Abstract
Background
Vestibular Evoked Myogenic Potential (VEMP) testing has gained increased interest in the diagnosis of a variety of vestibular etiologies. Comparisons of P13 / N23 latency, amplitude and threshold response curves have been used to compare pathologic groups to normal controls. Appropriate characterization of these etiologies requires normative data across the frequency spectrum and age range.
Purpose
The objective of the current study was to test the hypothesis that significant changes in VEMP responses occur as a function of increased age across all test stimuli as well as characterize the VEMP threshold response curve across age.
Research Design
This project incorporated a prospective study design using a sample of convenience. Openly recruited subjects were assigned to groups according to age.
Study Sample
Forty-six normal controls ranging between 20 and 76 years of age participated in the study. Participants were separated by decade into 5 age categories from 20 to 60 plus years. Normal participants were characterized by having normal hearing sensitivity, no history of neurologic or balance/dizziness involvement and negative results on a direct office vestibular examination.
Intervention
VEMP responses were measured at threshold to click and 250, 500, 750, and 1000 Hz tone burst stimuli and at a suprathreshold level to 500 Hz toneburst stimuli at123 dBSPL.
Data Collection and Analysis
A mixed group factorial ANOVA and linear regression were performed to examine the effects of VEMP characteristics upon age.
Results
There were no significant differences between ears for any of the test parameters. There were no significant differences between age groups for n23 latency or amplitude in response to any of the stimuli. Significant mean differences did exist between age groups for p13 latency (250, 750, and 1000 Hz) and threshold (500 and 750 Hz). Age was significantly correlated with VEMP parameters. VEMP threshold was positively correlated (250, 500, 750, 1000 Hz); and amplitude was negatively correlated (500 Hz Maximum). The threshold response curves revealed best frequency tuning at 500 Hz with the highest thresholds in response to click stimuli. However, this best frequency tuning dissipated with increased age. VEMP response rates also decreased with increased age.
Conclusion
We have demonstrated that minor differences in VEMP responses occur with age. Given the reduced response rates and flattened frequency tuning curve for individuals over the age of 60, frequency tuning curves may not be a good diagnostic indicator for this age group.
Keywords: Vestibular, age, VEMP
Introduction
Vestibular evoked myogenic potential (VEMP) testing is currently being utilized in the assessment of a variety of vestibular etiologies. The VEMP response is obtained by measuring the release of the sternocleidomastoid (SCM) muscle from a contracted state provoked by delivering auditory stimuli to the ipsilateral ear (Colebatch et al., 1994). VEMP responses are considered to be a reflection of vestibulospinal projections to the neck, which give rise to information regarding saccule and inferior vestibular nerve integrity (Colebatch and Halmagyi, 1992; Robertson and Ireland, 1995). The pathway of the VEMP response is projected to begin in the saccule and extend along the inferior branch of the vestibular nerve to the vestibular nuclei and project to motor neurons in the SCM muscle causing a release from the contracted state (Colebatch and Halmagyi, 1992; Colebatch et al, 1994; Robertson and Ireland, 1995; Halmagyi and Colebatch, 1995).
This reflex pathway was originally studied in animal models, which indicated afferent vestibular hair cells in the saccule to be responsive to click and tonal auditory stimulation at high intensity levels in cats and guinea pigs (Didier and Cazals, 1989; McCue and Guinan, 1994; McCue and Guinan, 1997; Merchant, 1999). Auditory sensitive neurons have also been located in the lateral and descending vestibular nuclei of guinea pigs (Merchant, 1999). These afferent vestibular hair cells of the saccule have been found to have an increased firing rate in response to toneburst and click acoustic stimuli above 90 dB SPL in cats (McCue and Guinan, 1994). Further research indicated these fibers to be most responsive to frequencies between 500 and 1000 Hz, with little to no responsiveness occurring to auditory stimuli above 3000 Hz (McCue and Guinan, 1997).
Likewise, the VEMP response in humans has been found to be present at high presentation levels. VEMP thresholds have been reported between 120–135 dB SPL (Welgampola and Colebatch, 2001a) and 75–105 dBnHL (Ochi and Ohashi, 2003) in response to click stimuli, 105–120 dB SPL in response to 1000 Hz toneburst stimuli (Welgampola and Colebatch, 2001a) and 60 to 75 dBnHL in response to 250 Hz toneburst stimuli (Zapala and Brey, 2004). Like the animal models, VEMP responses in humans have been found to show optimal frequency sensitivity. Optimal stimulus frequencies have been reported at 300–350 Hz (Todd et al, 2000), 500 Hz (Rauch et al, 2004) and 700 Hz in normal subjects (Welgampola and Colebatch, 2001a). Because the VEMP responds optimally at specific frequencies, VEMP threshold response curves can be generated. These threshold response curves are showing promise in the identification of Meniere’s disease and discrimination between the symptomatic and asymptomatic ear (Rauch et al, 2004; Lin et al, 2006).
Normative data across all stimulus frequencies is necessary in order to correctly characterize VEMP responses in populations with vestibular disorders. Current investigations have documented age related changes in the VEMP response (Su et al, 2001; Welgampola and Colebatch, 2001b; Ochi and Ohashi, 2003; Zapala and Brey, 2004; Basta et al, 2005; Basta et al, 2007; Lee et al, 2008). Research indicates a decrease in VEMP amplitude and an increase in VEMP threshold with increased age, however these studies have only evaluated VEMP responses to click, 250 and 500 Hz toneburst stimuli (Su et al, 2001; Welgampola and Colebatch, 2001b; Ochi and Ohashi, 2003; Zapala and Brey, 2004; Basta et al, 2005; Basta et al, 2007; Lee et al, 2008). Of importance, is a lack of a uniform means of SCM muscle contraction strength monitoring between these studies (Welgampola and Colebatch, 2001b; Ochi and Ohashi, 2003; Zapala and Brey, 2004). Because the amplitude of the VEMP response is contingent upon the degree of SCM muscle contraction (Colebatch et al, 1994; Akin, et al, 2004), investigations assessing age related changes in VEMP responses must compare responses among populations with a uniform means of SCM muscle contraction.
The objective of the current study was to test the hypothesis that significant changes in VEMP responses occur as a function of increased age assessing both click and 250, 500, 750 and 1000 Hz toneburst stimuli as well as to characterize the VEMP threshold response curve across age. In order to test this hypothesis, age related VEMP threshold response curves were generated on a control population.
