Abstract
Sphingolipids are not only major lipid components of all eukaryotic cell membranes, but they also comprise an important family of bioactive signaling molecules that regulate a diverse array of biological responses. The sphingolipid metabolite sphingosine-1-phosphate (S1P), is a key regulator of immune responses. Cellular levels of S1P are determined by the balance between its synthesis, involving two sphingosine kinases (SphK1 and SphK2), and its degradation, involving S1P lyase and S1P phosphatases. S1P mainly signals through its cell-surface receptors and may also have intracellular functions. S1P has important functions in mast cells – the major effectors of allergic responses. Antigen triggering of IgE receptors on mast cells activates both SphKs resulting in the production of S1P that is released and regulates and amplifies mast cell functions, including degranulation as well as cytokine and chemokine release.
Keywords: allergy, anaphylaxis, inflammation, mast cells, sphingolipid metabolites, sphingosine-1-phosphate, sphingosine kinase
Over the last decade or so, the work of many investigators has established the importance of the bioactive lipid mediator sphingosine-1-phosphate (S1P) in regulating numerous and diverse cellular processes in various cell types, including proliferation, cell survival, motility and cytoskeletal rearrangements as wells as angiogenesis [1,2]. S1P exerts the majority of its effects as an extracellular ligand for a family of five specific G proteincoupled receptors, denoted S1P1–5 [1]. These receptors all bind S1P with similar affinity but are able to couple to a variety of G proteins, thus enabling S1P to regulate numerous downstream signaling pathways [2]. In addition, every cell in the body expresses at least one of the S1P receptors, which are differentially expressed from cell to cell, further complicating the understanding of the wide ranging yet distinct actions of S1P. S1P has also demonstrated some actions that are independent of its receptors [1], although its intracellular targets have not yet been unequivocally identified. This review is focused on the emerging importance of S1P functions in mast cells and discusses the potential for targeting sphingosine kinases (SphKs), S1P receptors, as well as S1P itself, to suppress mast cell-mediated inflammation and related pathological conditions.
Sphingolipid metabolism & sphingosine kinases
Intracellular levels of S1P are tightly regulated by the balance between its synthesis, which involves SphK1 and SphK2, and its degradation, which can occur either reversibly by two specific S1P phosphatases or irreversibly by S1P lyase (Figure 1). Therefore, this balance between S1P and its precursors – sphingosine and ceramide – and their overall regulation of opposing signaling pathways is instrumental in determining cell fate and has been termed the ‘sphingo-lipid rheostat’ [3]. SphK1 was the first isozyme discovered and characterized and is, therefore, the most well studied. It is activated by numerous stimuli, including many growth factors and cytokines and crosslinking of immuno globulin receptors [1]. Activation of SphK1, which requires its phosphorylation by ERK1/2 [4,5], is accompanied by its translocation to the plasma membrane where its substrate sphingosine resides [6,7]. Much less is known about the regulation of SphK2. Its subcellular localization is cell-type specific, that is cytosolic in some types and mainly nuclear in others, and it can translocate between these compartments in response to specific stimuli [8]. In rodent mast cells, SphK1 and SphK2 are largely cytosolic under basal conditions and translocate to the plasma membrane following IgE-receptor engagement [6,7].
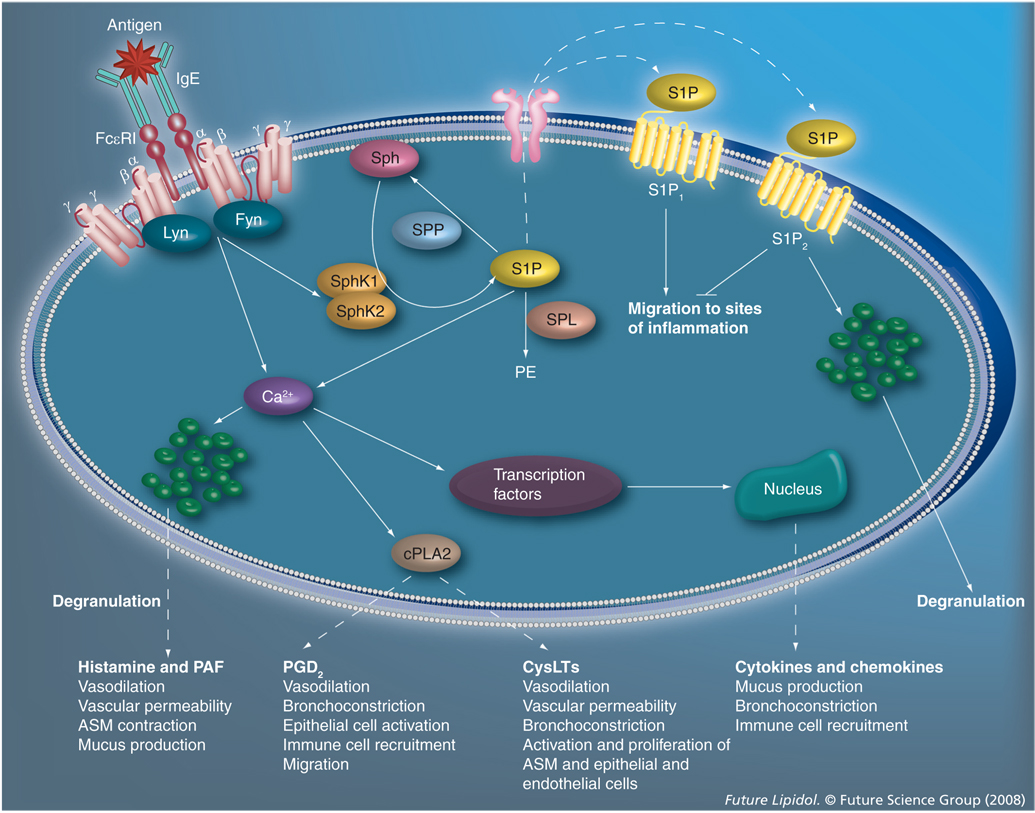
Figure 1. The role of S1P in mast cell-mediated allergic responses and anaphylaxis.
