Abstract
PURPOSE
Microglia in the central nervous system display a marked structural dynamism in their processes in the resting state. This dynamic behavior, which may play a constitutive surveying role in the uninjured neural parenchyma, is also highly responsive to tissue injury. The role of CX3CR1, a chemokine receptor expressed in microglia, in regulating microglia morphology and dynamic behavior in the resting state and after laser-induced focal injury was examined.
METHODS
Time-lapse confocal imaging of retinal explants was used to evaluate the dynamic behavior of retinal microglia labeled with green fluorescent protein (GFP). Transgenic mice in which CX3CR1 signaling was ablated (CX3CR1GFP/GFP/CX3CR1−/−) and preserved (CX3CR1+/GFP/CX3CR1+/−) were used.
RESULTS
Retinal microglial density, distribution, cellular morphology, and overall retinal tissue anatomy were not altered in young CX3CR1−/− animals. In the absence of CX3CR1, retinal microglia continued to exhibit dynamic motility in their processes. However, rates of process movement were significantly decreased, both under resting conditions and in response to tissue injury. In addition, microglia migration occurring in response to focal laser injury was also significantly slowed in microglia lacking CX3CR1.
CONCLUSIONS
CX3CR1 signaling in retinal microglia, though not absolutely required for the presence of microglial dynamism, plays a role in potentiating the rate of retinal microglial process dynamism and cellular migration. CX3CL1 signaling from retinal neurons and endothelial cells likely modulates dynamic microglia behavior so as to influence the level of microglial surveillance under basal conditions and the rate of dynamic behavior in response to tissue injury.
Microglial cells, the resident macrophages of the central nervous system (CNS), are distributed throughout the neural parenchyma of the brain and the retina. These cells play important roles in development,1 in tissue responses to injury and infection,2,3 and in the pathogenesis of neurodegenerative disease.4 In the basal state, microglia in the CNS display a continuous, dynamic behavior in the form of rapid process extensions and retractions while maintaining stable soma positions. 5–7 After a focal tissue insult, microglial process movements lose their random nature and polarize themselves toward the site of injury. Microglia in this context increase in their overall rate of process motility and may acquire a whole cell migratory behavior and translocate through neural tissue.5–9 These behaviors have been documented in ex vivo6,9 and in vivo preparations5–9 and likely reflect endogenous mechanisms used by microglia to survey the CNS milieu for appropriate signals and to mobilize in response to insults.5–7 This capability is analogous to the “patrolling” behavior also described in peripheral monocytes on the endothelial surfaces of blood vessels.10 Although the patrolling behavior of peripheral monocytes has been shown to be highly dependent on chemokine signaling mediated through the receptor CX3CR1, the relationship of chemokine signaling with constitutive microglial surveillance behavior in the CNS has not been characterized.
CX3CR1 is a chemokine receptor expressed by microglia in the brain and the retina.11–13 Its only known ligand, CX3CL1 (also known as fractalkine or neurotactin), is constitutively expressed by neurons in the CNS13 and by neurons and endothelial cells in the retina.14 CX3CR1-CX3CL1 signaling has been implicated in neuron-to-microglia communications underlying the processes of microglial chemotaxis,13,15 microglia-mediated neurotoxicity,11,16 and microglial activation.17 CX3CL1 uniquely exists as both a membrane-bound ligand and a soluble chemokine that is proteolytically cleaved from the bound form.18,19 The bimodal property allows CX3CL1 to function within cell-to-cell contacts and at a distance. As such, CX3CR1-CX3CL1 signaling is a suitable candidate for the chemokine-based regulation of dynamic microglial behavior under resting conditions and in response to nearby tissue injury.
We have previously described the nature of dynamic microglial behavior using live ex vivo imaging of intact retina explants6 and demonstrated that the features of microglial response to injury in this preparation were consistent with low-magnification observations of microglia in vivo.8 In the present study, we used this ex vivo system to evaluate the potential role of CX3CR1 signaling in regulating different aspects of microglial behavior in the CNS. The mammalian retina, in addition to serving as a useful model system for examining neuronal-microglial interactions in the CNS, has also been implicated as a locus of disease caused by defective CX3CR1 signaling. In mice lacking CX3CR1, retinal microglia accumulate aberrantly in the subretinal space with advancing age.12,20 We reasoned that though defective CX3CR1 signaling in the long term culminates in the dislocation of microglia in the aged retina, CX3CR1 signaling in the short term may also influence constitutive dynamic microglial behavior in the young adult retina under resting conditions and in response to focal injury.
We explored this hypothesis by performing live imaging of microglia in retinal explants isolated from CX3CR1+/GFP and CX3CR1GFP/GFP transgenic mice in which CX3CR1 signaling was preserved and ablated, respectively. We first confirmed that in the absence of CX3CR1 signaling, morphologic features of the retina, including microglia distribution, vascular patterning, and neuronal lamination, all develop normally to adulthood.21 We then used live imaging to continuously monitor individual microglial processes, overall microglial morphology, and whole cell cellular migration. Our results demonstrated that though chemokine signaling through CX3CR1 is not necessary for the presence and nature of constitutive “surveying” behavior in resting microglia, it plays a significant role in regulating their magnitude. In addition, in response to focal injury, microglia lacking CX3CR1 are still able to polarize their processes toward injured tissue and to acquire a migratory phenotype, but the resultant rate of migration without CX3CR1 signaling is significantly slowed. Thus, neuronal cells and vascular endothelial cells, the main sources of CX3CL1 in the CNS and the retina,14,22 are likely to use this mode of signaling to communicate with neighboring microglia and to exert a modulatory influence on their behavior. Our findings demonstrate a specific role for constitutive CX3CR1-mediated signaling in the CNS and illustrate a mechanism by which microglial behavior, at rest and in response to injury, may be shaped by neuronally derived influences.
MATERIALS AND METHODS
Experimental Animals
Homozygous CX3CR1GFP/GFP transgenic mice on a C57BL/6 background, in which the coding sequence of the CX3CR1 has been replaced by the gene coding for enhanced green fluorescent protein, were obtained from The Jackson Laboratory (Bar Harbor, ME). Heterozygous CX3CR1+/GFP animals were created by breeding to wild-type mice of a C57BL/6 background. Young adult mice 3 to 5 weeks of age were used. In the remainder of this article, CX3CR1+/GFP and CX3CR1GFP/GFP genotypes are referred to as CX3CR1+/− and CX3CR1−/−, respectively, reflecting the presence and absence of CX3CR1 signaling. All mice were housed and bred in National Institutes of Health animal facilities. Experiments were conducted according to protocols approved by a local Institutional Animal Care and Use Committee and adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Immunohistochemistry
Retinal tissue, fixed in 4% paraformaldehyde in 1× PBS (pH 7.3), was either flatmounted, embedded in 7% agarose, and cut into 120-µm-thick sections with a vibrating microtome (VT1000S; Leica, Wetzlar, Germany) or embedded in paraffin and sectioned into 5-µm-thick sections. Immunohistochemical staining was performed on the sections with Griffonia simplicifolia IB4 lectin (1:100) to reveal vascular staining and with 4′,6-diamidino-2-phenylindole (DAPI; 1:1000) to act as a nuclear marker (Invitrogen, Carlsbad, CA).