Materials and Methods
Forty-six normal controls ranging between 20 and 76 (mean 44.2) years of age participated in the research study. Participants were recruited from the local community and the Human Subjects Research Core, a database of individuals who have indicated their interest in participating in research at Boys Town National Research Hospital. Participants were separated into the following age categories (N = the number of subjects):
Group 1 included subjects between 20–29 (mean 23.4) years (N = 10).
Group 2 included subjects between 30–39 (mean 32.38) years (N = 8).
Group 3 included subjects between 40–49 (mean 42.6) years (N = 10).
Group 4 included subjects between 50–59 (mean 55.25) years (N = 8).
Group 5 included subjects age 60 + (mean 67.2) years (N = 10).
Normal participants were characterized by having normal hearing sensitivity and no history of balance disorders, dizziness, or neurologic involvement. In order to rule out the presence of hearing loss, all subjects completed a hearing screening at 25 dB from 250 to 8000 Hz. Evidence of age related hearing loss was permissible for individuals 65 years or older if hearing loss was symmetrical, sensorineural in nature and not of sudden onset. In order to rule out neurologic involvement or the presence of central and/or peripheral vestibular dysfunction, all subjects completed a short case history and bedside exam including ocular range of motion, saccade, smooth pursuit, head thrust, gaze with and without fixation and head shake testing without fixation.
All subjects completed VEMP testing in a seated position in response to rarefaction broadband clicks (100 μsec) and tone burst stimuli (250, 500, 750, and 1000 Hz) with a two-cycle rise/fall and no plateau (Blackman gated). SCM contraction was obtained by having each participant generate (via head turn) 25 mmHg of additional resistance with a blood pressure cuff pre-inflated to 20 mmHg during acoustic stimulation (Vanspauwen et al, 2006b). The blood pressure cuff was pre-inflated around the subject’s flat hand which was then held against the face at the zygomatic arch area. The subject monitored the blood pressure meter in order to obtain constant feedback regarding the amount of pressure being applied into the cuff with a head turn. Participants were instructed to rest between trials. All stimuli were presented via insert phones at a repetition rate of 13.3 per second. The responses of 200 stimuli were recorded and averaged per trial. EMG signals were amplified and band-pass filtered from 20 to 1500 Hz by a Biologic Auditory Evoked Potentials unit. The active electrode (non-inverting) was placed on the ipsilateral SCM muscle belly with a ground electrode on the forehead and a reference electrode (inverting) on the manubrium of the sternum. The order of stimuli presentation and selection of right or left SCM muscle was randomized.
Two control trial conditions were completed during VEMP testing to ensure that the VEMP response was contingent on SCM muscle contraction and auditory stimulus presentation. First, VEMP responses were recorded while subjects contracted the SCM via the above mentioned method with no auditory stimulus present. Secondly, VEMP responses were recorded while subjects looked straight ahead with no SCM muscle contraction while an auditory stimulus was presented.
VEMP threshold, amplitude and P13 and N23 latency were recorded for each stimuli as well as the amplitude and P13 and N23 latency in response to maximum auditory stimulation (123 dB peak SPL) to 500 Hz toneburst stimuli. VEMP threshold was defined as the lowest level at which both the P13 and N23 were clearly definable and replicable. Amplitude, P13 and N23 latency were calculated on the average of two replicable trials at threshold and in response to 123 dB SPL to 500 Hz toneburst stimuli. The maximum level tested for each toneburst frequency was 123 dB SPL and for click stimuli was 119 dB SPL. When a clearly definable and replicable VEMP response was not obtained at this level, VEMP responses were considered absent.
Results
Response rates varied according to stimulus type and age. As shown in Table 1, the highest response rates occurred in the younger groups (Groups 1 – 4; 20 to 59 years) in response to 500, 750, and 1000 Hz toneburst stimuli. In terms of stimulus type, poorer response rates were noted in response to click and 250 Hz toneburst stimuli. VEMP responses were present bilaterally in only 15.6% (7 of 45) of participants in response to click stimuli and in 65.9% (29 of 44) of participants in response to 250 Hz toneburst stimuli; whereas VEMP responses were present bilaterally in 94% (43 of 46), 94% (43 of 46), and 89% (41 of 46) of participants in response to 500, 750 and 1000 Hz tone burst stimuli respectively. For both click and 250 Hz tone burst stimuli, absent responses predominately occurred in Groups 4 (50 – 59 years) and 5 (60 + years). While these age groups accounted for 28 of 60 (46.7%) ears with absent VEMP in response to click stimuli and 14 of 21 (66.6%) ears with absent VEMP in response to 250 Hz tone burst stimuli, there was no significant difference in response rates between the age groups for any of the stimulus types.
Table 1.
Vestibular Evoked Myogenic Potential response rates by age group and stimuli
| Age Group |
STIMULUS | ||||
|---|---|---|---|---|---|
| click | 250 Hz | 500 Hz | 750 Hz | 1000 Hz | |
| 20 – 29 | 50% | 95% | 100% | 100% | 100% |
| 30 – 39 | 50% | 75% | 100% | 100% | 100% |
| 40 – 49 | 30% | 89% | 95% | 100% | 95% |
| 50 – 59 | 31% | 50% | 100% | 100% | 100% |
| 60 + | 6% | 67% | 90% | 83% | 78% |
| Overall | 33% | 75% | 97% | 97% | 95% |
A mixed group factorial Analysis of Variance (ANOVA) was performed to examine the effects of age categories upon VEMP parameters (threshold, P13 latency, N23 latency, and amplitude) and side (right versus left). There was no main effect for age across stimuli: click, 250, 500, 750, and 1000 Hz toneburst, with the exception of a few parameters. A significant difference in VEMP threshold between age groups was found in response to 500 Hz (F 4,38=3.063, p = .028) and 750 Hz (F 4,38=3.184, p = .024) toneburst stimuli. In response to 500 Hz tone burst stimuli, post hoc analysis using Tukey’s honest significant difference (HSD) indicated that Groups 2 (30–39 years) and 3 (40–49 years) had significantly lower VEMP thresholds than Groups 4 (50–59 years) and 5 (60+ years). In response to 750 Hz tone burst stimuli, Group 1 (20–29 years) had significantly lower VEMP thresholds than Group 5 (60+ years), while Group 2 (30–39 years) had significantly lower VEMP thresholds than both Groups 4 (50–59 years) and 5 (60+ years).
