Abstract
Background
Infection with Vibrio cholerae induces durable immunity against subsequent disease, a process hypothesized to reflect anamnestic immune responses at the intestinal mucosa. The presence of antigen-specific memory B cells may therefore be a more direct measure of protection than serum antibody responses.
Methods
We measured immunoglobulin (Ig) G memory B cells specific to cholera toxin B subunit (CTB) in 14 patients up to 90 days after V. cholerae O1 infection, by polyclonal stimulation of peripheral blood mononuclear cells followed by standard enzyme-linked immunospot assay.
Results
All patients generated CTB-specific IgG memory B cell responses by day 30 (mean, 0.10% of total circulating IgG memory B cells; range, 0.037%−0.28%), which persisted to day 90 (mean, 0.07%; range, 0.003%−0.27%). In contrast, circulating CTB-specific IgG antibody-secreting cells and serum vibriocidal and anti-CTB antibody responses peaked on day 7 and declined to undetectable or significantly lower levels by day 90.
Conclusions
CTB-specific IgG memory B cell responses are detectable in the circulation at least 3 months after V. cholerae O1 infection and remain measurable even after serum antibody titers have declined to undetectable or considerably lower levels. This suggests that antigen-specific memory B cells may be an important long-term marker of the immune response to cholera.
Vibrio cholerae is an important cause of diarrheal morbidity and mortality. The vast majority of human disease is attributed to V. cholerae serogroups O1 and O139, both of which are noninvasive pathogens that colonize the small intestine and cause secretory diarrhea [1]. Studies in areas of endemicity and in volunteers have demonstrated that infection with V. cholerae provides long-term protection against subsequent disease [2, 3]. However, little is known about the nature of protective immunity to cholera.
Patients with cholera develop humoral immune responses to several V. cholerae antigens, including cholera toxin B subunit (CTB), lipopolysaccharide (LPS), and the toxin-coregulated pilus (TCP). However, levels of serum anti-LPS and anti-CTB IgG antibodies have not been shown to correlate with protection in humans [2], and it is not known whether anti-TCP antibodies play a role in immunity. The only known correlate of protection from V. cholerae O1 infection is the serum vibriocidal antibody, a complement-fixing bactericidal antibody response. In areas endemic for V. cholerae O1, the vibriocidal titer increases with age and is inversely related to colonization and disease with V. cholerae [2-5]. However, the role played by a complement-fixing antibody in protection against a noninvasive pathogen has not been elucidated, and there is no threshold vibriocidal antibody titer at which complete protection is achieved. This suggests that the vibriocidal antibody may be a marker of other protective immune responses occurring at the mucosal surface [6].
Because V. cholerae is noninvasive, it is hypothesized that a protective mucosal response is mediated by the secretory IgA (sIgA) system of the gut-associated lymphoid tissue (GALT) [7-9]. Studies of gastrointestinal lavage samples from volunteers receiving CTB orally demonstrate a potent induction of anti-CTB sIgA that peaks 7 days after ingestion and declines to baseline within 15 months. However, after boosting at 15 months, these volunteers mount anamnestic responses, with a rapid return to peak titers in as short a time as 3 days [7, 9]. These observations of mucosal immunologic memory support a model in which protection from cholera may be mediated by rapid anamnestic responses of memory B cells in the GALT, to CTB or other V. cholerae antigens.
Despite their potential importance, however, memory B cell responses in cholera have not been characterized. An assay recently described by Crotty et al. has made it possible to quantify small populations of antigen-specific memory B cells in the peripheral circulation; in this approach, memory B cells are polyclonally stimulated to proliferate and differentiate into antibody-secreting cells (ASCs), which can be quantified by isotype and antigen specificity using a standard enzyme-linked immunospot (ELISPOT) assay [10-12]. This assay has been used to characterize immunologic memory after vaccination against smallpox, anthrax, and influenza and after exposure to Bacillus anthracis [10, 11, 13-15]. However, the development of antigen-specific memory B cell populations in naturally acquired noninvasive infections at the mucosal surface has not been studied.
In the present study, we characterized the generation of antigen-specific memory B cells in patients with cholera and examined the relationship between these responses and other previously characterized immunologic markers of V. cholerae infection. The antigen CTB was chosen because it is a potent immunogen and has been shown to induce robust anamnestic responses in V. cholerae infection on rechallenge of previously infected individuals. We present here evidence of the development and maintenance of a circulating CTB-specific memory B cell population after V. cholerae O1 infection.
