Abstract
Several studies have reported that reproductive hormones can alter baseline sleep-wake states, however, no studies in mice have examined whether reproductive hormone replacement in adult females and males influences sleep. In this study, we determined whether androgen replacement in males and estrogen replacement in females alter sleep-wake amount and sleep rebound after extended wakefulness. The gonads from adult male and female C57BL/6J mice were removed and animals were implanted with continuous release hormone or placebo pellets. Male mice received testosterone and females received 17β-estradiol. Recording electrodes were implanted to monitor sleep-wake states under baseline conditions and in response to six hours of sleep deprivation. During baseline recording estradiol-treated females exhibited a reduction in NREM sleep amount that was predominant during the dark phase. Testosterone-treated males conversely, exhibited an increase in NREM sleep amount. After sleep deprivation, hormone-treated males and females exhibited similar amounts of recovery sleep however both groups exhibited slightly more sleep than placebo-treated controls. The results of these experiments demonstrate that the androgens and estrogens are primarily responsible for sex differences in baseline sleep-wake amount but do not have substantial effects on homeostatic sleep rebound after extended wakefulness.
Keywords: sex, hormones, estradiol, testosterone, mouse, sleep deprivation
Introduction
Women and men sleep differently and recover differently from sleep deprivation (for a review see [11]). Though several sex differences in sleep are attributable to reproductive hormones, little information is available about how they affect sleep. This remains an important area of investigation since women bear the majority of hormone-associated sleep disruptions, particularly those related to pregnancy, postpartum recovery, and menopause. One solution to this lack of mechanistic information is to continue to uncover new facts in animal models about how reproductive hormones influence sleep and the ability to recover sleep after extended wakefulness.
Mice are becoming increasingly valuable to sleep research as advancements in genetics and molecular biology are yielding mutant mice with a variety of unique phenotypes [14]. However, without a better understanding of how basic traits such as sex influence sleep, the potential of genetic models to provide new information about sleep mechanisms is limited. We previously reported several sex differences in baseline sleep-wake architecture that were eliminated upon removal of the gonads from female and male mice [10]. Specifically, gonadally intact males had more daily sleep than intact females, but these baseline sex differences did not carry-over to recovery from sleep deprivation during which males and females exhibited virtually identical absolute amounts of sleep and wakefulness. Gonadectomy in male and female mice eliminated the baseline sex differences in sleep amount, but did not have substantial influences on rebound after sleep deprivation. These studies demonstrated that endogenous hormones are likely responsible for baseline sex differences in sleep, and suggest that sleep homeostatic regulatory mechanisms are insulated against the effects of hormones on sleep-wake amount. In the current study, we restored estradiol in ovariectomized females and testosterone in castrated males to physiological levels to determine whether hormone replacement restored baseline sex differences and altered the homeostatic response to sleep deprivation.
Materials and Methods
Adult male (n=16) and female (n=16) mice (C57BL/6J, 6-8 weeks of age) were purchased from Jackson Laboratories (Bar Harbor, ME) and maintained on a 14-hr light:10-hr dark (14L:10D) schedule throughout the study. Food and water were available ad libitum, and mice were housed for at least two weeks in the Northwestern University Animal Care Facility prior to experimental use. All protocols and procedures were approved by the Northwestern University Animal Care and Use Committee. Male and female mice were gonadectomized as previously described [10]. Immediately following gonadectomy while still under anesthesia, mice were implanted with electroencephalograph (EEG) and electromyograph (EMG) electrodes for polysomnographic recording of sleep and wake states (for complete methods, see [10]).
