Abstract
The gene–environment interaction effect in the development of conduct disorder is one of the most important discoveries of the past decade, but the mechanisms through which this effect operates remain elusive. I propose a model of these processes that focuses on the individual’s response to a threatening stimulus in ongoing social interaction. The individual’s response coordinates three interrelated systems: neural, autonomic, and information-processing. In each system, adaptive, evolutionarily selected response patterns characterize normal responding, but in psychopathology these patterns have gone awry. Antecedents of individual differences in these response patterns arise from genetic polymorphisms, adverse environmental experiences early in life, and their interaction. Programs of research are proposed to test hypotheses in the model through longitudinal, experimental, and clinical intervention methods. This model can serve as a template for inquiry in other forms of developmental psychopathology.
We have learned more in the past 2 decades about the development of conduct disorder than we had in the previous 2 millennia. We now know that the fundamental building blocks of this debilitating condition involve an interaction effect between one’s genetic context and threatening life experiences. That is, early experiences of physical maltreatment and harsh parenting have been found to predict later conduct disorder, but only among those individuals who are born with a polymorphism in the monoamine oxidase A (MAOA) gene.
Although important, this discovery does not provide an understanding of how conduct disorder develops. What remains to be discovered are the mechanisms through which this gene–environment interaction effect occurs. Answering this question will enhance treatment efficacy for adolescents with this disorder, foster novel preventive interventions for high-risk children, and provide a template for understanding the development of psychopathology more generally. Thus, the question of mechanisms in gene–environment interaction effects is one of the most important questions to be answered in psychology in the next 2 decades.
THE PROBLEM OF CONDUCT DISORDER
The fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV; American Psychiatric Association, 1994) defines conduct disorder as a repetitive and persistent pattern of behavior in which an individual violates the basic rights of others while also significantly impairing one’s own social, academic, or occupational functioning (p. 85). Prevalence is estimated at 6%–16% of adolescent males and 2%–9% of adolescent females (Farrington, 2008). Conduct disorder is the most common of all child psychiatric disorders, and its lifetime costs have been estimated at over $2 million per case (Cohen, 2005). In spite of terrific scientific attention, prevention and treatment remain elusive (Dodge, Coie, & Lynam, 2006).
Complicating matters are recent findings that conduct disorder is a heterogeneous set (Rutter, 2008). Moffitt (1993) has hypothesized a distinction between lifecourse-persistent and adolescence-limited antisocial behavior. Hodgins (2008) has suggested distinctions in aggressive behavior between persons with psychosis or substance misuse and persons without comorbidity. Viding, Larsson, and Jones (2008) have suggested a distinction in antisocial behavior itself, between callous or unemotional, psychopathic traits and highly emotional aggression. Rutter (2008) has suggested that these subtypes may have different epidemiologies and mechanisms. For example, Viding et al. (2008) found greater heritability for psychopathic traits than for emotional aggression.
Still another distinction within conduct disorder is between individual aggressive behaviors and a general pattern of conduct disorder. Scientific inquiry into neural, molecular genetic, and cognitive mechanisms is likely to address processes in individual behaviors, whereas behavior–genetic and epidemiologic studies will address patterns and disorders. The term conduct disorder is used in this article, but the distinction between a disorder and a single behavior must be retained.
THE GENE–ENVIRONMENT INTERACTION EFFECT IN CONDUCT DISORDER
Cloninger, Sigvardsson, Bohrnan, and von Knorring’s (1982) seminal study of 862 Scandinavian male adoptees was among the first to demonstrate an interaction between heritability and environment in disruptive behavior outcomes. He found that under conditions of low genetic risk (i.e., having a biological parent who was not a criminal), the impact of the environment (i.e., having an adoptive parent who was or was not a criminal) on later criminality was rather small (rate of .03 for children of noncriminal adoptive parents vs. .07 for children of criminal adoptive parents). However, under conditions of high genetic risk, the impact of the environment was strong (.12 for children of noncriminal adoptive parents vs. .40 for children of criminal adoptive parents). These findings can be interpreted to indicate that the role of genetic risk is to make the individual susceptible to the adverse influence of the environment.
