Abstract
Purpose
Current standard chemotherapeutic regimens for malignant melanoma are unsatisfactory. Although in vitro studies of arsenic trioxide (ATO) have demonstrated promise against melanoma, recent phase II clinical trials have failed to show any significant clinical benefit when used as a single agent. To enhance the efficacy of ATO in the treatment of melanoma, we sought to identify compounds that potentiate the cytotoxic effects of ATO in melanoma cells. Through a screen of 2000 marketed drugs and naturally occurring compounds, a variety of antibiotic inhibitors of mitochondrial protein translation were identified.
Methods
The mechanism of action for the most effective agent identified, thiostrepton, was examined in a panel of melanoma cells. Effects of combinatorial ATO and thiostrepton treatment on cytotoxicity, apoptosis, mitochondrial protein content, and reactive oxygen species (ROS) were assessed.
Results
Thiostrepton (1μM) sensitized 3 out of 5 melanoma cell lines to ATO-mediated growth inhibition. Treatment with thiostrepton resulted in reduced levels of the mitochondrial-encoded protein cytochrome oxidase I (COX1). Exposure to thiostrepton in combination with ATO resulted in increased levels of cleaved poly (ADP-ribose) polymerase and cellular ROS. The growth inhibitory and pro-apototic effects of addition of the ATO/thiostrepton combination were reversed by the free radical scavenger N-acetyl-l-cysteine.
Conculsions
Our data suggest that thiostrepton enhances the cytotoxic effects of ATO through a ROS-dependent mechanism. Co-administration of oxidative stress-inducing drugs such as thiostrepton in order to enhance the efficacy of ATO in the treatment of melanoma warrants further investigation.
Keywords: Melanoma, Arsenic trioxide, Thiostrepton, Reactive oxygen species, Chemoresistance
Introduction
The incidence of melanoma is increasing at an alarming rate (11). Furthermore, metastatic melanoma has a poor prognosis, with a median survival of 6 months (7). Current chemotherapeutic regimens for malignant melanoma provide limited clinical benefit with response rates ranging from 10–20% and fail to improve patient survival (24). The need for new effective therapies in the treatment of metastatic melanoma is clear.
Arsenic trioxide (ATO) is an anti-cancer agent whose use originated in traditional Chinese medicine. More recently, the discovery of its profound efficacy in acute promyelocytic leukemia (APL) has renewed interest in ATO as an antineoplastic agent (6, 25). Clinical studies have demonstrated the utility of ATO in other hematologic malignancies including multiple myeloma (3) and myelodysplastic syndrome (20). ATO has also shown promise in the treatment of solid tumors including neuroblastoma, head and neck cancer, gastric cancer, prostate and ovarian cancers, and renal cell carcinoma (1, 12, 23, 27, 31). While in vitro studies reportedly demonstrated ATO-mediated induction of apoptosis in a variety of melanoma cell lines (13), ATO showed only modest activity in patients with metastatic melanoma when administered as a single agent (17, 26).
In the present study we sought to identify therapeutic agents that augment the cytotoxic effects of ATO on melanoma. Through a screen of 2000 marketed drugs and naturally occurring compounds and subsequent mechanism-based testing, we have identified a group of antibiotics including tetracyclines and thiostrepton that significantly enhance ATO-mediated growth inhibition and apoptosis in melanoma cells. Our results reveal that these agents-which inhibit eukaryotic mitochondrial translation – sensitize melanoma cells to ATO through a mechanism dependent upon the induction of reactive oxygen species.
Materials and Methods
Reagents
ATO, meclocycline, minocycline, thiostrepton, chloramphenicol, fusidic acid, and N-acetyl-l-cysteine (NAC) were purchased from Sigma-Aldrich (St. Louis, MO).
Cell lines
M-14, SK-Mel-19, SK-Mel-94, SK-Mel-173, and Yusac2 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 2mM L-glutamine, and 1% penicillin-streptomycin (5). All cells were cultured at 37°C in a 10% CO2 humidified atmosphere.
Chemical library screening
The Spectrum library (Microsource Discovery Systems, Gaylordsville, CT), containing 2000 marketed drugs and naturally occurring compounds, was used to screen effects on SK-Mel-19 cells. Cells were seeded at a density of 50,000 cells/cm2 in 96-well plates and allowed to adhere overnight. Library compounds were added to cell cultures at a final concentration of 1μM, either alone or in combination with 1μM ATO. After a 72 hour incubation period, cellular proliferation was measured using the CellTiter 96® AQueous non-radioactive cell proliferation assay (Promega, Madison, WI), according to manufacturer’s instructions. Absorbances were measured using a microplate reader (Biorad model 550) at 495 nm. The absorbance of control wells exposed to vehicle alone defined 100% viability and the effect of drugs on cellular proliferation was expressed as a percentage of cell viability relative to untreated cells.
