Abstract
The thiopurines, azathioprine and 6-mercaptopurine, are effective immune-modulators and cytotoxic agents extensively used in the treatment of autoimmune diseases, graft rejection, and cancer. There is compelling epidemiologic evidence that thiopurine treatment increases the risk for a variety of tumors by mechanisms that are unclear. We investigated the in vivo mutagenicity of long-term thiopurine treatment by determining the frequency and spectra of somatic mutation events at the HPRT locus in peripheral T lymphocytes as well as the prevalence of mutant clonal proliferation in a cross-sectional analysis of data from 119 children and adults with inflammatory bowel disease (IBD). Analyses of variance and regression were performed to assess relationships among the frequency and spectra of HPRT mutations with disease, duration of illness, duration of treatment and total therapeutic dose of azathioprine and 6-mercaptopurine. We observed a significant increase in the frequency of somatic mutations in 56 subjects treated with thiopurines for IBD compared to 63 subjects not treated with thiopurines. This increase was related to both total dose (p<0.001) and duration of treatment (p<0.001). Comparative mutation spectra analysis of 1,020 mutant isolates revealed a significant increase in the proportion of all transitions (p <0.001), in particular G:C to A:T transitions (p<0.001). Combined analyses of two signatures for mutant clonality, HPRT mutation and TCRβ CDR3 region unique gene sequence also demonstrated a significant thiopurine-dependent increase in mutant cell clonal proliferation (p<0.001). These findings provide in vivo evidence for mutation induction as a potential carcinogenic mechanism associated with chronic thiopurine intervention.
Keywords: Thiopurine, mutagenicity, cancer risk, inflammatory bowel disease, HPRT, proliferation
INTRODUCTION
Azathioprine and 6-mercaptopurine (6-MP) are effective immune modulators and cytotoxic agents used extensively for the treatment of autoimmune diseases (1), transplant graft rejection (2), and cancer (3). Their pharmacologic effects are complex and involve both inhibition of purine biosynthesis and incorporation of 2-deoxy-6-thioguanine nucleotides into DNA (4, 5). Cytotoxicity following thiopurine incorporation into DNA appears to require methylation of the thiol group, mispairing of S-methylthioguanine with thymine, with subsequent recognition and signaling by the mismatch repair system (4).
A major continued concern is the broad-based epidemiologic evidence for duration (6) and dose-dependent (7-9) increases in the incidences of mesenchymal and solid tumors following thiopurine treatment (6-17). This associated cancer risk has resulted in azathioprine being classified as genotoxic and a human carcinogen (18, 19). While the carcinogenicity of thiopurines has been attributed to their immunosuppressive effects, comparative epidemiologic studies with other immune modulators also suggest a thiopurine-specific contribution to cancer risk (9, 14, 15, 17). In particular, in vitro and animal investigations indicate these agents are directly mutagenic (4, 20-25). In this regard, human biomonitoring investigations have observed a significant increase in the frequency of mutant T cells following thiopurine treatment for transplant rejection (26), systemic lupus erythematosus (27) and diabetes mellitus (28), but the etiology of these increases was not discerned. To date, the specific mechanism(s) responsible for the in vivo carcinogenicity of thiopurine treatment has not been elucidated.
In this study, we investigated potential in vivo carcinogenic mechanisms associated with thiopurine treatment by conducting a cross-sectional analysis of its mutagenicity by examining the frequency and spectra of somatic mutations at the hypoxanthine phosphoribosyltransferase (HPRT) locus in peripheral T cells, as well as determining the prevalence of in vivo clonal expansion of mutant isolates in subjects with inflammatory bowel disease (IBD) in relation to disease type, duration of illness, duration of treatment, and total thiopurine dose.
Material and Methods
Study Population
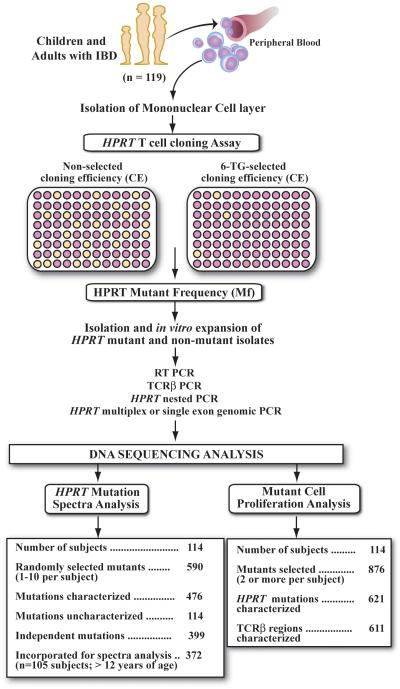
The overall research design employed is illustrated in Figure 1. In vivo mutagenicity of thiopurine treatment was examined in 119 children and adults with either ulcerative colitis (UC) or Crohn’s disease (CD). Both of these chronic inflammatory bowel syndromes are associated with increased cancer risk at affected sites of inflammation (29) and lymphoma following thiopurine intervention (13). We elected to study the potential mutagenic effects of thiopurine treatment in subjects with IBD since only a portion of subjects receive thiopurine intervention, and in comparison to subjects with cancer or status post transplantation, these subjects do not receive long-term treatment with other established mutagenic agents. Subjects with IBD were recruited from the pediatric and adult gastroenterology clinics at Fletcher Allen Health Care, the tertiary care facility at the University of Vermont, and consented with protocols approved by the Committee on Human Research. Diagnosis and therapeutic intervention were confirmed by participating gastroenterologists and a review of medical records. Heparinized blood samples were collected from 39 children (≤18 years old) and 80 adults (>18 years old). Fifty-six subjects comprised a group of thiopurine treated (TP) subjects who received azathioprine (n=40), 6-MP (n=11), or both (n=5), including 19 children and 17 adults with CD and 7 children and 13 adults with UC (Table 1). Sixty-three subjects comprise a group of non-thiopurine treated (non-TP) subjects, including 4 children and 13 adults with CD and 9 children and 37 adults with UC. All subjects previously or concurrently received other treatment modalities that included: 5-aminosalicylic acid derivatives (n=98), corticosteroids (n=72), infliximab (n=21), metronidazole (n=30) and/or ciprofloxacin (n=7). The total equivalent dose (moles, converted to mmoles) of thiopurine exposure for subjects was determined by summing the total amount of thiopurines prescribed (grams) up to sample acquisition, divided by the molecular weight of each drug.