Jaffee et al. (2005) analyzed data from the 1,116 twin pairs in the British E-Risk study. Four rank-ordered groups of increasing heritable risk were identified, following a procedure used by Kendler et al. (1995), with the lowest heritable-risk group being children whose monozygotic twin was not conduct disordered (CD), the next lowest group being children whose dizygotic twin was not CD, the next highest group being children whose dizygotic twin was CD, and the highest group being children whose monozygotic twin was CD. The experience of child physical maltreatment was determined by clinical interview with the mother, following procedures by Dodge, Bates, and Pettit (1990). Among the children at lowest heritable risk, the experience of physical maltreatment had little effect on conduct disorder outcomes (for nonmaltreated children, rate = .02; for maltreated children, rate = .04). At the next highest level of heritable risk, the effect of maltreatment was small (.06 vs. .13), and it grew larger at the next highest level (.19 vs. .37). Finally, at the highest level, the effect of maltreatment was largest (.46 vs. .70).
Caspi et al, (2002) identified the specific gene implicated in this interaction effect in the MAOA gene, which resides on the X chromosome. MAOA is an enzyme that selectively degrades serotonin, norephinephrine, and dopamine following reuptake from the synaptic cleft. When humans are faced with threat or provocation, we naturally experience rage and an impulse to react aggressively, but activation of the MAOA enzyme enables us to withhold that response. MAOA therefore plays a key role in regulating behavior following threatening stimuli (Shih, Chen,& Ridd, 1999). In a context of statistically normative high MAOA activation, we can cope effectively in response to threatening experiences such as physical maltreatment, but in a context of low MAOA activation, a severe threat such as physical maltreatment is devastating and leads to out-of-control reactive aggressive behavior. High MAOA activation is thus a protective adaptation of evolution. Using the 1,037 children in the Dunedin Multidisciplinary Health and Development Study, Caspi et al. (2002) found a significant Gene × Environment interaction effect on conduct disorder outcomes, with the adverse effect of maltreatment being larger among youth with low MAOA activity (rates of .23 vs. .81 for non-maltreatment and maltreatment, respectively) than that found among youth with high MAOA activity (.22 vs. .41).
Foley et al, (2004) replicated this pattern in their sample of 514 male twins. Instead of physical maltreatment, their measure of environmental risk was a composite score of parental neglect, exposure to interparental conflict, and inconsistent parental discipline (0 or 1 = low adversity; 3 or 4 = high adversity). They found a significant interaction effect, with the effect of childhood adversity on conduct disorder being larger among youth with low MAOA activity (.06 vs. .50) than it was among youth with high MAOA activity (.01 vs. .07).
Kim-Cohen et al. (2006) analyzed the data of Caspi and Moffitt’s E-Risk study in Britain and found that about one third of boys have a polymorphism that yields low MAOA activity. They assessed physical maltreatment using the interview protocol developed by Dodge et al. (1990) and found that 6.4% had experienced physical maltreatment. They found that among boys with normal high MAOA activity, maltreatment increased the antisocial behavior score by about two thirds of a standard deviation relative to boys who had not experienced maltreatment, whereas among boys with the polymorphism the impact of maltreatment was about twice as strong, at 1.3 standard deviations. Furthermore, they determined that the interaction effect could not be accounted for by passive or evocative gene–environment correlation.
Meta-analyses support the robustness of the interaction effect. Dodge and Sherrill (2007) evaluated 30 studies of biological–environmental interaction effects and found strong support. Taylor and Kim-Cohen (2007) meta-analyzed seven studies of MAOA × Maltreatment interactions and found a .30 pooled estimate of maltreatment effect within low MAOA activity groups, in contrast with a .13 pooled estimate within high MAOA activity groups.
These findings lead to several conclusions. First, the environmental psycho-toxin of early physical maltreatment robustly predicts later chronic antisocial behavior. It appears that the extreme threatening experience of early-life physical abuse leads a boy to hyper-react aggressively in new situations, although the basis for this conclusion is correlational evidence that requires stronger empirical support from better research designs. Second, the impact is moderated by the genetic context: The effect is twice as strong among boys who have genetically based MAOA difficulty in regulating responses to threat. This finding has been replicated multiple times and appears to be a robust effect (in spite of several nonreplications). The genetic moderation finding buttresses the hypothesis of a causal impact of maltreatment on a subgroup of youth, although the possibility that maltreatment is merely a noncausal marker of a hidden gene (and therefore a Gene × Gene interaction) looms. Third, because MAOA appears to operate on reactions to threat, it is likely to mediate reactive aggression and not psychopathic traits; thus, the empirical findings might be even stronger for reactive aggression outcomes than for a disorder that combines heterogeneous characteristics. Fourth, the population base rate of this polymorphism is high (about one third in the E-Risk sample). This is a rate that has been sustained across evolution, suggesting that it is not a “bad” gene and may have adaptive value in some circumstances.
