Abstract
Traditional biochemistry of contact activation of blood coagulation suggesting that anionic hydrophilic surfaces are specific activators of the cascade is inconsistent with known trends in protein adsorption. To investigate contact activation reactions, a chromogenic assay was used to measure prekallikrein (PK) hydrolysis to kallikrein (Kal) by activated factor XII (FXIIa) at test hydrophilic (clean glass) and hydrophobic (silanized glass) surfaces in the presence of bovine serum albumin (BSA). Hydrolysis of PK by FXIIa is detected after contact of the zymogen FXII with a test hydrophobic surface only if putatively-adsorbed FXIIa is competitively displaced by BSA. By contrast, FXIIa activity is detected spontaneously following FXII activation by a hydrophilic surface and requires no adsorption displacement. These results (i) show that an anionic hydrophilic surface is not a necessary cofactor for FXIIa-mediated hydrolysis of PK, (ii) indicate that PK hydrolysis does not need to occur by an activation complex assembled directly on an anionic, activating surface, (iii) confirms that contact activation of FXII (autoactivation) is not specific to anionic hydrophilic surfaces, and (iv) demonstrates that protein-adsorption competition is an essential feature that must be included in any comprehensive mechanism of surface-induced blood coagulation.
Introduction
The use of blood-contacting medical devices for treatment of cardiovascular disease leads to the activation of factors in response to materials that initiate blood coagulation and thrombosis. These activated enzymes also perturb the immune system, the complement system, and fibrinolytic pathways besides generating vascular permeability factors that are all detrimental to patient health [1]. Despite decades of research, development of improved hemocompatible materials has remained a largely unrealized objective [2]. Towards engineering improved blood-compatible materials there is a need to investigate the role of the surface in influencing these responses.
It is widely believed that the intrinsic coagulation cascade has little physiological significance and that the extrinsic cascade is the predominant pathway of coagulation in vivo [3]. Some recent studies indicate that the intrinsic pathway may play a role during in thrombosis in vivo via a heretofore-unknown mechanism [4, 5]. Elevated levels of activated enzymes of the intrinsic pathway from blood-biomaterial contact associated with the use of cardiopulmonary bypass-oxygenators, haemodialysis membranes and left-ventricular-assist-devices underscore the significance of this pathway with the use of such medical devices [6–9].
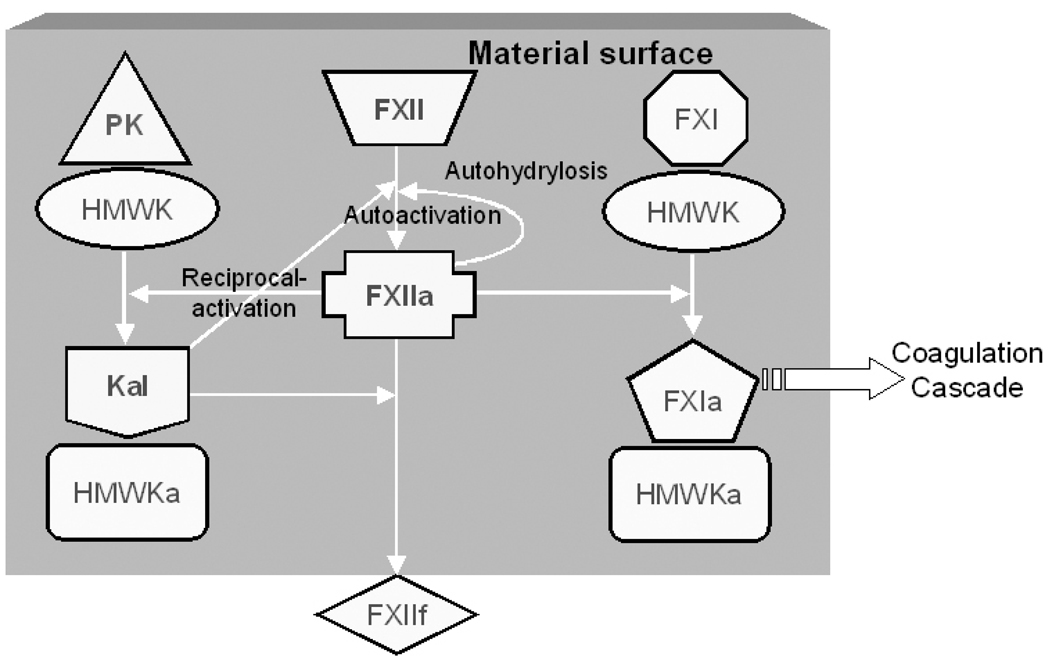
The intrinsic pathway is essentially a series of limited proteolytic conversion of inactive zymogens to active enzymes [3]. It is widely believed that negatively-charged surfaces, such as glass, are specific initiators of the coagulation cascade [10, 11]. The intrinsic pathway is thought to be initiated by the “binding” of coagulation factor XII (FXII, Hageman factor) to the negatively-charged surface via “specific interactions” [12, 13]. Binding to the anionic surface causes autoactivation of FXII [14, 15]. It also facilitates the assembly of a contact activation complex essential for enzyme amplification via reciprocal-activation of FXII and prekallikrein (PK, Fletcher factor) [16] and cascade propagation by FXIIa-mediated factor XI (FXI, Plasma Thromboplastin Antecedent) hydrolysis [17–19]. Figure 1 schematically shows these interactions as they occur at a procoagulatn surface.
Figure 1.
Schematic representation of the proposed interactions of contact activation proteins at a biomaterial surface. According to the traditional biochemical theory, all of these interactions are believed to occur on a negatively–charged surface, and the surface plays an integral role as a cofactor in the reactions. Factor XII (FXII) is activated to FXIIa by surface-mediated autoactivation. In reciprocal-activation, Prekallikrein (PK) present as a complex with high-molecular-weight-kininogen (HMWK) is hydrolyzed to Kallikrein (Kal) by FXIIa, and Kal activates additional FXII molecules to FXIIa. Factor XI (FXI) activation to FXIa by FXIIa helps in propagating the cascade. Kal also cleaves FXIIa to smaller fragments (FXIIf).