Figure 1. Research overview for investigating in vivo mutagenicity and mutant cell clonal expansion in subjects treated with thiopurines for IBD.
Mononuclear cells from peripheral blood are plated at limiting dilutions in the absence (non-selection) and higher density in the presence of 6-thioguanine (selection). HPRT Mf is determined by calculating the ratio of mean cloning efficiency (CE) of selected mutant isolates over mean non-selected CE of T cells in the absence of selection. Both HPRT mutations and rearranged TCRβ CDR3 gene regions are utilized to determine in vivo mutant cell expansion, while only unique HPRT mutations are utilized to determine mutation frequency (MutFreq) and mutation spectra changes.
Table 1. Duration of illness, total dose and frequency of HPRT T cell mutants in subjects with IBD.
| Subjects | Number | Age (yrs)* | Duration of Illness (yrs)* | Total thiopurine dose (mmoles)* | Mf+ (x 10-6)* | lnMf + (x 10-6)* |
|---|---|---|---|---|---|---|
| Normal Controls | 280 | |||||
| Children | 49 | 5.7 (4.6) | 2.3 (2.2) | 0.4 (1.0) | ||
| Adults | 231 | 52.8 (16.0) | 13.7 (9.0) | 2.4 (0.7) | ||
| No Thiopurine Treatment | ||||||
| Ulcerative colitis | 46 | |||||
| Children | 9 | 14.4 (2.1) | 1.3 (1.3) | 0.0 | 6.8 (4.2) | 1.7 (0.7) |
| Adults | 37 | 39.7 (15.9) | 7.8 (8.2) | 0.0 | 23.7 (38.3) | 2.5 (1.1) |
| Crohn’s Disease | 17 | |||||
| Children | 4 | 15.8 (1.2) | 1.2 (2/0) | 0.0 | 3.9 (1.4) | 1.3 (0.4) |
| Adults | 13 | 42.9 (10.4) | 11.2 (9.3) | 0.0 | 12.6 (7.3) | 2.4 (0.5) |
| Thiopurine Treatment | ||||||
| Ulcerative colitis | 20 | |||||
| Children | 7 | 14.9 (4.4) | 4.1 (3.1) | 458.1 (354.9) | 6340.4 (12287) | 5.5 (3.7) |
| Adults | 13 | 30.7 (11.1) | 4.3 (3.5) | 459.7 (697.8) | 6246.6 (20612.6) | 4.0 (3.3) |
| Crohn’s Disease | 36 | |||||
| Children | 19 | 15.0 (3.3) | 3.9 (3.6) | 264.4 (314.7) | 368.2 (617.2) | 4.1 (2.1) |
| Adults | 17 | 33.6 (13.4) | 9.4 (7.9) | 501.2 (548.7) | 15768.2 (39664.8) | 6.0 (3.6) |
Mean (standard deviation)
Mf and lnMf data summarized here has not been adjusted for CE and age. Mean lnMf is computed from the individual log-transformed Mf value
HPRT T cell cloning assay for determining somatic mutant frequency
The HPRT T cell cloning assay has been extensively used as a mutagenicity biomarker for determining the frequency and spectra of preexisting and exogenously induced somatic mutation events in peripheral human T lymphocytes (30, 31). Briefly, since HPRT encodes a phosphoribosylation enzyme in the purine salvage pathway, analysis of in vivo mutation events is accomplished by isolating HPRT deficient T cells from peripheral blood through in vitro selection with 6-thioguanine (6-TG). This approach allows only cells with inactivating gene mutations to proliferate in the presence of 6-TG. The HPRT T cell cloning assay has a number of additional characteristics that has made it a valuable human mutation biomarker system. HPRT mutant T cells arise in a normal T cell milieu, uncomplicated by metabolic and genetic derangements inherent in diseased or malignant cells, allowing for the evaluation of mutagenic signatures related to exposure. Since the HPRT locus is located on the X- chromosome, a single mutation event can result in a selectable mutant phenotype, and is thus more sensitive than selectable autosomal gene loci. In addition, HPRT T cell mutant isolates can be expanded in vitro and used for a both mutation spectra and clonality analyses. The determination and statistical analysis for in vivo somatic HPRT mutant frequency (Mf) in peripheral T cells by cloning HPRT mutants following limiting dilution in the absence and presence of 6-TG have been previously described (32, 33).
Molecular analysis of HPRT mutations and determination of mutant cell proliferation
The HPRT biomarker system also allows for the analysis of mutation spectra changes associated with exogenous/iatrogenic exposures, as well as the identification of independent in vivo sequential mutation events (34) and mutant clonal proliferations by simultaneously analyzing two independent clonality indicators, HPRT mutations and unique T cell receptor β (TCR β) CDR3 variable gene region sequences (35, 36). Experimental approaches, methods and interpretations of mutation spectra, and comparison of HPRT mutations and TCRβ CDR3 variable gene regions of mutant isolates for determining mutant proliferation have been previously described (34-36). HPRT mutation spectra were determined by analyzing up to 10 mutants per TP treated subject and up to 5 mutants per non-TP treated subject. HPRT mutations were characterized by performing reverse transcriptase-PCR amplification (RT-PCR) from approximately 1 × 104 cells to identify mutations within coding regions, exon exclusions or intron inclusions. Single exon or multiplex genomic PCR of HPRT gene regions (37) was performed to identify splice mutations, deletions or insertions. HPRT mutants that showed exclusion of exons 2-3 in cDNA were screened for V(D)J recombinase-mediated deletions of HPRT exons 2-3 with specific primers spanning that region (38). PCR products were either separated by gel electrophoresis, extracted and purified via Qiagen’s QIAquick gel extraction kit, or treated with 3.75 units of exonuclease I and 1.25 units of shrimp alkaline phosphatase, prior to DNA sequencing.