Moffitt, Caspi, and Rutter (2006) have argued that a gene–environment interaction effect in psychopathology should not be considered rare or anomalous. They point out that such interactions are common in other domains, such as agriculture (e.g., crops’ genotypes moderate resistance to disease), and that they are increasingly common in somatic medicine and human infectious disease (e.g., genotypes moderate susceptibility to malaria). They further argue that genetically based individual variation in response to the environment is the “raw material” (p. 6) for natural selection and could be adaptive for the species. Boyce and Ellis (2005) have argued that high susceptibility to the environment’s impact has been selected through evolution and that individual variation in susceptibility is adaptive for the species. Givers that human survival depends on goodness-of-fit between the individual and the environment, rejection of gene–environment interaction effects seems implausible.
THE NEED TO UNDERSTAND MECHANISMS IN THE GENE–ENVIRONMENT INTERACTION EFFECT
Once a gene–environment interaction effect has been robustly identified in epidemiologic research, the next step in scientific progress is to understand its mechanisms of action. Several scholars have called for research in such mechanisms. Rutter, Moffitt, and Caspi (2006) asserted that “the study and elucidation of the mechanisms involved in the different forms of gene–environment interplay should cast important light on basic causal mechanisms for psychopathology” (p. 252); however, they did not discuss specific hypotheses about mechanisms nor which systems to examine. Caspi and Moffitt (2006) have suggested that the major mechanism will be a “neural substrate reactivity measure” (p. 584), although they are skeptical that we know which substrate is involved in any effect: “At present … evidence concerning environmental and genotypic effects in relation to neural substrate measures is sparse, and therefore gene–environment interaction hypotheses are likely to be circumstantial at best, and flimsy at worst” (p. 585).
Three Interrelated Systems
The field moves forward by testing circumstantial and flimsy hypotheses that get refined through empirical tests. Where do we look for hypotheses? I suggest that hypotheses should be translated from the body of basic psychological inquiry in the development of aggressive behavior (Dodge et al., 2006). The findings of this field suggest that the mechanisms for aggression involve three interrelated systems: the neural system; the autonomic arousal system; and the information-processing system. A general model, presented in Figure 1, asserts that the process through which the environment exerts its influence on conduct-disorder-related aggressive behavior is the way that the brain processes environmental pathogenic stimuli during social interaction episodes. These pathogens include provocations, threats to the self, frustrations, and goal blocking (Berkowitz, 2008). The brain is the processor of these stimuli and the mediator of environmental impact. Individual differences in the vulnerability of the brain are shaped by genetic variation, early threatening environments, and their interaction and interplay.
Fig. 1.
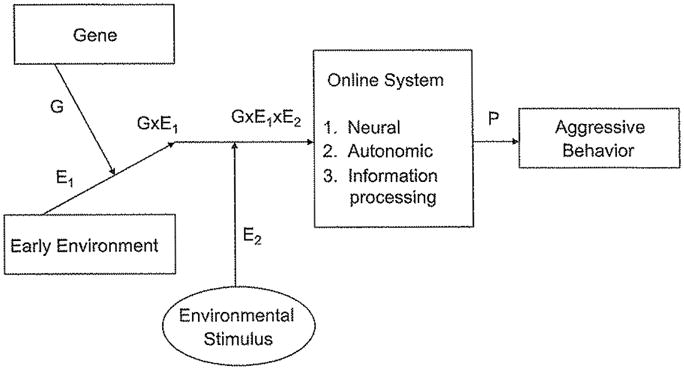
Proposed model of the mechanisms through which gene–environment interaction affects aggressive behavior.