Mice were allowed to recover for at least 20 days after which they were implanted with 60-day continuous release hormone pellets (Innovative Research, Sarasota, FL). Mice were anesthetized by inhalation of isoflurane (0.2ml) in a transparent cylindrical induction chamber (10″×6″ diam) and a small incision was made dorsally and slightly lateral to the nape of the neck approximately 3mm caudal to the ear. Each hormone pellet (1/8″ diameter) was subcutaneously implanted. Females were implanted with 60-day continuous release 17β-estradiol (1,3,5-Estratriene-3,17β-diol; 0.72mg total; 0.012mg/day; n=8) or placebo (n=8) and male mice were implanted with testosterone (17β-hydroxyandrost-4-en-3-one; 12.5mg total; 0.21mg/day; n=8) or placebo (n=8). After an additional seven days of recovery, mice were placed in a sleep-recording chamber and connected to a lightweight rotating tether system (Plastics One, Roanoke, VA), which enabled complete freedom of movement throughout the cage. Except for the recording tether, conditions in the recording chamber were identical to those in the home cage. Mice were allowed a minimum of seven additional days to acclimate to the tether after which time they underwent 48 hrs of baseline sleep-wake recording beginning at light onset (zeitgeber time (ZT) 0). The second day of baseline recording was analyzed in this study. Recording of polysomnographic waveforms has been previously described [10]. EEG signals were amplified approximately 10,000 times, with -6dB/oct high-pass and low-pass filter settings at 1.0Hz and 30Hz (3dB), respectively. EMG signals were amplified 5,000 times and low-pass filtered at 100Hz. Both signals were then digitized at 100Hz by an analog-to-digital converter (Data Translation model DT-01EZ) and stored on an IBM AT computer system. Waveforms were collected using Multisleep (Actimetrics, Evanston, IL), a software system designed specifically for gathering and analyzing rodent sleep data.
After baseline recording mice were subjected to a standard homeostatic challenge of 6 hrs of sleep deprivation (beginning at ZT 0) and then allowed 18 hrs of recovery sleep. Mice were sleep deprived by a gentle handling procedure (previously described in [10]). After sleep-wake recording, mice were returned to their home cages and were allowed seven days of recovery. On day 8, mice were decapitated and trunk blood was collected in heparinized tubes, stored at 4°C overnight, and centrifuged the following day at 1,500 g for 20 min at 8°C, and the serum was stored at −80°C until assayed. Levels of testosterone and luteinizing hormone (an indicator of estradiol levels [2]) were determined using radioimmunoassay (RIA). Hormone levels in pellet-implanted females (luteinizing hormone: 2.4±0.2ng/ml;) and males (testosterone: 0.12±.02ng/ml) were in the physiological range.
All waveforms were classified into 10-second epochs of wake (low-voltage, high-frequency EEG; high-amplitude EMG), NREM sleep (high-voltage, mixed-frequency EEG; low-amplitude EMG) or REM sleep (low-voltage EEG with a predominance of theta activity (6-10 Hz); very low amplitude EMG). For power spectral analysis, each epoch was divided into five 2-second subepochs, and the EEG was subjected to fast Fourier transformation. All 10-second NREM epochs were included in the spectral analysis. The average power density in the delta (1-4 Hz) frequency range was determined. Data were analyzed using analysis of variance (ANOVA) to detect between-(male vs female; placebo vs hormone-replaced) and within-(14-h light vs 10-h dark; baseline vs recovery) factor differences. A repeated measures ANOVA was used to detect the effect of time (light vs dark and 2-h intervals) on experimental variables. Posthoc multiple comparison analysis (Tukey's) was used to follow-up significant main effects. Significance was set at p<.05 for all analyses.
Results
Estradiol replacement in females decreased NREM sleep amount
Estradiol-treated females exhibited approximately 100 min less NREM sleep than the placebo-treated females during baseline recording. The entire reduction of NREM sleep occurred during the dark phase. When the baseline recording period was subdivided into light and dark phases (Figure 1), there was an effect of estradiol replacement (F1,14=7.3, p=.001) and time (F1,14=168.9, p<.001) on percentage of time spent in NREM sleep and also a treatment × time interaction (F1,14=5.5, p=.004). During the light phase, the percentage of NREM sleep was not different between the two groups. There was a significant dark phase decrease in REM sleep in estradiol-treated females, although the effect looked relatively small in terms of absolute time (shown in Figure 1).
Figure 1.