The physical chemistry of protein adsorption in biomaterial surface science remains a contentious subject. It is broadly agreed that hydrophobic surfaces (defined here as a surface having a water contact angle greater than 65º) adsorb more protein than hydrophilic surfaces (those surfaces having a water contact angle less than 65º) [20, 21]. These observations would imply that hydrophobic surfaces that are more efficient in concentrating plasma proteins at the interface should be more efficient in activating the cascade. There are clear contradictions between the sequence of events proposed in traditional blood biochemistry and the surface science of protein adsorption. This has resulted in a patchwork of mechanistic fixes that are not supported in subsequent research into the interfacial activity of blood proteins [22, 23]. These unresolved issues have been addressed extensively in a recent review article addressing blood coagulation [24].
The overarching motivation for our work is to develop a better understanding of the role of the surface in contact activation consistent with theories of protein adsorption to material surfaces. In sharp contrast to the aforementioned traditional theory of activation by anionic surfaces, our group has observed nearly equal levels of autoactivation at both hydrophobic and hydrophilic surfaces in neat buffer solutions of FXII. However, FXIIa generation in plasma was found to be attenuated at hydrophobic surfaces rather than accentuated at hydrophilic surfaces [25]. In more recent work, we quantified the effects of competitive-protein adsorption in contact activation at hydrophilic and hydrophobic surfaces [26]. FXIIa generation at hydrophilic surfaces from solutions containing a cocktail of proteins including FXII was greater than that obtained under corresponding activation conditions in neat-buffer solutions of FXII. By contrast, FXIIa yield at hydrophobic surfaces was significantly lowered in the presence of other proteins compared to neat solution. These observations implicate protein-adsorption competition as an important mediator of contact activation. We report herein on the role of protein-adsorption competition at surfaces in modulating interactions between FXII and prekallikrien. These two proteins are components of the reciprocal-activation reactions [27] and the interactions were assessed by measuring FXII-mediated PK hydrolysis at test hydrophilic and hydrophobic surfaces using a simplified in vitro system. This simplified test system allows for analysis of the molecular interactions in the absence of cofactors such as high molecular weight kininogen that might otherwise confound the molecular reactions.
Materials and methods
Preparation of model material surfaces
Model hydrophobic and hydrophilic surfaces were prepared from 5 mL borosilicate glass vials (12 mm X 75 mm, Kimble Glass Inc, Vineland, NJ) using procedures reported previously [28, 29]. Vials were rinsed with chloroform, dried, and treated in a glow-discharge chamber at 100 W power for 30 min. Freshly-cleaned glass vials served as model hydrophilic surfaces. Model hydrophobic surfaces were prepared by silanization of glow-discharge-cleaned vials with n-octadecyltrichlorosilane (OTS, Gelest Inc., Morrisville, PA) by filling the vials with 5 % (v/v) OTS in chloroform (Sigma-Aldrich, St. Louis, MO) for 60 min. Vials were rinsed twice with chloroform and annealed overnight at 110°C in a vacuum-oven. OTS-modification of borosilicate glass particles of diameter 500 µm (Sigma-Aldrich) was performed using similar procedures. Water-wettability of these model surfaces was estimated by measuring water contact angles on identically-treated 12 mm diameter glass coverslip witness samples (VWR). Contact angles on the surfaces were measured by the horizontal sessile drop method on a Krüss goniometer using 18 MΩ water (Millipore Simplicity 185 System) as the probe liquid. Polystyrene vials (VWR) having identical dimensions to the glass vials were used after rinsing with 18 MΩ water and drying.
Proteins and plasma
Human PK, human Kal, human FXII, human FXIIa and corn trypsin inhibitor (CTI) were obtained commercially from Enzyme Research Lab (South Bend, IN) and used as-received without further purification. Solutions were prepared by serial dilution of supplier-provided stock concentrations with 0.01 M phosphate-buffered saline (PBS, pH 7.4, Sigma-Aldrich). Bovine serum albumin (BSA) solutions in PBS were freshly prepared from lyophilized BSA (fraction V, Sigma-Aldrich).
Human platelet-poor plasma (PPP) was prepared by pooling 5 units of anti-coagulated salvaged human plasma (outdated less than 5 days prior to receipt) from the Blood Bank at the Pennsylvania State University Milton S. Hershey Medical Center. PPP preparation and storage has been described previously [28, 29]. Pooled plasma was centrifuged 10 min at 500X g and 35°C. PPP was stored in 12 mL aliquots at –20°C and thawed at 37°C for 40 min prior to use.
Coagulation assay
An in vitro assay was used to measure the coagulation response of the model surfaces following procedures reported previously [28–30]. Briefly, 0.5 mL of PPP was mixed with 0.1 mL of 0.1 M calcium chloride (Sigma-Aldrich). The volume was adjusted with PBS to obtain 1 mL solution resulting in a 1:1 dilution of plasma in buffer. The vials were capped with parafilm, rotated at 8 rpm on a hematology mixer, and the time required from activation of the intrinsic pathway of the coagulation cascade to the first appearance of a visible clot was designated as the coagulation time (CT).
Kallikrein generation assay
The Kal assay was designed to closely mimic the conditions of the coagulation assay in the test procogulant vials. Each surface-treated vial contained 1 mL protein solution consisting of PK (20 µg/mL, ∼ 50% dilution of normal plasma concentration [31]) with either FXIIa (at 5 × 10−2 µg/mL) or FXII (at 5 × 10−2 or 4 µg/mL as noted in the text) in PBS. The FXIIa concentration used was determined to elicit a measurable response in the plasma coagulation assay but without reaching the minimum CT. Kal production at hydrophilic and hydrophobic surfaces was compared to that at the same surfaces when pre-incubated with BSA. These “blocked” surfaces were prepared by overnight incubation with 100 mg/mL BSA. Prior to use, the BSA was drained and vials were rinsed with PBS. These surfaces were designated as OTS-BSA and glass-BSA and used immediately without drying.