METHODS
Study subjects
The study was approved by the institutional review boards of the International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B) and Massachusetts General Hospital. Informed consent was obtained from 15 patients admitted to Dhaka Hospital of the ICDDR,B with severe acute watery diarrhea and stool cultures positive for V. cholerae O1 between December 2006 and May 2007. All patients were treated with intravenous fluid resuscitation and doxycycline. Blood samples were obtained on the second day of hospitalization and again on days 7, 30, and 90. Blood samples at each time point were assayed for vibriocidal antibody, serum anti-CTB IgG and IgA, and circulating CTB-specific IgG ASCs. The CTB-specific IgG memory B cell ELISPOT assay and ELISA for antibody in memory B cell culture supernatants were performed on study days 2, 30, and 90. One patient opted out of the study after the baseline assessment and was excluded from the analysis.
Isolation of peripheral blood mononuclear cells (PBMCs)
Heparinized blood was diluted in PBS, and PBMCs and serum were isolated by centrifugation on Ficoll-Isopaque (Pharmacia). Serum was frozen at −70°C before use in serologic assays. Isolated PBMCs were resuspended to a concentration of 1 × 106 cells/mL in RPMI complete medium (Gibco) with 10% heat-inactivated fetal bovine serum (FBS; HyClone). Resuspended cells were used immediately for the circulating CTB-specific IgG ASC ELISPOT assay as well as for the memory B cell culture and memory B cell culture supernatant assays.
Serum vibriocidal antibody assay
Vibriocidal antibody assays were performed by standardized methods described elsewhere [7], using guinea pig complement and the homologous serotype of V. cholerae O1 El Tor Ogawa (X25049) or El Tor Inaba (T-19479) as the target organism. The vibriocidal titer was defined as the reciprocal of the highest serum dilution resulting in >50% reduction in optical density (OD) compared with the OD of control wells without serum.
Serum and memory B cell culture supernatant anti-CTB IgG and IgA ELISA
Anti-CTB IgG and IgA in serum samples and in memory B cell culture supernatants were quantified using ELISA protocols described elsewhere [16]. ELISA plates were coated with ganglioside GM1 (0.3 nmol/mL) followed by recombinant CTB (2.5 μg/mL) (gifts from A. M. Svennerholm, Göteborg University). Serum (100 μL/well, diluted 1:200 in 0.1% bovine serum albumin [BSA] in PBS-Tween) or memory B cell culture supernatant (100 μL/well, diluted 1:5 in 0.1% BSA in PBS-Tween) was added. Horseradish peroxidase–conjugated antibodies to either human IgG and IgA were applied, and plates were developed with o-phenylenediamine substrate (Sigma) in 0.1 mol/L sodium citrate buffer and 0.1% hydrogen peroxide. Plates were read kinetically at an OD of 450 nm for 5 min. The maximal rate of OD change was expressed in milliabsorbance units per minute, and ELISA units were standardized by calculating the ratio of the test sample to a sample of pooled convalescent-stage serum from patients who had recovered from cholera, run as a positive control on each plate.
Circulating IgG antibody ASC ELISPOT assay
Nitrocellulose-bottomed plates (MAHAN 4550; Millipore) were coated with GM1 (3 nmol/mL) or affinity-purified goat anti–human IgG (5 μg/mL) (Jackson Immunology Research) and incubated overnight at 4°C. Recombinant CTB was applied to the GM1-coated plates, 5 × 105 PBMCs/well were added to the CTB-coated plates, and 1 × 105 PBMCs were added to each starting well of the total IgG–coated plates and serially diluted. After a 4-h incubation, plates were washed, and IgG ASCs were detected using horseradish peroxidase–conjugated mouse anti–human IgG (Hybridoma Laboratories) diluted 1:1000. After overnight incubation, plates were developed with 30% hydrogen peroxide in 3-amino-9-ethyl carbazole substrate. Red spots on the nitrocellulose membranes, representing individual ASCs, were enumerated independently by 2 individuals, and the data were averaged. The number of CTB-specific IgG ASCs was divided by the number of total IgG ASCs, with the result expressed as a percentage.