Confocal Time-Lapse Imaging in Living Retinal Explants
Retinal explants were prepared and ex vivo time-lapse imaging was performed as previously described.6 For imaging at baseline conditions, oxygenated Ringer solution was continuously superfused through the chamber. To study the effect of CX3CL1 on microglial morphology and behavior, Ringer solution containing 10 to 15 µM CX3CL1 (AF537; R&D Systems, Minneapolis, MN) was superfused into the recording chamber, replacing its contents. After 7 to 10 minutes of CX3CL1 exposure, the chamber was once again superfused with oxygenated Ringer solution for the “washout” phase of the recording. Time-lapse imaging was performed under identical conditions for CX3CR1+/− and CX3CR1−/− retinas. The application of focal injury to retinal explants was performed with similar settings, as previously described.6
RT-PCR and Enzyme-Linked Immunosorbent Assay Analysis of CX3CL1 mRNA and Protein Expression after Laser Injury
Retinal explants were prepared as for live-imaging analysis; explants were subject to focal laser injury or were left untreated. Explants from both groups were maintained in Ringer solution in an oxygenated chamber for 1 and 6 hours after laser injury. For RT-PCR analysis, the treated and untreated explants were transferred to a storage solution (RNAlater solution; Ambion, Austin, TX), and total RNA was isolated with a commercial kit (RNeasy Mini kit; Qiagen, Valencia, CA). First-strand cDNA was synthesized with a cDNA synthesis kit (Ambion) using oligo-dT as the primer. After dilution with Tris-EDTA, 1 µL cDNA was added to each 20-µL PCR reaction. CX3CL1-specific cDNA amplification was performed for 30 cycles at an annealing temperature of 56°C using the oligonucleotides 5′ ccgtttggccgagtcctgc 3′ (forward) and 5′ cactggcaccaggacgtatg3′ (reverse). Amplification of glyceraldehyde 3′-phosphate dehydrogenase (GAPDH) was included in each assay as an internal control to normalize the amount of cDNA with the primers 5′ cctctggaaagctgtggct 3′ (forward) and 5′ gttgctgtagccgtattcatt 3′ (reverse). The mRNA concentration of CX3CL1 was normalized against that of GAPDH. Relative expression data were averaged from three biological repeats.
For ELISA of CX3CL1 expression, similarly treated retinal explants were collected in 200 µL lysis buffer (RIPA buffer; Sigma, St. Louis, MO) and were incubated on ice for 20 minutes in the presence of protease inhibitors (Pierce, Rockford, IL). After centrifugation at 14,000g for 20 minutes, the supernatant was collected, and the concentration of total protein was determined with the use of a protein assay kit (BCA Protein Assay Reagent; Pierce). CX3CL1 protein levels were measured 6 hours after laser injury with sandwich immunoassay (R&D Systems) according to the manufacturer’s protocol.
Image and Statistical Analyses
Image processing was performed with ImageJ software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html), as previously described.6 Statistical analyses were performed using computer software (Prism; GraphPad, San Diego, CA).
Statistical significances for comparisons between genotypes were analyzed with an unpaired, two-tailed t-test, whereas comparisons before and after interventions (e.g., the application of CX3CL1) were analyzed with a paired, two-tailed t-test. One-way ANOVA was used to compare expression levels of CX3CL1.
RESULTS
Effect of CX3CR1 Signaling on Dynamic Behavior of Resting Microglia
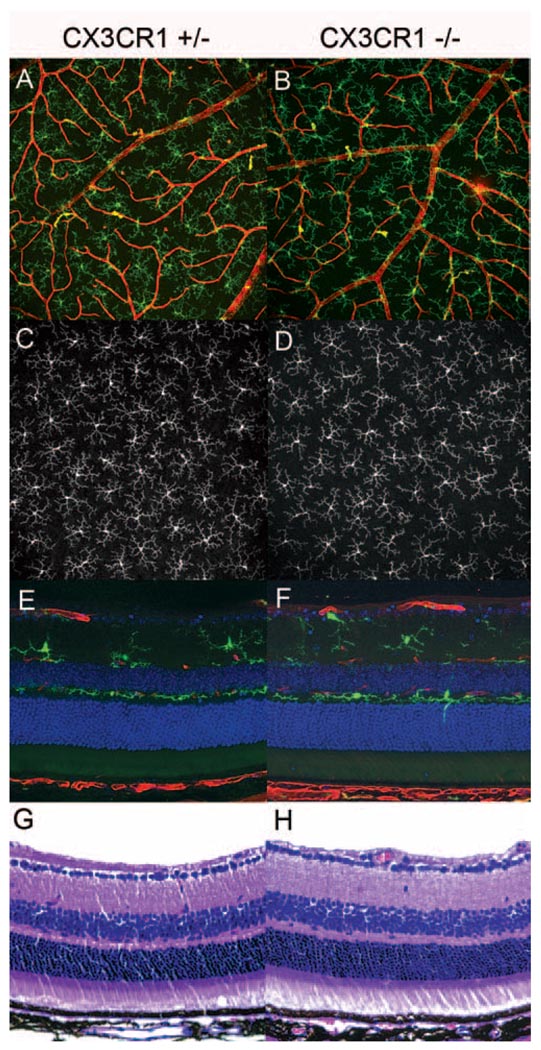
Although previous studies have described the presence of abnormal subretinal accumulations of microglia in older CX3CR1-deficient mice,12 the development and homing of microglia to the retina in early adulthood (6–12 weeks of age) occur independently of CX3CR1 signaling.21 We verified that CX3CR1+/− and CX3CR1−/− animals from the age group studied here (3–5 weeks of age) had similar microglia density, morphology (Figs. 1A–D), and location in the stratified layers of the inner retina (Figs. 1E, F) as those found in wild-type mice.23 The architecture of the retinal vasculature, neuronal components and plexiform layers also appeared to develop normally and independently of CX3CR1 signaling (Figs. 1G, H).
Figure 1.
