Abstract
During the recent decade, the periodontal attachment apparatus has become one of the premier areas of the body for the development of novel tissue-engineering strategies. In the present review, we describe a developmental biology approach to characterize current concepts in periodontal regeneration and to discuss strategies for future applications in periodontal therapies. To decipher the developmental make-up of the periodontal region, we have followed the path of the migratory neural crest, since it gives rise to periodontal progenitor tissues, which in turn are subjected to the influence of diverse craniofacial extracellular matrices and peptide growth factors. Based on this developmental perspective, we have conducted a systematic analysis of the factors, progenitor cells, and matrices used in current periodontal tissue-engineering approaches. We propose that the developmental history of a tissue is a highly instructive design template for the discovery of novel bioengineering tools and approaches.
Keywords: stem cells, neural crest, periodontal, progenitors, scaffolds
From the Neural Crest to the Periodontal Region: A Developmental Biography of Periodontal Progenitors
Many tissues of the craniofacial region, including the facial skeleton, the teeth, and associated tissues, are derived from a migratory, multipotent cell population formed in the border regions of the neural plate and the embryonic ectoderm through an epithelial-to-mesenchymal transition (Knecht and Bronner-Fraser, 2002) [Editor’s note: The preceding is one of 200 references to be found in the online Appendix.] (Fig. 1). These multipotent migratory cells are called neural crest cells and are capable of self-renewing decisions and therefore are often considered stem cells or stem-cell-like (Trainor, 2005). In the developing head, the facial neural crest cells migrate from the posterior midbrain and hindbrain regions to the branchial arches, where they populate almost the entire mesenchyme adjacent to the oral epithelium (Couly and Le Douarin, 1987, 1990; Chai et al., 2000). Interactions of ectomesenchymal neural crest cells with surrounding tissues derived from paraxial mesoderm, ectoderm, and endoderm induce the differentiation of neural crest cells into osteoblasts, chondrocytes, odontoblasts, and cementoblasts and lead to the formation of maxillary and mandibular bones, cartilage, dentin, cementum, and other connective tissues (Tan and Morriss-Kay, 1986; Serbedzija et al., 1992; Chai et al., 2000; Sharpe, 2001; Helms and Schneider, 2003). Some of the transcription factors important for odontogenic neural crest cell specification include Msx (Satokata and Maas, 1994), Dlx family members (Qiu et al., 1997; Acampora et al., 1999; Ferguson et al., 2000), goosecoid (Rivera-Perez et al., 1999), and Barx 1 (Barlow et al., 1999). These transcription factors are responsible for the timing and spatial deposition of structural proteins which, in turn, define the development and shape of odontogenic tissues, including the differentiation of neural-crest-derived stem cells and the deposition of tissue-specific extracellular matrices. The exact mechanisms of how neural crest cells terminally differentiate into individual lineages or specific target tissues remain to be defined. However, the common origin of osteoblasts, cementoblasts, and odontoblasts from the craniofacial neural crest suggests similarities in gene expression patterns, based on their common lineage, in tandem with unique molecular signatures that specify the differentiation of individual target tissues.
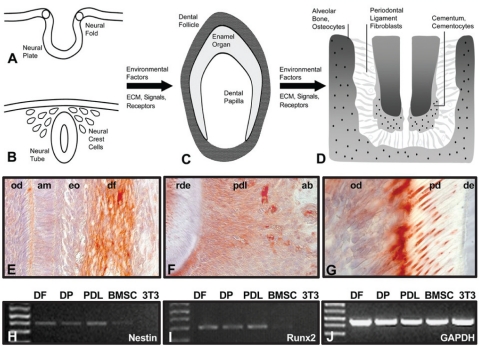
Figure 1.
Neural crest origin and lineage specification of periodontal progeny. During neural tube closure, ectoderm from the lips of the neural fold transforms into ectomesenchyme and emigrates into the space between the neural tube and the surface ectoderm (A, B). Cranial neural crest migrates into the craniofacial periphery and contributes to the formation of bones, cartilage, and teeth. Odontogenic neural crest gives rise to two intermediate pluripotent progenitor populations, dental papilla and dental follicle (C). Environmental factors from the extracellular matrix or signaling factors trigger subsequent differentiation of dental follicle progenitors into alveolar bone osteoblasts, periodontal ligament fibroblasts, and cementoblasts (D). The fibrous attachment of the tooth root to the surrounding alveolar bone is maintained by periodontal ligament fibroblasts. Connective tissue fibers such as Sharpey’s fibers are embedded in an extracellular matrix composed mostly of collagen and enriched with proteoglycans and periostin. The socket of the tooth contains osteoblasts and osteocytes that are involved in the maintenance of alveolar bone. Immunostaining for the intermediate filament and neural crest marker Nestin provides evidence for the neural crest origin of dental follicle (df, E), periodontal ligament (pdl, F), alveolar bone osteoblasts (ab), and odontoblasts (od, E, G). Ameloblasts (am), enamel organ (en), root dentin (rde), alveolar bone (ab), predentin (pd), and dentin (de) were labeled for orientation purposes, but did not stain. Signals for Nestin (H) and for the mineralized tissue marker Runx2 (I) were detected in DF, DP, and PDL cells by RT-PCR. Weak signals for Nestin and Runx2 were also recorded in BMSC cells, but not in NIH 3T3 cells (H, I). (J) The internal control, GAPDH. Combined Nestin and Runx2 gene expression suggests that DF, DP, and PDL cells were neural-crest-derived progenitors, but are already committed toward a mineralized tissue-related lineage.
In the odontogenic region, craniofacial stem cells of neural crest origin interact with the oral epithelium to form complex tooth organs that secrete enamel and dentin mineralized tissues. In addition, a neural-crest-derived connective tissue sheath surrounding the tooth organ, the dental follicle, gives rise to the periodontal tissues root cementum, periodontal ligament, and alveolar bone (Fig. 1). True to their neural crest origin, periodontal progenitors from the dental follicle remain in a migratory state, even after the completion of tooth crown formation (Diekwisch, 2002), retaining their ability to penetrate Hertwig’s Epithelial Root Sheath and to invade adjacent tissues such as the developing root surface soon after the onset of root development (Luan et al., 2006b). Several recent studies have indicated that periodontal progenitor cell populations continue to express neural crest cell markers such as nestin, Notch-1, and LNGFR (Low Affinity Nerve Growth Factor Receptor, p75 neurotrophin receptor), and are able to differentiate into glial cells, a characteristic of neural crest lineage (Le Douarin, 2004). Specifically, dental follicle (DF) and periodontal ligament (PDL) progenitors express the neural crest markers, LNGFR and nestin (Kawano et al., 2004; Morsczeck et al., 2005; Coura et al., 2008; Stevens et al., 2008), but not hematopoietic markers, CD34 and CD45 (Luan et al., 2006a; Stevens et al., 2008). DF and PDL also express the neural stem cell and putative dental stem cell marker Notch-1 (Harada et al., 1999; Johansson et al., 1999; Morsczeck et al., 2005; C Zhang et al., 2008) that has been shown to maintain progenitor cells and induce neurogenesis (Tsarovina et al., 2008).