Six effects, plus numerous subeffects, are asserted in this model, including (a) G, the main effect of individual variation in genes; (b) E1, the main effect of variation in the early environment; (c) G × E1, the gene-by-early-environment interaction effect; (d) E2, the main effect of the proximal environmental stimulus during social interaction; (e) G × E1 × E2, the interaction effect between the gene × Early Environment interaction and the proximal environmental stimulus; and (f) the total effect of the individual’s intrapersonal response system on aggressive behavior.
The model focuses on the individual’s online, real-time responding to proximal environmental stimuli involving threats, provocation, and adversity. At a neural level, monoamine oxidase A (MAOA) is the enzyme that acts on brain synapses during social interaction by degrading norepinephrine during threatening experiences. Failure in regulation of norepinephrine degradation leads to uncontrolled aggressive reactions. Throughout evolution, self-defensive aggressive reactions were, no doubt, adaptive, and still are; however, dysregulated over-reactions are socially dysfunctional and a defining symptom of conduct disorder. The same process has an autonomic system correlate (Crozier et al., 2008). Psychophysiologically, threat leads to increases in autonomic arousal (i.e., increases in heart rate) that prepare the organism for heightened, focused, cognitive awareness of central threat cues and readiness for necessary self-defensive behavioral response. Again, this system is highly adaptive when regulated and socially dysfunctional when out of control. Finally, these two processes have an information-processing correlate as well (Dodge, 2006). Phenomenologically, humans experience these brain actions as cognition and emotion. They selectively attend to threat cues and interpret cues as hostile-intentional threats or benign actions by others. They experience anger, and they readily access self-defensive goals and aggressive behavioral scripts from memory. They evaluate aggressive responses as leading to favorable consequences, and they impulsively react aggressively without consideration of long-term ill effects. These patterns are adaptive under conditions of true threat but are maladaptive when applied indiscriminately.
The proposition is that, in response to possible threat, three systems coactivate: Low MAOA activity is associated with a pattern of autonomic arousal and defensive information-processing that is characterized by hypervigilance to hostile cues, hostile attributional biases, selection of self-defensive goals, and the experience of self-righteous anger. All of these processing responses lead to highly reactive aggressive behavior (Link P in Figure 1).
The antecedents of action in these three interrelated systems include genes, early environments, proximal environments, and their interaction effects. The general thesis is that psycho-environmental toxins poke at the brain and that the brain processes the environmental stimuli partly as a function of genetically influenced neurochemical actions that have autonomic and cognitive-emotional correlates. These cognitive, emotional, and neurochemical brain processes are the mechanisms through which the environment causes psychopathology.
How to Test the Mechanisms
Neural Mechanisms
Caspi and Moffitt (2006) have proposed a plan to identify neural mechanisms in the gene–environment interaction effect: “First, evidence is needed about which neural substrate is involved in the disorder. Second, evidence is needed that an environmental cause of the disorder has effects on variables indexing the same neural substrate. Third, evidence is needed that a candidate gene has functional effects on variables indexing that same neural substrate. It is this convergence of environmental and genotypic effects within the same neural substrate that allows for the possibility of gene–environment interactions” (p. 585). Caspi and Moffitt’s three steps correspond to Links P, E1, and G in Figure 1, respectively.
Neurobiological deficits have long been implicated in antisocial behaviors in children (Van Goozen, Fairchild, & Harold, 2008); however, most of this inquiry has been directed toward identifying main-effect structural traitlike characteristics that are correlated with antisocial behavior. Recent work has attempted to link genotypes with neural substrates (Link G in Fig. 1). Using MRI to identify brain structural differences associated with the low expression variant of the polymorphism in MAOA, Meyer-Lindenberg et al. (2006) found that, relative to MAOA-high males, MAOA-low males (but not females) showed an 8% reduction in gray-matter volume that encompassed the cingulate gyrus and bilateral amygdalae. Perhaps a larger effect would be observed if early maltreatment histories of these males were known, to capture the G × E1 effect from Figure 1 on neural structure.
Figure 1 suggests that even more powerful discoveries will be made through dynamic assessments that account for online responses to proximal environmental stimuli, such as functional magnetic resonance imaging (fMRI). Such tests could capture G × E2 effects as well as G × E1 × E2 effects. Meyer-Lindenberg et al. (2006) presented angry and fearful faces to adults and found that MAOA-low adults showed significant increased reactivity in the left amygdala and decreased reactivity of the subgenual and supragenual ventral cingulate cortex in comparison with the results seen in MAOA-high adults. During experimenter-manipulated recall of aversive emotional information, MAOA-low males showed increased left amygdala and hippocamopal reactivity relative to MAOA-high males. Likewise, during a no-go task that required response inhibition, MAOA-low males showed deficient activation of the dorsal anterior cingulate. These exciting findings indicate the power of bringing genetic analyses into experimental psychopathology. Again, even stronger findings might be observed by using maltreatment histories to test the G × E1 × E2 effect.