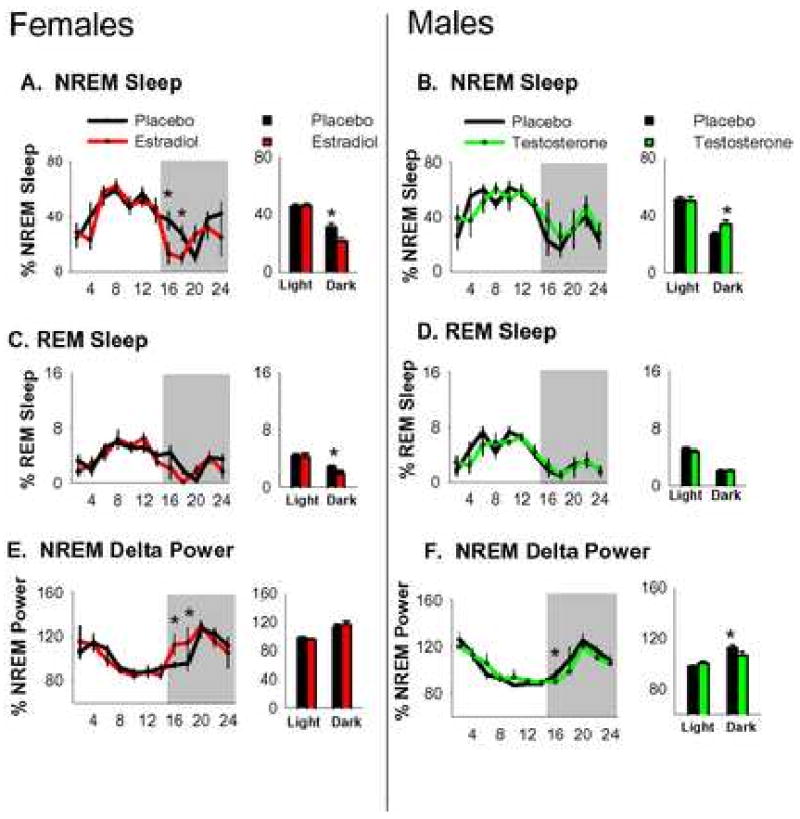
Baseline NREM sleep (A&B) REM sleep (C&D) and NREM delta power (E&F) in gonadectomized (placebo) and hormone replaced females and males. Sleep data is depicted as percentage of total recording time. Delta power is normalized as a percentage of total power during recording period (24 hrs). Line graphs on the left depict 24 hrs of baseline sleep states (14L:10D) averaged into 2-hr intervals. Shaded area represents the 10-hr dark phase. Bar graphs on the right depict the same data averaged into the light and dark phases. Error bars represent mean±s.e.m. *p<.05.
The reduction of sleep amount was accompanied by a concomitant increase in wake amount. Females that received estradiol exhibited 112 min more wakefulness than those that received placebo. The majority of the additional wakefulness occurred in the dark phase during which females that received estradiol exhibited approximately 100 min more wakefulness than those that received placebo. When baseline recording was subdivided into the light and dark phases, there was an effect of estradiol replacement (F1,14=9.7, p<.001) on percentage of time awake and a treatment × time interaction (F1,14=4.2, p=.015). During the dark phase, estradiol-treated females displayed more wakefulness (p<.005, Tukey's) than placebo-treated controls.
Testosterone replacement in males increased NREM sleep amount
Testosterone-treated males exhibited approximately 20 min more NREM sleep than the placebo-treated males during baseline recording. The increase of NREM sleep occurred completely during the dark phase. When the baseline recording period was subdivided into light and dark phases (Figure 1), there was an effect of time (F1,14=174.6, p<.001) on percentage of NREM sleep and also a treatment × time interaction (F1,14=6.7, p=.006). There were no differences (p>.05; Tukey's) in REM sleep amount between the two groups during 24 hrs of recording or when the recording period was subdivided into the light phase and dark phase (Figure 1).
The increase of NREM sleep by testosterone was accompanied by a concomitant decrease in wake amount. Males that received testosterone exhibited approximately 20 min less wakefulness than those that received placebo and the reduction in wakefulness occurred during the dark phase. When baseline recording was subdivided into the light phase and the dark phase, there was a treatment × time interaction (F1,14=4.7, p=.027). During the dark phase, testosterone-treated males displayed less wakefulness (p<.05, Tukey's) than the placebo-treated males. A main effect of time (F1,14=155.7, p<.001) indicated that both groups exhibited a normal diurnal distribution of wakefulness with a rapid increase at the transition to darkness and more wakefulness (p<.005, Tukey's) during the dark phase than the light. The combination of hormone replacement in females and males restored sex differences in baseline sleep amount (Table 1).
Table 1.
