Summary
In the presence of unattached/weakly attached kinetochores, the spindle assembly checkpoint (SAC) delays exit from mitosis by preventing the anaphase-promoting complex (APC)-mediated proteolysis of cyclin B, a regulatory subunit of cyclin-dependent kinase 1 (Cdk1). Like all checkpoints, the SAC does not arrest cells permanently, and escape from mitosis in the presence of an unsatisfied SAC requires that cyclin B/Cdk1 activity be inhibited. In yeast [1–5], and likely Drosophila [6–8], this occurs through an “adaptation” process involving an inhibitory phosphorylation on Cdk1 and/or activation of a cyclin-dependent kinase inhibitor (Cdki). The mechanism that allows vertebrate cells to escape mitosis when the SAC cannot be satisfied is unknown. To explore this issue, we conducted fluorescence microscopy studies on rat kangaroo (PtK) and human (RPE1) cells dividing in the presence of nocodazole. We find that in the absence of microtubules (MTs), escape from mitosis occurs in the presence of an active SAC and requires cyclin B destruction. We also find that cyclin B is progressively destroyed during the block by a proteasome-dependent mechanism. Thus, vertebrate cells do not adapt to the SAC. Rather, our data suggest that in normal cells, the SAC cannot prevent a slow but continuous degradation of cyclin B that ultimately drives the cell out of mitosis.
Results and Discussion
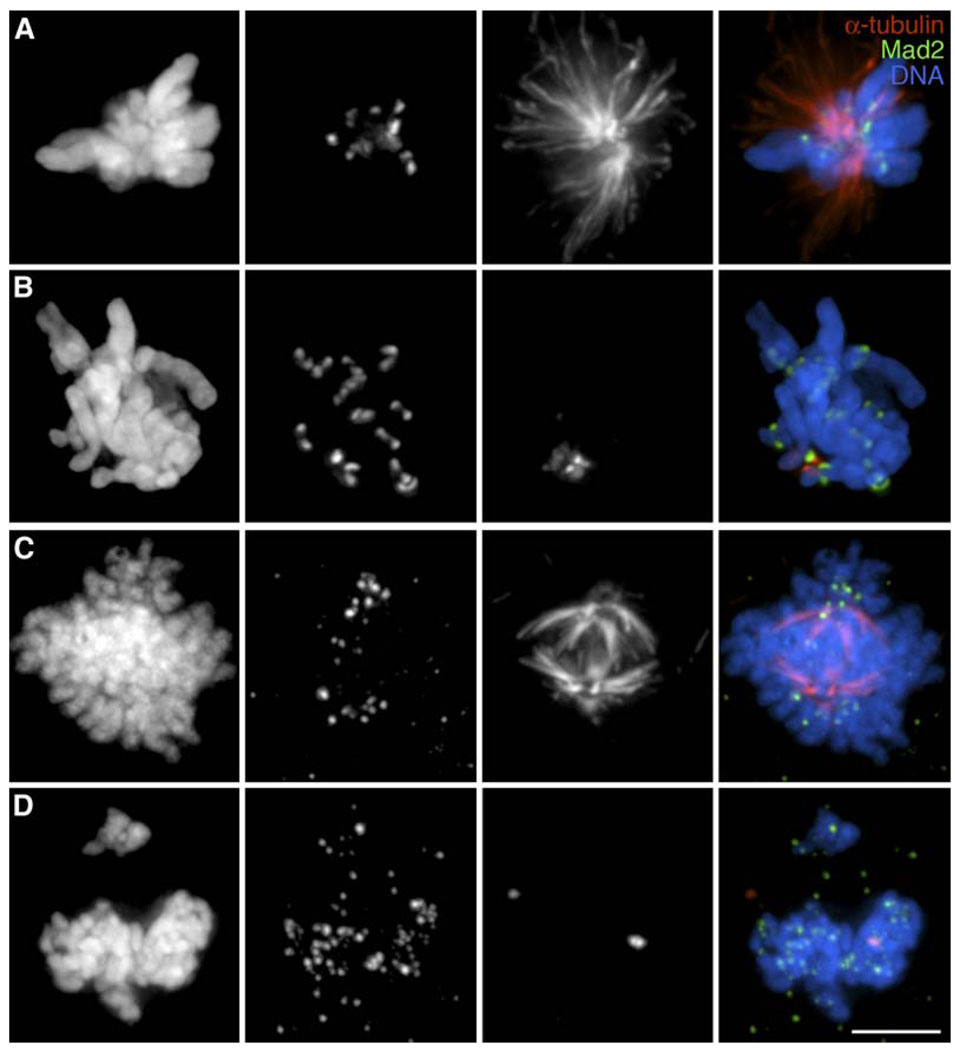
At 37°C, the duration of mitosis in PtK1 cells (2N = 11), defined from nuclear envelope breakdown to nuclear envelope reformation, is 33 ± 7 min (n = 23). When PtK1 cells enter mitosis in the presence of 400 nM nocodazole, they form a monopolar spindle (Figure 1A) and remain in mitosis for 191 ± 52 min (n = 22). When PtK1 enter mitosis in 10 µM nocodazole, no spindle MTs are formed (Figure 1B) and they remain in mitosis for 287 ± 79 min (n = 38), after which they enter the next G1 in the presence of 22 unattached kinetochores. Similarly, although RPE1 (2N = 46) cells normally complete mitosis in 17 ± 6 min at 37°C (n = 22), they require 546 ± 96 min (n = 18) in the presence of 200 nM nocodazole and 875 ± 130 min (n = 13) in the presence of 3.2 µM nocodazole. As with PtK1 cells, RPE1 cells form rudimentary spindles at the lower (200 nM) but not at the higher (3.2 µM) drug concentration (Figures 1C and 1D). When the duration of mitosis in the presence or absence of MTs is compared for each cell type, by means of the two-tailed Student’s t test, they are found to be statistically different with p values below the 0.0001 level.