A significant difference in P13 latency did exist between age groups in response to 250 Hz (F 4,24=3.361, p = .026) 750 Hz (F 4,38=8.49, p = .001) and 1000 Hz (F 4,36=3.709, p = .013) toneburst stimuli. For 250 Hz toneburst stimuli, post hoc analysis indicated that Group 1 (20–29 years) had significantly longer P13 latencies in comparison to all age groups (HSD minimum mean difference = 2.286). For both 750 and 1000 Hz toneburst stimuli, post hoc analysis indicated that Group 1 (20–29 years) had significantly longer P13 latencies than Group 3 (40–49 years) and Group 5 (60+ years).
In response to 500 Hz toneburst stimuli at the maximum level (123 dB SPL), there were no statistically significant differences regarding age on any of the VEMP parameters: P13 latency, N23 latency and amplitude. The latency and amplitude parameters in response to maximal stimulation (123 dB SPL) at 500 Hz are shown in Table 2 for each of the age categories. These parameters are shown to characterize the response at a suprathreshold level.
Table 2.
Normative Mean Values (across all ages for right and left ears combined). All parameters are reported at threshold with the exception of 500 Hz toneburst maximum presentation level (123 dBSPL).
| Click | 250 Hz | 500 Hz | 750 Hz | 1000 Hz | 500 Hz | MAX | |
|---|---|---|---|---|---|---|---|
| THRESHOLD | |||||||
| OVERALL | 122.17 (4.09) | 116.87 (6.45) | 114.16 (6.45) | 115.75 (5.53) | 116.65 (5.2) | NA | |
| 20–29 | 123 (2.58) | 116.58 (5.01) | 113.25 (3.35) | 113.75 (3.58) | 115.5 (3.2) | NA | |
| 30–39 | 121.25 (4.43) | 114.58 (7.22) | 110.31 (6.45) | 112.5 (4.47) | 114.69 (4.64) | NA | |
| 40–49 | 119.17 (5.85) | 114.69 (6.94) | 111.84 (6.08) | 116 (7.36) | 116.05 (6.36) | NA | |
| 50–59 | 125 | 119.38 (5.63) | 117.81 (5.15) | 117.81 (4.46) | 119.06 (5.83) | NA | |
| 60+ | 125 | 120.83 (5.97) | 117.78 (7.52) | 119.33 (4.17) | 118.57 (4.57) | NA | |
| AMPLITUDE | |||||||
| OVERALL | 27.17 (9.13) | 27.86 (11.61) | 27.65 (11.13) | 29.96 (13.36) | 29.83 (13.37) | 57.34 (34.32) | |
| 20–29 | 27.88 (9.87) | 29.59 (9.56) | 32.76 (10.92) | 35.46 (16.35) | 33.57 (15.22) | 66.12 (31.49) | |
| 30–39 | 26.71 (8.33) | 29.9 (12.63) | 25.75 (12) | 26.13 (11.8) | 24.39 (11.18) | 74.54 (40.96) | |
| 40–49 | 30.57 (11.20) | 28.15 (11.87) | 27.64 (11.32) | 27.14 (9.81) | 27.02 (8.39) | 56.13 (30.21) | |
| 50–59 | 22.1 (7.47) | 23.10 (9.25) | 20.63 (6.76) | 27.42 (15.58) | 31.93 (16.77) | 42.37 (35.16) | |
| 60+ | 28.69 | 25.89 (14.92) | 29.92 (10.94) | 33.19 (10.22) | 32.16 (13.05) | 50.61 (29.3) | |
| P13 LATENCY | |||||||
| OVERALL | 13.62 (2.88) | 20.99 (2.45) | 16.24 (2.42) | 15.2 (2.26) | 14.58 (2.49) | 15.92 (1.92) | |
| 20–29 | 14.53 (2.51) | 22.86 (1.96) | 17.67 (3.38) | 17.45 (2.42) | 16.41 (2.57) | 17 (2.58) | |
| 30–39 | 12.15 (0.94) | 19.24 (0.9) | 16.42 (1.71) | 14.97 (1.67) | 14.47 (1.98) | 15.88 (0.97) | |
| 40–49 | 12.78 (4.13) | 21.04 (3.15) | 15.09 (1.25) | 14.04 (1.62) | 13.1 (2.05) | 15.06 (1.39) | |
| 50–59 | 14.43 (3.35) | 20.16 (2.01) | 16.78 (2.06) | 15.01 (1.96) | 14.99 (2.23) | 16.43 (2.18) | |
| 60+ | 17.42 | 20.25 (1.5) | 15.23 (2.08) | 14.19 (1.55) | 13.6 (2.2) | 15.35 (1.52) | |
| N23 LATENCY | |||||||
| OVERALL | 20 (2.66) | 28.49 (2.69) | 22.97 (2.62) | 22.12 (2.32) | 21.41 (2.36) | 23.22 (2.16) | |
| 20–29 | 20.75 (2.27) | 29.77 (2.26) | 24.12 (3.05) | 24.17 (1.84) | 22.35 (2.59) | 23.66 (2.33) | |
| 30–39 | 19.09 (1.82) | 27.79 (1.81) | 23.33 (2.62) | 21.82 (2.26) | 21.12 (2.32) | 23.84 (2.22) | |
| 40–49 | 19.36 (3.66) | 28.61 (3.46) | 22.05 (2.45) | 20.74 (2.39) | 20.73 (2.69) | 22.85 (2.07) | |
| 50–59 | 19.66 (2.5) | 28.29 (1.83) | 22.81 (2.2) | 22.29 (1.87) | 21.52 (1.48) | 23.29 (2.22) | |
| 60+ | 25.33 | 27.16 (2.8) | 22.47 (2.35) | 21.35 (1.36) | 21.19 (2.29) | 22.64 (2.03) | |
When examining the effects of ear differences (right versus left), there was no main effect for ear on threshold, P13 latency, N23 latency, and amplitude, for right versus left ear across stimuli: click, 250, 500, 750, and 1000 Hz tone burst. There was also no interaction between age category and ear difference (right versus left) as they relate to threshold, P13 latency, N23 latency, and amplitude across stimuli: click, 250, 500, 750, and 1000 Hz toneburst. Lastly, no subject displayed a VEMP response in either of the control conditions.
