Summary
The pathways for air and food cross in the pharynx. In breathing, air may flow through either the nose or the mouth, it always flows through the pharynx. During swallowing, the pharynx changes from an airway to a food channel. The pharynx is isolated from the nasal cavity and lower airway by velopharyngeal and laryngeal closure during the pharyngeal swallow. During mastication, the food bolus accumulates in the pharynx prior to swallow initiation. The structures in the oral cavity, pharynx and larynx serve multiple functions in breathing, speaking, mastication and swallowing. Thus, the fine temporal coordination of feeding among breathing, mastication and swallowing is essential to provide proper food nutrition and to prevent pulmonary aspiration. This review paper will review the temporo-spatial coordination of the movements of oral, pharyngeal, and laryngeal structures during mastication and swallowing, and temporal coordination between breathing, mastication, and swallowing.
Keywords: Eating, Mastication, Swallowing, Respiration, Pharynx, Process model of feeding
1 Introduction
The pathways for food and air cross in the pharynx. In breathing, air may flow through either the nose or the mouth, it always flows through the pharynx. When we swallow, the pharynx becomes a passage for food. This passage is separated from the lower airway and nasal cavity during the pharyngeal swallow to prevent aspiration of foreign materials into the trachea before or during swallowing. When eating solid food, mastication occurs in the oral cavity respiration continues through the nasal cavity and pharynx. The two functions, mastication and breathing, appear to occur in different areas. In reality, however, food is collected in the pharynx during eating [1, 2]. Chewed food is gradually transported through the fauces and is collected in the oropharynx or valleculae, where the bolus is formed prior to swallowing. Bolus transport and positioning prior to swallow initiation vary depending on physical characteristics of the food [3, 4]. While the bolus is collecting in the pharynx, breathing continues in the same anatomical space. When the swallow is initiated, the collection of food in the pharynx is combined with the transported food bolus from the mouth, and propelled through the pharynx to the esophagus. Because feeding and breathing share the same anatomical space, the fine temporal coordination of feeding and breathing is essential to provide proper nutrition and to prevent pulmonary aspiration and its sequelae. The aim of this review is to describe current understandings of the temporo-spatial coordination of the movements of the structures of the oral cavity, larynx, and pharynx during mastication and swallowing, and define the coordination of breathing and eating. This review does not address the neural control or coordination of eating and breathing or animal models of feeding. Several other review articles are available. [5, 6]
2 Anatomy
Feeding and breathing share the same anatomy. The pharynx is a route for breathing and feeding (mastication and swallowing) but is used in different ways. The pharyngeal cavity consists of the muscles of the soft palate, tongue, epiglottis, and pharyngeal walls, and its shape is altered dynamically for breathing, eating or vocalization. The pharynx is dilated to maintain airway patency for breathing, but is constricted to push the food bolus down to the esophagus for swallowing.
2.1. Pharynx for breathing
The muscle activities of the soft palate, tongue and pharyngeal wall are critical to maintaining pharyngeal airway patency for proper ventilation and gas exchange [7–15]. Pharyngeal muscles counteract negative transluminal air pressure that tends to collapse the airway during inspiration. In obstructive sleep apnea, the activities of pharyngeal muscle activities are suppressed during sleep, allowing the phaynrgeal airway to collapse during inspiration. There are extensive studies investigating control mechanisms of pharyngeal muscles and their role in the pathogenesis of breathing disorders [15, 16].
The genioglossus, which is the main muscle for tongue protrusion, has a critical role in controlling pharyngeal airway patency. It is tonically active to maintain tongue position, and is phasically active to counteract intraluminal negative pressure during inspiration [11]. Therefore, the majority of the studies related to obstructive sleep apnea have focused on motor control of the genioglossus in animals or humans [15, 16]. Several studies have also reported muscle activities of the other extrinsic tongue (hyoglossus or styloglossus) muscles or the intrinsic tongue muscles [12, 13].
The soft palate has an important role in determining the route of respiration [9]. During nasal breathing, the soft palate is lowered and apposed to the tongue, dilating the velopharyngeal isthmus (retro-palatal airway) [17]. During oral breathing, in contrast, the soft palate elevates to open the fauces, separating the nasal cavity from the pharyngeal airway [18]. Complex activities of several palatal muscles determine the position of the palate for the route of respiration. The two main muscles for determining palatal position are the levator veli palatini and the palatoglossus. Both muscles are active during oral and nasal breathing. However, the levator palatini is more active during oral breathing and the palatoglossus during nasal breathing [19].
2.2. Pharynx for mastication and swallowing
Food processing occurs in the oral cavity and the chewed food bolus is transported to the oropharyngeal surface of the tongue and the valleculae during mastication. Thus, the pharynx becomes the space for bolus aggregation prior to the pharyngeal swallow. Pharyngeal bolus aggregation continues for a few seconds in healthy individuals eating solid food while breathing continues [4]. During this period of bolus aggregation, the pharyngeal space is shared by feeding and breathing.
During swallowing, the pharynx is used only for the food passage and is completely separated from the airway in healthy individuals. Velopharyngeal closure separates the nasal cavity from the pharynx, and laryngeal closure, including glottal closure, arytenoid adduction, and epiglottal folding, seals the lower airway. The backward movement of the tongue and gradual progressive contraction of the pharyngeal wall contraction (starting at the level of the soft palate and proceeding downward) squeeze the food bolus into the esophagus through the upper esophageal sphincter.
3 A sequence from Mastication to Swallowing
3.1. Process model of feeding
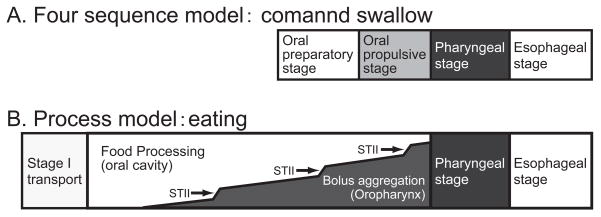
The normal swallow in humans is previously described with a four-stage sequential model. In this model, the swallowing process was depicted as having oral preparatory, oral propulsive, pharyngeal, and esophageal stages based primarily on the location of the bolus in the food pathway [20, 21] (Fig. 1). According to this model, the four stages progress sequentially, with minimal temporal overlap among stages. The swallowing process may be appropriately described as a five-stage process adding pre-oral (anticipatory) stage in case of considering clinical diagnosis and treatment of dysphagia [22]. Pre-oral stage accounts for pre-oral motor, cognitive, psychosocial and somataesthetic factors. These factors have significant influence on the following stages, and impairment of the pre-oral stage has significant influence on oral or oropharyngeal dysphagia.
Fig. 1.
Four sequential model and process model are illustrated in diagrams showing progession from left to right, and aligning the common elements of the two models. A) In the conventional sequential model, the four stages have minimum overlap so that oral propulsive stage starts after oral preparatory stage is completed. B) In the Process Model, food processing (in the oral cavity) and bolus aggregation (in the pharynx) can occur at the same time. After food is ingested into the mouth, it is carried to the post-canine teeth for mastication (stage I transport). The food is reduced in size by chewing and mixed with saliva until it is ready to swallow (Food Processing). A portion of the chewed food is propelled into the oropharynx (stage II transport, ST II), where the bolus gradually accumulates while food processing continues in the mouth. Subsequent stage II transport cycles bring additional food to the oropharynx, and the bolus gradually accumulates there. Arrows indicate stage II transport cycles. Pharyngeal and esophageal stages have essentially the same mechanisms in the two models.
