Abstract
Glutamate is the major excitatory neurotransmitter in the mammalian central nervous system. Disturbed glutamate signaling resulting in hypofunction of NMDA receptors has been implicated in the pathophysiology of schizophrenia. Glutamate Carboxypeptidase II (GCP II) hydrolyzes N-acetyl-alpha L-aspartyl-L-glutamate (NAAG) into glutamate and N-acetyl-aspartate (NAA). NAAG is a neuropeptide that is an NMDA receptor antagonist as well as an agonist for the metabotropic glutamate receptor-3 (mGluR3), which inhibits glutamate release. The aggregate effect of NAAG is thus to attenuate NMDA receptor activation. To manipulate the expression of GCP II, loxP sites were inserted flanking exon 1 and 2, which were excised by crossing with a Cre-expressing mouse. The mice heterozygous for this deletion showed a 50% reduction in the expression level of protein and functional activity of GCP II in brain samples. Heterozygous mutant crosses did not yield any homozygous null animals at birth or as embryos (N >200 live births and fetuses). These data are consistent with the previous report that GCP II homozygous mutant mice generated by removing exons 9 and 10 of GCP II gene were embryonically lethal and confirm our hypothesis that GCP II plays an essential role early in embryonic development. Heterozygous mice, however, developed normally to adulthood and exhibited increased locomotor activity, reduced social interaction, and a subtle cognitive deficit in working memory.
Keywords: Glutamate carboxypeptidase II, N-acetyl-aspartyl-glutamate (NAAG), glutamate, N-methyl-D-aspartate receptor (NMDAR), knockout mice, schizophrenia
Introduction
N-acetyl aspartyl glutamate (NAAG) is a neuropeptide, which is concentrated in storage vesicles and released in a calcium-dependent manner upon neuronal depolarization (Williamson and Neale., 1988; Tsai et al., 1988; Zollinger et al., 1994; Renno et al., 1997). NAAG has a highly selective co-localization with classical neurotransmitters such as the cholinergic acid motor neurons, the glutamatergic retinal ganglionic cell projections, the noradrenergic locus coeruleus neurons and the glutamatergic cortical pyramidal neurons, especially in primates (Moffett et al., 1989, 1993, 1994, 1995; Frondoza et al., 1990; Slusher et al., 1992; Tsai et al., 1993; Berger et al., 1995; Passani et al., 1997). NAAG antagonizes glutamate activated NMDA receptor responses in CA1 pyramidal cells and GABAergic interneurons (Bergeron et al., 2005, 2007; Grunze et al., 1996). NAAG also appears to be a potent and specific agonist at the mGluR3 receptor (Wroblewska et al., 1997; Bischofberger and Schild, 1996).
Robinson et al. (1987) first described a quisqualate sensitive brain peptidase activity with highly affinity for NAAG that was activated by metal ions. It was designated N-Acetylated-Alpha-Linked-Acidic-Dipeptidase (NAALADase). Using antibodies developed against homogenously purified rat brain NAALADase, Carter et al. (1996) identified the cDNA encoding NAALADase in a rat brain cDNA library. It showed remarkable homology to prostate specific membrane antigen (PSMA), a cell surface glycoprotein highly expressed on metastatic human prostate cancer cells. Subsequent studies demonstrated that on the basis of cDNA sequence, enzymatic activity and antigenic characteristics, NAALADase was identical to PSMA. Amino acid similarities between peptides derived from duodenal folate hydrolase and amino acid sequence in NAALADase led to the demonstration that NAALADase/PSMA was also identical to folate hydrolase 1 (FOLH 1) (Halsted et al., 1998). The current enzyme designation for NAALADase/PSMA is Glutamate Carboxypeptidase II (GCP II) and the human gene is referred to as Folh 1(Carter and Coyle, 2004)
Considerable evidence supports the notion that GCP II regulates the synaptic concentrations of NAAG (Slusher et al., 1990; Robinson et al., 1987; Berger et al., 1999; Stauch et al., 1989; Bergeron et al., 2007). The enzyme is expressed almost exclusively in astrocytes where it is concentrated in the synaptoneurosomes and in the glomeruli in the cerebellum (Berger et al., 1995, 1999; Sácha et al., 2007). In an in vivo dialysis study, Slusher et al. (1999) demonstrated that perfusion of the cerebral cortex with the potent GCP II inhibitor, 2-phosphonomethyl-pentanedoic acid (2-PMPA), markedly reduced the extracellular concentrations of glutamate and subsequent lesion size following middle cerebral artery occlusion in the rat. Similar reductions in glutamate and increases in extracellular NAAG were observed following inhibition of GCP II in the hippocampus in a rat model of traumatic brain injury (Zhong et al., 2005). In the acute hippocampal slice preparation, Bergeron et al. (2007) showed that perfusion with 2-PMPA attenuated NMDA receptor (NMDAR) currents on CA1 pyramidal cells following stimulation of the presynaptic Schaffer collaterals whereas perfusion with a soluble GCP II construct enhanced these NMDAR responses, thus establishing that GCP II modulates the concentration of released endogenous NAAG at synaptic NMDARs.
Considerable evidence from genetic and postmortem studies support the hypothesis that hypofunction of the NMDA receptor plays an important role in the pathophysiology of schizophrenia (Lisman et al., 2008). In this regard, several postmortem studies have demonstrated reduced expression of GCP II in cortico-limbic regions of the brain in schizophrenia, which would enhance the synaptic actions of NAAG, thereby interfering with NMDA receptor function
Bacich et al. (2002, 2005) previously described a null mutation of GCP II in which stop codons were inserted in exons 1 and 2. Mice homozygous for the null mutation did not express GCP II as demonstrated by RT-PCR of GCP II mRNA and Western blots. However, a residual 7-18 percent of wild type NAAG peptidase activity was detected in brain. Notably, no differences in the endogenous levels of NAAG, N-acetyl aspartate or glutamate, and very modest and subtle differences in behavior were noted in the homozygous versus wild type. In contrast, Tsai et al. (2003) knocked out the zinc ligand domain essential for enzyme activity of GCP II by deleting exons 9 and 10. Mouse fetuses homozygous for the null mutation did not survive past eight days gestation, indicating that GCP II is essential for embryogenesis prior to brain development. In an attempt to resolve the strikingly different outcomes for the two null mutations of GCP II, we have exploited the Cre/Lox P system to remove exons 1 and 2, the exons targeted by Bacich et al. (2002) to determine the impact of reduced or absent GCP II.