Another step in testing Figure 1 will be the examination of the relation between neural processing, as measured by fMRI, and behavioral outcomes. Two parallel research designs will be important. First, researchers must associate fMRI responses such as those noted above with individual differences in behavior as measured by psychiatric diagnosis or aggressive behaviors (Link P). Second, researchers must manipulate neural functioning experimentally in order to observe effects on behavior. Crockett, Clark, Tabibnia, Lieberman, and Robbins (2008) administered a procedure as a way to manipulate 5-HT function during a laboratory game in which subjects are provoked unfairly. They found that stronger retaliatory (aggressive) behavioral responses occurred following acute tryptophan depletion than following a placebo.
Social–Cognitive Mechanisms
A parallel history characterizes research in social-cognitive mechanisms in aggressive behavior. Following a period of examining traitlike social–cognitive characteristics of aggressive children (reviewed by Dodge et al., 2006), scholars have studied social–cognitive responses to experimentally manipulated online social stimuli involving provocation and threat. Dodge (2006) has summarized the findings indicating that aggressive behaviors are likely to follow a series of information-processing actions that include hypervigilant selective attention to hostile cues, hostile attributions of others’ behavior, experience of heightened anger, aggressive response access from memory, and favorable evaluations of the likely consequences of aggressing. These same patterns characterize chronically aggressive children.
Longitudinal studies indicate that these aggressogenic information-processing patterns develop partly as a consequence of early adverse life experiences of physical maltreatment (link E1). Dodge et al, (1990), and Weiss, Dodge, Bates, and Pettit (1992) found that children with a history of physical maltreatment become hypervigilant to hostile cues, likely to attribute hostile intent to ambiguous peer provocateurs, likely to access aggressive responses from memory in response to provocation, and likely to evaluate the consequences of aggressing favorably. Hill, Murray, Leidecker, and Sharp (2008) found that having a mother with postpartum depression and an insecure attachment at 18 months of age alters intentionality attributions in response to threat at 5 years of age. Figure 1 suggests that these modest-sized findings would be stronger if a genetic analysis were added (i.e., the G × E1 × E2 effect).
Autonomic Arousal Mechanisms
Various autonomic measures indicate the relevance of this system to conduct problem-related behavior. A meta-analysis by Ortiz and Raine (2004) indicates robust findings indicating that chronically antisocial individuals display a static trait of relatively low resting heart rate. Lorber’s review (2004) indicates the added value of the dynamic interaction with the proximal environment (E2): Aggressive individuals display high heart rate reactivity when responding to adverse stimuli but not when they respond to positive stimuli. Crozier et al. (2008) found that both resting heart rate and high heart rate reactivity in response to provocation predict growth in aggressive behavior across time. Patrick (2008) has hypothesized that both low resting heart rate and high heart reactivity to adverse stimuli may be related to impairments in affective regulatory circuits in the brain.
The study of sympathetic system mechanisms in aggressive behavior could well benefit from the same kind of methods that are beginning to be used in the study of neural mechanisms. First, longitudinal studies could identify environmental experiences that are associated with the development of autonomic arousal patterns. Second, integration of genetic information in these studies and testing of G × E1 and G × E2 effects might increase effect sizes. Third, experimental manipulation of autonomic arousal could test the causal role of heart rate reactivity in aggressive behavior. Fourth, one could test the hypothesis that acquired autonomic arousal patterns mediate the impact of G, E1, and G × E1 effects on the development of aggressive behavior. Finally, distinguishing between reactive and callous aggressive behavior might refine empirical findings (i.e., if low resting heart rate is related to callous psychopathy and high heart rate reactivity is related to reactive aggression).