Mean±s.e.m. of wake, NREM, and REMS sleep in minutes in estradiol-treated females and testostereone-treated males. *Different from values in estradiol-treated females (p<.05 Tukey's).
| Light | Dark | |||||
|---|---|---|---|---|---|---|
| Wake | NREMS | REMS | Wake | NREMS | REMS | |
| Estradiol | 368.1 ± 13.5 | 422.8 ± 13.6 | 42.5 ± 1.7 | 421.5 ± 12.2 | 165.8 ± 11.5 | 12.8 ± 1.2 |
| Testosterone | 350.7 ± 17.7 | 432.4 ± 9.5 | 36.6 ± 2.5 | 353.0 ± 15.0* | 230.0 ± 15.6* | 16.6 ± 1.0 |
Estradiol and testosterone alter baseline sleep pressure
In order to identify changes in homeostatic sleep pressure during baseline sleep, we analyzed the 24-hr distribution of NREM delta power, a quantitative indicator of sleep pressure. Absolute NREM delta power during baseline recording was averaged into 2-hr intervals and normalized by taking the quotient of each 2-hr average over the 24-hr average for each mouse.
The distribution of NREM delta power was altered by estradiol replacement. When normalized NREM delta power was divided 2-hr intervals, a repeated-measures 2-way ANOVA revealed an effect of estradiol replacement (F1,14=1.9, p=.03) and time (F11,14=23.1, p=.002) and a treatment × time interaction (F11,154=2.3, p=.05; Figure 1). Though both groups began the light phase with elevated delta power, posthoc analysis revealed that delta power was higher in estradiol-treated females (p<.05, Tukey's) after the light to dark transition (ZT 14-18). These differences in delta power suggest an increase sleep pressure during this period which is consistent with the peak levels of wakefulness observed in estradiol-treated females at the same time intervals. At all other time-points, delta power between the two groups was similar.
The distribution of normalized NREM delta power was not substantially altered by testosterone replacement. When delta power was averaged into 2-hr intervals, a repeated-measures 2-way ANOVA revealed an effect of time (F11,14=1.8, p=.05) and a treatment × time interaction (F11,154=1.9, p=.05). Posthoc analysis revealed that delta power was lower (p<.05) in testosterone-treated males (p<.05, Tukey's) after the light to dark transition (ZT 14-16). At all other time-points, delta power between the two groups was similar.
Hormone replacement had few effects on recovery from sleep deprivation
Since total sleep and wake amounts during the first 6 hrs of the light phase are similar between hormone-treated and placebo-treated groups, during sleep deprivation all groups lost similar amounts of sleep. When recovery sleep was averaged into 2-hr intervals a repeated-measures there were effects of estradiol replacement (F1,14=14.5, p=.003), and time (F11,14=113.6, p<.001), and a treatment × time interaction (F11,154=94.5, p=.002; Figure 2). Posthoc analysis revealed that during the portion of the recovery period which extended into the dark phase, estradiol-treated females exhibited less NREM sleep than placebo-treated females at ZT 14-18 and again during the last two hours of the dark phase. Placebo-treated females exhibited less NREM sleep at ZT 18-20.
Figure 2.
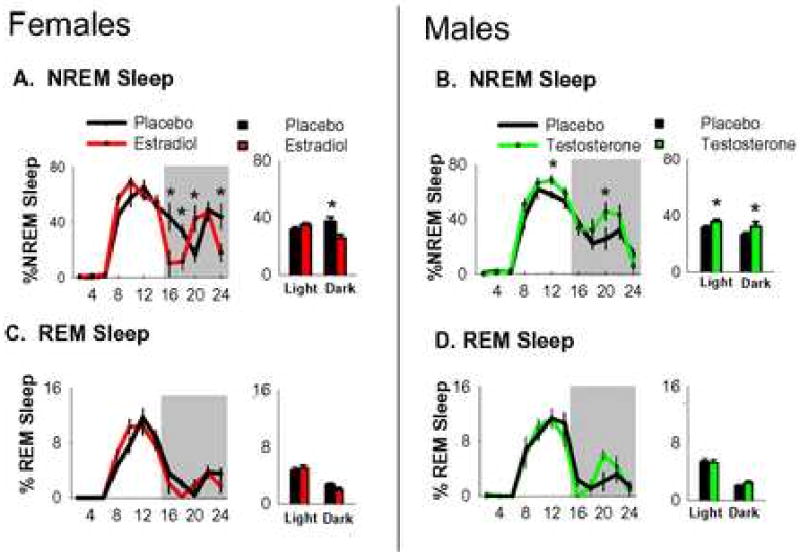
NREM (A&B) and REM (C&D) sleep in gonadectomized and hormone replaced females and males after 6 hrs of sleep deprivation. Sleep data is depicted as percentage of total recording time. Line graphs on the left depict 24 hrs of sleep states averaged into 2-hr intervals. Shaded area represents the 10-hr dark phase. Bar graphs on the right depict the same data averaged into the light and dark phases. Light phase bars include sleep deprivation. Error bars represent mean±s.e.m. *p<.05.
