Abstract
We transformed an alkaloid biosynthetic gene with reengineered substrate specificity into Catharanthus roseus. The resulting transgenic plant cell culture produced a variety of unnatural alkaloid compounds when cocultured with simple, achiral, commercially available precursors that the reengineered enzyme was designed to accept. This work demonstrates the power of genetic engineering to retailor the structures of complex alkaloid natural products in plant culture.
Modification of natural products can yield analogs of natural products, or ‘unnatural products’, with improved or new medicinal properties1. Introduction of genes with altered substrate specificity into existing natural product biosynthetic pathways has resulted in fermentation of unnatural microbe-derived compounds from non-natural starting substrates2. Noteworthy achievements in plant metabolic engineering have enabled tuning of natural product production levels in plants3 and heterologous expression of natural compounds in plants4. However, biosynthesis of unnatural plant-derived compounds has primarily focused on relatively short flavonoid5 or carotenoid6 pathways that can be heterologously expressed in microbial hosts. Production of unnatural products in plants is largely unprecedented. It is imperative to explore the capacity, scope and limitations for metabolic engineering of unnatural products within the plant cell environment. Here we describe the introduction of a reengineered alkaloid biosynthetic gene into the flowering plant Catharanthus roseus (Madagascar periwinkle) cell culture. The resulting culture produces numerous unnatural alkaloid compounds when cocultured with commercially available precursors that the reengineered enzyme has been designed to accept. This work demonstrates the power of genetic engineering to harness the potential of complex plant-derived natural product pathways.
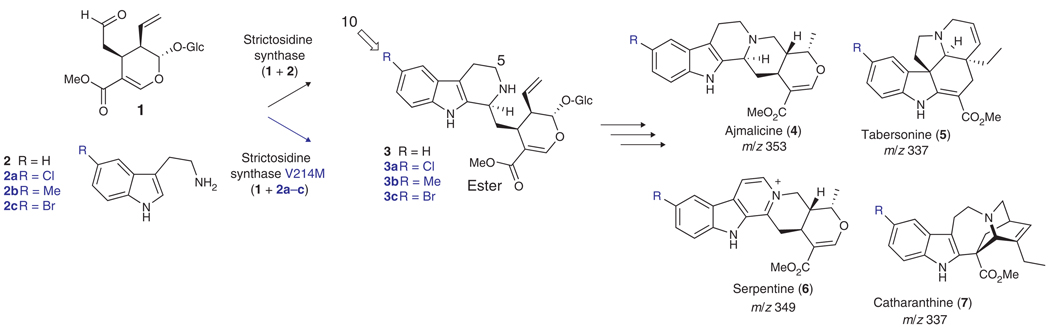
Monoterpene indole alkaloid biosynthesis in C. roseus is complicated and only partially characterized7. This pathway produces a variety of alkaloids with diverse biological activities and complex molecular architecture from the iridoid terpene secologanin (1) and tryptamine (2) (Scheme 1). Some successes have been recently reported for heterologous expression of isoquinoline-type alkaloids in microbes8,9. However, the paucity of cloned biosynthetic genes makes heterologous expression of monoterpene indole alkaloids impossible at this time. Furthermore, the complexity of this pathway makes complex monoterpene indole alkaloid expression in microbes a challenging undertaking. For example, biosynthesis of tabersonine, an early precursor for the anticancer bisindole alkaloids, requires an estimated 15 distinct enzymatic steps from the geraniol terpene and tryptamine precursors7. Monoterpene indole alkaloid biosynthesis is therefore a compelling system for exploring unnatural product biosynthesis in plant cell culture.
Scheme 1.
Biosynthesis of monoterpene indole alkaloids. Secologanin (1), a complex terpenoid derived from geraniol, and tryptamine (2) form monoterpene indole alkaloids 4, 5, 6 and 7 via the biosynthetic intermediate strictosidine (3). Strictosidine synthase, the enzyme that catalyzes formation of strictosidine, has expanded substrate specificity enabling turnover of tryptamine analogs 2a, 2b and 2c when the point mutation V214M is incorporated into the protein sequence.
