Abstract
Obesity-related disorders are closely associated with the pathogenesis of cardiovascular disease. Adiponectin is a circulating adipose tissue-derived hormone that is down-regulated in obese individuals. Hypoadiponectinemia has been identified as an independent risk factor for type 2 diabetes, coronary artery disease, and hypertension, and experimental studies show that adiponectin plays a protective role in the development of insulin resistance, atherosclerosis, and inflammation. More recent findings have shown that adiponectin directly affects signaling in myocardial cells and exerts beneficial actions on the heart after pressure overload and ischemia–reperfusion injury. This review focuses on the role of adiponectin in the regulation of myocardial remodeling and acute cardiac injury.
Obesity is strongly associated with type 2 diabetes, hypertension, and heart disease (Friedman 2003, Reilly and Rader 2003). However, the link between excess fat accumulation and the development of obesity-linked disease is poorly understood at a molecular level. Accumulating evidence suggests that adipose tissue is not simply an energy storage tissue but that it also functions as a secretory organ producing a variety of bioactive molecules that are referred to as adipokines. Adipokines include leptin, tumor necrosis factor-α (TNF-α), plasminogen activator inhibitor type 1, interleukin (IL) 1β, IL-6, IL-8, IL-10, resistin, retinol binding protein-4, and Adipokines are believed to directly or indirectly affect the pathophysiology of various obesity-linked disorders and biologic processes that involve dysregulated immune responses.
Adiponectin and Obesity
Adiponectin, also referred to as ACRP30, AdipoQ, and gelatin-binding protein-28 (Hu et al. 1996, Maeda et al. 1996, Scherer et al. 1995), is an adipocyte-derived protein that is abundantly present in plasma (range, 3–30 μg/mL) and accounts for 0.01% of total plasma protein (Arita et al. 1999, Ouchi et al. 2003a). Adiponectin exists in three major oligomeric forms: trimer, hexamer, and a high-molecular-weight form in both human and mouse plasma (Kishida et al. 2003, Pajvani et al. 2003). Adiponectin is also processed by proteolysis, and fragments including the globular domain can also be detected in plasma (Fruebis et al. 2001).
Plasma concentrations of total adiponectin can be measured by an enzyme-linked immunosorbent assay system. Although adiponectin is secreted by adipose tissue, plasma levels of this adipokine are significantly lower in obese subjects than in nonobese subjects (Arita et al. 1999). A significant negative correlation is found between body mass index (BMI) and plasma adiponectin levels (Arita et al. 1999, Cnop et al. 2003), and adiponectin levels are negatively correlated with percent body fat, waist-to-hip ratio, and intra-abdominal fat (Cnop et al. 2003, Weyer et al. 2001). The paradoxical down-regulation of adiponectin during obesity results from complex cross-regulatory interactions between adiponectin and inflammatory cytokines (Fasshauer et al. 2003, Ouchi et al. 2003a).
Plasma adiponectin concentrations have also been studied in various obesity-related diseases. In patients with type 2 diabetes, plasma adiponectin concentrations are lower than in age-and BMI-matched nondiabetic men and women (Hotta et al. 2000). Conversely, individuals with high adiponectin concentrations were found to be at lower risk of developing type 2 diabetes than those with low concentrations (Lindsay et al. 2002, Spranger et al. 2003). Plasma adiponectin concentrations are also lower in patients with clinical manifestations of coronary artery disease than in age- and BMI-adjusted control subjects (Kumada et al. 2003, Ouchi et al. 1999). Finally, circulating adiponectin levels are inversely correlated with other cardiovascular risk factors, including hyperlipidemia, high blood pressure, and C-reactive protein (CRP) levels (Iwashima et al. 2004, Ouchi et al. 2003a). Collectively, these epidemiologic studies suggest that adiponectin may play a pivotal role in the development of vascular and metabolic diseases that are prevalent in obese individuals.