Figure 1. Abnormal Spindles with Mad2-Positive Kinetochores Form in the Presence of Nocodazole.
PtK1 (A, B) and RPE1 (C, D) cultures were incubated with 400 nM (A), 10 µM (B), 200 nM (C), or 3.2 µM nocodazole for 6 hr (A and B), 9 hr (C), or 15 hr (D), respectively, prior to fixation and immunostaining for Mad2 and α-tubulin. First column, DNA; second column, Mad2; third column, α-tubulin; fourth column, merged images. Note that a rudimentary spindle forms in both cell types at the lower but not higher nocodazole concentrations. See text for details. Scale bar equals 5 µm.
As both cell types escape mitosis in the absence of MTs, they begin to violently bleb and thereafter reflatten with multiple micronuclei (e.g., see Figures 2B, 2C, and 2E). These findings are consistent with previous reports that PtK1 and RPE1 cells can exit mitosis without satisfying the SAC and also that the duration of the delay is related to the drug concentration (reviewed in [6]).
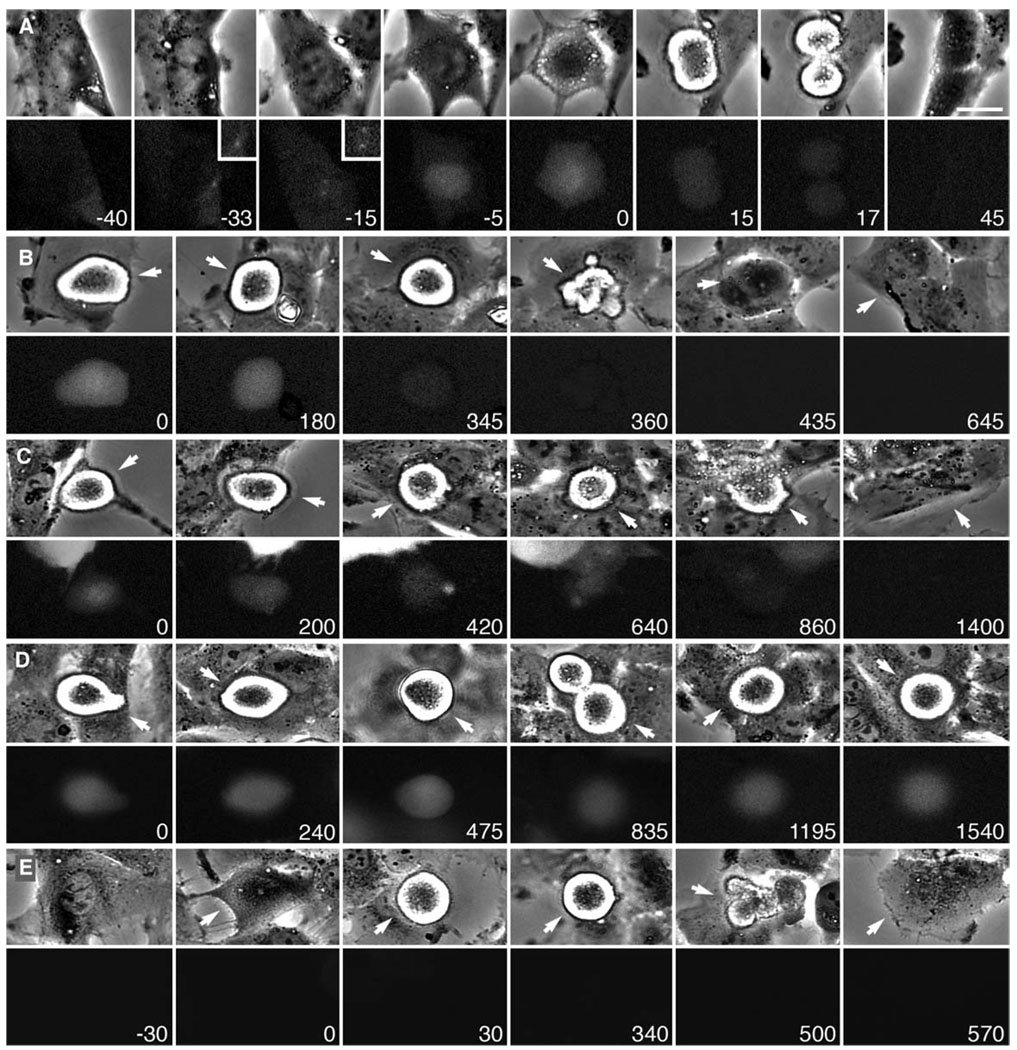
Figure 2. Cyclin B Degradation Is Required for RPE1 Cells to Exit Mitosis in the Presence of an Unsatisfied SAC.
Selected phase-contrast (top) and fluorescence (bottom) frames from time-lapse video recordings.
(A–C) RPE1 cells were transfected with a cyclin B-GFP plasmid and GFP-expressing cells were followed.
(A) Non-drug-treated control cell in which cyclin B-GFP accumulates in the nucleus during prophase (−5 min) and is then destroyed after the SAC is satisfied and the cell enters anaphase (15–17 min). Insets show centrosomes at a higher magnification. Time, relative to nuclear envelope breakdown, is shown in minutes. Scale bar equals 5 µm.
(B and C) GFP-expressing mitotic cells in the presence of 200 nM (B) or 3.2 µM (C) nocodazole. The arrow in each frame marks the cell of interest. Time is shown in minutes. Note that the cyclin B-GFP fluorescence begins to visibly decline throughout the block and is barely detectable near the time the cells finally begin to exit mitosis (360 min in [B] and 860 min in [C]).