Further differentiation of periodontal progenitors results in the formation of a complex attachment apparatus that allows for the elastic anchorage of mammalian teeth within the jaw bone (Fig. 1; Luan et al., 2006b). This attachment between tooth root cementum and the surrounding alveolar bone is accomplished by a fibrous periodontal ligament consisting mostly of Sharpey’s fibers (Fig. 1; Johnson, 2005). The regeneration of this complex tooth attachment apparatus is the goal of periodontal tissue engineering. In the present review, we propose that the key to the improved healing and regeneration of periodontal tissues might reside within the periodontium itself and might be found through the extraction of informational molecules that originate in the periodontal tissues and its connective tissue matrices, as previously proposed (Pitaru et al., 1993, 1994; Dereka et al., 2006; Foster et al., 2007). As advances in proteomics and genomics continue to decipher signaling events during periodontal development, it seems logical that mimicking these events would result in ideal tools and strategies for tissue engineering. However, as much as a faithful recapitulation of developmental events might appear desirable, this might be neither practical nor feasible, considering the extraordinary complexity of the periodontal tissues. Further studies will be needed to identify ideal combinations of progenitor cells, factors, and matrices, to aid in the regeneration of the periodontal attachment apparatus under various clinical conditions.
Hierarchies in Neural Crest Lineage Specification as a Tool for Tissue Engineering
The developing cranial neural crest is a highly invasive and migratory population of cells that interacts with pharyngeal endoderm, paraxial mesoderm, and facial ectoderm to construct the tissues and organs of the vertebrate head (Le Douarin, 2004). Equipped with a high degree of plasticity upon emigration from the neural primordium, neural crest cells encounter a plethora of environmental signals on their way into the craniofacial periphery (Trainor and Krumlauf, 2001; Le Douarin, 2004). These signals affect the progress of migratory neural crest cells toward regional lineage specification and result in the formation of unique target tissues, such as teeth and periodontal tissues. It is believed that the regional specification of craniofacial structures is controlled by the nested expression of related genes, resulting in a regional code to instruct organismal end-point designs (Depew et al., 2005). Following this concept, one can envision a unique sequence of genetic and environmental events resulting in the differentiation and development of each individual craniofacial target tissue. While both cranial and trunk neural crest cells have the ability to develop into pigment cells, glial cells, and several types of peripheral neurons, only cephalic neural crest cells give rise to mesectodermal derivatives such as cartilaginous cells and the membranous bones of the head (Knight and Schilling, 2006). The ability of the cranial neural crest to develop into the facial skeleton is dependent on the absence of Hox genes (Couly et al., 2002), a requirement that may be overwritten by in vitro conditions (McGonnell and Graham, 2002).
Recent studies have shown that even when neural crest cells have reached their individual homing destinations, several of them remain undifferentiated, pluripotent, and even endowed with the stem cell capacity of self-renewal (Le Douarin, 2004). These peripheral, tissue-derived, or adult stem cells are believed to be exclusively derived from the neural crest (Pierret et al., 2006). Multipotential neural-crest-derived progenitor populations have been identified in several odontogenic lineages, including the dental follicle (Morsczeck et al., 2005), dental pulp (Gronthos et al., 2000), periodontal ligament (Seo et al., 2004), and mandibular processes (Zhang et al., 2006). While maintaining an ability to self-renew and transdifferentiate, DF, PDL, and cementoblast progenitors are somewhat more fate-restricted than pre-migratory neural crest progenitors, since they express Runx 2, a master regulator of skeletogenesis that allows these cells to readily differentiate and form mineralized tissues. Moreover, our preliminary data (not published) indicated that dental progenitors appeared to be at different stages of the progressive fate restriction process from stem cell to differentiated endpoint cell. Analysis of these data revealed that DF cells demonstrated high alkaline phosphatase (ALP) activity prior to cell confluence in culture, likely to be indicative of stem cell characteristics, while PDL cells exhibited higher ALP activity after 14 days of cell confluence, which we interpret to indicate the onset of mineralization. The association between high ALP expression in DF cells and undifferentiated stem cell characteristics had been established in a previous study with the highly undifferentiated cell line DF2 (Luan et al., 2006a). Differences in differentiation state among DF, PDL, and cementoblast progenitor cells will contribute to their usefulness for specific applications in periodontal tissue engineering.
Similar to other organ systems, the differentiation of periodontal tissues from the pre-migratory neural crest is likely to occur through progressive restriction in the potentialities of a putative multipotent NC stem cell (Lo and Anderson, 1995; Le Douarin, 2004; Trentin et al., 2004; Fig. 2). When applied to periodontal lineage differentiation from neural crest progenitors, the dental follicle appears to contain the population of intermediate pluripotent progenitors that are frequently found during continuous neural crest lineage segregation of other tissues (Le Douarin, 2004; Fig. 2). In support of this concept, earlier studies from our laboratory have provided evidence for the presence of heterogenic cell populations in the dental follicle (DF1, DF2, and DF3; Luan et al., 2006a), resembling heterogenic progenitor populations in migratory neural crest progeny of other tissues (Baker, 2005). In such a model, the dental follicle contains several intermediate pluripotent progenitors that, under the influence of unique periodontal environments, differentiate into periodontal target populations such as periodontal ligament, cementoblasts, and alveolar bone osteoblasts (Figs. 1, 2). In addition, the periodontal ligament also contains uncommitted progenitors that serve as a reservoir for the continuous regeneration and remodeling of periodontal tissues (Fig. 2). Here, we propose that the hierarchical model of periodontal lineage segregation from migratory neural crest through dental follicle intermediates may be used as a blueprint for the engineering of specific tissues for periodontal regeneration. Once the genetic profile between intermediate periodontal progenitor populations and target tissue precursors has been compared, ideal combinations of progenitor populations and inducing factors may be developed that will aid in the regeneration of individual periodontal tissues.
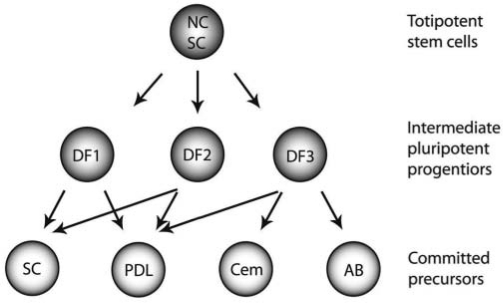
Figure 2.
Hierarchical model of periodontal lineage segregation from migratory neural crest through dental follicle intermediates. The differentiation of periodontal tissues from the pre-migratory neural crest is likely to occur through progressive restriction in the potentialities of a putative multipotent NC stem cell (modified after Lo and Anderson, 1995, and Le Douarin, 2004). In such a model, the dental follicle contains several intermediate pluripotent progenitors (DF1, DF2, DF3; from Luan et al., 2006a) that, under the influence of unique periodontal environments, differentiate into periodontal target populations such as periodontal ligament fibroblasts (PDL), cementoblasts (Cem), and alveolar bone osteoblasts (AB). Pluripotent progenitors (SC) are also found in adult periodontal tissues. Here we propose that the hierarchical model of periodontal lineage segregation from the migratory neural crest through dental follicle intermediates may be used as a blueprint for the engineering of specific tissues for periodontal regeneration.
Precursor and Progenitor Populations for Periodontal Regeneration
From a developmental perspective, periodontal tissues originate from a migratory population of neural crest cells called the dental follicle. The dental follicle forms a dense connective tissue sheath surrounding the developing tooth. While differentiating during root formation, the dental follicle gives rise to alveolar bone, root cementum, and periodontal ligament. A second source of periodontal tissue progenitors has been identified, in the immediate proximity of blood vessels of the periodontium, that might play a role in periodontal tissue renewal after the completion of root formation (Melcher, 1970, 1976; Melcher et al., 1987). There might be a relationship between this second group of progenitors and the extremely high turnover of periodontal tissues and matrix proteins (Sodek et al., 1989; Beertsen et al., 1997), allowing these cells to contribute to the continuous renewal of the periodontal ligament. The two tissues that naturally contribute to the formation of the periodontium, dental follicle and periodontal ligament progenitors, emerge as logical first-choice resources in the quest for ideal cell populations for periodontal tissue engineering.