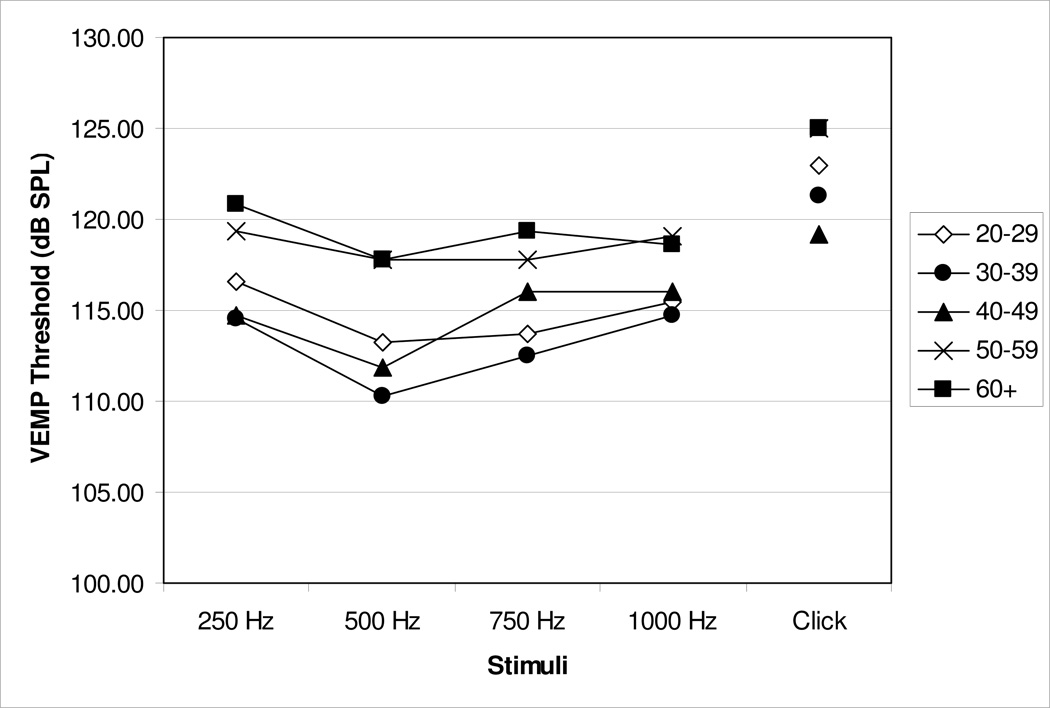
Because no statistically significant difference between the right and left ears was noted, data from the right and left ears has been collapsed for descriptive statistics. Data in Table 2 shows the overall VEMP characteristics across stimuli and age. Threshold response curves across stimuli and as a function of age grouping, shown in Figure 1, indicate that the best frequency threshold responses occurred at 500 Hz regardless of age category with the exception of a flat threshold response curve for Group 5 (60+ years). This frequency tuning appears to be more pronounced in Groups 1 – 3 (20 – 49 years). Of the 46 subjects who completed VEMP testing, 65% (30 of 46) demonstrated the lowest VEMP threshold in response to 500 Hz, 26% (12 of 46) in response to 750 Hz, 7% (3 of 46) in response to 1000 Hz, and 2% (1 of 46) in response to 250 Hz. No subjects demonstrated best threshold responses to click stimuli.
Figure 1.
Mean VEMP threshold responses for all stimuli across age groups. For means and standard deviations, refer to Table 2.
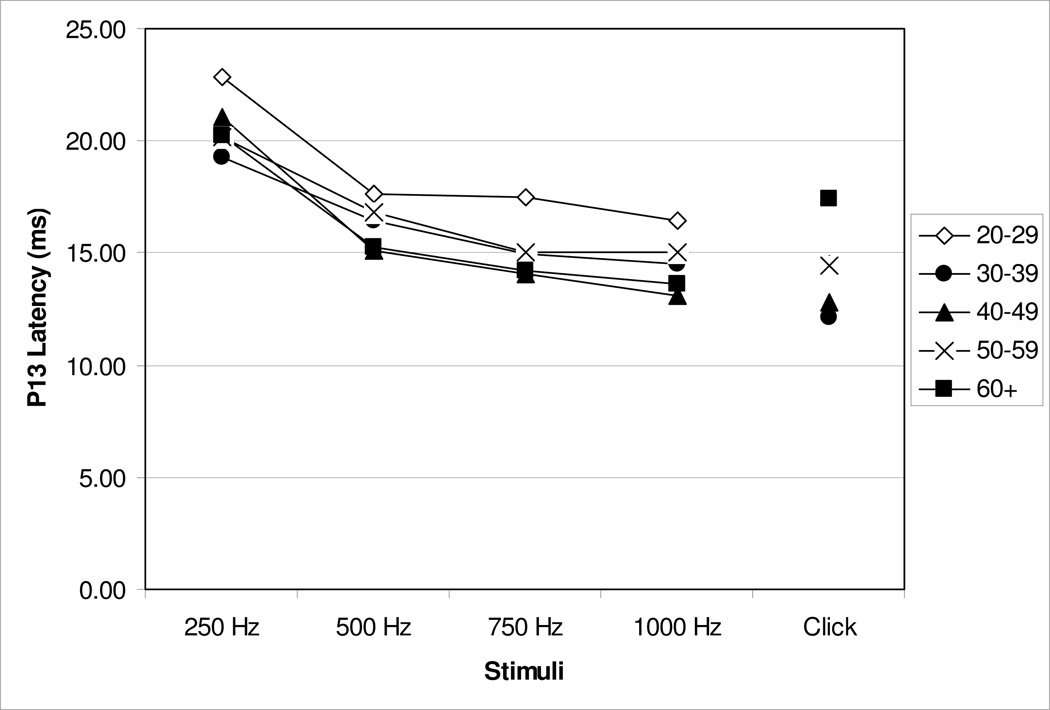
Response curves of mean P13 latencies at threshold across all frequencies as a function of age grouping are shown in Figure 2 for all subjects. These response curves indicate that as the frequency of stimuli decreases the latency of the response is prolonged.
Figure 2.
Mean latency response curve for all stimuli across age groups. For means and standard deviations, refer to Table 2.