The vials were capped with parafilm and rotated at 8 rpm on a hematology mixer. Kal production for each test condition was determined by removal of 15 µL aliquots of the protein solution from the test vials at discrete time points between 0 and 10 min. The corresponding Kal concentration was determined using a chromogenic assay described below. Proteins putatively adsorbed to hydrophobic OTS surfaces were displaced by spiking vials with BSA solutions at t = 10.5 min. 38 µL of BSA solution appropriate to yield concentrations of either 1 mg/mL or 5 mg/mL in the vials were added to the 910 µL of solution remaining in the OTS vials, and Kal generation was measured up to 30 min.
Chromogenic assay
The concentration of Kal at each time point was determined using the commercially-available chromogenic substrate Pefachrome-PK® (Centerchem Inc, Norwalk, CT). Supplier-provided vials containing 10 µmoles of the reagent were stored at 4°C and reconstituted to 0.5 mM in 18 MΩ Millipore water prior to use. 15 µL of protein solution from the Kal generation assay was added to a 1.5 mL disposable polystyrene cuvette (VWR) containing 1066 µL of 50 mM tris-immidazole buffer (pH 7.8, 0.15 mM NaCl), 150 µL of 0.5 mM Pefachrome-PK solution, and CTI at 25X the molar concentration of FXII/FXIIa used. CTI is a FXIIa inhibitor [32] used to inhibit additional FXIIa hydrolysis of PK during the 10 min incubation period with the chromogenic substrate. The reaction was carried out for 10 min at 37°C and then stopped by adding 240 µL of 10% glacial acetic acid (Mallinckrodt Baker, Paris, KY). Absorbance was measured at 405 nm using a Lambda 25® UV/Vis spectrometer (Perkin-Elmer Instruments, Wellesley, MA), and compared to blanks prepared using protein-free PBS aliquots. A standard curve (not shown) prepared at 405 nm was linear over 0 < [Kal] < 50 µg/mL (R2 = 96%). Control experiments (not shown) indicated that FXIIa, BSA and CTI did not elicit any measurable effects on the reaction of Kal with the chromogenic substrate.
Statistical analyses
Statistical analyses were performed by parametric ANOVA (Tukey’s test) using InStat software (GraphPad Software). Means of experimental Kal concentration in test vials at a given time were compared pair-wise and the differences were considered statistically significant for p < 0.05. Significant differences are denoted by one symbol (p < 0.05), two symbols (p < 0.01), or three symbols (p < 0.001).
Results and Discussion
The observation that blood and blood plasma clot faster in glass vials than in plastic vials is a classic observation in hematology. The traditional biochemical theory explaining this routinely-observed phenomenon is that certain coagulation factors adsorb specifically to negatively-charged surfaces to initiate and propagate coagulation. In this sense, anionic hydrophilic surfaces are viewed as specific activators and facilitators of contact activation reactions (Figure 1). In sharp contradiction to the traditional theory, our recent results underscore the need for a new paradigm to incorporate protein adsorption and protein-adsorption competition into a comprehensive model of contact activation [25, 26].
The results in this study illustrate in molecular terms how protein-adsorption competition at hydrophobic surfaces moderates the FXII-PK interactions of reciprocal-activation. FXII-mediated PK hydrolysis to Kallikrein (Kal) in buffer solutions was measured at test hydrophilic and hydrophobic surfaces. Putatively adsorbed enzymes at the hydrophobic surfaces were displaced by protein-adsorption competition to demonstrate that adsorption to hydrophobic surfaces reduces the availability of activated molecules. Thus, adsorption to hydrophobic surfaces attenuates enzyme-amplification reactions leading to an apparent specificity for hydrophilic surfaces. An alternate model for the interaction of the proteins at the biomaterial surface is proposed based on the reported results.
Coagulation response to model surfaces
Sessile drop water contact angles measured on coverslip witness samples confirmed the hydrophilic and hydrophobic character of the glass and OTS surfaces, respectively (Table 1). The choice of these hydrophilic and hydrophobic surfaces was intentional and represents the two near-extremities of the full span of observable water-wettability (a measure of surface energy) of solid surfaces. The coagulation response of recalcified human PPP to surfaces was assayed and expressed quantitatively in terms of coagulation time (CT), also listed in Table 1. Lower CT for glass indicates that it is a more efficient procoagulant than an equivalent surface area of hydrophobic material. CT for OTS materials were similar to those measured in response to an equivalent surface area of polystyrene and consistent with previously-reported results [28–30].
Table 1.
Water contact angles and the measured CT response to PPP.