Apoptosis assay
Cells were treated with 1μM ATO, 1μM thiostrepton, or both for 18 hours. Cells were harvested and cellular lysate was obtained from lysis buffer. Thirty μg of total protein was heat denatured and resolved on 10% SDS-PAGE gels. Following protein transfer to PVDF membrane, samples were probed with primary antibodies to poly (ADP-ribose) polymerase (PARP) and cleaved PARP (rabbit polyclonal, Cell Signaling Technology, Boston, MA). Following wash cycles, membranes were probed with secondary donkey anti-rabbit IgG (Amersham, Piscataway, NJ). Immunoreactive bands were visualized using ECL detection reagent (PerkinElmer, Waltham, MA) and X-OMAT processing. Densitometry values were calculated using ImageQuant TL software.
Mitochondrial translation assay
Cells were incubated with 1μM thiostrepton for 0, 6, 12, 18, 24, or 48 hours. Protein was harvested and western blotting was performed as described above, using primary antibody against COXI (mouse monoclonal, Santa Cruz Biotechnologies, Santa Cruz, CA) and secondary goat anti-mouse IgG (Accurate Chemical and Scientific Corporation, Westbury, NY).
Measurement of intracellular ROS generation
Intracellular ROS generation was directly visualized by fluorescent microscopic examination of cells following staining with the oxidant-sensitive probe H2DCFDA. Cells were incubated with indicated compound(s) for 18 hours. Intracellular ROS levels were determined using Image-iT™ LIVE green reactive oxygen species detection kit (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. DNA was counterstained with Hoechst 33342. Cells were examined under microscopy using fluorescein filter sets (Zeiss Axiophot upright phase contrast).
Results
Tetracyclines and thiostrepton enhance ATO-mediated growth inhibition
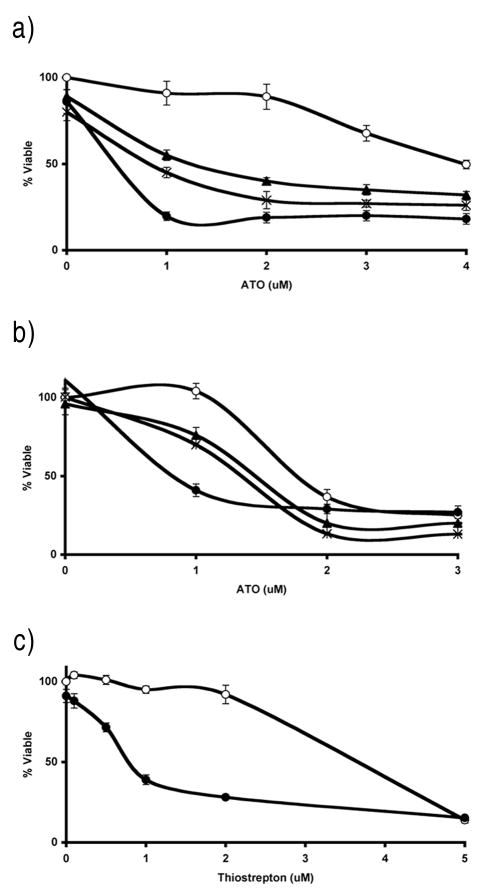
We found that a number of melanoma cell lines demonstrated substantial resistance to the cytotoxic effects of ATO (data not shown). In order to screen for agents that sensitize otherwise-resistant melanoma cells to ATO, we chose highly ATO-resistant SK-Mel-19 melanoma cells, in which the IC50 for growth inhibition by ATO is 4–7 μM, and moderately ATO-resistant M-14 melanoma cells, which display an IC50 of approximately 2 μM (Figure 1). We screened the commercially available Spectrum Collection in SK-Mel-19 cells in the presence and absence of 1 μM ATO, a clinically achievable concentration that is minimally growth inhibitory to the cells in vitro. We sought compounds that lacked growth inhibitory effect when used alone, but which showed a greater than 30% increase in growth inhibition when combined with 1 μM ATO.
Figure 1.
Inhibitors of mitochondrial protein synthesis enhance ATO-mediated growth inhibition in melanoma cells. A,B, Cellular proliferation was measured at indicated ATO and test compound concentrations. ATO alone (○), ATO + 1μM meclocycline (×), ATO + 1μM minocycline (▲), ATO + 1μM thiostrepton (●) in SK-Mel-19 (a) and M-14 (b) melanoma cells. C, Thiostrepton alone (○) or in combination with 1μM ATO (●) in SK-Mel-19 melanoma cells.