Mastication was regarded as a part of oral preparatory stage in the four-stage sequential model. During the oral preparatory stage, the food was thought to be retained in the oral cavity by closure of the lips anteriorly and the faucial isthmus posteriorly. Firm contact between the soft palate and the posterior surface of the tongue was believed necessary to prevent premature leakage of the food into the pharynx and prevent aspiration prior to the swallow [21].
The Process Model of Feeding provides an alternative description for mastication and swallowing in which triturated food is propelled through the fauces for bolus formation in the oropharynx (including the valleculae) during chewing. The Process Model has its origin in studies of mammalian feeding [6, 23–27] and was later adapted to feeding in humans [1]. When eating solid food, the food is first transported to the occlusal surfaces of the postcanine dentition (stage I transport, Fig. 1). Then, the food is reduced in size and lubricated with saliva (food processing). Triturated food is transported to the oropharynx (stage II transport) and collects there (bolus aggregation) until swallow onset. Food processing continues during stage II transport and bolus aggregation in the oropharynx. Thus, the oral preparatory phase (food processing) and oral propulsive phase (stage II transport and bolus aggregation) are can overlap in time (Fig. 1). This is a significant difference of the process model from the conventional model of swallowing.
After ingestion and stage I transport, the food is processed until it is ready for swallowing. During processing, food particles are reduced in size and softened by mastication and mixed with saliva to optimize bolus consistency for swallowing. Cyclic jaw movement is temporo-spatially coordinated with the movements of the tongue, cheek, and soft palate during food processing [28–31].
With aging, there is an increase in the number of chewing cycles and the myoelectric activity of the jaw adductor musculature during mastication [32, 33]. These factors result in increased mastication time. But despite the increase in mastication time, bolus size at the time of swallow onset is larger in the elderly than in the young, primarily because of the decrease in masticatory function.
Tongue movement is temporally linked with cyclic jaw movement in food processing [28]. During chewing, the tongue (pushing laterally) and the cheek (pushing medially) reposition food on the occlusal surfaces before each closing stroke of the teeth [29]. The tongue moves anteroposteriorly, mediolaterally, and rotates on its three axes during chewing. Those tongue movements can carry all or part of the food piece to the opposite side of the mouth during food processing, thus reversing the working and balancing sides. Bilateral chewing is also seen frequently in healthy adults [29].
The soft palate moves cyclically during feeding, and this movement is temporally (but not mechanically) linked to jaw movement [30] (Fig. 2). The soft palate always elevates during pharyngeal swallowing, and often elevates during processing and stage II transport. However, the temporal relationships between jaw and soft palate movements during food processing are totally different from the relationships during swallowing [34]. During processing, the soft palate moves upward as the jaw opens and downward as the jaw closes (Fig. 3). During swallowing, however, the soft palate elevates after the jaw is closed and (for swallows intercalated during chewing) descends during jaw opening (Fig. 3). The prevalence of palatal movement varies greatly among individuals but the timing of palatal movement relative to jaw movement is highly consistent [30]. Rhythmic movements of the soft palate and tongue open the fauces during food processing to provide open communication between the oral cavity and pharynx.
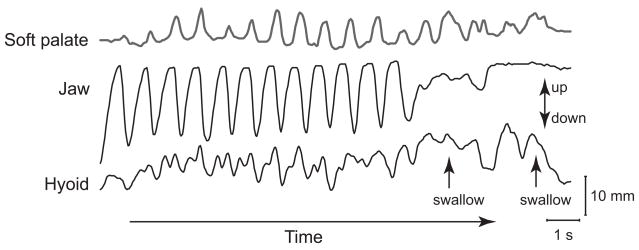
Fig. 2.
Vertical movement of the jaw, hyoid bone, and soft palate over time while eating a piece of banana. This is an actual recording of a healthy adult volunteer. Motions were measured with videofluorography and an image analysis system. Movement towards the top of the figure is upwards. Rhythmic movements of the soft palate and hyoid bone are temporally associated with cyclic jaw movement. (Source: Matsuo et al. Jpn J Dysphagia Rehabil 2008;12(1):20–30 [34]).
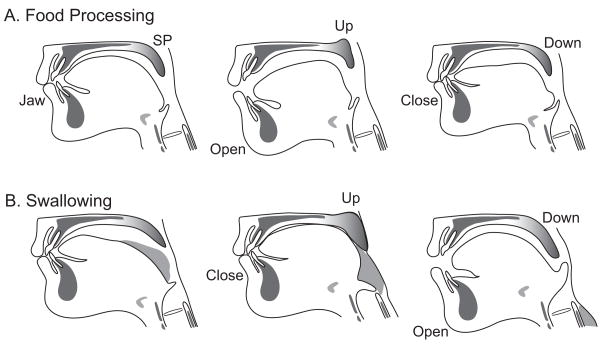
Fig. 3.
Food processing and swallowing: drawings based on a videofluorographic recording. The temporal relationships between the soft palate and jaw are strikingly different between the two behaviors. A) Food Processing. The soft palate moves upward as the jaw opens, and moves downward as the jaw closes B) Swallowing. The soft palate elevates after the jaw has closed, and moves downward as the jaw opens. (Source: Matsuo et al., J Dent Res 2005 Jan;84(1):39–42 [30]).
When a portion of the food is suitable for swallowing, the triturated food is segregated from the particles on the occlusal surface and gathered on the dorsal surface of the tongue. The food is propelled back through the fauces to the oropharynx by the tongue squeezing it back along the palate. This propulsion of the food is called stage II transport [4, 35]. Stage II transport is primarily driven by the tongue, and does not require gravity, though it is assisted by gravity in the upright position [3, 36]. During stage II transport cycles, the tongue squeezes the food bolus back along the palate during jaw opening, and the palate elevates briefly after that squeeze-back action [30](Fig. 4).
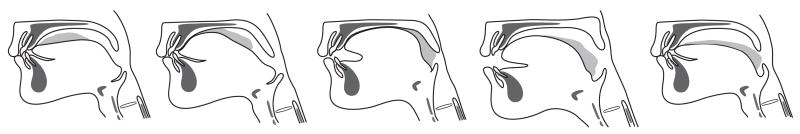
Fig. 4.
Stage II transport: Drawings based on a videofluorographic recording. The anterior tongue surface first contacts the hard palate just behind the upper incisors. The area of tongue-palate contact gradually expands backward, squeezing the triturated food to the oropharynx (Squeeze-back mechanism). The food bolus is positioned on the oropharyngeal surface of the tongue where it will accumulate during subsequent stage II transport cycles.
Stage II transport occurs intermittently during food processing [30] but the frequency of stage II transport cycles increases toward the swallow. The transported food accumulates on the oropharyngeal surface of the tongue and the valleculae until swallow initiation. Chewing continues, and the bolus in the oropharynx is increased incrementally by subsequent stage II transport cycles. The duration of bolus aggregation in the oropharynx ranges from a fraction of a second to about ten seconds, and has substantial inter-individual variation [4].