Memory B cell culture and ELISPOT assay
Memory B cell assays were performed on days 2, 30, and 90, as described by Crotty et al. [10, 11]. Under sterile conditions, 5 × 105 PBMCs/well were placed in 24-well cell culture plates (BD Biosciences) containing culture medium optimized to stimulate antigen-independent proliferation and terminal differentiation of memory B cells into ASCs. The stimulation medium consisted of RPMI 1640, 10% FBS, 200 U/mL penicillin, 200 μg/mL streptomycin, 200 mmol/L l-glutamine, 50 mmol/L β-mercaptoethanol, and a mixture of 3 B cell mitogens: 6 μg/mL CpG oligonucleotide (Operon), a 1:100,000 dilution of crude pokeweed mitogen extract, and a 1:10,000 dilution of fixed Staphylococcus aureus Cowan strain (Sigma). As a negative control, PBMCs were placed in RPMI medium alone. Plates were incubated at 37°C in 5% CO2 for 5−6 days, after which cells were harvested and washed and CTB-specific and total IgG ELISPOT assays were performed as described above. From each culture well, 20% of cells were used for total IgG ELISPOT assays, and 80% were used for CTB-specific IgG ELISPOT assays. ELISPOT counts were expressed as the percentage of CTB-specific IgG memory B cells among the total IgG memory B cells. Plates coated with keyhole limpet hemocyanin (KLH; 2.5 μg/mL; Pierce Biotechnology) were used as negative controls. We defined appropriate stimulation of PBMCs in our assay as stimulation generating >4500 total IgG memory B cells after culture and stimulation of 5 × 105 PBMCs. This approximates the definition of standard stimulation described elsewhere and represented the 10% percentile for all stimulated cultures [10]. Individual wells that did not have appropriate stimulation were excluded from the analysis.
Antibody in memory B cell culture supernatant ELISA
We assayed supernatants of PBMCs cultured in the memory B cell assay for anti-CTB IgG and IgA secreted by memory B cell–derived ASCs. Supernatants were collected after 5−6 days of incubation, using the mitogen cocktail described above, and mixed with a protease inhibitor cocktail consisting of aprotinin (0.15 μmol/L), leupeptin (10 μmol/L), sodium azide (15 μmol/L), and 4-(aminoethyl)benzenesulfonyl fluoride (0.2 μmol/L). Supernatants were then aliquoted and frozen at −70°C until assayed for anti-CTB IgG and IgA by ELISA as described above.
Statistical analyses
Differences in immunologic responses were tested for significance by paired nonparametric (Wilcoxon signed rank) tests, and correlations were analyzed by Spearman's ρ statistic. Analyses were performed with GraphPad Prism (version 4.0) and SPSS (version 12.0) software.
RESULTS
Study population
Fourteen of 15 patients completed follow-up to day 90 and were included in the analysis. Demographic, epidemiologic, and clinical features of these 14 patients are provided in table 1.
Table 1.
Demographic, serologic, and clinical features of patients who completed follow-up to day 90 (n = 14).
| Feature | Value |
|---|---|
| Age, mean ± SD, years | 30.1 ± 10.7 |
| Sex | |
| Female | 8 |
| Male | 6 |
| Vibrio cholerae O1 serotype | |
| Inaba | 7 |
| Ogawa | 7 |
| Duration of diarrhea at presentation, mean ± SD, h | 18.6 ± 20.0 |
| Duration of hospitalization, mean ± SD, h | 38.9 ± 26.4 |
NOTE. Data are no. of patients, unless otherwise indicated.
Vibriocidal and serum anti-CTB responses
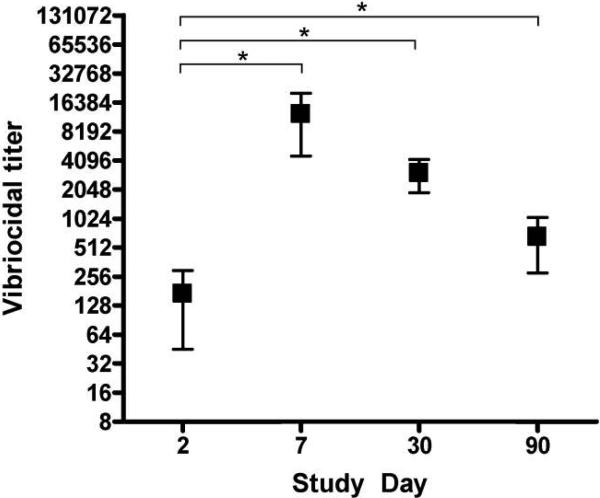
V. cholerae O1 Inaba or Ogawa serotype–specific vibriocidal antibody responses were measured on study days 2, 7, 30, and 90 (figure 1). The geometric mean vibriocidal titer on day 2 was 78.4 (95% confidence interval [CI], 35.71−172.0), and, as expected, all patients mounted strong vibriocidal responses by day 7 (geometric mean titer, 6547) (P < .001). Seroconversion, represented by a 4-fold or greater rise in vibriocidal titer, was seen in all 14 patients. The vibriocidal titer declined to a geometric mean of 2207 (95% CI, 1276−3816) on day 30 and further to 430.7 (95% CI, 240.8−770.2) by day 90, although the 90-day titer remained higher than at baseline (P = .006).