| Surface | Sessile Drop Contact Angle | CT (min) |
|---|---|---|
| Glass | <5° | 5.6 ±0.1 |
| OTS | 108° ±3° | 53.3 ±4.5 |
| Polystyrene | - | 43.1 ±7.5 |
Values are displayed as mean ± S.D. for n ≥3
Kallikrein generation in solutions of PK and FXIIa
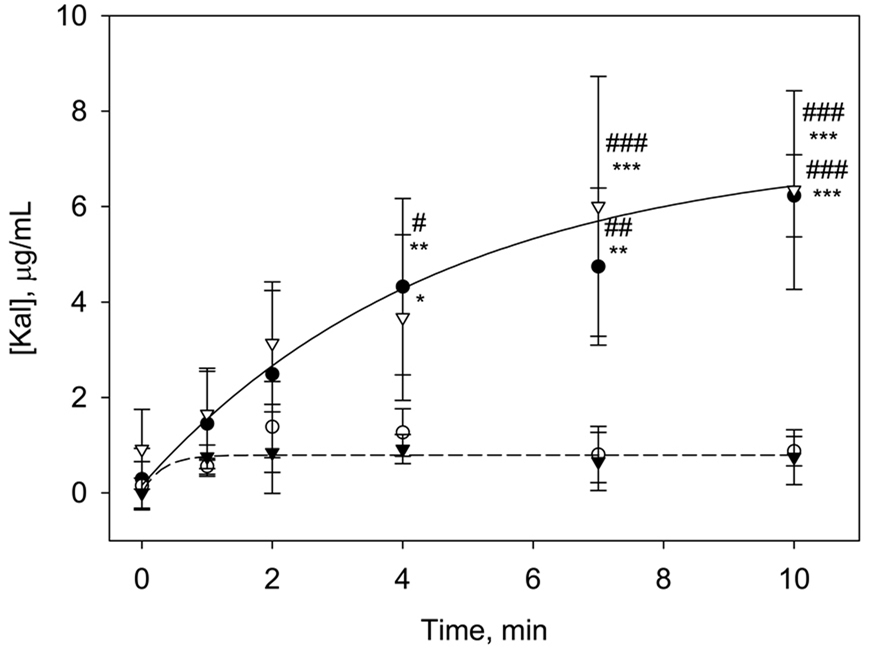
The time course of FXIIa-induced hydrolysis of PK in buffer solutions following contact with hydrophobic or hydrophilic procoagulant surfaces was measured using a Kal-specific chromogenic assay. The experiment was designed to investigate the interactions of the two proteins at test surfaces without the overwhelming presence of numerous other plasma proteins. In this case, the autoactivation step was bypassed by direct addition of FXIIa enzyme. Figure 2 reports results obtained under four different experimental conditions (mean ± S.D. from at least 3 individual measurements). In hydrophobic vials, a small initial increase in Kal concentration was followed by a steady-state Kal yield. Kal rate and yield was significantly higher in glass vials (p < 0.05 at all t > 4 min). We attribute the lower Kal generation from PK by FXIIa in the hydrophobic OTS vials to protein adsorption attenuating interaction of the proteins in solution. Kal production was significantly higher in the poorly-adsorbent hydrophilic glass vials.
Figure 2.
Temporal changes in Kal generation in solutions of 20 µg/mL PK and 5 × 10−2 µg/mL FXIIa at glass (closed circles, ●), OTS (closed triangles, ▼) and OTS-BSA (open triangles, ∇) surfaces up to 10 min. Also shown is Kal generation in glass vials containing 500 mm2 OTS beads (open circles, ○). Guidelines are drawn to highlight the profound differences in trends. The symbols # and * represent statistical significance with respect to Kal concentrations in glass vials containing OTS beads and OTS vials, respectively, at each time point. (Data expressed as mean ± S.D. for n≥3).
To minimize loss of proteins to adsorption at hydrophobic surfaces, the OTS vials were blocked by overnight incubation with BSA. As plotted in Figure 2, Kal production in OTS vials preblocked with BSA was no different than that measured in hydrophilic glass vials. Blocking with BSA reduced adsorption of the contact activation proteins thereby preventing the attenuation of the reactions at the hydrophobic surface. Note also that Kal generation in glass vials blocked with BSA was not different than that in non-blocked glass surfaces (data not shown). This lack of an effect for BSA blocking is consistent with the hydrophilic surface being a poorly protein-adsorbent substrate, and therefore there was no adsorption event to be blocked.
Furthermore, Figure 2 also shows that addition of 500 mm2 hydrophobic OTS particles to the glass vials leads to Kal production that is the same as that seen in hydrophobic vials. Adsorption of the proteins to the hydrophobic particles introduced in the glass vials leads to an adsorption of enzymes from solution, thereby reducing Kal yield. Control experiments showed no Kal production in the absence of FXIIa for either hydrophobic or hydrophilic vials (data not shown).
The trends in Figure 3 are in sharp contrast to the conventional theory stating that PK→Kal conversion by FXIIa occurs through assembly of a contact activation complex on a negatively-charged surface, where the surface serves as a “cofactor” [19]. In the case of BSA-blocked OTS, there was no negatively-charged surface to serve as a cofactor, yet PK→Kal conversion identical to a glass surface was observed. Therefore, these results demonstrate that the PK→Kal activation arising from FXIIa neither occurs by the direct assembly of proteins on the surface nor is the reaction specific to negatively-charged hydrophilic surfaces. Rather, it appears that this reaction is attenuated by protein adsorption at hydrophobic surfaces. Further supporting evidence of this response is seen in the observation that addition of OTS beads to glass vials caused a sharp reduction in Kal generation, ostensibly due to protein adsorption to the hydrophobic surfaces. Note that despite the presence of a negatively-charged glass surface in the test system, PK→Kal conversion could not be not detected.
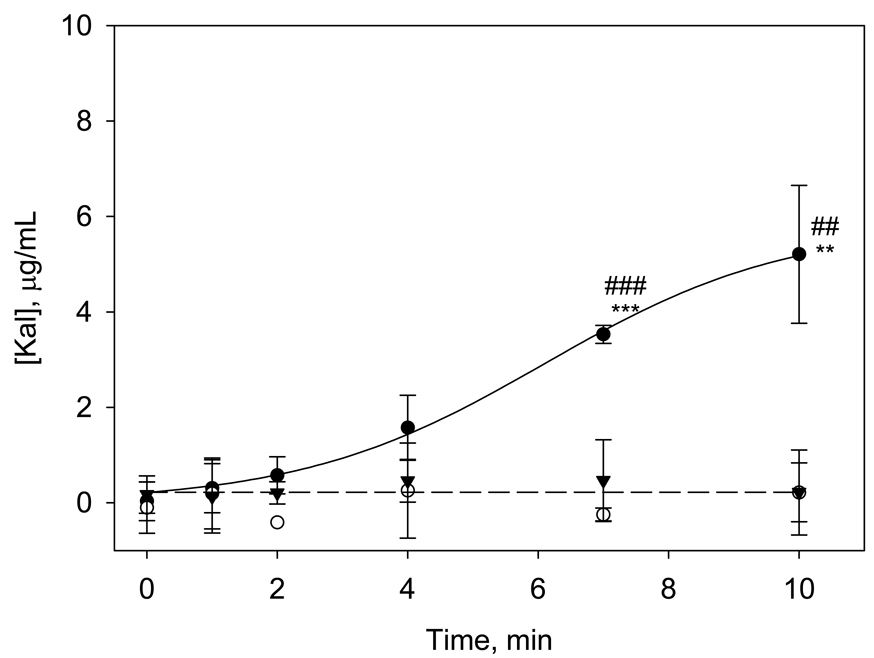
Figure 3.