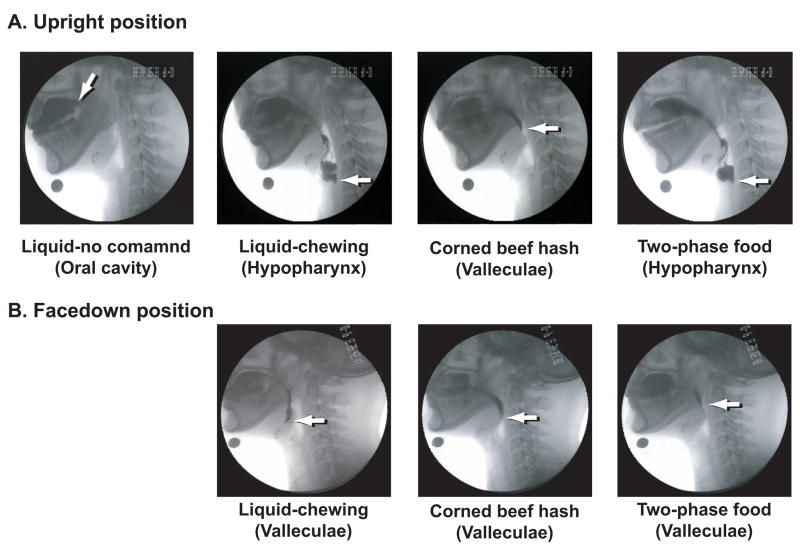
The relationships among food transport, bolus aggregation, and swallow initiation are modified by the physical characteristics of the food and by coupling these activities with mastication [2–4]. When drinking liquid, the bolus is commonly held in the oral cavity until just before the initiation of the pharyngeal swallow; this is believed to be important for preventing aspiration of the liquid. When eating solid food, however, food is propelled to the oropharynx where it accumulates and bolus is formed prior to swallowing. Saitoh et al. [3] studied normal subjects eating a complex food containing both liquid and solid phases. They reported that the leading edge (liquid component) of the food often reached the hypopharynx before swallow initiation (Fig. 5). Gravity had a significant impact on the position of the leading edge of the food at swallow initiation with these two-phase foods. When eating in a facedown posture (upper body and head parallel to the ground), the leading edge of the food never entered the hypopharynx before swallow initiation.
Fig. 5.
Example of VFG images showing the oral cavity and pharynx at the moment of swallow initiation with various food consistencies in (A) upright and (B) facedown positions. Images in (B) are rotated counter-clockwise by 90 degrees to simplify comparisons. Arrows point to the leading edge of the barium in each image. (A) In upright position with liquid, the leading edge is normally in the oral cavity at swallow onset without chewing, but moves to the hypopharynx before swallowing when the subject is instructed to chew and then swallow. With a complex two-phase food including both soft solid and liquid phases, the leading edge is in the hypopharynx at swallow onset. (B) With facedown position, for each foods, the leading edge was in the valleculae at swallow onset but did not enter the hypopharynx. This suggests that propulsion to the oropharynx and to the hypopharynx had different mechanisms, the latter dependent on gravity (Source: Saitoh et al., Dysphagia 2007 Apr;22(2):100–7 [3]).
4 Coordination between Breathing and Swallowing
4.1. Airway protective mechanism during swallowing
Safe bolus passage through the pharynx without tracheal aspiration is critical to human swallowing. Thus, swallowing has two essential physiologic aspects: (1) passage of food from the oral cavity to the stomach and (2) airway protection to prevent contamination of the trachea, bronchi, and lungs. There are several airway protective mechanisms preventing aspiration of foreign materials into the trachea before, during, and after swallowing.
There are three gate keepers to prevent tracheopulmonary aspiration: the epiglottis, arytenoids, and vocal folds. There is fine temporal coordination among these structures, resulting in laryngeal closure that starts at the bottom and progresses upward. First in sequence is adduction of the true vocal folds. This closes the glottis (space between the vocal folds). Next is closure of the laryngeal vestibule. The false vocal folds adduct and the arytenoid cartilages adduct and tilt forward to contact the base of the epiglottis prior to opening of the UES. [37, 38] The epiglottis is the most superficial shield for lower airway protection. The mechanism of posterior epiglottic tilting in human swallowing remains unclear, but is probably related to hyo-laryngeal elevation, pharyngeal constriction, bolus movement, and tongue base retraction [39]. During swallowing, the hyoid bone and larynx are pulled upward and forward by contraction of the suprahyoid muscles and thyrohyoid muscle. This displacement tucks the larynx under the base of the tongue, helping the epiglottic tilting backward to seal the laryngeal vestibule. (Fig. 6).
Fig. 6.
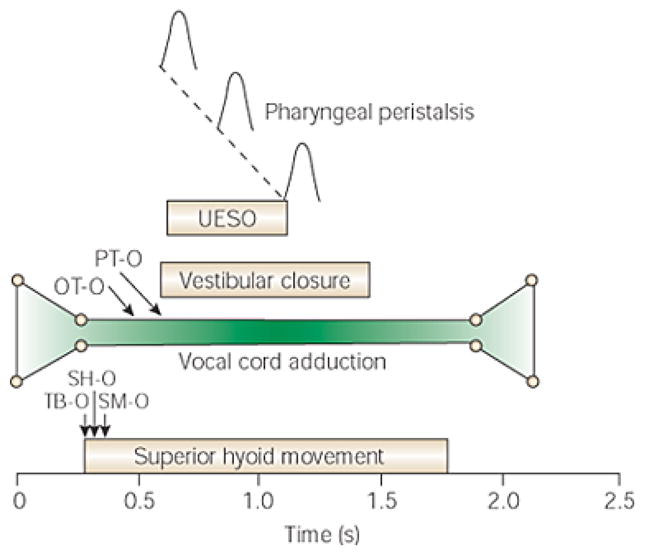
Average temporal relationships of vocal cord adduction to other critical events during 5-mL barium swallows. Bolus transit through the pharynx and across the upper esophageal sphincter (UES) begins and ends while the vocal cords are fully adducted. TB-O, onset of tongue base movement; SH-O, onset of superior hyoid movement; SM-O, onset of submental myoelectrical activity; UESO, UES opening; OT-O, onset of bolus movement from the mouth; PT-O, arrival of bolus into pharynx. (Source: Shaker et al. Gastroenterology 1990;98(6):1478–84 [37])
The vocal folds closure reflex occurs not only in swallowing but also in belching and esophageal distention [40, 41]. During belching, the vocal folds close and the arytenoids adduct before the UES opening [40]. These reflexes may also play an important role in preventing aspiration of the materials regurgitated to the pharynx after gastro-esophageal reflux.
4.2. Swallowing and respiratory phase
Swallowing and respiration have tight temporal coordination in adult humans. A number of studies, using various measurement systems and different food consistencies, confirm that swallowing usually occurs during expiration. Martin-Harris [42] presented a excellent summary table in her online review of studies on temporal coordination of breathing and swallowing in adult humans. In those studies, respiratory inductive plethysmography and/or nasal air pressure manometry was used to monitor respiratory phase (inspiration vs. expiration). Physiologic events during swallowing were analyzed with indirect (submental EMG or swallow sounds), or direct visualization (videofluorography or videoendoscopy).