In addition to dental follicle and periodontal ligament cells, other types of progenitor populations have been used successfully for periodontal regeneration approaches, including bone marrow mesenchymal stem cells (BMMSCs) and cementoblast progenitors (CBs). The suitability of other progenitor populations for periodontal regeneration illustrates the concept of plasticity, which implies that adult stem cells can be transformed into multiple target tissues under suitable inductive conditions (Ballas et al., 2002; Jiang et al., 2002). Cellular plasticity during periodontal regeneration also eloquently underscores the instructive capabilities of periodontal environments, providing for a defining milieu of matrices, surfaces, growth factors, and cytokines that readily promote lineage commitment of a variety of progenitors. While the ability of adult progenitor and stem cells to commit to various periodontal lineages is well-established, it is not clear to what extent these regenerates represent a “true periodontium” on biological and functional levels.
Dental Follicle Progenitor Cells (DFPCs)
The dental follicle (DF) is a transient connective tissue sac surrounding the developing tooth organ that gives rise to the periodontal tissues cementum, alveolar bone, and periodontal ligament (Paynter and Pudy, 1958; Lester, 1969a,b; Ten Cate, 1969; Freeman and Ten Cate, 1971; Ten Cate et al., 1971; Owens, 1978; Schroeder, 1986; Palmer and Lumsden, 1987; Lumsden, 1988; Schroeder et al., 1992; Chai et al., 2000; Diekwisch, 2001, 2002). From a developmental perspective, the DF contains a unique population of migratory neural crest cells that gives rise to the periodontium (Palmer and Lumsden, 1987; Lumsden, 1988; Chai et al., 2000; Diekwisch, 2001, 2002). While histologically a uniform structure, it contains several heterogeneous cell populations, ranging from osteogenic lineages to highly undifferentiated mesenchymal cells (Jiang et al., 2002). As the immediate tissue of origin, the DF constitutes an ideal precursor tissue for periodontal tissue engineering (Morsczeck et al., 2005). Transplanted DFPCs have formed fibrillar tissues that resembled mammalian periodontal ligaments (Morsczeck et al., 2005; Yokoi et al., 2007). While DFPCs readily form periodontal-ligament-like fibers, it is not clear whether they are suitable for mineralized tissue engineering such as alveolar bone or cementum, even though individual experiments report the formation of bone-like morphologies or alizarin red staining (Handa et al., 2002; Morsczeck et al., 2005; Yokoi et al., 2007). We found that DFPCs formed mineralized deposits when implanted into hydrogels and after culture under osteogenic conditions (Fig. 3), but were less susceptible to osteogenic induction than BMMSCs or PDLPGs.
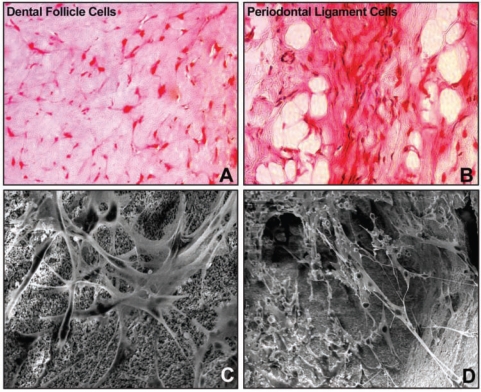
Figure 3.
Behavior of periodontal progenitor cells in matrices. In this study, dental follicle (DF) (A, C) and periodontal ligament (PDL) (B, D) cells were grown in collagen gels (A, B) and inside hydroxyapatite-tricalcium phosphate cubes (C, D). (A, B) Combined Safranin O staining and von Kossa post-staining of DF (A) and PDL (B) cells encapsulated in collagen gels at a concentration of 2 x 105 cells/mL. Either DF or PDL cells were first cultured for one week in osteogenic differentiation medium (Cambrex) and thereafter implanted subcutaneously into nude mice for a period of 4 weeks. Note the elongated mineralization sites in implanted PDL cells compared with the lack of mineralized foci in DF cells. (C, D) Scanning electron micrographs of DF (C) and PDL (D) cells seeded onto 4mm x 4mm x 4mm blocks of 20/80 HA/TCP at a concentration of 2 x 106 and cultured in vitro for 2 weeks. The images in (C, D) illustrate elongated and stretched DF (C) and PDL (D) cells with long processes at a depth of 2000 microns within 20/80 HA/TCP cubes.
Periodontal Ligament Progenitor Cells (PDLPGs)
Next to the dental follicle as a developmental precursor tissue, a second source for periodontal progenitor cells resides immediately within the periodontium. In comparison with dental follicle progenitors, PDLPGs appear to display osteogenic and cementogenic mineralization patterns more readily, especially after treatment with either growth factors or protein extracts (Somerman et al., 1988; Liu et al., 1997; Hou et al., 2000). We found that PDLPGS treated under osteogenic conditions and then implanted subcutaneously retained their ability to form mineral deposits as visualized by von Kossa’s stain, while DF cells subjected to the same conditions did not (Fig. 3). From a developmental perspective, it is conceivable that at least some periodontal ligament progenitor populations might be originating from migratory cells located in the alveolar bone (Dereka et al., 2006; Foster et al., 2007), which would explain, at least in part, the greater susceptibility of PDLPGs to mineralizing conditions. In comparison with DF progenitor cells, PDLPGs appear to be more suitable to form extracellular matrices such as alveolar bone and cementum. A pilot study in dogs has demonstrated increased alveolar bone regeneration in furcation defects, once more underscoring the usefulness of PDLPGs for the regeneration of periodontal mineralized tissues (Dogan et al., 2002). Together, these studies indicate that progenitor cells residing inside the periodontium have the ability to differentiate and form periodontal tissues, such as root cementum or alveolar bone.
Cementum Precursor Cells (CPCs)
Cementoblasts are unique cells involved in the deposition of root or crown cementum (Diekwisch, 2001). It is not clear whether the cells that are involved in the deposition of cellular and acellular cementum are of different origin, but there are distinct differences between both types of cementoblasts in terms of gene expression and modes of cementum deposition (Thomas, 1995; Berkovitz et al., 2002). Notably, cementoblasts from cellular cementum are incorporated into the forming cementum matrix and eventually converted into cementocytes, while cementoblasts that secrete acellular cementum typically reside on the surface of the secreted cementum layer (Berkovitz et al., 2002). From a tissue engineering perspective, acellular and cellular cementum might have different biochemical compositions, each requiring unique cells and factors to mimic their biological function.
Several cell lines from cementum-derived cells have been established, some of them capable of forming mineralized tissue distinct from bone when transplanted into immunodeficient mice (Grzesik et al., 1998, 2000; D’Errico et al., 1999, 2000). It has also been shown that parathyroid-hormone-related protein affects extracellular matrix gene expression and inhibits cementoblast-mediated mineralization in vitro (Ouyang et al., 2000a,b). Moreover, the expression of mineral-associated genes in cementoblasts is regulated by growth factors such as IGF-1, PDGF-BB, and TGF-β (Saygin et al., 2000). These studies point to the complex modes of regulation of the mineralized status of the periodontal region. Cementum-derived cell lines and cementoblasts are excellent models to decipher the factors that contribute to the specific mineralization of cementum during cementogenesis. These factors might be useful for cementum-specific tissue regeneration, even though it may remain a challenging task to stimulate cementum mineralization selectively while keeping the adjacent periodontal ligament in an un-mineralized state.