Materials and methods
Generation of GCP II knockout mice by use of the Cre-loxP system
We identified a positive ~ 100 Kb genomic clone containing mouse GCP II gene as described previously (Tsai et al. 2003). A 20.7 Kb gene fragment encoding exons 1-3 was subcloned and analyzed by restriction enzyme mapping. To construct the knock-out targeting vector, a loxP site was inserted upstream of exon 1, and a Neomycin resistance (neo) cassette flanked by two loxP sites was inserted the downstream of exon 2 (Figure1). The linearized vector was electroporated into embryonic stem cells (ES) derived from the 129SvJ strain. Neomycin resistant colonies were isolated, expanded, and screened by PCR with primers specific for mouse GCP II and the neo cassette. PCR primers for genotyping are: P1, 5' -GGATATGCATGGTATATAATCAC- 3' ; P2, 5' - GCATCAGCAATGGTGTCAGA- 3' ; P3, 5' - CATTGAGTAAGAGGCATTAGGCT- 3' ; P4, 5' - CTTACCTCATTTCCATCTTCATT- 3' ; Neo P1, 5' - CATTCCTCCACTCATGATCTA- 3' ; Neo P2, 5' - GCCTTGGGAAAAGCGCCTCC- 3' . P1, P2, P3, and P4 were designed to annealing within the GCP II gene. Neo P1 and P2 were neo cassette sequences. PCR was executed using 40 ng of each primer, 0.2 mM dNTPs and 0.4 U of Taq polymerase (Promega Inc., Madison, WI) in a total volume of 20 μl in the buffer supplied by the manufacturer. Initial denaturation was 5 min at 94°C followed by 35 cycles of amplification (94°C for 30 sec, 60°C for 30 sec, and 72°C for 1 min).
Fig. 1.

Knockout targeting strategy. In the targeting construct, LoxP1 and neo cassette flanked by two LoxP(LoxP2 and LoxP3) sites were inserted into upstream sequences of exon 1 and downstream sequences of exon2. Chimeric mice were bred with 129S6/SvEv Tac mice. The F1 mice with targeted germ line transmission of GCP II mutation were crossed with Cre recombinase-expressing mice. After Cre/LoxP-mediated recombination, neo cassette, exon 1 and exon 2 were excised, yielding GCP II knockout mice. PCR primers used for initial screening of ES and targeted mice are indicated by numbered arrows.
Targeted ES cells were injected into C57BL/B6 blastocytes and transferred to pseudopregnant mothers. Chimeric mice were bred with 129S6/SvEv Tac mice. The F1 mice with targeted germ line transmission of GCP II mutation were crossed with Cre recombinase-expressing mice, yielding GCP II exon 1-2 and 5'-UTR knockout mice. Genotypes were determined by PCR analysis of DNA obtained from the tail biopsy as for the ES cells. The mice used in experiments described here were back-crossed to C57BL/B6 wild type animals.
Real-time PCR
Mouse brain samples were harvested. Total RNA was extracted using RNeasy Mini Kit (Qiagen). Equal amount of total RNA (400 ng) were reverse transcribed using the Transcriptor First Strand cDNA synthesis kit (Roche Diagnostics) following manufacturer's protocols. The PCR amplifications were performed with iQ™ SYBR Green PCR Kit (BIO-RAD) using SYBR green as fluorescent dye on a MiniOpticon MJ Mini Personal Thermal Cycler (BIO-RAD). The amplification conditions were as follows: initial incubation at 95°C for 3 min, followed by 40 cycles of denaturation at 94°C for 15 s, annealing for 30 sec (at 62°C for β-actin, 60°C for GCP3, and 58°C for mGluR3, respectively), and extension at 72°C for 30 s. Subsequently, melting curve analysis was performed to verify the specificity of the PCR products.
The quantification of target RNA was achieved in triplicate according to the standard curve method with β-actin as a calibrator. The primer sequences were as follows: mGCP3 sense, 5' - CACGACCAGCAATTGAGAAA- 3'; mGCP3 antisense, 5' - TGCTTGGAGCAAAGATGATG- 3'; mGluR3 sense, 5' - GAAAGGACAAAGGCAGCAAG- 3'; mGluR3 antisense , 5' - GGGTCCAATGTTTCCTGCTA- 3'; β-actin sense, 5' - AGTGTGACGTTGACATCCGTA- 3'; β-actin antisense, 5' - GCCAGAGCAGTAATCTCCTTCT- 3'. All primer pairs delivered gene single products, which were sequenced to confirm specificity.
GCP II protein and enzyme activity analysis
Mouse brain tissues were homogenized in ice-cold 50 mM Tris-HCl buffer (pH 7.4) and centrifuged at 4000g for 30 min at 4°C. The resulting supernatant was removed. The membrane pellets were resuspended in 50 mM Tris-HCl buffer and washed by centrifugation (4000g at 4°C for 30 min). GCP II protein expression in tissues was detected by chemiluminescence using rabbit antiserum against human GCP II (generated by us) as the primary antibody, horseradish peroxidase-conjugated goat anti-rabbit IgG H& L (ab6721, Abcam Inc. Cambridge, MA) as secondary antibody and Western Lightning Chemiluminescence Reagent (PerkinElmer LAS, Inc. Boston , MA). Comparable levels of loaded protein were confirmed by re-probing membranes with a β-actin-specific antibody (Abcam ab8227). Semi-quantitative assessment of protein bands from the fluorograms was executed by computerized densitometry on a MacIntosh G4 computer (Apple, Cupertino, CA) using Quantity One Quantitation Software (Bio-Rad, Hercules, CA).
GCP II radioenzymatic assays were performed as described by Robinson et al. (1987) by using 4 nM N-acetylaspartyl [3H]glutamate (PerkinElmer LAS, Inc, Boston , MA) and determining the [3H]glutamate liberated by GCP II. The specificity of GCP II-hydrolyzing activity was assessed by using the inhibitor quisqualic acid.
Behavioral assays
All mice were maintained on a 12/12 h light/dark cycle with food and water available ad libitum. All procedures were approved by the Wellesley College Institutional Animal Care and Use Committee and conformed to the standards set forth in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All heterozygous mice used for behavioral assays were back-crossed at least seven generations to the C57BL/B6 background. Ten heterozygous dams were mated with wild-type C57BL/B6 male mice, and birth was considered postnatal day (P1). Sixty three mice from 14 litters were tested in total (see individual experiments for specific details). Testing was conducted from weeks 7 to 6 months. No mortality was noted during behavioral testing, and the experimenters were blind to genotypes for all testing. In all experiments, apart from prepulse inhibition of startle, only males were used. This was in order to avoid confounding sex differences (Berger-Sweeny et al., 1995). Physical and neurological parameters were tested using standard protocols as well as monitoring for general health by observation (Crawley, 2000). In adulthood (> 8 weeks), locomotor, social, and cognitive behaviors were assessed using the battery of tests described below.
Locomotor activity
To measure baseline activity levels, each mouse was placed in a 25 × 47 × 21 cm activity monitoring chamber, and activity was measured by an automated recording system connected to three pairs of infrared photobeams. Beam breaks, as the mice moved through the chamber, were counted for 12 hours across the dark cycle to assess baseline motor activity. Mice had access to food and water throughout the testing session. Activity levels were measured in male wild type (MWt) n = 12 and male GCP II heterozygotes (MH) n = 12.