Testosterone-treated males also exhibited more NREM recovery sleep than placebo-treated males, though the additional sleep occurred later during recovery. A repeated-measures 2-way ANOVA detected effects of testosterone replacement (F1,14=49.9, p<.001), time (F11,14=116.0, p<.001), and a treatment × time interaction (F11,154=1.2, p=.05). Posthoc analysis revealed that testosterone-treated males exhibited more NREM sleep than placebo-treated males at ZT 10-12. During the remainder of the recovery period which extended into the dark phase, testosterone-treated males exhibited more NREM sleep than placebo-treated males at ZT 18-22 (Figure 2). Testosterone replacement had negligible effects on REM recovery from homeostatic challenge. When REM recovery sleep was averaged into 2-hr intervals, there were no effects of testosterone treatment (p>.05). Interestingly, there was a sex difference in NREM sleep in gonadectomized mice during the dark phase of recovery sleep. Specifically, gonadectomized females exhibited approximately 51 min more NREM sleep during the dark phase (t14=3.07, P=.013) than gonadectomized males (both groups received placebo).
Absolute NREM delta power during recovery sleep was averaged into 2-hr intervals and normalized by taking the quotient of each 2-hr average over the 24-hr baseline average for each mouse. Normalized NREM delta power was not different between male placebo and testosterone treated groups or female placebo and estradiol treated groups at any time during 18 hrs of recovery from sleep deprivation (ANOVA; Figure 3).
Figure 3.
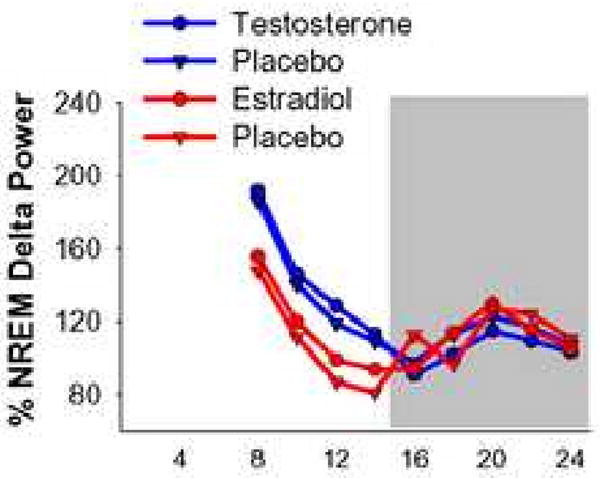
NREM delta power and NREM sleep amount during recovery from 6 hrs of sleep deprivation. NREM delta power (normalized as a percentage of total baseline delta power) average into 2-hr intervals in gonadectomized and hormone replaced females and males. Shaded area represents the 10-hr dark phase.
Discussion
Our first finding was that the baseline sex differences in sleep and wake amount that were eliminated by gonadectomy [10] were almost completely restored by hormone replacement. Estradiol replacement in females caused a decrease in sleep amount that predominated during the dark phase and was sufficient to restore baseline sleep in ovariectomized females to levels observed in intact females. Testosterone replacement in males caused an increase in sleep amount during the dark phase which resulted in slight reductions in wake amount during the 24-hr baseline recording period. The combined effects of estradiol replacement in females and testosterone replacement in males restored sex differences in baseline sleep and wakefulness.
