Abstract
Background
Infection with Vibrio cholerae induces protection from subsequent severe disease, suggesting that an effective vaccine could be an important preventive strategy. Available vaccines provide less protection against cholera than natural infection, particularly in children.
Methods
We examined a cohort of 121 children (2 years-12 years of age) and 276 older patients (>12 years of age) hospitalized with cholera in Dhaka, Bangladesh over a 4-year period, to compare clinical features in older patients and children and immune responses to key antigens.
Results
Older patients had more severe disease. Children with cholera were more commonly retinol deficient, while zinc deficiency was equally prevalent in both groups. Children developed higher vibriocidal and serum immune responses to the B subunit of cholera toxin (CTB). In contrast, older patients mounted higher immune responses to 2 other key V. cholerae antigens, the lipopolysaccharide (LPS) and toxin coregulated pilus antigens (TcpA). We compared immune responses following infection with those occurring after receipt of a live, oral vaccine in both children and older patients in Bangladesh, during a similar time period. The response rates for vibriocidal and LPS antibodies were higher after infection than after vaccination. Both vaccinated older patients and children responded poorly to CTB and TcpA.
Conclusions
Although children developed vigorous vibriocidal and CTB-specific responses following infection, they had lessened responses to LPS and TcpA compared with older patients, as well as lessened responses to vaccination. More studies need to be carried out to determine factors, including micronutrient interventions that can improve responses in children to both natural infection and vaccination.
Keywords: cholera, Vibrio cholerae O1/O139, children, older patients, immunological responses
Vibrio cholerae is classically associated with a severe acute secretory diarrhea, although a spectrum of symptoms, ranging from severe dehydrating illness to mild or asymptomatic infections, may occur.1 Both V. cholerae serogroups O1 and O139 are responsible for epidemic cholera.2 Since its initial emergence, V. cholerae O139 accounts for only a minority of cases of cholera, with the majority due to the El Tor biotype of V. cholerae O1, either the Inaba or Ogawa serotypes. In areas of the world endemic for cholera, V. cholerae O1 infection is more common in children than older individuals, likely reflecting acquired immunity in the aging population, while V. cholerae O139 infection is more common in older patients,3 likely because of the low prevalence of this infection overall, and therefore the lack of acquired immunity in a substantial proportion of the aging population.4,5 An appropriate vaccine would be an important public health tool to prevent or decrease epidemics of severe disease due to V. cholerae in both epidemic and endemic settings.6-9 However, available oral vaccines have been found to be more immunogenic and protective in older patients than in children,6 while the global burden of cholera falls disproportionately on children in developing countries. Numerous studies in endemic areas demonstrate that the highest incidence of cholera occurs in children younger than 9-12 years of age.2 In 2 recent studies, the peak incidence was highest in children <5 years of age or <1 year of age, respectively.10,11 Epidemiological studies in Bangladesh have shown that the case fatality rate of cholera in children ages 1-5 years old is 10 times higher than that in older patients.12 If promptly recognized and treated appropriately, the mortality should be very low but recognition and treatment may not always happen in the resource-limited settings in which cholera occurs.