Several tetracycline derivatives were found to sensitize SK-Mel-19 and M-14 melanoma cells to the growth inhibitory effects of ATO. As shown in Figure 1, meclocycline and minocycline significantly inhibit cellular proliferation when combined with ATO, compared to either agent alone, in both SK-Mel-19 and M-14 melanoma cells. Because previous studies showed that the antiproliferative effects of tetracyclines in certain mammalian cells results from binding to, and inhibition of, mitochondrial ribosomes (28), we hypothesized that the sensitization to ATO was due to inhibition of mammalian mitochondrial protein synthesis by the tetracyclines (18).
To test this hypothesis, a literature search was performed to identify other inhibitors of mitochondrial protein synthesis structurally unrelated to tetracyclines. Thiostrepton is reported to be a potent inhibitor of both the mitochondrial and prokaryotic ribosome (30). We found that thiostrepton at 1 μM, a concentration previously shown to inhibit mitochondrial protein translation (30), strongly enhanced ATO-mediated inhibition of cellular proliferation in SK-Mel-19 and M-14 cells, despite minimal growth inhibition as a single agent (Figure 1). Furthermore, a range of thiostrepton concentrations enhanced the growth inhibitory effects of 1uM ATO (Figure 1). The addition of 1 μM thiostrepton resulted in a 50% or greater decrease in ATO IC50 for 3 out of 5 melanoma cell lines tested (Table 1).
Table 1.
IC50 for ATO alone and ATO/thiostrepton in a panel of melanoma cell lines. Error values calculated using SEM from at least 4 independent experiments. Ths = thiostrepton.
| Cell line | ATO IC50 | ATO + 1μM Ths IC50 |
|---|---|---|
| SK-Mel-19 | 4.1 ± 0.5 μM | 0.3 ± 0.03 μM |
| M-14 | 1.9 ± 0.03 μM | 0.8 ± 0.1 μM |
| SK-Mel-94 | 1.6 ± 0.1 μM | 0.6 ± 0.03 μM |
| Yusac2 | 1.8 ± 0.1 μM | 1.5 ± 0.2 μM |
| SK-Mel-173 | 1.3 ± 0.1 μM | 1.1 ± 0.1 μM |
We also tested a number of antibiotics described in the literature as less potent inhibitors of mitochondrial protein translation when compared to tetracyclines or thiostrepton. Neither chloramphenicol (18) nor fusidic acid (30) sensitized melanoma cells to ATO-induced inhibition of cellular proliferation when administered at a concentration of 1 μM (data not shown).
ATO in combination with thiostrepton enhances apoptosis
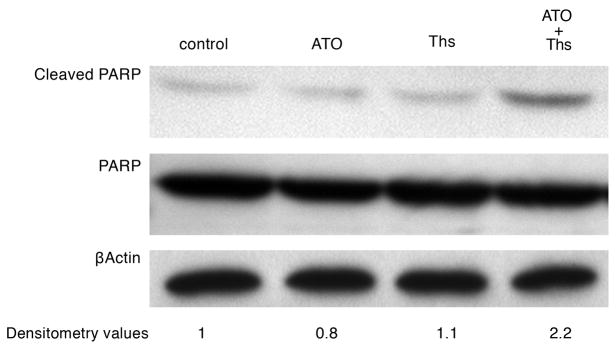
Because combination treatment resulted in reduced cellular proliferation, we evaluated the induction of apoptosis in treated cells via assessment of poly-ADP ribose polymerase (PARP) cleavage. Cleavage of full length PARP protein (116 kDa) into a stable fragment (85 kDa) is an important cellular marker of apoptosis (16). As shown in Figure 2, significantly higher levels of cleaved PARP protein were present in M-14 melanoma cells exposed to the combination of ATO/thiostrepton than with either agent alone, indicating enhanced levels of apoptosis. The addition of ATO or thiostrepton alone had no effect on cleaved PARP levels compared to the control sample.
Figure 2.
Combination of ATO/thiostrepton induces PARP cleavage in M-14 cells. Cells were incubated with ATO 1 μM, thiostrepton 1 μM, or both for 18 hours followed by western blot analysis of cell lysates with antibodies against cleaved PARP (85 kDa), full-length PARP (116 kDa), and β-actin (45 kDa). Densitometry values for cleaved PARP are included. Ths = thiostrepton.