The characterization of the TCRβ CDR3 variable gene regions and HPRT mutations in mutant isolates provide two independent measures for clonal proliferation that can be followed longitudinally (34, 35) (supplemental Figure 1). For TCRβ variable gene sequence analysis, RT-PCR generated cDNA was amplified using a primer to the TCRβ constant region and a mix of 26 TCRβ V region primers (39). The PCR products were then processed and sequenced as described above. The prevalence of in vivo clonal expansion was determined by analyzing 621 HPRT mutations and 611 TCRβ CDR3 variable gene region sequences from 114 subjects from whom two or more mutants were available (supplemental Figure 1; supplemental Table 2). The in vivo frequency of independent mutation events (MutFreq) was ascertained by multiplying each subject’s HPRT Mf by the ratio of independent mutation events to the total analyzed mutants.
Statistical Analysis
Because HPRT Mf has a lognormal distribution, all statistical analyses were based on its natural logarithm (lnMf), which has been shown to be related to age and non-selected cloning efficiency (CE) (32). We therefore adjusted for age and CE by including them as covariates in all analyses of lnMf (40). A summary of each subject’s gender, age, duration of illness, type and treatment duration, total dose of thiopurine, CE, Mf, lnMf, and MutFreq is provided (supplemental Table 1). Analysis of covariance was used to compare differences in lnMf among controls, non-TP and TP treated subjects, as well as between children and adults with UC and CD. The Student-Newman-Keuls test was used to adjust for multiple pair-wise comparisons. In TP treated subjects, the effects of treatment duration and total dose on Mf and MutFreq were assessed by regression analyses. A Chi-square test was used to compare the percents of TP and non-TP treated subjects with mutant cell proliferation, and a Mantel-Haenzel test for linear association was used to assess the dose-dependence of increases in the percent of subjects with mutant cell expansions. Mutation spectra data were analyzed using logistic regression to assess the statistical significance of differences in the proportions of specific mutations among controls (supplemental Table 3), non-TP and TP treated subjects. Subjects under the age of 12 were excluded from mutation spectra analyses because differences in the age distributions of normal controls and IBD subjects precludes adjustment for age-related spectra changes in this group. Age group (12-18 or ≥19 years) and sex were included in the regression models as covariates. Although mutations from the same subjects may be correlated, we could not use a hierarchical regression model in these analyses because the spectra data for adult controls did not contain unique subject identifiers. The effects of treatment were determined by comparing data only from the TP and non-TP treated subjects. Hierarchical logistic regression models with age group (12-18 or ≥ 19 years), thiopurine treatment (yes or no) and treatment by age group interaction were fitted to determine if the effect of treatment differed in adults and children. Similar models were fitted using thiopurine treatment categories based on the total dose (0, 1-133, 134-289, 290-727 and ≥ 728 mmoles) to determine if the proportion of a particular class of mutation displayed a dose-response relationship. The non-zero categories correspond to dose quartiles for all TP treated subjects. All of these models included study subject as a random effect to account for potential correlation between mutations from the same individual.
Results
In vivo HPRT mutant frequency in subjects with IBD
Unadjusted HPRT Mf and lnMf data with respect to type of IBD and thiopurine treatment for the 119 children and adults studied as well as previously reported data utilized for controls (32, 40, 41) are summarized in Table 1. The effects of IBD and non-TP treatment on the in vivo background HPRT Mf were initially assessed by comparing lnMf in non-TP treated subjects to controls after adjustment for age and CE. A statistically higher lnMf was observed in one sub-group, non-TP treated children with UC (p<0.05), while lnMf in all the other non-TP treated IBD groups were not significantly elevated. Analysis of lnMf for the non-TP treated IBD group as a whole showed no statistical difference from controls, indicating that IBD itself and non-thiopurine interventions did not significantly affect the background HPRT Mf in non-TP treated IBD subjects.
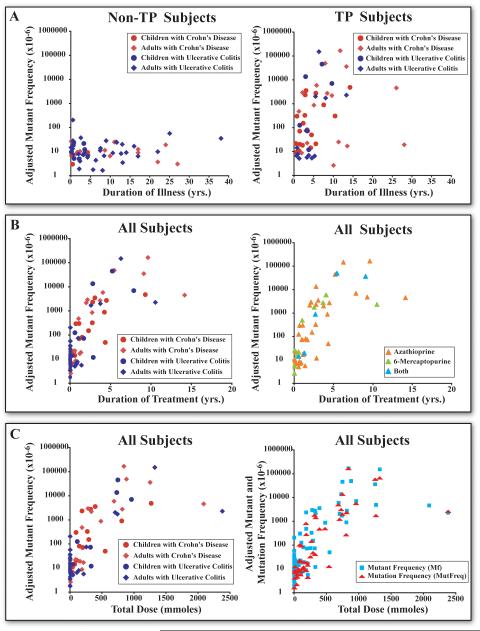
For the 56 TP treated subjects, total thiopurine dose ranged between 3.8 and 2,379 mmoles taken over 0.04 to14.1 years. We observed a significantly higher mean lnMf in TP treated subjects compared to controls and non-TP treated subjects (Table 1, p < 0.05). Importantly our sub-analysis revealed no significant difference in lnMf between TP treated IBD children or adults or between TP treated IBD subjects with UC or CD. Regression analysis revealed no significant increases in lnMf associated with duration of illness for non-TP treated subjects, Figure 2 (Panel A), (supplemental Table 1). In contrast, we observed a significant increase in lnMf in TP treated subjects associated with duration of illness (p=0.004), Figure 2 (Panel A), but of importance, this association was not significant after adjustment for either duration of treatment or total thiopurine dose. Specifically, the significant increases in lnMf in TP treated subjects were related to both duration of treatment (p<0.001), Figure 2 (Panel B)(supplemental Table 1) and total thiopurine dose (p<0.001), Figure 2 (Panel C), independent of duration of illness. The estimated rate of increase in lnMf in TP treated subjects was 0.776 per year of treatment, corresponding to a doubling in Mf. The estimated increase in lnMf with each 100 mmole increase in total dose was 0.467, corresponding to a 67% increase in Mf. The rates of increase in lnMf in TP treated subjects did not differ significantly between treated children and adults, or treated UC and CD subjects. The rates of increase were also not drug specific since there were no significant differences in lnMf among subjects treated with only azathioprine, 6-MP or both agents, Figure 2 (Panel B), (supplemental Table 1).
Figure 2. Comparative analysis of adjusted HPRT mutant frequency (Mf) with respect to duration of illness, duration of treatment, and total dose of thiopurines.