In the present study, all groups exhibited a rebound after sleep deprivation. Interestingly, both estradiol and testosterone replacement had positive effects on NREM recovery sleep. Therefore, though estradiol and testosterone both altered recovery, the alterations were not sex- or hormone- specific. Conversely, after sleep deprivation gonadectomized females exhibited more NREM sleep during the dark phase than gonadectomized males. In a previous study, when sleep deprivation was administered during the latter half of the light phase, gonadectomized females and males exhibited identical amounts of recovery sleep [10]. However, in this study when sleep deprivation was administered during the first half of the light phase in gonadectomized mice, subsequent NREM recovery sleep exhibited sex differences during the dark phase. This result is an example of a sex difference in mice that does not appear to be dependent on gonadal hormones and may potentially be dependent on sex-specific organization of sleep regulatory mechanisms or on sex chromosome complement. These results suggest that non-gonadal determinants of sex have influences on sleep rebound that may interact with gonadal hormones and provide further evidence that the effects of sex on homeostatic recovery to sleep loss are complex and appear to involve gonadal and non-gonadal factors.
Recent studies have suggested a potential mechanism through which gonadal hormones may regulate sleep. Hadjimarkou et al, [5] found that estradiol replacement in female rats decreases protein levels of the immediate early gene product fos in the ventrolateral preopitc nucleus (VLPO) of the hypothalamus. Since VLPO neurons send inhibitory projections to wake promoting areas of the brain, it is likely that decreases in fos expression signal a reduction of wake inhibition. The VLPO of estradiol treated rats also exhibit decreases in the lipocalin-prostaglandin D synthase (L-PGDS), a lipid enzyme that has sleep promoting properties. These results suggest that L-PGDS or its catalytic product prostaglin D2 may mediate the ability of estradiol to increase wakefulness. Similar to mice, in rats the ability of estrogen to alter sleep occurs primarily during the dark phase, suggesting a conservation of mechanism between these species. It is important to note however, that the effects of estradiol in mice are primarily on NREM sleep while in rats, estradiol decreases REM sleep amount [5]. That the same treatment alters different sleep stages suggests that estradiol could be acting on different sleep regulatory pathways in mice and rats.
In the current study, most of the baseline sex differences in sleep amount in hormone-replaced mice occurred during the active (dark) phase. Therefore, the diurnal distribution of sex differences in sleep amount was conserved and was similar to what has been reported in intact mice [10]. Since each hormone was released continuously, these results demonstrate that the secretion of endogenous gonadal hormones need not be rhythmic to produce diurnal sex differences. One possible explanation is that the circadian timing system may act downstream of gonadal hormones to restrict their ability to alter sleep to the active phase. Evidence of this potential arrangement include findings that: 1) the suprachiasmatic nucleus (SCN) of the hypothalamus, the location of the primary circadian clock, is immunoreactive for androgen and estrogen receptors [7,12], and 2) estrogen receptor mRNA levels exhibit a circadian rhythm of expression in the SCN in rats [13]. Studies in primates and mice suggest that the SCN has wake promoting abilities during the active phase [3,4], though other studies contradict this viewpoint (for a review, see [9]). Therefore, a circadian rhythm of gonadal hormone receptor expression in the SCN (or other sleep regulatory nucleus) could be responsible for the selective ability of gonadal hormones to alter sleep during the dark phase.
This study demonstrates that reproductive hormone changes can alter baseline sleep-wake states but do not have substantial effects on the homeostatic response to sleep loss. Therefore, it appears that the ability to recover lost sleep may be hard-wired and resistant to changes in reproductive hormone levels. We have found that acute sleep responses to stress are sensitive to reproductive hormone levels (unpublished results). Taken together, a model emerges in which sex differences in daily sleep amount and quality may result from sex differences in sleep responses to stress. In other words, sex differences in the ability to recover from sleep loss appear to be dependent upon whether the sleep depriving stimulus evokes a biological stress response.
In humans, mood and anxiety disorders are commonly associated with sleep impairments. Furthermore sex differences in the incidence of insomnia are strikingly similar to sex disparities in the risk for mood and anxiety disorders [1,6,8]. Since stress, and perhaps the sleep loss that often accompanies stress, is a potential causative element of both sleep disorders and mood disorders, it is important to determine how the biological determinants of sex alter sleep and influence the ability to recover from sleep loss. The results from the current study support the hypothesis that sex differences in sleep and the ability to recover from sleep loss are mediated at least in part by biological determinants of sex.