Thiostrepton decreases the level of COXI in M-14 cells
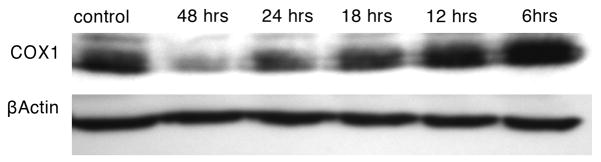
To examine whether thiostrepton inhibits mitochondrial protein translation in melanoma cell lines, levels of COXI were assessed. The cytochrome c oxidase I gene (COXI) is encoded in the mitochondrial genome, and the mRNA product subsequently translated by mitochondrial ribosomes. Incubation of M-14 cells with thiostrepton resulted in a time-dependent decrease in COXI protein levels (Figure 3). A noticeable decrease in COXI protein levels is evident by 18 hours. These results are consistent with inhibition of mitochondrial protein translation by thiostrepton in melanoma cells.
Figure 3.
Thiostrepton decreases the level of COXI in M-14 cells in a time-dependent manner. Cells were incubated with 1 μM thiostrepton for multiple time periods (0–48 hours) followed by western blotting for COXI (38 kDa). An untreated sample was used as a negative control. Afterwards, the membrane was stripped and reprobed with anti-β-actin antibody (45 kDa) as a loading control.
Thiostrepton enhances the cytotoxic effects of ATO through a ROS dependent mechanism
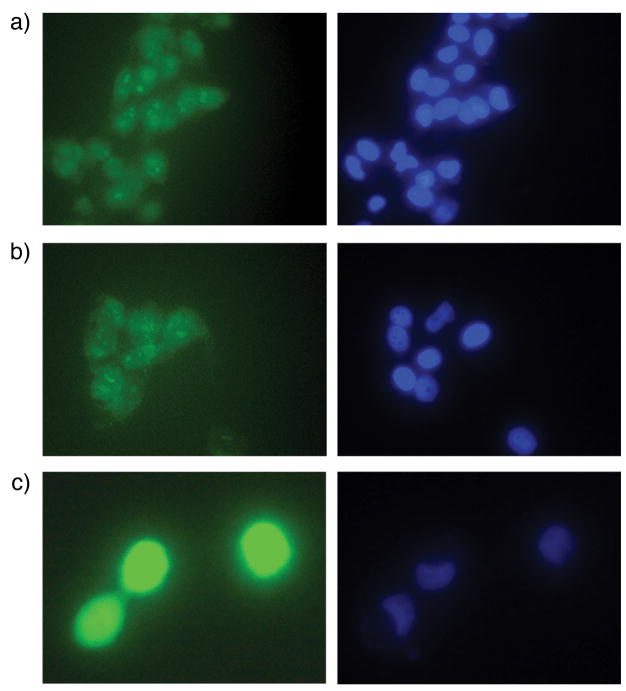
Mitochondrial dysfunction and perturbations in mitochondrial gene expression have been associated with enhanced production of ROS (4). Furthermore, cytotoxic effects of ATO on tumor cells has been postulated to occur through activation of ROS and modulation of glutathione redox systems (8). This led us to investigate the role of ROS in thiostrepton-mediated enhancement of ATO toxicity in melanoma cells. We hypothesized that the combination of ATO and a mitochondrial protein translation inhibitor would increase intracellular ROS levels. To evaluate the role of ROS in ATO/thiostrepton cytotoxicity, we used the oxidant sensitive probe carboxy-H2DCFDA. Carboxy-H2DCFDA enters live cells where esterases deacetylate the molecule. In the presence of ROS, the reduced fluorescein compound is oxidized and emits a bright green fluorescent signal. The ATO/thiostrepton combination resulted in significantly higher levels of intracellular ROS compared to treatment with either drug alone (Figure 4), suggesting that the combined effect of ATO/thiostrepton is mediated by ROS.
Figure 4.
The combination of ATO/thiostrepton induces higher levels of intracellular ROS than either drug alone in M-14 cells. Following incubation with 1μM ATO, 1μM thiostrepton, or both for 18 hours, cells were stained with carboxy-H2DCFDA (green) for intracellular ROS and Hoechst 33342 (blue) for DNA. A, cells treated with ATO. B, cells treated with thiostrepton. C, cells treated with both ATO and thiostrepton. Similar results were obtained in three separate experiments.