Figure 1.
Serum vibriocidal antibody responses. *P < .05 (Wilcoxon signed rank test).
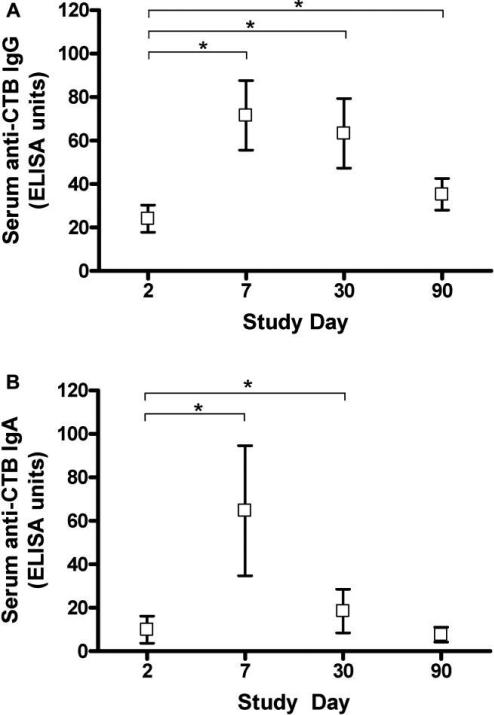
Serum anti-CTB IgA and IgG levels were measured on days 2, 7, 30, and 90. Nine patients demonstrated peak IgA and IgG levels on day 7, and 5 patients had peak IgG levels on day 30 (figure 2A and 2B). On day 90, the anti-CTB IgG level was higher than on day 2 (P = .017), whereas the IgA level had returned to baseline.
Figure 2.
Serum cholera toxin B subunit (CTB)–specific IgG and IgA responses. *P < .05 (Wilcoxon signed rank test).
Circulating CTB-specific IgG ASCs
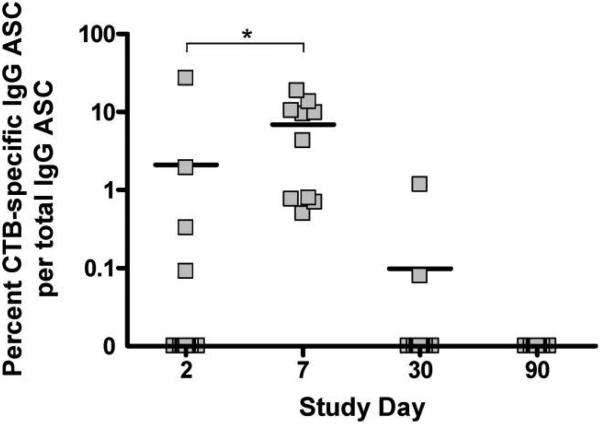
The ASC assay quantifies GALT-activated ASCs as they transiently circulate before returning to mucosal effector sites, and it is therefore considered to measure acute and recent mucosal immune responses [17-19]. CTB-specific IgG ASC ELISPOT assays were performed on days 2, 7, 30, and 90 (figure 3). On day 2, 10 patients had no detectable circulating CTB-specific IgG ASCs and 4 had detectable ASCs, possibly representing an early primary or anamnestic response to infection. In all patients, ASC counts peaked on day 7, to a mean of 6.9% of total IgG ASCs. Only 2 patients still had detectable ASC responses on day 30, and responses were undetectable in all patients by day 90.
Figure 3.
Circulating cholera toxin B subunit (CTB)–specific IgG antibody-secreting cell (ASC) responses. *P < .05 (Wilcoxon signed rank test).