Integration Across Systems
Figure 1 posits three systems as mediators of gene–environment effects but does not yet articulate the nature of the relation among these systems. Yet another task for scholars will be to understand how neural, information-processing, and autonomic systems relate to each other during social interactions. Crozier et al. (2008) found that social-information-processing responses mediated the effect of heart rate reactivity on aggressive behavior, suggesting that these systems may follow a similar path. Integrating neural responses to both of these systems seems to be a logical next step.
Mediation of Gene–Environment Effects Through Systems Analysis
Two complementary methods are available to test the hypothesis that the process through which gene–environment effects operate (i.e., the “how” of conduct disorder development) involves the three proposed systems. With longitudinal data, mediation tests can be applied using contemporary methods (MacKinnon, Lockwood, Hoffman, West, & Sheets, 2002). Second, experimental manipulations of antecedent steps can be used to examine causal influence on subsequent processes. So, environments (E1 and E2) can be manipulated through laboratory experiments or clinical interventions to determine the impact on each of the three systems and conduct disorder outcomes. Also, each of the three systems can be experimentally altered through pharmacologic or cognitive manipulations to determine the impact on conduct disorder and aggressive behavior outcomes.
GENERALIZATION TO OTHER DISORDERS
The same template that is depicted in Figure 1 could be applied to the study of other disorders in developmental psychopathology. It has long been known that stressful life experiences, especially those involving threat, loss, humiliation, or rejection, predict the onset of depression but that not all people respond to stressors in this manner. It turns out that, in response to the stressful life experience of childhood maltreatment, individuals with a short-short or short-long allele polymorphism in the promoter region of the serotonin transporter (5-HTT) gene are more likely to exhibit a major depressive episode than are individuals who are homozygous for the long allele (Caspi et al., 2003).
Why might persons with this polymorphism be susceptible to depression? Through evolution, humans naturally respond to stressors with fear and anxiety, but we also respond with high serotonergic activity that titrates the anxiety response. Individuals with a short-allele polymorphism in 5HTT are unable to regulate this response to stressors. The findings indicate that the severe stressor of early maltreatment will lead to depression in a genetic context in which healthy serotonergic activity is not available.
Future studies of this diathesis-stress model in depression could integrate other features of the model in Figure 1, namely, incorporation of G × E1 × E2 effects and examination of mechanisms in neural, information-processing, and autonomic systems. Certainly, the rich body of knowledge in depression already incorporates much of this analysis.
Caspi and Moffitt (2006) note that one contribution of Gene × Environment analyses in the study of psychopathology will be a greater understanding of the supposed nonspecific effects of the environment on psychopathology (called multifinality effects). For example, it is known that childhood maltreatment is a devastating life event that causes all sorts of psychiatric disorder outcomes in different people. Which disorder results for a given person? It depends on the genetic context in which it occurs and the mechanisms that operate. If maltreatment occurs in a genetic context of low MAOA activation, maltreatment will likely cause reactive aggression and conduct disorder. If maltreatment occurs in the genetic context of a short–short or short–long allele polymorphism in serotonergic activity, it will likely cause a major depressive episode. These outcomes are not mutually exclusive. In fact, many maltreated children experience both reactive aggression and depression. Likewise, these genetic contexts are not mutually exclusive and may co-occur. Furthermore, the path from genetic context to neural mechanisms is not linear and may be affected by interactions among genes (Meyer-Lindenberg et al., 2008).
It is as if this horrible environmental pathogen of physical abuse is thrust upon a child, and the child succumbs at her or his weakest link, which is determined by a genetic context. Likewise, it is known that chronic stressors affect both depressive disorder and cardiovascular function, but they do so differently in different individuals. Perhaps the genetic variable and analyses of neural, information-processing, and autonomic mechanisms could distinguish outcomes.
RESEARCH CAUTIONS AND THE FUTURE PATH
The proposed model offers plenty of research questions to pursue in the coming decade. However, the model presumes that these recent findings are robust and have no alternate explanation. A major caution must be expressed that some of the presumed gene–environment interaction effects could be masked gene–gene interactions. That is, just because we identify a correlation between an environmental variable such as maltreatment and an outcome such as depression or antisocial behavior does not mean that we can conclude cause. We must develop stronger methods, data, and theories to buttress this case. Likewise, discovery of a correlation between a particular gene and a behavioral outcome does not mean that the gene is causal. It is plausible that genes covary. One gene might be correlated with a behavior outcome because of its correlation with another gene that is actually causal.