Studies of an oral, killed whole cell-cholera toxin B subunit vaccine showed a short-term protective efficacy of 26% in children in Bangladesh, a substantial drop off from the 63% efficacy seen in older patients6; similar results were seen in studies carried out in Peru.13 The live oral cholera vaccines, CVD103-HgR and Peru-15, also showed lower immunogenicity in children than in older patients in different settings.7,14,15
Because cholera affects children in endemic areas and because of the disparities in vaccine efficacy between children and older patients, increased efforts are now being given to understanding the problems associated with the lower “take rates” to oral vaccines in the younger age groups. Despite this need, the differences in immunologic responses to natural infection in children versus older patients with cholera have not been extensively characterized. To gain better understanding of this issue, we examined a cohort of older patients and children hospitalized with cholera in Dhaka, Bangladesh over a 4-year period, and compared clinical features as well as immunologic responses to key cholera antigens. We also compared the immunologic responses seen in natural infection with those reported in phase I/II trials of the live, oral attenuated cholera vaccine, Peru-15, in Bangladesh during the same time period.14,15
MATERIALS AND METHODS
Study Design and Subject Enrollment
The hospital at the Clinical Research and Service Centre (CRSC) of the International Centre for Diarrheal Disease Research (ICDDR, B) cares for approximately 10,000-20,000 cholera patients annually. From January 2001 to December 2005, we enrolled patients presenting to the hospital with acute watery diarrhea. The degree of dehydration ranged from mild to severe, as assessed according to World Health Organization guidelines.16 We enrolled cholera patients based on our case definition in a prospective way and followed this cohort of patients for clinical and immunologic parameters for a period of 21 days after onset of illness. Enrollment was based on a convenience sample in accord with inclusion and exclusion criteria described earlier.1 Inclusion criteria included a positive stool culture for V. cholerae O1 or O139 in patients age 2 years and older, and absence of significant comorbid conditions. Exclusion criteria included current enrollment in an unrelated interventional study, or having a family member who had received care at the ICDDR, B within the preceding 2 months. We only had 2 patients in the cohort who were <2 years of age (under 1 year), but we excluded them from the analysis because the numbers were too few for any meaningful analysis. We wished to compare features of cholera in children (2-12 years) with those in older patients (>12 years of age). We included in our study 276 adolescents and older patients (>12 years) and 121 younger children (2-12 years) who received care for cholera at the ICDDR, B. We collected blood and stool specimens for immunologic measurements on days 2, 7, and 21 after the onset of illness. We compared clinical features of children and older patients, as well as vibriocidal antibody responses and immune responses to the cholera toxin B subunit (CTB), toxin-coregulated pilus subunit (TcpA), and lipopolysaccharide (LPS) in sera (days 2, 7, and 21), and antibody responses to CTB in feces (days 2 and 7). Study day 1 was defined as the day of identification of the patient; these patients were enrolled on day 2 if they had a stool culture that was positive for V. cholerae O1 or O139. Information regarding clinical features and demographics were collected from patients at enrollment after clinical stabilization. Specimens for zinc and retinol estimation were only available from a subsample of patients. We analyzed sera from 151 patients, of whom 75 were children aged 2-12 years, and 76 were older than 12 years of age. Serum retinol levels were assayed by high-performance liquid chromatography (Shimadzu, SPD-10Avp), and serum zinc levels were measured by atomic absorption spectrophotometer (Shimadzu, AA-65015). Retinol deficiency for all age groups was defined as a serum level <20 μg/dL and zinc deficiency as <0.6 mg/L.17
Informed consent for participation in this research study was obtained from all patients or their guardians, and approval for this study was obtained from the Research and the Ethical Review Committees of the ICDDR, B, as well as from the Institutional Review Board of the Massachusetts General Hospital.
Confirmation of Bacterial Strains
All cases of cholera were confirmed by culturing stool for V. cholerae O1 or O139 using standard procedures.18 All isolates recovered were either El Tor V. cholerae O1 or O139.
Measurement of Vibriocidal and Antigen-Specific Antibody Responses in Serum and Fecal Extracts
We performed vibriocidal antibody assays as previously described, using guinea pig complement and the homologous serotype of V. cholerae O1 or O139 as the target organism.19 Serum and fecal antibodies specific to CTB, TcpA, and LPS were measured by a kinetic enzyme-linked immunosorbent assay (ELISA) method.20 Briefly, 96-well microtiter plates were coated with either purified homologous LPS serotype (El Tor O1 Ogawa/Inaba or O139) (250 ng/well), or sequentially with GM1 ganglioside (100 ng/well) followed by recombinant CTB (50 ng/well) (gifts of A.M. Svennerholm), or with recombinant TcpA21 (150 ng/well); note that CTB and TcpA are identical between the V. cholerae O1 El Tor biotype and V. cholerae O139, and we used purified El Tor proteins in our assays. Plates were incubated with diluted patient sera, washed, and secondary, horseradish peroxidase-conjugated antihuman IgG or antihuman IgA antibodies were applied (Jackson Laboratories, Bar Harbor, ME). Plates were developed using 0.1% orthophenylene diamine (Sigma, St. Louis, MO) in 0.1 M sodium citrate buffer with 0.1% hydrogen peroxide; optical densities were measured kinetically at 450 nm for 5 minutes at 19-second intervals and values expressed as mAb/min. Antigen specific-IgA in fecal extracts was expressed as the fraction of total IgA in fecal extracts, which was determined by ELISA compared with a standard (1 mg/mL) derived from human colostrums obtained as previously described.22 Each ELISA plate had a positive control derived from pooled, convalescent sera from patients previously studied with cholera, and this positive control was used to standardize results across different ELISA plates. Immune responses in patients were measured as a ratio between convalescence (day 7 or 21) compared with that observed at the acute stage (day 2).