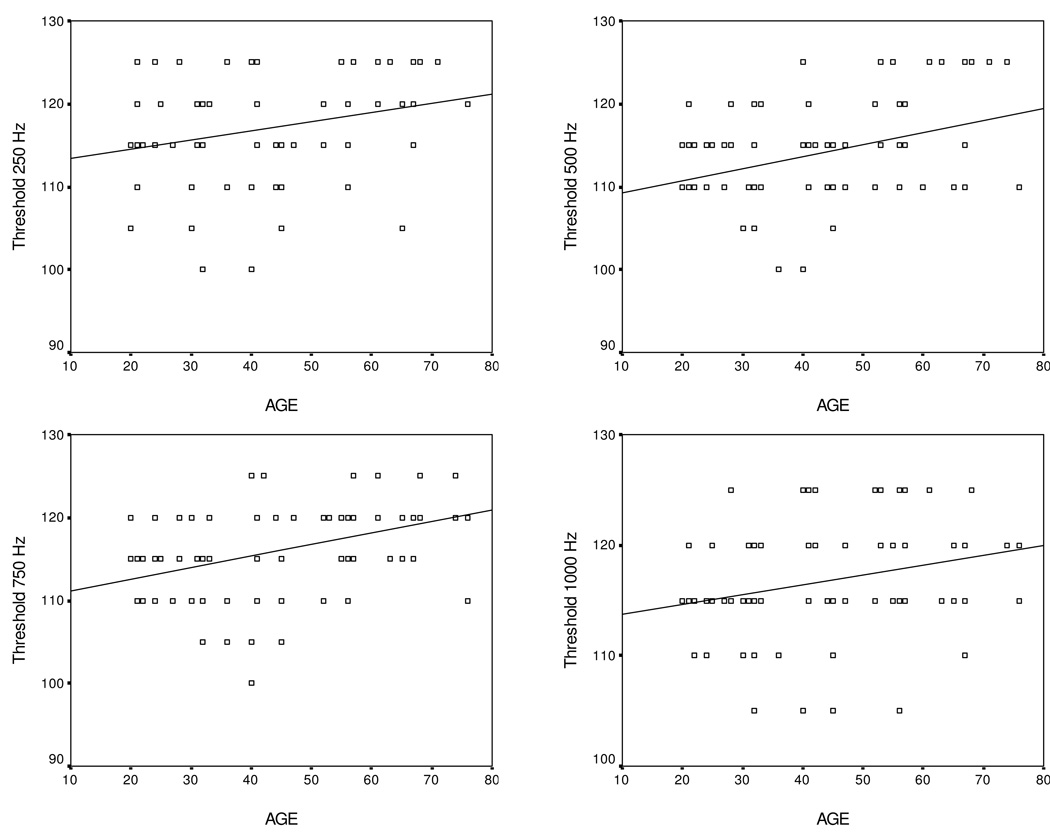
Scatterplots depicting VEMP threshold as a function of stimuli across age groups are shown in Figure 3. While significant differences were noted between age groups in response to 500 and 750 Hz, there was a marginally significant positive correlation between age and VEMP threshold for all toneburst stimuli: 250 Hz (r = 0.275, p = 0.024), 500 Hz (r = 0.362, p = 0.001), 750 Hz (r = 0.4, p = 0.001), and 1000 Hz (r = 0.272, p = 0.012) indicating increased VEMP threshold with increased age. There was no significant correlation between age and VEMP threshold in response to click stimuli.
Figure 3.
Scatterplots of age and VEMP threshold for toneburst frequencies of 250, 500, 750 and 1k Hz.
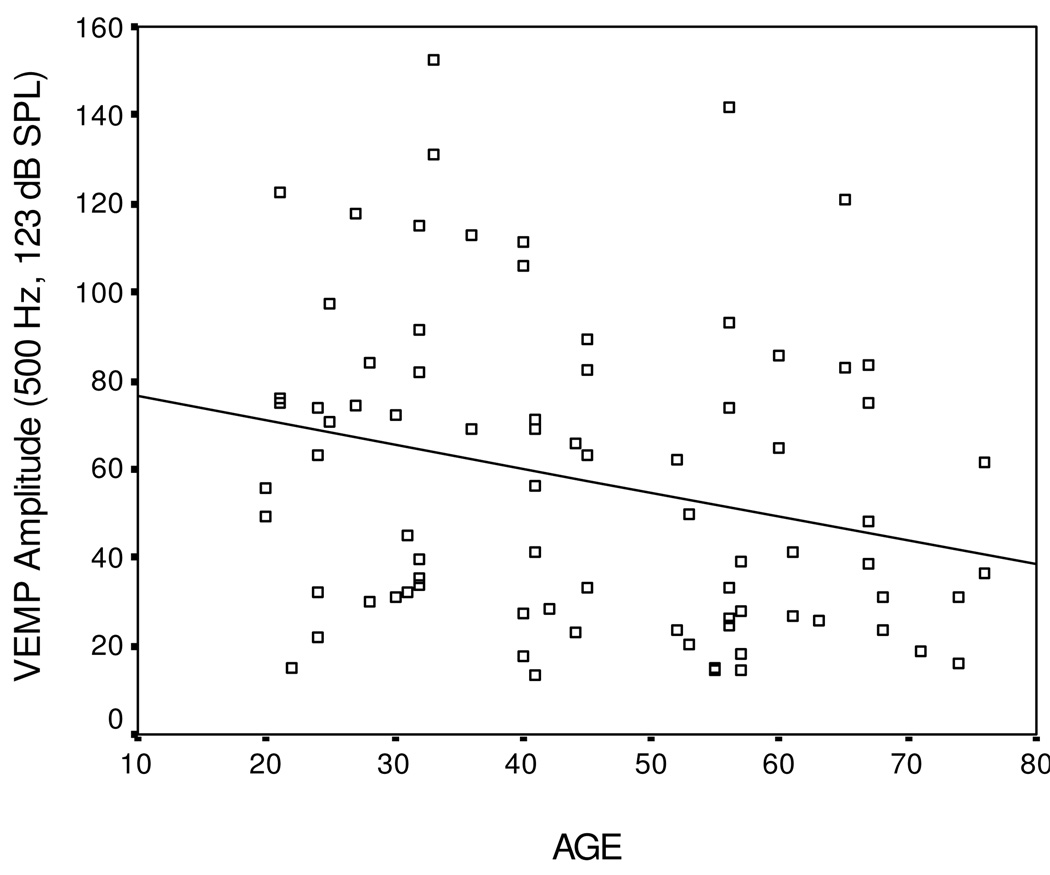
While there were no significant mean differences noted between age groups for VEMP amplitude, a marginally significant negative correlation was noted between age and 500 Hz tone burst stimuli at the maximum level (123 dB SPL) (r = −0.255, p = 0.021) indicating reduced VEMP amplitudes with increased age as shown in Figure 4.
Figure 4.