Measurement of CTB-specific IgG memory B cells
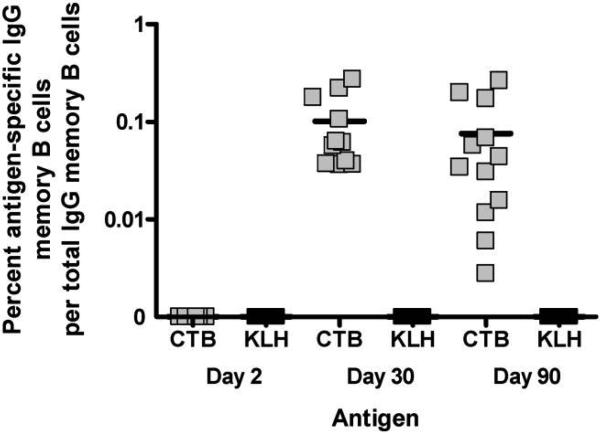
Memory B cell cultures and ELISPOT assays were performed on days 2, 30, and 90 (figure 4). On day 2, during acute illness, we observed that polyclonal stimulation with B cell mitogens resulted in limited proliferation of detectable memory B cells after 5 days of culture, with 7 of 14 patients exhibiting little or no proliferation (average total IgG memory B cells, 4416 cells/5 × 105 PBMCs; range, 390−9320). One of the 7 patients who responded to stimulation on day 2 had detectable CTB-specific memory B cells, but this patient also had circulating CTB-specific IgG ASCs detectable at this time and was therefore excluded from the analysis on day 2. None of the patients without circulating CTB-specific IgG ASCs demonstrated detectable CTB-specific IgG memory B cells on day 2. However, on days 30 and 90, almost all patients responded with a significant proliferation of lymphocytes to stimulation (on day 30, 11/14 patients; on day 90, 13/14 patients; average total IgG memory B cells, 18,461 cells/5 × 105 PBMCs; range, 247−52,500), and all patients had measurable CTB-specific IgG memory B cells in stimulated cultures. There was no correlation between the degree of stimulation of total IgG memory B cells achieved and the proportion of CTB-specific memory B cells detected (Spearman's ρ = −0.28; P = .20). No antigen-specific responses were detected against the negative control antigen KLH.
Figure 4.
Cholera toxin B subunit (CTB)–specific (gray squares) and keyhole limpet hemocyanin (KLH)–specific (black squares) IgG memory B cell kinetics. On all days, data points were excluded if peripheral blood mononuclear cells (PBMCs) were poorly stimulated (<4500 total IgG memory B cells/5 × 105 PBMCs cultured). Additionally, data points were excluded if a CTB-specific antibody-secreting cell response was already present before culture and stimulation.
CTB-specific IgG memory B cells on day 30 comprised 0.10% of total IgG ASCs (range, 0.037%−0.28%). On day 90, this proportion was 0.07%, and the range widened to 0.003%−0.27% (figure 4). Frequencies on days 30 and 90 were not significantly different from each other (P = .58). Furthermore, there was no significant correlation between the CTB-specific memory B cell responses of individuals on days 30 and 90 (Spearman's ρ = 0.13; P = .70).
Measurement of CTB-specific IgA and IgG in supernatants of memory B cell cultures
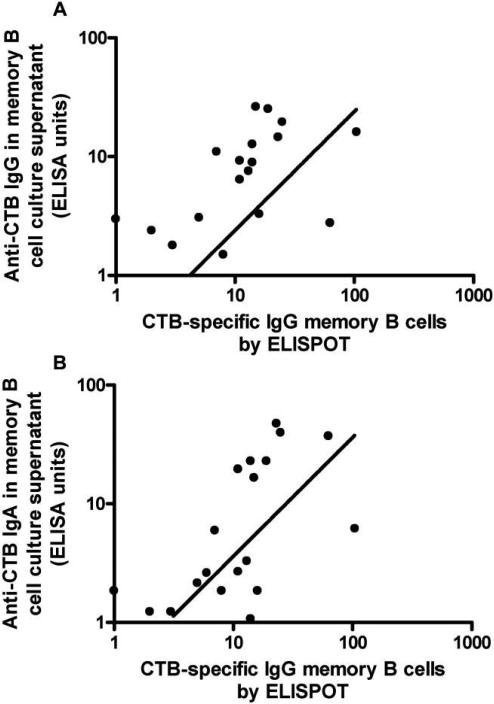
Anti-CTB IgA and IgG levels in memory B cell culture supernatants were assayed on days 2, 30, and 90. Both IgA and IgG levels peaked on day 30 and declined significantly from day 30 to 90 (for IgA, P = .004; for IgG, P = .012). To evaluate whether measurement of anti-CTB IgA and IgG in supernatants of memory B cell cultures might serve as a proxy measure of memory B cells in the same cultures quantified by ELISPOT assay, we examined the correlation between ELISA antibody measurements in culture supernatants and ELISPOT measurements of CTB-specific IgG memory B cells. We found that CTB-specific IgG memory B cell counts were strongly correlated with both anti-CTB IgG (Spearman's ρ = 0.7042; P < .001) and anti-CTB IgA (Spearman's ρ = 0.6733; P < .001) in culture supernatants (figure 5A and 5B).
Figure 5.
Correlation between cholera toxin B subunit (CTB)–specific IgG (A) or IgA (B) in memory B cell culture supernatant and the nos. of memory B cells in the same cultures, as demonstrated by standard enzyme-linked immunospot (ELISPOT) assay. For panel A, Spearman's ρ = 0.7042 and P < .001; for panel B, Spearman's ρ = 0.6733 and P < .001.