Role of Adiponectin in Obesity-Linked Diseases
Increasing evidence from experimental models indicates that adiponectin plays a causal role in the development of obesity-linked disorders, including insulin resistance and vascular diseases. Adiponectin-deficient (APN-KO) mice exhibit diet-induced insulin resistance when fed high-fat/sucrose (Maeda et al. 2002) or high-fat (Nawrocki et al. 2006) diets. Adiponectin-deficient mice are also reported to exhibit moderate insulin resistance when fed a normal chow diet (Kubota et al. 2002). In contrast, another strain of adiponectin-deficient mice does not display an insulin-resistant phenotype (Ma et al. 2002). The reason for the phenotype differences between the two lines is unknown. In another study, administration of the globular domain fragment of adiponectin was found to increase fatty acid oxidation in muscle and cause weight loss in mice that had been fed a high-fat/sucrose diet (Fruebis et al. 2001). These effects were accompanied by reductions in plasma levels of glucose, free fatty acids, and triglycerides. It has also been shown that adiponectin treatment reduces plasma glucose levels in mice without affecting insulin levels (Berg et al. 2001). Collectively, these data suggest that adiponectin may act as a protective factor against the development of insulin resistance and diabetes.
Adiponectin stimulates glucose metabolism by promoting the phosphorylation and activation of adenosine monophosphate-activated protein kinase (AMPK), a stress-responsive kinase, in skeletal muscle (Tomas et al. 2002, Yamauchi et al. 2002), liver (Yamauchi et al. 2002) and adipocytes (Wu et al. 2003). AMPK activation is believed to be mediated, at least in part, by adiponectin binding to the cell surface receptors AdipoR1 and AdipoR2 (Yamauchi et al. 2003). Recently, T-cadherin was also identified as an adiponectin receptor (Hug et al. 2004), but its role in intracellular signaling remains unclear.
Several studies have shown that low adiponectin levels are associated with development of vascular disease. APN-KO mice exhibit increased neointimal hyperplasia after acute vascular injury (Kubota et al. 2002, Matsuda et al. 2002), impaired endothelium-dependent vasodilation on an atherogenic diet (Ouchi et al. 2003b), and impaired neovascularization in response to ischemia (Shibata et al. 2004b). Conversely, adiponectin overexpression was found to reduce atherosclerotic lesion formation in a mouse model of atherosclerosis (Okamoto et al. 2002) and promote angiogenesis response to ischemia (Shibata et al. 2004b).
Consistent with its vasculoprotective actions, in vitro experiments show that adiponectin protein reduces TNF-α-stimulated expression of adhesion molecules and IL-8 in endothelial cells (Kobashi et al. 2005, Ouchi et al. 2000), inhibits macrophage-to-foam cell transformation (Ouchi et al. 2001), and suppresses growth factor-stimulated proliferation of smooth muscle cells (Arita et al. 2002). Consistent with its angiogenic actions, adiponectin stimulates endothelial cell migration and differentiation into capillary-like structures in vitro through activation of AMPK signaling (Ouchi et al. 2004). AMPK signaling also mediates adiponectin-stimulated nitric oxide production in endothelial cells through its ability to phosphorylate endothelial nitric oxide synthase (Chen et al. 2003). Finally, adiponectin has antiapoptotic actions in human endothelial cells that are dependent on the induction of AMPK signaling (Kobayashi et al. 2004). Collectively, these findings suggest that down-regulation of the adiponectin–AMPK regulatory pathway may participate in endothelial cell dysfunction and vessel rarefaction that are observed in obese states (Al Suwaidi et al. 2001, Lind and Lithell 1993, Steinberg et al. 1996, Yilmaz et al. 2003).
Adiponectin and Hypertrophic Cardiomyopathy
Pathologic cardiac remodeling is associated with many obesity-related conditions (Ilercil et al. 2001, Rutter et al. 2003), and diastolic dysfunction is one of the earliest clinical manifestations of insulin resistance (Schannwell et al. 2002). Recent studies have shown that adiponectin influences cardiac remodeling in pathologic states. Pressure overload in APN-KO mice results in enhanced concentric cardiac hypertrophy and increased mortality (Liao et al. 2005, Shibata et al. 2004a). Conversely, adenovirus-mediated delivery of adiponectin attenuates cardiac hypertrophy in response to pressure overload in APN-KO, wild-type, and diabetic db/db mice (Shibata et al. 2004a). Adiponectin over-expression also attenuates angiotensin II-induced cardiac hypertrophy, suggesting that adiponectin functions as a general repressor of pathologic cardiac growth. These findings suggest that low adiponectin levels may contribute to the development of hypertrophic cardiomyopathy in obese individuals (Figure 1).