Mf values have been adjusted for the effects of age and CE by normalizing to the average age and CE for each subject (35). Panel A) Relationships between adjusted Mf and duration of illness in non-TP and TP treated subjects. Panel B) Relationships between adjusted Mf and duration of thiopurine treatment in IBD subjects, as well as the relationships between adjusted Mf and treatment duration of different thiopurine interventions. Panel C) Relationships between adjusted Mf and MutFreq and total thiopurine dose in IBD subjects.
Prevalence of in vivo mutant cell clonal proliferation and frequency of independent somatic HPRT mutations (MutFreq)
To gain insight into the potential effects of in vivo mutant clonal expansion and selection for HPRT mutants on the treatment-specific increases in mutant frequencies (Mf), we investigated the relationship between the prevalence of clonal mutant proliferation and the calculated MutFreq in TP and non-TP treated IBD subjects.
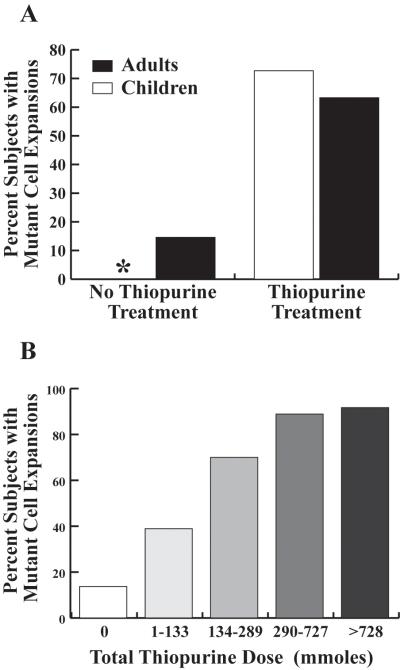
We determined whether thiopurine treatment results in an increase in the prevalence of clonal proliferation of HPRT mutants by comparing 621 characterized HPRT mutations and 611 TCRβ regions from 876 HPRT mutants from 114 non-TP and TP treated IBD subjects (supplemental Table 2 and supplemental Figure 1). The percents of non-TP treated children and adults in which there were two or more HPRT mutant isolates associated with mutant clonal expansions were 0% and 14.6% respectively, Figure 3 (Panel A). In contrast, there were significant increases in the percents of TP treated children (72.2%) and adults (63.3%) with mutant clonal expansions compared to non-TP treated subjects (p< 0.001) Figure 3 (Panel A). There were also significant dose-dependent increases in the proportion of subjects with mutant clonal expansions (p<0.001), Figure 3 (Panel B).
Figure 3. Prevalence of in vivo mutant cell proliferation in TP and non-TP IBD treated subjects.
Panel A) The proportion of subjects with evidence of in vivo expansion of mutant cells in TP and non-TP treated subjects. (*) 0% of non-TP treated children had evidence of mutant cell expansions. Panel B) The proportion of subjects with evidence of in vivo mutant cell expansion compared to total thiopurine dose.
The prevalence of in vivo mutant clonal expansion in these subjects was then used to correct for the possible contribution of in vivo mutant proliferation on the treatment-specific increases in lnMf to estimate the frequency of independent in vivo somatic mutation events (MutFreq). For TP treated subjects, the MutFreq was significantly lower than Mf (p<0.001) as a consequence of mutant clonal expansions associated with treatment. But of importance, we also observed significant increases in MutFreq in TP treated subjects as seen with Mf which correlated specifically with treatment duration and total dose (p<0.001) Figure 2 (Panel C), (supplemental Table 1). This strongly suggests that the significant increases in MutFreq following thiopurine treatment are not the result of selective in vivo expansion of preexisting HPRT mutants.
Mutation spectra analysis
The in vivo mutagenicity of thiopurine treatment was further examined by comparing 648 independent mutations from a composite of controls and 372 independent mutations from 105 IBD subjects 12 years and older (supplemental Tables 2 and 3). Nineteen percent of the randomly selected HPRT mutants from IBD subjects remained uncharacterized, which is consistent with previous human biomonitoring HPRT spectra studies (36, 42). Reasons for the uncharacterized mutants include: 1) exon exclusions in mutants from female subjects in which splice alterations and deletions could not be confirmed or detected; 2) no cDNA detected, with genomic PCR products of exons 1 and 9 showing no mutations; 3) cDNA revealed no mutation, and 4) incomplete analysis.
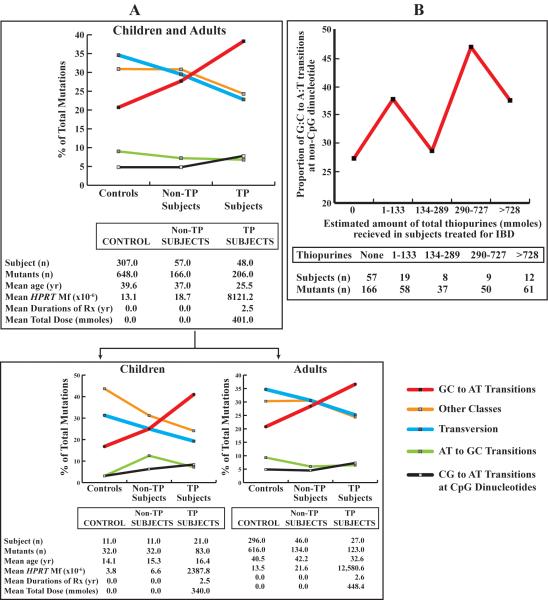
We observed no significant differences in the spectra of mutations between non-TP treated subjects compared to controls, Figure 4 (Panel A), (supplemental Tables 2 and 3). This establishes that there is no detectable IBD disease or non-TP treatment specific differences in the proportions of mutations. In contrast, there was a significant increase in the proportion of all transitions in TP treated subjects (52.9%) compared to non-TP treated subjects (39.8%; p=0.016), and controls (34.4%; p<0.001) after adjustment for age and multiple observations per subject. In particular there was a significant increase in the proportion of G:C to A:T transitions at non-CpG dinucleotides in TP treated subjects (38.3%) compared to non-TP treated subjects (27.7%; p=0.037), and controls (20.7%; p<0.001), Figure 4 (Panel A), (supplemental Tables 2 and 3). The increase in transitions was also accompanied by a concomitant significant decrease in the proportion of all transversions among TP treated subjects (22.8%) compared to controls (34.6%), p=0.021.