Rotorod
The rotorod apparatus is designed to assess motor coordination and consists of a plastic roller (3 cm in diameter) with small grooves running along its turning axis (Lalond et al., 2005). On the first day, mice were given a training session during which every mouse was placed on the rotarod at a constant speed (4 rpm) for a maximum of 60 s. Afterwards, mice received three trials. During the test session, animals were placed on the still rod. Rotation was started at 4 rpm and accelerated at a constant rate to 40 rpm over 180 s. The latency to fall off the rotarod was recorded automatically as the mice fell through an infrared photobeam. (male wildtype (MWt) n = 12; male heterozygotes (MH), n = 13)
Water Maze
The apparatus and procedure follows published protocols (Frick et al., 2000; Fredricksson et al., 2004). The swim maze is a 150 cm diameter black acrylic pool, 50 cm deep, with depth of water maintained at 31 cm and thermostatically controlled at 24 °C. A clear platform (12 cm diameter) invisible to the mice, was placed in a constant position 15 cm from the edge of the pool 1 cm below the surface of the water. Each mouse was given four trials on each of 8 consecutive days. During each trial the mouse was placed at one of the four defined compass points at the edge of the pool and given 60 seconds to locate the submerged platform. On reaching the platform, the mouse was allowed to remain on it for 15 s (orientation period) before being dried and returned to its home cage prior to the next trial 10 minutes later. For the spatial navigation trials, latency to the platform was measured as an assessment of the animal's spatial learning. During the probe trial, the platform was removed entirely, and the mouse was given 60 s to search for the platform and then removed from the pool by the experimenter. The time each mouse spent in the platform quadrant during the probe trial was used to evaluate its memory of the platform location. During working memory trials, the platform was moved to a different quadrant each day and the mouse was given 4 trials to learn the new platform location. Latency and the percentage of time spent in the old platform quadrant in comparison to the new platform quadrant were used to assess the mouse's ability to unlearn the previous platform location and learn a new position. For every trial, all mice were started in the same location and were allotted a maximum of 60 seconds to find the platform (MWt, n = 12; MH, n = 12). The mice were tracked and recorded using Water 2020 (HVS Imaging, UK) video tracking system, which provided latency, path length, percentage time in each quadrant, and speed data.
Prepulse inhibition (PPI) of acoustic startle
A mouse was placed in the startle chamber (San Diego Instruments; San Diego, CA). Each chamber was composed of a ventilated and illuminated sound-attenuating enclosure with a high-frequency loudspeaker that produces both background 70 dB noise and the various acoustic stimuli. Mice are held in a nonrestrictive Plexiglas cylinder resting on a platform fitted with a piezoelectric motion transducing device. Vibrations caused by whole-body startle responses of the mouse were converted to analog signals by the piezoelectric transducer attached to the underside of the platform and then digitized and stored by a computer for analysis. Output data were given in mV. The 70-dB white noise background was presented alone for 5 min to acclimate the mice to the chamber and then continued throughout the remainder of the test session. Four blocks of trials were presented with 15 s inter-trial intervals. Blocks 1 and 4 consisted of six pulse-alone (a 40-msec 120-dB broadband burst) trials. Blocks 2 and 3 each contained five trials, presented in a pseudo-randomized sequence comprising: a 40-msec broadband, 120-dB burst, as pulse-alone or, preceded by 50 msec with 20-msec-long prepulses of 73-dB, 76-dB, or 82-dB (3, 6, and 12 dB above background stimuli); and a no-stimulus trial (Aubert et al., 2006). Startle magnitude was calculated as the average response to the startle trials presented during block(s) two and three of the session. PPI was calculated as a percentage of the pulse-alone scores (Geyer and Swerdlow, 1998). This group contained 13 MWt, 15 MH, 16 female wild-type (FWt), and 18 female heterozygous (FH) mice.
Social Interaction
The apparatus consisted of a 40.5 cm W × 60 cm L × 22 cm H Plexiglas chamber divided into three equal compartments. Small 4 cm × 4 cm openings were located on the dividing walls allowing access to the side compartments. These openings could be opened and closed. The test consisted of three parts as previously described in Moy et al. (2004). In the first session, the subject mouse was habituated to the testing apparatus by placing it in the center compartment and allowing it to explore for 5 min with the doorways to the side compartments closed. The subject mouse was then returned to its home cage while the apparatus was cleaned with 70% Ethanol. In the second session, testing sociability, an unfamiliar male C57BL/6J (Stranger 1), with no prior contact to the subject mouse, was enclosed in a small wire cage (Galaxy Cup, Spectrum Diversified Designs, Inc., Streetsboro, OH) in either the left or the right chamber and allowed to habituate for 1 minute. The location of Stranger 1 was systematically alternated between the left and right chamber between subject mice to control for location effects. The subject mouse was then returned to the center compartment, with both doorways open, and allowed to explore for 10 min. Following the 10 min session, both the subject mouse and Stranger 1 were removed from the apparatus and returned to their home cages while the chamber was cleaned. In the third session, testing preference for social novelty, Stranger 1 was returned to its enclosure and a new male C57BL/6J mouse, Stranger 2, was enclosed in the other side compartment. Both strangers are allowed to habituate for 1 min before the subject mouse was placed in the center compartment and allowed to explore the apparatus for another 10 min. Both sessions two and three were videotaped and measurements were obtained of the time the subject mouse spent in each of the three compartments (with Stranger 1, in the center, and either alone or with Stranger 2 in sessions two and three, respectively) as well as the number of entries into each of the side chambers. The social interaction test was performed on 13 MWt and 12 MH animals.
Statistical Analysis
Statistical analysis on all behavioral tasks was performed using either an ANOVA or a repeated-measures ANOVA with p < 0.05 considered significant. Post hoc analysis using two-tailed students t-tests were performed on all ANOVAs found to be significant.
Results
Generation of GCP II exon 1 and 2 knockout mice
We crossed mice with germ line transmission of the GCP II targeting construct with mice expressing Cre enzyme to generate GCP II exon 1 and 2 knockout mice. Cre catalyzes a loxP site-dependent recombination that excises the selection marker sequences from the targeted allele, leaving a single loxP site instead of exon 1, exon 2, 5'-UTR of GCP II gene, and neo cassette, yielding GCP II exon 1-2 and 5'-UTR knockout mice (Figure 1).
Wildtype (Wt or GCP II+/+) and heterozygous mice (GCP II+/-) were born at expected frequencies and appeared normal and healthy. Heterozygous mice were crossed to produce Wt, heterozygous, and homozygous mice (GCP II-/-). However, no homozygous null animals (42 expected) were observed in a total of 170 live births from GCP II +/- intercrosses. Furthermore, we analyzed embryos from heterozygous intercrosses at E11 to term (expected 1:2:1 ratio of GCP II-/- to GCP II +/- to GCP II+/+) ). None of 87 embryos examined were homozygous null. We concluded that GCP II is an essential gene in mice prior to this point in embryonic development.