Correlation between quantitative levels of CTB-specific IgG memory B cells and traditional immunologic markers in cholera
We next correlated numbers of memory B cells with previously described measures of systemic and mucosal immune responses to cholera. Neither day 30 nor 90 memory B cell responses correlated with the peaks of traditional immunologic measurements in cholera, the vibriocidal titer on day 7, the serum anti-CTB IgG or IgA levels on day 7, or the circulating CTB-specific IgG ASCs on day 7.
DISCUSSION
The development of immunologic memory by the GALT sIgA system requires antigen-specific activation of naive B cells in the GALT, which can be detected as transiently circulating ASCs before they return to the intestinal mucosa. The detection of these transiently circulating antigen-committed ASCs (the ASC assay) serves as a marker of mucosal immune responses to recent infection. Using this assay, V. cholerae infection has been shown to induce CTB- and LPS-specific ASCs that peak in circulation a week after the onset of illness [17, 20]. Furthermore, the gut-homing potential of ASCs has been demonstrated by their expression of α4β7 integrin, the receptor of the gut vascular addressin MAdCAM-1 (mucosal vascular addressin cell adhesion molecule 1) [21-23]. However, although these assays are useful in measuring mucosal responses in recent illness or immunogenicity in vaccine recipients, they are limited in their ability to quantify anamnestic responses that may confer long-term protective immunity to V. cholerae infection.
Current understanding of memory B cell generation suggests that antigens such as CTB lead to the activation and proliferation of naive B cells in a CD4 T cell–mediated fashion. Activated B cells may then differentiate into short-lived, antigen-committed ASCs or undergo a germinal center reaction with CD4 T cell help, where somatic hypermutation, affinity maturation, and class switching generate high-affinity, antigen-specific memory B cells. Thus, compared with naive B cells, the memory B cell phenotype is characterized by the absence of surface IgD and IgM, the presence of CD27, and, in the case of protection at the intestinal mucosa, the expression of gut-homing receptors, such as α4β7 and CCR9 [24]. Memory B cells can persist for decades in an antigen-independent manner and can be further boosted by subsequent antigenic exposure [11, 25].
However, no measurement of antigen-specific memory B cell responses has been reported in cholera or other noninvasive intestinal mucosal infections. Therefore, the goals of the present study were to test the hypothesis that an antigen-specific memory B cell population is generated after V. cholerae O1 infection, to track this population up to 3 months after infection, and to investigate its relationships to the currently used surrogate measures of mucosal immune responses.
Our results characterize the generation and maintenance of an antigen-specific memory B cell population after cholera. In our study subjects, we demonstrate that CTB-specific IgG memory B cell populations are undetectable in acute illness but become detectable and persist in all patients for ≥3 months after infection. The frequencies of antigen-specific memory B cells ranged between 0.003% and 0.28% of total IgG memory B cells during this period, and the proportion of CTB-specific IgG memory B cells remained stable between 1 and 3 months after acute illness. In contrast, 3 months after acute illness, other immunologic markers of infection either declined significantly or were no longer detectable. Although the vibriocidal titer—the only known predictor of protection against cholera—remained higher on day 90 than on day 2, it declined significantly between 1 and 3 months after infection. Proxy measurements of the mucosal immune response declined more rapidly, including the circulating ASCs, and serum anti-CTB IgA, which declined to baseline levels by day 90. Taken together, these results suggest that the measurement of CTB-specific memory B cells generated after V. cholerae O1 infection may provide a more durable marker of immunity to cholera than traditional immunologic markers.
The quantity of memory B cells did not correlate strongly with several other immunologic markers of recent V. cholerae infection, including the peak vibriocidal titer or peak number of circulating CTB-specific IgG ASCs. However, because all patients enrolled had severe cholera and uniformly mounted very strong vibriocidal and ASC responses, the evaluation of only the most symptomatic individuals may have masked possible correlations between these measurements and subsequent memory B cell responses. Alternatively, given the perceived predominance of the IgA system in both acute and protective immune responses to cholera, it is plausible that IgG memory B cells are less well correlated with the magnitude of acute mucosal responses than are IgA memory B cells.
We found a very strong correlation between the numbers of IgG memory B cells measured by ELISPOT assay and anti-CTB IgG and IgA measured by ELISA in supernatants of memory B cell cultures. This suggests that the measurement of antibodies in memory B cell culture supernatants may be a proxy measurement for memory B cell quantification. Use of ELISAs for examining culture supernatants is simpler than the ELISPOT procedure and also allows simultaneous detection of memory responses to multiple antigens mediated by different antibody isotypes. Measurement of antibody in memory B cell culture supernatants may therefore be a useful alternative to study the development of memory to multiple antigens. Furthermore, the correlation seen between IgG memory B cells measured by ELISPOT assay and IgA in memory B cell culture supernatant measured by ELISA suggests that IgG memory B cells may reflect the kinetics of IgA memory B cells as well.