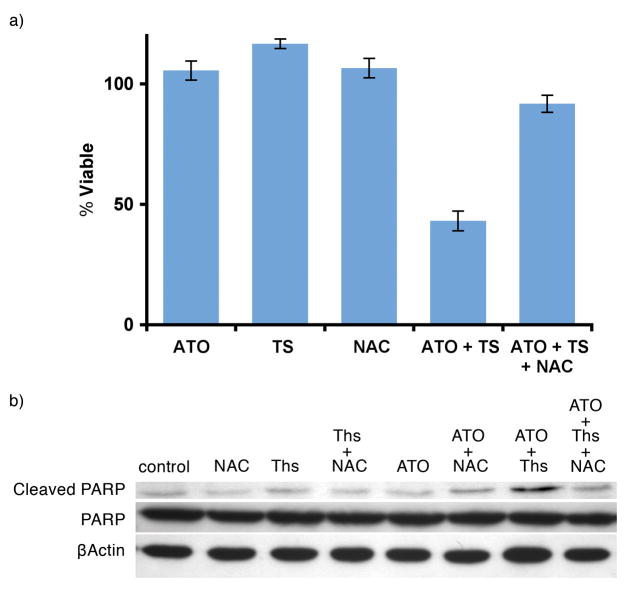
To further demonstrate the involvement of ROS in ATO/thiostrepton cytotoxicity, we repeated cellular proliferation and apoptosis assays with the addition of the free radical scavenger, N-acetyl-l-cysteine (NAC). At a concentration of 100 μM, NAC reversed ATO/thiostrepton-induced growth inhibition and restored levels of cleaved PARP to those observed in untreated cells (Figure 5). Taken together, these data suggest that the cytotoxic effects of ATO/thiostrepton on melanoma cells are mediated by oxidative stress.
Figure 5.
ATO/thiostrepton cytotoxicity is reversed by the free radical scavenger, NAC, in M-14 cells. A, cellular proliferation assay was performed under the following treatment conditions: 1μM ATO, 1μM thiostrepton, 100 μM of NAC or indication combinations. Experiments were performed in triplicate and error bars were calculated using SEM. B, PARP based apoptosis assay. Cells were incubated with ATO 1 μM, thiostrepton 1 μM, 100 μM of NAC or indication combinations for 18 hours. Ths = thiostrepton.
Discussion
The efficacy of ATO in the treatment of APL as well as evidence supporting the promise of this agent in the treatment of other solid and hematological tumors prompted clinical trials evaluating the utility of ATO in the treatment of metastatic melanoma (17, 26). These studies revealed only modest effects of ATO when administered as a single agent, although it was noted that treatment was well-tolerated. Studies that involve co-administration of ATO with other agents in order to enhance its efficacy in the treatment of metastatic melanoma are reportedly in progress (2).
The highly effective antitumor action of ATO in APL occurs at low concentrations of drug (less than 0.5μM) (21). In contrast, most studies of solid tumor cells require strikingly higher doses in order to induce substantial apoptosis or growth inhibition. However, the incidence of ATO-mediated side effects makes the administration of the higher doses needed to achieve these levels clinically impractical. Although not specifically discussed in reports on the clinical trials of ATO in metastatic melanoma, the relative lack of sensitivity of melanoma cell lines in vitro suggests that the modest effect observed in clinical trials may be due in large part to dosing limitations. In order to overcome this clinical limitation, we sought to identify agents that sensitize melanoma cells to ATO, thereby potentially allowing for the enhancement of antitumor effects to be achieved in patients without raising the concentration of ATO administered to toxic levels. In this study, treatment of melanoma cells with the antibiotic thiostrepton in combination with ATO resulted in robust growth inhibition at low concentrations of ATO that are readily achievable in patients (Figure 1).
We have found that thiostrepton-mediated sensitization of melanoma cells to ATO occurs through a ROS dependent mechanism. Thiostrepton inhibits eukaryotic mitochondrial protein translation (Figure 3). Specifically, thiostrepton inhibits both prokaryotic and mammalian mitochondrial ribosomes through binding to the large ribosomal subunit, thereby inhibiting the translocation step of protein translation (19, 22). Although it has been well documented that mitochondrial dysfunction results in altered ROS production, a recent report by Bonawitz et al. (4) demonstrates that defective mitochondrial gene expression leads to increased ROS production in eukaryotes. This correlation led us to investigate the role of ROS in thiostrepton-mediated enhancement of ATO cytotoxicity. We demonstrate that combinatorial treatment of melanoma cells with ATO and thiostrepton results in a significant increase in intracellular ROS levels as compared to treatment with either agent alone (Figure 4). Furthermore, the growth inhibitory effects of ATO/thiostrepton treatment are reversed when melanoma cells are also treated with the free radical scavenger NAC (Figure 5). These data support the notion that increased ROS production (possibly resulting from impaired mitochondrial translation) contributes to thiostrepton–mediated enhancement of ATO cytotoxicity in melanoma.