GCP II protein expression and enzyme activity are decreased
We used Western blot analysis to measure the expression of GCP II. The results revealed that the antibody against human GCP II protein bound a band of ~100 kDa (Figure 2). The expression level of GCP II protein in GCP II mutant heterozygous mice was approximately 50% less than that in Wt animals. To determine whether reduced GCP II protein expression in heterozygous mutant mice affects the enzyme function, membrane preparations from the animals were examined for GCP II hydrolysis activity. GCP II activity in the brain of heterozygous mutant mice was decreased 51+/- 4%, compared to that in wild type mice (N=9; p<0.001). The [3H] NAAG hydrolysis was completely inhibited by the GCP II inhibitor quisqualic acid in all samples assessed, indicating that the degradation of [3H] NAAG was GCP II specific (data not shown).
Fig. 2.

Western blot analysis of GCP II protein from representative prefrontal cortex of brain tissues from Wt and GCP II+/- mice using rabbit antiserum against human GCP II as primary antibodies. The membrane protein from a prostate cancer cell line, LNCaP, was used as a positive control. Semi-quantitative analysis of band density showed that the level of GCP II protein in GCP II+/- mice was approximately half that in Wt mice.
GCP III and mGluR3 transcript level are similar in GCP II heterozygous mutant and wild type mice
In order to determine whether the disruption of the GCP II gene resulted in compensatory changes related to NAAG metabolism and function in the nervous system, GCP III and mGluR3 mRNA transcript levels in brain tissues obtained from wild type and GCP II heterozygous mutant mice were examined. The result showed no differences in message transcript levels of GCP III and mGluR3 between wild type and GCP II heterozygous mutant mice (N=9).
Behavioral analysis
We completed an initial behavioral characterization of the GCP II mice focusing on tasks, which are behaviorally relevant to the symptoms exhibited by patients with schizophrenia. Behavioral tasks included two measures of motor function, a social interaction test to assess both sociability and preference for social novelty, a swim maze task to assess cognitive function, and prepulse inhibition (PPI) to assess sensorimotor gating. GCP II+/- mice exhibited no motor deficits compared to Wt controls. Motor coordination was normal in the heterozygous mice as assessed by the rotorod. Mean time to fall was 102 ± 27 s for Wt and 92 ± 26 s for GCP II+/- mice (F(1,23) = 1.17 p = 0.29). Baseline locomotor activity levels revealed that the GCP II heterozygous mice were hyperactive compared to Wt mice measured over a 12 hour period (Fig. 3). The average number of beam breaks/hour for GCP II+/- was 483 ± 143 and for Wt mice was 399 ± 60 (F(1,22) = 5.02, p < 0.05). However, a repeated-measures ANOVA revealed no significant interaction between genotype and time interval (F(11,22) = 1.27, p = 0.52) suggesting that the GCP II heterozygous mice habituated to the chamber similar to control animals.
Fig. 3.

GCP II heterozygous mice are hyperactive compared to controls. Each data point represents the total number of photobeam breaks ± S.E.M. within a single hour interval during the dark cycle. GCP II+/- mice were significantly more active than Wt mice but showed a similar pattern of habituation across time.
The social interaction test (Moy et al., 2004) was designed to assess sociability as well as a preference for social novelty both of which have been shown to be impaired in rodent models of reduced NMDAR function and schizophrenia (Rung et al., 2005). The results from both sessions of this test were suggestive of abnormal social interactions in the GCP II heterozygous mutant mice. A two-way repeated-measures ANOVA revealed a significant interaction between compartments and genotype across the two sessions (F(1,22) = 5.96, p < 0.05). Post hoc tests revealed that, during the first session, the Wt mice showed a preference for the compartment in which an unknown stranger mouse, Stranger 1, resided (t(22) = 4.61, p < 0.001) whereas the GCP II+/- mice showed no preference for compartment (t(22) = 0.29, p = 0.78)(Fig. 4A). These data were indicative of social withdrawal in the GCP II heterozygous mice. During the second session, Wt mice appeared to have switched their preference to a second unknown stranger, Stranger 2, rather than spending time with the now familiar Stranger 1. However, post hoc analysis comparing time spent with Stranger 1 versus Stranger 2 indicated that this difference was not statistically significant (t(24) = 1.80, p = 0.08). Similar to the first session, GCP II heterozygous mutant mice showed no preference for compartment (t (22) = 0.82, p = 0.42) (Fig 4B). Data from the second session, while not conclusive, were suggestive of a deficit in the ability of the GCP II heterozygous mutant mice to recognize the presence of a novel mouse.
Fig. 4.
GCP II heterozygous mice exhibit abnormal social behavior. The duration of time spent in each compartment + SEM for Session 1 (A) and Session 2 (B) of a social interaction task. (A) GCP II heterozygous mice exhibited signs of social withdrawal. While Wt mice preferred to spend time with a Stranger mouse (black bars) rather than alone (grey bars), GCP II+/- mice spent equal amounts of time in both compartments. (B) Neither Wt nor GCP II+/- mice showed a preference for interacting with a new Stranger mouse (grey bars) in comparison to the Stranger (black bars) in which they had previously interacted. *** P < 0.001.
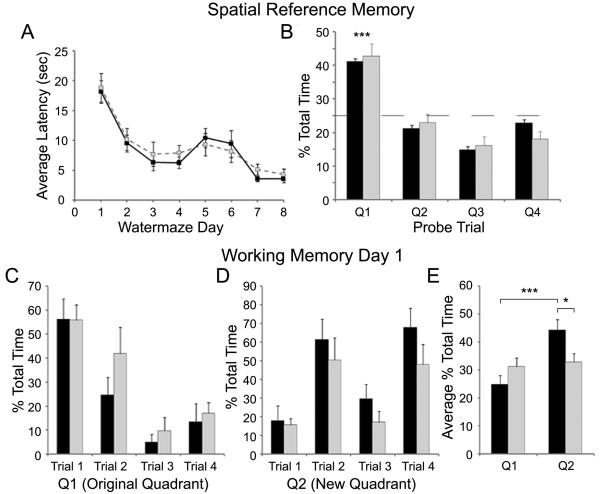
The water maze was chosen as a cognitive task because it is sensitive to subtle cognitive impairments, and the mouse's performance can be assessed relatively quickly (Frick et al., 2000). GCP II heterozygous mutant mice showed a subtle cognitive deficit on the working memory portion, but not the spatial reference portion of a spatial water maze task.