Antigens induce allergic responses via crosslinking of IgE-bound FcεRI receptors on the surface of mast cells, triggering elaborate signaling cascades that result in degranulation and immediate release of a plethora of inflammatory mediators. One such pathway is the activation of SphK1 and SphK2 and the subsequent production of S1P. S1P can be degraded by SPP or SPL, leading to the production of PE. Intracellular S1P can then be secreted from mast cells via the ABCC1 transporter to bind its receptors extracellularly, or may act intracellularly to induce calcium release independent of phosphatidylinositol (3,4,5)-trisphosphate. Extracellular signaling through S1P1 is important for migration of mast cells to sites of inflammation, while S1P2 inhibits motility, probably to resolve cellular movement upon arrival at target sites and also aids in degranulation. Calcium influx is necessary for processes that are critical to the induction of allergic responses and anaphylaxis, such as degranulation and activation of cPLA2, and various transcription factors. Histamine and PAF induce vasodilation, ASM contraction, and increase vascular permeability and mucus production. Activation of cPLA2 is the rate-limiting step in the production of all eicosanoids, including PGD2 and CysLTs, which themselves induce vasodilation, bronchoconstriction vascular permeability, epithelial and endothelial cell activation and proliferation, immune cell recruitment, migration and ASM activation and proliferation. Release of immediate mediators of inflammation from mast cells is followed by increased transcription of various cytokine and chemokine factors that induce mucus production, bronchoconstriction and immune cell recruitment. Collectively, mast-cell mediators promote inflammation and, furthermore, function to activate and recruit other immune cells, thereby exacerbating the symptoms of allergy and anaphylaxis. ASM: Airway smooth muscle; cPLA: Cytosolic phospholipase A2; CysLT: Cysteinyl leukotriene; PAF: Platelet-activating factor; PE: Phosphatidylethanoloamine; PGD2: Prostaglandin D2; PG: Prostaglandin; SphK: Sphingosine kinase; S1P: Sphingosine-1-phosphate; SPL: S1P lyase; SPP: Specific phosphatase.
Interestingly, although SphK1 and SphK2 are highly homologous and utilize the same substrate to produce the same product, they exhibit both functional and experimental differences. In contrast to the growth and survival promoting actions of SphK1, overexpression of SphK2 in many cells induces cell death and growth arrest. Very few studies to date have examined the role of endogenous SphK2. It has recently been demonstrated that SphK2 is also activated by phosphorylation [9], as well as by antigen crosslinking of IgE receptors [7]. Mice with knockouts of either SphK1 or SphK2 are viable, have only slightly decreased S1P levels and exhibit no obvious phenotypes [10]. However, knockout of both SphK1 and SphK2 results in complete loss of S1P and is embryonically fatal [10], demonstrating the necessity of S1P for life. Together, these observations also suggest that SphK1 and SphK2 may have some redundant, overlapping and/or compensatory functions.
FTY720
While both SphK1 and SphK2 can phosphorylate sphingosine and sphinganine (dihydrosphingosine), only SphK2 can efficiently catalyze the phosphorylation of the immunosuppressive drug FTY720 (Fingolimod) [11] – a sphingosine analog – forming FTY720-phosphate, which is a S1P mimetic capable of binding with high affinity to all of the S1P receptors except S1P2. Its immunosuppresive action as a potent ligand of S1P1 on lymphocytes leads to prolonged downregulation of this receptor [12,13]. Since S1P1 is required for lymphocyte egress from secondary lymph nodes and lymphoid organs, these cells are sequestered in this location by FTY720 administration, resulting in lymphopenia and rendering them incapable of responding to inflammation [14,15].
Sphingosine-1-phosphate receptors
As all mast cells express two of the five S1P receptors (S1P1 and S1P2) [6,16], the following section focuses on these two receptors, and the reader is referred to more comprehensive reviews for information about the other S1P receptors [17,18].
S1P1
S1P1 is widely expressed, with predominant expression found in brain, kidney, spleen, lung and the cardiovascular system [19]. S1P1 was first demonstrated to be important in angiogenesis when mice lacking this receptor were found to have incomplete vascular development and consequently died in utero [20]. This receptor is also a key player in the maintenance of vascular integrity, which is important for inflammation and asthmatic lung remodeling [21–23]. Importantly, S1P1 is also critical in lymphocyte egress from the thymus and peripheral lymphoid organs. Indeed, mice lacking expression of S1P1 in hematopoietic cells exhibit lymphopenia since mature T and B cells are unable to exit the thymus [13]. Furthermore, S1P1 signaling is strongly upregulated prior to the exit of T cells from the thymus, suggesting a role in the chemotactic responsiveness of these cells [13].
S1P2
S1P2 is also expressed in a variety of cell types. In contrast to S1P1−/− mice, mice lacking S1P2 are viable and only display a defect in proper development of auditory and vestibular systems, resulting in complete deafness [24–26]. In mast cells, S1P2 is important for effective degranulation [6]. In the vascular system, activation of S1P2 also increases vascular permeability, similar to S1P1 [23]. Furthermore, S1P2 is considered to be a ‘repellant’ receptor as binding of S1P to S1P2 decreases motility of many cell types, including mast cells [6].
Roles of SphK1 & SphK2 in mast cells
Mast cells express the high-affinity receptor for IgE – FcεRI – which is an important component of allergic diseases. Its crosslinking by monomeric IgE bound to multivalent antigens initiates an elaborate and complicated cascade of signaling events that leads to the release of preformed granules (degranulation) containing histamine and other mediators of immediate responses as well as the subsequent production and secretion of cytokines and chemokines and lipid mediators, such as eicosanoids (leukotrienes and prostaglandins) and S1P [27,28]. These mast cell mediators promote inflammation by enhancing vascular permeability while initiating the recruitment and activation of other immune cells involved in allergic and inflammatory responses.
Crosslinking of IgE receptors on mast cells results in activation of several key regulators, including Lyn, Fyn and Syk, which are initiators of intricate pathways involving numerous downstream signaling molecules that ultimately co ordinate and control mast cell responsiveness [29]. Loss of Fyn or Lyn in mast cells has widespread effects, impairing degranulation and cytokine production. While Fyn and Lyn tyrosine kinases are associated with SphK1 and SphK2 in murine mast cells, activation of SphK1 requires Fyn but Lyn is partly dispensable [7]. Both Lyn and Fyn contribute to SphK2 translocation to the plasma membrane upon FcεRI triggering. Interestingly, SphK2 was reported to be the major contributor of S1P in murine mast cells derived from embryonic liver progenitors [30]. Mast cells derived from SphK2-knockout mice demonstrated impaired degranulation and production of certain cyto kines, primarily due to reductions in intracellular calcium levels and PKC activation. Impairment of degranulation in SphK2-deficient mast cells was partially restored by the addition of exogenous S1P. This confirmed that SphK2 is necessary, but not sufficient, for IgE-mediated responses, at least in murine mast cells [7]. By contrast, in human mast cells, SphK1 but not SphK2 is critical for antigen-induced degranulation, chemokine secretion and migration, while both isozymes are important for cytokine secretion [31]. Furthermore, downregulation of SphK1 reduced the rapid and transient increase in intracellular calcium induced by FcεRI crosslinking, which is necessary for mast cell degranulation [32]. In addition to the engagement of FcεRI, several other stimuli are capable of triggering secretion of inflammatory mediators from activated mast cells, including the anaphylatoxin C5a [33]. With regard to the actions of C5a, SphK1 expression is required for its ability to trigger calcium release, chemotaxis, degranulation and cytokine release from human macrophages [34]. However, neutrophils isolated from SphK1-knockout mice showed normal responses to C5a [35].
