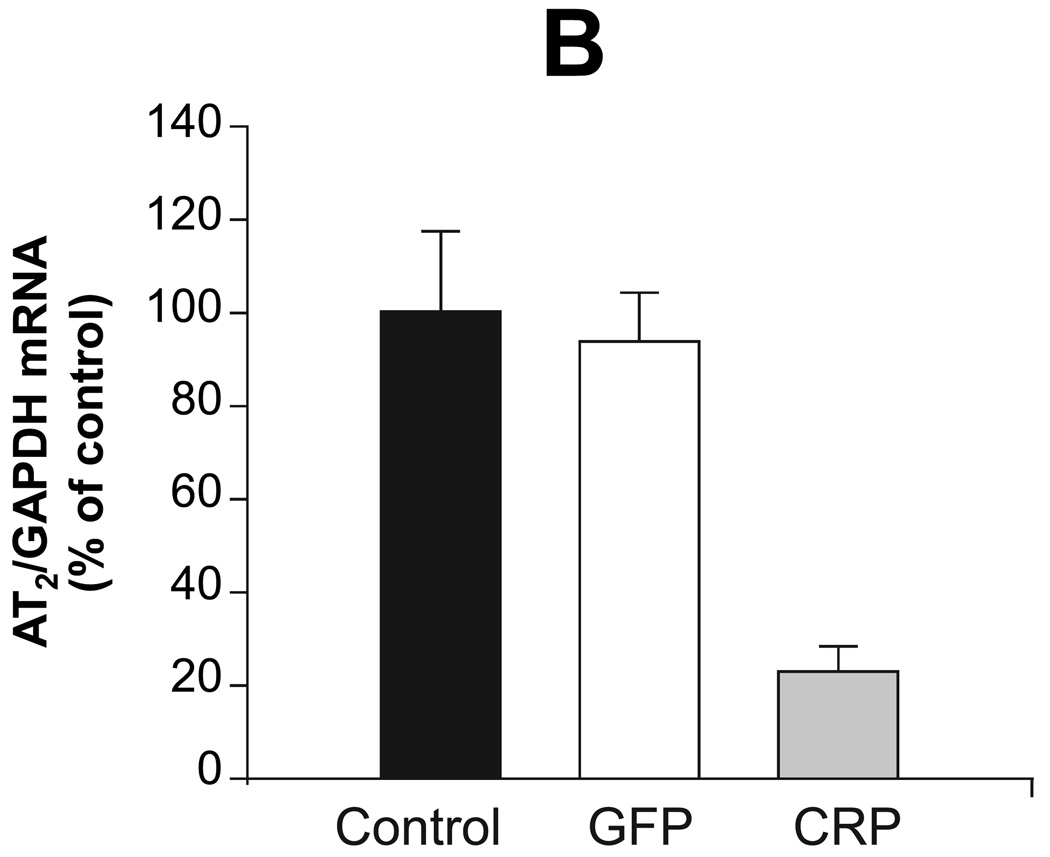
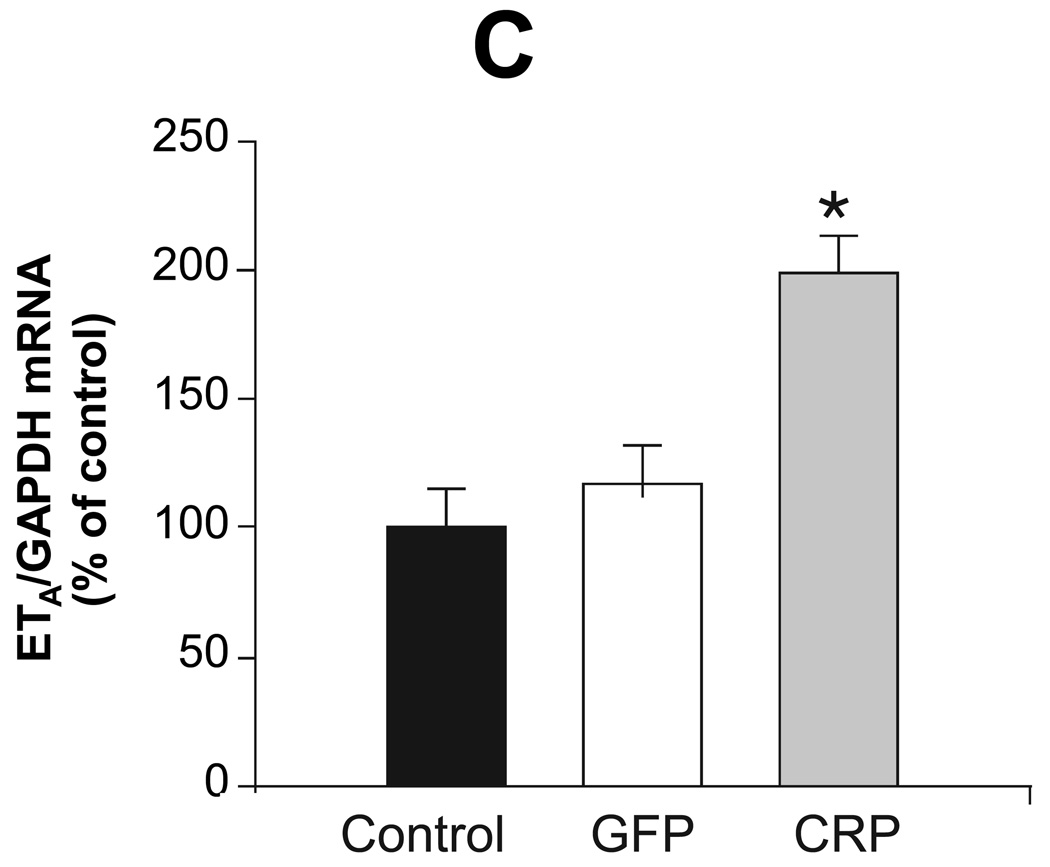
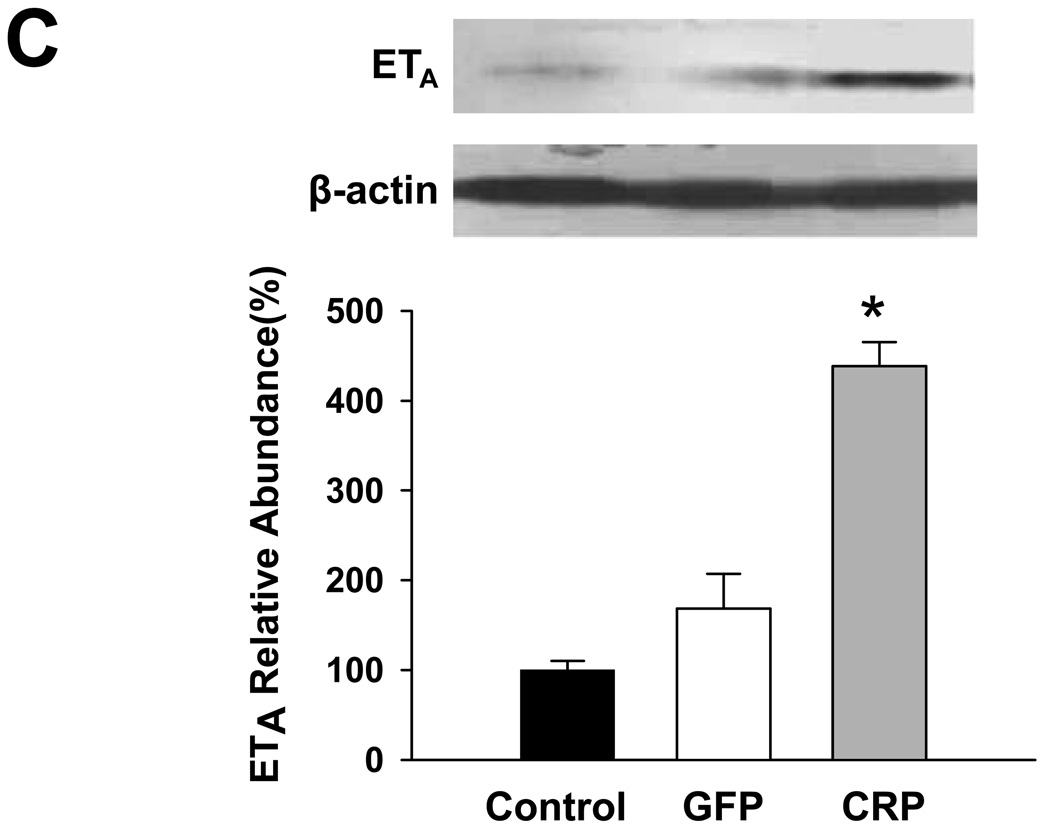
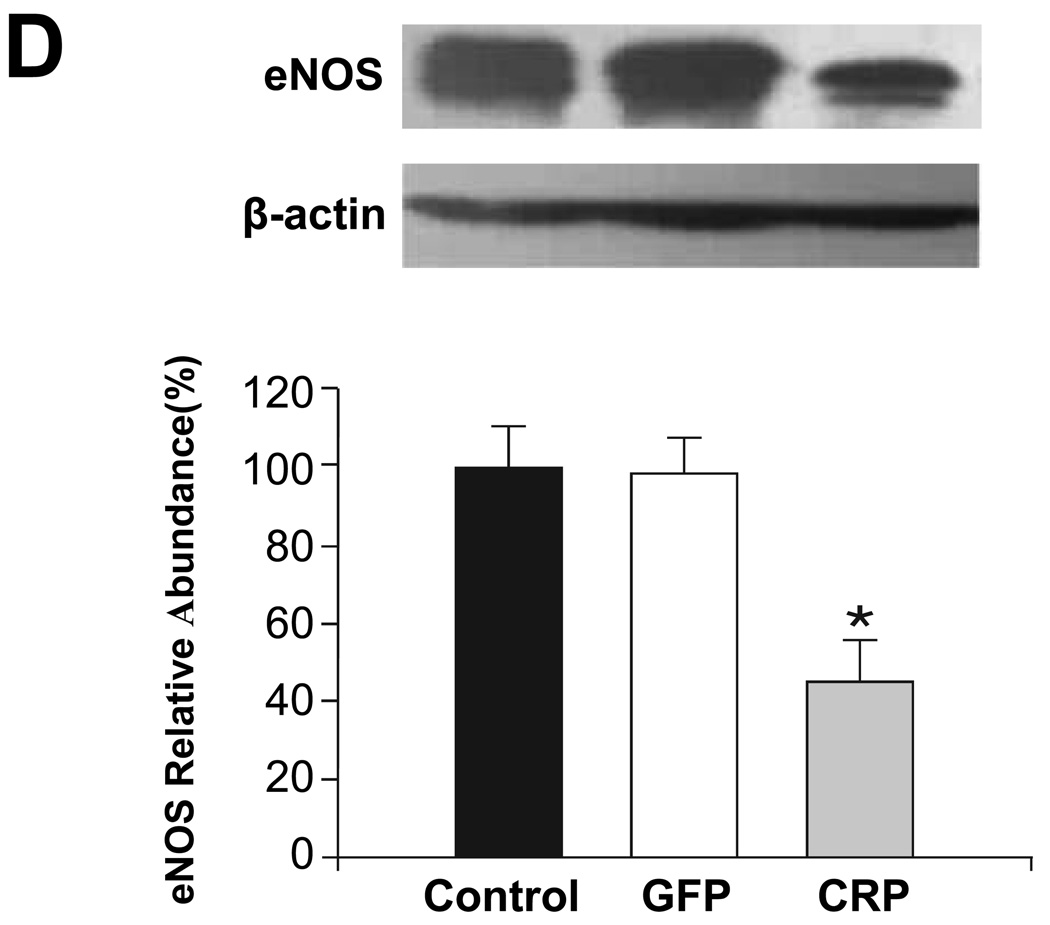
Previous studies have demonstrated that CRP downregulates eNOS and attenuates NO production 8, suggesting that inflammation and/or CRP induce endothelial dysfunction. We examined aortic expression of eNOS, ET-1, ETA, AT1 and AT2 to explore potential mechanisms underlying the effect of CRP gene delivery on vessel function and blood pressure. AAV-hCRP treatment significantly increased AT1, ETA and ET-1 mRNA concentrations while the treatment decreased AT2 and eNOS mRNA concentrations in aortic samples collected 4 months following gene delivery (Figure 4). In contrast, AAV-GFP had little or no effect on these parameters (Figure 4). Protein expression was assessed in the same aortic samples by western blotting and revealed the same pattern of changes and lack of effect of AAV-GFP compared to the saline control group (Figure 5A–D). Likewise, serum ET-1 concentrations increased following AAV-hCRP injection but were unchanged following AAV-GFP injection (Figure 5E). The reduction in eNOS mRNA and protein expression following AAV-hCRP injection (Figure 4D and Figure 5D) is consistent with the reduction in serum NO concentration and urine cGMP excretion (Figure 3).
Figure 4.