Temporal changes in Kal generation in solutions of 20 µg/mL PK and 5 × 10−2 µg/mL of FXII at glass (closed circles, ●), OTS (closed triangles, ▼) and OTS-BSA (open circles, ○) surfaces. Data are plotted using same y-scale as Figure 2 for ease of comparison. Guidelines are drawn to highlight the differences in trends. The symbols * and # represent statistical significance with respect to OTS vials and OTS-BSA surfaces, respectively, at a given time point. (Data expressed as mean ± S.D. for n≥3).
Kallikrein generation in solutions of PK and FXII
The Kal chromogenic assay was next used to measure Kal arising from PK hydrolysis by agents produced through interactions of FXII with either hydrophobic or hydrophilic procoagulant surfaces (putatively FXIIa but possibly including FXIIf and other fragments). The objective was to elucidate the role of adsorption to the surface on the contact activation and enzyme amplification reactions of FXII and PK. Results in Figure 3 show that there was no measurable Kal production in either hydrophobic vials or hydrophobic vials blocked with BSA. In glass vials however, there was an initial lag period followed by a steady rise in Kal concentration that was statistically greater than Kal concentrations for the OTS and OTS-BSA surfaces at t ≥ 7 min. Similar trends were observed for the glass-BSA vials (data not shown).
Significantly higher amounts of Kal generated at glass surfaces are indicative of increased FXIIa activity towards PK than is found at OTS or OTS-BSA surfaces. This is not surprising because FXIIa generated by (surface-mediated) autoactivation is necessary for PK hydrolysis and also leads to more rapid coagulation as observed at the hydrophilic surfaces. Control experiments verified that Kal generation did not occur at glass or OTS surfaces in neat PK solutions, confirming that Kal production required the presence of FXIIa. BSA-blocking of the OTS surface minimized FXII autoactivation so that subsequent reciprocal-activation processes did not occur, demonstrated by the low PK→Kal conversion. This is also consistent with weaker coagulation induced by surfaces blocked with BSA as observed previously [28]. At the hydrophobic surfaces, we believe that FXIIa molecules putatively generated via autoactivation [25] were adsorbed to the surface, and these active enzyme molecules were unable to participate in subsequent reciprocal-activation reactions, resulting in low Kal production.
In hydrophilic vials, the FXIIa molecules generated via autoactivation near the poorly-adsorbent surface could more readily participate in the subsequent PK→Kal conversion as the molecules were not confined to the surface region by adsorption. This resulted in a rapid increase in measured Kal yield (Figure 3). When glass vials were pre-incubated with BSA (data not shown) the response was similar to that in unblocked glass because once again there was no adsorption process to be blocked.
Displacement of adsorbed enzymes at OTS surfaces using BSA
The results in the preceding sections strongly suggest that adsorption of proteins to the hydrophobic OTS surfaces prevents the molecular interactions necessary for reciprocal-activation, resulting in low Kal generation. These results do not, however, directly address the question of whether FXII can be activated by the hydrophobic surface to yield a functional FXIIa enzyme capable of initiating the amplification reactions. If such activation did indeed occur, then the enzyme could potentially be displaced from the hydrophobic surface. These displaced molecules would then be available for the amplification reactions to proceed resulting in increased Kal generation. As a test of this hypothesis, BSA, a molecule not involved in the coagulation cascade and for which we have found no effect on the Kal assay, was employed as a displacing protein to induce competitive desorption of adsorbed enzyme from the OTS surfaces. Vogler and co-workers have proposed that protein adsorption to hydrophobic surfaces is essentially the partitioning of proteins from the bulk solution into an interphase. The extent of partitioning is expressed as partition coefficients, and the protein concentration in the interphase scales with bulk-solution concentration up to a saturating maximum [23, 33, 34]. At the same time, some of the FXIIa formed at the interface would be expected to partition back out into solution. For the experiments herein, BSA at a very high concentration relative to both FXII and PK was used to displace the adsorbed zymogens/enzymes from the hydrophobic surface by competing for adsorption sites.