Figure 1.
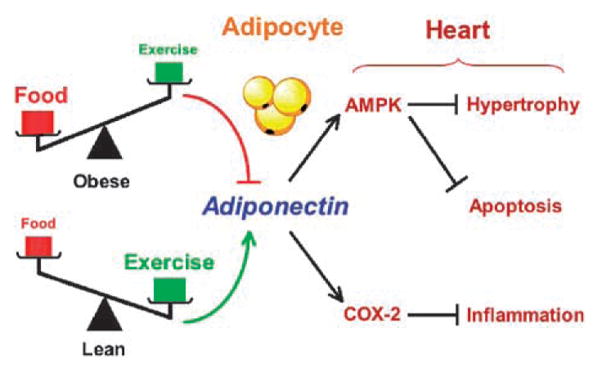
Cardioprotective actions of adiponectin. Plasma adiponectin level is negatively regulated by adiposity, which is influenced by the levels of food intake and physical activity. Adiponectin stimulates myocardial AMPK signaling, leading to a suppression of myocyte hypertrophy and apoptosis. Adiponectin also stimulates COX-2 expression, resulting in reduction in cardiac inflammation.
Adiponectin receptors are expressed by cardiac myocytes and heart tissues (Ivanov et al. 2001, Lord et al. 2005, Takahashi et al. 2005, Yamauchi et al. 2003), and recent studies have shown that adiponectin will modulate intracellular signaling and growth in these cells. In rat neonatal cardiac myocytes, adiponectin activates AMPK and inhibits both extracellular signal-regulated kinase (ERK) activation and the hypertrophic response to α-adrenergic receptor stimulation (Shibata et al. 2004a). The inhibition of ERK and hypertrophy by adiponectin can be reversed by transduction with dominant-negative AMPK. Consistent with these findings in myocytes, AMPK is reported to suppress angiotensin II-stimulated ERK phosphorylation and proliferation in vascular smooth muscle cells (Nagata et al. 2004). It has also been shown that activation of AMPK inhibits protein synthesis and hypertrophic responses in cardiac myocytes via decreased p70S6 kinase phosphorylation and increased phosphorylation of eukaryotic elongation factor-2 (Chan et al. 2004). Activation of AMPK by adiponectin also increases glucose and fatty acid uptake in cardiac myocytes (Pineiro et al. 2005), and these metabolic effects will influence cardiac remodeling. AMPK phosphorylation is attenuated in APN-KO mice after pressure overload (Shibata et al. 2004a), whereas AMPK activity is increased as hearts undergo pressure overload hypertrophy (Tian et al. 2001). Collectively, these findings suggest that the adiponectin-AMPK regulatory axis negatively regulates changes in intracellular signaling and metabolism that are linked to the progression of myocardial hypertrophy (Figure 1).
Adiponectin and Myocardial Ischemia–Reperfusion Injury
Obesity-related disorders have a major impact on the incidence, severity, and outcome of ischemic heart disease (Orlander et al. 1994, Wolk et al. 2003). In a recent clinical study, high plasma adiponectin levels were associated with a lower risk of myocardial infarction, independent of CRP levels and glycemic status (Pischon et al. 2004). Furthermore, adiponectin levels rapidly decline after acute myocardial infarction (Kojima et al. 2003). The reduction of plasma adiponectin levels after acute myocardial infarction negatively correlates with plasma CRP levels, suggesting that hypoadiponectinemia is associated with an increased inflammatory response to acute myocardial ischemia.
Recent work from our group demonstrates that after ischemia–reperfusion, APN-KO mice develop larger infarcts than wild-type mice (Shibata et al. 2005). These larger infarcts were associated with increased myocardial cell apoptosis and TNF-α expression in the APN-KO mice. Conversely, adenovirus-mediated delivery of adiponectin diminished infarct size, myocardial apoptosis, and TNF-α production in both APN-KO and wild-type mice. Of note, this study showed that the one-time administration of recombinant adiponectin protein, injected either 30 min before the induction of ischemia, during ischemia, or 15 min after reperfusion, results in a reduction in infarct size. Thus, short-term administration of adiponectin may have practical clinical utility in the treatment of acute myocardial infarction.