Effects of CRP gene transfer on mRNA concentrations in aortic tissue 4 months after gene delivery. Aortic mRNA concentrations of (A) AT1, (B) AT2, (C) ETA, (D) eNOS and (E) ET-1 are shown. Data are represented as the mean ± SEM of five independent experiments relative to GAPDH mRNA levels. * P < 0.05 vs. control group.
Figure 5.
Effects of CRP gene transfer on protein expression in aortic tissue 4 months after gene delivery. Aortic protein concentrations of (A) AT1, (B) AT2, (C) ETA and (D) eNOS (E) are shown. The upper portion is a representative western blot of the protein of interest while the lower graph is of the mean densitometric data of protein values normalized to β-actin. (E) Serum ET-1 concentrations as measured by ELISA. Data are presented as the mean ± SEM of three independent experiments. * P < 0.05 vs. control group.(AT1: P=0.000, AT2: P=0.000, ETA: P=0.000, eNOS: P=0.009, ET-1: P=0.001)
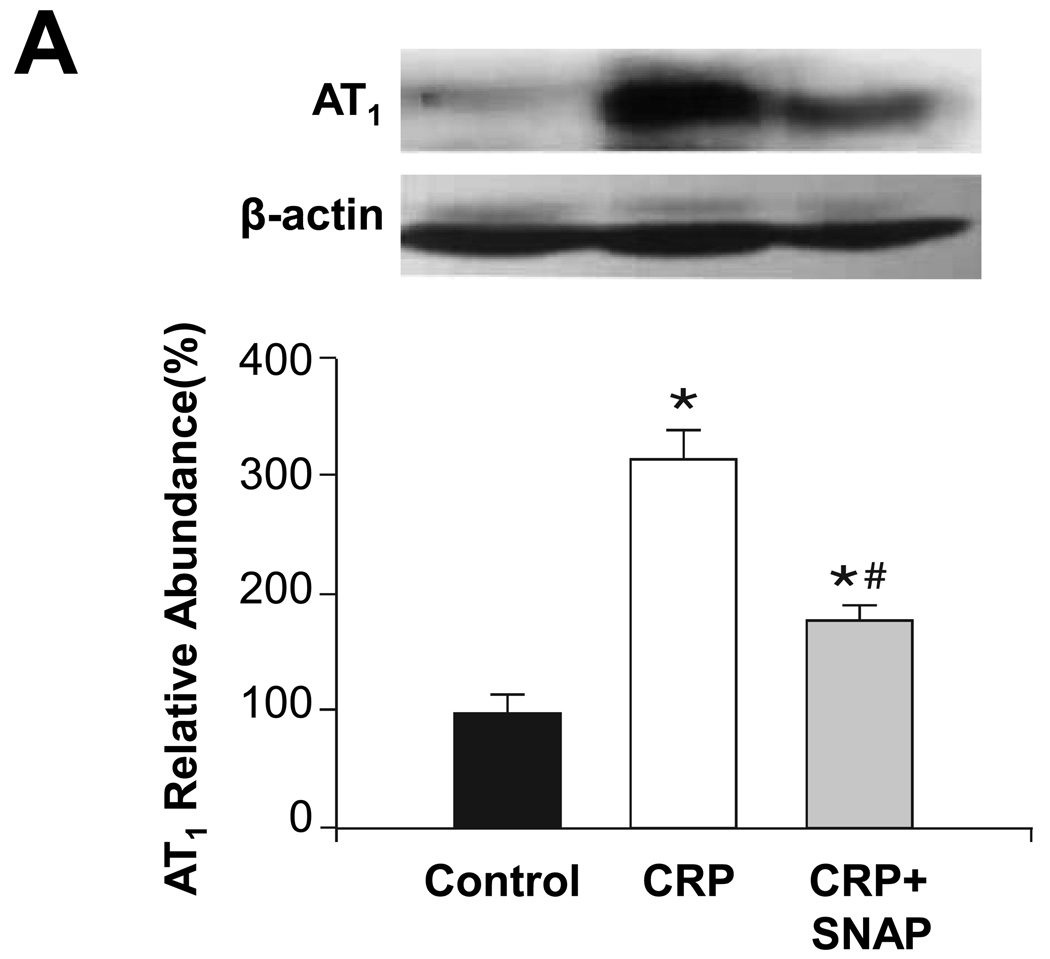
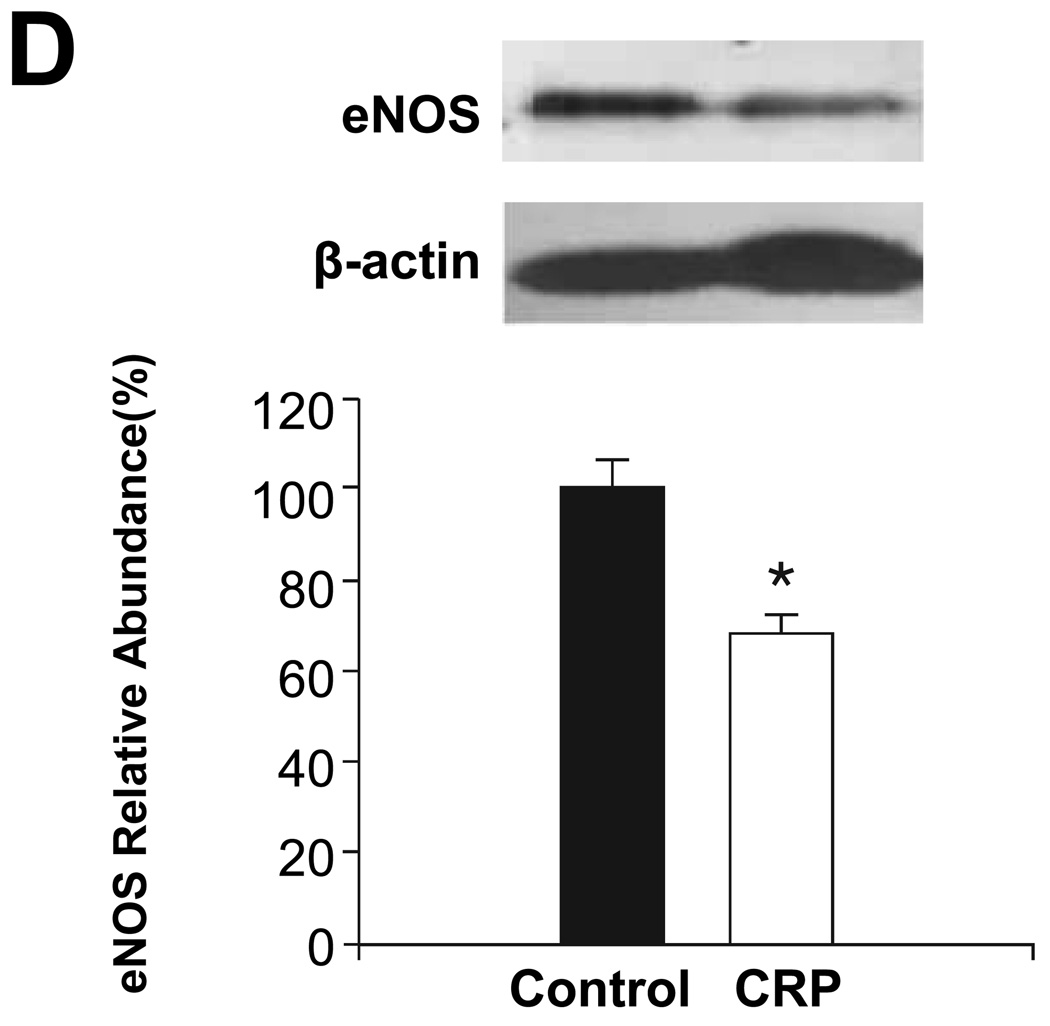
To determine whether these changes in AAV-hCRP-treated rats were induced directly by effects of CRP on vascular tissues, isolated thoracic aortas were incubated with hCRP and the ensuing effects on expression of eNOS, ET-1, ETA, AT1 and AT2 were evaluated. Similar to the findings from tissues isolated from intact animals, ex vivo incubation of aortas with hCRP was found to enhance AT1 and ETA and mRNA concentrations of ET-1, while AT2 and eNOS were decreased (Figure 6). Moreover, the changes observed in AT1, ETA, ET-1, and AT2 were partially reversed by the NO donor SNAP, suggesting a role for reduced NO production in the observed effects of hCRP (Figure 6). Thus, CRP appears to directly influence the expression of these parameters in the aorta in a NO-dependent manner.