Figure 4. Mutation spectra in Non-TP and TP treated IBD subjects (≥ 12 years of age) and normal controls.
Panel A) Mutation spectra distribution in non-TP and TP treated children and adults combined and separate compared to normal controls. The “Other Classes” of mutations include: exon deletions, micro-deletions, nucleotide duplications or losses, insertions, and complex insertion/deletion mutations. Panel B) The relationship between the proportion of G:C to A:T transitions at non-CpG dinucleotides and total dose of thiopurine treatment.
We also observed a significant dose-dependent increase in the proportion of all transitions (p=0.013), in particular G:C to A:T transitions (p=0.045) at non-CpG dinucleotides after adjustment for age and multiple observations per subject, which supports our overall spectra findings (Figure 5; Supplemental Table 2). This increase in G:C to A:T transitions is consistent with the in vitro mutagenic signature observed for these drugs (4, 20, 22).
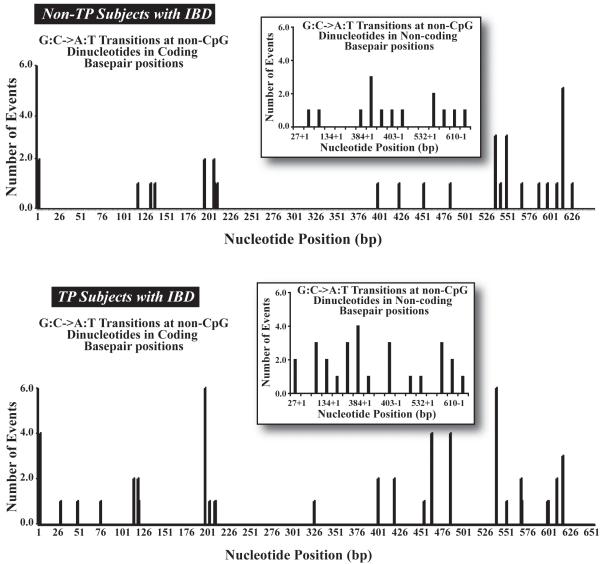
Figure 5. Distribution and frequency of G:C to A:T transitions at non-CpG dinucleotides mutations in TP and non-TP treated subjects at both coding and non-coding (splice-site) base pair positions in the HPRT locus.
Thiopurine treatment also resulted in a distinct distribution of unique G:C to A:T transitions at non-CpG dinucleotides at both coding and non-coding/splice-site base pair positions in TP treated subjects compared to non-TP treated subjects (Supplemental Table 4, Figure 5). There was an increase in the occurrence of unique G:C to A:T transitions at non-CpG dinucleotides (n=38 compared to n=32) at both coding and non-coding sites and in the number of unique sites (n=18 compared to n=12) in TP treated subjects compared to non-TP treated subjects. There were 20 common sites (coding and non-coding) in which these transitions occurred in both non-TP treated and TP treated subjects. The most frequent mutation coding sites (≥ 3 events) for G:C to A:T transitions at non-CpG dinucleotides in non-TP treated subjects were at positions 538, 551, and 617. For TP subjects the most frequent coding sites were at positions 3, 197, 463, 485, 539, and 617. Of interest is that G:C to A:T transitions at non-CpG dinucleotides at positions 463 and 539 in TP treated subjects was not observed in non-TP treated subjects. Analogous findings were also observed at splice-site sequences. In addition, the distribution of these transitions at coding and non-coding regions is consistent in each treatment group and distribution subcategory. These data suggest that the distribution of G:C to A:T transitions at non-CpG dinucleotides in TP subjects represents both thiopurine-specific and preexisting mutations and not simply an increase in spontaneous or hotspot mutation events.
Discussion
Investigations attempting to link treatment-specific mutations to carcinogenic risk in humans are extraordinarily difficult because of the necessity to control for a multitude of complex variables including the genetic and cellular effects of the underlying disease(s), duration and total dose of each therapeutic intervention as well as other environmental exposures. Furthermore, tumors themselves are genomically unstable, making potential primary and secondary cancer-relevant mutations difficult to discern. With respect to this study, definitively linking thiopurine mutation induction in IBD subjects to the formation of specific tumors is currently not possible since data for cancer specific mutations in tumors from subjects who only received thiopurine treatment does not exist. Hence, identifying the in vivo induction of treatment-specific mutations in non-transformed cells is an extremely important approach for elucidating potential carcinogenic mutagenic events directly in subjects receiving this treatment.
In this study we provide evidence that chronic thiopurine treatment results in a dose-dependent increase in the in vivo frequency of somatic mutations, in particular a treatment-specific increase in G:C to A:T transitions at non-CpG dinucleotides, which is consistent with the in vitro mutation signature of these drugs (4, 22). These data strongly suggest that thiopurine intervention is mutagenic in humans.
These findings were ascertained by determining the frequency and spectra of somatic mutations at the HPRT locus in peripheral human T cells, and an examination of in vivo preexisting or induced mutant cell clonal proliferation by combining the analysis of two distinct clonality signatures (35). Since the mutation events observed were investigated in subjects receiving chronic thiopurine treatment, the possibility exists for the in vivo selection and enrichment of preexisting HPRT mutants with potential growth advantages that may influence the relative contribution of thiopurine therapy on the observed Mf. This effect is unlikely since we observed a significant dose-dependent increase in MutFreq, that have been corrected for both preexisting and treatment induced clonal expansion of mutant cells. The potential in vivo cytotoxic effects of thiopurines on proliferating non-HPRT mutants with the concomitant survival of HPRT mutants could also theoretically have influenced our results. This appears unlikely as well since we concurrently observed significant dose-dependent mutation spectra changes. The latter observations establish the in vivo mutagenicity of thiopurine intervention, since significant spectra changes would not occur if this treatment simply enriched for the expansion of preexisting HPRT mutants. It is also important to note that all of these findings were independent of the underlying disease, duration of disease, and other therapeutic interventions.
We were unable to specifically evaluate the effects of other potential environmental exposures because of limited data. The most important exogenous exposure likely to influence our observations would be environmental tobacco smoke (ETS), which has previously been shown to result in an increase in V(D)J recombinase mediated deletions in newborns (43), and no spectra changes or an increase in the proportion of G:C to T:A transversions in adults (44, 45). This is in contrast to the significant proportional decrease in transversions, and an increase in G:C to A:T transitions at non-CpG dinucleotides observed in TP treated subjects.