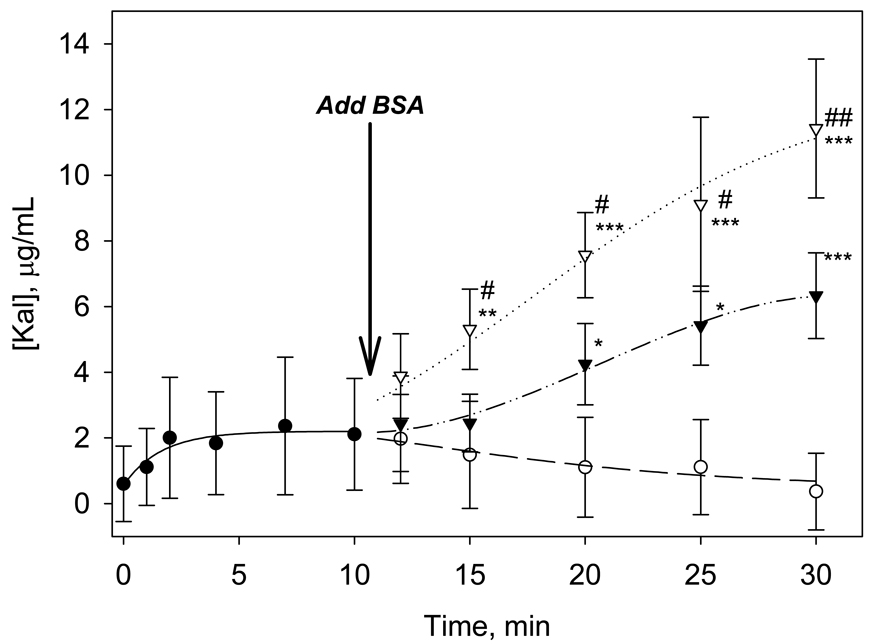
When BSA at 1 mg/mL was added to the Kal assays in OTS vials containing solutions of PK at 20 µg/mL and FXII at 5 × 10−2 µg/mL, there was a modest increase in Kal concentration, but the increase was not statistically different from PBS controls. Subsequently, the concentration of FXII in the test solution was increased to 4 µg/mL (still ∼ an order of magnitude lower than physiologic FXII concentration). Immediately after protein-surface interaction, there was a noticeable, although not statistically significant, increase in the amount of kallikrien seen in solution (Figure 4). This is consistent with a fraction of the FXIIa formed at the interface partitioning back into solution where it acts on the PK substrate. Addition of BSA to these solutions at t=10.5 min caused rapid increases in Kal measured in solution (Figure 4). Statistically greater Kal concentrations were obtained for both 1 and 5 mg/mL BSA spikes compared to the 0 mg/mL PBS control for t ≥ 20 min total reaction time, with the 5 mg/ml spike reaching significance at t ≥15 min. The data reveals that addition of BSA at 1 mg/mL resulted in significant increases in Kal in the OTS vials compared to negative controls in which an equivalent volume of buffer was added. BSA addition at 5 mg/mL caused an even sharper rise in Kal with statistically greater amounts measured with respect to both the 1 mg/mL BSA and the PBS control within 5 min of introducing the competing protein. Addition of the competing protein to the test vials results in rapid Kal production in these solutions presumably through displacement of adsorbed enzymes and/or zymogens from the surface. These displacements increase the availability of molecules in solution facilitating reciprocal-activation reactions that generate Kal.
Figure 4.
Temporal changes in Kal generation in solutions of 20 µg/mL PK and 4 µg/mL FXII in OTS vials (closed circles, ●). The effects of addition of BSA at 5 mg/mL (open triangles, ∇), 1 mg/mL (closed triangles, ▼), or 0 mg/mL, (PBS alone, open circles, ○) at 10.5 min are also presented. The symbols * and # represent statistical significance with respect to 0 mg/mL and 1 mg/mL BSA, respectively, at given time points. (Data expressed as mean ± S.D. for n≥3).
A wide series of control experiments showed that addition of BSA to the protein solutions had no effect on either PK→Kal conversion or on FXII→FXIIa activation. Furthermore, results reported above were corroborated in a limited series of experiments conducted using highly purified human serum albumin. From these extensive control experiments we conclude that there is no proteolytic activity associated with the displacing protein and that the activation steps occur as a result of surface-induced activation of FXIIa followed by FXIIa-mediated activation of Kal.
To the best of our knowledge, the only report on the effect of adsorption on contact activation is the work of Scott et al [35]. They observed a loss of activity of FXII-fragments towards PK cleavage when exposed to surfaces of plastic tubes, but this effect was reduced in the presence of other proteins (high-molecular-weight-kininogen, BSA, fibrinogen) as well as phospholipid leading to an “enhancement effect” on Kal generation. The results are consistent with what was observed in the experiments presented in this manuscript, with adsorption of enzymes being reduced in the presence of other biosurfactants thereby leading to the enhancement effect observed. However, we believe the explanation offered here is consistent with known trends in protein adsorption, where adsorption to surfaces leads to losses in the number of protein molecules available for reactions but not necessarily their enzymatic activity.
Role of the material surface in contact activation
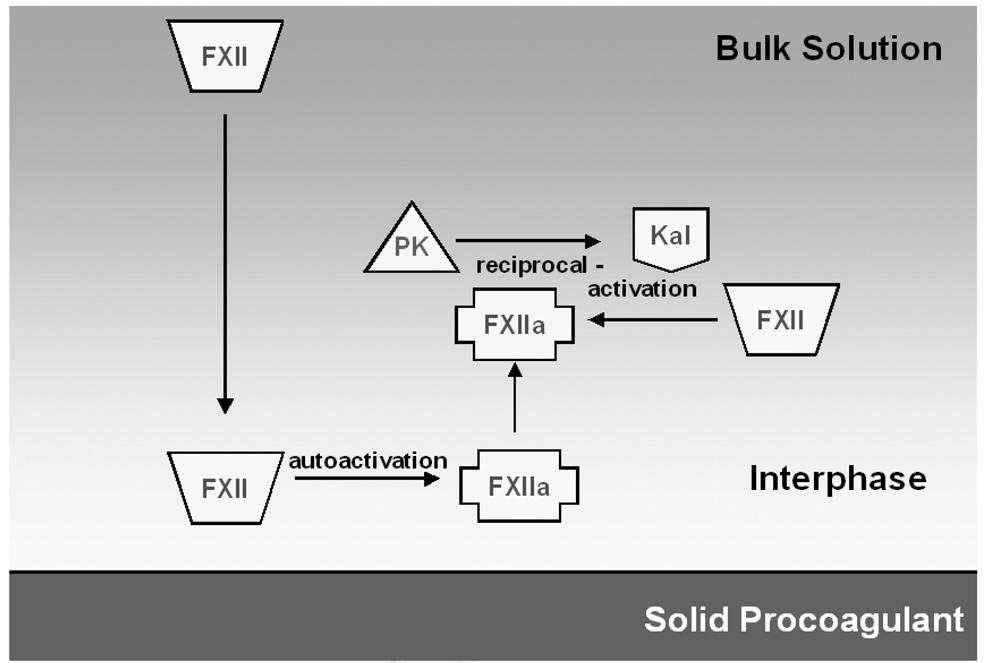
Based on a series of recent results, including data presented here, we propose a modified sequence of contact activation events (Figure 5). Autoactivation of FXII is proposed to occur in close vicinity to a solid surface and does not require direct adsorption onto the procoagulant surface nor the presence of an anionic hydrophilic surface. The subsequent interactions necessary for reciprocal-activation of PK and FXII do not occur by assembly of molecules directly on the procoagulant surface. Rather, it appears that adsorption of FXIIa molecules at hydrophobic surfaces renders these proteins unavailable to participate in the reciprocal-activation reactions necessary for enzyme amplification. In contrast, FXIIa generated at poorly-adsorbent hydrophilic surfaces are readily able to participate in reciprocal-activation reactions. This results in an apparent specificity for contact activation events at hydrophilic surfaces relative to the attenuated response seen at hydrophobic surfaces as a product of protein adsorption events. Moreover, this scheme of events is consistent with protein adsorption studies that have eliminated special adsorption properties for the contact activation proteins [22, 23]. Collectively, all our results would suggest that protein-adsorption competition at procoagulant surfaces plays a key moderating role on activation of the coagulation cascade.