In adult humans, swallowing usually starts during the expiratory phase of breathing, and respiration resumes with continued expiration after swallowing. Many studies investigate the temporal coordination between breathing and swallowing of a liquid bolus. The predominant respiration-swallowing pattern in adult humans is the “exhale – swallow – exhale ” pattern (67 –79%), followed by “inhale – swallow – exhale” pattern (18 – 21%) [43–45]. The “exhale – swallow –inhale” and “inhale – swallow – inhale” patterns occur rarely in healthy adults. The resumption with expiration is regarded as an airway protective mechanism as it can prevent inhalation of residual material left in thwe pharynx after swallowing [46].
The swallow respiratory temporal coordination varies with the conditions of swallowing, including method of ingestion, body position, and food consistency. When performing sequential swallows while drinking from a cup, respiration can resume in inspiration [47]. When drinking liquids while positioned down on hands and knees, swallow onset is more likely to occur during early expiration while swallows in upright position tend to occur late in the expiratory phase [48]. With eating solid food, temporal coordination persists with the “exhale – swallow – exhale” relationships [49–51].
Swallow-respiratory coordination varies across the human lifespan. The temporal coordination in infants is different from that in adults. In infants, the temporal pattern of respiratory phase and swallowing is more variable, and swallows are more likely to occur during inspiration [52–54]. This may be due to anatomical differences between infants and adults and/or maturation of the neural control mechanisms. In infants, the larynx is in positioned higher in the neck, posterior to the oral cavity. It is opened to the nasopharynx, sealed off from the food pathway (between swallows) by contact between the soft palate and epiglottis. The larynx descends in the neck during infancy [55], and the temporal coordination between breathing and swallowing shifts to “exhale – swallow –exhale” relationship [56–58].
Swallow-respiratory coordination is altered in the elderly as well. Respiratory patterns are characterized by increased incidence of inspiration both before and after the swallow [46, 59–62]. Swallow-respiratory coordination is further altered by disease. Swallows occur more frequently during inspiration in individuals with cerebrovascular disease, Parkinson’s disease and other neurological diseases [60, 63–66]. This occurrence of swallowing during inspiration could be a causal factor in the high incidence of aspiration pneumonia in these diseases [67, 68].
4.3. Pause in breathing during swallowing
Breathing ceases briefly during swallowing. The pause in breathing is due to inhibition of respiration at neural control centers in the brainstem, and not simply due to closure of the upper airway [49, 69, 70]. Indeed, inhibition of breathing during swallowing persists after endotracheal intubation or laryngectomy (in which the airway and foodway are separated anatomically [70, 71]. The onset of the pause in breathing for swallowing is approximately synchronous initiation of bolus propulsion in the oral cavity during a swallow of a single liquid bolus [59]. The duration of repiratory pause is 0.5 to 1.5 s in healthy adults [43, 45, 69]. The effect of bolus volume on duration of respiratory pause is still controversial. Some studies report an increase in duration with bolus volume increase [61, 72]. Hiss et al. suggested that this prolongation is due to earlier onset of swallowing apnea with larger volume [72]. The other studies reported no difference in swallowing apnea duration among different bolus volumes [73, 74]. The duration of the pause in breathing for swallowing is prolonged with aging [59].
When eating solid foods, the timing and duration of the pause in breathing are highly variable [49, 50, 75, 76]. The pause in breathing often begins substantially before the swallow, and the swallow occurs during these extended pauses [49, 50]. This increases respiratory cycle duration substantially [50, 51, 73]. Matsuo et al. reported that the prolongation of the respiratory cycle duration exceeds the duration of the swallow itself [50]. They suggested that this prolongation was due, in part, to the period of pre-swallow bolus aggregation in the pharynx, however, it could begin during food processing, before the onset of stage II transport.
5 Coordination between Breathing and Mastication
It was long believed that the oral cavity is separated from the pharynx during food processing by posterior tongue-palate contact that closes the fauces until the food is ready for swallowing. More recent studies reveal that the fauces are typically open during food processing and triturated food is propelled to the oropharynx (stage II transport) well before swallow initiation. These studies indicate that the food accumulating in the oropharynx is located in the path of respiratory airflow during mastication. Respiration and mastication must be controlled so as to prevent aspiration during this period of bolus aggregation given the overlapping anatomy of airway and foodway.
5.1. Change of respiratory cycle duration
Eating solid food alters the respiratory rhythm. The rhythm is perturbed with onset of mastication. Respiratory frequency increases during mastication, but decreases with swallowing [49, 50, 77] (Fig. 7). Fontana et al. reported that total airway resistance increases during mastication, suggesting a reduction in upper airway patency during mastication due to the activities of the masticatory muscles. Prolonged periods of respiratory pause associated with feeding are reported in normal individuals [49, 50, 75] and several laryngectomized individuals [76]. Those results may represent an exaggerated physiological response to the presence of food in the oral cavity and pharynx.
Fig. 7.
Respiratory cycle total duration (TRC) and duration of inspiratory (TI) and expiratory (TE) phases, by respiratory cycle type, defined as: pre-feeding cycles (Pre-F), feeding cycles with no swallow (F-noSW), swallow cycles (F-SW) and post-feeding cycles (Post-F). The number of cycles in each group is shown in parentheses. Asterisks indicate statistically significant differences (P < 0.001). TE and TRC durations were shortest in feeding cycles with no swallow and longest in swallow cycles. TI duration was longer for pre- and post-feeding cycles than for feeding or swallowing cycles. (Source: Matsuo et al., J Appl Physiol 2008;104(3):674–81 [50]).
5.2. Air movement during mastication
During food processing in the mouth, cyclical movements of the soft palate and tongue moves are temporally linked with masticatory jaw movement [28, 30, 34]. This opens the fauces during food processing, and provides a route for communication between the oral cavity and nasal cavity through the pharynx. While eating of solid food, breathing is through the nose, not the mouth, since the oral cavity is used for food processing and the lips are sealed to prevent the food from escaping anteriorly [50]. Nasal air manometry captures the changes in air pressure associated with breathing. It also demonstrates oscillations in nasal air pressure associated with masticatory jaw movement [75, 78, 79]: Nasal air pressure is positive during jaw closing and negative (relative to atmospheric pressure) during jaw opening. Finding from these studies suggest that jaw closing pumps the air from the mouth to the nasal cavity, through the pharynx. Air movement associated with masticatory jaw movement may also deliver aroma of the chewed food to nasal chemoreceptors [79].
5.3. Airway protection during bolus formation in the pharynx
Triturated food is aggregated in the oropharynx for a period of time before swallow, while the larynx is open. The presence of the food in the pharynx can present a risk for pre-swallow aspiration, if respiration continues and the airway remains open. Indeed, air can be inhaled through the pharyngeal airway while food is but a few millimeters from the laryngeal aditus. The mechanisms which prevent aspiration during the period of bolus aggregation in the oropharynx are not well understood. Palmer and his colleagues have investigated the relationship between the location and timing of pharyngeal bolus aggregation and breathing pattern [50, 75]. It is well known that there is a pause in breathing during the pharyngeal swallow, as discussed above. A preliminary investigation by Palmer and Hiiemae [75], using nasal air pressure manometry, suggested that the period of respiratory inhibition may be longer than the swallow itself, to incorporate the pre-swallow period of bolus aggregation and that respiration is inhibited during bolus aggregation. They conducted further investigation of breathing patterns during the mastication, aggregation and swallowing, using both nasal manometry and respiratory plethysmography [50]. Matsuo et al., found that there can be inspiration, expiration or a pause in breathing during bolus aggregation, and in some cases there are frequently multiple respiratory cycles during bolus aggregation. These findings suggested that the presence of the food in the valleculae did not directly influence control of respiration.