Retinal development occurs normally in the presence and absence of CX3CR1. Retinas of CX3CR1-deficient animals do not exhibit structural differences in retinal lamination, vasculature, or overall morphology and distribution of microglia (A, B). Extended-focus confocal images of retinal microglia (green) and blood vessels (red) in the inner retina (extending from the ganglion cell layer to the inner plexiform layer) in wholemount preparations of CX3CR1+/− (A) and CX3CR1−/− (B) retinas demonstrate normal structure and distribution. (C, D) Extended-focus confocal images of the outer retina (outer plexiform layer) in wholemount preparations show normal mosaic tiling by microglia in CX3CR1+/− and CX3CR1−/− animals. Laminar distribution of microglia (green) in agarose-embedded vibratome sections (E, F) and structural lamination of the retina in paraffin sections (G, H) are comparable in CX3CR1+/− and CX3CR1−/− animals (red, lectin; blue, DAPI).
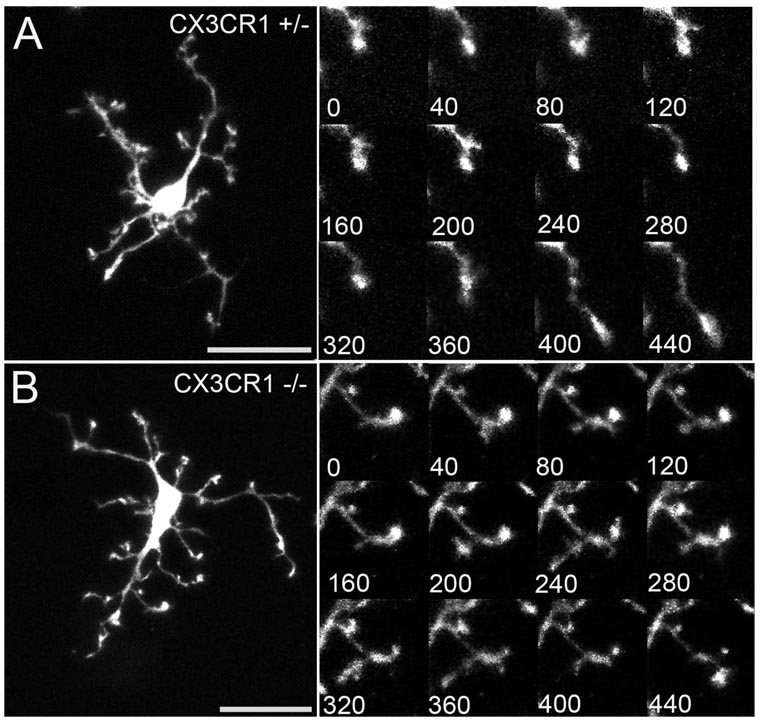
The normal development of neuronal, vascular, and microglial components of the young adult retinas of CX3CR1+/− and CX3CR1−/− animals suggests that any differences in microglial behavior are secondary to altered microglial communication rather than to developmentally induced changes. We have previously reported that resting retinal microglia from CX3CR1+/− animals with preserved CX3CR1 signaling display a dynamic and continuous “surveying” behavior.6 Here we show that CX3CR1+/− and CX3CR1−/− microglial cellular processes repeatedly extend and retract in an apparently random manner. New processes are also continuously produced, and existing processes are retracted (Figs. 2A, B; Movies S1, S2, http://www.iovs.org/cgi/content/full/50/9/4444/DC1). However, in resting microglia, overt cellular migration is not observed, and cellular somata remain fixed in position with uniform spacing between cells.
Figure 2.
Dynamic behavior in resting retinal microglia is evidenced by rapidly remodeling processes. Cellular processes of CX3CR1+/− (A) and CX3CR1−/− (B) microglia exhibit similar patterns of motility that include extensions and retractions of existing processes, de novo creation of processes, and elimination of existing processes. (A, B, insets) Time-lapse confocal images demonstrate the nature and rate of these movements. Consecutive time-lapse images were taken 40 seconds apart. Scale bar, 25 µm.
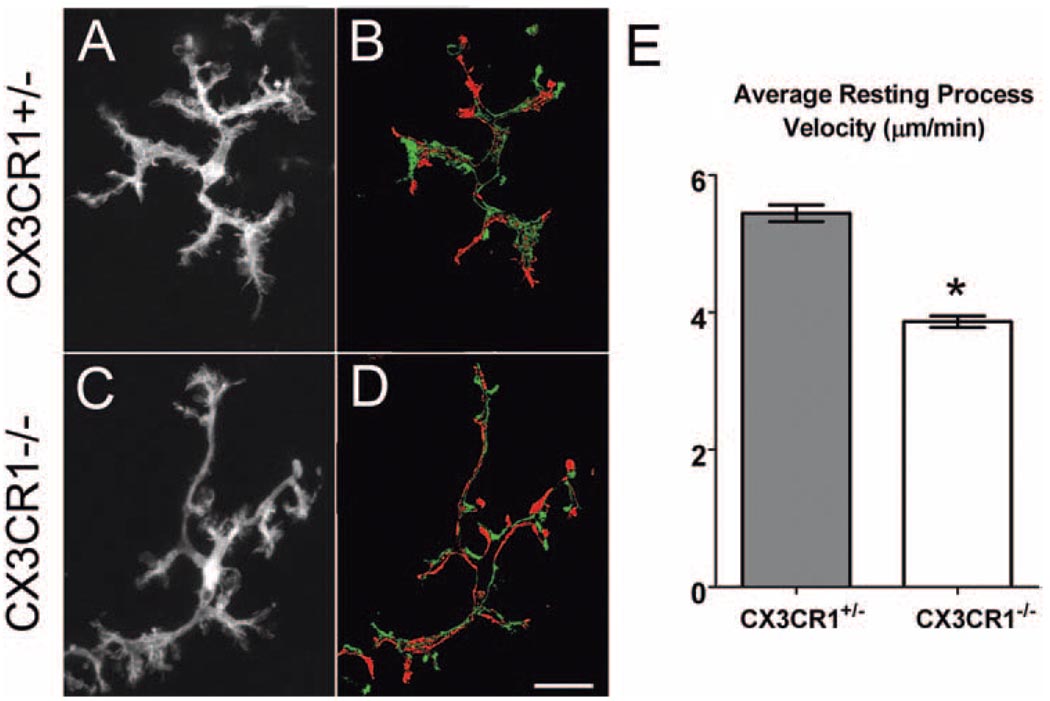
CX3CR1−/− and CX3CR1+/− microglia were also able to move their processes through their extracellular environment to similar extents. Maximum intensity projections of time-lapse recordings showed that CX3CR1+/− and CX3CR1−/− microglia were capable of covering an extended extracellular space (Figs. 3A, C). The balance between positive (process extensions and creation of new processes) and negative (process retractions and elimination of existing processes) structural changes allowed for the maintenance of overall cellular morphologic homeostasis in both genotypes. Like CX3CR1+/− microglia, CX3CR1−/− microglia were nonmigratory in the resting state. Although the qualitative features of microglial process dynamics appeared to be independent of CX3CR1 signaling, quantification of the average process velocity revealed that the processes of CX3CR1+/− microglia were significantly slower (3.9 ± 0.08 µm/min) than the processes of CX3CR1+/− microglia (5.4 ± 0.12 µm/min) (P < 0.05; Fig. 3E).