GCP II heterozygous mutant mice performed similarly to controls during the acquisition phase of spatial learning. A repeated-measures ANOVA revealed that both GCP II+/- and Wt mice learned to find the hidden platform as evidenced by a decrease in latency to the platform over the 8 days of testing (F(7,16) = 28.208, p << 0.001) (Fig. 5A) with no significant interaction between latency and genotype (F(7,16) = 0.5, p = 0.821). During the probe trial, an ANOVA revealed a significant effect of quadrant (F(3,20) = 14.262, p << 0.001) with both genotypes spending significantly more time in the platform quadrant (wt 42.8 ± 3.4 %; GCP II+/- 41.0 ± 3.6 %) than the other three quadrants (all p's << 0.001) and no significant interaction with genotype (F(3,20) = 1.305, p = 0.300) suggesting that the GCP II+/- mice had learned the location of the platform similarly to the Wt mice (Fig. 5B).
Fig. 5.
GCP II heterozygous mice are impaired on the working memory portion of a spatial water maze task. (A) The average latency + SEM to find the platform each day of the 8 day water-maze task. Both Wt (black boxes) and GCP II+/- (grey boxes) mice learned to find the platform across days. (B) The percentage of 60 seconds + SEM on the probe trial spent within the platform quadrant (Q1) in comparison to the quadrants in which the platform was not located (Q2-Q4). Both Wt (black bars) and GCP II+/- (grey bars) mice spent significantly more time in Q1 than each of the other 3 quadrants with the dashed line representing chance performance level. There were no significant differences between the genotypes for any quadrant. (C, D) The percentage of time + SEM spent in either the original platform quadrant, Q1 (C) or the new platform quadrant, Q2 (D) across the four trials for Wt (black bars) and heterozygous (grey bars) mice on first day of the working memory task. (E) The average percentage of time across the 4 trials + SEM spent in the original platform quadrant (Q1) or the new platform quadrant (Q2). Wt mice spent significantly more time in the new quadrant (Q2) than they did in Q1 or than the GCP II+/- mice spent in Q2. * p < 0.05, *** p < 0.001.
The GCP II heterozygous mice were subtly impaired on the first day of the working memory portion of the water maze task. During this task, mice were given four trials to learn the new location of the hidden platform. A repeated-measures ANOVA of latency to platform revealed that both WT and GCP+/- mice learned the new platform location across trials (F(3,20) = 3.85, p < 0.05); however, there was no significant interaction between latency to platform across the four trials and genotype (F(3,20) = 0.57, p = 0.64; data not shown). Although, a comparison of the percentage of total time spent in the original and new quadrants between Wt and GCP II+/- mice revealed a difference in how well the mice learned the new platform location (Fig. 5C, D). A two-way repeated measures ANOVA comparing the time spent in the original quadrant to the time spent in the new platform quadrant across trials revealed a significant interaction between quadrant and genotype (F(1,22) = 5.632, p < 0.05). Post hoc analysis revealed that, on average, Wt mice spent significantly more time in the new platform quadrant, Q2, than GCP II heterozygous mutant mice (t(22) = 2.41, p < 0.05) (Fig. 7E). Wt mice also spent significantly more time in the new platform quadrant, Q2, in comparison to the old platform quadrant, Q1 (t(22) = 4.05, p << 0.001) while the GCP II+/- mice spent similar amounts of time in both quadrants (t(22) = 0.40, p = 0.70). Both genotypes spent similar amounts of time in quadrants Q3 and Q4, in which the platform had not been located. GCP II heterozygous mutant mice performed similarly to Wt mice on all other days of the working memory task (data not shown).
Prepulse inhibition of acoustic startle was examined in the GCP II mice because impaired PPI has been demonstrated in patients with schizophrenia (Braff et al., 2001). GCP II+/- mice exhibited no deficits compared to Wt animals on PPI. The response to the startle alone measured in the GCP II+/- mice (268 ± 126 mV) was not significantly different from wildtype animals (234 ± 134 mV; F(1,55) = 1.54, p = 0.22). The PPI was measured for prepulses given at three different intensities (3, 6, and 12 dB) above background. A two-way ANOVA showed that GCP II+/- mice responded similarly to Wt at all three intensities (F(2,55) = 0.57, p = 0.64) suggesting that sensorimotor gating in these animals is intact (Fig. 6).
Fig. 6.

GCP II heterozygous mice are not impaired in PPI of the acoustic startle response. The % PPI + S.E.M. for 3 pre-pulse intensities in Wt (black bars) and GCP II+/- mice (grey bars). % PPI increases with increasing decibel level of the prepulse in both the Wt and GCP II+/- mice as expected. There were no significant differences between the genotypes at any prepulse.
Discussion
Two mouse lines with a loss of functional GCP II protein had been developed previously; however, they led to very divergent phenotypes. Bacich et al. (2002) reported that mice homozygous for stop mutations in exons 1 and 2 exhibited a minimal phenotype in adulthood. In contrast, Tsai et al (2003) found early embryonic lethality for mice homozygous for an exon 9-10 excision, which deletes the cofactor binding site in GCP II. The new model described here, in which exon 1-2 and the 5' UTR are deleted appears to be consistent with that of Tsaiet al. (2003). It remains unclear why there is such a marked phenotypic difference between knockout strategies. It is notable that embryonic death in the Tsai et al. (2003) knockout and the knockout described here occurs prior to meaningful development of the central nervous system including the neural tube, which indicates that neuronally mediated events are unlikely to account for the lethality. We have detected GCP II expression in embryonic stem cells, suggesting that it may play an important role at very early stages of embryonic development (Tsai et al., 2003). GCP II also catalyzes the removal of the polyglutamate tail from dietary polyglutamyl folates (Halsted et al., 1998). Perhaps this early role in development may involve the disposition of folic acid. The dramatic phenotype of GCP II null mice clearly demonstrated the essential role of GCP II in embryonic development and survival.
Bacich et al. (2002) reported that mice homozygous for a null mutation in GCP II had a subtle behavioral phenotype including differences in anxiety measures and food intake. They reported a 7 to 18 percent residual “NAAG peptidase activity in brain, kidney and liver.” When GCP II was originally characterized as a quisqualate sensitive NAAG hydrolase, virtually no activity was observed in the rat liver and no immunoreactive protein was found on Western blot. Shafizadeh and Halstead (2007) in characterizing folate hydrolase activity in the rat intestine found that the bulk of the activity was due to γ-glutamyl hydrolase and not GCP II, and they observed a very faint GCP II transcript in the liver after RT-PCR. They noted subtle differences in the kinetics, inhibitor sensitivity and affects of metal ions on activity of wild type as compared to residual NAAG peptidase activity in brain membranes. Interestingly, the wild type and residual brain membrane activity in the mutants also exhibited several differences from cloned GCP II transfected into CHO cells. Bzdega et al. (2004) identified another NAAG hydrolase with a μM Km that they designated GCP3. Northern blots did not show GCP3 mRNA in brain, in contrast to the ovary, testes and lung. However, RT-PCR revealed weak brain expression with regional brain differences. Bzdega et al. (2004) argued that GCP3 is unlikely to account for the residual NAAG hydrolase activity in the GCP II knockout mice because that activity was much less sensitive to inhibition by 2-PMPA whereas GCP II and GCP3 are equally sensitive to 2-PMPA. O'Keefe et al. (2004) described a PSMA-like gene that possesses 98% identify to the PSMA gene at the nucleotide level but is under the control of a different promoter than the PSMA gene and lacks the exon 1 encoding transmembrane domain. As the transmembrane domain is essential for glycosylation, and glycosylation is indispensable for enzymatic activity (Speno et al., 1999), it would seem that this protein, if expressed, would not result in active enzyme.