(D and E) RPE1 cultures were transfected with a GFP-cyclin B-Δ85 plasmid. 24 hr after transfection, the cultures were incubated with 200 nM nocodazole and areas were subsequently followed by dual-mode time-lapse microscopy. In the same field of view, we could find GFP-expressing and nonexpressing cells.
(D) GFP-expressing cells (e.g., arrow) remained in mitosis until filming was terminated after more than 24 hr, and throughout this time the GFP fluorescence remained relatively level (see Figure 3).
(E) In contrast, cells in the same culture that were not transfected with the plasmid (e.g., arrows) escaped mitosis after ~8 hr. See also Figure 3.
Bypassing an Unsatisfied SAC Requires Cyclin B Degradation
If escaping an unsatisfied SAC involves a Cdki or an inhibitory phosphorylation on cyclin B/Cdk1, then early G1 cells produced by this process should contain high cyclin B levels. To test this, we followed RPE1 cells in the presence of 200 nM nocodazole, fixed them shortly after they had exited mitosis, and stained them for the indirect immunofluorescence (IMF) localization of cyclin B. When compared to G2 controls in the same cultures, cells that had escaped mitosis and entered early G1 without satisfying the SAC appeared depleted of cyclin B (see Figure S1 in the Supplemental Data available with this article online). To confirm this qualitative finding, we transfected RPE1 cells with a cyclin B-GFP plasmid and followed them, in the presence of 200 nM (n = 5) or 3.2 µM (n = 3) nocodazole, as they escaped mitosis. Under both conditions, and in all cases, cyclin B fluorescence slowly decayed during the block until it was no longer detectable by eye, after which the cells exited mitosis (Figures 2A–2C). This decay occurred more rapidly in cells treated with 200 nM nocodazole than with 3.2 µM nocodazole (Figure 3). Clute and Pines [9] reported a similar progressive decay in GFP-cyclin B fluorescence intensity when HeLa cells were delayed in mitosis by 10 µM taxol, a drug that stabilizes MTs. In our study, as in theirs, the fluorescence decay was not due to photobleaching, since the fluorescence intensity of GFP-cyclin B containing bystander cells in the same fields did not change (Figure 3). Thus, in vertebrates, escape from mitosis when the SAC cannot be satisfied correlates with the progressive destruction of cyclin B.
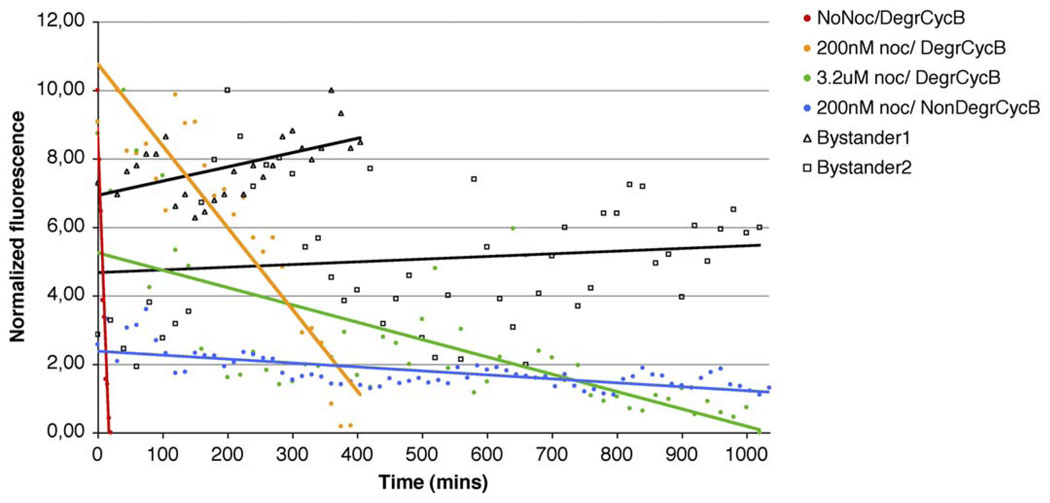
Figure 3. Cyclin B Proteolysis Occurs throughout a Nocodazole-Induced Block in Mitosis.
RPE1 cultures were transfected with a plasmid carrying either a degradable or nondegradable (Δ85) GFP-cyclin B, and progress through mitosis was followed in GFP-expressing cells. Red line, GFP-cyclin B-expressing control cell not treated with nocodazole; orange line, the GFP-cyclin B-expressing cell shown in Figure 2B that was treated with 200 nM nocodazole; green line, the GFP-expressing cell shown in Figure 2C that was treated with 3.2 µM nocodazole. Areas containing mitotic cells were then followed by dual-mode time-lapse microscopy. Fluorescence was measured at each time point in a fixed size area of the cell corresponding to the mitotic spindle region in mitotic cells or the cytoplasm in interphase cells. Fluorescence levels of both mitotic and interphase cells were normalized to the background. Fluorescence of the mitotic cells was further normalized to a transfected interphase bystander cell (black lines) to allow comparison of intensities from independent recordings. The data shown here are representative of 11 control cells, 5 cells expressing GFP-cyclin B and incubated in 200 nM nocodazole, 3 cells expressing GFP-cyclin B and incubated in 3.2 µM nocodazole, and 3 cells expressing nondegradable GFP-cyclin B-Δ85 and incubated with 200 nM nocodazole (blue line). Note that the higher the nocodazole concentration, the slower cyclin B is degraded and the longer the cells take to exitmitosis (cf. orange and green lines). Also, degradation of the GFP fluorescence associated with the nondegradable form of cyclin B (blue line) was largely inhibited. Cells transfected with this construct were unable to escape mitosis during the recording interval (up to 1740 min).