Factors for Periodontal Regeneration
On their long path through the regional micro-environments of the first branchial arch, neural crest cells are subjected to a multitude of ever-changing concentrations of growth factors and various extracellular matrix surfaces, which continuously influence their lineage and differentiated state. Even during the final episode of their journey, when these neural-crest-derived travelers emerge from the dental follicle to become committed cementoblasts, alveolar bone osteoblasts, and periodontal ligament fibroblasts (Fig. 1), the factors within their surrounding extracellular matrix environment exert pivotal control over their lineage, cellular behavior, and, ultimately, fate. It seems logical that the ultimate goal of successful periodontal tissue engineering would be to faithfully mimic the defining molecular environments that contribute to the cellular commitment and functional behavior of the adaptable progenitor cell populations. Experimental exploitation of this thought confirmed the susceptibility of periodontal progenitors and tissues to classic connective tissue growth factors such as IGFs and FGFs (Giannobile, 1996; Kitamura et al., 2008). These studies also shed light on the complexity of extracellular matrix micro-environments featuring a multitude of growth factors of various concentrations that contribute to an intricately balanced homeostasis unique to the regional state of development and defining for the cells that surround them. Mimicking the complexities of such unique periodontal micro-environments emerges as a new challenge as the science of periodontal tissue engineering moves into its second generation.
Deciphering and mimicking the interactions that occur during root development will be of great benefit not only for periodontal regeneration, but also for the development of tools for root regeneration. Tooth root development involves a series of epithelial-mesenchymal interactions among Hertwig’s Epithelial Root Sheath (HERS), adjacent dental papilla mesenchyme, and dental follicle (Foster et al., 2007). Among the candidate molecules involved in such interaction are bone morphogenic proteins (BMPs), which regulate the development of calcified tissues by directing mesenchymal progenitor differentiation. From post-natal days (PND) 6 to 23, BMP2 and BMP7 were detected in HERS and in DF cells and in differentiated periodontal cells. BMP receptors (BMPR)-Ib and -II and the activin receptor-1 (ActR-1) were detected in DF and HERS at D6 and later more diffusely in the periodontium, suggesting that DF cells may be a target of BMPs secreted by HERS (Kemoun et al., 2007). Fibroblast growth factors (FGFs) are another group of genes which have been identified as potential signals for cell communication between dental epithelia and cranial NC-derived cells of the dental papilla and dental follicle. FGF2 was localized in the dental pulp and periodontal ligament on post-natal day (PND) 9 and in cementoblasts on PND 16, 20, and 24. The pattern of localization of FGF2 in the development root suggests that this growth factor may participate in the signaling network associated with root development (Madan and Kramer, 2005). Platelet-derived growth factor receptor alpha (PDGFR-a) and PDGF ligands are key regulators for embryonic development. PDGFRa and PDGF are specifically and consistently expressed in the cranial NC-derived odontogenic mesenchyme and the dental epithelium, respectively, through all stages of tooth development, suggesting a paracrine function of PDGF signaling in regulating tooth morphogenesis (Xu et al., 2005). Connective tissue growth factor (CTGF/CCN) is a more recently described growth factor which was found to be expressed in the dental follicle of developing molar and incisal tooth germs during the perinatal stage (Yamaai et al., 2005). Together, these factors are likely to contribute to the epithelial-mesenchymal signaling that occurs during tooth root formation. Related to similarities in signaling cues between development and regeneration, these factors have also demonstrated significant effects on the regeneration of periodontal tissues. During regeneration, peptide factors are once more likely to target periodontal progenitors, residing in periodontal stem cell niches, to proliferate and differentiate along pathways similar to those to which developmental progenitors were subjected during root development.
One of the unique features of the mammalian periodontium is the presence of a non-mineralized periodontal ligament connecting the alveolar bone and root surface to each other. The thickness of the periodontal ligament remains effectively unchanged during tooth movement (Luan et al., 2007) and decreases only gradually with age. This non-mineralized periodontal ligament is essentially a mammalian characteristic, while teeth are ankylosed to the jawbone in most reptiles, amphibians, and fish (Luan et al., 2006b). Changes in genes such as ank1 (Ho et al., 2000) or ameloblastin (Fukumoto et al., 2004) alter the width and the mineralization status of the ligament, illustrating that the width of the non-mineralized zone between alveolar bone and root cementum is controlled by several factors and molecules. To maintain the long-term structural and biochemical integrity of the periodontium, periodontal tissue engineering must seek to restore the complex signaling environment that strengthens the mineralized tissues bone and cementum, and at the same time monitors and renews the non-mineralized periodontal fiber attachment apparatus.
Platelet-derived Growth Factors (PDGFs)
Platelet-derived growth factors (PDGF) belong to a family of cystine-knot growth factors that include several PDGF isoforms and the VEGF subfamily (vascular endothelial growth factor). PDGF has been one of the first growth factors implicated in periodontal wound repair and might be one of the most significant (Howell and Chisholm, 1997; Zaman et al., 1999; Giannobile et al., 2001; Anusaksathien et al., 2004; Sarment et al., 2006). Recent PDGF gene therapy studies in which PDGF delivery resulted in significant alveolar bone and cementum regeneration have provided a strong testimony to PDGF’s powerful potential in the regeneration of lost periodontal tissues (Jin et al., 2004). The significant effects of PDGF administration on tissue regeneration and re-growth might be at least partly a result of similar effects during development, since PDGF affects several key cellular functions such as gene expression, cell proliferation, neural crest cell migration, and angiogenesis (Heldin and Westermark, 1999; Hoch and Soriano, 2003; Li et al., 2003; Andrae et al., 2008). It is known, however, that PDGFs drive pathological mesenchymal responses in vascular disorders and tumorigenesis (Andrae et al., 2002), suggesting that its application should be explored with care.
Fibroblast Growth Factors (FGFs)
Fibroblast growth factors (FGFs) are cytokines with powerful roles related to cell migration and proliferation (Folkman and Klagsbrun, 1987; Klagsbrun, 1989; Terranova et al., 1989). In vitro, FGFs inhibit the induction of alkaline phosphatase activity and mineralized nodule formation by periodontal ligament cells (Takayama et al., 1997). Two mechanisms might be responsible for the positive effects of FGFs in periodontal regeneration: (i) their role in angiogenesis and promotion of wound healing, and (ii) their effect on the growth of immature periodontal ligament cells (Takayama et al., 1997; Murakami et al., 1999). In addition, it has been hypothesized that FGFs might participate in the prevention of PDL calcification and spatial maintenance (Shimono et al., 2003). There have been several reports of the successful use of basic fibroblast growth factor (bFGF) for periodontal regeneration (Murakami et al., 1999; Rossa et al., 2000; Takayama et al., 2001; Murakami et al., 2003). During regeneration of periodontal tissues, and similar to its role in development, basic FGF might act as a cytokine that controls elastin expression, in addition to affecting chemotaxis and mitogenesis (Palmon et al., 2001).