Multiple cellular actions of ATO have been described, including effects on cellular signaling and alteration of cellular redox systems. Evidence from studies on a range of malignancies has shown that ATO-induced apoptosis is determined in part by intracellular redox status. For example, the susceptibility of various leukemic cell lines to ATO-induced growth inhibition and apoptosis is inversely correlated with glutathione (GSH) content (8). Furthermore, experimental modulation of GSH content can alter the cellular response to ATO. Increasing the GSH content with NAC or lipoic acid rendered leukemic cells more resistant to the cytotoxic effects of ATO (8). Arsenic-resistant NB4 cell clones demonstrated susceptibility to ATO when GSH levels were depleted with L-buthionine-(S,R)-sulfoximine (BSO) (9). In another study, pretreatment with selenite, which increases the activity of glutathione peroxidase (GPx) and therefore lowers H2O2, rendered APL cells resistant to otherwise toxic levels of ATO (15). Similar data exists for solid tumors as well. BSO enhanced ATO-induced apoptosis in renal cell carcinoma (29) and formation of free radicals by the antioxidant trolox enhanced ATO-mediated oxidative stress in breast cancer cells (10). Previous research in our laboratory has shown that redox status is also important in determining melanoma cell response to ATO. BSO sensitized various melanoma cell lines to ATO-induced growth inhibition and apoptosis (unpublished data). The data suggesting that redox status is an important determinant of the cellular response to ATO have led to a number of clinical trials exploring this relationship. For example, ascorbic acid - thought to auto-oxidize in the cell, decreasing the cellular pool of GSH - has shown beneficial effects when administered with ATO to patients with multiple myeloma (3). Indeed, 2 of the 5 melanoma cell lines examined in the current study did not show thiostrepton-mediated sensitization to ATO. Characterization of the redox status of thiostrepton-responsive versus non-responsive melanoma cell lines might provide further insight into the role of ROS in ATO sensitization.
In the present study, we demonstrate that thiostrepton sensitizes melanoma cells to ATO cytotoxicity through an ROS dependent mechanism. Thiostrepton is commercially available as part of a topical antibiotic mixture used to treat bacterial infections in animals and has also been used to effectively treat murine bacterial systemic infections (14). However, to date, it has not been approved for human use. The data presented here warrant further characterization of ATO sensitization by thiostrepton through in vivo studies in order to assess the possible clinical effectiveness of this drug combination in the treatment of metastatic melanoma. Additionally, toxicology studies in animal models will be performed to determine the safest and most beneficial mode of human administration. Other antibiotic inhibitors of mitochondrial protein translation addressed in this study including minocycline may serve as a better candidate for further in vivo study and clinical development given its extensive history of safe use in man as well as oral availability.
While efficacy of ATO against melanoma cells can be demonstrated in vitro, there has been difficulty in translating these results to the clinic. Combination therapy is a potential method to increase the efficacy of ATO in melanoma. Consistent with data from studies of other malignancies, our research suggests that increasing oxidative stress in melanoma cells by inhibition of mitochondrial protein translation sensitizes them to ATO. In future trials of ATO in metastatic melanoma, co-administration of a drug that increases oxidative stress, either by inhibition of mitochondrial protein translation or by some other means, may increase the efficacy of this promising and well-tolerated agent.
Acknowledgments
This work was supported by grants from the NIH (R21AR050645) and by a grant from Jacklyn and Miguel Bezos to SJO.