The induction of G:C to A:T transition mutations by thiopurine treatment is likely the result of incorporation of 6-thioguanine nucleotides into DNA and subsequent mispairing with thymine in replicating cells (4, 20, 22, 25). Support for the carcinogenicity of a thiopurine specific mutagenic mechanism can be inferred from epidemiologic evidence correlating azathioprine treatment with significantly higher incidences of solid tumors, lymphoproliferative disorders, and skin cancers when compared to other immune modulators, including mycophenolate mofetil which closely resembles thiopurines in its mechanism of action (9, 14, 15, 17, 25, 46). Mycophenolate mofetil primarily inhibits inosine monophosphate dehydrogenase resulting in the inhibition of purine synthesis, while azathioprine and 6-mercaptopurine exert their cytotoxicity by inhibiting purine synthesis and by incorporation into DNA. This suggests the increased cancer risk associated with thiopurine treatment is associated with the mutagenicity of these agents following DNA incorporation.
The significant increase in the prevalence of treatment-specific cell proliferation may represent an additional mechanism for accumulating G:T mismatches from unrepaired replication errors (47), as well as by enhancing incorporation of 6-thioguanine nucleotides during DNA replication. Clonal proliferation among mutants, with the associated failure to find similar patterns among non-mutant cells, further implicates cell proliferation as a significant in vivo mutagenic mechanism.
Cellular resistance to thiopurines has been shown in vitro to select for cells defective in mismatch repair (MMR), while microsatellite instability has been observed in acute myelogenous leukemia from transplant patients following azathioprine treatment (8). It is not known whether our findings could also be attributed to the in vivo selection of MMR defective cells and the in vivo emergence of cells with genomic instability.
Our data indicate that thiopurine treatment could potentially lead to somatic mutations at disease-specific loci in actively replicating tissue cell populations including: 1) the bone marrow, which has been shown to incorporate higher levels of 6-thioguanine nucleotides (48), and 2) activated immune cells and epithelial tissues undergoing repeated cycles of repair and cell regeneration, especially in subjects with IBD and chronic graft versus host disease (29, 49). Lymphocytes and skin epithelium of patients who received thiopurine treatment have also been shown to incorporate 6-thioguanine nucleotides (24, 50). In this study, we also observed an increase in G:C to A:T transitions at non-CpG dinucleotides in clonally proliferating activated T cells, some of which may have a recent precursor origin (data not shown). Altogether, these observations provide an important connection between the mutagenicity of thiopurine intervention in these cell populations and the increased risk of leukemia (8), lymphoma (7, 12, 13) and skin cancer (9, 10, 17).
In this cross-sectional study, we provide evidence that suggest thiopurine intervention is mutagenic, resulting in a dose-dependent increase in the frequency of somatic mutations and treatment-specific mutation induction of G:C to A:T transitions at non-CpG dinucleotides. These findings provide direct in vivo evidence for mutation induction as a potential carcinogenic mechanism associated with chronic thiopurine intervention.
Supplementary Material
Acknowledgements
We would like to thank our patients and the pediatric and adult gastroenterology staff at Fletcher Allen Health Center, in particular Dr. Michael D’Amico, Elizabeth Robinson, Dr. James Vecchio, Dr. Peter Moses, Dr. Doris Strader, Dr. Nicholas Ferrentino, Dr. Richard Zubarick, Dr. Stephen Willis, Dr. Eric Ganguly, Lisa Evans, Lynn Metcalf, and Lisa Shephard, who contributed to subject recruitment. We would like to thank Terri Messier, Jami Rivers and Dr. Heather Kendall for their technical assistance, Dr. John Curry for providing us his adult HPRT mutation database, and Dr. Nick Heintz for his insightful comments. All of the authors of this manuscript reviewed and or had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the analysis of these data.
Financial Support: This work was supported by the National Cancer Institute grant CA09094013, grant 1003312 from the Burroughs Wellcome Fund, and support from the University of Vermont College of Medicine M.D., Ph.D. Program. The automated DNA sequencing was performed in the Vermont Cancer Center DNA Analysis Facility and was supported in part by grant P30CA22435 from the NCI.
Footnotes
Conflicts of Interest: None
REFERENCES
- 1.Teml A, Schaeffeler E, Herrlinger KR, Klotz U, Schwab M. Thiopurine treatment in inflammatory bowel disease: clinical pharmacology and implication of pharmacogenetically guided dosing. Clin Pharmacokinet. 2007;46:187–208. doi: 10.2165/00003088-200746030-00001. [DOI] [PubMed] [Google Scholar]
- 2.Jorga A, Johnston A. Novel therapies in transplantation. Expert Opin Investig Drugs. 2005;14:295–304. doi: 10.1517/13543784.14.3.295. [DOI] [PubMed] [Google Scholar]
- 3.Elion GB. The purine path to chemotherapy. Science. 1989;244:41–7. doi: 10.1126/science.2649979. [DOI] [PubMed] [Google Scholar]
- 4.Swann PF, Waters TR, Moulton DC, et al. Role of postreplicative DNA mismatch repair in the cytotoxic action of thioguanine. Science. 1996;273:1109–11. doi: 10.1126/science.273.5278.1109. [DOI] [PubMed] [Google Scholar]