Extracellular functions of S1P in mast cells
Inside-out signaling, whereby S1P generated intracellularly by activation of SphKs is secreted and activates S1P1 and S1P2 receptors on the same or nearby mast cells, plays important roles in mast cell responses [36]. For example, activation of S1P1 is critical for migration of mast cells toward antigens and might be involved in the movement of mast cells up an antigen gradient to sites of inflammation [6]. Furthermore, expression of the motility-inhibiting S1P2 receptor in mast cells is upregulated by crosslinking of FcεRI by antigens [6], suggesting that mast cells are attracted to an inflammation site by a S1P1-dependent motility process and halt upon reaching their destination owing to upregulation of S1P2. Here, activation by inside-out signaling also enhances their degranulation. Thus, there appears to be an exquisite interplay of S1P controlled responses following FcεRI activation in mast cells.
Secretion of S1P from mast cells
The mechanism by which intracellularly produced S1P can exit from cells to interact with its receptors located on the extracellular leaflet of the plasma membrane has been a long standing mystery. It has been proposed that SphK1 may be secreted from cells and catalyzes the conversion of sphingosine to S1P in the extracellular milieu [37,38], although no evidence has been found for this in mast cells [6]. A partial answer has now been provided by our recent discovery that the ATP-binding cassette transporter ABCC1 promotes the export of S1P across the plasma membrane of activated rodent and human mast cells independent of their degranulation [39]. It is possible that other ABC transporters may also participate in export of S1P.
Blood levels of S1P
The concentration of S1P in blood is maintained at high levels. Plasma levels range from 0.1 to 0.6 µM, while serum levels range from 0.4 to 1.1 µM [40,41]. S1P mainly circulates as a complex with albumin and lipoproteins. Platelets that produce, store and secrete large amounts of S1P, were long considered to be the major source of circulating S1P. However, recent studies suggest that erythrocytes may be the major source of S1P in blood [42,43]. The vascular endothelium, in addition to the hematopoietic system, has also been suggested to be an important contributor of plasma S1P [44].
Levels of S1P in tissues are significantly lower than in blood, possibly owing to the presence of S1P phosphatase and S1P lyase, which are absent or low in platelets and erythrocytes [45]. This leads to the establishment of a concentration gradient of S1P between blood and tissues, which is important for lymphocyte trafficking. Intriguingly, deletion of either isoform of SphK in mice does not abolish this blood–tissue gradient of S1P [30], while loss of S1P lyase activity does so [45]. Similarly, secretion of S1P by mast cells may also serve to establish a gradient that aids in the recruitment of other immune cells whose chemotactic motility is stimulated by S1P. However, susceptibility to in vivo anaphylaxis correlated with circulating S1P generated by SphK1 that was predominantly from a non-mast cell source(s) [30].
Intracellular actions of S1P in mast cells
Although intracellular targets of S1P have yet to be identified, S1P has intracellular second messenger actions that regulate calcium levels in mast cells independently of phosphatidylinositol (3,4,5)-trisphosphate (InsP3) [46]. This calcium mobilization was recently demonstrated to be dependent on clathrin [47]. It has also been suggested that both InsP3 and S1P contribute to FcεRI-induced calcium release from the endoplasmic reticulum and that production of InsP3 is necessary for S1P to cause calcium mobilization from the endoplasmic reticulum [48].
Intriguingly, fetal liver-derived mast cells from mice lacking SphK2 display impaired calcium mobilization upon IgE-receptor activation, even when S1P is added exogenously [30]. Additionally, exogenous S1P only partially restored degranulation to mast cells isolated from mice lacking Fyn kinase [7]. Collectively, these data suggest that S1P may be a bona fide second messenger in mast cells, although acting in a manner that still requires clarification.
Anaphylaxis
Anaphylaxis is a severe and potentially fatal immediate systemic allergic reaction that occurs suddenly after contact with an allergy-causing substance and is triggered by rapid, IgE-mediated immune release of potent mediators from tissue mast cells and peripheral basophils [49]. Mast cells reside at mucosal, submucosal and perivascular locations in close proximity to epithelial surfaces, near blood vessels, nerves and glands, where they are able to detect invading pathogens and changes in their environment [28]. In humans, mast cell-derived mediators contribute to the pathophysiology of allergic diseases, inducing tissue edema, broncho constriction, increased vascular permeability, influx of inflammatory cells and mucus secretion. In addition, mast cells express numerous receptors for cytokines, chemokines and eicosanoids, as well as Toll-like receptors, which enable them to recognize diverse allergic stimuli. The diversity in cellular location, as well as the repertoire of receptors expressed and mediators released, permits mast cells to be key regulators of innate and adaptive immunity.
Murine and human immune systems are reasonably similar and so animal models of anaphylaxis may provide information that is potentially relevant to human anaphylaxis. Systemic anaphylaxis in the mouse can be mediated via two independent mechanisms; a classical pathway mediated by IgE, FcεRI, mast cells, histamine and platelet-activating factor (PAF), and an alternative pathway mediated by IgG, FcγRIII, macrophages and PAF [50]. Most human systemic anaphylaxis is probably IgE-dependent, although there is some evidence for IgE-independent anaphylaxis [51]. Some potent food allergens, particularly peanuts and tree nuts, can stimulate an anaphylactic-like, non-IgE-mediated response, thereby synergizing with IgE-induced mast cell activation to exacerbate anaphylaxis.
Role of S1P in anaphylaxis
Recent studies indicate that SphKs are also determinants of anaphylaxis. SphK2 is the main isoform required for generation of S1P, calcium influx and degranulation of rodent mast cells [7]. However, susceptibility to anaphylaxis in mice was correlated with circulating S1P generated by SphK1, predominantly from a non-mast cell source [30]. Mast cells do not contribute to basal circulating levels of S1P as mast cell-deficient mice have similar levels of plasma S1P compared with their counterparts engrafted with normal mast cells [30]. Mice deficient in SphK1 have reduced levels of circulating S1P and are resistant to anaphylaxis. They also have impaired histamine responses despite normal mast cell function. However, mice deficient in SphK2 have enhanced levels of S1P in the blood and respond normally to anaphylactic challenge with normal histamine release [30].
Intestinal anaphylaxis (allergic diarrhea) is almost totally IgE- and mast cell-dependent, but is mediated predominantly by PAF and serotonin. In a murine intestinal anaphylaxis model, S1P1 expression was preferentially associated with pathogenic CD4+ T cells induced by allergen challenge in the large intestine. FTY720 prevented allergic diarrhea by inhibiting the migration of these cells and decreased mast cell infiltration into the large intestine, but did not affect eosinophil infiltration or serum IgE production [52].