Figure 3.
Resting dynamics of CX3CR1+/− and CX3CR1−/− microglia have similar patterns of behavior but differ in their rates of movement. Dynamic behavior of microglia allows extensive sampling of the surrounding environment, as demonstrated in z-projections of 50 time-lapse images captured 20 seconds apart (A, heterozygous; C, homozygous). Microglia maintain an overall balance of process extensions and retractions so that the overall cellular area remains relatively constant, as shown in the subtraction confocal images (B, heterozygous; D, homozygous). Numbers of processes newly formed (green) and eliminated (red) between t = 0 seconds and t = 500 seconds are relatively equal. (E) Comparison of the average velocity of microglial processes in CX3CR1+/− (n = 363 processes in 37 cells) and CX3CR1−/− (n = 255 processes in 47 cells) microglia shows an overall degree of motility that is significantly lower in the absence of CX3CR1 signaling. *P < 0.05. Scale bar, 25 µm.
Effect of CX3CR1 Signaling on Microglial Process Dynamics and Morphology in Response to Focal Injury
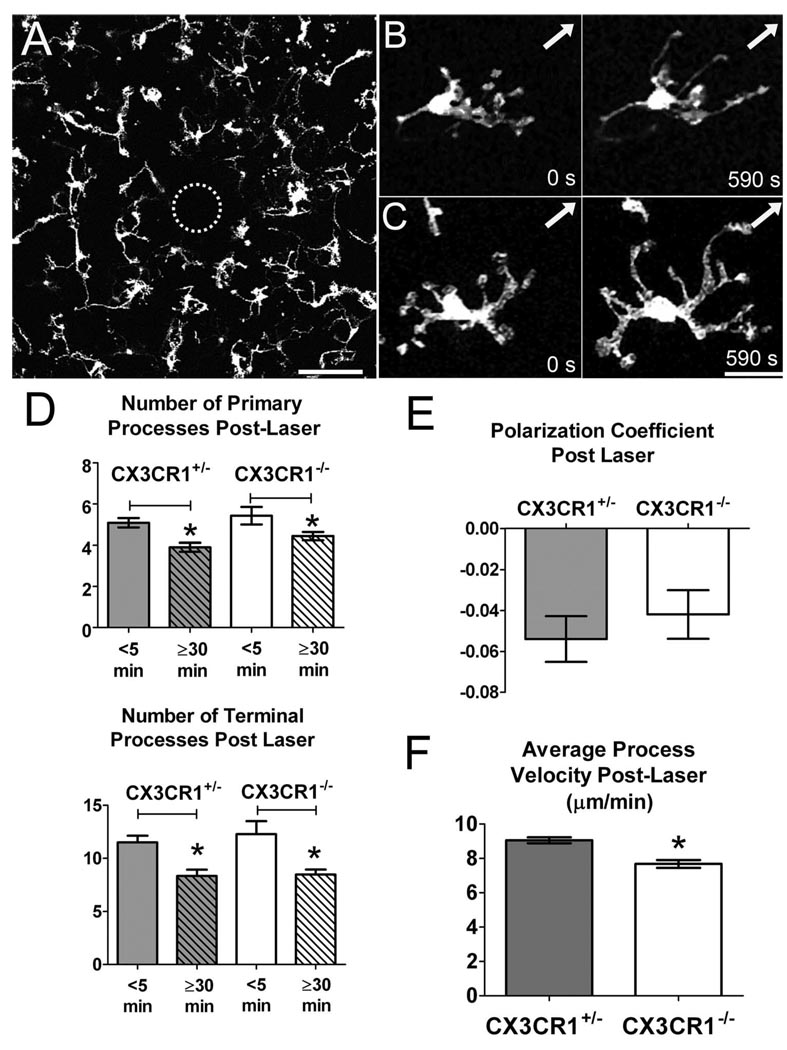
Retinal microglia undergo prominent changes in morphology and behavior after tissue injury. Our experiments used a photocoagulative argon laser to induce focal retinal tissue injury. Here, small laser burns, approximately 50 to 100 µm in diameter, were delivered in a controlled manner to the full thickness of the retina tissue (Fig. 4A). We previously reported that CX3CR1+/− microglia in the vicinity of the laser burn (<500 µm) undergo distinct morphologic changes shortly (5–70 minutes) after injury.6 The cells first direct their processes preferentially toward the injury site, which leads to polarization of the microglial arbor. At the same time, these cells reduce their overall number of primary and secondary processes. The average dynamism of individual processes also increases, with processes moving at a higher average velocity than in the resting state.
Figure 4.
Morphologic and dynamic changes of CX3CR1+/− and CX3CR1−/− retinal microglia in response to focal laser injury. (A) Confocal image taken at low magnification (20×) of a wholemount CX3CR1−/− retina 5 minutes after the induction of focal laser injury. Dotted circle: Locus of laser burn with underlying ablation of microglia. Scale bar, 100 µm. (B, C) After focal laser injury, surrounding CX3CR1+/− (B) and CX3CR1−/− (C) microglia progressively orient their processes toward the site of focal injury (arrow). This occurs over a time scale of approximately 10 minutes. Scale bar, 25 µm. (D) Retinal microglia in CX3CR1+/− and CX3CR1−/− animals undergo similar morphologic changes after laser injury, simplifying their branched structure and decreasing the number of primary and terminal processes. Significant differences were found within each genotype when comparing the number of processes at 5 minutes and 30 minutes after injury (analysis of a total of 150 cells in 24 laser-induced injuries; *P < 0.05). (E) Polarization coefficients of CX3CR1+/− (n = 153 cells) and CX3CR1−/− (n = 136 cells) microglia after laser injury were not statistically different, which suggests that microglia polarization develops independently of CX3CR1 signaling. (F) Average process velocity increased after focal injury in CX3CR1+/− and CX3CR1−/− retinal microglia. However, the postinjury velocity of CX3CR1+/− microglial processes (n = 119 processes in 28 cells) was statistically higher than in CX3CR1−/− microglia (n = 181 processes in 51 cells). *P < 0.05.
To evaluate the role of CX3CR1 signaling on these dynamic responses to injury, we performed similar laser injuries on CX3CR1−/− retinas. We found that microglia lacking CX3CR1 responded to laser injury in a manner qualitatively similar to that seen in microglia with preserved CX3CR1 signaling (Figs. 4B, 4C; Movies S3, S4). Quantification of the primary and terminal process number per cell in CX3CR1+/− and CX3CR1−/− microglia indicated that microglia of both genotypes decrease their process number in a time-dependent manner after injury. Microglia of both genotypes also acquired polarized morphologies of similar polarization coefficient values (Fig. 4E). CX3CR1+/− and CX3CR1−/− microglia increased their average process motility in response to injury; however the post-injury velocity of CX3CR1+/− microglial processes remained significantly higher (9.1 ± 0.2 µm/min) than that of CX3CR1−/− microglial processes (7.7 ± 0.2 µm/min; P < 0.0001; Fig. 4F).