Figure 5.
Proposed model for the sequence of events involving PK and FXII at solid procoagulant surfaces. Contact activation is initiated by autoactivation of FXII at the interphase and is not specific to negatively-charged surfaces. Subsequent interactions involving reciprocal-activation of PK and FXII do not occur by direct assembly on the surface but rather require displacement from the surface.
Conclusion
A chromogenic assay was used to measure FXIIa mediated conversion of PK to Kal following contact with model procoagulant materials. Results showed that the presence of a hydrophobic surface inhibited the apparent activity of FXIIa, but displacement of the active enzyme by albumin resulted in the rapid production of Kal indicative of enzymatic activity. Taken together, the results presented here (i) show that an anionic hydrophilic surface is not a necessary cofactor for FXIIa-mediated hydrolysis of PK, (ii) indicate that PK hydrolysis does not occur in an activation complex assembled directly on an activating surface, (iii) confirm that contact activation of FXII (autoactivation) is not specific to anionic hydrophilic surfaces, and (iv) demonstrate that protein-adsorption competition is an essential component of blood plasma coagulation that must be included in any comprehensive descriptive mechanism.
Acknowledgements
This work was supported by NIH (RO1HL69965), and the Summer Undergraduate Research Internship Program at the Penn State College of Medicine. The authors thank Drs. T.L. Lowe and E. Gil for assistance with spectrophotometric measurements.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Janatova J. Activation and control of complement, inflammation, and infection associated with the use of biomedical polymers. ASAIO Journal. 2000;46(6):S53–S62. doi: 10.1097/00002480-200011000-00038. [DOI] [PubMed] [Google Scholar]
- 2.Ratner BD. Blood compatibility- a perspective. Journal of Biomaterials Science: Polymer edition. 2000;11(11):1107–1119. doi: 10.1163/156856200744219. [DOI] [PubMed] [Google Scholar]
- 3.Colman RW, Clowes AW, George JN, Hirsh J, Marder VJ. Overview of Hemostasis. In: Colman RW, Hirsh J, Marder VJ, Clowes AW, George JN, editors. Hemostasis and Thrombosis-Basic Principles and Clinical Practice. Philadelphia: Lippincott William and William; 2001. pp. 103–122. [Google Scholar]
- 4.Kleinschnitz C, Stoll G, Bendszus M, Schuh K, Pauer HU, Burfeind P, et al. Targeting coagulation factor XII provides protection from pathological thrombosis in cerebral ischemia without interfering with hemostasis.[see comment] Journal of Experimental Medicine. 2006;203(3):513–518. doi: 10.1084/jem.20052458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Renne T, Pozgajova M, Gruner S, Schuh K, Pauer HU, Burfeind P, et al. Defective thrombus formation in mice lacking coagulation factor XII. Journal of Experimental Medicine. 2005;202(2):271–281. doi: 10.1084/jem.20050664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell DJ, Dixon B, Kladis A, Kemme M, Santamaria JD. Activation of the kallikrein-kinin system by cardiopulmonary bypass in humans. American Journal of Physiology -Regulatory Integrative & Comparative Physiology. 2001;281(4):R1059–R1070. doi: 10.1152/ajpregu.2001.281.4.R1059. [DOI] [PubMed] [Google Scholar]
- 7.Koster A, Loebe M, Hansen R, Potapov EV, Noon GP, Kuppe H, et al. Alterations in coagulation after implantation of a pulsatile Novacor LVAD and the axial flow MicroMed DeBakey LVAD. Annals of Thoracic Surgery. 2000;70(2):533–537. doi: 10.1016/s0003-4975(00)01404-1. [DOI] [PubMed] [Google Scholar]
- 8.Paparella D, Brister SJ, Buchanan MR. Coagulation disorders of cardiopulmonary bypass: a review. Intensive Care Medicine. 2004;30(10):1873–1881. doi: 10.1007/s00134-004-2388-0. [DOI] [PubMed] [Google Scholar]
- 9.Svensson M, Friberger P, Lundstrom O, Stegmayr B. Activation of FXII during haemodialysis. Scandinavian Journal of Clinical & Laboratory Investigation. 1996;56(7):649–652. doi: 10.3109/00365519609090600. [DOI] [PubMed] [Google Scholar]
- 10.Ratnoff OD, Rosenblum JM. Role of Hageman factor in the initiation of clotting by glass. American Journal of Medicine. 1958;25:160–168. doi: 10.1016/0002-9343(58)90023-8. [DOI] [PubMed] [Google Scholar]
- 11.Revak SD, Cochrane CG, Griffin JH. The binding and cleavage characteristics of human Hageman factor during contact activation. The Journal of Clinical Investigation. 1977;59:1167–1175. doi: 10.1172/JCI108741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirby EP, McDevitt PJ. The binding of bovine factor XII to kaolin. Blood. 1983;61(4):652–659. [PubMed] [Google Scholar]
- 13.Wiggins RC, Cochrane CG. The autoactivation of rabbit Hageman factor. Journal of Experimental Medicine. 1979;150(5):1122–1133. doi: 10.1084/jem.150.5.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samuel M, Pixley RA, Villanueva MA, Colman RW, Villanueva GB. Human factor XII (Hageman factor) autoactivation by dextran sulfate. Circular dichroism, fluorescence, and ultraviolet difference spectroscopic studies. Journal of Biological Chemistry. 1992;267(27):19691–19697. [PubMed] [Google Scholar]