Bypassing a Functional SAC Does Not Involve Adaptation to the Checkpoint
Our finding that cyclin B is destroyed as vertebrate cells bypass a functional SAC implies that escaping mitosis requires cyclin B destruction rather than activation of a Cdki or an inhibitory phosphorylation on cyclin B/Cdk1. However, it is possible that these other escape routes are present but masked by cyclin B destruction. To test this possibility, we followed RPE1 and PtK1 cells as they entered mitosis in the presence of nocodazole and MG132, a potent proteasome inhibitor. Under this condition, both RPE1 (n = 7) and PtK1 (n = 9) cells failed to exit mitosis and ultimately died in division after a prolonged (>12–15 hr) arrest (Figure S2). To determine whether the failure of cells treated with both nocodazole and MG132 to escape mitosis is due to toxic effects caused by inhibiting proteolysis, we transfected RPE1 cultures with GFP-cyclin B-Δ85. This cyclin lacks the N-terminal D-box required for APC-mediated proteolysis [10], yet it binds to Cdk1 and forms an active kinase complex [11]. We then followed GFP-cyclin B-Δ85- containing cells by phase/fluorescence video LM as they entered mitosis in the presence of 200 nM nocodazole. Not surprisingly, we found that cells expressing nondegradable cyclin B invariably remained in mitosis for >24 hr (Figure 2D and Figure 3), whereas neighboring cells that were not expressing the GFP construct exited mitosis within 8–10 hr (Figure 2E). Thus, in vertebrates, bypassing an unsatisfied SAC requires cyclin B destruction, and in these cells, other upstream proteolysis-independent “adaptation” pathways are not operative.
Mad2 and BubR1 Remain Associated with Kinetochores as Cells Bypass a Functional SAC
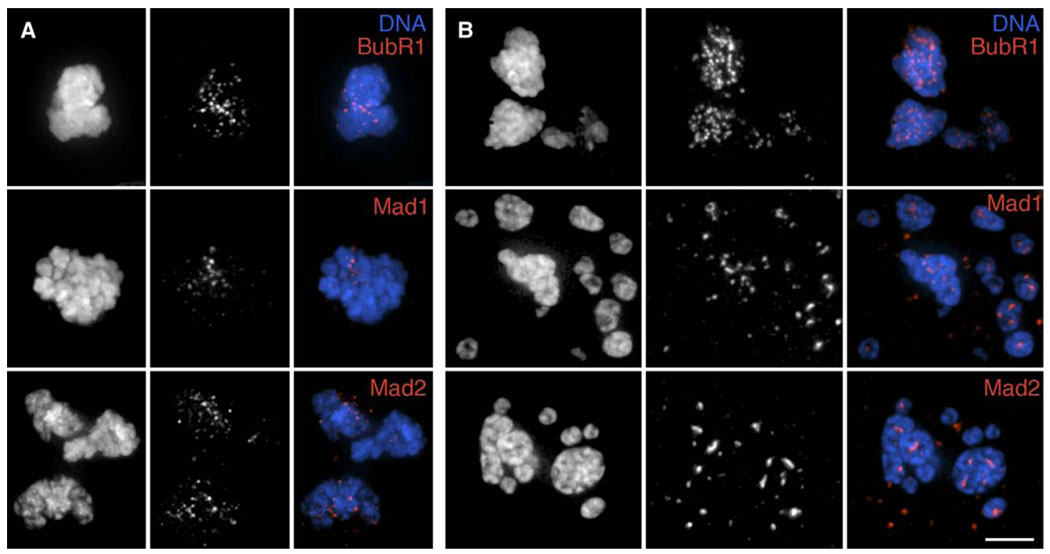
Cells could escape mitosis without satisfying the SAC if kinetochores became progressively modified over time, until they no longer bound one or more kinetochore-associated checkpoint-signaling proteins (like Mad2 or BubR1), or if one or more of these checkpoint proteins is degraded during the block. To evaluate this idea, we asked whether escape from mitosis in the presence of nocodazole correlates with the disappearance of one or more SAC proteins from kinetochores, as occurs during a normal mitosis. To this end, we followed nocodazole- treated YFP-Mad2-expressing PtK2 cells as they entered and exited mitosis. As expected, in control cells Mad2 fluorescence was not detected on kinetochores just prior to anaphase (n = 14; data not shown). In contrast, we always found a variable number of Mad2- positive kinetochores in PtK2 cells (n = 17) as they exited mitosis in the presence of 10 µM nocodazole (Figure S3). To expand this finding, we treated RPE1 cultures with 200 nM or 3.2 µM nocodazole for 9 or 15 hr, respectively, to ensure the presence in each of a cell population that had overridden the SAC. After fixation, the cultures were then stained with antibodies against SAC proteins (BubR1, Mad1, and Mad2). Under both conditions, we invariably found a dot-like staining pattern associated with the chromatin in cells that had escaped mitosis, as judged by the presence of decondensing chromosomes and/or micronuclei (Figures 4A and 4B). When compared to cells treated with 200 nM nocodazole, the kinetochore regions of cells that had escaped mitosis in the presence of 3.2 µM nocodazole stained very intensely for all checkpoint proteins (cf. Figures 4A and 4B). This difference can be attributed to the fact that the formation of rudimentary spindles and kinetochore fibers at the lower drug concentration (Figure 1C) depletes checkpoint proteins from some of the kinetochores. Thus, in vertebrates, exit from mitosis when the SAC cannot be satisfied is not due to global changes in kinetochores and/or the destruction of SAC proteins.
Figure 4. BubR1, Mad1, and Mad2 Remain Associated with RPE1 Kinetochores as Cells Exit Mitosis in the Presence of an Unsatisfied SAC.