Connective Tissue Growth Factor (CTGF)
Another growth factor that has been used for periodontal regeneration is connective tissue growth factor (CTGF), which plays a potent role in tooth germ development, periodontal tissue remodeling, mesenchymal tissue regeneration, and wound healing (Shimo et al., 2002; CG Lin et al., 2003; Friedrichsen et al., 2005; BR Lin et al., 2005; Yamaai et al., 2005). Connective tissue growth factor (CTGF) is a member of the recently described CCN gene family (Connective tissue growth factor, Cyr61/cef10, and Neuroblastoma overexpressed gene) and acts to promote fibroblast proliferation, migration, adhesion, and extracellular matrix formation (Moussad and Brigstock, 2000; Takigawa, 2003). CCN proteins are adhesive matricellular proteins that might function as signaling molecules because of the structural similarity observed between CCN and ECM proteins, their localization in the ECM, and their ability to interact with several types of receptors and regulatory proteins (Planque and Perbal, 2003). It has been suggested that CTGF expression is regulated by TGF-β1/BMP-2 and results in enhanced collagen I and alkaline phosphatase expression during PDL homeostasis in vitro (Asano et al., 2005). CTGF is also involved in chondrogenesis and plays an important role in angiogenesis in vivo (Shimo et al., 2002). Studies from our laboratory indicate that CTGF significantly enhances periostin extracellular matrix synthesis and is a more potent factor than FGF in promoting gene expression of key periodontal matrix genes (not shown).
Transforming Growth Factor Beta (TGF-β) Superfamily
The transforming growth factor beta (TGF-β) superfamily is a large family of growth and differentiation factors that includes the TGF-β subfamily, the activin subfamily, and bone morphogenetic proteins. TGF-β family members typically affect the transcriptional regulation of target genes by activating SMAD protein-related intracellular signaling pathways (Massagué and Wotton, 2000). The unique effects of TGF-β family members on cell proliferation, differentiation, and death place these cytokines in an ideal position to facilitate tissue engineering and to alter the proliferative or differentiated state of individual target tissues prior to or during implantation. In PDL cells, TGF-β1 enhances mitogenesis while down-regulating its osteoblast-like features (Brady et al., 1998). TGF-β3 in Matrigel constructs promotes periodontal-fiber-like formation in class II furcation defects in non-human primates after 8 weeks of implantation (Teare et al., 2008).
While other TGF-β family members might alter individual cell function or fiber formation, several bone morphogenetic proteins, such as BMP-2 and BMP-7, hold the promise of inducing new hard-tissue formation. From a clinical perspective, regeneration of alveolar bone and cementum is a much desired therapeutic goal, since bone loss is one of the decisive parameters of advanced periodontal disease (Genco and Löe, 1993). There has been ample evidence from periodontal regeneration studies that both BMP-2 and BMP-7 (OP-1) are capable of inducing formation of new alveolar bone and cementum (Giannobile et al., 1998; Talwar et al., 2001; Wikesjö et al., 2004). However, since classic bone morphogenetic proteins induce cells to differentiate along an osteogenic pathway (Knippenberg et al., 2006; Sumanasinghe et al., 2006), ankylosis is a frequent side-effect of BMP-2-based therapies, and physiological alveolar bone–periodontal ligament–cementum relationships are rarely found (Selvig et al., 2002). This study illustrates that non-targeted delivery of a single growth factor does not necessarily result in the regeneration of a complex periodontal attachment apparatus, even though clinically acceptable results might be achieved at first sight.
Enamel Matrix Derivatives
There have been several reports suggesting that epithelial cells secrete proteins that contribute to cementogenesis (Stahl and Slavkin, 1972; Hammarström et al., 1996). These reports have been questioned in several other studies (Luo et al., 1991; Ten Cate, 1996; Zeichner-David et al., 2003). The presumed presence of enamel gene products on the root surface has served as the scientific rationale for the use of porcine enamel matrix extracts (EMD, Emdogain®) for the purpose of periodontal regeneration. Following its introduction more than a decade ago, several clinical studies indicated that treatment with EMD positively influenced periodontal wound healing in humans, and that clinical results were comparable with those obtained with Guided Tissue Regeneration (Heijl et al., 1997; reviewed in Sculean et al., 2005). However, others reported that Emdogain® regenerated only a little more tissue than surgical cleaning alone, and that Emdogain® did not save more periodontally compromised teeth (Baelum and Lopez, 2003; Esposito et al., 2005). Cell biological studies have demonstrated that EMD shifted mesenchymal cells toward the osteoblast and/or chondroblast lineage (Ohyama et al., 2002), up-regulated osteopontin while down-regulating osteocalcin (Hakki et al., 2001), and affected matrix proteoglycan synthesis (versican, biglycan, decorin) (Haase and Bartold, 2001). Based on its successful market introduction as a novel biomimetic, Emdogain® and its parent company Biora® have received much attention as a European biotech venture.
From a biological perspective, it is not clear to what extent EMD truly mimics the formative environment of a developing periodontium, because a layer of enamel matrix proteins, as is used in the clinical application of EMD, does not occur during tooth root development. In fact, the protein matrix that provides the basis for Emdogain® is found only during tooth crown development and consists mostly of amelogenins (Hamamoto et al., 2002), which are the major protein component of tooth enamel formation (Fincham and Simmer, 1997). So far, evidence for amelogenin proteins in Hertwig’s Epithelial Root Sheath or on the developing root sheath remains questionable (Luo et al., 1991; Diekwisch, 2001; Zeichner-David et al., 2003). As much as it might appear desirable to reference clinical application as evidence for the overall concepts of using EMD as a biomimetic of early epithelial events in root formation and cementogenesis, a causal relationship remains to be established. Recent studies have shed new light on the many functions of enamel proteins as extracellular matrix intermediaries in the growth and differentiation of odontogenic tissues (Veis, 2003; Gibson, 2008). For future tissue-engineering approaches, it will be useful to dissect, understand, and apply individual components of the periodontal signaling environment that can be manufactured in a synthetic fashion.
Scaffolds for Periodontal Regeneration
On their migratory path from the free regions of the neural fold to their target tissues, neural crest cells are subjected to a multitude of factors and micro-environments presented by local substrates and surface conditions. The majority of these signals and structural cues are provided by a unique protein environment surrounding living cells, the extracellular matrix. The extracellular matrix (ECM) is an intercellular protein network that exerts profound control over cells by regulating gene expression associated with cell growth, attachment, differentiation, and survival (Buckley et al., 1998; Boudreau and Jones, 1999; Giancotti and Ruoslahti, 1999). In the developing craniofacial region, the extracellular matrix provides both a three-dimensional scaffold for the migration and anchorage of emigrating neural crest cells and a signaling micro-environment that induces originally multipotent craniofacial neural crest cells to commit to increasingly differentiated and tissue-specific lineages. By establishing a balance between various substrate components, ECM heterogeneity contributes to the guidance and targeting of neural crest cells (Henderson and Copp, 1997). While migrating from the neural fold toward the craniofacial periphery, neural crest cells encounter basement membrane matrices such as fibronectin and laminin, growth factors and mitogens such as BMPs and FGFs, and a variety of different surface topographies provided by bones, fluids, or muscles. As such, craniofacial stem cell lineage specification is a continuous process orchestrated by extracellular matrix components, matrix structure, and soluble growth factors (Semino, 2003; English, 2006).