Figure 6.
Effect of in vitro treatment with CRP on protein or mRNA concentrations in aortic tissues. Aortic segments excised from 2 month old rats were treated with CRP (50 mg/l) and SNAP (100 mg/l) as indicated for 6 hours. Protein concentrations of (A) AT1, (B) AT2, (C) ETA and (D) eNOS are shown. The upper portion is a representative western blot of the protein of interest, while the lower graph is of the mean densitometric data of protein values normalized to β-actin. (E) ET-1 mRNA values are shown normalized to GAPDH. Data are presented as the mean ± SEM of three independent experiments. * P < 0.05 vs. control group; # P < 0.05 vs. CRP group. (AT1: * P=0.002, #P=0.023 AT2: * P=0.000, #P=0.002 ETA: * P=0.003, #P=0.039 eNOS: * P=0.02, ET-1: * P=0.016, #P=0.003)
Discussion
There has been little direct investigation of the effect of CRP on blood pressure. Therefore, the present study was undertaken to examine the effects of CRP overexpression on blood pressure using an AAV delivery system in rats. A single injection of AAV-hCRP was found to result in efficient and sustained expression of hCRP and to cause a significant increase in blood pressure that lasted for up to 4 months. CRP overexpression was associated with impaired endothelial-dependent relaxation, increased arterial stiffness, and decreased NO production. Furthermore, ex