Experimental manipulation of environments seems to be the strongest method available to test environmental variables. Both E1 and E2 environments can be subjected to manipulation. Laboratory experiments such as those described above already manipulate E2 environments regularly. The impact of E1 environments can also be studied through natural experiments, such as the studies of children adopted from Eastern European orphanages (Rutter & The English and Romanian Adoptees Study Team, 1998). Preventive intervention studies also provide a strong test of E1 environments. Prevention science thus is poised to play an important role in genetic and neuroscientific inquiry.
Perhaps the most important assertion to be made in this brief article is that the greatest contributions to science in the coming decade will be made by scientific teams that are able to combine multiple disciplinary perspectives and methods to understand how psychopathology develops. Genetics, neuroscience, developmental psychology, social psychology, cognitive science, psychophysiology, and prevention science must be combined to understand how psychopathology develops and can be prevented. Training of future scholars, therefore, must blend the appropriate need for depth with a healthy dose of breadth.
Acknowledgments
The author is grateful for the support of Senior Scientist Award K05DA015226 and the Transdisciplinary Prevention Research Center Award P20DA017589, both from the National Institute on Drug Abuse.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Berkowitz L. On the consideration of automatic as well as controlled psychological processes in aggression. Aggressive Behavior. 2008;34:117–129. doi: 10.1002/ab.20244. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Development and Psychopathology. 2005;17:271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE. Gene-environment interactions in psychiatry: Joining forces with neuroseience? Nature Reviews Neuroscience. 2006;7:583–590. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington HL, et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–399. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Cloninger R, Sigvardsson S, Bohman M, von Knorring A. Predisposition to petty criminality in Swedish adoptees. Archives of General Psychiatry. 1982;39:1242–1247. doi: 10.1001/archpsyc.1982.04290110010002. [DOI] [PubMed] [Google Scholar]
- Cohen MA. The costs of crime and justice. New York: Routledge; 2005. [Google Scholar]
- Crockett MJ, Clark L, Tabibnia G, Lieberman MD, Robbins TW. Serotonin modulates behavioral reactions to unfairness. Science. 2008;320:1739. doi: 10.1126/science.1155577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozier JC, Dodge KA, Fontaine EG, Lansford JE, Bates JE, Pettit GS, Levenson RW. Social information processing and cardiac predictors of adolescent antisocial behavior. Journal of Abnormal Psychology. 2008;117:253–267. doi: 10.1037/0021-843X.117.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge KA. Translational science in action: Hostile attributional style and the development of aggressive behavior problems. Development and Psychopathology. 2006;18:791–814. doi: 10.1017/s0954579406060391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge KA, Bates JE, Pettit GS. Mechanisms in the cycle of violence. Science. 1990;250:1678–1683. doi: 10.1126/science.2270481. [DOI] [PubMed] [Google Scholar]
- Dodge KA, Coie JD, Lynam D. Aggression and antisocial behavior in youth. In: Damon W, Eisenberg N, editors. Handbook of child psychology: Vol. 3. Social, emotional, and personality development. 6. New York: Wiley; 2006. pp. 719–788. [Google Scholar]
- Dodge KA, Sherrill MR. The interaction of nature and nurture in antisocial behavior. In: Flannery D, Vazonsyi A, Waldman I, editors. The Cambridge handbook of violent behavior. New York: Cambridge University Press; 2007. pp. 215–242. [Google Scholar]
- Farrington DP. Conduct disorder, aggression, and delinquency. In: Lerner RM, Steinberg L, editors. Handbook of adolescent psychology. 3. Hoboken, NJ: Wiley; 2008. pp. 324–345. [Google Scholar]
- Foley D, Eaves L, Wormley B, Silberg JL, Maes H, Kuhn J, et al. Childhood adversity, monoamine oxidase A genotype, and risk for conduct disorder. Archives of General Psychiatry. 2004;61:738–744. doi: 10.1001/archpsyc.61.7.738. [DOI] [PubMed] [Google Scholar]