Adiponectin inhibits apoptosis in cardiac myocytes and fibroblasts that are exposed to hypoxia-reoxygenation stress (Shibata et al. 2005). Transduction with dominant-negative AMPK blocks the prosurvival actions of adiponectin, indicating that adiponectin inhibits cardiac cell apoptosis through AMPK-dependent signaling. In this regard, activation of AMPK in heart after ischemia–reperfusion is markedly attenuated in APN-KO mice compared with wild-type mice. Consistent with these findings, transgenic mice expressing a kinase-dead mutant of AMPK display increased apoptosis and cardiac dysfunction after ischemic–reperfusion injury ex vivo (Russell et al. 2004). AMPK signaling may be beneficial for the ischemic heart through its ability to stimulate glucose transport (Li et al. 2005, Russell et al. 1999) and decrease endoplasmic reticulum stress (Terai et al. 2005). Taken together, these studies suggest that activation of AMPK signaling plays an important role in the favorable actions of adiponectin on cardiac damage after ischemia–reperfusion.
The protective action of adiponectin against myocardial ischemia–reperfusion injury is also mediated by its ability to activate cyclooxygenase-2 (COX-2) in cardiac cells (Shibata et al. 2005). The up-regulation of COX-2 by adiponectin leads to an increase in prostaglandin E2 synthesis and inhibition of lipopolysaccharide (LPS)-induced TNF-α production, consistent with findings in other cell types (Meja et al. 1997, Pruimboom et al. 1994). Pharmacologic inhibition of the COX-2–prostaglandin E2 pathway reverses the inhibitory effects of adiponectin on LPS-induced TNF-α production, and COX-2 inhibition partially reversed the protective actions of adiponectin on infarct size in vivo (Shibata et al. 2005). Of interest, COX-2 inhibition had no effect on adiponectin-mediated AMPK activation in cultured cardiac cells. Conversely, AMPK-inhibition had no effect on COX-2 induction by adiponectin or on the suppressive effect of adiponectin on TNF-α production caused by LPS. These findings suggest that adiponectin protects the heart from ischemia–reperfusion injury through the regulation of independent signaling pathways involving both AMPK-mediated antiapoptotic actions and COX-2-mediated anti-inflammatory actions (Figure 1).
Cyclooxygenase-2 and its metabolites have been shown to be required for late preconditioning and play important protective roles in myocardial ischemia–reperfusion damage (Bolli et al. 2002, Camitta et al. 2001, Hohlfeld et al. 2000, Xiao et al. 2001, Xiao et al. 2004). Recent clinical trials reveal that treatment with selective COX-2 inhibitors results in an increased risk for cardiovascular complications including myocardial infarction (Bresalier et al. 2005, Solomon et al. 2005). Therefore, interference with the protective actions of adiponectin on the heart could partly explain why COX-2 inhibition contributes to adverse cardiovascular events.
Adiponectin and Heart Failure
Adiponectin may also influence the development of chronic heart failure, but at this time, little is known about the effects of this adipokine on left ventricular remodeling. In this regard, epidemiologic data do not provide straightforward explanations. Paradoxically, although obesity is a risk factor for the development of heart failure, a higher BMI is associated with an improved prognosis in patients with established chronic heart failure because wasting is strongly associated with the increased risk of death in this patient population (Anker et al. 1997). Therefore, high adiponectin levels are a predictor of mortality in patients with heart failure because high body mass, hence low adiponectin, improves survival once heart failure is established (Kistorp et al. 2005). Similarly, high serum cholesterol is also associated with improved survival in these patients because it probably serves as a surrogate marker of body mass (Rauchhaus et al. 2000). Therefore, the results of epidemiologic studies are complicated and counterintuitive due to the confounding pathologic responses that are associated with heart failure. Thus, detailed biochemical and mouse genetic studies are required to better understand the roles of adipokines in this disease.
Conclusion
Adiponectin is an adipose-derived hormone that exhibits protective properties on the heart and blood vessels. In heart, adiponectin serves as a regulator of cardiac injury through modulation of anti-inflammatory and prosurvival reactions, and it also functions to inhibit hypertrophic remodeling. Future work will be required to identify the oligomeric isoform(s) of adiponectin that confer cardioprotection and clarify the receptor-mediated signaling mechanisms that inhibit myocardial apoptosis, inflammation, and hypertrophy. Evaluation of the molecular and cellular mechanisms of adiponectin action should lead to better understanding of how obesity affects the heart and permit the development of novel approaches to treat heart disease.