- 5.Tidd DM, Paterson AR. A biochemical mechanism for the delayed cytotoxic reaction of 6-mercaptopurine. Cancer Res. 1974;34:738–46. [PubMed] [Google Scholar]
- 6.Asten P, Barrett J, Symmons D. Risk of developing certain malignancies is related to duration of immunosuppressive drug exposure in patients with rheumatic diseases. J Rheumatol. 1999;26:1705–14. [PubMed] [Google Scholar]
- 7.Silman AJ, Petrie J, Hazleman B, Evans SJ. Lymphoproliferative cancer and other malignancy in patients with rheumatoid arthritis treated with azathioprine: a 20 year follow up study. Ann Rheum Dis. 1988;47:988–92. doi: 10.1136/ard.47.12.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Offman J, Opelz G, Doehler B, et al. Defective DNA mismatch repair in acute myeloid leukemia/myelodysplastic syndrome after organ transplantation. Blood. 2004;104:822–8. doi: 10.1182/blood-2003-11-3938. [DOI] [PubMed] [Google Scholar]
- 9.Curtis RE, Metayer C, Rizzo JD, et al. Impact of chronic GVHD therapy on the development of squamous-cell cancers after hematopoietic stem-cell transplantation: an international case-control study. Blood. 2005;105:3802–11. doi: 10.1182/blood-2004-09-3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maddox JS, Soltani K. Risk of nonmelanoma skin cancer with azathioprine use. Inflamm Bowel Dis. 2008 doi: 10.1002/ibd.20444. [DOI] [PubMed] [Google Scholar]
- 11.Penn I. De novo malignances in pediatric organ transplant recipients. Pediatr Transplant. 1998;2:56–63. [PubMed] [Google Scholar]
- 12.Rosh JR, Gross T, Mamula P, Griffiths A, Hyams J. Hepatosplenic T-cell lymphoma in adolescents and young adults with Crohn’s disease: a cautionary tale? Inflamm Bowel Dis. 2007;13:1024–30. doi: 10.1002/ibd.20169. [DOI] [PubMed] [Google Scholar]
- 13.Kandiel A, Fraser AG, Korelitz BI, Brensinger C, Lewis JD. Increased risk of lymphoma among inflammatory bowel disease patients treated with azathioprine and 6-mercaptopurine. Gut. 2005;54:1121–5. doi: 10.1136/gut.2004.049460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.David KM, Morris JA, Steffen BJ, Chi-Burris KS, Gotz VP, Gordon RD. Mycophenolate mofetil vs. azathioprine is associated with decreased acute rejection, late acute rejection, and risk for cardiovascular death in renal transplant recipients with pre-transplant diabetes. Clin Transplant. 2005;19:279–85. doi: 10.1111/j.1399-0012.2005.00338.x. [DOI] [PubMed] [Google Scholar]
- 15.Deeg HJ, Socie G, Schoch G, et al. Malignancies after marrow transplantation for aplastic anemia and fanconi anemia: a joint Seattle and Paris analysis of results in 700 patients. Blood. 1996;87:386–92. [PubMed] [Google Scholar]
- 16.Relling MV, Rubnitz JE, Rivera GK, et al. High incidence of secondary brain tumours after radiotherapy and antimetabolites. Lancet. 1999;354:34–9. doi: 10.1016/S0140-6736(98)11079-6. [DOI] [PubMed] [Google Scholar]
- 17.Kasiske BL, Snyder JJ, Gilbertson DT, Wang C. Cancer after kidney transplantation in the United States. Am J Transplant. 2004;4:905–13. doi: 10.1111/j.1600-6143.2004.00450.x. [DOI] [PubMed] [Google Scholar]
- 18.(IARC) IAfRoC, editor. IARC Monographs on the evaluation of the carcinogenic risk of chemicals to humans. IARC; Lyon: 1987. Azathioprine (Group1) [Google Scholar]
- 19.Grosse Y, Baan R, Straif K, et al. A review of human carcinogens-Part A: pharmaceuticals. Lancet Oncol. 2009;10:13–4. doi: 10.1016/s1470-2045(08)70286-9. [DOI] [PubMed] [Google Scholar]
- 20.Yuan B, Wang Y. Mutagenic and cytotoxic properties of 6-thioguanine, S6-methylthioguanine and guanine-S6-sulfonic acid. J Biol Chem. 2008 doi: 10.1074/jbc.M804047200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karran P. Thiopurines, DNA damage, DNA repair and therapy-related cancer. Br Med Bull. 2006;79-80:153–70. doi: 10.1093/bmb/ldl020. [DOI] [PubMed] [Google Scholar]
- 22.Uribe-Luna S, Quintana-Hau JD, Maldonado-Rodriguez R, et al. Mutagenic consequences of the incorporation of 6-thioguanine into DNA. Biochem Pharmacol. 1997;54:419–24. doi: 10.1016/s0006-2952(97)00200-1. [DOI] [PubMed] [Google Scholar]
- 23.Smith CC, Archer GE, Forster EJ, Lambert TR, Rees RW, Lynch AM. Analysis of gene mutations and clastogenicity following short-term treatment with azathioprine in MutaMouse. Environ Mol Mutagen. 1999;34:131–9. [PubMed] [Google Scholar]
- 24.O’Donovan P, Perrett CM, Zhang X, et al. Azathioprine and UVA light generate mutagenic oxidative DNA damage. Science. 2005;309:1871–4. doi: 10.1126/science.1114233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karran P, Attard N. Thiopurines in current medical practice: molecular mechanisms and contributions to therapy-related cancer. Nat Rev Cancer. 2008;8:24–36. doi: 10.1038/nrc2292. [DOI] [PubMed] [Google Scholar]
- 26.Ansari AA, Mayne A, Sundstrom JB, et al. Frequency of hypoxanthine guanine phosphoribosyltransferase (HPRT-) T cells in the peripheral blood of cardiac transplant recipients. A noninvasive technique for the diagnosis of allograft rejection. Circulation. 1995;92:862–74. doi: 10.1161/01.cir.92.4.862. [DOI] [PubMed] [Google Scholar]
- 27.Dawisha SM, Gmelig-Meyling F, Steinberg AD. Assessment of clinical parameters associated with increased frequency of mutant T cells in patients with systemic lupus erythematosus. Arthritis Rheum. 1994;37:270–7. doi: 10.1002/art.1780370217. [DOI] [PubMed] [Google Scholar]
- 28.Falta MT, Atkinson MA, Allegretta M, Vacek PM, Albertini RJ. Azathioprine associated T-cell mutations in insulin-dependent diabetes mellitus. Scand J Immunol. 2000;51:626–33. doi: 10.1046/j.1365-3083.2000.00742.x. [DOI] [PubMed] [Google Scholar]