The study of precursor-directed biosynthesis in C. roseus has revealed that one key bottleneck in the production of unnatural alkaloids is the stringent substrate specificity of strictosidine synthase, the enzyme that catalyzes formation of the biosynthetic intermediate strictosidine (3) from secologanin and tryptamine (Scheme 1)10. Although some analogs of tryptamine and secologanin starting materials can be turned over by the strictosidine synthase enzyme, many substrates are not recognized by this enzyme, such as tryptamine analogs with substituents at the 5 and α positions. The recently reported crystal structure of strictosidine synthase11 has enabled design of enzyme mutants with broadened substrate specificities, thereby allowing enzymatic production of a greater variety of strictosidine analogs as previously reported10,12,13. These strictosidine analogs, synthesized in vitro from secologanin (isolated from Lonicera tatarica leaves10) and tryptamine analogs, were fed to wild-type C. roseus cultures. While strictosidine analogs substituted at the ester and 5 positions were incorporated only into heteroyohimine-type alkaloids (for example, ajmalicine)10,12, a strictosidine analog substituted at the 10 position, 3c, appeared to be incorporated into many branches of the pathway (Scheme 1)10. The strictosidine synthase mutant (V214M) that is able to catalyze the formation of 3a, 3b and 3c was therefore chosen for transformation into C. roseus to explore the prospects of metabolic reprogramming in this medicinal plant.
Hairy root cell culture, which results after infection of a plant with Agrobacterium rhizogenes, produces a range of alkaloids including ajmalicine (4), tabersonine (5) and serpentine (6) (Scheme 1)14. Using previously established transformation protocols15, the strictosidine synthase mutant gene containing the point mutation V214M was transformed into hairy root culture under the control of the 35S cauliflower mosaic virus (CaMV) promoter (pCAMBIA vector, Supplementary Methods online). PCR analysis of genomic DNA confirmed incorporation of the strictosidine synthase sequence encoding the V214M point mutation, the 35S CaMV promoter and the hygromycin antibiotic selection marker, thereby indicating that the strictosidine synthase mutant was incorporated into the C. roseus genome (Supplementary Methods).
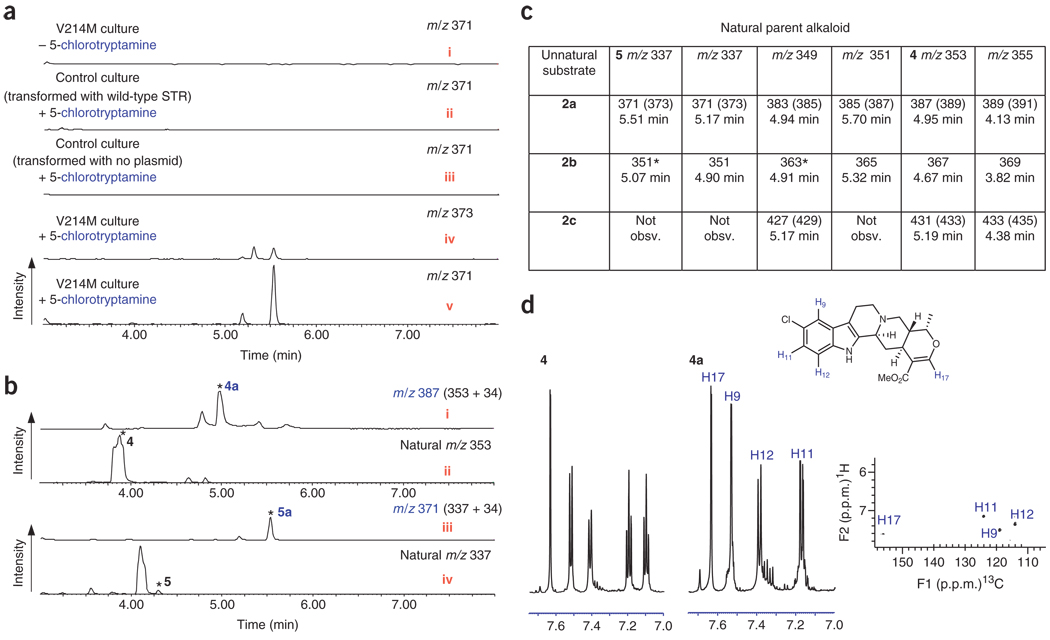
Transgenic hairy root lines harboring the V214M mutant enzyme were cultured in liquid Gamborg’s B5 medium (Sigma) in the presence of 0.5 mM 5-chlorotryptamine (2a), 5-methyltryptamine (2b) or 5-bromotryptamine (2c). These substrate analogs are only turned over by the V214M mutant enzyme and are not recognized by natural strictosidine synthase10. After one week of culture, the plant material was lysed and alkaloids were extracted into organic solvent. LC-MS analysis of these extracts indicated appearance