Several mechanisms for airway protection during bolus aggregation have been proposed [2, 80]. Dua et al. [2] reported that the vocal folds close briefly when the bolus is propelled to the pharynx. They suggested that this brief adduction of the vocal folds may help to prevent aspiration before swallowing. Another proposal is the optimization model for mastication and swallowing by Prinz and Lucas [80]. They hypothesize that there is an optimal cohesiveness of the food bolus for swallowing that depends on the size of particles and the quantity of saliva. During food processing, food particles are reduced in size by mastication and mixed with saliva. Salivation lubricates the food particles to provide optimized cohesiveness for bolus formation in the pharynx. Accordingly, they suggested that the resulting bolus has optimized particle size and cohesiveness for swallowing. This enables the bolus to remain stick together rather than falling apart during bolus aggregation and swallowing. They also suggested that if swallowing is delayed, excessive saliva can flood the bolus, separating particles and reducing cohesion.
Palmer and his colleagues revealed that stage II transport may be inhibited volitionally during feeding [81]. When healthy subjects are instructed to chew food and hold it in the mouth until they think it is ready for swallowing, stage II transport is delayed, and transport to the valleculae inhibited. Volition alters swallow initiation in both timing of swallow onset and positioning of the food bolus relative to the airway. If the pre-swallow bolus entry in the pharynx increases risk of aspiration, the volitional control of bolus transport could be a useful dysphagia rehabilitation technique to inhibit premature bolus transport to the pharynx.
6 Directions for Future Research
The soft palate and tongue serve multiple functions in feeding and breathing. During mastication and swallowing, movements of the tongue and soft palate are temporally coordinated with jaw movement. During food processing, the tongue and soft palate move cyclically in association with masticatory jaw movement. During stage II transport, the tongue and the palate squeezes the food bolus to the oropharynx. During pharyngeal swallow, the soft palate elevates and the tongue retracts, closing the pharyngeal space. The tongue and soft palate also play an important role in controlling upper airway patency. During nasal breathing, the soft palate is lowered and the tongue base pulled forward to dilate the velopharyngeal isthmus [17]. These motions needed to dilate the phaynx during nasal breathing can conflict with those needed for mastication and food transport during eating. Large gaps remain in our understanding of the neural control of the soft palate and tongue related to breathing and mastication, and in the mechanisms for preventing aspiration during mastication and bolus aggregation.
Delayed swallow initiation or premature leakage of the food into the foodway before swallowing is regarded as a frequent characteristic of abnormal swallowing [21, 82, 83]. However, delayed swallow initiation is not simply defined, since pre-swallow bolus entry into the pharynx occurs in healthy individuals drinking liquids [84–86] or eating solid food [1–3]. The presence of the food in the pharynx before swallowing is not necessarily abnormal, but it can increase risk of aspiration in dysphagic individuals with poor airway protection. Further investigations are needed to determine the significance of food remaining in the pharynx for an extended period before swallowing in individuals with dysphagia.
Acknowledgments
The late Dr. Karen Hiiemae contributed immeasurably to this work. We could not have done this work without her support and instruction. Supported in part by USPHS Award R01 DC02123 from the National Institute on Deafness and other Communication Disorders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Palmer JB, Rudin NJ, Lara G, Crompton AW. Coordination of mastication and swallowing. Dysphagia. 1992;7(4):187–200. doi: 10.1007/BF02493469. [DOI] [PubMed] [Google Scholar]
- 2.Dua KS, Ren J, Bardan E, Xie P, Shaker R. Coordination of deglutitive glottal function and pharyngeal bolus transit during normal eating. Gastroenterology. 1997;112(1):73–83. doi: 10.1016/s0016-5085(97)70221-x. [DOI] [PubMed] [Google Scholar]
- 3.Saitoh E, Shibata S, Matsuo K, Baba M, Fujii W, Palmer JB. Chewing and food consistency: effects on bolus transport and swallow initiation. Dysphagia. 2007 Apr;22(2):100–7. doi: 10.1007/s00455-006-9060-5. [DOI] [PubMed] [Google Scholar]
- 4.Hiiemae KM, Palmer JB. Food transport and bolus formation during complete feeding sequences on foods of different initial consistency. Dysphagia. 1999;14(1):31–42. doi: 10.1007/PL00009582. [DOI] [PubMed] [Google Scholar]
- 5.Miller AJ. Oral and pharyngeal reflexes in the mammalian nervous system: their diverse range in complexity and the pivotal role of the tongue. Crit Rev Oral Biol Med. 2002;13(5):409–25. doi: 10.1177/154411130201300505. [DOI] [PubMed] [Google Scholar]
- 6.Hiiemae KM. Feeding in Mammals. In: Schwenk K, editor. Feeding: Form, Function, and Evolution in Tetrapod Vertebrates. 1. San Diego, CA: Academic Press; 2000. pp. 411–48. [Google Scholar]
- 7.Launois SH, Remsburg S, Yang WJ, Weiss JW. Relationship between velopharyngeal dimensions and palatal EMG during progressive hypercapnia. J Appl Physiol. 1996 Feb;80(2):478–85. doi: 10.1152/jappl.1996.80.2.478. [DOI] [PubMed] [Google Scholar]
- 8.Olson LG, Fouke JM, Hoekje PL, Strohl KP. A Biomechanical view of upper airway function. In: Mathew OP, Sant’Ambrogio G, editors. Respiratory Function of the Upper Airway. New York: Dekker; 1988. pp. 359–90. [Google Scholar]
- 9.Rodenstein DO, Stanescu DC. The soft palate and breathing. Am Rev Respir Dis. 1986 Aug;134(2):311–25. doi: 10.1164/arrd.1986.134.2.311. [DOI] [PubMed] [Google Scholar]