Effect of CX3CR1 on Microglial Cell Migration in Response to Focal Injury
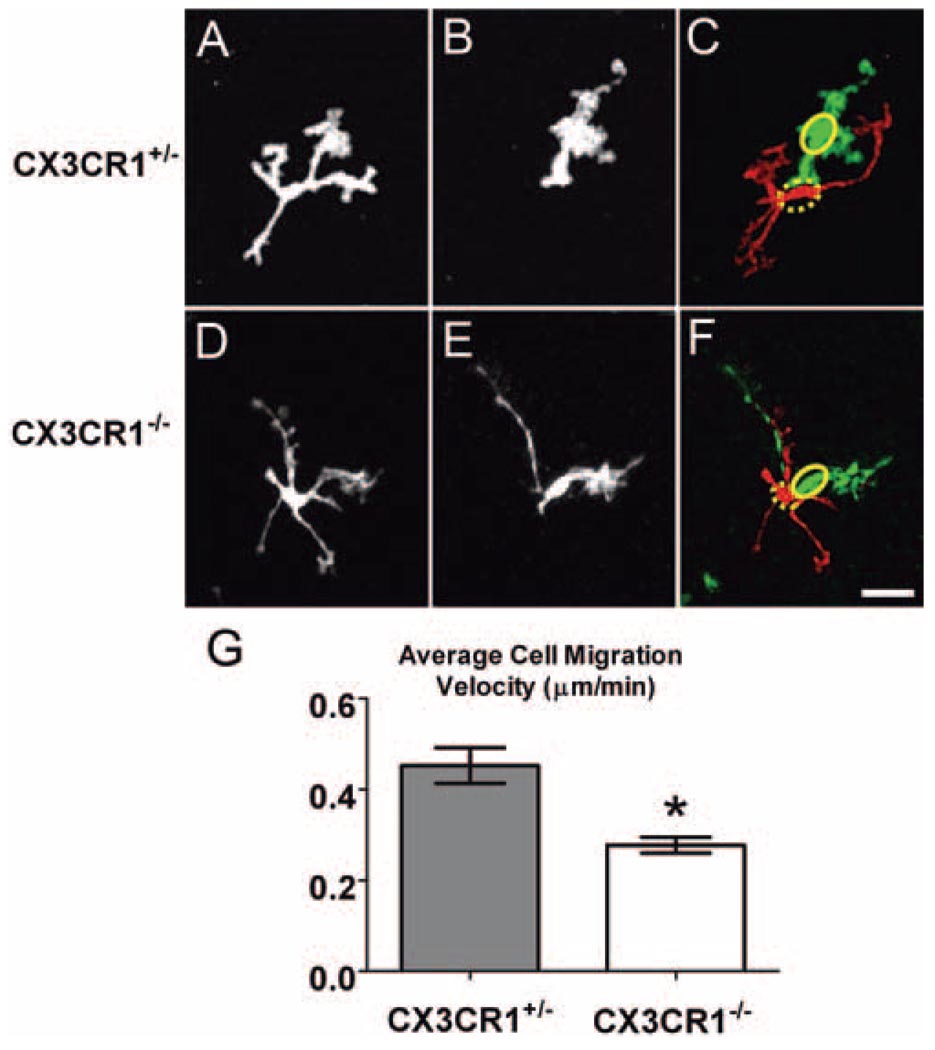
CX3CR1+/− retinal microglia in the vicinity of the laser burn acquired a migratory capability, confirming our previous findings. However, CX3CR1−/− microglia demonstrated significantly reduced rates of migration and soma translocation compared with CX3CR1+/− microglia (Figs. 5A–F; Movies S5, S6). The average cell migration velocity for CX3CR1−/− microglia (0.28 ± 0.02 µm/min) was significantly lower than that for CX3CR1+/− microglia (0.45 ± 0.04 µm/min; P < 0.05; Fig. 5G).
Figure 5.
CX3CR1+/− and CX3CR1−/− retinal microglia exhibited migratory behavior after focal laser injury. Representative examples of CX3CR1+/− and CX3CR1−/− retinal microglia at t = 0 (A, D) and at t = 495 seconds (B, E). Subtraction images (C, F) show the initial (red, with soma position indicated by dashed ellipse) and final (green, with soma position indicated by solid ellipse) positions of migrating microglia. Scale bar, 25 µm. (G) The average soma migration rate in CX3CR1+/− microglia (n = 150 cells in 11 recordings) was significantly higher than in CX3CR1−/− microglia (n = 157 cells in 12 recordings). *P < 0.05.
Effect of Exogenous CX3CL1 on Retinal Microglial Morphology and Behavior
In addition to assessing the effects of the presence and absence of endogenous CX3CR1 signaling, we also evaluated the effect of exogenously applied CX3CL1 on retinal microglia. CX3CL1, when exogenously applied at concentrations in the range of 5 to 100 nM to cultured microglia, have been demonstrated to induce calcium mobilization, activate intracellular signaling, induce microglial chemotaxis,15 and result in the release of adenosine.24 Here we show that the application of CX3CL1 (10–15 nM) to CX3CR1+/− microglia in retinal explants resulted in a rapid change in microglia morphology in most (60%–71%) microglia imaged. In responding microglia, after 1 to 2 minutes of application, cell processes were replaced by shorter and more highly branched counterparts that were flatter and more lamellipodia-like in structure (Figs. 6A–C). These morphologic changes were readily and rapidly reversible on washout with normal Ringer solution (Fig. 6D; Movie S7). Unlike CX3CR1+/− microglia, CX3CR1−/− microglia did not show a similar morphologic response (Figs. 6E, F). Despite the morphologic transformation, CX3CR1+/− microglial processes retained their dynamic, bidirectional movements, maintained a similar overall rate of process movement (Fig. 6G), and did not adopt a migratory phenotype.