RPE1 cultures were incubated with 200 nM (A) or 3.2 µM(B) nocodazole for 9 or 15 hr, respectively, before fixation and immunostaining for BubR1 (top), Mad1 (middle), or Mad2 (bottom). Cells that had just exitedmitosis (see text for details) were then located and photographed. Left columns in each panel, DNA; middle columns, BubR1, Mad1, or Mad2; right columns, merged images. Scale bar equals 5 µm.
When spindle MT formation is prevented, sister chromatids in some but not all vertebrate cells disjoin just before the cell escapes mitosis [12]. Although this is true for PtK1 cells [13], our data imply that it does not occur in RPE1 cells. This is suggested from the fact that many of the sister kinetochores contained in the restitution nuclei formed in cells treated with 3.2 µM nocodazole appeared closely linked through a single centromere region (Figure 4B). Since chromatid disjunction normally occurs via the APCCdc20-mediated destruction of securin, this finding suggests that APCCdc20 is inactive as RPE1 cells exit mitosis without forming MTs.
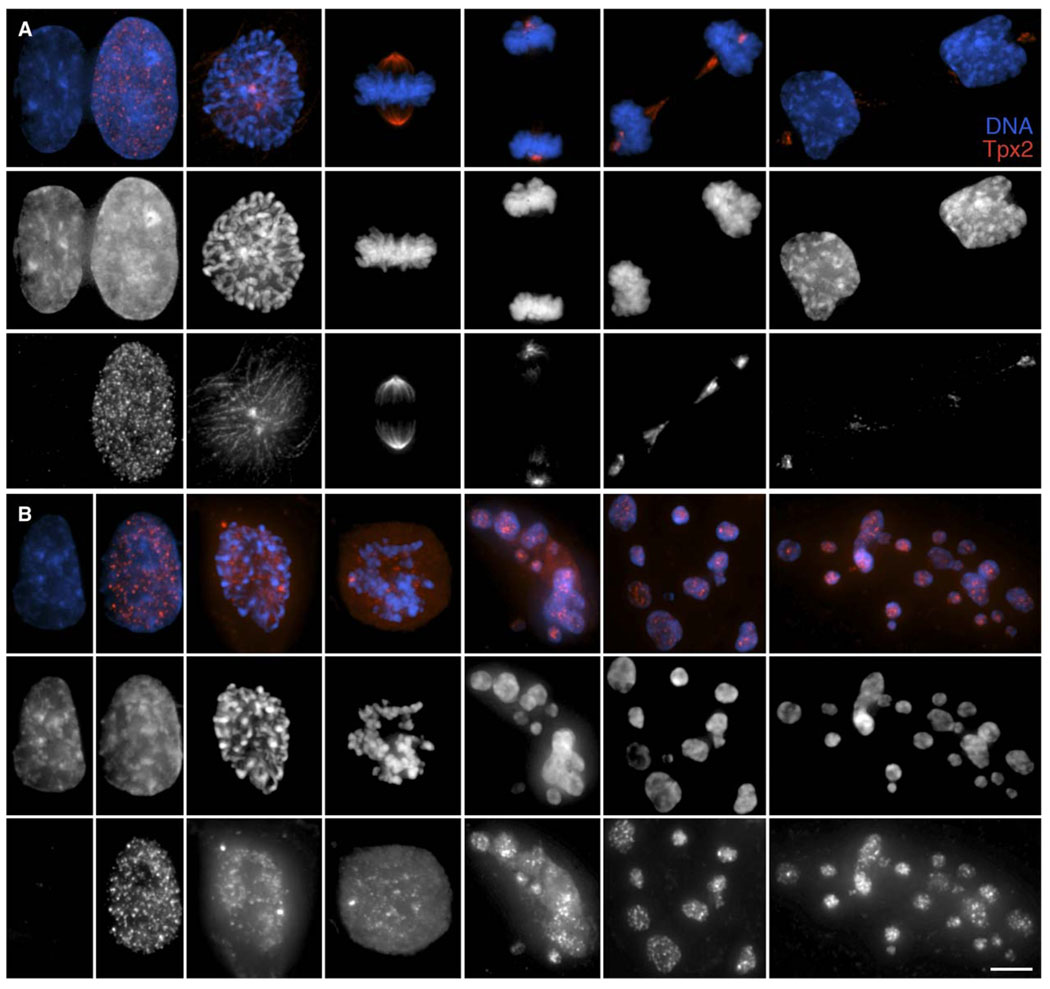
Tpx2, a MT-associated protein required for spindle formation, is a conspicuous component of S, G2, and mitotic, but not G1, cells. Like cyclin B, the level of Tpx2 peaks during spindle formation and then rapidly declines during anaphase/telophase until it is gone in early G1 [14]. It was recently shown that Tpx2 degradation is controlled by APCCdh1, which, unlike APCCdc20, does not normally become active until anaphase [15]. We therefore asked whether Tpx2 is degraded in cells that have exited mitosis without satisfying the SAC. For this study, we treated RPE1 cultures with 3.2 µM no-codazole for 15 hr and fixed and stained them for Tpx2 immunofluorescence. As expected, we found intense Tpx2 staining in mitotic cells (Figure 5A). However, unlike in early G1 cells in nontreated control cultures that are largely depleted of Tpx2 (Figure 5A), high levels of this protein were always seen in the micronuclei of cells that had reflattened after bypassing an unsatisfied SAC (Figure 5B). This result suggests that when the SAC cannot be satisfied, APCCdh1 activity remains depressed as cells exit mitosis.
Figure 5. Tpx2, an APC Substrate, Is Not Degraded in Cells that Exit Mitosis without Satisfying the SAC.
(A) In untreated RPE1 cells, Tpx2 (bottom) is found in S/G2 but not G1 nuclei. During mitosis, this MT-associated protein localizes to spindle MTs, while during anaphase it is found on midbody MTs and centrosomes. Tpx2 is largely degraded by the time the nuclear envelope reforms around daughter nuclei during late telophase, although some remains associated with the centrosomes (far right row).