In addition to growth factors and cytokines, developing neural-crest-derived periodontal progenitors experience an enormous diversity of matrix chemistries and surface microtopographies in their surrounding micro-environments. Recent studies have established that chemical composition and surface microtopographies exert profound control over gene expression profiles and lineage commitment of stem cell populations (Tan and Desai, 2003). To mimic the diversity of biological scaffold materials, advances in chemical engineering have resulted in a plethora of new scaffold materials and fabrication techniques, all of which aimed to provide an optimum temporary framework to facilitate cellular growth and tissue regeneration. Progress in materials engineering and design has yielded novel bioinspired scaffolds that more closely resemble natural matrices than their predecessors and mimic the complexity of biological extracellular environments (Langer and Vacanti, 1993; Park et al., 2007). Unfortunately, few microtopographies, in their diversity, compare with the stark contrast between inorganic apatite surfaces of alveolar bone and root cementum that define the lateral margins of the periodontium and the tensile soft-tissue environments rich in collagen and glycoproteins that form the central region of the periodontal ligament. The enormous structural and chemical differences among these three principal periodontal building blocks explain the challenges that periodontal tissue engineering faces in the design of optimum scaffolds for the regeneration of all three periodontal tissues.
The quest for the ultimate periodontal scaffold, from a purely materials point of view, is hampered by the fact that the periodontium is a living tissue subjected to continuous remodeling. Nevertheless, recent improvements in scaffold fabrication strategies have greatly enhanced the biocompatibility and remodeling capacity of synthetic scaffolds. Traditional scaffold fabrication techniques include particle leaching, freeze-drying, phase separation, fiber mesh assembly, melt processing, batch foaming, and electrospinning (reviewed in Weigel et al., 2006; Mano et al., 2007). In recent years, novel scaffold fabrication techniques have emerged, such as rapid prototyping, 3D printing, and electronically controlled solid free-form fabrication (Hutmacher et al., 2004; Yeong et al., 2004), enabling materials engineers to mimic naturally occurring fibro-osseous tissues of the periodontium and tailor them to a desired size and shape.
Collagen, Chitosan, and Other Scaffolds from Natural Extracellular Matrices
Natural extracellular matrices provide ideal scaffold materials (Badylak, 2007), since they mimic aspects of the physiological micro-environment to which progenitor cells are subjected during maintenance and differentiation. While many of the components of naturally occurring extracellular matrices are well-known, engineering the exacting composition and surface structure of the biological matrix, its degradation chemistry, and its display of growth factors to cell surfaces remains as perhaps one of the greatest challenges of tissue engineering today. Advantages of scaffolds derived from natural matrices include a presentation of physiological cues for the induction and maintenance of cell machinery components and an ability to degrade enzymatically along natural pathways. On the downside, some naturally occurring ECMs suffer from immunogenicity and weak mechanical properties (Badylak, 2002). To overcome these problems, novel designer scaffolds have been fabricated, including synthetic polymer/collagen hybrid scaffolds (Chen et al., 2001, 2004) and nano-hydroxyapatite/collagen composites (Fukui et al., 2008; Stanishevsky et al., 2008).
Collagen is one of the major extracellular matrix proteins of the periodontium (Fong et al., 2005), and as a result, collagen gel scaffolds provide ample clues for biological recognition important for cell machinery survival. Collagen hydrogels have been extensively used for tissue engineering, featuring several desirable attributes, such as biocompatibility, mechanical strength, and suitability as a carrier for growth factor release (Hayashi, 1994; Meikle, 2007; Cen et al., 2008). In periodontal regeneration, BMP2-treated collagen gels have improved periodontal wound healing (King et al., 1998). A collagen sponge sandwich membrane impregnated with bFGF resulted in periodontal tissue regeneration in an animal experiment (Nakahara et al., 2003). Thus, collagen carriers are useful for growth factor delivery in periodontal regeneration. In the present study, we successfully used collagen scaffolds for the growth of dental follicle and periodontal ligament progenitors (Fig. 3). The collagenous scaffolds provided a nearly ideal micro-environment for PDL cells to attach, proliferate, form a PDL spindle-like morphology (Fig. 3), and subsequently differentiate into a PDL-like tissue by secreting its extracellular matrix proteins.
The second most prominent natural scaffold material for periodontal regeneration is a polysaccharide originally derived from invertebrate shells. Chitin, the source material for chitosan, is found in the exoskeletons of invertebrates such as crustaceans, mollusks, and insects (Kim et al., 2008). Chitin’s acid-soluble deacetylation product, chitosan, is a linear polysaccharide composed of glucosamine and N-acetyl glucosamine units linked by β(1-4) glycosidic bonds (Kim et al., 2008). Several useful properties contribute to the suitability of chitosan and chitosan/polymer composite carriers for periodontal regeneration, including antimicrobial properties (Ikinci et al., 2002; Akıncıbay et al., 2007), improved wound healing (Okamoto et al., 1995; Yeo et al., 2005), growth factor release (Park et al., 2003), and enhanced bone and cementum formation (Mukherjee et al., 2003; Park et al., 2003; Yeo et al., 2005). Implantation of a three-dimensional nanohydroxyapatite/chitosan scaffold seeded with human periodontal ligament cells into athymic mice demonstrated tissue integration and recruitment, lending further support to the suitability of chitosan for periodontal regeneration (Y Zhang et al., 2007, 2008).
Other scaffold components based on naturally occurring polymers have also frequently been used for bioengineering applications and might hold promise as scaffolds for periodontal regeneration. Silk and silk-fibroin scaffolds are used for guided bone regeneration and as suture materials (Kim et al., 2005; Kino et al., 2007), and fibrin or fibrin-fibronectin sealants find application as adhesives (Fabris et al., 1998; Barbosa et al., 2007). There are other naturally occurring polymers, such as glycosaminoglycans, hyaluronic acid, cellulose, and plant and algal polysaccharides (reviewed in Mano et al., 2007), that are also biodegradable and display a broad spectrum of biological and mechanical properties that might be of use for future periodontal engineering applications.
Calcium Phosphate Scaffolds
The periodontal ligament is surrounded by two adjacent mineralized tissues, root cementum and alveolar bone, which enhance and provide for the stability of the periodontal attachment apparatus. These mineralized tissues are highly specialized extracellular matrices composed mostly of calcium hydroxyapatite and collagen. In an attempt to mimic and regenerate these unique extracellular matrices, investigators have developed several calcium-phosphate-containing scaffold materials. For example, calcium phosphate cements (CPC) have been used in periodontal bone repair (Xu et al., 2006), and nanohydroxyapatite/chitosan scaffolds have been developed as a substrate for periodontal tissue engineering (Zhang et al., 2007). Originally developed as an alternative to bone grafts, several calcium phosphate biomaterials have been successfully used as bone replacement materials, allowing for control of porosity, particle size, structure, and mineral phase composition (reviewed in Kretlow and Mikos, 2007). Numerous calcium phosphate preparations have shown promise for bone augmentation and engineering, including Bonesource hydroxyapatite cement (Friedman et al., 1998), tricalcium phosphate (Lange et al., 1986), porous calcium metaphosphate matrices (Lee et al., 2001), injectable calcium phosphate cements (Larsson and Bauer, 2002), biphasic calcium phosphate (Daculsi and Layrolle, 2004; Manjubala et al., 2005), and octacalcium phosphate (Suzuki et al., 2006). The first generation of calcium phosphates used in bone regeneration suffered from poor mechanical strength and low macroporosity, resulting in the development of composites containing both CaP and synthetic polymers with improved biomechanical properties (Ignatius et al., 2001; Sachlos and Czernuszka, 2003; Kretlow and Mikos, 2007). In our studies, blocks of hydroxyapatite-tricalcium phosphate (HAP-TCP) were a suitable scaffold for the growth of both dental follicle and periodontal progenitor cells (Fig. 3).