Scatterplot of age and VEMP amplitude in response to maximum stimulation (123 dB SPL at 500 Hz).
Discussion
In the literature to date, only minor age differences in VEMP responses have been reported. Overall, there has been agreement that regardless of stimulus type, VEMP amplitudes decrease with increased age. This trend has been reported in response to click stimuli (Su et al, 2001; Welgampola and Colebatch, 2001b; Ochi and Ohashi, 2003; Lee et al, 2008), 250 Hz tone burst stimuli (Zapala and Brey, 2004) and 500 Hz tone burst stimuli (Basta et al, 2005; Basta et al, 2007). We did not demonstrate an age effect for VEMP amplitude at any of the test frequencies. Our comparisons for amplitude were calculated at threshold where we expect amplitudes to be reduced for all ages, while others calculated VEMP amplitude differences at a suprathreshold level (Su et al, 2001; Welgampola and Colebatch, 2001b; Ochi and Ohashi, 2003, Zapala and Brey, 2004, Basta et al, 2005; Basta et al, 2007; Lee et al, 2008). In this study, we did analyze VEMP responses to 500 Hz tone bursts at a suprathreshold level (123 dBSPL at 500 Hz). We found no overall mean differences between age groups however we did find a significant negative correlation for VEMP amplitude and age for the suprathreshold responses at 500 Hz.
Previous studies have also documented higher VEMP thresholds with increased age (Welgampola and Colebatch, 2001b; Ochi and Ohashi, 2003). These studies have only investigated threshold differences across age in response to click stimuli. We found significant mean threshold differences with respect to age groups in response to 500 and 750 Hz tone burst stimuli and significant positive correlations between age and VEMP threshold in response to all toneburst stimuli. However we did not find differences in VEMP threshold among age groups in response to click stimuli. This may be attributed to our poor response rates. In response to click stimuli, we demonstrated poor response rates among all participants. This was especially true of the age 60+ group in which only one subject exhibited a VEMP response to click stimuli in one ear. These response rates are in agreement with Su et al (2004) and Welgampola et al (2001b), who also report a decrease in response rate for individuals over 60 years of age.
Few studies have reported other age related changes in the VEMP response. There is little agreement regarding the effect of age on P13 and N23 latencies. An increase in both P13 and N23 latencies has been reported with age (Su et al, 2001; Zapala and Brey, 2004; Lee et al, 2008). However, Basta et al (2005) report no difference in either latency measurement across age groups. While both Welgampola et al (2001b) and Su et al (2001) report no difference in p13 latency as related to age, Welgampola et al (2001b) report a weak negative correlation for N23 latency and age and Su et al (2001) report a positive correlation between N23 latency and age. Similarly, we did not find a uniform relationship between P13 or N23 latencies and age. Our findings suggest significantly longer P13 latencies with younger age. We know that degree of SCM contraction does not affect VEMP latencies (Akin et al, 2004), therefore the disagreement among reported findings may be related to differences in recording techniques such as variations in stimuli, rise / fall and filter setting. In relation to the frequency of the stimuli, P13 latencies were shown to be prolonged as the frequency of the stimulus decreases. This has been attributed by others to occur as a result of the tone burst rise time (Akin et al., 2003; Rauch et al, 2004).
Other confounds potentially effecting VEMP responses and their direct comparison to other investigations include differences in methodology such as maximum output levels, repetition rate, stimulus duration, and method of SCM muscle activation and monitoring. In comparison to other studies investigating both click evoked and 250 Hz tone burst VEMP, the response rates obtained in the current study are far lower than those reported in the literature (Su et al, 2001; Rauch et al, 2004; Zapala and Brey, 2004). One explanation for our low response rate of click evoked VEMP is the low intensity presentation level of the stimuli. For the current study, the maximum click stimulus presentation level allowed by the equipment was measured to be at 119 dB SPL (90 dB nHL). In contrast, other studies obtained VEMP responses from clicks at 95 dB nHL (Su et al, 2001), 100 dB nHL (0 dBnHL = 45 dB peak SPL) (Welgampola and Colebatch, 2001b) and 90 dBnHL (133 dB peak sound pressure) (Rauch et al, 2004). Maximum output levels however do not explain our low response rates to 250 Hz toneburst stimuli. Zapala and Brey (2004) report similar output levels of 250 Hz toneburst stimuli (90 dBnHL (123 dB peak SPL)) as compared to our presentation levels (123 dB SPL (80 dBnHL)) therefore we propose other differences in methodology discussed below, may be responsible for our low response rates.
Other investigations reporting normative VEMP data have used repetition rates of approximately 5 /s while we used a repetition rate of 13.3 /s (Basta et al, 2005; Ochi and Ohashi, 2003; Su et al, 2001; Welgampola and Colebatch, 2001b, Zapala and Brey, 2004). While lower VEMP amplitudes have been reported with increased repetition rates, specifically for repetition rates greater than 10 Hz in response to click stimuli (Wu and Murofushi, 1999), we chose to use a repetition rate of 13.3/s similar to that also used by Rauch et al (2004) for a better comparison to their threshold response curves. While a higher repetition rate increases speed of data collection, its effect on VEMP amplitude should not go unrecognized. The underlying mechanism accounting for the amplitude decrease in response to a higher repetition rate is not understood and is currently attributed to possible adaptation of the vestibular end organ (Wu and Murofushi, 1999). Regardless of the underlying mechanism, it is unknown whether this possible adaptation effect resulting in reduced VEMP amplitude is greater for older populations and might possibly account for our low response rates in Group 5 (60+ years). It is also unknown whether the effect of a higher repetition rate is selective for stimulus type and might account for our differences in response rates across stimulus type.
Another factor potentially affecting response rates, specifically for 250 Hz, is that of stimulus duration. We used a two-cycle rise/fall and no plateau (Blackman gated) while other investigators used a one-cycle rise/fall and no plateau (Blackman gated) (Zapala and Brey, 2004). While no studies have been completed investigating the effect of rise/fall time of the toneburst stimulus on VEMP responses, investigations looking at click duration note an increase in VEMP amplitude with an increase in stimulus duration and decreased response rates with decreased stimulus duration (Huang et al, 2005). If a similar pattern holds true for toneburst stimuli, our longer duration stimulus should not have resulted in lower VEMP amplitudes or decreased response rates. However, it may be postulated that the lower rate of rise / fall time by using 2 cycles versus 1 cycle could have effected the synchronous neural stimulation and the effect of reduced neural synchrony would be most likely a reduction in amplitude of the averaged response.