- Hill J, Murray L, Leidecker V, Sharp H. The dynamics of threat, fear and intentionality in the conduct disorders: Longitudinal findings in the children of women with post-natal depression. Philosophical Transactions of the Royal Society of London. 2008;363:2529–2541. doi: 10.1098/rstb.2008.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgins S. Violent behaviour among people with schizophrenia: A framework for investigations of causes, and effective treatment, and prevention. Philosophical Transactions of the Royal Society of London. 2008;363:2505–2518. doi: 10.1098/rstb.2008.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffee SR, Caspi A, Moffitt TE, Dodge KA, Rutter M, Taylor A, Tully LA. Nature × Nurture: Genetic vulnerabilities interact with physical maltreatment to promote conduct problems. Development and Psychopathology. 2005;17:67–84. doi: 10.1017/s0954579405050042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Kessler RC, Walters EE, MacLean C, Neale MC, Heath AC, Eaves LJ. Stressful life events, genetic liability and onset of an episode of major depression in women. American Journal of Psychiatry. 1995;152:833–842. doi: 10.1176/ajp.152.6.833. [DOI] [PubMed] [Google Scholar]
- Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, Moffitt TE. MAOA, maltreatment, and gene–environment interaction predicting children’s mental health: new evidence and a meta-analysis. Molecular Psychiatry. 2006;11:903–1004. doi: 10.1038/sj.mp.4001851. [DOI] [PubMed] [Google Scholar]
- Lorber MF. Psychophysiology of aggression, psychopathy, and conduct problems: A meta-analysis. Psychological Bulletin. 2004;130:531–552. doi: 10.1037/0033-2909.130.4.531. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test the significance of the mediated effect. Psychological Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Buckholtz JW, Kolachana B, Hariri AR, Pezawas L, Blasi G, et al. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proceedings of the National Academy of Sciences, USA. 2006;103:6269–6274. doi: 10.1073/pnas.0511311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE. Adolescence-limited and lifecourse-persistent antisocial behavior: A developmental taxonomy. Psychological Review. 1993;100:674–701. [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Rutter M. Measured gene–environment interactions in psychopathology: Concepts, research strategies, and implications for research, intervention, and public understanding of genetics. Perspectives on Psychological Science. 2006;1:5–27. doi: 10.1111/j.1745-6916.2006.00002.x. [DOI] [PubMed] [Google Scholar]
- Ortiz J, Raine A. Heart rate level and antisocial behavior in children and adolescents: A meta-analysis. Journal of the American Academy of Child & Adolescent Psychiatry. 2004;43:154–162. doi: 10.1097/00004583-200402000-00010. [DOI] [PubMed] [Google Scholar]
- Patrick CJ. Psychophysiological correlates of aggression and violence; An integrative review. Philosophical Transactions of the Royal Society of London. 2008;363:2543–2555. doi: 10.1098/rstb.2008.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M. Introduction. Philosophical Transactions of the Royal Society of London. 2008;363:2485–2489. doi: 10.1098/rstb.2008.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M The English and Romanian Adoptees Study Team. Developmental catch-up and deficit following adoption after severe global early privation. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1998;39:465–476. [PubMed] [Google Scholar]
- Rutter M, Moffitt TE, Caspi A. Gene-environment interplay and psychopathology: Multiple varieties but real effects. Journal of Child Psychology and Psychiatry. 2006;47:226–261. doi: 10.1111/j.1469-7610.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- Shih J, Chen K, Ridd M. Monoamine oxidase: From genes to behavior. Annual Review of Neuroscience. 1999;22:197–217. doi: 10.1146/annurev.neuro.22.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A, Kim-Cohen J. Meta-analysis of gene-environment interactions in developmental psychopathology. Development and Psychopathology. 2007;19:1029–1037. doi: 10.1017/S095457940700051X. [DOI] [PubMed] [Google Scholar]
- Van Goozen SHM, Fairchild G, Harold GT. The role of neurobiological deficits in childhood antisocial behavior. Current Directions in Psychological Science. 2008;17:224–228. [Google Scholar]
- Viding E, Larsson H, Jones AP. Quantitative genetic studies of antisocial behaviour. Philosophical Transactions of the Royal Society of London. 2008;363:2519–2527. doi: 10.1098/rstb.2008.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B, Dodge KA, Bates JE, Pettit GS. Some consequences of early harsh discipline: Child aggression and a maladaptive social information processing style. Child Development. 1992;63:1321–1335. doi: 10.1111/j.1467-8624.1992.tb01697.x. [DOI] [PubMed] [Google Scholar]