Synthetic Polymer Scaffolds
In contrast to their biological counterparts, mechanical properties, scaffold architecture, porosity, and degradation properties of synthetic polymer scaffolds can be individually designed to suit a broad variety of applications (Gunatillake and Adhikari, 2003; Stavropoulos et al., 2004). In particular, polyesters (e.g., polyglycolic acid, PGA; polylactic acid, PLA; polylactic-co-glycolic acid, PLGA) have provided popular scaffold materials for tissue engineering, because of the ease of degradation through random hydrolysis of ester bonds (Gunatillake and Adhikari, 2003). Other synthetic polymers used for tissue-engineering applications include polyanhydrytes (Laurencin et al., 1990; Kohn et al., 1996), polycarbonates (Muggli et al., 1998), polylactones (PCL, Hayashi, 1994), polypropylene fumarates (PPF, Kharas et al., 1997; Temenoff and Mikos, 2000), and polyurethanes (PU, Pinchuk, 1994; Gunatillake et al., 2001). While some of the degradation products of synthetic polymers naturally occur in the human body, there have been concerns about the biocompatibility of synthetic polymer scaffolds because of the cytotoxicity of acidic breakdown products, inflammatory responses to the release of small particles, or other local and systemic host reactions (Taylor et al., 1994).
Through their application as barrier membranes, synthetic polymer scaffolds have gained prominence as successful aids in periodontal tissue regeneration. The use of barrier membranes was originally pioneered in long bones to prevent connective tissue cells from invading bony defects (Hurley et al., 1959; Bassett and Creighton, 1961; Boyne and Mikels, 1968). These early studies relied on microporous cellulose acetate membranes found in standard laboratory filters (Millipore®, Billerica, MA, USA). Subsequently, the concept of using Millipore filters as a barrier between adjacent tissue types was applied in the first successful report on guided periodontal tissue regeneration (Nyman et al., 1982). Soon thereafter, the Millipore filter was replaced by an expanded polytetrafluorethylene membrane (ePTFE, Goretex®, W.L. Gore & Co., Flagstaff, AZ, USA; Gottlow et al., 1984, 1986). In these studies, non-resorbable barrier membranes (either cellulose acetate or ePTFE) were used to prevent epithelial tissues from invading the periodontal wound and allowed slower-healing connective tissues to regenerate the physiological connective tissue attachment between root surface and periodontal ligament. The use of non-resorbable membranes in the early days of periodontal tissue regeneration required a re-entry surgery to remove the barrier membrane following successful regeneration after a period of 6-8 weeks.
To avoid the second surgical procedure, investigators introduced innovative resorbable membranes, using a variety of biological and synthetic polymer materials, including collagen, polylactic acid (Guidor®), polyglycolic and polylactic acid (Resolut®, W.L. Gore & Co.), and a synthetic liquid polymer of lactic acid (Atrisorb®). These newly designed resorbable membranes have been successfully used for periodontal guided tissue regeneration applications (Laurell et al., 1994; Hardwick et al., 1995; Nyman et al., 1995; Wang and MacNeil, 1998). In addition to their use in barrier membranes, synthetic polymer scaffolds have also been successfully used in other applications of periodontal tissue engineering. PLGA scaffolds seeded with periodontal ligament fibroblasts and subjected to osteogenic medium have demonstrated osteogenic induction in vitro (Inanc et al., 2006), and PLGA membranes impregnated with an antibiotic enhanced periodontal regeneration in an animal model (Kurtis et al., 2002; Kim et al., 2007).
RGD Peptides
While the detailed biochemistry and mechanics of periodontal fiber attachment to mineralized cementum and alveolar bone remain to be explored, several studies have focused on integrins as molecular mediators of periodontal attachment (Grzesik et al., 1998; Lallier et al., 2001). High levels of integrin subunits α1-5, α11, β1, β5, and β8 (Lallier et al., 2001) are found in periodontal ligament fibroblasts. Moreover, synthetic integrin-binding peptides containing the RGD recognition sequence have been shown to promote periodontal ligament cell attachment in vitro (Grzesik et al., 1998). The Arg-Gly-Asp (RGD) motif is a three-amino-acid motif that has been originally identified as the minimal amino acid sequence promoting cell adhesion (Pierschbacher and Ruoslahti, 1984). In conjunction with the integrins that serve as RGD receptors, RGD-containing peptides and other RGD mimics activate several integrin-related functions in biological systems, including cell migration, growth, differentiation, apoptosis, and adhesion (Ruoslahti, 1996; Giancotti and Ruoslahti, 1999). Together, these findings emphasize the pivotal role of the periodontal extracellular matrix surface peptide chemistry in affecting cell behavior and tooth attachment.
Delivery of Factors
To ensure a steady release, growth factors are commonly conjugated to matrices, and as a result, several growth factor delivery systems have been developed. Some of the successful periodontal growth factors delivery systems include dextran-co-gelatin hydrogel and gelatin microspheres for IGF-1 and FGF-2 delivery (Murakami et al., 2003; Chen et al., 2006), and collagen sponge and gels for BMP-12 and BMP-2 delivery (King et al., 1998; Wikesjö et al., 2003). In traditional matrix-conjugated delivery systems, quantity and time of release are difficult to predict. To circumvent this issue, investigators have developed controlled-release microparticles that help to fine-tune the timing and dosage of a variety of factors in living tissues (Varde and Pack, 2004). In recent years, gene transfer techniques with adenovirus strategies have become increasingly popular to deliver therapeutic doses of proteins over extended periods of time (Kozarsky and Wilson, 1993; Baum and Mooney, 2000). In periodontal therapy, gene transfer techniques have been successfully used to transduce cells derived from the periodontium and promote biological activity by using a recombinant adenovirus encoding PDGF-AA (Zhu et al., 2001).
Perspective
A Systems Approach to Periodontal Regeneration Based on Neural Crest Lineage Segregation
The recent decade of advances in computational biology and bioinformatics has resulted in the availability of data-mining strategies for large datasets that can readily be exploited to identify novel genes and proteins for regenerative therapies, select cell types suitable for specific types of matrix production, and optimize scaffolds and materials for improved biological compatibility. As a first step toward bioinformatics-based strategies for periodontal regeneration, the establishment of a robust genomic/proteomic expression profile database for periodontal development would be highly desirable. Strategies to establish the unique genomic and proteomic signatures of cell lineages involved in the development and differentiation of neural crest progenitors into periodontal target populations may include in silico subtractive hybridization experiments and comparative matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) spectrometry. As a next step, data-mining algorithms can be used to establish day-to-day, tissue-specific profiles of gene expression throughout the formation of the tooth attachment apparatus. In addition, ChIP-chip and computational strategies can be used to analyze cis-elements in known and newly identified genes. This approach will establish direct connectivities within transcription networks involved in periodontal lineage determination and facilitate the alignment and in-depth bioinformatic analysis of large subsets of lineage-specific cis-elements such as promoters, enhancers, and silencers. Such an analysis will define the combinatorial codes of cis-regulatory elements that promote periodontal lineage-specific patterns of gene expression. Once this network of transcription factors, growth factors, and matrix proteins involved in periodontal development has been characterized in detail, it is then possible to infer molecular network-based strategies for periodontal regeneration in a systematic fashion, and transfer and apply this information toward controlled factor release during tissue regeneration.