We hypothesized that with approximately 50% the Wt levels of GCP II, the GCP II heterozygous mutant mice would display a behavioral phenotype characteristic of animals with reduced NMDAR function. Furthermore, this reduction recapitulates the decreased expression of GCP II observed in post-mortem brain in schizophrenia (Tsai et al., 1995; Hakak et al., 2001; Tkachev et al., 2007; Guilarte et al., 2008). Previous behavioral studies show that mice with genetic or pharmacological disruptions of NMDA receptors exhibit several behavioral abnormalities including hyperactivity, abnormal social behavior, impaired PPI, and cognitive deficits in working memory tasks (Duncan et al., 2004, Enomoto et al., 2007, Mohn et al., 1999). Behavioral testing of the GCP II mice revealed alterations in three of these four behavioral domains. Specifically, GCP II heterozygous mutant mice were hyperactive exhibited signs of social withdrawal, and showed a subtle cognitive deficit on the working memory portion of a spatial water maze task compared to Wt mice.
The cognitive deficit in the GCP II heterozygous mice was present only on the first day of the working memory swim maze task suggesting that while the GCP II mice are initially worse at unlearning the platform location, they do eventually unlearn the old position and learn the new platform position. During the four trials, both genotypes had similar latencies to find the platform; however, after the first trial, these times were so short (between 3 and 5 seconds) and the variation between animals too large to find significant differences in performance. Instead, by comparing amount of time spent in the two quadrants of interest, the original platform quadrant, Q1, and the new platform quadrant, Q2, we believe that this uncovered a difference in the strategy the two genotypes employed to find the platform. The GCP II heterozygous mice spend significantly more time in the original platform quadrant, Q1, rather than switching preference to the new platform quadrant, Q2, across the 4 trials unlike the Wt animals who immediately swam to the new platform location. These data suggest that the GCP II+/- mice were more likely to be perseverating on the previous platform location. Thus, an impairment in the reversal of spatial reference memory likely underlies the initial impairment in working memory observed during the first day with a new platform position.
While the GCP II heterozygous mice do not exhibit all of the behavioral deficits implicated previously with NMDAR hypofunctioning, they do show several behavioral abnormalities that are associated with NMDAR malfunctioning. The subtle behavioral phenotype described here resembles that of two other recombinant models of schizophrenia: the DISC1 dominant-negative knock-in (Hikida et al., 2007) and the serine racemase knock-out (Basu et al., 2008). Notably, PPI was not disrupted in all three models, consistent with the observation that sensory gating abnormalities are an endophenotype not shared by all individuals, or expected to be present in all animal models with schizophrenia.(Swerdlow et al., 2008).
In conclusion, we report here that GCP II homozygous mutant mice, in which exon 1-2 and 5'-UTR of GCP II were removed, exhibit early embryonic lethality. This data corroborates earlier findings that GCP II homozygous mutants, generated by removing exons 9 and 10 of GCP II gene, were embryonic lethal and confirms our hypothesis that GCP II plays an essential role in embryonic development. In addition, GCP II heterozygous mice exhibit behavioral abnormalities reminiscent of those described in mouse models of NMDA receptor hypofunction and humans with schizophrenia. Thus the GCP II knockout appears to be both a good model to study NMDA receptor hypofunction as well as some of the behavioral characteristics of schizophrenia.
Acknowledgment
We thank Daniel Liu for assistance in the real-time PCR experiments; Julie Kurek, Pat Carey, and Val LePage for assistance with animal colony maintenance, and Loren N. Saulsberry for assistance with the prepulse inhibition behavioral experiments. This research was supported by RO1 MH051290-12 and P50 MH060450-09 to JTC.
References
- Aubert L, Reiss D, Ouagazzal AM. Auditory and visual prepulse inhibition in mice: parametric analysis and strain comparisons. Genes Brain Behav. 2006;5:423–431. doi: 10.1111/j.1601-183X.2005.00178.x. [DOI] [PubMed] [Google Scholar]
- Bacich DJ, Ramadan E, O'Keefe DS, Bukhari N, Wegorzewska I, Ojeifo O, Olszewski R, Wrenn CC, Bzdega T, Wroblewska B, Heston WD, Neale JH. Deletion of the glutamate carboxypeptidase II gene in mice reveals a second enzyme activity that hydrolyzes N-acetylaspartylglutamate. J Neurochem. 2002;83:20–9. doi: 10.1046/j.1471-4159.2002.01117.x. [DOI] [PubMed] [Google Scholar]
- Bacich DJ, Wozniak KM, Lu XC, O'Keefe DS, Callizot N, Heston WD, Slusher BS. Mice lacking glutamate carboxypeptidase II are protected from peripheral neuropathy and ischemic brain injury. J Neurochem. 2005;95:314–23. doi: 10.1111/j.1471-4159.2005.03361.x. [DOI] [PubMed] [Google Scholar]
- Basu AC, Tsai GE, Ma C-L, Ehmsen JT, Mustafa AK, Han L, Jiang ZI, Benneyworth MA, Froimowitz MP, Lange N, Snydre SH, Bergeron R, Coyle JT. Targeted disruption of serine racemase affects glutamatergic neurotransmission and behavior. Mol Psychiatry, epub. 2008 doi: 10.1038/mp.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger UV, Carter RE, Coyle JT. The immunocytochemical localization of N-acetylaspartyl glutamate, its hydrolysing enzyme NAALADase, and the NMDAR-1 receptor at a vertebrate neuromuscular junction. Neuroscience. 1995;64:847–50. doi: 10.1016/0306-4522(95)92578-8. [DOI] [PubMed] [Google Scholar]