of new compounds that appeared to be derived from the exogenous substrates 2a, 2b and 2c (Fig. 1a–c; Supplementary Fig. 1 and Supplementary Table 1 online). Unnatural alkaloids could be rapidly assigned by LC-MS as deriving from 2a and 2c, as chlorine and bromine exhibit a characteristic isotopic signature (Fig. 1a, iv,v and Supplementary Fig. 1). Control experiments clearly indicated that these compounds were not present when the tryptamine analog was absent from the medium (Fig. 1a, i). Hairy root lines transformed with the wild-type strictosidine synthase gene also failed to produce chlorinated alkaloids when cultivated with 2a (Fig. 1a, ii and Supplementary Fig. 1). Hairy root cultures infected with A. rhizogenes lacking the V214M mutant did not produce these compounds when cultivated in the presence of tryptamine analogs 2a, 2b and 2c (Fig. 1a, iii and Supplementary Fig. 1).
Figure 1.
Unnatural alkaloid production in C. roseus hairy root culture expressing reengineered strictosidine synthase V214M. (a) Extracted LC-MS traces for a representative unnatural alkaloid (m/z 337+34) derived from 2a. This compound is not observed when 2a is absent from the medium (i), or in control hairy root cultures transformed with wild-type strictosidine synthase gene (ii), or in control cultures transformed with no plasmid (iii). The compound displays the isotopic distribution expected for a chlorinated compound (iv,v). (b) LC-MS traces showing production of two unnatural alkaloids derived from 2a (i,iii), along with the traces showing production of the parent natural alkaloids from the same cultures (ii, iv). Asterisked compounds 4a (i), 4 (ii) and 5a (iii) were isolated and characterized by NMR. Asterisked compound 5 (iv) was assigned based on coelution with an authentic standard (Bestchemistry, Zhjiang, China). LC-MS spectra of i and ii are normalized to the same y axis scale to allow comparison of the relative amounts of 4 and 4a; iii and iv are normalized to allow comparison of the relative amounts of 5 and 5a. See Supplementary Figure 1 for isotopic peaks associated with chlorinated and brominated compounds. MS data of the additional peaks in trace i suggest that these compounds are chlorinated isomers of 4a (See Supplementary Fig. 1). (c) Tabulated MS data of the major unnatural alkaloids produced from transgenic hairy root lines and 2a, 2b and 2c. Asterisked masses indicate that compounds with identical mass and retention time are present in low amounts in the wild-type control cocultured with tryptamine analog. Asterisked compounds therefore cannot be definitively assigned as unnatural compounds. See Supplementary Figure 1 for MS traces and Supplementary Table 1 for exact mass data. (d) Aromatic region of 1H NMR spectrum of 4 and 1H NMR and 1H-13C HSQC spectra of 4a isolated from transgenic C. roseus hairy root culture expressing V214M cultured with 500 µM 2a.