vivo and in vivo studies revealed that CRP increased aortic ET-1, ETA, and AT1 expression and decreased AT2 and eNOS expression, and that the effects of CRP on ET-1, ETA, AT1 and AT2 were likely mediated by decreased NO production.
Previous studies have revealed that a single injection of CRP does not result in significant changes in blood pressure in rats 10 or mice 11. Of note, CRP was administered acutely in these studies, which poorly models the chronic increase in CRP that is observed clinically. More recently, Vongpatanasin et al9 evaluated blood pressure in CRP transgenic mice and found that CRP caused a sustained increase in blood pressure in vivo. However, other CRP transgenic mice do not show increased blood pressure 12. In the current study, we used an AAV vector to obtain sustained CRP expression in rats and found that increased CRP expression led to a significant increase in blood pressure compared with controls. Importantly, the blood pressure increase observed by the tail-cuff method was confirmed by invasive measurements of aortic and maximum left ventricular pressure via indwelling catheters.
Endothelial-derived NO has vasodilatory activity and the decreased availability of NO in the endothelium may be implicated in the development of hypertension. Indeed, mice with a disrupted eNOS gene have a significant increase in baseline blood pressure13. Moreover, a recent study reported that endogenous endothelial-derived NO tonically restrains blood pressure in normal human subjects by more than 30 mmHg 14. These and other data suggest that endothelial-derived NO may be an important regulator of blood pressure in multiple species.
Our observation of CRP-induced hypertension led us to hypothesize that the effect of CRP on blood pressure may have been mediated through NO deficiency. To address this possibility, we used two strategies to assess NO activity in vivo; we determined NO-dependent vasodilation and measured the nitrite/nitrate metabolites and a second messenger of NO (cGMP) as biochemical surrogates for NO production15. Gene transfer of CRP impaired endothelial-dependent relaxation and decreased serum nitrite/nitrate and urine cGMP concentrations. Furthermore, a previous study demonstrated that CRP did not influence NO independent relaxation in different species. Thus, we presume that NO synthesis deficiency likely contributed to hypertension in rats overexpressing CRP. We also observed no difference between the treatment groups in the in vitro assessment of NE-induced constriction of thoracic aortic rings, a result that differed from those reported in a recent study in which repeated administration of native CRP to mice induced hyporeactivity of isolated aortic rings to NE7. In addition to potential species differences, this difference between studies may be attributable to loss of the inhibitory effect of basal NO or to expression of other endothelium-derived contracting factors in the present study.