(B) After treatment with 3.2 µM nocodazole, Tpx2 (bottom row) is found in the nuclei of G2 and prophase cells. In the absence of spindle MT formation, this protein then remains diffuse in the cytoplasm throughout the prolonged mitosis. However, once the cells exitmitosis and reflatten on the substrate (last three images in each row), it is found concentrated in the G1 micronuclei. Top row, merged images; middle row, DNA; bottom row, Tpx2. Scale bar equals 5 µm.
How Vertebrate Cells Bypass a Functional SAC
In the presence of drugs that inhibit MT formation, the SAC cannot be satisfied, yet many cells ultimately escape mitosis and enter the next G1. Here we show that this escape is not due to adaptation pathways that suppress cyclin B/Cdk1 activity by, e.g., inhibitory phosphorylations or activation of a Cdki. Importantly, we also show that this escape is not due to the depletion of Mad2 or BubR1, which remain associated with unattached kinetochores as the SAC is bypassed. Instead, we find that in vertebrates, escaping mitosis when in the presence of a functional SAC requires proteolysis and, more specifically, the destruction of cyclin B. As first reported for treatments that stabilize MTs [9], we also found that cyclin B levels progressively decline during a mitotic block induced by drugs that prevent MT assembly. Thus, the key issue for how a vertebrate cell escapes mitosis when it cannot satisfy the SAC is how it degrades its cyclin B during the block.
Several recent reports suggest that the checkpoint proteins Bub1 and BubR1 are degraded in HeLa in a caspase-dependent manner during a mitosis prolonged by drugs [16–18]. This, by itself, would lead to checkpoint inactivation, APC-mediated cyclin B destruction, and escape from mitosis. However, these claims are not supported by our data that BubR1 remains associated with kinetochores even after cells have escaped the mitotic block (Figure 4). Moreover, they are based on sorting analyses of cell populations, arrested in mitosis for 24–48 hr, in which the cells are likely dying during mitosis via apoptosis [19, 20]. Instead, our data appear to rule out the possibility that vertebrates escape mitosis in the absence of MTs by suddenly inactivating the SAC and thus activating the APCs near the end of the block. Indeed, when the SAC cannot be satisfied, the levels of cyclin B decline gradually and not suddenly. Furthermore, during and after escaping mitosis in the absence of MTs, the kinetochores retain high levels of checkpoint signaling proteins (e.g., Mad2 and BubR1), the APC substrate Tpx2 is not degraded, and RPE1 cells do not appear to disjoin their chromatids. Concerning the last point, it is noteworthy that in vertebrates chromatid disjunction does not occur even in untreated cells containing a fully functional spindle until ~95% of the cyclin B has been degraded [9, 11]. Moreover, evidence is accumulating that this event requires cyclin B destruction since it does not occur, or is considerably delayed, in non-drug-treated cells expressing a nondegradable cyclin B that have presumably satisfied the SAC [11, 21].
In lieu of checkpoint inactivation, there are two non-mutually exclusive mechanisms for how cells can escape mitosis in the presence of a functional SAC. The first is that cyclin B is slowly and constantly degraded in an APC-independent manner until it falls below the threshold for maintaining the mitotic state. For several reasons this scenario is highly unlikely. First, our data reveal that the degradation of cyclin B is specific to mitosis, since it is not seen in G2 GFP-cyclin B-expressing bystander cells. Second, the degradation of GFP-cyclin B lacking a D-box, and therefore its APC recognition site, occurs extremely slowly even in the presence of fully functional spindles (Figure 3; [9, 11]). Presumably, in the absence of MTs, any general cyclin denaturation/degradation pathway not involving APCs would also degrade GFP-cyclin B-Δ85 at the same rate as degradable cyclin. We evaluated this idea by treating RPE1 cells expressing GFP-cyclin B with 200 nM nocodazole and 5 µM MG132, and then by asking how quickly GFP-cyclin B is degraded in the absence of proteasome function. Although this combination of drugs is lethal over prolonged periods (12–15 hr; see Figure S2), we found that the GFP-cyclin B fluorescence intensity did not decay (and actually slowly increased) over the 5–10 hr we followed the cells (Figure S4). Thus, when the SAC cannot be satisfied, cyclin B degradation is not due to a steady general denaturation of the protein but instead requires proteasome-mediated proteolysis. Finally, we found that cells that form rudimentary spindles in nocodazole escape mitosis significantly faster than those that cannot generate spindle MTs (Figure 3; [6, 22]). This positive correlation between the presence of spindle MTs and an accelerated escape from mitosis is difficult to explain by a nonspecific cyclin B destruction mechanism.
The more attractive mechanism forhow cells exit mitosis in the presence of a functional SAC is simply that the SAC is not normally 100% effective at blocking all APCs from ubiquinating cyclin B. Under this condition, a constant low level of ubiquination and subsequent proteolysis would gradually drop the cyclin B level below that needed to maintain the mitotic state. This route provides a simple and elegant mechanism for ultimately overcoming the mitotic condition before the cell dies, which, at least under some conditions, may be important (e.g., although the value of this feature to multicellular organisms is not readily apparent, in plants it leads to changes in ploidy levels and new strains of commercially valuable crops). This mechanism also provides a straightforward explanation for why the nocodazole-induced mitotic delay seen in normal [23, 24] and some cancer [25–27] cells containing abnormally low levels of Mad2 or BubR1 is greatly attenuated relative to cells containing normal levels of these checkpoint proteins: since even with normal Mad2 or BubR1 concentrations the SAC cannot prohibit a low level ubiquination of cyclin B by the APCCdc20 complex, lowering the concentration further makes the SAC even less effective at inhibiting the APCs, and the more rapidly cyclin B levels would be degraded below that needed to maintain the mitotic state.