Asthma
In asthma, mast cells infiltrate the bronchial epithelium and, upon activation, release inflammatory mediators that influence bronchial epithelial function. Mast cell numbers are greater in the mucosal epithelium of patients with asthma and allergic diseases compared with disease-free controls, with no substantial change in the numbers of mast cells in the adjacent connective tissues [53]. Abnormal airway smooth muscle function is a key feature in the pathophysiology of asthma, with a positive correlation between mast cell numbers and bronchial hyper-responsiveness [54].
Role of S1P in asthma
Previous studies demonstrated that S1P was elevated in the airways of asthmatic individuals after antigen challenge and that S1P modulates human airway smooth muscle cell functions that promote inflammation and airway remodeling in asthma [55] and can induce contraction of airway smooth muscle [56]. S1P can also amplify and enhance mast cell functions and may regulate their arrival to sites of inflammation [6,57]. Rodent models in which asthma-like symptoms are introduced by sensitization and challenge with antigen in an adjuvant are characterized by airway eosinophilia, which contributes to the observed airway hyper-responsiveness (AHR). It has recently been demonstrated that S1P induces dose-dependent contraction of bronchi and increases AHR in ovalbumin (OVA)-sensitized mice [58]. These events were associated with increased expression of SphK1 and SphK2, as well as S1P2 and S1P3 receptors. Local administration of S1P caused inflammation and eosinophil recruitment in a rat-paw inflammation model [59]. Furthermore, S1P and the kinases that produce it play important roles in many types of immune cells involved in allergic responses and asthma (Table 1), implicating S1P as a pleiotropic lipid mediator important in the inflammatory and allergic reactions and asthma.
Table 1.
Involvement of SphKs in response to stimulation of various types of immune cells.
| Cell type | Source | Stimulus | SphK involved | Effects | Ref. |
|---|---|---|---|---|---|
| Mast | Rat RBL-2H3 | IgE/Ag | SphK1 | Initial rise in calcium from internal stores, degranulation, cytokine production and/or smigration towards antigen | [6] |
| Murine BMMC | IgE/Ag | SphK2 | Production of S1P, calcium influx, PKC activation, degranulation and/or cytokine production | [30] | |
| Human LAD2/CB-MC | IgE/Ag | SphK1 | Degranulation, CCL2 secretion and/or migration towards antigen | [31] | |
| Human LAD2/CB-MC | IgE/Ag | SphK1/SphK2 | TNF secretion | [31] | |
| Macrophages | Human blood | C5a | SphK1 | Intracellular calcium signaling, degranulation, cytokine generation (TNF, Il-6 and IL-8) and/or chemotaxis | [34] |
| Murine RAW264 | LPS | SphK1 | ERK1/2 and NF-κB activation | [67] | |
| Human U937 | IFN-γ | SphK1 | Vesicular trafficking | [68] | |
| Murine bone marrow | RANKL | SphK1 | Osteoclastogenesis via regulation of p38, ERK, NFATc1 and/or cFos | [69] | |
| Human blood | Apoptosis Inducers | SphK2 | Polarization to M2 phenotype, decreased TNF and IL-12-p70 production, increased IL-8 and IL-10 production and/or decreased NF-κB signaling | [70] | |
| Murine in vivo | C5a | SphK1 | Acute peritonitis, systemic inflammation, multiorgan damage and/or release of proinflammatory mediators | [71] | |
| B cells | Human lymphoblasts | S1P | SphK1 | Resistance to Fas-mediated cell death | [72] |
| T cells | Murine CD4+ | IL-2 | SphK2 | Regulation of proliferation, secretion of cytokines and/or STAT5 activation | [73] |
| Murine | T-cell receptor | SphK1 | Negative regulation of chemokine expression s(IFN-ã, TNF and IL-2) | [74] | |
| Murine 2D6 clone | IL-12 | SphK2 | Promotes Th1 differentiation and cell-mediated immune responses | [75] | |
| Neutrophils | Human blood | C5a | SphK1 | Calcium release, degranulation, chemotaxis and/or sactivation of NADPH oxidase | [76] |
| Eosinophils | Murine in vivo | OVA | SphKs* | Inflammatory cell infiltration, eosinophilia, increased bronchoalveolar lavage fluid, IL-4, IL-5 and eotaxin production and/or increased serum sIgE levels | [65] |
| Murine in vivo | MeCh | SphKs* | Mucus production and/or airway hyper-responsiveness | [65] | |
Isozyme not identified.
BMMC: Bone marrow-derived mast cells; CB-MC: Cord blood-derived mast cells; CCL2: CC chemokine ligand 2; ERK: Extracellular signal-regulated kinase; LPS: Lipopolysaccharide; MeCh: Methacholine; OVA: Ovalbumin; S1P: Sphingosine-1-phosphate; SphK: Sphingosine kinase.
FTY720 is highly effective in reducing the severity of autoimmune diseases in several animal models [60]. Neither FTY720 nor FTY720-phosphate, despite its similarity to S1P, affect mast cell degranulation, yet both significantly reduce antigen-induced secretion of prosta glandin D2 and cysteinyl leukotrienes [61]. FTY720 was suggested to be a direct inhibitor of cytosolic phospholipase A2, the rate-limiting enzyme in the production of all eicosanoids [61]. Indeed, oral treatment of mice with FTY720 inhibits AHR induced by adoptive transfer of Th1 and Th2 cells and asthma induced by active immunization and challenge with OVA [62]. In addition, inhalation administration of FTY720 prior to, or during, ongoing allergen challenge suppressed Th2-dependent eosinophilic airway inflammation and bronchial AHR by inhibition of migration of lung dendritic cells to the mediastinal lymph nodes, thus preventing the formation of allergen-specific Th2 cells in the lymph nodes [63].
SphK inhibitors: new targeted antiallergic therapies?
Current therapeutic strategies for these unfortunately most common diseases are often of poor efficacy and associated with undesirable side effects. Complete allergen avoidance is very difficult to achieve. Desensitizing immunotherapy has been performed for many years, with doubtful efficacy in many cases and has occasionally even been hazardous. Thus, new approaches for the development of novel inhibitors of allergic diseases have great potential. Modulating the production of S1P and/or specifically targeting its receptors are attractive novel approaches for the modulation of mast cell-mediated allergic diseases. Promising observations in murine models of allergic disease provide proof of concept for the importance of SphKs and production of S1P as targets in inflammatory responses. For example, increased levels of proinflammatory cytokines in the peritoneal cavity of mice administered C5a were substantially decreased by treatment with the pan SphK inhibitor, N,N-dimethylsphingosine (DMS) [64]. This was accompanied by suppression of C5a-induced neutropenic responses, as well as by increased vascular permeability [64]. Moreover, C5a activates SphK in human neutrophils and the SphK inhibitor DMS largely blocked C5a-stimulated calcium mobilization, chemotaxis and cytokine production [34]. However, although an in vivo model of bacterial lung infection revealed an accelerated p rogression of disease in SphK2 but not SphK1-knockout mice, effector functions of SphK1- or SphK2-deficient neutrophils and their capacity to kill bacteria were normal [35].