- 10.Mortimore IL, Mathur R, Douglas NJ. Effect of posture, route of respiration, and negative pressure on palatal muscle activity in humans. J Appl Physiol. 1995 Aug;79(2):448–54. doi: 10.1152/jappl.1995.79.2.448. [DOI] [PubMed] [Google Scholar]
- 11.Tsuiki S, Ono T, Ishiwata Y, Kuroda T. Functional divergence of human genioglossus motor units with respiratory-related activity. Eur Respir J. 2000 May;15(5):906–10. doi: 10.1034/j.1399-3003.2000.15e16.x. [DOI] [PubMed] [Google Scholar]
- 12.Bailey EF, Fregosi RF. Coordination of intrinsic and extrinsic tongue muscles during spontaneous breathing in the rat. J Appl Physiol. 2004 Feb;96(2):440–9. doi: 10.1152/japplphysiol.00733.2003. [DOI] [PubMed] [Google Scholar]
- 13.Bailey EF, Jones CL, Reeder JC, Fuller DD, Fregosi RF. Effect of pulmonary stretch receptor feedback and CO(2) on upper airway and respiratory pump muscle activity in the rat. The Journal of physiology. 2001 Apr 15;532(Pt 2):525–34. doi: 10.1111/j.1469-7793.2001.0525f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kokawa T, Saigusa H, Aino I, Matsuoka C, Nakamura T, Tanuma K, et al. Physiological studies of retrusive movements of the human tongue. J Voice. 2006 Sep;20(3):414–22. doi: 10.1016/j.jvoice.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Bailey EF, Fregosi RF. Modulation of upper airway muscle activities by bronchopulmonary afferents. J Appl Physiol. 2006 Aug;101(2):609–17. doi: 10.1152/japplphysiol.00204.2006. [DOI] [PubMed] [Google Scholar]
- 16.Horner RL. Respiratory motor activity: influence of neuromodulators and implications for sleep disordered breathing. Can J Physiol Pharmacol. 2007 Jan;85(1):155–65. doi: 10.1139/y06-089. [DOI] [PubMed] [Google Scholar]
- 17.Hairston LE, Sauerland EK. Electromyography of the human palate: discharge patterns of the levator and tensor veli palatini. Electromyogr Clin Neurophysiol. 1981 Feb–Mar;21(2–3):287–97. [PubMed] [Google Scholar]
- 18.Rodenstein DO, Stanescu DC. Soft palate and oronasal breathing in humans. J Appl Physiol. 1984 Sep;57(3):651–7. doi: 10.1152/jappl.1984.57.3.651. [DOI] [PubMed] [Google Scholar]
- 19.Tangel DJ, Mezzanotte WS, White DP. Respiratory-related control of palatoglossus and levator palatini muscle activity. J Appl Physiol. 1995 Feb;78(2):680–8. doi: 10.1152/jappl.1995.78.2.680. [DOI] [PubMed] [Google Scholar]
- 20.Dodds WJ, Stewart ET, Logemann JA. Physiology and radiology of the normal oral and pharyngeal phases of swallowing [see comments] AJR Am J Roentgenol. 1990;154(5):953–63. doi: 10.2214/ajr.154.5.2108569. [DOI] [PubMed] [Google Scholar]
- 21.Logemann JA. Evaluation and treatment of swallowing disorders. 2. Austin Texas: ProEd; 1998. [Google Scholar]
- 22.Leopold NA, Kagel MC. Dysphagia--ingestion or deglutition?: a proposed paradigm. Dysphagia. 1997 Fall;12(4):202–6. doi: 10.1007/PL00009537. [DOI] [PubMed] [Google Scholar]
- 23.German RZ, Saxe SA, Crompton AW, Hiiemae KM. Food transport through the anterior oral cavity in macaques. Am J Phys Anthropol. 1989 Nov;80(3):369–77. doi: 10.1002/ajpa.1330800310. [DOI] [PubMed] [Google Scholar]
- 24.Franks HA, Crompton AW, German RZ. Mechanism of intraoral transport in macaques. Am J Phys Anthropol. 1984 Nov;65(3):275–82. doi: 10.1002/ajpa.1330650307. [DOI] [PubMed] [Google Scholar]
- 25.Franks HA, German RZ, Crompton AW, Hiiemae KM. Mechanism of intra-oral transport in a herbivore, the hyrax (Procavia syriacus) Arch Oral Biol. 1985;30(7):539–44. doi: 10.1016/0003-9969(85)90054-8. [DOI] [PubMed] [Google Scholar]
- 26.Thexton A, Hiiemae KM. The effect of food consistency upon jaw movement in the macaque: a cineradiographic study. J Dent Res. 1997;76(1):552–60. doi: 10.1177/00220345970760010501. [DOI] [PubMed] [Google Scholar]
- 27.Hylander WL, Johnson KR, Crompton AW. Loading patterns and jaw movements during mastication in Macaca fascicularis: a bone-strain, electromyographic, and cineradiographic analysis. Am J Phys Anthropol. 1987 Mar;72(3):287–314. doi: 10.1002/ajpa.1330720304. [DOI] [PubMed] [Google Scholar]
- 28.Palmer JB, Hiiemae KM, Liu J. Tongue-jaw linkages in human feeding: a preliminary videofluorographic study. Arch Oral Biol. 1997;42(6):429–41. doi: 10.1016/s0003-9969(97)00020-4. [DOI] [PubMed] [Google Scholar]
- 29.Mioche L, Hiiemae KM, Palmer JB. A postero-anterior videofluorographic study of the intra-oral management of food in man. Arch Oral Biol. 2002 Apr;47(4):267–80. doi: 10.1016/s0003-9969(02)00007-9. [DOI] [PubMed] [Google Scholar]
- 30.Matsuo K, Hiiemae KM, Palmer JB. Cyclic motion of the soft palate in feeding. J Dent Res. 2005 Jan;84(1):39–42. doi: 10.1177/154405910508400106. [DOI] [PubMed] [Google Scholar]
- 31.Hiiemae KM, Palmer JB. Tongue movements in feeding and speech. Crit Rev Oral Biol Med. 2003;14(6):413–29. doi: 10.1177/154411130301400604. [DOI] [PubMed] [Google Scholar]
- 32.Peyron A, Lassauzay C, Woda A. Effects of increased hardness on jaw movement and muscle activity during chewing of visco-elastic model foods. Exp Brain Res. 2002 Jan;142(1):41–51. doi: 10.1007/s00221-001-0916-5. [DOI] [PubMed] [Google Scholar]
- 33.Kohyama K, Mioche L, Bourdiol P. Influence of age and dental status on chewing behaviour studied by EMG recordings during consumption of various food samples. Gerodontology. 2003 Jul;20(1):15–23. doi: 10.1111/j.1741-2358.2003.00015.x. [DOI] [PubMed] [Google Scholar]
- 34.Matsuo K, Metani H, Mays KA, Palmer JB. Tempospatial Linkage of soft palate and jaw movements in feeding [Japanese] Jpn J Dysphagia Rehabil. 2008;12(1):20–30. [Google Scholar]
- 35.Matsuo K, Palmer JB. Anatomy and physiology of feeding and swallowing: normal and abnormal. Physical medicine and rehabilitation clinics of North America. 2008 Nov;19(4):691–707. doi: 10.1016/j.pmr.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palmer JB. Bolus aggregation in the oropharynx does not depend on gravity. Arch Phys Med Rehabil. 1998;79(6):691–6. doi: 10.1016/s0003-9993(98)90046-6. [DOI] [PubMed] [Google Scholar]