Another potential confound of the current study is our lack of monitoring unrectified EMG activity. This factor is of importance given the VEMP amplitude is related to the amount of muscle activation (Colebatch et al, 1994; Akin, et al, 2004). However we feel this was controlled for by use of the blood pressure cuff to monitor muscle contraction. Vanspauwen et al (2006a) demonstrated that with the use of a blood pressure cuff, SCM muscle contraction could reliably be monitored and maintained, thus resulting in smaller variability of VEMP amplitudes as compared to using no feedback method. These investigators reliably obtained VEMP responses with a minimum subsequent cuff pressure of 40 and 50 mmHg (Vanspauwen et al, 2006b) therefore our use of 45 mmHg (25 mmHg of additional resistance with a blood pressure cuff pre-inflated to 20 mmHg) was felt to be adequate for SCM muscle contraction. It is not felt that this method of SCM contraction accounts for our low response rates. If this were the case, a uniform reduction in response rates across stimuli would be anticipated as opposed to a selective reduction in response to clicks and 250 Hz toneburst stimuli. While measurement of mean rectified voltage is the optimal method for monitoring muscle contraction during VEMP testing, use of a blood pressure cuff has been shown to be a reliable alternative thus allowing for use in everyday clinical practice (Vanspauwen et al, 2006).
Investigating the effects of age on the VEMP response is essential considering age related changes to both the neuroanatomy as well as other tests of vestibular function have been documented. Given the large discrepancy in reported findings of P13/N23 VEMP latency as related to age, there does not appear to be an obvious relationship between these two parameters. However, reductions in VEMP amplitude with increased age, has been a consistent finding across investigations (Su et al, 2001; Welgampola and Colebatch, 2001b; Ochi and Ohashi, 2003; Zapala and Brey, 2004; Basta et al, 2005; Lee et al, 2008). The age related changes documented in the VEMP response may be attributed to the subsequent decline in overall neuroanatomy and physiological function as studies indicate that with an increase in age a decrease in the number of otoconia, specifically within the saccule as well as a decreased number of neurons within the medial vestibular nucleus occurs (Ross et al, 1976; Tang et al, 2001/2002). Welgampola et al (2001b) noted reductions in click evoked VEMP amplitude in the 6th decade with reductions in galvanic VEMP in the 7th decade and propose degeneration of the peripheral system prior to degeneration of the neural supply. Similar to VEMP findings, other studies measuring vestibular function have documented age related changes. Decreased VOR gain and increased phase on rotary chair testing have been documented with increased age (Peterka et al, 1990; Baloh et al, 1993). The semicircular canal-otolith interaction and otolith ocular reflex have also been found to show age related changes (Furman and Redfern, 2001). Other potential explanations for reductions in VEMP amplitude have been proposed such as a reduction in cervical muscle tonicity (Lee et al, 2008). However Basta et al (2007) report no significant differences in overall muscle tonicity regardless of age coupled with decreased VEMP amplitudes; attributing the decreased VEMP amplitude to a decline in physiologic function.
The normative data generated in this investigation would be helpful in the assessment criteria of specific vestibular etiologies in every day clinical practice. In particular, the threshold response curves would be helpful for potential use in the identification of Meniere’s disease (Rauch et al, 2004). In comparison to the threshold responses of normal controls as obtained by Rauch et al (2004), our threshold response curves reveal similar best frequency tuning at 500 Hz with the highest thresholds in response to click stimuli. However, it appears that this best frequency tuning dissipates with age and may not be a good diagnostic indicator for individuals over the age of 60. The second phase of the current investigation (presently under way) is to compare VEMP responses of the developed clinical normative ranges to those obtained from two pathologic groups; Meniere’s disease and non-specific unilateral peripheral vestibular hypofunction.
Overall, our findings are consistent with the literature to date in that VEMP thresholds increase with increased age. It is therefore suggested from the results of this study that age should be taken into account when interpreting VEMP threshold and that a comparison of threshold response curves may not be appropriate for individuals greater than 60 years of age. We did not demonstrate an age effect for VEMP amplitude at threshold for any of the test frequencies however we did find a significant negative correlation for VEMP amplitude and age in response to suprathreshold stimuli.
Acknowledgements
Subject recruitment partially supported by a grant from the Nebraska Speech Language Hearing Association and Human Research Subjects Core Grant P30DC004662 Michael Gorga PI, Boys Town National Research Hospital.
Abbreviations
- VEMP
Vestibular Evoked Myogenic Potential
- SCM
sternocleidomastoid
- HSD
honest significant difference
Footnotes
Poster presentation of preliminary data presented at The Association for Research in Otolaryngology Meeting, February 2006, Denver, CO
Contributor Information
Kristen L. Janky, University of Nebraska-Lincoln, Boys Town National Research Hospital
Neil Shepard, Mayo Clinic-Rochester.