- Berger UV, Luthi-Carter R, Passani LA, Elkabes S, Black I, Konradi C, Coyle JT. Glutamate carboxypeptidase II is expressed by astrocytes in the adult rat nervous system. J Comp Neurol. 1999;415:52–64. doi: 10.1002/(sici)1096-9861(19991206)415:1<52::aid-cne4>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Bergeron R, Imamura Y, Frangioni JV, Greene RW, Coyle JT. Endogenous N-acetylaspartylglutamate reduced NMDA receptor-dependent current neurotransmission in the CA1 area of the hippocampus. J Neurochem. 2007;100:346–57. doi: 10.1111/j.1471-4159.2006.04253.x. [DOI] [PubMed] [Google Scholar]
- Bergeron R, Coyle JT, Tsai G, Greene RW. NAAG reduces NMDA receptor current in CA1 hippocampal pyramidal neurons of acute slices and dissociated neurons. Neuropsychopharmacology. 2005;30:7–16. doi: 10.1038/sj.npp.1300559. [DOI] [PubMed] [Google Scholar]
- Bischofberger J, Schild D. Glutamate and N-acetylaspartylglutamate block HVA calcium currents in frog olfactory bulb interneurons via an mGluR2/3-like receptor. J Neurophysiol. 1996;76:2089–92. doi: 10.1152/jn.1996.76.3.2089. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology. 2001;156:234–58. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Bzdega T, Crowe SL, Ramadan ER, Sciarretta KH, Olszewski RT, Ojeifo OA, Rafalski VA, Wroblewska B, Neale JH. The cloning and characterization of a second brain enzyme with NAAG peptidase activity. J Neurochem. 2004;89:627–35. doi: 10.1111/j.1471-4159.2004.02361.x. [DOI] [PubMed] [Google Scholar]
- Carter RE, Feldman AR, Coyle JT. Prostate-specific membrane antigen is a hydrolase with substrate and pharmacologic characteristics of a neuropeptidase. Proc Natl Acad Sci U S A. 1996;93:749–53. doi: 10.1073/pnas.93.2.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RE, Coyle JT. Glutamate carboxypeptidase II. Handbook of Proteolytic Enzymes. (2nd Edn) 2004;290:960–963. [Google Scholar]
- Cassidy M, Neale JH. Localization and transport of N-acetylaspartylglutamate in cells of whole murine brain in primary culture. J Neurochem. 1993;60:1631–8. doi: 10.1111/j.1471-4159.1993.tb13385.x. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Moy SS, Perez A, Eddy DM, Zinzow WM, Lieberman JA, Snouwaert JN, Koller BH. Deficits in sensorimotor gating and tests of social behavior in a genetic model of reduced NMDA receptor function. Behav. Brain Res. 2004;153:507–519. doi: 10.1016/j.bbr.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Egan MF, Straub RE, Goldberg TE, Yakub I, Callicott JH, Hariri AR, Mattay VS, Bertolino A, Hyde TM, Shannon-Weickert C, Akil M, Crook J, Vakkalanka RK, Balkissoon R, Gibbs RA, Kleinman JE, Weinberger DR. Variation in GRM3 affects cognition, prefrontal glutamate, and risk for schizophrenia. Proc Natl Acad Sci U S A. 2004;101:12604–9. doi: 10.1073/pnas.0405077101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto T, Noda Y, Nabeshima T. Phencyclidine and genetic animal models of schizophrenia developed in relation to the glutamate hypothesis. Methods Find Exp Clin Pharmacol. 2007;29:291–301. doi: 10.1358/mf.2007.29.4.1075358. [DOI] [PubMed] [Google Scholar]
- Fredriksson A, Archer T, Alm H, Gordh T, Eriksson P. Neurofunctional deficits and potentiated apoptosis by neonatal NMDA antagonist administration. Behavioural brain research. 2004;153:367–76. doi: 10.1016/j.bbr.2003.12.026. [DOI] [PubMed] [Google Scholar]
- Frick KM, Burlingame LA, Arters JA, Berger-Sweeney J. Reference memory, anxiety and estrous cyclicity in C57BL/6NIA mice are affected by age and sex. Neuroscience. 2000;95:293–307. doi: 10.1016/s0306-4522(99)00418-2. [DOI] [PubMed] [Google Scholar]
- Frondoza CG, Logan S, Forloni G, Coyle JT. Production and characterization of monoclonal antibodies to N-acetyl-aspartyl-glutamate. J Histochem Cytochem. 1990;38:493–502. doi: 10.1177/38.4.2319120. [DOI] [PubMed] [Google Scholar]
- Grunze HC, Rainnie DG, Hasselmo ME, Barkai E, Hearn EF, McCarley RW, Greene RW. NMDA-dependent modulation of CA1 local circuit inhibition. J Neurosci. 1996;16:2034–43. doi: 10.1523/JNEUROSCI.16-06-02034.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte TR, Hammoud DA, McGlothan JL, Caffo BS, Foss CA, Kozikowski AP, Pomper MG. Dysregulation of glutamate carboxypeptidase II in psychiatric disease. Schizophr Res. 2008 Feb;99(1-3):324–32. doi: 10.1016/j.schres.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakak Y, Walker JR, Li C, Wong WH, Davis KL, Buxbaum JD, Haroutunian V, Fienberg AA. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci U S A. 2001 Apr 10;98(8):4746–51. doi: 10.1073/pnas.081071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikida T, Jaaro-Peled H, Seshadri S, Oishi K, Hookway C, Kong S, Wu D, Xue R, Andradé M, Tankou S, Mori S, Gallagher M, Ishizuka K, Pletnikov M, Kida S, Sawa A. Dominant-negative DISC1 transgenic mice display schizophrenia-associated phenotypes detected by measures translatable to humans. Proc Natl Acad Sci U S A. 2007 Sep 4;104(36):14501–6. doi: 10.1073/pnas.0704774104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halsted CH, Ling EH, Luthi-Carter R, Villanueva JA, Gardner JM, Coyle JT. Folylpoly-gamma-glutamate carboxypeptidase from pig jejunum. Molecular characterization and relation to glutamate carboxypeptidase II. J Biol Chem. 1998;273:20417–24. doi: 10.1074/jbc.273.32.20417. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, Grace AA. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett JR, Cassidy M, Namboodiri MA. Selective distribution of N-acetylaspartylglutamate immunoreactivity in the extrapyramidal system of the rat. Brain Res. 1989;494:255–66. doi: 10.1016/0006-8993(89)90594-5. [DOI] [PubMed] [Google Scholar]
- Moffett JR, Namboodiri MA, Neale JH. Enhanced carbodiimide fixation for immunohistochemistry: application to the comparative distributions of N-acetylaspartylglutamate and N-acetylaspartate immunoreactivities in rat brain. J Histochem Cytochem. 1993;41:559–70. doi: 10.1177/41.4.8450195. [DOI] [PubMed] [Google Scholar]