References
- 1.Akao Y, Nakagawa Y, Akiyama K. Arsenic trioxide induces apoptosis in neuroblastoma cell lines through the activation of caspase 3 in vitro. FEBS Lett. 1999;455:59–62. doi: 10.1016/s0014-5793(99)00841-8. [DOI] [PubMed] [Google Scholar]
- 2.Bael TEPB, Thoreson M, Richmond T, Eyer J, Gollob JA. Presented at the Journal of Clinical Oncology, 2005 ASCO annual meeting proceedings; 2005. p. 7553. Part I of II (June 1 Supplement), 2005. [Google Scholar]
- 3.Berenson JR, Boccia R, Siegel D, Bozdech M, Bessudo A, Stadtmauer E, Talisman Pomeroy J, Steis R, Flam M, Lutzky J, Jilani S, Volk J, Wong SF, Moss R, Patel R, Ferretti D, Russell K, Louie R, Yeh HS, Swift RA. Efficacy and safety of melphalan, arsenic trioxide and ascorbic acid combination therapy in patients with relapsed or refractory multiple myeloma: a prospective, multicentre, phase II, single-arm study. Br J Haematol. 2006;135:174–83. doi: 10.1111/j.1365-2141.2006.06280.x. [DOI] [PubMed] [Google Scholar]
- 4.Bonawitz ND, Rodeheffer MS, Shadel GS. Defective mitochondrial gene expression results in reactive oxygen species-mediated inhibition of respiration and reduction of yeast life span. Mol Cell Biol. 2006;26:4818–29. doi: 10.1128/MCB.02360-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chee DO, Boddie AW, Roth JA, Holmes EC, Morton DL. Production of melanoma-associated antigen(s) by a defined malignant melanoma cell strain grown in chemically defined medium. Cancer Res. 1976;36:1503–9. [PubMed] [Google Scholar]
- 6.Chen GQ, Zhu J, Shi XG, Ni JH, Zhong HJ, Si GY, Jin XL, Tang W, Li XS, Xong SM, Shen ZX, Sun GL, Ma J, Zhang P, Zhang TD, Gazin C, Naoe T, Chen SJ, Wang ZY, Chen Z. In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with downregulation of Bcl-2 expression and modulation of PML-RAR alpha/PML proteins. Blood. 1996;88:1052–61. [PubMed] [Google Scholar]
- 7.Cummins DL, Cummins JM, Pantle H, Silverman MA, Leonard AL, Chanmugam A. Cutaneous malignant melanoma. Mayo Clin Proc. 2006;81:500–7. doi: 10.4065/81.4.500. [DOI] [PubMed] [Google Scholar]
- 8.Dai J, Weinberg RS, Waxman S, Jing Y. Malignant cells can be sensitized to undergo growth inhibition and apoptosis by arsenic trioxide through modulation of the glutathione redox system. Blood. 1999;93:268–77. [PubMed] [Google Scholar]
- 9.Davison K, Cote S, Mader S, Miller WH. Glutathione depletion overcomes resistance to arsenic trioxide in arsenic-resistant cell lines. Leukemia. 2003;17:931–40. doi: 10.1038/sj.leu.2402876. [DOI] [PubMed] [Google Scholar]
- 10.Diaz Z, Colombo M, Mann KK, Su H, Smith KN, Bohle DS, Schipper HM, Miller WH., Jr Trolox selectively enhances arsenic-mediated oxidative stress and apoptosis in APL and other malignant cell lines. Blood. 2005;105:1237–45. doi: 10.1182/blood-2004-05-1772. [DOI] [PubMed] [Google Scholar]
- 11.Edwards BK, Brown ML, Wingo PA, Howe HL, Ward E, Ries LA, Schrag D, Jamison PM, Jemal A, Wu XC, Friedman C, Harlan L, Warren J, Anderson RN, Pickle LW. Annual report to the nation on the status of cancer, 1975–2002, featuring population-based trends in cancer treatment. J Natl Cancer Inst. 2005;97:1407–27. doi: 10.1093/jnci/dji289. [DOI] [PubMed] [Google Scholar]
- 12.Hyun Park W, Hee Cho Y, Won Jung C, Oh Park J, Kim K, Hyuck Im Y, Lee MH, Ki Kang W, Park K. Arsenic trioxide inhibits the growth of A498 renal cell carcinoma cells via cell cycle arrest or apoptosis. Biochem Biophys Res Commun. 2003;300:230–5. doi: 10.1016/s0006-291x(02)02831-0. [DOI] [PubMed] [Google Scholar]
- 13.Islam M, Kirkwood JM. Arsenic trioxide induces apoptosis of human melanoma cell lines in vitro. Proc Am Soc Clin Oncol. 2001 2001 (abstr 1435) [Google Scholar]
- 14.Jambor WP, Steinberg BA, Suydam LO. Thiostrepton, a new antibiotic. III. In vivo studies. Antibiot Annu. 1955;3:562–5. [PubMed] [Google Scholar]
- 15.Jing Y, Dai J, Chalmers-Redman RM, Tatton WG, Waxman S. Arsenic trioxide selectively induces acute promyelocytic leukemia cell apoptosis via a hydrogen peroxide-dependent pathway. Blood. 1999;94:2102–11. [PubMed] [Google Scholar]