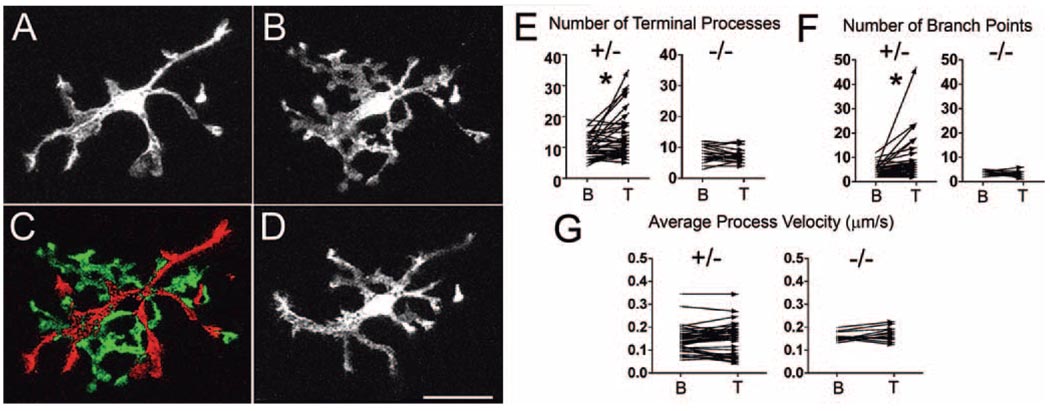
Figure 6.
Exogenously applied CX3CL1 leads to changes in microglial morphology. Representative example of a CX3CR1+/− retinal microglial cell (A) under baseline conditions and (B) after application of exogenous CX3CL1 (12.5 nM for 10 minutes). (C) In response to CX3CL1, microglia retract their longer processes (red) and produce multiple highly branched lamellipodia-like processes (green), as shown in the subtraction image. These morphologic changes were reversible, with microglia reverting back to their usual morphology after washout of CX3CL1 (D, 12 minutes after washout). No migratory behavior involving the translocation of the soma was observed during or after CX3CL1 treatment over approximately 30 minutes. (E) Quantification of terminal processes in retinal microglia under baseline conditions (indicated as “B” on the x-axis) and during CX3CL1 treatment (10–15 nM; indicated as “T”). After CX3CL1 application, the number of their processes significantly increased in CX3CR1+/− microglia, whereas little change was seen with CX3CR1−/− microglia. *P < 0.05. (F) The number of branch points similarly increased significantly with CX3CL1 application in CX3CR1+/− microglia, but not in CX3CR1−/− microglia. *P < 0.05. (G) Average process velocity was not significantly affected by CX3CL1 treatment in CX3CR1+/− or CX3CR1−/− retinal microglia.
Expression of CX3CL1 in the Retina after Laser Injury
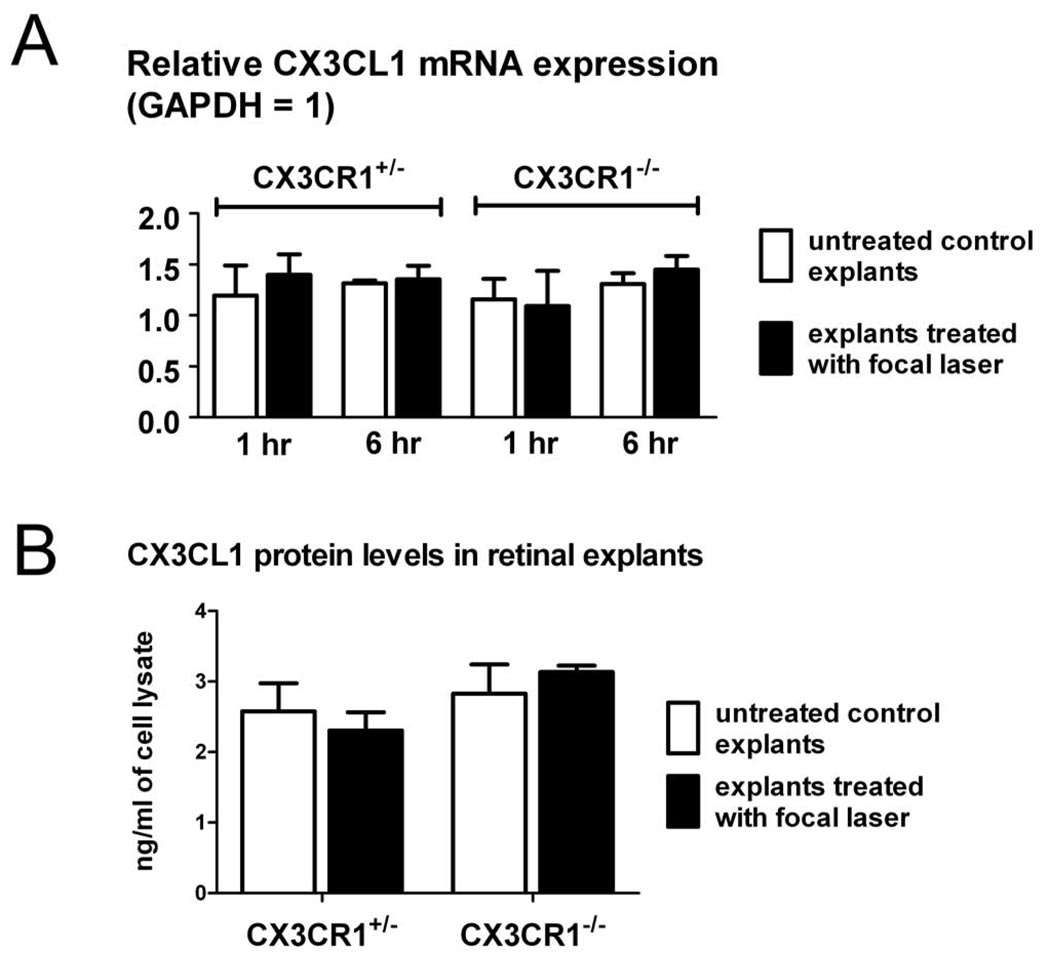
The expression of CX3CL1 after focal laser injury was evaluated on an mRNA level by RT-PCR and on a protein level by ELISA in CX3CR1+/− and CX3CR1−/− retinal explants up to 6 hours after laser injury. CX3CL1 levels were not statistically distinct between CX3CR1+/− and CX3CR1−/− retinas (Figs. 7A, B). In addition, no significant changes in CX3CL1 expression level were induced acutely in either genotype by the application of focal laser injury.
Figure 7.
Expression of CX3CL1 in the retina is not acutely increased after focal laser injury. (A) Expression of CX3CL1 mRNA was assessed by semiquantitative RT-PCR in CX3CR1+/− and CX3CR1−/− retinal explants 1 hour and 6 hours after focal laser injury. Retinal explants not treated with focal laser were used as controls. For all comparisons, CX3CL1 mRNA expression levels were not significantly changed between genotypes or between laser explants and controls (P > 0.05 for all comparisons). (B) Expression of CX3CL1 on a protein level as evaluated by ELISA was also not significantly different (P < 0.05) when evaluated 6 hours after focal laser treatment. Retinal explants not treated with focal laser were used as controls.