The frequencies of CTB-specific IgG memory B cells measured in our study were lower than the frequencies described after vaccination against smallpox, anthrax, influenza, and rotavirus or after inhalational anthrax infection [10, 11, 13-15]. This difference may be explained in at least 3 ways. First, given that anamnestic responses in cholera appear to be largely mounted at the intestinal mucosa, memory B cells quantified from circulation may underestimate the magnitude of an intestinal mucosa–committed memory B cell population. Second, in this preliminary study, we have characterized only IgG isotype memory B cells. In a protective immune response that is thought to be characterized mainly by sIgA, it is possible that memory B cells of the IgG isotype play a less important role than do those of the IgA isotype and are generated at lower frequencies. Finally, it is possible that infections with noninvasive pathogens such as V. cholerae simply result in a relatively diminished generation of memory B cells compared with systemic infections.
There are 2 important limitations of this study of memory B cell responses after V. cholerae O1 infection. First, all patients enrolled in our study had severe dehydrating disease. Both acute and memory responses after less-severe disease may differ from those seen in our patients. Second, we did not study immune responses to other V. cholerae O1 antigens, such as LPS and TCP, or quantify memory B cells of the IgA isotype. Given these considerations, ongoing studies are aimed at further characterizing the generation and long-term maintenance of memory B cells of both IgG and IgA isotypes, with specificity to CTB, LPS, TCP, and other V. cholerae O1 antigens, as well as directly measuring antigen-specific memory B cells in mucosal biopsy specimens in patients after V. cholerae infection.
Acknowledgments
We thank John Glidewell for technical support. We thank the study participants as well as the dedicated field and laboratory workers of the Cholera Immune Response Study at the International Centre for Diarrhoeal Disease Research, Bangladesh.
Financial support: ICDDR,B: Centre for Health and Population Research; National Institutes of Health (NIH; grants U01 AI058935 to S.B.C., R03 AI063079 to F.Q., and R01 AI40725 to E.T.R.); International Research Scientist Development Awards K01 TW07144 to R.C.L. and K01 TW07409 to J.B.H.); Fogarty International Center, NIH (Fogarty/Ellison Fellowship in Global Health [D43 TW005572] to C.R.J., M.S.B., and A.I.K.).
Footnotes
Potential conflicts of interest: none reported.
Presented in part: 42nd Panel Meeting on Cholera and Other Bacterial Infections, United States–Japan Cooperative Medical Science Program, Austin, Texas, December 2007.
References
- 1.Sack DA, Sack RB, Nair GB, Siddique AK. Cholera. Lancet. 2004;363:223–33. doi: 10.1016/s0140-6736(03)15328-7. [DOI] [PubMed] [Google Scholar]
- 2.Glass RI, Svennerholm AM, Khan MR, Huda S, Huq MI, Holmgren J. Seroepidemiological studies of El Tor cholera in Bangladesh: association of serum antibody levels with protection. J Infect Dis. 1985;151:236–42. doi: 10.1093/infdis/151.2.236. [DOI] [PubMed] [Google Scholar]
- 3.Glass RI, Becker S, Huq MI, et al. Endemic cholera in rural Bangladesh, 1966−1980. Am J Epidemiol. 1982;116:959–70. doi: 10.1093/oxfordjournals.aje.a113498. [DOI] [PubMed] [Google Scholar]
- 4.Mosley WH, McCormack WM, Ahmed A, Chowdhury AK, Barui RK. Report of the 1966−67 cholera vaccine trial in rural East Pakistan. II. Results of the serological surveys in the study population: the relationship of the case rate to antibody titre and an estimate of the inapparent infection rate with Vibrio cholerae. Bull World Health Organ. 1969;40:187–97. [PMC free article] [PubMed] [Google Scholar]
- 5.Mosley WH, Benenson AS, Barui R. A serological survey for cholera antibodies in rural East Pakistan. II. A comparison of antibody titres in the immunized and control population of a cholera-vaccine field-trial area and the relation of antibody titre to cholera case rate. Bull World Health Organ. 1968;38:335–46. [PMC free article] [PubMed] [Google Scholar]
- 6.Saha D, LaRocque RC, Khan AI, et al. Incomplete correlation of serum vibriocidal antibody titer with protection from Vibrio cholerae infection in urban Bangladesh. J Infect Dis. 2004;189:2318–22. doi: 10.1086/421275. [DOI] [PubMed] [Google Scholar]