Acknowledgments
This work was supported by NIH grants AR40197, HL77774, HL81587, and AG15052 to K Walsh. N Ouchi was supported by a Department of Medicine Pilot Project Grant from Boston University. R Shibata was supported by grants from the American Heart Association Postdoctoral Fellowship Award, Northeast Affiliate, and the Uehara Memorial Foundation.
References
- Al Suwaidi J, Higano ST, Holmes DR, et al. Obesity is independently associated with coronary endothelial dysfunction in patients with normal or mildly diseased coronary arteries. J Am Coll Cardiol. 2001;37:1523–1528. doi: 10.1016/s0735-1097(01)01212-8. [DOI] [PubMed] [Google Scholar]
- Anker SD, Ponikowski P, Varney S, et al. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349:1050–1053. doi: 10.1016/S0140-6736(96)07015-8. [DOI] [PubMed] [Google Scholar]
- Arita Y, Kihara S, Ouchi N, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- Arita Y, Kihara S, Ouchi N, et al. Adipocyte-derived plasma protein adiponectin acts as a platelet-derived growth factor-BB-binding protein and regulates growth factor-induced common postreceptor signal in vascular smooth muscle cell. Circulation. 2002;105:2893–2898. doi: 10.1161/01.cir.0000018622.84402.ff. [DOI] [PubMed] [Google Scholar]
- Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- Berg AH, Combs TP, Du X, et al. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- Bolli R, Shinmura K, Tang XL, et al. Discovery of a new function of cyclooxygenase (COX)-2: COX-2 is a cardioprotective protein that alleviates ischemia/reperfusion injury and mediates the late phase of preconditioning. Cardiovasc Res. 2002;55:506–519. doi: 10.1016/s0008-6363(02)00414-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresalier RS, Sandler RS, Quan H, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352:1092–1102. doi: 10.1056/NEJMoa050493. [DOI] [PubMed] [Google Scholar]
- Camitta MG, Gabel SA, Chulada P, et al. Cyclooxygenase-1 and -2 knockout mice demonstrate increased cardiac ischemia/reperfusion injury but are protected by acute preconditioning. Circulation. 2001;104:2453–2458. doi: 10.1161/hc4401.098429. [DOI] [PubMed] [Google Scholar]
- Chan AY, Soltys CL, Young ME, et al. Activation of AMP-activated protein kinase inhibits protein synthesis associated with hypertrophy in the cardiac myocyte. J Biol Chem. 2004;279:32771–32779. doi: 10.1074/jbc.M403528200. [DOI] [PubMed] [Google Scholar]
- Chen H, Montagnani M, Funahashi T, et al. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem. 2003;278:45021–45026. doi: 10.1074/jbc.M307878200. [DOI] [PubMed] [Google Scholar]
- Cnop M, Havel PJ, Utzschneider KM, et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46:459–469. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- Fasshauer M, Kralisch S, Klier M, et al. Adiponectin gene expression and secretion is inhibited by interleukin-6 in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2003;301:1045–1050. doi: 10.1016/s0006-291x(03)00090-1. [DOI] [PubMed] [Google Scholar]
- Friedman JM. A war on obesity, not the obese. Science. 2003;299:856–858. doi: 10.1126/science.1079856. [DOI] [PubMed] [Google Scholar]
- Fruebis J, Tsao TS, Javorschi S, et al. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci U S A. 2001;98:2005–2010. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohlfeld T, Meyer-Kirchrath J, Vogel YC, et al. Reduction of infarct size by selective stimulation of prostaglandin EP(3)receptors in the reperfused ischemic pig heart. J Mol Cell Cardiol. 2000;32:285–296. doi: 10.1006/jmcc.1999.1072. [DOI] [PubMed] [Google Scholar]
- Hotta K, Funahashi T, Arita Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- Hug C, Wang J, Ahmad NS, et al. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci U S A. 2004;101:10308–10313. doi: 10.1073/pnas.0403382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilercil A, Devereux RB, Roman MJ, et al. Relationship of impaired glucose tolerance to left ventricular structure and function: the Strong Heart Study. Am Heart J. 2001;141:992–998. doi: 10.1067/mhj.2001.115302. [DOI] [PubMed] [Google Scholar]