DISCUSSION
The marked changes in CNS microglia seen in different pathologic states have been traditionally construed as a transformative process in which a microglial cell converts from a dormant, inactive state to an active state (i.e., “activated” microglia). However, recent evidence5–7 has shown that so-called “resting” microglia are not dormant at all but exhibit rapid, scanning movements that effectively cover the surrounding extracellular space. In a recent review, Hanisch and Kettenmann25 retermed resting microglia in the steady state as surveying microglia, postulating that they dynamically seek out nearby signals in the surrounding environment, interpret them, and then modulate their phenotype in a diverse and graded manner. This proposed surveillance function of microglia is supported by their rapid response to microlesions in neuronal tissue. Microglial processes rapidly polarize toward the injury site and increase their rates of motility, in some cases acquiring a slow migratory phenotype.5–7
Constitutive surveying behavior of microglia has been documented in the cerebral cortex,5,7 hippocampus,26 and retina6 and may be common to all CNS microglia. However, its regulation remains unclear. Neurotransmission may be one such mode of regulation, but varied results have been found. Exogenous application of glutamate and GABA in hippocampal slices had little effect on microglial motility in one study,26 whereas blockade of endogenous GABAergic transmission increased process motility in another.7 Electrical activity in nearby neurons also did not exert a significant influence on microglial motility as either decreasing activity using tetrodotoxin7 or increasing activity by applied tetanic stimulation.26 Both had little effect on microglia behavior.
The role of chemokine signaling in regulating microglia process motility, however, has not been extensively addressed in previous studies. In the retina, CX3CL1 is constitutively expressed in retinal neurons and vascular endothelial cells and is upregulated under conditions of inflammation.14 Given that CX3CR1 is expressed only by microglia in the retina, CX3CL1-CX3CR1 signaling represented a potential mode of constitutive neuron-to-microglia communication relevant to modulating microglial behavior. We have addressed this question in the present study by using ex vivo live imaging in retinal explants that allow intact neuronal tissue to be isolated and manipulated without the physical injury associated with tissue slicing. Retinal explants afford good optical access to retinal microglia so that individual processes can be imaged reliably at high spatial and temporal resolution. Previous studies using similar explant preparations have demonstrated that multiple endogenous physiological processes (e.g., neuronal electrical activity, neurotransmission, 27,28 and dynamic neuronal plasticity29,30) are maintained in the explanted retina. Although studies of dynamism in retinal microglia have been previously conducted in vivo8,31 and have provided support for some of our ex vivo observations,6 current in vivo imaging approaches lack the spatial and temporal resolution needed to monitor individual microglial processes continuously. Although it is possible that CX3CL1 levels in explant retinal tissue may vary from those found in vivo, our results indicated that CX3CL1 expression in retinal explants was detectable and was maintained over the duration of our imaging studies.
Although CX3CL1 has been previously shown to induce morphologic changes in dissociated cells in vitro,15,32 we demonstrated here that CX3CR1+/− microglia in the context of retinal tissue are also responsive to exogenous CX3CL1. The direct involvement of CX3CR1 is supported by our observations that CX3CR1-deficient microglia fail to show similar morphologic changes. Interestingly, exogenous bath application of CX3CL1, while converting microglial processes from a long filopodia-like form to a flat, more highly branched, lamellipodia-like structures, did not alter the velocity of processes. It is possible that the effects of CX3CR1-mediated signaling may depend on how this ligand is presented. Whether CX3CL1 is presented in its secreted or membrane-bound form or within a concentration gradient, may contribute to differing microglial effects. In addition, the rapid response of retinal microglia to CX3CL1 application and the equally rapid recovery after washout seen in our experiments suggested that the effects of CX3CL1-CX3CR1 signaling are short-lived, allowing microglia to constantly adjust their dynamism and morphology according to current levels of signaling.
Our results indicate that CX3CR1 signaling in microglia contributes to the rate of resting microglial dynamism and to the rate of dynamic microglial responses to focal tissue injury. Although the presence of CX3CR1 signaling did not appear to be absolutely required for the presence of these responses, it significantly potentiated the rate of these responses. Given that retinal development and microglial morphology and distribution are normal in the CX3CR1-deficient retina, it is more likely that these quantitative differences in dynamic motility arise from differences in CX3CR1-mediated signaling rather than from a nonspecific deleterious effect on the health of CX3CR1-deficient microglia.
In our expression studies, we did not observe an acute overall upregulation of CX3CL1 in the retina on an mRNA or a protein level in the hours after focal laser injury. In addition, locally elevated levels of CX3CL1 in perilesional areas were not detected on immunohistochemical staining (data not shown). As a result, the polarization of microglia processes and the increase in process motility observed acutely after laser injury are unlikely to be directly induced by chemotaxis toward increased CX3CL1 expression in the lesion zone. Rather, preexisting overall levels of CX3CL1-CX3CR1 signaling to microglia at the time of injury set the overall “tone” for microglial behavior. Chronic levels of retinal inflammation can elevate CX3CL1 levels14 and, in so doing, may influence the vigor of microglial surveying behavior and prompt a more rapid dynamic microglial response to tissue insult. In this context, the level of CX3CL1-CX3CR1 signaling may serve as a mechanism by which immune vigilance and microglial responsiveness may be regulated across the retina in functionally useful ways.
In the absence of all CX3CR1 signaling, though no developmental or anatomic abnormalities in the retina are seen in young animals, it is possible that the reduced ability of microglia to screen the neural parenchyma and respond rapidly to insults may have long-term consequences. These may be related to the abnormal accumulation of microglia in the subretinal space, as seen with aging or after neuronal injury.12,20,33 In addition, the increased neurotoxicity associated with CX3CR1-deficient brain microglia11,12 may also be related to aberrations in microglial immune surveillance and response.
Why microglia are so prominently dynamic in their behavior is an interesting question whose answer remains incomplete. Studying the details of retinal microglial behavior and their regulatory mechanisms may provide insight into microglial function in the retina and into the nature of intercellular communications that microglia have with other retinal cell types. Our findings here provide an initial demonstration that microglial process motility in intact neuronal tissue may be the subject of chemokine regulation and may present an example by which signals arising from neurons may modulate the dynamic behavior and injury response of microglia.
Acknowledgments
The authors thank Lian Zhao and the National Eye Institute Histology Core for technical assistance.
Supported by the National Eye Institute Intramural Research Program and by the Intramural Division of the National Eye Institute and the Howard Hughes Medical Institute (JEL).