- 7.Svennerholm AM, Jertborn M, Gothefors L, Karim AM, Sack DA, Holmgren J. Mucosal antitoxic and antibacterial immunity after cholera disease and after immunization with a combined B subunit-whole cell vaccine. J Infect Dis. 1984;149:884–93. doi: 10.1093/infdis/149.6.884. [DOI] [PubMed] [Google Scholar]
- 8.Levine MM, Kaper JB, Black RE, Clements ML. New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol Rev. 1983;47:510–50. doi: 10.1128/mr.47.4.510-550.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Svennerholm AM, Gothefors L, Sack DA, Bardhan PK, Holmgren J. Local and systemic antibody responses and immunological memory in humans after immunization with cholera B subunit by different routes. Bull World Health Organ. 1984;62:909–18. [PMC free article] [PubMed] [Google Scholar]
- 10.Crotty S, Aubert RD, Glidewell J, Ahmed R. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J Immunol Methods. 2004;286:111–22. doi: 10.1016/j.jim.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 11.Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171:4969–73. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 12.Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298:2199–202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 13.Sasaki S, Jaimes MC, Holmes TH, et al. Comparison of the influenza virus-specific effector and memory B-cell responses to immunization of children and adults with live attenuated or inactivated influenza virus vaccines. J Virol. 2007;81:215–28. doi: 10.1128/JVI.01957-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quinn CP, Dull PM, Semenova V, et al. Immune responses to Bacillus anthracis protective antigen in patients with bioterrorism-related cutaneous or inhalation anthrax. J Infect Dis. 2004;190:1228–36. doi: 10.1086/423937. [DOI] [PubMed] [Google Scholar]
- 15.Rojas OL, Caicedo L, Guzman C, et al. Evaluation of circulating intestinally committed memory B cells in children vaccinated with attenuated human rotavirus vaccine. Viral Immunol. 2007;20:300–11. doi: 10.1089/vim.2006.0105. [DOI] [PubMed] [Google Scholar]
- 16.Qadri F, Ahmed F, Karim MM, et al. Lipopolysaccharide- and cholera toxin-specific subclass distribution of B-cell responses in cholera. Clin Diagn Lab Immunol. 1999;6:812–8. doi: 10.1128/cdli.6.6.812-818.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qadri F, Ryan ET, Faruque AS, et al. Antigen-specific immunoglobulin A antibodies secreted from circulating B cells are an effective marker for recent local immune responses in patients with cholera: comparison to antibody-secreting cell responses and other immunological markers. Infect Immun. 2003;71:4808–14. doi: 10.1128/IAI.71.8.4808-4814.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forrest BD. Indirect measurement of intestinal immune responses to an orally administered attenuated bacterial vaccine. Infect Immun. 1992;60:2023–9. doi: 10.1128/iai.60.5.2023-2029.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qadri F, Jonson G, Begum YA, et al. Immune response to the mannose-sensitive hemagglutinin in patients with cholera due to Vibrio cholerae O1 and O0139. Clin Diagn Lab Immunol. 1997;4:429–34. doi: 10.1128/cdli.4.4.429-434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qadri F, Wenneras C, Albert MJ, et al. Comparison of immune responses in patients infected with Vibrio cholerae O139 and O1. Infect Immun. 1997;65:3571–6. doi: 10.1128/iai.65.9.3571-3576.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erle DJ, Briskin MJ, Butcher EC, Garcia-Pardo A, Lazarovits AI, Tidswell M. Expression and function of the MAdCAM-1 receptor, integrin alpha 4 beta 7, on human leukocytes. J Immunol. 1994;153:517–28. [PubMed] [Google Scholar]
- 22.Qadri F, Makela PH, Holmgren J, et al. Enteric infections in an endemic area induce a circulating antibody-secreting cell response with homing potentials to both mucosal and systemic tissues. J Infect Dis. 1998;177:1594–9. doi: 10.1086/515306. [DOI] [PubMed] [Google Scholar]
- 23.Berlin C, Berg EL, Briskin MJ, et al. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74:185–95. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- 24.Rott LS, Briskin MJ, Butcher EC. Expression of alpha4beta7 and E-selectin ligand by circulating memory B cells: implications for targeted trafficking to mucosal and systemic sites. J Leukoc Biol. 2000;68:807–14. [PubMed] [Google Scholar]
- 25.Maruyama M, Lam KP, Rajewsky K. Memory B-cell persistence is independent of persisting immunizing antigen. Nature. 2000;407:636–42. doi: 10.1038/35036600. [DOI] [PubMed] [Google Scholar]