- Ivanov D, Philippova M, Antropova J, et al. Expression of cell adhesion molecule T-cadherin in the human vasculature. Histochem Cell Biol. 2001;115:231–242. doi: 10.1007/s004180100252. [DOI] [PubMed] [Google Scholar]
- Iwashima Y, Katsuya T, Ishikawa K, et al. Hypoadiponectinemia is an independent risk factor for hypertension. Hypertension. 2004;43:1318–1323. doi: 10.1161/01.HYP.0000129281.03801.4b. [DOI] [PubMed] [Google Scholar]
- Kishida K, Nagaretani H, Kondo H, et al. Disturbed secretion of mutant adiponectin associated with the metabolic syndrome. Biochem Biophys Res Commun. 2003;306:286–292. doi: 10.1016/s0006-291x(03)00940-9. [DOI] [PubMed] [Google Scholar]
- Kistorp C, Faber J, Galatius S, et al. Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation. 2005;112:1756–1762. doi: 10.1161/CIRCULATIONAHA.104.530972. [DOI] [PubMed] [Google Scholar]
- Kobashi C, Urakaze M, Kishida M, et al. Adiponectin inhibits endothelial synthesis of interleukin-8. Circ Res. 2005;97:1245–1252. doi: 10.1161/01.RES.0000194328.57164.36. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Ouchi N, Kihara S, et al. Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circ Res. 2004;94:e27–e31. doi: 10.1161/01.RES.0000119921.86460.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S, Funahashi T, Sakamoto T, et al. The variation of plasma concentrations of a novel, adipocyte derived protein, adiponectin, in patients with acute myocardial infarction. Heart. 2003;89:667. doi: 10.1136/heart.89.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota N, Terauchi Y, Yamauchi T, et al. Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem. 2002;277:25863–25866. doi: 10.1074/jbc.C200251200. [DOI] [PubMed] [Google Scholar]
- Kumada M, Kihara S, Sumitsuji S, et al. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003;23:85–89. doi: 10.1161/01.atv.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- Li J, Miller EJ, Ninomiya-Tsuji J, et al. AMP-activated protein kinase activates p38 mitogen-activated protein kinase by increasing recruitment of p38 MAPK to TAB1 in the ischemic heart. Circ Res. 2005;97:872–879. doi: 10.1161/01.RES.0000187458.77026.10. [DOI] [PubMed] [Google Scholar]
- Liao Y, Takashima S, Maeda N, et al. Exacerbation of heart failure in adiponectin-deficient mice due to impaired regulation of AMPK and glucose metabolism. Cardiovasc Res. 2005;67:705–713. doi: 10.1016/j.cardiores.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Lind L, Lithell H. Decreased peripheral blood flow in the pathogenesis of the metabolic syndrome comprising hypertension, hyperlipidemia, and hyperinsulinemia. Am Heart J. 1993;125:1494–1497. doi: 10.1016/0002-8703(93)90446-g. [DOI] [PubMed] [Google Scholar]
- Lindsay RS, Funahashi T, Hanson RL, et al. Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet. 2002;360:57–58. doi: 10.1016/S0140-6736(02)09335-2. [DOI] [PubMed] [Google Scholar]
- Lord E, Ledoux S, Murphy BD, et al. Expression of adiponectin and its receptors in swine. J Anim Sci. 2005;83:565–578. doi: 10.2527/2005.833565x. [DOI] [PubMed] [Google Scholar]
- Ma K, Cabrero A, Saha PK, et al. Increased beta-oxidation but no insulin resistance or glucose intolerance in mice lacking adiponectin. J Biol Chem. 2002;277:34658–34661. doi: 10.1074/jbc.C200362200. [DOI] [PubMed] [Google Scholar]
- Maeda K, Okubo K, Shimomura I, et al. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1) Biochem Biophys Res Commun. 1996;221:286–289. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- Maeda N, Shimomura I, Kishida K, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- Matsuda M, Shimomura I, Sata M, et al. Role of adiponectin in preventing vascular stenosis. The missing link of adipo-vascular axis. J Biol Chem. 2002;277:37487–37491. doi: 10.1074/jbc.M206083200. [DOI] [PubMed] [Google Scholar]