A recent study by Lai et al. utilized the pan-SphK inhibitor DMS and downregulation of SphK1 expression to demonstrate that they both effectively suppressed airway eosinophilia, pulmonary inflammation and secretion of Th2 cytokines and chemokines, and markedly attenuated OVA-induced AHR in sensitized mice [65]. Serum levels of OVA-specific IgE were reduced by SphK1 siRNA, suggesting that production of S1P may regulate B-cell trafficking and IgE production. In another study, OVA inhalation caused S1P release into bronchial alveolar lavage (BAL) and expression of SphK1 around bronchial epithelial walls. Inhalation of pan-SphK inhibitors decreased S1P in BAL, accompanied by decreased eosinophil infiltration and eotaxin expression. Furthermore, bronchial hyper-responsiveness to inhaled methacholine and goblet cell hyperplasia were improved by SphK inhibitors [66].
Executive summary.
Sphingolipid metabolism & sphingosine kinases
Sphingosine kinases (SphK) 1 and 2 are highly homologous, but have distinct functions.
Antigens and many other external stimuli activate SphK1, inducing its phosphorylation and translocation to the plasma membrane.
Although antigen also stimulates SphK2 in rodent mast cells, much less is known about the regulation of SphK2.
FTY720
Only SphK2 catalyzes the phosphorylation of the immunosuppressive drug FTY720 (Fingolimod) producing a mimetic of sphingosine-1-phosphate (S1P) that induces lymphopenia.
S1P receptors
The S1P1 receptor regulates migration of mast cells toward antigen and sites of inflammation and is necessary for T- and B-lymphocyte egress from thymus and peripheral lymphoid organs.
The S1P2 receptor is important for proper mast cell degranulation and the resolution of their migration at sites of antigen challenge.
Roles of SphK1 & SphK2 in mast cells
S1P generated by activation of SphK1 and SphK2 in mast cells promotes inflammation by stimulating calcium influx, degranulation and cytokine production. This increases vascular permeability, inducing airway smooth muscle contraction and triggering airway hyper-responsiveness, while simultaneously signaling the recruitment and activation of other immune cells involved in allergic and inflammatory responses.
SphK1 is required for optimal histamine release and plays a key role in allergic responses.
SphK2 is important for degranulation, calcium influx and production of certain cytokines in murine mast cells.
Extracellular functions of S1P in mast cells
Inside-out signaling, whereby S1P generated intracellularly by activation of SphKs in response to antigen is secreted and activates S1P1 and S1P2 receptors on the same or nearby mast cells, plays important roles in amplification of mast cell responses.
Secretion of S1P from mast cells
The ABC transporter ABCC1 exports S1P in addition to cysteinyl leukotriene from mast cells.
Blood levels of S1P
Major sources of blood S1P are platelets and erythrocytes, and possibly endothelial cells.
Intracellular actions of S1P in mast cells
S1P generated intracellularly may have unique functions in mast cells acting by mechanisms that are not fully understood.
Role of S1P in anaphylaxis
Susceptibility to anaphylaxis in mice correlates with circulating S1P generated by SphK1, predominantly from a non-mast cell sources.
Role of S1P in asthma
S1P is elevated in the airways of asthmatic individuals after antigen challenge.
S1P and SphKs are involved in murine models of airway hyper-responsiveness.
Local application of FTY720 to the lung abrogates experimental asthma by altering dendritic cell function.
SphK inhibitors: new targeted antiallergic therapies?
Activation of SphK1 and SphK2, and subsequent production of S1P, exacerbate the symptoms of allergic responses, asthma and anaphylaxis. SphKs, as well as S1P and its receptors, are attractive targets for the development of therapeutics for treatment of these human disorders.
Promising observations in murine models of allergic disease provide proof-of-concept for the importance of SphKs and production of S1P as targets in inflammatory responses.
Conclusion & future perspective
Although S1P has been implicated as an important component of the regulation of immune responses, there are still many questions waiting to be answered. The lack of SphK isozyme-specific inhibitors has made it challenging to assign specific functions to SphK1 and SphK2. However, the recent development and availability of specific S1P receptor agonists and antagonists will likely encourage more preclinical and clinical trials to target effects mediated by S1P. In conclusion, the relevance of mast cells, S1P, SphKs and S1P receptors for the maintenance of normal physiology, or in disease states, constitutes an outstanding and intricate combination of players important for immune responses at the cellular, signaling and molecular levels. The need for specific SphK inhibitors is driving the development of new compounds by many pharmaceutical companies. Combining knowledge gained from molecular strategies and conditional gene knockouts to interfere with expression of enzymes that regulate S1P levels with pharmacological approaches will surely aid in this quest.
Acknowledgements
This work was supported by grants from the NIH R01AI50094, U19AI077435 and R37GM043880 (awarded to Sarah Spiegel), and KO1AR053186 (awarded to Carole Oskeritzian). Sheldon Milstien was supported by the Intramural Research Program of the National Institute of Mental Health.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Megan M Price, Department of Biochemistry & Molecular Biology, Virginia Commonwealth University School of Medicine, Richmond, VA 23298, USA, Tel.: +1 804 828 9332, Fax: +1 804 828 8999.
Carole A Oskeritzian, Department of Biochemistry & Molecular Biology, Virginia Commonwealth University School of Medicine, Richmond, VA 23298, USA, Tel.: +1 804 828 9332, Fax: +1 804 828 8999.
Sheldon Milstien, Intramural Research Program, National Institute of Mental Health (NIMH), Bethesda, MD 20892, USA, Tel.: +1 804 828 4988, Fax: +1 804 828 8999.
Sarah Spiegel, Department of Biochemistry & Molecular Biology, Virginia Commonwealth University School of Medicine, Richmond, VA 23298, USA, Tel.: +1 804 828 9330, Fax: +1 804 828 8999, sspiegel@vcu.edu.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat. Rev. Mol. Cell. Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 2.Rosen H, Goetzl EJ. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat. Rev. Immunol. 2005;5:560–570. doi: 10.1038/nri1650. [DOI] [PubMed] [Google Scholar]
- 3.Cuvillier O, Pirianov G, Kleuser B, et al. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381:800–803. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- 4.Pitson SM, Moretti PA, Zebol JR, et al. Activation of sphingosine kinase 1 by ERK1/2-mediated phosphorylation. EMBO J. 2003;22:5491–5500. doi: 10.1093/emboj/cdg540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pitson SM, Xia P, Leclercq TM, et al. Phosphorylation-dependent translocation of sphingosine kinase to the plasma membrane drives its oncogenic signalling. J. Exp. Med. 2005;201:49–54. doi: 10.1084/jem.20040559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jolly PS, Bektas M, Olivera A, et al. Transactivation of sphingosine-1-phosphate receptors by FcεRI triggering is required for normal mast cell degranulation and chemotaxis. J. Exp. Med. 2004;199:959–970. doi: 10.1084/jem.20030680. ▪▪ Demonstrates the role of sphingosine-1-phosphate (S1P) and its receptors in mast cell functions.