- 29.Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G7–17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 30.Albertini RJ. HPRT mutations in humans: biomarkers for mechanistic studies. Mutat Res. 2001;489:1–16. doi: 10.1016/s1383-5742(01)00064-3. [DOI] [PubMed] [Google Scholar]
- 31.Albertini RJ, Nicklas JA, O’Neill JP, Robison SH. In vivo somatic mutations in humans: measurement and analysis. Annual Review of genetics. 1990;24:305–26. doi: 10.1146/annurev.ge.24.120190.001513. [DOI] [PubMed] [Google Scholar]
- 32.Finette BA, Sullivan LM, O’Neill JP, Nicklas JA, Vacek PM, Albertini RJ. Determination of hprt mutant frequencies in T-lymphocytes from a healthy pediatric population: statistical comparison between newborn, children and adult mutant frequencies, cloning efficiency and age. Mutat Res. 1994;308:223–31. doi: 10.1016/0027-5107(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 33.Yoshioka M, Vacek PM, Poseno T, Silver R, Finette BA. Gender-specific frequency of background somatic mutations at the hypoxanthine phosphoribosyltransferase locus in cord blood T lymphocytes from preterm newborns. Proc Natl Acad Sci U S A. 1999;96:586–91. doi: 10.1073/pnas.96.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finette BA, Homans AC, Albertini RJ. Emergence of genetic instability in children treated for leukemia. Science. 2000;288:514–7. doi: 10.1126/science.288.5465.514. [DOI] [PubMed] [Google Scholar]
- 35.Finette BA, Homans AC, Rivers J, Messier T, Albertini RJ. Accumulation of somatic mutations in proliferating T cell clones from children treated for leukemia. Leukemia. 2001;15:1898–905. doi: 10.1038/sj.leu.2402306. [DOI] [PubMed] [Google Scholar]
- 36.Kendall HE, Vacek PM, Rivers JL, Rice SC, Messier TL, Finette BA. Analysis of genetic alterations and clonal proliferation in children treated for acute lymphocytic leukemia. Cancer Res. 2006;66:8455–61. doi: 10.1158/0008-5472.CAN-05-4015. [DOI] [PubMed] [Google Scholar]
- 37.Gibbs RA, Nguyen PN, Edwards A, Civitello AB, Caskey CT. Multiplex DNA deletion detection and exon sequencing of the hypoxanthine phosphoribosyltransferase gene in Lesch-Nyhan families. Genomics. 1990;7:235–44. doi: 10.1016/0888-7543(90)90545-6. [DOI] [PubMed] [Google Scholar]
- 38.Finette BA, Poseno T, Albertini RJ. V(D)J recombinase-mediated HPRT mutations in peripheral blood lymphocytes of normal children. Cancer Res. 1996;56:1405–12. [PubMed] [Google Scholar]
- 39.Abramson LS, Albertini RJ, Pachman LM, Finette BA. Association among somatic HPRT mutant frequency, peripheral blood T-lymphocyte clonality, and serologic parameters of disease activity in children with juvenile onset dermatomyositis. Clin Immunol. 1999;91:61–7. doi: 10.1006/clim.1998.4675. [DOI] [PubMed] [Google Scholar]
- 40.Finette BA, Poseno T, Vacek PM, Albertini RJ. The effects of maternal cigarette smoke exposure on somatic mutant frequencies at the hprt locus in healthy newborns. Mutat Res. 1997;377:115–23. doi: 10.1016/s0027-5107(97)00069-9. [DOI] [PubMed] [Google Scholar]
- 41.Branda RF, Sullivan LM, O’Neill JP, et al. Measurement of HPRT mutant frequencies in T-lymphocytes from healthy human populations. Mutat Res. 1993;285:267–79. doi: 10.1016/0027-5107(93)90115-v. [DOI] [PubMed] [Google Scholar]
- 42.Finette BA, Kendall H, Vacek PM. Mutational spectral analysis at the HPRT locus in healthy children. Mutat Res. 2002;505:27–41. doi: 10.1016/s0027-5107(02)00119-7. [DOI] [PubMed] [Google Scholar]
- 43.Finette BA, O’Neill JP, Vacek PM, Albertini RJ. Gene mutations with characteristic deletions in cord blood T lymphocytes associated with passive maternal exposure to tobacco smoke. Nat Med. 1998;4:1144–51. doi: 10.1038/2640. [DOI] [PubMed] [Google Scholar]
- 44.Curry J, Karnaoukhova L, Guenette GC, Glickman BW. Influence of sex, smoking and age on human hprt mutation frequencies and spectra. Genetics. 1999;152:1065–77. doi: 10.1093/genetics/152.3.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hackman P, Hou SM, Nyberg F, Pershagen G, Lambert B. Mutational spectra at the hypoxanthine-guanine phosphoribosyltransferase (HPRT) locus in T-lymphocytes of nonsmoking and smoking lung cancer patients. Mutat Res. 2000;468:45–61. doi: 10.1016/s1383-5718(00)00039-5. [DOI] [PubMed] [Google Scholar]
- 46.Tage-Jensen U, Schlichting P, Thomsen HF, Hoybye G, Thomsen AC. Malignancies following long-term azathioprine treatment in chronic liver disease. A report from the Copenhagen Study Group for Liver Diseases. Liver. 1987;7:81–3. doi: 10.1111/j.1600-0676.1987.tb00321.x. [DOI] [PubMed] [Google Scholar]
- 47.Mark SC, Sandercock LE, Luchman HA, Baross A, Edelmann W, Jirik FR. Elevated mutant frequencies and predominance of G:C to A:T transition mutations in Msh6(-/-) small intestinal epithelium. Oncogene. 2002;21:7126–30. doi: 10.1038/sj.onc.1205861. [DOI] [PubMed] [Google Scholar]
- 48.LePage GA, Whitecar JP., Jr. Pharmacology of 6-thioguanine in man. Cancer Res. 1971;31:1627–31. [PubMed] [Google Scholar]
- 49.Ratanatharathorn V, Ayash L, Lazarus HM, Fu J, Uberti JP. Chronic graft-versus-host disease: clinical manifestation and therapy. Bone Marrow Transplant. 2001;28:121–9. doi: 10.1038/sj.bmt.1703111. [DOI] [PubMed] [Google Scholar]
- 50.Cuffari C, Li DY, Mahoney J, Barnes Y, Bayless TM. Peripheral blood mononuclear cell DNA 6-thioguanine metabolite levels correlate with decreased interferon-gamma production in patients with Crohn’s disease on AZA therapy. Dig Dis Sci. 2004;49:133–7. doi: 10.1023/b:ddas.0000011614.88494.ee. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.