Footnotes
Disclosure: K.J. Liang, None; J.E. Lee, None; Y.D. Wang, None; W. Ma, None; A.M. Fontainhas, None; R.N. Fariss, None; W.T. Wong, None
References
- 1.Das S, Basu A. Inflammation: a new candidate in modulating adult neurogenesis. J Neurosci Res. 2008;86:1199–1208. doi: 10.1002/jnr.21585. [DOI] [PubMed] [Google Scholar]
- 2.Falsig J, van Beek J, Hermann C, Leist M. Molecular basis for detection of invading pathogens in the brain. J Neurosci Res. 2008;86:1434–1447. doi: 10.1002/jnr.21590. [DOI] [PubMed] [Google Scholar]
- 3.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 4.Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 5.Davalos D, Grutzendler J, Yang G, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 6.Lee JE, Liang KJ, Fariss R, Wong WT. Ex vivo dynamic imaging of retinal microglia using time-lapse confocal microscopy. Invest Ophthalmol Vis Sci. 2008;49:4169–4176. doi: 10.1167/iovs.08-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 8.Eter N, Engel D, Meyer L, et al. In vivo visualization of dendritic cells, macrophages and microglial cells responding to laser-induced damage in the fundus of the eye. Invest Ophthalmol Vis Sci. 2008;49:3649–3658. doi: 10.1167/iovs.07-1322. [DOI] [PubMed] [Google Scholar]
- 9.Kurpius D, Nolley EP, Dailey ME. Purines induce directed migration and rapid homing of microglia to injured pyramidal neurons in developing hippocampus. Glia. 2007;55:873–884. doi: 10.1002/glia.20509. [DOI] [PubMed] [Google Scholar]
- 10.Auffray C, Fogg D, Garfa M, et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 11.Cardona AE, Pioro EP, Sasse ME, et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- 12.Combadiere C, Feumi C, Raoul W, et al. CX3CR1-dependent sub-retinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J Clin Invest. 2007;117:2920–2928. doi: 10.1172/JCI31692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrison JK, Jiang Y, Chen S, et al. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci U S A. 1998;95:10896–10901. doi: 10.1073/pnas.95.18.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silverman MD, Zamora DO, Pan Y, et al. Constitutive and inflammatory mediator-regulated fractalkine expression in human ocular tissues and cultured cells. Invest Ophthalmol Vis Sci. 2003;44:1608–1615. doi: 10.1167/iovs.02-0233. [DOI] [PubMed] [Google Scholar]
- 15.Maciejewski-Lenoir D, Chen S, Feng L, Maki R, Bacon KB. Characterization of fractalkine in rat brain cells: migratory and activation signals for CX3CR-1-expressing microglia. J Immunol. 1999;163:1628–1635. [PubMed] [Google Scholar]
- 16.Mizuno T, Kawanokuchi J, Numata K, Suzumura A. Production and neuroprotective functions of fractalkine in the central nervous system. Brain Res. 2003;979:65–70. doi: 10.1016/s0006-8993(03)02867-1. [DOI] [PubMed] [Google Scholar]
- 17.Zujovic V, Benavides J, Vige X, Carter C, Taupin V. Fractalkine modulates TNF-alpha secretion and neurotoxicity induced by microglial activation. Glia. 2000;29:305–315. [PubMed] [Google Scholar]
- 18.Bazan JF, Bacon KB, Hardiman G, et al. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385:640–644. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- 19.Pan Y, Lloyd C, Zhou H, et al. Neurotactin, a membrane-anchored chemokine upregulated in brain inflammation. Nature. 1997;387:611–617. doi: 10.1038/42491. [DOI] [PubMed] [Google Scholar]
- 20.Tuo J, Bojanowski CM, Zhou M, et al. Murine ccl2/cx3cr1 deficiency results in retinal lesions mimicking human age-related macular degeneration. Invest Ophthalmol Vis Sci. 2007;48:3827–3836. doi: 10.1167/iovs.07-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kezic J, McMenamin PG. Differential turnover rates of monocyte-derived cells in varied ocular tissue microenvironments. J Leukoc Biol. 2008;84:721–729. doi: 10.1189/jlb.0308166. [DOI] [PubMed] [Google Scholar]
- 22.Tarozzo G, Bortolazzi S, Crochemore C, et al. Fractalkine protein localization and gene expression in mouse brain. J Neurosci Res. 2003;73:81–88. doi: 10.1002/jnr.10645. [DOI] [PubMed] [Google Scholar]
- 23.Santos AM, Calvente R, Tassi M, et al. Embryonic and postnatal development of microglial cells in the mouse retina. J Comp Neurol. 2008;506:224–239. doi: 10.1002/cne.21538. [DOI] [PubMed] [Google Scholar]
- 24.Lauro C, Di Angelantonio S, Cipriani R, et al. Activity of adenosine receptors type 1 Is required for CX3CL1-mediated neuroprotection and neuromodulation in hippocampal neurons. J Immunol. 2008;180:7590–7596. doi: 10.4049/jimmunol.180.11.7590. [DOI] [PubMed] [Google Scholar]
- 25.Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 26.Wu LJ, Zhuo M. Resting microglial motility is independent of synaptic plasticity in mammalian brain. J Neurophysiol. 2008;99:2026–2032. doi: 10.1152/jn.01210.2007. [DOI] [PubMed] [Google Scholar]
- 27.Wong WT, Myhr KL, Miller ED, Wong RO. Developmental changes in the neurotransmitter regulation of correlated spontaneous retinal activity. J Neurosci. 2000;20:351–360. doi: 10.1523/JNEUROSCI.20-01-00351.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong WT, Sanes JR, Wong RO. Developmentally regulated spontaneous activity in the embryonic chick retina. J Neurosci. 1998;18:8839–8852. doi: 10.1523/JNEUROSCI.18-21-08839.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong WT, Faulkner-Jones BE, Sanes JR, Wong RO. Rapid dendritic remodeling in the developing retina: dependence on neurotransmission and reciprocal regulation by Rac and Rho. J Neurosci. 2000;20:5024–5036. doi: 10.1523/JNEUROSCI.20-13-05024.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong WT, Wong RO. Changing specificity of neurotransmitter regulation of rapid dendritic remodeling during synaptogenesis. Nat Neurosci. 2001;4:351–352. doi: 10.1038/85987. [DOI] [PubMed] [Google Scholar]
- 31.Paques M, Simonutti M, Roux MJ, et al. High resolution fundus imaging by confocal scanning laser ophthalmoscopy in the mouse. Vision Res. 2006;46:1336–1345. doi: 10.1016/j.visres.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 32.Gevrey JC, Isaac BM, Cox D. Syk is required for monocyte/macrophage chemotaxis to CX3CL1 (Fractalkine) J Immunol. 2005;175:3737–3745. doi: 10.4049/jimmunol.175.6.3737. [DOI] [PubMed] [Google Scholar]
- 33.Raoul W, Keller N, Rodero M, Behar-Cohen F, Sennlaub F, Combadiere C. Role of the chemokine receptor CX3CR1 in the mobilization of phagocytic retinal microglial cells. J Neuroimmunol. 2008;198:56–61. doi: 10.1016/j.jneuroim.2008.04.014. [DOI] [PubMed] [Google Scholar]