References
- Akin FW, Murnane OD, Panus PC, Caruther SK, Wilkinson AE, Proffitt TM. The influence of voluntary tonic EMG level on the vestibular-evoked myogenic potential. J Rehabil Res Dev. 2004;41:473–480. doi: 10.1682/jrrd.2003.04.0060. [DOI] [PubMed] [Google Scholar]
- Akin FW, Murnane OD, Proffitt TM. The effects of click and tone-burst stimulus parameters on the vestibular evoked myogenic potential (VEMP) J Am Acad Audiol. 2003;14:500–509. doi: 10.3766/jaaa.14.9.5. [DOI] [PubMed] [Google Scholar]
- Baloh RW, Jacobson KM, Socotch TM. The effect of aging on visual-vestibuloocular responses. Exp Brain Res. 1993;95:509–516. doi: 10.1007/BF00227144. [DOI] [PubMed] [Google Scholar]
- Basta D, Todt I, Ernst A. Characterization of age-related changes in vestibular evoked Myogenic potentials. J Vestib Res. 2007;17:93–98. [PubMed] [Google Scholar]
- Basta D, Todt I, Ernst A. Normative data for P1/N1-latencies of vestibular evoked myogenic potentials induced by air- or bone-conducted tone bursts. Clin Neurophysiol. 2005;116:2216–2219. doi: 10.1016/j.clinph.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Colebatch JG, Halmagyi GM. Vestibular evoked potentials in human neck muscles before and after unilateral vestibular deafferentation. Neurology. 1992;42:1635–1636. doi: 10.1212/wnl.42.8.1635. [DOI] [PubMed] [Google Scholar]
- Colebatch JG, Halmagyi GM, Skuse NF. Myogenic potentials generated by a click-evoked vestibullocollic reflex. J Neurol Neruosurg Psychiatry. 1994;57:190–197. doi: 10.1136/jnnp.57.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didier A, Cazals Y. Acoustic responses recorded from the saccular bundle on the eighth nerve of the guinea pig. Hear Res. 1989;37:123–128. doi: 10.1016/0378-5955(89)90034-8. [DOI] [PubMed] [Google Scholar]
- Furman JM, Redfern MS. Effect of aging on the otolith-ocular reflex. J Vestib Res. 2001;11:91–103. [PubMed] [Google Scholar]
- Halmagyi GM, Colebatch JG. Vestibular evoked myogenic potentials in the sternomastoid muscle are not of lateral canal origin. Acta Otolaryngol (Stockh) 1995;520 Suppl:1–3. doi: 10.3109/00016489509125174. [DOI] [PubMed] [Google Scholar]
- Huang TW, Su HC, Cheng PW. Effect of click duration on vestibular-evoked myogenic potentials. Acta Otolaryngol. 2005;125:141–144. doi: 10.1080/00016480410016900. [DOI] [PubMed] [Google Scholar]
- Lee SK, Cha CI, Jung TS, Park DC, Yeo SG. Age-related differences in parameters of vestibular evoked Myogenic potentials. Acta Otolaryngol. 2008;128:66–72. doi: 10.1080/00016480701387108. [DOI] [PubMed] [Google Scholar]
- Lin MY, Timmer FC, Oriel BS, Guangwei Z, Guinan JJ, Kujawa SG, Herrmann BS, Merchant SN, Rauch SD. Vestibular evoked myogenic potentials (VEMP) can detect asymptomatic saccular hydrops. Laryngoscope. 2006;116:987–992. doi: 10.1097/01.mlg.0000216815.75512.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCue MP, Guinan JJ. Acoustically responsive fibers in the vestibular nerve of the cat. J Neurosci. 1994;14:6058–6070. doi: 10.1523/JNEUROSCI.14-10-06058.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCue MP, Guinan JJ. Sound-evoked activity in primary afferent neurons of a mammalian vestibular system. Am J Otol. 1997;18:355–360. [PubMed] [Google Scholar]
- Merchant SN. A Method for quantitative assessment of vestibular otopathology. Laryngoscope. 1999;109:1560–1569. doi: 10.1097/00005537-199910000-00004. [DOI] [PubMed] [Google Scholar]
- Murofushi T, Curthoys IS, Gilchrist D. Response of guinea pig vestibular nucleus neurons to clicks. Exp Brain Res. 1996;111:149–152. doi: 10.1007/BF00229565. [DOI] [PubMed] [Google Scholar]
- Ochi K, Ohashi T. Age-related changes in the vestibular-evoked myogenic potentials. Otolaryngol Head Neck Surg. 2003;129:655–659. doi: 10.1016/s0194-5998(03)01578-x. [DOI] [PubMed] [Google Scholar]
- Peterka RJ, Black FO, Schoenhoff MB. Age-related changes in human vestibulo-ocular reflexes: sinusoidal rotation and caloric tests. J Vestib Res. 1990;1:49–59. [PubMed] [Google Scholar]
- Rauch SD, Zhou G, Kujawa SG, Guinan JJ, Herrmann BS. Vestibular evoked myogenic potentials show altered turning in patients with Meniere’s disease. Otol Neurotol. 2004;25:333–338. doi: 10.1097/00129492-200405000-00022. [DOI] [PubMed] [Google Scholar]
- Robertson DD, Ireland DJ. Vestibular evoked myogenic potentials. J Otolaryngol. 1995;24:3–8. [PubMed] [Google Scholar]
- Ross MD, Peacor D, Johnsson LG, Allard LF. Observations on normal and degenerating human otoconia. Ann Otol Rhinol Laryngol. 1976;85:210–226. doi: 10.1177/000348947608500302. [DOI] [PubMed] [Google Scholar]
- Su HC, Huang TW, Young YH, Cheng PW. Aging effect on vestibular evoked myogenic potential. Otol Neurotol. 2001;25:977–980. doi: 10.1097/00129492-200411000-00019. [DOI] [PubMed] [Google Scholar]
- Tang Y, Lopez I, Baloh R. Age-related change of the neuronal number in the human medial vestibular nucleus: a stereological investigation. J Vestib Res. 20012002;11:357–363. [PubMed] [Google Scholar]
- Todd NP, Cody FW, Banks JR. A saccular origin of frequency tuning in myogenic vestibular evoked potentials? Implications for human responses to loud sounds. Hear Res. 2000;141:180–188. doi: 10.1016/s0378-5955(99)00222-1. [DOI] [PubMed] [Google Scholar]
- Vanspauwen R, Wuyts FL, Van de Heyning PH. Improving vestibular evoked myogenic potential reliability by using a blood pressure manometer. Laryngoscope. 2006a;116:131–135. doi: 10.1097/01.mlg.0000187405.57567.ae. [DOI] [PubMed] [Google Scholar]
- Vanspauwen R, Wuyts FL, Van de Heyning PH. Validity of a new feedback method for the VEMP test. Acta Oto-Laryngol. 2006b;126:796–800. doi: 10.1080/00016480500527227. [DOI] [PubMed] [Google Scholar]
- Welgampola MS, Colebatch JG. Characteristics of tone burst-evoked myogenic potentials in the sternocleidomastoid muscles. Otol Neurotol. 2001a;22:796–802. doi: 10.1097/00129492-200111000-00014. [DOI] [PubMed] [Google Scholar]
- Welgampola MS, Colebatch JG. Vestibulocollic reflexes: normal values and the effect of age. Clin Neurophysiol. 2001b;112:1971–1979. doi: 10.1016/s1388-2457(01)00645-9. [DOI] [PubMed] [Google Scholar]
- Wu C, Murofushi T. The effect of click repetition rate on vestibular evoked myogenic potential. Acta Otolaryngol. 1999;119:29–32. doi: 10.1080/00016489950181891. [DOI] [PubMed] [Google Scholar]
- Zapala DA, Brey RH. Clinical experience with the vestibular evoked myogenic potential. J Am Acad Audiol. 2004;15:198–215. doi: 10.3766/jaaa.15.3.3. [DOI] [PubMed] [Google Scholar]