- 7.Olivera A, Urtz N, Mizugishi K, et al. IgE-dependent activation of sphingosine kinases 1 and 2 and secretion of sphingosine 1-phosphate requires Fyn kinase and contributes to mast cell responses. J. Biol. Chem. 2006;281:2515–2525. doi: 10.1074/jbc.M508931200. ▪▪ Highlights the involvement of sphingosine kinases (SphKs) in the FcεRI signaling pathway. Lyn is important for SphKs translocation, while Fyn is important for their activation.
- 8.Igarashi N, Okada T, Hayashi S, et al. Sphingosine kinase 2 is a nuclear protein and inhibits DNA synthesis. J. Biol. Chem. 2003;278:46832–46839. doi: 10.1074/jbc.M306577200. [DOI] [PubMed] [Google Scholar]
- 9.Hait NC, Bellamy A, Milstien S, et al. Sphingosine kinase type 2 activation by ERK-mediated phosphorylation. J. Biol. Chem. 2007;282:12058–12065. doi: 10.1074/jbc.M609559200. [DOI] [PubMed] [Google Scholar]
- 10.Mizugishi K, Yamashita T, Olivera A, et al. Essential role for sphingosine kinases in neural and vascular development. Mol. Cell. Biol. 2005;25:11113–11121. doi: 10.1128/MCB.25.24.11113-11121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paugh SW, Payne SG, Barbour SE, et al. The immunosuppressant FTY720 is phosphorylated by sphingosine kinase type 2. FEBS Lett. 2003;554:189–193. doi: 10.1016/s0014-5793(03)01168-2. [DOI] [PubMed] [Google Scholar]
- 12.Brinkmann V, Davis MD, Heise CE, et al. The immune modulator, FTY720, targets sphingosine 1-phosphate receptors. J. Biol. Chem. 2002;277:21453–21457. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- 13.Matloubian M, Lo CG, Cinamon G, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. ▪▪ Indicates, together with [42,45], the importance of an S1P gradient and the role that S1P1 plays in lymphocyte egress.
- 14.Allende ML, Sasaki T, Kawai H, et al. Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J. Biol. Chem. 2004;279:52487–52492. doi: 10.1074/jbc.M406512200. [DOI] [PubMed] [Google Scholar]
- 15.Zemann B, Kinzel B, Muller M, et al. Sphingosine kinase type 2 is essential for lymphopenia induced by the immunomodulatory drug FTY720. Blood. 2006;107:1454–1458. doi: 10.1182/blood-2005-07-2628. [DOI] [PubMed] [Google Scholar]
- 16.Olivera A, Rivera J. Sphingolipids and the balancing of immune cell function: lessons from the mast cell. J. Immunol. 2005;174:1153–1158. doi: 10.4049/jimmunol.174.3.1153. [DOI] [PubMed] [Google Scholar]
- 17.Kluk MJ, Hla T. Signaling of sphingosine-1-phosphate via the S1P/EDG-family of G-protein-coupled receptors. Biochim. Biophys. Acta. 2002;1582:72–82. doi: 10.1016/s1388-1981(02)00139-7. [DOI] [PubMed] [Google Scholar]
- 18.Ishii I, Fukushima N, Ye X, et al. Lysophospholipid receptors: signaling and biology. Annu. Rev. Biochem. 2004;73:321–354. doi: 10.1146/annurev.biochem.73.011303.073731. [DOI] [PubMed] [Google Scholar]
- 19.Lee MJ, Van Brocklyn JR, Thangada S, et al. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science. 1998;279:1552–1555. doi: 10.1126/science.279.5356.1552. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Wada R, Yamashita T, et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J. Clin. Invest. 2000;106:951–961. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McVerry BJ, Garcia JG. Endothelial cell barrier regulation by sphingosine 1-phosphate. J. Cell. Biochem. 2004;92:1075–1085. doi: 10.1002/jcb.20088. [DOI] [PubMed] [Google Scholar]
- 22.Singleton PA, Dudek SM, Chiang ET, et al. Regulation of sphingosine 1-phosphate-induced endothelial cytoskeletal rearrangement and barrier enhancement by S1P1 receptor, PI3 kinase, Tiam1/Rac1 and α-actinin. FASEB J. 2005;19:1646–1656. doi: 10.1096/fj.05-3928com. [DOI] [PubMed] [Google Scholar]
- 23.Sanchez T, Skoura A, Wu MT, et al. Induction of vascular permeability by the sphingosine-1-phosphate receptor-2 (S1P2R) and its downstream effectors ROCK and PTEN. Arterioscler. Thromb. Vasc. Biol. 2007;27:1312–1318. doi: 10.1161/ATVBAHA.107.143735. [DOI] [PubMed] [Google Scholar]
- 24.Maclennan AJ, Benner SJ, Andringa A, et al. The S1P2 sphingosine 1-phosphate receptor is essential for auditory and vestibular function. Hear. Res. 2006;220:38–48. doi: 10.1016/j.heares.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 25.Herr DR, Grillet N, Schwander M, et al. Sphingosine 1-phosphate (S1P) signaling is required for maintenance of hair cells mainly via activation of S1P2. J. Neurosci. 2007;27:1474–1478. doi: 10.1523/JNEUROSCI.4245-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kono M, Belyantseva IA, Skoura A, et al. Deafness and stria vascularis defects in S1P2 receptor null mice. J. Biol. Chem. 2007;282:10690–10696. doi: 10.1074/jbc.M700370200. [DOI] [PubMed] [Google Scholar]
- 27.Rivera J, Gilfillan AM. Molecular regulation of mast cell activation. J. Allergy Clin. Immunol. 2006;117:1214–1225. doi: 10.1016/j.jaci.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 28.Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat. Rev. Immunol. 2008;8:478–486. doi: 10.1038/nri2327. ▪ Comprehensive review on the immunomodulatory functions of mast cells.