- 37.Shaker R, Dodds WJ, Dantas RO, Hogan WJ, Arndorfer RC. Coordination of deglutitive glottic closure with oropharyngeal swallowing. Gastroenterology. 1990;98(6):1478–84. doi: 10.1016/0016-5085(90)91078-k. [DOI] [PubMed] [Google Scholar]
- 38.Ohmae Y, Logemann JA, Kaiser P, Hanson DG, Kahrilas PJ. Timing of glottic closure during normal swallow. Head Neck. 1995;17(5):394–402. doi: 10.1002/hed.2880170506. [DOI] [PubMed] [Google Scholar]
- 39.Logemann JA, Kahrilas PJ, Cheng J, Pauloski BR, Gibbons PJ, Rademaker AW, et al. Closure mechanisms of laryngeal vestibule during swallow. Am J Physiol. 1992;262(2 Pt 1):G338–44. doi: 10.1152/ajpgi.1992.262.2.G338. [DOI] [PubMed] [Google Scholar]
- 40.Shaker R, Ren J, Kern M, Dodds WJ, Hogan WJ, Li Q. Mechanisms of airway protection and upper esophageal sphincter opening during belching. Am J Physiol. 1992 Apr;262(4 Pt 1):G621–8. doi: 10.1152/ajpgi.1992.262.4.G621. [DOI] [PubMed] [Google Scholar]
- 41.Shaker R, Dodds WJ, Ren J, Hogan WJ, Arndorfer RC. Esophagoglottal closure reflex: a mechanism of airway protection. Gastroenterology. 1992 Mar;102(3):857–61. doi: 10.1016/0016-5085(92)90169-y. [DOI] [PubMed] [Google Scholar]
- 42.Martin-Harris B. Coordination of respiration and swallowing. GI Motility online. 2006 doi: 10.1038/gimo10. [DOI] [Google Scholar]
- 43.Selley WG, Flack FC, Ellis RE, Brooks WA. Respiratory patterns associated with swallowing: Part 1. The normal adult pattern and changes with age. Age Ageing. 1989 May;18(3):168–72. doi: 10.1093/ageing/18.3.168. [DOI] [PubMed] [Google Scholar]
- 44.Klahn MS, Perlman AL. Temporal and durational patterns associating respiration and swallowing. Dysphagia. 1999 Summer;14(3):131–8. doi: 10.1007/PL00009594. [DOI] [PubMed] [Google Scholar]
- 45.Martin-Harris B, Brodsky MB, Price CC, Michel Y, Walters B. Temporal coordination of pharyngeal and laryngeal dynamics with breathing during swallowing: single liquid swallows. J Appl Physiol. 2003 May;94(5):1735–43. doi: 10.1152/japplphysiol.00806.2002. [DOI] [PubMed] [Google Scholar]
- 46.Shaker R, Li Q, Ren J, Townsend WF, Dodds WJ, Martin BJ, et al. Coordination of deglutition and phases of respiration: effect of aging, tachypnea, bolus volume, and chronic obstructive pulmonary disease. Am J Physiol. 1992;263(5 Pt 1):G750–5. doi: 10.1152/ajpgi.1992.263.5.G750. [DOI] [PubMed] [Google Scholar]
- 47.Dozier TS, Brodsky MB, Michel Y, Walters BC, Jr, Martin-Harris B. Coordination of swallowing and respiration in normal sequential cup swallows. Laryngoscope. 2006 Aug;116(8):1489–93. doi: 10.1097/01.mlg.0000227724.61801.b4. [DOI] [PubMed] [Google Scholar]
- 48.McFarland DH, Lund JP, Gagner M. Effects of posture on the coordination of respiration and swallowing. J Neurophysiol. 1994;72(5):2431–7. doi: 10.1152/jn.1994.72.5.2431. [DOI] [PubMed] [Google Scholar]
- 49.McFarland DH, Lund JP. Modification of mastication and respiration during swallowing in the adult human. J Neurophysiol. 1995;74(4):1509–17. doi: 10.1152/jn.1995.74.4.1509. [DOI] [PubMed] [Google Scholar]
- 50.Matsuo K, Hiiemae KM, Gonzalez-Fernandez M, Palmer JB. Respiration during Feeding on Solid Food: Alterations in Breathing during Mastication, Pharyngeal Bolus Aggregation and Swallowing. J Appl Physiol. 2008 Dec 27;104(3):674–81. doi: 10.1152/japplphysiol.00527.2007. [DOI] [PubMed] [Google Scholar]
- 51.Smith J, Wolkove N, Colacone A, Kreisman H. Coordination of eating, drinking and breathing in adults. Chest. 1989 Sep;96(3):578–82. doi: 10.1378/chest.96.3.578. [DOI] [PubMed] [Google Scholar]
- 52.Kelly BN, Huckabee ML, Jones RD, Frampton CM. The early impact of feeding on infant breathing-swallowing coordination. Respiratory physiology & neurobiology. 2007 May 14;156(2):147–53. doi: 10.1016/j.resp.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 53.Wilson SL, Thach BT, Brouillette RT, Abu-Osba YK. Coordination of breathing and swallowing in human infants. J Appl Physiol. 1981;50(4):851–8. doi: 10.1152/jappl.1981.50.4.851. [DOI] [PubMed] [Google Scholar]
- 54.Bamford O, Taciak V, Gewolb IH. The relationship between rhythmic swallowing and breathing during suckle feeding in term neonates. Pediatr Res. 1992 Jun;31(6):619–24. doi: 10.1203/00006450-199206000-00016. [DOI] [PubMed] [Google Scholar]
- 55.Lieberman DE, McCarthy RC, Hiiemae KM, Palmer JB. Ontogeny of postnatal hyoid and larynx descent in humans. Arch Oral Biol. 2001;46(2):117–28. doi: 10.1016/s0003-9969(00)00108-4. [DOI] [PubMed] [Google Scholar]
- 56.Laitman JT, Reidenberg JS. Specializations of the human upper respiratory and upper digestive systems as seen through comparative and developmental anatomy. Dysphagia. 1993;8(4):318–25. doi: 10.1007/BF01321770. [DOI] [PubMed] [Google Scholar]
- 57.German RZ, Crompton AW, Thexton AJ. The coordination and interaction between respiration and deglutition in young pigs. J Comp Physiol [A] 1998 Apr;182(4):539–47. doi: 10.1007/s003590050201. [DOI] [PubMed] [Google Scholar]
- 58.Morris SE, Dunn-Klein M. Pre feeding skills. Tucson: Therapy Skills Builders; 1987. [Google Scholar]
- 59.Martin-Harris B, Brodsky MB, Michel Y, Ford CL, Walters B, Heffner J. Breathing and swallowing dynamics across the adult lifespan. Arch Otolaryngol Head Neck Surg. 2005 Sep;131(9):762–70. doi: 10.1001/archotol.131.9.762. [DOI] [PubMed] [Google Scholar]
- 60.Leslie P, Drinnan MJ, Ford GA, Wilson JA. Swallow respiration patterns in dysphagic patients following acute stroke. Dysphagia. 2002 Summer;17(3):202–7. doi: 10.1007/s00455-002-0053-8. [DOI] [PubMed] [Google Scholar]
- 61.Hiss SG, Treole K, Stuart A. Effects of age, gender, bolus volume, and trial on swallowing apnea duration and swallow/respiratory phase relationships of normal adults. Dysphagia. 2001 Spring;16(2):128–35. doi: 10.1007/s004550011001. [DOI] [PubMed] [Google Scholar]