The NO deficiency could be due to a decrease in the synthesis of NO from endothelial cells and/or to an increase in NO elimination. Considerable experimental evidence suggests that CRP may act via the former mechanism. For example, pharmacological interventions that combine eNOS upregulation and reversal of eNOS uncoupling can markedly increase bioactive NO in the vasculature and reduce blood pressure in hypertensive rats 16, and CRP has been shown to cause downregulation of eNOS expression in cultured endothelial cells and to subsequently inhibit angiogenesis8. In the present study, we observed that CRP gene transfer attenuated eNOS expression at both the mRNA and protein levels, similar to previous findings. In addition, ex vivo experiments demonstrated that eNOS protein expression in aortic tissue was decreased after incubation with CRP. Our data indicate a direct inhibitory effect of CRP on eNOS expression. Additionally, Jialal et al have investigated the possible mechanisms of the inhibition of eNOS by CRP and found that CRP decreases BH4 due to inhibition of GTPCH1. CRP also causes the activation of NADPH oxidase resulting in superoxide production. Both of these effects will induce eNOS uncoupling by altering eNOS dimerization. These authors also reported the involvement of FcγR I(CD64) and FcγR II(CD32) in mediating the effects of CRP on eNOS inhibition17, plus the fact that CRP can bind to CD64 and CD32 on human aortic endothelial cells and be internalized to mediate its biological activity.18 Together, the inhibition of CRP on eNOS expression and activity caused subsequent NO deficiency.
ET-1, one of the most potent endogenous vasoconstrictors, is involved in endothelial dysfunction, cellular proliferation, and augments the vascular actions of Ang-II, NE and serotonin and is downregulated by activators of the NO/cGMP pathway 19. There is a consensus that ET-1 is involved in increaseing mean arterial pressure, and ET antagonists have been demonstrated to lower blood pressure in a hypertension model20. Furthermore, Verma and coworkers reported on a role for ET-1 in mediating the direct pro-inflammatory actions of CRP 21. These data led us to suspect a role for ET-1 in the mechanism of CRP-induced hypertension. As predicted, CRP gene transfer evoked the production of ET-1 both in plasma and aortic tissue. In addition, our study indicated that ET-1 expression was increased in aorta following CRP incubation, suggesting that upregulation of ET-1 was induced by CRP per se, and not as a response to CRP-induced hypertension.
Decreased NO activity can increase expression of ETs and enhance the contraction response to these peptides.22. We observed that the increase in ET-1 expression in aorta incubated with CRP was reversed by co-incubation with the NO donor. These findings, combined with our observation that CRP decreased NO production and eNOS expression, suggest that the effect of CRP on ET-1 was likely the result of decreased NO production. We also found that CRP significantly increased expression of the ETA receptor that mediates most of the vascular constrictive and cell proliferative actions of ET-1. Therefore, the upregulated ETA receptor expression may have enhanced the effect of the CRP-induced increase in ET-1 on blood pressure elevation.
Ang-II, the main effector peptide molecule of the renin-angiotensin system, is also implicated in the pathogenesis of hypertension. The biological effects of Ang-II are mediated by two major receptors, AT1 and AT2, and we found that CRP gene transfer induced AT1 expression increase while AT2 expression was decreased. Our ex vivo analyses indicated that co-incubation with the NO donor blunted the effects of CRP on AT1 and AT2 expression, and that impairment of NO biosynthesis is also involved in the effect of CRP on AT1 and AT2. In addition, activation of AT1 inhibits NO production 23 while activation of AT2 stimulates NO production 24. Taken together, these effects may represent a positive feedback cycle that enhances the effects of CRP and promotes the development of hypertension.
Several studies have recently shown that increased arterial stiffness is associated with an increased risk of cardiovascular events, although the relationship between arterial stiffness and hypertension has not been fully clarified. In the present study, we used Ea as a marker to reflect arterial stiffness 25 and found that CRP overexpression resulted in increased Ea. Previous studies have demonstrated that hypertension produces arterial stiffness by both functional and structural mechanisms, and it has been suggested that the relationship between hypertension and arterial stiffness may be bidirectional. In a population of normotensive subjects, aortic stiffness was found to be an important determinant of future increases in blood pressure 26, while Yasmin et al. recently reported that pulse wave velocity were related to concentrations of CRP in healthy individuals, suggesting that inflammatory processes may be involved in arterial stiffness 27. Furthermore, acute systemic inflammation produced by vaccination leads to deterioration of arterial stiffness, supporting a cause-and-effect relationship between inflammation and arterial stiffness 28. Considering these studies, we speculate that CRP-induced arterial stiffness may have contributed to the hypertension observed in the current study. However, further studies will be required to determine the actual mechanism(s) of CRP-induced impairment of arterial elasticity.