- 16.Kaufmann SH, Desnoyers S, Ottaviano Y, Davidson NE, Poirier GG. Specific proteolytic cleavage of poly(ADP-ribose) polymerase: an early marker of chemotherapy-induced apoptosis. Cancer Res. 1993;53:3976–85. [PubMed] [Google Scholar]
- 17.Kim KB, Bedikian AY, Camacho LH, Papadopoulos NE, McCullough C. A phase II trial of arsenic trioxide in patients with metastatic melanoma. Cancer. 2005;104:1687–92. doi: 10.1002/cncr.21386. [DOI] [PubMed] [Google Scholar]
- 18.McKee EE, Ferguson M, Bentley AT, Marks TA. Inhibition of mammalian mitochondrial protein synthesis by oxazolidinones. Antimicrob Agents Chemother. 2006;50:2042–9. doi: 10.1128/AAC.01411-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan D, Kirillov SV, Cooperman BS. Kinetically competent intermediates in the translocation step of protein synthesis. Mol Cell. 2007;25:519–29. doi: 10.1016/j.molcel.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raza A, Buonamici S, Lisak L, Tahir S, Li D, Imran M, Chaudary NI, Pervaiz H, Gallegos JA, Alvi MI, Mumtaz M, Gezer S, Venugopal P, Reddy P, Galili N, Candoni A, Singer J, Nucifora G. Arsenic trioxide and thalidomide combination produces multi-lineage hematological responses in myelodysplastic syndromes patients, particularly in those with high pre-therapy EVI1 expression. Leuk Res. 2004;28:791–803. doi: 10.1016/j.leukres.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 21.Rego EM, He LZ, Warrell RP, Jr, Wang ZG, Pandolfi PP. Retinoic acid (RA) and As2O3 treatment in transgenic models of acute promyelocytic leukemia (APL) unravel the distinct nature of the leukemogenic process induced by the PML-RARalpha and PLZF-RARalpha oncoproteins. Proc Natl Acad Sci U S A. 2000;97:10173–8. doi: 10.1073/pnas.180290497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodnina MV, Savelsbergh A, Matassova NB, Katunin VI, Semenkov YP, Wintermeyer W. Thiostrepton inhibits the turnover but not the GTPase of elongation factor G on the ribosome. Proc Natl Acad Sci U S A. 1999;96:9586–90. doi: 10.1073/pnas.96.17.9586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seol JG, Park WH, Kim ES, Jung CW, Hyun JM, Kim BK, Lee YY. Effect of arsenic trioxide on cell cycle arrest in head and neck cancer cell line PCI-1. Biochem Biophys Res Commun. 1999;265:400–4. doi: 10.1006/bbrc.1999.1697. [DOI] [PubMed] [Google Scholar]
- 24.Serrone L, Zeuli M, Sega FM, Cognetti F. Dacarbazine-based chemotherapy for metastatic melanoma: thirty-year experience overview. J Exp Clin Cancer Res. 2000;19:21–34. [PubMed] [Google Scholar]
- 25.Soignet SL, Maslak P, Wang ZG, Jhanwar S, Calleja E, Dardashti LJ, Corso D, DeBlasio A, Gabrilove J, Scheinberg DA, Pandolfi PP, Warrell RP., Jr Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N Engl J Med. 1998;339:1341–8. doi: 10.1056/NEJM199811053391901. [DOI] [PubMed] [Google Scholar]
- 26.Tarhini AA, Kirkwood JM, Gooding WE, Stuckert JJ, Agarwala SS. Presented at the American Society of Clinical Oncology; 2007. (abstr 8569) [DOI] [PubMed] [Google Scholar]
- 27.Uslu R, Sanli UA, Sezgin C, Karabulut B, Terzioglu E, Omay SB, Goker E. Arsenic trioxide-mediated cytotoxicity and apoptosis in prostate and ovarian carcinoma cell lines. Clin Cancer Res. 2000;6:4957–64. [PubMed] [Google Scholar]
- 28.van den Bogert C, Dontje BH, Holtrop M, Melis TE, Romijn JC, van Dongen JW, Kroon AM. Arrest of the proliferation of renal and prostate carcinomas of human origin by inhibition of mitochondrial protein synthesis. Cancer Res. 1986;46:3283–9. [PubMed] [Google Scholar]
- 29.Wu XX, Ogawa O, Kakehi Y. Enhancement of arsenic trioxide-induced apoptosis in renal cell carcinoma cells by L-buthionine sulfoximine. Int J Oncol. 2004;24:1489–97. [PubMed] [Google Scholar]
- 30.Zhang L, Ging NC, Komoda T, Hanada T, Suzuki T, Watanabe K. Antibiotic susceptibility of mammalian mitochondrial translation. FEBS Lett. 2005;579:6423–7. doi: 10.1016/j.febslet.2005.09.103. [DOI] [PubMed] [Google Scholar]
- 31.Zhang TC, Cao EH, Li JF, Ma W, Qin JF. Induction of apoptosis and inhibition of human gastric cancer MGC-803 cell growth by arsenic trioxide. Eur J Cancer. 1999;35:1258–63. doi: 10.1016/s0959-8049(99)00106-9. [DOI] [PubMed] [Google Scholar]