Cells that form rudimentary spindles in response to low nocodazole concentrations are unable to satisfy the SAC as evidenced by the continuous presence of Mad2 and BubR1 on some kinetochores after they escape mitosis (Figure 4A). However, as noted above, such cells exit mitosis significantly faster than those that cannot generate spindle MTs (see also [6, 22, 28]). Not surprisingly, we find that nocodazole-treated cells containing rudimentary spindles degrade their cyclin B much faster than cells lacking MTs(Figure 3). This means that when the SAC cannot be satisfied, the mere presence of MTs somehow accelerates the destruction of cyclin B. Although the reasons for this are unknown, it is possible that APCs, which are normally associated with the spindle [29, 30], work more efficiently when attached to MTs. Alternatively, at the lower drug concentrations, many kinetochores become attached to spindle MTs, which makes them poor checkpoint signalers because they possess reduced amounts of checkpoint proteins (e.g., cf. Figures 1A and 1C with 1B and 1D; Figures 4A and 4B; see [31]). Under this condition, the amount of the APC inhibitor ultimately generated may be significantly less than that formed in the complete absence of all MTs, and the higher residual APC activity would produce a more rapid degradation of cyclin B and a corresponding shorter delay in mitosis.
Supplementary Material
Acknowledgments
We thank Dr. Jonathon Pines (University of Cambridge, UK) for the pCMX cyclin B1-GFP plasmid, Dr. Tim Yen (Fox Chase Cancer Center, Philadelphia, PA) for the anti-BubR1 antibody, Dr. Edward Salmon (University of North Carolina, Chapel Hill, NC) for the PtK2 YFP-Mad2-expressing cell line and anti-Mad2 antibody, and Dr. Naihan Xu (Hong Kong University, Clear Water Bay, Kowloon) for the GFP-cyclin B1-Δ85 construct. We also thank Drs. Helder Maiato and Alexey Khodjakov for help with this project. Supported by NIH/GMS 40198 to C.L.R. D.A.B. is the recipient of a doctoral research fellowship SFRH/BD/13663/2003 from Fundação para a Ciência e a Tecnologia of Portugal.
Footnotes
Supplemental Data
Supplemental Data include four figures and Supplemental Experimental Procedures and can be found with this article online at http://www.current-biology.com/cgi/content/full/16/12/1194/DC1/.
References
- 1.Rudner AD, Murray AW. The spindle assembly checkpoint. Curr. Opin. Cell Biol. 1996;8:773–780. doi: 10.1016/s0955-0674(96)80077-9. [DOI] [PubMed] [Google Scholar]
- 2.Minshull J, Straight A, Rudner AD, Dernburg AF, Belmont A, Murray AW. Protein phosphatase 2A regulates MPF activity and sister chromatid cohesion in budding yeast. Curr. Biol. 1996;6:1609–1620. doi: 10.1016/s0960-9822(02)70784-7. [DOI] [PubMed] [Google Scholar]
- 3.Rudner AD, Hardwick KG, Murray AW. Cdc28 activates exit from mitosis in budding yeast. J. Cell Biol. 2000;149:1361–1376. doi: 10.1083/jcb.149.7.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Novak B, Toth A, Csikasz-Nagy A, Gyorffy B, Tyson JJ, Nasmyth K. Finishing the cell cycle. J. Theor. Biol. 1999;199:223–233. doi: 10.1006/jtbi.1999.0956. [DOI] [PubMed] [Google Scholar]
- 5.Wolfe BA, Gould KL. Fission yeast Clp1p phosphatase affects G(2)/M transition and mitotic exit through Cdc25p inactivation. EMBO J. 2004;23:919–929. doi: 10.1038/sj.emboj.7600103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rieder CL, Maiato H. Stuck in division or passing through: what happens when cells cannot satisfy the spindle assembly checkpoint. Dev. Cell. 2004;7:637–651. doi: 10.1016/j.devcel.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Foley E, O’Farrell PH, Sprenger F. Rux is a cyclin-dependent kinase inhibitor (CKI) specific for mitotic cyclin-Cdk complexes. Curr. Biol. 1999;9:1392–1402. doi: 10.1016/s0960-9822(00)80084-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foley E, Sprenger F. The cyclin-dependent kinase inhibitor Roughex is involved in mitotic exit in Drosophila. Curr. Biol. 2001;11:151–160. doi: 10.1016/s0960-9822(01)00050-1. [DOI] [PubMed] [Google Scholar]
- 9.Clute P, Pines J. Temporal and spatial control of cyclin B1 destruction in metaphase. Nat. Cell Biol. 1999;1:82–87. doi: 10.1038/10049. [DOI] [PubMed] [Google Scholar]
- 10.Yamano H, Gannon J, Mahbubani H, Hunt T. Cell cycle-regulated recognition of the destruction box of cyclin B by the APC/C in Xenopus egg extracts. Mol. Cell. 2004;132:137–147. doi: 10.1016/s1097-2765(03)00480-5. [DOI] [PubMed] [Google Scholar]
- 11.Chang DC, Xu N, Luo KQ. Degradation of cyclin B is required for the onset of anaphase in mammalian cells. J. Biol. Chem. 2003;278:37865–37873. doi: 10.1074/jbc.M306376200. [DOI] [PubMed] [Google Scholar]
- 12.Rieder CL, Palazzo RE. Colcemid and the mitotic cycle. J. Cell Sci. 1992;102:387–392. doi: 10.1242/jcs.102.3.387. [DOI] [PubMed] [Google Scholar]