In summary, our study showed that AAV-mediated delivery of hCRP resulted in sustained hCRP expression and hypertension in rats. The effect of CRP on hypertension was related to vascular dysfunction, including decreased NO production, subsequent alteration in the ET and renin-angiotensin systems, and impaired arterial elasticity. These results support a causal role for CRP in the pathogenesis of hypertension. Moreover, they suggest the possibility that anti-inflammatory treatments such as statins may be beneficial in the management of hypertension. Prospective trials will be neeeded to assess whether lowering CRP can prevent and/or reduce the risk of hypertension development in humans.
Acknowledgments
Sources of Funding
This work was supported by grants from National Nature Science Foundation Committee of China (No. 30430320 and 30540087), National “973” projects (No. 2007CB512004) and International Collaboration project (No. 2005DFA30880), and by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
Footnotes
Disclosures
None
References
- 1.Chrysohoou C, Pitsavos C, Panagiotakos DB, Skoumas J, Stefanadis C. Association between prehypertension status and inflammatory markers related to atherosclerotic disease: The ATTICA Study. Am J Hypertens. 2004;17:568–573. doi: 10.1016/j.amjhyper.2004.03.675. [DOI] [PubMed] [Google Scholar]
- 2.Lakoski SG, Cushman M, Palmas W, Blumenthal R, D'Agostino RB, Jr, Herrington DM. The relationship between blood pressure and C-reactive protein in the Multi-Ethnic Study of Atherosclerosis (MESA) J Am Coll Cardiol. 2005;46:1869–1874. doi: 10.1016/j.jacc.2005.07.050. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107:363–369. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- 4.Sesso HD, Buring JE, Rifai N, Blake GJ, Gaziano JM, Ridker PM. C-reactive protein and the risk of developing hypertension. Jama. 2003;290:2945–2951. doi: 10.1001/jama.290.22.2945. [DOI] [PubMed] [Google Scholar]
- 5.Niskanen L, Laaksonen DE, Nyyssonen K, Punnonen K, Valkonen VP, Fuentes R, Tuomainen TP, Salonen R, Salonen JT. Inflammation, abdominal obesity, and smoking as predictors of hypertension. Hypertension. 2004;44:859–865. doi: 10.1161/01.HYP.0000146691.51307.84. [DOI] [PubMed] [Google Scholar]
- 6.Xiao X, Li J, Samulski RJ. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol. 1998;72:2224–2232. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwedler SB, Kuhlencordt PJ, Ponnuswamy PP, Hatiboglu G, Quaschning T, Widder J, Wanner C, Potempa LA, Galle J. Native C-reactive protein induces endothelial dysfunction in ApoE−/− mice: implications for iNOS and reactive oxygen species. Atherosclerosis. 2007;195:e76–e84. doi: 10.1016/j.atherosclerosis.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 8.Verma S, Wang CH, Li SH, Dumont AS, Fedak PW, Badiwala MV, Dhillon B, Weisel RD, Li RK, Mickle DA, Stewart DJ. A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation. 2002;106:913–919. doi: 10.1161/01.cir.0000029802.88087.5e. [DOI] [PubMed] [Google Scholar]
- 9.Vongpatanasin W, Thomas GD, Schwartz R, Cassis LA, Osborne-Lawrence S, Hahner L, Gibson LL, Black S, Samols D, Shaul PW. C-reactive protein causes downregulation of vascular angiotensin subtype 2 receptors and systolic hypertension in mice. Circulation. 2007;115:1020–1028. doi: 10.1161/CIRCULATIONAHA.106.664854. [DOI] [PubMed] [Google Scholar]
- 10.Clapp BR, Hirschfield GM, Storry C, Gallimore JR, Stidwill RP, Singer M, Deanfield JE, MacAllister RJ, Pepys MB, Vallance P, Hingorani AD. Inflammation and endothelial function: direct vascular effects of human C-reactive protein on nitric oxide bioavailability. Circulation. 2005;111:1530–1536. doi: 10.1161/01.CIR.0000159336.31613.31. [DOI] [PubMed] [Google Scholar]
- 11.Bisoendial RJ, Kastelein JJ, Peters SL, Levels JH, Birjmohun R, Rotmans JI, Hartman D, Meijers JC, Levi M, Stroes ES. Effects of CRP infusion on endothelial function and coagulation in normocholesterolemic and hypercholesterolemic subjects. J Lipid Res. 2007;48:952–960. doi: 10.1194/jlr.P600014-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.Paul A, Ko KW, Li L, Yechoor V, McCrory MA, Szalai AJ, Chan L. C-reactive protein accelerates the progression of atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2004;109:647–655. doi: 10.1161/01.CIR.0000114526.50618.24. [DOI] [PubMed] [Google Scholar]