Although a variety of unnatural alkaloids seem to be formed from 2a, 2b and 2c in these transgenic cultures (Fig. 1c and Supplementary Fig. 1), analysis of the flux of 2a, 2b and 2c into downstream metabolites will require extensive characterization of all alkaloid products. Only a limited analysis is addressed in this study. When 2a (500 µM) was added to the culture medium, approximately equal amounts of natural ajmalicine and chlorinated ajmalicine (4a) were produced (Fig. 1b and Supplementary Fig. 2 online). Compound 4a was purified and characterized by 1H NMR, 1H-13C heteronuclear single quantum coherence (HSQC) NMR, UV spectroscopy and highresolution MS (m/z 387.1483, isolated yield of approximately 1.5 mg per 150 ml culture) (Fig. 1d and Supplementary Fig. 3 online). Cultivation with 2b (500 µM) resulted in production of methylated ajmalicine (4b) at levels greater than 4 (Supplementary Fig. 2). Compound 4b could not be readily separated from a contaminating product, but 1H NMR, UV spectroscopy and high-resolution MS (m/z 367.2015) of the partially purified compound supported a structural assignment of 4b (Supplementary Fig. 4 online).
When hairy root cultures were cultivated with 2b (500 µM), higher levels of methylated tabersonine (5b) relative to natural tabersonine were observed (Supplementary Fig. 2). Compound 5b was isolated and characterized by 1H NMR, 1H-13C HSQC, 1H-13C heteronuclear multiple bond correlation (HMBC) NMR, UV spectroscopy and highresolution MS (m/z 351.2064, isolated yield of approximately 2 mg per 150 ml culture) (Supplementary Fig. 4). Cultivation with 2a (500 µM) led to formation of chlorinated tabersonine (5a), also produced at higher levels than natural tabersonine (Fig. 1b and Supplementary Fig. 2). Although 5a could not be readily purified from contaminating alkaloid products, characterization of the partially purified compound by 1H NMR, UV spectroscopy and high-resolution MS (m/z 371.1528) supported a structural assignment of 5a (Supplementary Fig. 3).
Although hairy roots that have not been transformed with the mutant gene produce only trace amounts of catharanthine (7), for reasons that remain unclear, transformed cultures displayed enhanced production of 7, though the yields vary substantially from line to line (Supplementary Fig. 5 online). Low levels of a compound with molecular weight and UV spectrum consistent with a chlorinated catharanthine analog derived from 2a were also observed in transgenic lines expressing the V214M mutant (Supplementary Fig. 1 and Supplementary Fig. 2 and Supplementary Table 1). Efforts to purify and characterize these compounds, along with other putative unnatural alkaloids, are currently underway.
We quantified the levels of strictosidine synthase mRNA (mRNA encoding wild-type plus V214M mutant strictosidine synthase) in several hairy root lines using real-time reverse transcriptase PCR (rt-PCR). Three unnatural alkaloid–producing hairy root lines showed robust production of strictosidine synthase mRNA at levels approximately 10- to 30-fold higher than those observed in hairy root cultures not transformed with the pCAMBIA plasmid harboring the V214M mutant gene (Supplementary Fig. 6 online). rt-PCR of hairy root lines that were transformed with V214M under the control of an inducible promoter (pTA7002, Supplementary Methods)15 also showed low levels of strictosidine synthase mRNA in this particular system (Supplementary Fig. 6). Accordingly, no unnatural alkaloid production was observed after culturing these lines with 5-substituted tryptamine analog 2a (Supplementary Fig. 1).
Additionally, lysates from cultures transformed with V214M converted 2a and 2b to 3a and 3b in vitro (Supplementary Fig. 7 online). Lysates from control cultures that were transformed with wild-type strictosidine synthase or with wild-type A. rhizogenes lacking the V214M mutant gene failed to convert 2a and 2b into the corresponding strictosidine analogs (Supplementary Fig. 7). Collectively, these data indicate that production of unnatural strictosidine biosynthetic intermediates and the downstream unnatural alkaloid products strictly correlates with overexpression of the engineered enzyme displaying the desired unnatural substrate specificity.