- Meja KK, Barnes PJ, Giembycz MA. Characterization of the prostanoid receptor(s) on human blood monocytes at which prostaglandin E2 inhibits lipopolysaccharide-induced tumour necrosis factor-alpha generation. Br J Pharmacol. 1997;122:149–157. doi: 10.1038/sj.bjp.0701360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata D, Takeda R, Sata M, et al. AMP-activated protein kinase inhibits angiotensin II-stimulated vascular smooth muscle cell proliferation. Circulation. 2004;110:444–451. doi: 10.1161/01.CIR.0000136025.96811.76. [DOI] [PubMed] [Google Scholar]
- Nawrocki AR, Rajala MW, Tomas E, et al. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to PPARgamma-agonists. J Biol Chem. 2006;281:2654–2660. doi: 10.1074/jbc.M505311200. [DOI] [PubMed] [Google Scholar]
- Okamoto Y, Kihara S, Ouchi N, et al. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2002;106:2767–2770. doi: 10.1161/01.cir.0000042707.50032.19. [DOI] [PubMed] [Google Scholar]
- Orlander PR, Goff DC, Morrissey M, et al. The relation of diabetes to the severity of acute myocardial infarction and post-myocardial infarction survival in Mexican-Americans and non-Hispanic whites. The Corpus Christi Heart Project. Diabetes. 1994;43:897–902. doi: 10.2337/diab.43.7.897. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Kihara S, Arita Y, et al. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473–2476. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Kihara S, Arita Y, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–1301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Kihara S, Arita Y, et al. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation. 2001;103:1057–1063. doi: 10.1161/01.cir.103.8.1057. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Kihara S, Funahashi T, et al. Obesity, adiponectin and vascular inflammatory disease. Curr Opin Lipidol. 2003a;14:561–566. doi: 10.1097/00041433-200312000-00003. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Ohishi M, Kihara S, et al. Association of hypoadiponectinemia with impaired vasoreactivity. Hypertension. 2003b;42:231–234. doi: 10.1161/01.HYP.0000083488.67550.B8. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Kobayashi H, Kihara S, et al. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem. 2004;279:1304–1309. doi: 10.1074/jbc.M310389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajvani UB, Du X, Combs TP, et al. Structure–function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications for metabolic regulation and bioactivity. J Biol Chem. 2003;278:9073–9085. doi: 10.1074/jbc.M207198200. [DOI] [PubMed] [Google Scholar]
- Pineiro R, Iglesias MJ, Gallego R, et al. Adiponectin is synthesized and secreted by human and murine cardiomyocytes. FEBS Lett. 2005;579:5163–5169. doi: 10.1016/j.febslet.2005.07.098. [DOI] [PubMed] [Google Scholar]
- Pischon T, Girman CJ, Hotamisligil GS, et al. Plasma adiponectin levels and risk of myocardial infarction in men. Jama. 2004;291:1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- Pruimboom WM, van Dijk JA, Tak CJ, et al. Interactions between cytokines and eicosanoids: a study using human peritoneal macrophages. Immunol Lett. 1994;41:255–260. doi: 10.1016/0165-2478(94)90142-2. [DOI] [PubMed] [Google Scholar]
- Rauchhaus M, Koloczek V, Volk H, et al. Inflammatory cytokines and the possible immunological role for lipoproteins in chronic heart failure. Int J Cardiol. 2000;76:125–133. doi: 10.1016/s0167-5273(00)00224-2. [DOI] [PubMed] [Google Scholar]
- Reilly MP, Rader DJ. The metabolic syndrome: more than the sum of its parts? Circulation. 2003;108:1546–1551. doi: 10.1161/01.CIR.0000088846.10655.E0. [DOI] [PubMed] [Google Scholar]
- Russell RR, Bergeron R, Shulman GI, et al. Translocation of myocardial GLUT-4 and increased glucose uptake through activation of AMPK by AICAR. Am J Physiol. 1999;277:H643–H649. doi: 10.1152/ajpheart.1999.277.2.H643. [DOI] [PubMed] [Google Scholar]
- Russell RR, Li J, Coven DL, et al. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest. 2004;114:495–503. doi: 10.1172/JCI19297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter MK, Parise H, Benjamin EJ, et al. Impact of glucose intolerance and insulin resistance on cardiac structure and function: sex-related differences in the Framingham Heart Study. Circulation. 2003;107:448–454. doi: 10.1161/01.cir.0000045671.62860.98. [DOI] [PubMed] [Google Scholar]