- 62.Hirst LJ, Ford GA, Gibson GJ, Wilson JA. Swallow-induced alterations in breathing in normal older people. Dysphagia. 2002 Spring;17(2):152–61. doi: 10.1007/s00455-001-0115-3. [DOI] [PubMed] [Google Scholar]
- 63.Butler SG, Stuart A, Pressman H, Poage G, Roche WJ. Preliminary investigation of swallowing apnea duration and swallow/respiratory phase relationships in individuals with cerebral vascular accident. Dysphagia. 2007 Jul;22(3):215–24. doi: 10.1007/s00455-007-9077-4. [DOI] [PubMed] [Google Scholar]
- 64.Gross RD, Atwood CWJ, Ross SB, Eichhorn KA, Olszewski JW, Doyle PJ. The Coordination of Breathing and Swallowing in Parkinson’s Disease. Dysphagia. 2007 doi: 10.1007/s00455-007-9113-4. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 65.Selley WG, Flack FC, Ellis RE, Brooks WA. Respiratory patterns associated with swallowing: Part 2. Neurologically impaired dysphagic patients. Age Ageing. 1989 May;18(3):173–6. doi: 10.1093/ageing/18.3.173. [DOI] [PubMed] [Google Scholar]
- 66.Hadjikoutis S, Pickersgill TP, Dawson K, Wiles CM. Abnormal patterns of breathing during swallowing in neurological disorders. Brain. 2000 Sep;123(Pt 9):1863–73. doi: 10.1093/brain/123.9.1863. [DOI] [PubMed] [Google Scholar]
- 67.Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. 2005 Dec;36(12):2756–63. doi: 10.1161/01.STR.0000190056.76543.eb. [DOI] [PubMed] [Google Scholar]
- 68.Fall PA, Saleh A, Fredrickson M, Olsson JE, Granerus AK. Survival time, mortality, and cause of death in elderly patients with Parkinson’s disease: a 9-year follow-up. Mov Disord. 2003 Nov;18(11):1312–6. doi: 10.1002/mds.10537. [DOI] [PubMed] [Google Scholar]
- 69.Nishino T, Yonezawa T, Honda Y. Effects of swallowing on the pattern of continuous respiration in human adults. Am Rev Respir Dis. 1985 Dec;132(6):1219–22. doi: 10.1164/arrd.1985.132.6.1219. [DOI] [PubMed] [Google Scholar]
- 70.Nishino T, Hiraga K. Coordination of swallowing and respiration in unconscious subjects. J Appl Physiol. 1991;70(3):988–93. doi: 10.1152/jappl.1991.70.3.988. [DOI] [PubMed] [Google Scholar]
- 71.Hiss SG, Strauss M, Treole K, Stuart A, Boutilier S. Swallowing apnea as a function of airway closure. Dysphagia. 2003 Fall;18(4):293–300. doi: 10.1007/s00455-003-0021-y. [DOI] [PubMed] [Google Scholar]
- 72.Hiss SG, Strauss M, Treole K, Stuart A, Boutilier S. Effects of age, gender, bolus volume, bolus viscosity, and gustation on swallowing apnea onset relative to lingual bolus propulsion onset in normal adults. J Speech Lang Hear Res. 2004 Jun;47(3):572–83. doi: 10.1044/1092-4388(2004/044). [DOI] [PubMed] [Google Scholar]
- 73.Preiksaitis HG, Mills CA. Coordination of breathing and swallowing: effects of bolus consistency and presentation in normal adults. J Appl Physiol. 1996 Oct;81(4):1707–14. doi: 10.1152/jappl.1996.81.4.1707. [DOI] [PubMed] [Google Scholar]
- 74.Martin BJ, Logemann JA, Shaker R, Dodds WJ. Coordination between respiration and swallowing: respiratory phase relationships and temporal integration. J Appl Physiol. 1994 Feb;76(2):714–23. doi: 10.1152/jappl.1994.76.2.714. [DOI] [PubMed] [Google Scholar]
- 75.Palmer JB, Hiiemae KM. Eating and breathing: interactions between respiration and feeding on solid food. Dysphagia. 2003 Summer;18(3):169–78. doi: 10.1007/s00455-002-0097-9. [DOI] [PubMed] [Google Scholar]
- 76.Charbonneau I, Lund JP, McFarland DH. Persistence of respiratory-swallowing coordination after laryngectomy. J Speech Lang Hear Res. 2005 Feb;48(1):34–44. doi: 10.1044/1092-4388(2005/004). [DOI] [PubMed] [Google Scholar]
- 77.Fontana GA, Pantaleo T, Bongianni F, Cresci F, Viroli L, Sarago G. Changes in respiratory activity induced by mastication in humans. J Appl Physiol. 1992 Feb;72(2):779–86. doi: 10.1152/jappl.1992.72.2.779. [DOI] [PubMed] [Google Scholar]
- 78.Hodgson M, Linforth RS, Taylor AJ. Simultaneous real-time measurements of mastication, swallowing, nasal airflow, and aroma release. J Agric Food Chem. 2003 Aug 13;51(17):5052–7. doi: 10.1021/jf030118+. [DOI] [PubMed] [Google Scholar]
- 79.Buettner A, Beer A, Hannig C, Settles M. Observation of the swallowing process by application of videofluoroscopy and real-time magnetic resonance imaging-consequences for retronasal aroma stimulation. Chem Senses. 2001 Nov;26(9):1211–9. doi: 10.1093/chemse/26.9.1211. [DOI] [PubMed] [Google Scholar]
- 80.Prinz JF, Lucas PW. An optimization model for mastication and swallowing in mammals. Proc R Soc Lond B Biol Sci. 1997;264(1389):1715–21. doi: 10.1098/rspb.1997.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Palmer JB, Hiiemae KM, Matsuo K, Haishima H. Volitional Control of Food Transport and Bolus Formation during Feeding. Physiol Behav. doi: 10.1016/j.physbeh.2007.01.018. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Power ML, Hamdy S, Singh S, Tyrrell PJ, Turnbull I, Thompson DG. Deglutitive laryngeal closure in stroke patients. J Neurol Neurosurg Psychiatry. 2007 Feb;78(2):141–6. doi: 10.1136/jnnp.2006.101857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kahrilas PJ, Lin S, Rademaker AW, Logemann JA. Impaired deglutitive airway protection: a videofluoroscopic analysis of severity and mechanism. Gastroenterology. 1997 Nov;113(5):1457–64. doi: 10.1053/gast.1997.v113.pm9352847. [DOI] [PubMed] [Google Scholar]
- 84.Daniels SK, Foundas AL. Swallowing physiology of sequential straw drinking. Dysphagia. 2001 Summer;16(3):176–82. doi: 10.1007/s00455-001-0061-0. [DOI] [PubMed] [Google Scholar]
- 85.Martin-Harris B, Brodsky MB, Michel Y, Lee FS, Walters B. Delayed initiation of the pharyngeal swallow: normal variability in adult swallows. J Speech Lang Hear Res. 2007 Jun;50(3):585–94. doi: 10.1044/1092-4388(2007/041). [DOI] [PubMed] [Google Scholar]
- 86.Stephen JR, Taves DH, Smith RC, Martin RE. Bolus location at the initiation of the pharyngeal stage of swallowing in healthy older adults. Dysphagia. 2005 Fall;20(4):266–72. doi: 10.1007/s00455-005-0023-z. [DOI] [PubMed] [Google Scholar]