Computational technology may be of great benefit to optimize approaches for periodontal regeneration by identifying and using surface markers for the sorting of cell populations most suitable for the regeneration of unique periodontal lineages. Cell-surface markers have been successfully used for the selective sorting of many other types of stem cells to study their specialized biology (e.g., Lawson et al., 2007; Sugiyama et al., 2007). Both DNA microarray and proteomic analysis are useful strategies for the identification of membrane-associated gene products to establish specific molecular signatures for a series of periodontal progenitor cell lineages useful for periodontal regeneration. For proteomic analysis, cell membrane proteins can be separated by high-throughput protein identification strategies in which liquid chromatography separation devices are coupled with mass analyzers—for example, in gel-free liquid chromatography mass spectroscopy. In addition, peptide fractions can be analyzed by liquid chromatography electrospray ionization mass spectrometry. Once surface markers have been identified, they can then be used to screen for and enrich periodontal progenitor stem cells through fluorescence-activated cell-sorting (FACS).
Information obtained via bioinformatics approaches will need to be verified with mutant mouse models or other loss- and gain-of-function strategies. Second-generation shRNA libraries might be another valuable aid in the identification and verification of genes and factors useful for periodontal lineage differentiation. Such libraries can be used as a basis for high-throughput screens for newly identified factors or known factors that are implicated in regulating subsets of periodontal lineage-specific genes with periodontal differentiation reporter cell lines as cellular systems. Differentiated cells will be FACS-analyzed and expanded and the identity of the shRNA construct determined by PCR amplification and sequencing. This technology will establish and verify the relevance of individual genes and factors toward periodontal lineage differentiation and serve as a basis for the generation of genetically engineered dental progenitor cells.
To augment the aforementioned approaches, high-throughput microfluidic cell arrays can be used to generate large-scale data on optimum conditions to induce differentiation into target lineages suitable for periodontal tissue regeneration. High-throughput microfluidic screening is a recently developed technique that has been applied for drug screening, bioinformatics, and quantitative cell biology to study gene mutations, apoptosis, and inflammation (Hung and Chow, 2004; Valero et al., 2005; King et al., 2007). For periodontal regeneration, a microfluidic analysis will allow for quantitative detection of changes in fluorescence intensity or cellular parameters during progenitor differentiation. Differentiation reporter cell lines can be generated by stable transfection of periodontal progenitor cells with the coding region of Green Fluorescent Protein driven by the promoters of differentiation marker genes, and these lines can be used to obtain readouts of cell differentiation based on high-throughput screening. With high-throughput microfluidic cell arrays, a great variety of factors, progenitor cells, and scaffolds can be readily tested and this knowledge immediately applied toward the development of novel materials or inductive conditions for periodontal regeneration.
Regeneration of Diseased Periodontia
The present review sheds light on the many facets of periodontal tissue regeneration from a developmental biology perspective by portraying the periodontium as a neural-crest-derived tissue subjected to a multitude of factors and surfaces. At present, most of these approaches are geared toward the regeneration of an ideal, non-inflamed periodontium. However, in many cases, periodontal regeneration is of clinical benefit to persons suffering from periodontitis, an inflammatory disease of the tooth attachment apparatus that involves leukocyte-mediated loss of alveolar bone. In recent years, increased attention has been paid to developing strategies that reduce this inflammation as a prerequisite for successful periodontal therapy and tissue regeneration, since inflammation may lead to unintended immune responses, foreign body reactions, or reduced wound-healing ability. Among recently developed anti-inflammatory agents, eicosapentanoic-acid-derived Resolvin 1 (RvE1, Hasturk et al., 2006) and anti-inflammatory lipid mediators (Serhan and Levy, 2003) have shown promise in harnessing tissue inflammation and preparing tissues to receive regenerative agents. Together with improved growth factor and material combinations, novel anti-inflammatory mediators might greatly improve the clinical outcomes of periodontal regenerative treatments.
Supplementary Material
Footnotes
Support for these studies by NIDCR grants DE15425 and DE17447 is gratefully acknowledged. Synthetic bone substitute (20% HAP 80% TCT) was generously provided by Biomatlante, ZA Les Quatre Nations, 44360 Vigneux de Bretagne, France.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Badylak SF. (2007). The extracellular matrix as a biologic scaffold material. Biomaterials 28:3587-3593 [DOI] [PubMed] [Google Scholar]
- Giancotti FG, Ruoslahti E. (1999). Integrin signaling. Science 285:1028-1032 [DOI] [PubMed] [Google Scholar]
- Giannobile WV. (1996). Periodontal tissue engineering by growth factors. Bone 19(1 Suppl):23S-37S [DOI] [PubMed] [Google Scholar]
- Hasturk H, Kantarci A, Ohira T, Arita M, Ebrahimi N, Chiang N, et al. (2006). RvE1 protects from local inflammation and osteoclast-mediated bone destruction in periodontitis. Faseb J 20:401-403 [DOI] [PubMed] [Google Scholar]
- Ho AM, Johnson MD, Kingsley DM. (2000). Role of the mouse ank gene in control of tissue calcification and arthritis. Science 289:265-270 [DOI] [PubMed] [Google Scholar]
- Kretlow JD, Mikos AG. (2007). Review: mineralization of synthetic polymer scaffolds for bone tissue engineering. Tissue Eng 13:927-938 [DOI] [PubMed] [Google Scholar]
- Lallier TE, Yukna R, Moses RL. (2001). Extracellular matrix molecules improve periodontal ligament cell adhesion to anorganic bone matrix. J Dent Res 80:1748-1752 [DOI] [PubMed] [Google Scholar]
- Luan X, Ito Y, Dangaria S, Diekwisch TG. (2006a). Dental follicle progenitor cell heterogeneity in the developing mouse periodontium. Stem Cells Dev 15:595-608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan X, Ito Y, Diekwisch TG. (2006b). Evolution and development of Hertwig’s epithelial root sheath. Dev Dyn 235:1167-1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcher AH. (1976). On the repair potential of periodontal tissues. J Periodontol 47:256-260 [DOI] [PubMed] [Google Scholar]
- Nyman S, Gottlow J, Karring T, Lindhe J. (1982). The regenerative potential of the periodontal ligament. An experimental study in the monkey. J Clin Periodontol 9:257-265 [DOI] [PubMed] [Google Scholar]
- Tan W, Desai TA. (2003). Microfluidic patterning of cells in extracellular matrix biopolymers: effects of channel size, cell type, and matrix composition on pattern integrity. Tissue Eng 9:255-267 [DOI] [PubMed] [Google Scholar]
- Ten Cate AR, Mills C, Solomon J. (1971). The development of the periodontium: a transplantation and autoradiographic study. Anat Rec 170:365-380 [DOI] [PubMed] [Google Scholar]
- Trainor PA. (2005). Specification of neural crest cell formation and migration in mouse embryos. Semin Cell Dev Biol 16:683-693 [DOI] [PubMed] [Google Scholar]
- Varde NK, Pack DW. (2004). Microspheres for controlled release drug delivery. Expert Opin Biol Ther 4:35-51 [DOI] [PubMed] [Google Scholar]
- Weigel T, Schinkel G, Lendlein A. (2006). Design and preparation of polymeric scaffolds for tissue engineering. Expert Rev Med Devices 3:835-851 [DOI] [PubMed] [Google Scholar]
- Zhu Z, Lee CS, Tejeda KM, Giannobile WV. (2001). Gene transfer and expression of platelet-derived growth factors modulate periodontal cellular activity. J Dent Res 80:892-897 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.