- Schannwell CM, Schneppenheim M, Perings S, et al. Left ventricular diastolic dysfunction as an early manifestation of diabetic cardiomyopathy. Cardiology. 2002;98:33–39. doi: 10.1159/000064682. [DOI] [PubMed] [Google Scholar]
- Scherer PE, Williams S, Fogliano M, et al. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- Shibata R, Ouchi N, Ito M, et al. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med. 2004a;10:1384–1389. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata R, Ouchi N, Kihara S, et al. Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of AMP-activated protein kinase signaling. J Biol Chem. 2004b;279:28670–28674. doi: 10.1074/jbc.M402558200. [DOI] [PubMed] [Google Scholar]
- Shibata R, Sato K, Pimentel DR, et al. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med. 2005;11:1096–1103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon SD, McMurray JJ, Pfeffer MA, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352:1071–1080. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- Spranger J, Kroke A, Mohlig M, et al. Adiponectin and protection against type 2 diabetes mellitus. Lancet. 2003;361:226–228. doi: 10.1016/S0140-6736(03)12255-6. [DOI] [PubMed] [Google Scholar]
- Steinberg HO, Chaker H, Leaming R, et al. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest. 1996;97:2601–2610. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Saegusa S, Sumino H, et al. Adiponectin, T-cadherin and tumour necrosis factor-alpha in damaged cardiomyocytes from autopsy specimens. J Int Med Res. 2005;33:236–244. doi: 10.1177/147323000503300212. [DOI] [PubMed] [Google Scholar]
- Terai K, Hiramoto Y, Masaki M, et al. AMP-activated protein kinase protects cardiomyocytes against hypoxic injury through attenuation of endoplasmic reticulum stress. Mol Cell Biol. 2005;25:9554–9575. doi: 10.1128/MCB.25.21.9554-9575.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian R, Musi N, D’Agostino J, et al. Increased adenosine monophosphate-activated protein kinase activity in rat hearts with pressure-overload hypertrophy. Circulation. 2001;104:1664–1669. doi: 10.1161/hc4001.097183. [DOI] [PubMed] [Google Scholar]
- Tomas E, Tsao TS, Saha AK, et al. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci U S A. 2002;99:16309–16313. doi: 10.1073/pnas.222657499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyer C, Funahashi T, Tanaka S, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- Wolk R, Berger P, Lennon RJ, et al. Body mass index: a risk factor for unstable angina and myocardial infarction in patients with angiographically confirmed coronary artery disease. Circulation. 2003;108:2206–2211. doi: 10.1161/01.CIR.0000095270.85646.E8. [DOI] [PubMed] [Google Scholar]
- Wu X, Motoshima H, Mahadev K, et al. Involvement of AMP-activated protein kinase in glucose uptake stimulated by the globular domain of adiponectin in primary rat adipocytes. Diabetes. 2003;52:1355–1363. doi: 10.2337/diabetes.52.6.1355. [DOI] [PubMed] [Google Scholar]
- Xiao CY, Hara A, Yuhki K, et al. Roles of prostaglandin I(2) and thromboxane A(2) in cardiac ischemia-reperfusion injury: a study using mice lacking their respective receptors. Circulation. 2001;104:2210–2215. doi: 10.1161/hc4301.098058. [DOI] [PubMed] [Google Scholar]
- Xiao CY, Yuhki K, Hara A, et al. Prostaglandin E2 protects the heart from ischemia-reperfusion injury via its receptor subtype EP4. Circulation. 2004;109:2462–2468. doi: 10.1161/01.CIR.0000128046.54681.97. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Ito Y, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- Yang Q, Graham TE, Mody N, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- Yilmaz MB, Biyikoglu SF, Akin Y, et al. Obesity is associated with impaired coronary collateral vessel development. Int J Obes Relat Metab Disord. 2003;27:1541–1545. doi: 10.1038/sj.ijo.0802474. [DOI] [PubMed] [Google Scholar]