- 29.Rivera J, Olivera A. Src family kinases and lipid mediators in control of allergic inflammation. Immunol. Rev. 2007;217:255–268. doi: 10.1111/j.1600-065X.2007.00505.x. [DOI] [PubMed] [Google Scholar]
- 30.Olivera A, Mizugishi K, Tikhonova A, et al. The sphingosine kinase-sphingosine-1-phosphate axis is a determinant of mast cell function and anaphylaxis. Immunity. 2007;26:287–297. doi: 10.1016/j.immuni.2007.02.008. ▪▪ Demonstrates the importance of SphK2 in mast cell activation and function, including its role in calcium influx and PKC activation, and reveals that circulating levels of S1P produced by SphK1 are a determinant of anaphylactic responsiveness.
- 31.Oskeritzian CA, Alvarez SE, Hait NC, et al. Distinct roles of sphingosine kinases 1 and 2 in human mast-cell functions. Blood. 2008;111:4193–4200. doi: 10.1182/blood-2007-09-115451. ▪▪ Reveals that differential formation of S1P by SphK1 and SphK2 has distinct and important functions in human mast cells.
- 32.Melendez AJ, Khaw AK. Dichotomy of Ca2+ signals triggered by different phospholipids pathways in antigen stimulation of human mast cells. J. Biol. Chem. 2002;277:17255–17262. doi: 10.1074/jbc.M110944200. [DOI] [PubMed] [Google Scholar]
- 33.Galli SJ, Kalesnikoff J, Grimbaldeston MA, et al. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu. Rev. Immunol. 2005;23:749–786. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- 34.Melendez AJ, Ibrahim FB. Antisense knockdown of sphingosine kinase 1 in human macrophages inhibits C5a receptor-dependent signal transduction, Ca2+ signals, enzyme release, cytokine production, and chemotaxis. J. Immunol. 2004;173:1596–1603. doi: 10.4049/jimmunol.173.3.1596. [DOI] [PubMed] [Google Scholar]
- 35.Zemann B, Urtz N, Reuschel R, et al. Normal neutrophil functions in sphingosine kinase type 1 and 2 knockout mice. Immunol. Lett. 2007;109:56–63. doi: 10.1016/j.imlet.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 36.Oskeritzian CA, Milstien S, Spiegel S. Sphingosine-1-phosphate in allergic responses, asthma and anaphylaxis. Pharmacol. Ther. 2007;115:390–399. doi: 10.1016/j.pharmthera.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ancellin N, Colmont C, Su J, et al. Extracellular export of sphingosine kinase-1 enzyme: sphingosine 1-phosphate generation and the induction of angiogenic vascular maturation. J. Biol. Chem. 2002;277:6667–6675. doi: 10.1074/jbc.M102841200. [DOI] [PubMed] [Google Scholar]
- 38.Venkataraman K, Thangada S, Michaud J, et al. Extracellular export of sphingosine kinase-1a contributes to the vascular S1P gradient. Biochem. J. 2006;397:461–471. doi: 10.1042/BJ20060251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitra P, Oskeritzian CA, Payne SG, et al. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc. Natl Acad. Sci. USA. 2006;103:16394–16399. doi: 10.1073/pnas.0603734103. ▪▪ First demonstration that ABCC1 is important for the secretion of S1P from mast cells.
- 40.Yatomi Y, Igarashi Y, Yang L, et al. Sphingosine 1-phosphate, a bioactive sphingolipid abundantly stored in platelets, is a normal constituent of human plasma and serum. J. Biochem. 1997;121:969–973. doi: 10.1093/oxfordjournals.jbchem.a021681. [DOI] [PubMed] [Google Scholar]
- 41.Berdyshev EV, Gorshkova IA, Garcia JG, et al. Quantitative analysis of sphingoid base-1-phosphates as bisacetylated derivatives by liquid chromatography-tandem mass spectrometry. Anal. Biochem. 2005;339:129–136. doi: 10.1016/j.ab.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 42.Hanel P, Andreani P, Graler MH. Erythrocytes store and release sphingosine 1-phosphate in blood. FASEB J. 2007;21:1202–1209. doi: 10.1096/fj.06-7433com. ▪▪ Indicates, together with [13,45], the importance of an S1P gradient and the role that S1P1 plays in lymphocyte egress.
- 43.Pappu R, Schwab SR, Cornelissen I, et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 44.Venkataraman K, Lee YM, Michaud J, et al. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ. Res. 2008;102:669–676. doi: 10.1161/CIRCRESAHA.107.165845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwab SR, Pereira JP, Matloubian M, et al. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–1739. doi: 10.1126/science.1113640. ▪▪ Indicates, together with [13,42], the importance of an S1P gradient and the role that S1P1 plays in lymphocyte egress.
- 46.Choi OH, Kim J-H, Kinet J-P. Calcium mobilization via sphingosine kinase in signalling by the FcεRI antigen receptor. Nature. 1996;380:634–636. doi: 10.1038/380634a0. [DOI] [PubMed] [Google Scholar]
- 47.Ryu SD, Lee HS, Suk HY, et al. Cross-linking of FcεRI causes Ca2+ mobilization via a sphingosine kinase pathway in a clathrin-dependent manner. Cell Calcium. 2008 doi: 10.1016/j.ceca.2008.07.002. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee HS, Park CS, Lee YM, et al. Antigen-induced Ca2+ mobilization in RBL-2H3 cells: role of I(1,4,5)P3 and S1P and necessity of I(1,4,5)P3 production. Cell Calcium. 2005;38:581–592. doi: 10.1016/j.ceca.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 49.Schwartz LB. Mast cells and basophils. Clin. Allergy Immunol. 2002;16:3–42. [PubMed] [Google Scholar]
- 50.Finkelman FD, Rothenberg ME, Brandt EB, et al. Molecular mechanisms of anaphylaxis: lessons from studies with murine models. J. Allergy Clin. Immunol. 2005;115:449–457. doi: 10.1016/j.jaci.2004.12.1125. ▪ Complete overview of the pathways of systemic anaphylaxis.
- 51.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat. Immunol. 2005;6:135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 52.Kurashima Y, Kunisawa J, Higuchi M, et al. Sphingosine 1-phosphate-mediated trafficking of pathogenic Th2 and mast cells for the control of food allergy. J. Immunol. 2007;179:1577–1585. doi: 10.4049/jimmunol.179.3.1577. [DOI] [PubMed] [Google Scholar]
- 53.Boyce JA. Mast cells and eicosanoid mediators: a system of reciprocal paracrine and autocrine regulation. Immunol. Rev. 2007;217:168–185. doi: 10.1111/j.1600-065X.2007.00512.x. [DOI] [PubMed] [Google Scholar]
- 54.Brightling CE, Bradding P, Symon FA, et al. Mast-cell infiltration of airway smooth muscle in asthma. N. Engl. J. Med. 2002;346:1699–1705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]
- 55.Ammit AJ, Hastie AT, Edsall LC, et al. Sphingosine 1-phosphate modulates human airway smooth muscle cell functions that promote inflammation and airway remodeling in asthma. FASEB J. 2001;15:1212–1214. doi: 10.1096/fj.00-0742fje. ▪▪ Implicates S1P in the pathogenesis of airway inflammation and asthma and identifies it as an effective regulator of airway smooth muscle.