Acknowledgments
We wish to thank Ms. Marla Isaac, Ms. Brigitte Mann, and Dr. Jon Levine in the Neurobiology and Physiology department at Northwestern University for hormone assays. We also thank the NIA for funding support through grant R-01-AG-18200, and the Institute for Women's Health Research at the Feinberg School of Medicine, Northwestern University for supporting this research through the Pioneer Award competitive funding mechanism.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Accortt EE, Freeman MP, Allen JJ. Women and major depressive disorder: clinical perspectives on causal pathways. J Womens Health (Larchmt) 2008;17:1583–1590. doi: 10.1089/jwh.2007.0592. [DOI] [PubMed] [Google Scholar]
- 2.Bronson FH. Serum FSH, LH, and prolactin in adult ovariectomized mice bearing silastic implants of estradiol: responses to social cues. Biol Reprod. 1976;15:147–152. doi: 10.1095/biolreprod15.2.147. [DOI] [PubMed] [Google Scholar]
- 3.Easton AA, Meerlo P, Bergmann B, Turek FW. The suprachiasmatic nucleus regulates sleep timing and amount in mice. Sleep. 2004;27:1307–1318. doi: 10.1093/sleep/27.7.1307. [DOI] [PubMed] [Google Scholar]
- 4.Edgar DM, Dement WC, Fuller CA. Effect of SCN lesions on sleep in squirrel monkeys: evidence for opponent processes in sleep-wake regulation. J Neurosci. 1993;13:1065–1079. doi: 10.1523/JNEUROSCI.13-03-01065.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hadjimarkou MM, Benham R, Schwarz JM, Holder MK, Mong JA. Estradiol suppresses rapid eye movement sleep and activation of sleep-active neurons in the ventrolateral preoptic area. Eur J Neurosci. 2008;27:1780–1792. doi: 10.1111/j.1460-9568.2008.06142.x. [DOI] [PubMed] [Google Scholar]
- 6.Howell HB, Brawman-Mintzer O, Monnier J, Yonkers KA. Generalized anxiety disorder in women. Psychiatr Clin North Am. 2001;24:165–178. doi: 10.1016/s0193-953x(05)70212-4. [DOI] [PubMed] [Google Scholar]
- 7.Karatsoreos IN, Wang A, Sasanian J, Silver R. A role for androgens in regulating circadian behavior and the suprachiasmatic nucleus. Endocrinology. 2007;148:5487–5495. doi: 10.1210/en.2007-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krishnan V, Collop NA. Gender differences in sleep disorders. Curr Opin Pulm Med. 2006;12:383–389. doi: 10.1097/01.mcp.0000245705.69440.6a. [DOI] [PubMed] [Google Scholar]
- 9.Mistlberger RE. Circadian regulation of sleep in mammals: role of the suprachiasmatic nucleus. Brain Res Brain Res Rev. 2005;49:429–454. doi: 10.1016/j.brainresrev.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Paul KN, Dugovic C, Turek FW, Laposky AD. Diurnal sex differences in the sleep-wake cycle of mice are dependent on gonadal function. Sleep. 2006;29:1211–1223. doi: 10.1093/sleep/29.9.1211. [DOI] [PubMed] [Google Scholar]
- 11.Paul KN, Turek FW, Kryger MH. Influence of sex on sleep regulatory mechanisms. J Womens Health (Larchmt) 2008;17:1201–1208. doi: 10.1089/jwh.2008.0841. [DOI] [PubMed] [Google Scholar]
- 12.Vida B, Hrabovszky E, Kalamatianos T, Coen CW, Liposits Z, Kallo I. Oestrogen receptor alpha and beta immunoreactive cells in the suprachiasmatic nucleus of mice: distribution, sex differences and regulation by gonadal hormones. J Neuroendocrinol. 2008;20:1270–1277. doi: 10.1111/j.1365-2826.2008.01787.x. [DOI] [PubMed] [Google Scholar]
- 13.Wilson ME, Rosewell KL, Kashon ML, Shughrue PJ, Merchenthaler I, Wise PM. Age differentially influences estrogen receptor-alpha (ERalpha) and estrogen receptor-beta (ERbeta) gene expression in specific regions of the rat brain. Mech Ageing Dev. 2002;123:593–601. doi: 10.1016/s0047-6374(01)00406-7. [DOI] [PubMed] [Google Scholar]
- 14.Winrow CJ, Turek FW, Renger JJ. Genetic approaches for target identification in sleep/wake systems. IDrugs. 2008;11:811–816. [PubMed] [Google Scholar]