Fermentation is a powerful strategy for large-scale production of natural product analogs. Although plants have been previously engineered to produce greater amounts of a known natural product or a known biosynthetic intermediate by metabolic engineering strategies, use of plants or plant cell culture as a reactor for unnatural product production has not been widely demonstrated. This work shows that genetically reprogramming alkaloid metabolism can be achieved in medicinal plant cell culture, even though the genetic, biochemical and regulatory aspects of the pathway are incompletely characterized. In short, this study demonstrates the potential for reprogramming complex alkaloid pathways and sets the stage for further metabolic engineering efforts to improve the scope and practicality of unnatural product biosynthesis in plants.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge J. Shanks and C. Peebles (Iowa State) for detailed advice in the transformation procedure. CAMBIA is acknowledged for providing the pCAMBIA vectors, and N.-H. Chua (Rockefeller) is acknowledged for providing pTA7002. We thank L. Smeester (MIT) for assistance with rt-PCR, J.J. Maresh (MIT) for helpful discussions regarding the Agrobacterium transformation and N. Nims (MIT) for helpful suggestions regarding primer design for rt-PCR experiments. N. Yerkes (MIT) generously provided strictosidine standards. We thank T. Kutchan (Danforth Plant Science Center) for suggesting the pCAMBIA vector system. We gratefully acknowledge J. Simpson’s (MIT) assistance in obtaining two-dimensional NMR data. This work was supported by the US National Science Foundation (MCB0719120). We acknowledge the US National Institutes of Health and the American Cancer Society for additional support.
Footnotes
Note: Supplementary information and chemical compound information is available on the Nature Chemical Biology website.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/
References
- 1.Ganesan A. Curr. Opin. Chem. Biol. 2008;12:306–317. doi: 10.1016/j.cbpa.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 2.Menzella HG, Reeves CD. Curr. Opin. Microbiol. 2007;10:238–245. doi: 10.1016/j.mib.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Yun D-J, Hashimoto T, Yamada Y. Proc. Natl. Acad. Sci. USA. 1992;89:11799–11803. doi: 10.1073/pnas.89.24.11799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ye X, et al. Science. 2000;287:303–305. doi: 10.1126/science.287.5451.303. [DOI] [PubMed] [Google Scholar]
- 5.Katsuyama Y, Funa N, Miyahisa I, Horinouchi S. Chem. Biol. 2007;14:613–621. doi: 10.1016/j.chembiol.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt-Dannert C, Umeno D, Arnold FH. Nat. Biotechnol. 2000;18:750–753. doi: 10.1038/77319. [DOI] [PubMed] [Google Scholar]
- 7.O’Connor SE, Maresh JJ. Nat. Prod. Rep. 2006;23:532–547. doi: 10.1039/b512615k. [DOI] [PubMed] [Google Scholar]
- 8.Minami H, et al. Proc. Natl. Acad. Sci. USA. 2008;105:7393–7398. doi: 10.1073/pnas.0802981105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hawkins KM, Smolke CD. Nat. Chem. Biol. 2008;4:564–573. doi: 10.1038/nchembio.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernhardt P, McCoy E, O’Connor SE. Chem. Biol. 2007;14:888–897. doi: 10.1016/j.chembiol.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma X, et al. Plant Cell. 2006;18:907–920. doi: 10.1105/tpc.105.038018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen S, Galan MC, Coltharp C, O’Connor SE. Chem. Biol. 2006;13:1137–1141. doi: 10.1016/j.chembiol.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 13.Loris E, et al. Chem. Biol. 2007;14:979–985. doi: 10.1016/j.chembiol.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Toivonen L, Balsevich J, Kurz WGW. Plant Cell Tissue Organ Cult. 1989;18:79–93. [Google Scholar]
- 15.Hughes EH, Hong S-B, Shanks JV, San K-Y, Gibson SI. Biotechnol. Prog. 2002;18:1183–1186. doi: 10.1021/bp025603o. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.