- Moffett JR, Palkovits M, Namboodiri A, Neale JH. Comparative distribution of N-acetylaspartylglutamate and GAD67 in the cerebellum and precerebellar nuclei of the rat utilizing enhanced carbodiimide fixation and immunohistochemistry. J Comp Neurol. 1994;347:598–618. doi: 10.1002/cne.903470410. [DOI] [PubMed] [Google Scholar]
- Moffett JR, Namboodiri MA. Differential distribution of N-acetylaspartylglutamate and N-acetylaspartate immunoreactivities in rat forebrain. J Neurocytol. 1995;24:409–33. doi: 10.1007/BF01181604. [DOI] [PubMed] [Google Scholar]
- Mohn AR, Gainetdinov RR, Caron MG, Koller BH. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell. 1999;98:427–36. doi: 10.1016/s0092-8674(00)81972-8. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- O'Keefe DS, Bacich DJ, Heston WD. Comparative analysis of prostate-specific membrane antigen (PSMA) versus a prostate-specific membrane antigen-like gene. Prostate. 2004;58:200–10. doi: 10.1002/pros.10319. [DOI] [PubMed] [Google Scholar]
- Passani LA, Vonsattel JP, Coyle JT. Distribution of N-acetylaspartylglutamate immunoreactivity in human brain and its alteration in neurodegenerative disease. Brain Res. 1997;772:9–22. doi: 10.1016/s0006-8993(97)00784-1. [DOI] [PubMed] [Google Scholar]
- Robinson MB, Blakely RD, Couto R, Coyle JT. Hydrolysis of the brain dipeptide N-acetyl-L-aspartyl-L-glutamate. Identification and characterization of a novel N-acetylated alpha-linked acidic dipeptidase activity from rat brain. J Biol Chem. 1987;262:14498–506. [PubMed] [Google Scholar]
- Rung JP, Carlsson A, Rydén Markinhuhta K, Carlsson ML. MK-801 induced social withdrawal in rats; a model for negative symptoms of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:827–32. doi: 10.1016/j.pnpbp.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Sácha P, Zámecník J, Barinka C, Hlouchová K, Vícha A, Mlcochová P, Hilgert I, Eckschlager T, Konvalinka J. Expression of glutamate carboxypeptidase II in human brain. Neuroscience. 2007;144:1361–72. doi: 10.1016/j.neuroscience.2006.10.022. Epub 2006 Dec 5. [DOI] [PubMed] [Google Scholar]
- Shafizadeh TB, Halsted CH. gamma-Glutamyl hydrolase, not glutamate carboxypeptidase II, hydrolyzes dietary folate in rat small intestine. J Nutr. 2007;137:1149–53. doi: 10.1093/jn/137.5.1149. [DOI] [PubMed] [Google Scholar]
- Slusher BS, Robinson MB, Tsai G, Simmons ML, Richards SS, Coyle JT. Rat brain N-acetylated alpha-linked acidic dipeptidase activity. Purification and immunologic characterization. J Biol Chem. 1990;265:21297–301. [PubMed] [Google Scholar]
- Slusher BS, Tsai G, Yoo G, Coyle JT. Immunocytochemical localization of the N-acetyl- aspartyl-glutamate (NAAG) hydrolyzing enzyme N-acetylated alpha-linked acidic dipeptidase (NAALADase) J Comp Neurol. 1992;315:217–29. doi: 10.1002/cne.903150208. [DOI] [PubMed] [Google Scholar]
- Slusher BS, Vornov JJ, Thomas AG, Hurn PD, Harukuni I, Bhardwaj A, Traystman RJ, Robinson MB, Britton P, Lu XC, Tortella FC, Wozniak KM, Yudkoff M, Potter BM, Jackson PF. Selective inhibition of NAALADase, which converts NAAG to glutamate, reduces ischemic brain injury. Nat Med. 1999;5:1396–402. doi: 10.1038/70971. [DOI] [PubMed] [Google Scholar]
- Speno HS, Luthi-Carter R, Macias WL, Valentine SL, Joshi AR, Coyle JT. Site-directed mutagenesis of predicted active site residues in glutamate carboxypeptidase II. Mol Pharmacol. 1999;55:179–85. doi: 10.1124/mol.55.1.179. [DOI] [PubMed] [Google Scholar]
- Stauch BL, Robinson MB, Forloni G, Tsai G, Coyle JT. The effects of N-acetylated alpha-linked acidic dipeptidase (NAALADase) inhibitors on [3H]NAAG catabolism in vivo. Neurosci Lett. 1989;100:295–300. doi: 10.1016/0304-3940(89)90702-7. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Weber M, Qu Y, Light GA, Braff DL. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology (Berl) 2008 Aug;199(3):331–88. doi: 10.1007/s00213-008-1072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkachev D, Mimmack ML, Huffaker SJ, Ryan M, Bahn S. Further evidence for altered myelin biosynthesis and glutamatergic dysfunction in schizophrenia. Int J Neuropsychopharmacol. 2007 Aug;10(4):557–63. doi: 10.1017/S1461145706007334. [DOI] [PubMed] [Google Scholar]
- Tsai G, Forloni G, Robinson MB, Stauch BL, Coyle JT. Calcium-dependent evoked release of N-[3H]acetylaspartylglutamate from the optic pathway. J Neurochem. 1988;51:1956–9. doi: 10.1111/j.1471-4159.1988.tb01186.x. [DOI] [PubMed] [Google Scholar]
- Tsai G, Slusher BS, Sim L, Coyle JT. Immunocytochemical distribution of N-acetylaspartylglutamate in the rat forebrain and glutamatergic pathways. J Chem Neuroanat. 1993;6:277–92. doi: 10.1016/0891-0618(93)90033-z. [DOI] [PubMed] [Google Scholar]
- Tsai G, Dunham KS, Drager U, Grier A, Anderson C, Collura J, Coyle JT. Early embryonic death of glutamate carboxypeptidase II (NAALADase) homozygous mutants. Synapse. 2003;50:285–92. doi: 10.1002/syn.10263. [DOI] [PubMed] [Google Scholar]
- Williamson LC, Neale JH. Calcium-dependent release of N-acetylaspartylglutamate from retinal neurons upon depolarization. Brain Res. 1988;475:151–5. doi: 10.1016/0006-8993(88)90209-0. [DOI] [PubMed] [Google Scholar]
- Wroblewska B, Wroblewski JT, Pshenichkin S, Surin A, Sullivan SE, Neale JH. N-acetylaspartylglutamate selectively activates mGluR3 receptors in transfected cells. J Neurochem. 1997;69:174–81. doi: 10.1046/j.1471-4159.1997.69010174.x. [DOI] [PubMed] [Google Scholar]
- Zhong C, Zhao X, Sarva J, Kozikowski A, Neale JH, Lyeth BG. NAAG peptidase inhibitor reduces acute neuronal degeneration and astrocyte damage following lateral fluid percussion TBI in rats. J Neurotrauma. 2005;22:266–76. doi: 10.1089/neu.2005.22.266. [DOI] [PubMed] [Google Scholar]
- Zollinger M, Brauchli-Theotokis J, Gutteck-Amsler U, Do KQ, Streit P, Cuénod M. Release of N-acetylaspartylglutamate from slices of rat cerebellum, striatum, and spinal cord, and the effect of climbing fiber deprivation. J Neurochem. 1994;63:1133–42. doi: 10.1046/j.1471-4159.1994.63031133.x. [DOI] [PubMed] [Google Scholar]