- 15.Vroman L. Effects of hydrophobic surfaces upon blood coagulation. Thrombosis et Diathesis Haemorrhagica. 1964;10:455–493. [PubMed] [Google Scholar]
- 16.Griffin JH. Role of surface in surface-dependent activation of Hageman factor (blood coagulation Factor XII) Proceedings of the National Academy of Sciences USA. 1978;75(4):1998–2002. doi: 10.1073/pnas.75.4.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tankersley DL, Finlayson JS. Kinetics of activation and autoactivation of human factor XII. Biochemistry. 1984;23(2):273–279. doi: 10.1021/bi00297a016. [DOI] [PubMed] [Google Scholar]
- 18.Zhuo R, Vogler EA. Practical application of a chromogenic FXIIa assay. Biomaterials. 2006;27:4840–4845. doi: 10.1016/j.biomaterials.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan AP. Initiation of the intrinsic coagulation and fibrinolytic pathways of man: the role of surfaces, Hageman factor, prekallikrein, high molecular weight kininogen, and factor XI. Progress in Hemostasis and Thrombosis. 1978;4:127–175. [PubMed] [Google Scholar]
- 20.Wu Y, Simonovsky FI, Ratner BD, Horbett TA. The role of adsorbed fibrinogen in platelet adhesion to polyurethane surfaces: A comparison of surface hydrophobicity, protein adsorption, monoclonal antibody binding, and platelet adhesion. Journal of Biomedical Materials Research Part A. 2005;74A(4):722–738. doi: 10.1002/jbm.a.30381. [DOI] [PubMed] [Google Scholar]
- 21.Noh H, Vogler EA. Volumetric interpretation of protein adsorption: mass and energy balance for albumin adsorption to particulate adsorbents with incrementally increasing hydrophilicity. Biomaterials. 2006;27(34):5801–5812. doi: 10.1016/j.biomaterials.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Krishnan A, Sturgeon J, Siedlecki CA, Vogler EA. Scaled interfacial activity of proteins at the liquid-vapor interface. Journal of Biomedical Materials Research Part A. 2004;68(3):544–557. doi: 10.1002/jbm.a.20104. [DOI] [PubMed] [Google Scholar]
- 23.Krishnan A, Liu YH, Cha P, Allara D, Vogler EA. Scaled interfacial activity of proteins at a hydrophobic solid/aqueous-buffer interface. Journal of Biomedical Materials Research Part A. 2005;75(2):445–457. doi: 10.1002/jbm.a.30444. [DOI] [PubMed] [Google Scholar]
- 24.Vogler EA, Siedlecki CA. Contact activation of blood-plasma coagulation. Biomaterials. 2009;30(10):1857–1869. doi: 10.1016/j.biomaterials.2008.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhuo R, Siedlecki CA, Vogler EA. Autoactivation of blood factor XII at hydrophilic and hydrophobic surfaces. Biomaterials. 2006;27(24):4325–4332. doi: 10.1016/j.biomaterials.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Zhuo R, Siedlecki CA, Vogler EA. Competitive-protein adsorption in contact activation of blood factor XII. Biomaterials. 2007;28(30):4355–4369. doi: 10.1016/j.biomaterials.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chatterjee K, Guo Z, Vogler EA, Siedlecki CA. Contact activation pathways to activated blood factor XII. J Biomedical Materials Research A. 2008 doi: 10.1002/jbm.a.32076. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chatterjee K, Vogler EA, Siedlecki CA. Procoagulant activity of surface-immobilized Hageman factor. Biomaterials. 2006;27(33):5643–5650. doi: 10.1016/j.biomaterials.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 29.Guo Z, Bussard KM, Chatterjee K, Miller R, Vogler EA, Siedlecki CA. Mathematical modeling of material-induced blood plasma coagulation. Biomaterials. 2006;27:796–806. doi: 10.1016/j.biomaterials.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 30.Zhuo R, Miller R, Bussard KM, Siedlecki CA, Vogler EA. Procoagulant stimulus processing by the intrinsic pathway of blood plasma coagulation. Biomaterials. 2005;26(16):2965–2973. doi: 10.1016/j.biomaterials.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 31.Dobrovolsky AB, Titaeva EV. The fibrinolysis system: regulation of activity and physiologic functions of its main components. Biochemistry-Russia. 2002;67(1):99–108. doi: 10.1023/a:1013960416302. [DOI] [PubMed] [Google Scholar]
- 32.Rand MD, Lock JB, van't Veer C, Gaffney DP, Mann KG. Blood clotting in minimally altered whole blood. Blood. 1996;88(9):3432–3445. [PubMed] [Google Scholar]
- 33.Noh H, Vogler EA. Volumetric interpretation of protein adsorption: Partition coefficients, interphase volumes, and free energies of adsorption to hydrophobic surfaces. Biomaterials. 2006;27(34):5780–5793. doi: 10.1016/j.biomaterials.2006.07.038. [DOI] [PubMed] [Google Scholar]
- 34.Krishnan A, Siedlecki CA, Vogler EA. Traube-Rule Interpretation of Protein Adsorption at the Liquid-Vapor Interface. Langmuir. 2003;19(24):10342–10352. [Google Scholar]
- 35.Scott CF, Kirby EP, Schick PK, Colman RW. Effect of surfaces on fluid-phase prekallikrein activation. Blood. 1981;57(3):553–560. [PubMed] [Google Scholar]