- 13.Rieder CL, Schultz A, Cole R, Sluder G. Anaphase onset in vertebrate somatic cells is controlled by a checkpoint that monitors sister kinetochore attachment to the spindle. J. Cell Biol. 1994;127:301–310. doi: 10.1083/jcb.127.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gruss OJ, Wittmann M, Yokoyama H, Pepperkok R, Kuffer T, Sillje H, Karsenti E, Mattaj IW, Vernos I. Chromosome-induced microtubule assembly mediated by TPX2 is required for spindle formation in HeLa cells. Nat. Cell Biol. 2002;4:871–879. doi: 10.1038/ncb870. [DOI] [PubMed] [Google Scholar]
- 15.Stewart S, Fang G. Anaphase-promoting complex/cyclosome controls the stability of TPX2 during mitotic exit. Mol. Cell. Biol. 2005;25:10516–10527. doi: 10.1128/MCB.25.23.10516-10527.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baek KH, Shin HJ, Jeong SJ, Park JW, McKeon F, Lee CW, Kim CM. Caspases-dependent cleavage of mitotic checkpoint proteins in response tomicrotubule inhibitor. Oncol. Res. 2005;15:161–168. doi: 10.3727/096504005776367906. [DOI] [PubMed] [Google Scholar]
- 17.Kim M, Murphy K, Liu F, Parker SE, Dowling ML, Baff W, Kao GD. Caspase-mediated specific cleavage of BubR1 is a determinant of mitotic progression. Mol. Cell. Biol. 2005;25:9232–9248. doi: 10.1128/MCB.25.21.9232-9248.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin HJ, Baek KH, Jeon AH, Park MT, Lee SJ, Kang CM, Lee HS, Yoo SH, Chung DH, Sung YC, et al. Dual roles of human BubR1, a mitotic checkpoint kinase, in the monitoring of chromosomal instability. Cancer Cell. 2003;4:483–497. doi: 10.1016/s1535-6108(03)00302-7. [DOI] [PubMed] [Google Scholar]
- 19.Sherwood SW, Sheridan JP, Schimke RT. Induction of apoptosis by the anti-tubulin drug colcemid: relationship of mitotic checkpoint control to the induction of apoptosis in HeLa S3 cells. Exp. Cell Res. 1994;215:373–379. doi: 10.1006/excr.1994.1354. [DOI] [PubMed] [Google Scholar]
- 20.Panvichian R, Orth K, Day ML, Day KC, Pilat MJ, Pienta KJ. Paclitaxel-associated multimininucleation is permitted by the inhibition of caspase activation: a potential early step in drug resistance. Cancer Res. 1998;58:4667–4672. [PubMed] [Google Scholar]
- 21.Stemmann O, Zou H, Gerber SA, Gygi SP, Kirschner MW. Dual inhibition of chromatid separation at metaphase. Cell. 2001;107:715–726. doi: 10.1016/s0092-8674(01)00603-1. [DOI] [PubMed] [Google Scholar]
- 22.Andreassen PR, Margolis RL. Microtubule dependency of p34cdc2 inactivation and mitotic exit in mammalian cells. J. Cell Biol. 1994;127:789–802. doi: 10.1083/jcb.127.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michel LS, Liberal V, Chatterjee A, Kirchwegger R, Pasche B, Gerald W, Dobles M, Sorger PK, Murty VV, Benezra R. MAD2 haplo-insufficiency causes premature anaphase and chromosome instability in mammalian cells. Nature. 2001;409:355–359. doi: 10.1038/35053094. [DOI] [PubMed] [Google Scholar]
- 24.Dai W, Wang Q, Liu T, Swamy M, Fang Y, Xie S, Mahmood R, Yang YM, Xu M, Rao CV. Slippage of mitotic arrest and enhanced tumor development in mice with BubR1 haploinsufficiency. Cancer Res. 2004;64:440–445. doi: 10.1158/0008-5472.can-03-3119. [DOI] [PubMed] [Google Scholar]
- 25.Cahill DP, Lengauer C, Yu J, Riggins GJ, Willson JK, Markowitz SD, Kinzler KW, Vogelstein B. Mutations of mitotic checkpoint genes in human cancers. Nature. 1998;392:300–303. doi: 10.1038/32688. [DOI] [PubMed] [Google Scholar]
- 26.Kasai T, Iwanaga Y, Iha H, Jeang KT. Prevalent loss of mitotic spindle checkpoint in adult T-cell leukemia confers resistance to microtubule inhibitors. J. Biol. Chem. 2002;277:5187–5193. doi: 10.1074/jbc.M110295200. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi T, Haruki N, Nomoto S, Masuda A, Saji S, Osada H, Takahashi T. Identification of frequent impairment of the mitotic checkpoint and molecular analysis of the mitotic checkpoint genes, hsMAD2 and p55CDC, in human lung cancers. Oncogene. 1999;18:4295–4300. doi: 10.1038/sj.onc.1202807. [DOI] [PubMed] [Google Scholar]
- 28.Chen JG, Horwitz SB. Differential mitotic responses to microtubule-stabilizing and destabilizing drugs. Cancer Res. 2002;62:935–938. [PubMed] [Google Scholar]
- 29.Tugendreich S, Tomkiel J, Earnshaw W, Hieter P. Cdc27Hs colocalizes with Cdc16Hs to the centrosome and mitotic spindle and is essential for the metaphase to anaphase transition. Cell. 1995;81:261–268. doi: 10.1016/0092-8674(95)90336-4. [DOI] [PubMed] [Google Scholar]
- 30.Melloy PG, Holloway SL. Changes in the localization of the Saccharomyces cerevisiae anaphase-promoting complex upon microtubule depolymerizaton and spindle checkpoint activation. Genetics. 2004;167:1079–1094. doi: 10.1534/genetics.103.025478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weaver BAA, Cleveland DW. Decoding the links between mitosis, cancer, and chemotherapy: the mitotic checkpoint, adaptation, and cell death. Cancer Cell. 2005;8:7–12. doi: 10.1016/j.ccr.2005.06.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.