- 13.Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 14.Gamboa A, Shibao C, Diedrich A, Choi L, Pohar B, Jordan J, Paranjape S, Farley G, Biaggioni I. Contribution of endothelial nitric oxide to blood pressure in humans. Hypertension. 2007;49:170–177. doi: 10.1161/01.HYP.0000252425.06216.26. [DOI] [PubMed] [Google Scholar]
- 15.Boger RH, Bode-Boger SM, Thiele W, Junker W, Alexander K, Frolich JC. Biochemical evidence for impaired nitric oxide synthesis in patients with peripheral arterial occlusive disease. Circulation. 1997;95:2068–2074. doi: 10.1161/01.cir.95.8.2068. [DOI] [PubMed] [Google Scholar]
- 16.Li H, Witte K, August M, Brausch I, Godtel-Armbrust U, Habermeier A, Closs EI, Oelze M, Munzel T, Forstermann U. Reversal of endothelial nitric oxide synthase uncoupling and up-regulation of endothelial nitric oxide synthase expression lowers blood pressure in hypertensive rats. J Am Coll Cardiol. 2006;47:2536–2544. doi: 10.1016/j.jacc.2006.01.071. [DOI] [PubMed] [Google Scholar]
- 17.Singh U, Devaraj S, Vasquez-Vivar J, Jialal I. C-reactive protein decreases endothelial nitric oxide synthase activity via uncoupling. J Mol Cell Cardiol. 2007;43:780–791. doi: 10.1016/j.yjmcc.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devaraj S, Du Clos TW, Jialal I. Binding and internalization of C-reactive protein by Fcgamma receptors on human aortic endothelial cells mediates biological effects. Arterioscler Thromb Vasc Biol. 2005;25:1359–1363. doi: 10.1161/01.ATV.0000168573.10844.ae. [DOI] [PubMed] [Google Scholar]
- 19.Brunner F, Bras-Silva C, Cerdeira AS, Leite-Moreira AF. Cardiovascular endothelins: essential regulators of cardiovascular homeostasis. Pharmacol Ther. 2006;111:508–531. doi: 10.1016/j.pharmthera.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Kassab S, Miller MT, Novak J, Reckelhoff J, Clower B, Granger JP. Endothelin-A receptor antagonism attenuates the hypertension and renal injury in Dahl salt-sensitive rats. Hypertension. 1998;31:397–402. doi: 10.1161/01.hyp.31.1.397. [DOI] [PubMed] [Google Scholar]
- 21.Verma S, Li SH, Badiwala MV, Weisel RD, Fedak PW, Li RK, Dhillon B, Mickle DA. Endothelin antagonism and interleukin-6 inhibition attenuate the proatherogenic effects of C-reactive protein. Circulation. 2002;105:1890–1896. doi: 10.1161/01.cir.0000015126.83143.b4. [DOI] [PubMed] [Google Scholar]
- 22.Zhou Y, Mitra S, Varadharaj S, Parinandi N, Zweier JL, Flavahan NA. Increased expression of cyclooxygenase-2 mediates enhanced contraction to endothelin ETA receptor stimulation in endothelial nitric oxide synthase knockout mice. Circ Res. 2006;98:1439–1445. doi: 10.1161/01.RES.0000224120.52792.10. [DOI] [PubMed] [Google Scholar]
- 23.Zhao X, Li X, Trusa S, Olson SC. Angiotensin type 1 receptor is linked to inhibition of nitric oxide production in pulmonary endothelial cells. Regul Pept. 2005;132:113–122. doi: 10.1016/j.regpep.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 24.Savoia C, Ebrahimian T, He Y, Gratton JP, Schiffrin EL, Touyz RM. Angiotensin II/AT2 receptor-induced vasodilation in stroke-prone spontaneously hypertensive rats involves nitric oxide and cGMP-dependent protein kinase. J Hypertens. 2006;24:2417–2422. doi: 10.1097/01.hjh.0000251902.85675.7e. [DOI] [PubMed] [Google Scholar]
- 25.Kelly RP, Ting CT, Yang TM, Liu CP, Maughan WL, Chang MS, Kass DA. Effective arterial elastance as index of arterial vascular load in humans. Circulation. 1992;86:513–521. doi: 10.1161/01.cir.86.2.513. [DOI] [PubMed] [Google Scholar]
- 26.Dernellis J, Panaretou M. Aortic stiffness is an independent predictor of progression to hypertension in nonhypertensive subjects. Hypertension. 2005;45:426–431. doi: 10.1161/01.HYP.0000157818.58878.93. [DOI] [PubMed] [Google Scholar]
- 27.Yasmin, McEniery CM, Wallace S, Mackenzie IS, Cockcroft JR, Wilkinson IB. C-reactive protein is associated with arterial stiffness in apparently healthy individuals. Arterioscler Thromb Vasc Biol. 2004;24:969–974. doi: 10.1161/01.ATV.zhq0504.0173. [DOI] [PubMed] [Google Scholar]
- 28.Vlachopoulos C, Dima I, Aznaouridis K, Vasiliadou C, Ioakeimidis N, Aggeli C, Toutouza M, Stefanadis C. Acute systemic inflammation increases arterial stiffness and decreases wave reflections in healthy individuals. Circulation. 2005;112:2193–2200. doi: 10.1161/CIRCULATIONAHA.105.535435. [DOI] [PubMed] [Google Scholar]