- 56.Rosenfeldt HM, Amrani Y, Watterson KR, et al. Sphingosine-1-phosphate stimulates contraction of human airway smooth muscle cells. FASEB J. 2003;17:1789–1799. doi: 10.1096/fj.02-0836com. [DOI] [PubMed] [Google Scholar]
- 57.Jolly PS, Bektas M, Watterson KR, et al. Expression of SphK1 impairs degranulation and motility of RBL-2H3 mast cells by desensitizing S1P receptors. Blood. 2005;105:4736–4742. doi: 10.1182/blood-2004-12-4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roviezzo F, Di Lorenzo A, Bucci M, et al. Sphingosine-1-phosphate/sphingosine kinase pathway is involved in mouse airway hyper-responsiveness. Am. J. Respir. Cell Mol. Biol. 2007;36:757–762. doi: 10.1165/rcmb.2006-0383OC. ▪ Demonstrates a role for S1P and SphKs in airway hyper-reactivity in an asthma model.
- 59.Roviezzo F, Del Galdo F, Abbate G, et al. Human eosinophil chemotaxis and selective in vivo recruitment by sphingosine 1-phosphate. Proc. Natl Acad. Sci. USA. 2004;101:11170–11175. doi: 10.1073/pnas.0401439101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takabe K, Paugh SW, Milstien S, et al. ‘Inside-out’ signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol. Rev. 2008;60:181–195. doi: 10.1124/pr.107.07113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Payne SG, Oskeritzian CA, Griffiths R, et al. The immunosuppressant drug FTY720 inhibits cytosolic phospholipase A2 independently of sphingosine-1-phosphate receptors. Blood. 2007;109:1077–1085. doi: 10.1182/blood-2006-03-011437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sawicka E, Zuany-Amorim C, Manlius C, et al. Inhibition of Th1- and Th2-mediated airway inflammation by the sphingosine 1-phosphate receptor agonist FTY720. J. Immunol. 2003;171:6206–6214. doi: 10.4049/jimmunol.171.11.6206. [DOI] [PubMed] [Google Scholar]
- 63.Idzko M, Hammad H, van Nimwegen M, et al. Local application of FTY720 to the lung abrogates experimental asthma by altering dendritic cell function. J. Clin. Invest. 2006;116:2935–2944. doi: 10.1172/JCI28295. ▪▪ Demonstrates that FTY720 suppresses airway inflammation and bronchial hyper-responsiveness in a murine asthma model.
- 64.Vlasenko LP, Melendez AJ. A critical role for sphingosine kinase in anaphylatoxin-induced neutropenia, peritonitis, and cytokine production in vivo. J. Immunol. 2005;174:6456–6461. doi: 10.4049/jimmunol.174.10.6456. [DOI] [PubMed] [Google Scholar]
- 65.Lai WQ, Goh HH, Bao Z, et al. The role of sphingosine kinase in a murine model of allergic asthma. J. Immunol. 2008;180:4323–4329. doi: 10.4049/jimmunol.180.6.4323. ▪ Demonstrates that a pan SphK inhibitor reduces inflammation, bronchial alveolar lavage fluid, mucus production in the lungs and airway hyper-responsiveness in a murine model of allergic asthma.
- 66.Nishiuma T, Nishimura Y, Okada T, et al. Inhalation of sphingosine kinase inhibitor attenuates airway inflammation in asthmatic mouse model. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008;294:L1085–L1093. doi: 10.1152/ajplung.00445.2007. [DOI] [PubMed] [Google Scholar]
- 67.Wu W, Mosteller RD, Broek D. Sphingosine kinase protects lipopolysaccharide-activated macrophages from apoptosis. Mol. Cell. Biol. 2004;24:7359–7369. doi: 10.1128/MCB.24.17.7359-7369.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Melendez A, Floto RA, Gillooly DJ, et al. FcεRI coupling to phospholipase D initiates sphingosine kinase-mediated calcium mobilization and vesicular trafficking. J. Biol. Chem. 1998;273:9393–9402. doi: 10.1074/jbc.273.16.9393. [DOI] [PubMed] [Google Scholar]
- 69.Ryu J, Kim HJ, Chang EJ, et al. Sphingosine 1-phosphate as a regulator of osteoclast differentiation and osteoclast–osteoblast coupling. EMBO J. 2006;25:5840–5851. doi: 10.1038/sj.emboj.7601430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weigert A, Tzieply N, von Knethen A, et al. Tumor cell apoptosis polarizes macrophages: role of sphingosine-1-phosphate. Mol. Biol. Cell. 2007;8:3810–3819. doi: 10.1091/mbc.E06-12-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pushparaj PN, H’Ng SC, Melendez AJ. Refining siRNA in vivo transfection: silencing SPHK1 reveals its key role in C5a-induced inflammation in vivo. Int. J. Biochem. Cell Biol. 2008;40:1817–1825. doi: 10.1016/j.biocel.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 72.Tan SY, Xiao L, Pi X, et al. Aberrant Gi protein coupled receptor-mediated cell survival signaling in rheumatoid arthritis B cell lines. Front. Biosci. 2007;12:1651–1660. doi: 10.2741/2177. [DOI] [PubMed] [Google Scholar]
- 73.Samy ET, Meyer CA, Caplazi P, et al. Cutting edge: modulation of intestinal autoimmunity and IL-2 signaling by sphingosine kinase 2 independent of sphingosine 1-phosphate. J. Immunol. 2007;179:5644–5648. doi: 10.4049/jimmunol.179.9.5644. [DOI] [PubMed] [Google Scholar]
- 74.Yang J, Castle BE, Hanidu A, et al. Sphingosine kinase 1 is a negative regulator of CD4+ Th1 cells. J. Immunol. 2005;175:6580–6588. doi: 10.4049/jimmunol.175.10.6580. [DOI] [PubMed] [Google Scholar]
- 75.Yoshimoto T, Furuhata M, Kamiya S, et al. Positive modulation of IL-12 signaling by sphingosine kinase 2 associating with the IL-12 receptor 0β1 cytoplasmic region. J. Immunol. 2003;171:1352–1359. doi: 10.4049/jimmunol.171.3.1352. [DOI] [PubMed] [Google Scholar]
- 76.Ibrahim FB, Pang SJ, Melendez AJ. Anaphylatoxin signaling in human neutrophils. A key role for sphingosine kinase. J. Biol. Chem. 2004;279:44802–44811. doi: 10.1074/jbc.M403977200. [DOI] [PubMed] [Google Scholar]