Results in cholera patients were also compared with those obtained in previous trials in vaccinees who had received a live, oral attenuated cholera vaccine Peru-15 in studies carried out between 2002 and 2005 in participants from similar demographic and socioeconomic status as the cholera patients. The single dose V. cholerae O1 vaccine is of the El Tor biotype and Inaba serotype and was used at a 108 CFU dose. Immunologic data from recent studies carried out in Bangladesh with Peru-15 in older patients15 and children14 were compared with similar responses in patients. For this purpose, data from 40 older vaccinees aged 18-45 years and 50 children, 2-5 years of age, were compared with the subset of naturally infected patients of the same ages. The vibriocidal, CTB, TcpA, and LPS-specific responses were compared. Vaccinees showing a ≥4-fold increase in vibriocidal responses 7 days after administration of the vaccine were considered responders. For the CTB, TcpA, and LPS antigens, individuals with a ≥2-fold increase were considered significant responders.
Statistical Analyses
Data analyses were performed using SPSS for Windows (Version 11.5; SPSS Inc., Chicago, IL) and Epi Info (Version 6.0; USD, Stone Mountain, GA). A P value <0.05 was considered statistically significant. Strength of association was determined by calculating odds ratios (OR) and their 95% confidence intervals (CI). For groups of patients, antibody titers are presented as geometric mean titers (GMT) and standard deviations (SD) in Table 1. Log10 transformation of vibriocidal titers and associated standard errors of the mean (SEM) are shown in Figure 1. Comparisons were carried out using the Mann-Whitney U tests for non-normally distributed data. The χ2 test was used for comparison of categorical variables. All reported P values are 2-tailed. A linear regression/model was used to determine if the duration of illness before hospitalization was responsible for the differences in immune response (vibriocidal, CTB, TcpA, or LPS-specific) between children and adults on different study days.
TABLE 1.
Comparison of Immunological Responses in Patients With Cholera, Stratified by Age
| Responses on Different Study Days* | Patients ≤12 Yrs Mean (SD) | Patients >12 Yrs Mean (SD) | P† |
|---|---|---|---|
| Vibriocidal antibody titers | |||
| Day 2 | 39.52 (7.53) | 86.40 (4.85) | <0.001 |
| Day 7 | 5678.75 (1479) | 4137.42 (1862) | 0.03 |
| Day 21 | 3711.14 (588) | 2827.90 (2187) | 0.04 |
| CTB IgA | |||
| Day 2 | 32.03 (39.75) | 30.06 (32.42) | 0.39 |
| Day 7 | 146.48 (141) | 103.35 (204) | <0.001 |
| Day 21 | 83.15 (170) | 68.47 (170) | 0.01 |
| CTB IgG | |||
| Day 2 | 123.47 (81) | 92.69 (98) | <0.001 |
| Day 7 | 326.87 (46) | 252.93 (58) | <0.001 |
| Day 21 | 344.35 (43) | 274.65 (49) | <0.001 |
| CTB-IgA response in feces | |||
| Day 2 | 18.93 (3.08) | 11.54 (4.87) | <0.001 |
| Day 7 | 81.44 (4.41) | 24.24 (3.59) | <0.001 |
| LPS IgA | |||
| Day 2 | 10.82 (2.39) | 21.37 (2.32) | <0.001 |
| Day 7 | 45.30 (4.24) | 117.44 (2.97) | <0.001 |
| Day 21 | 32.38 (3.18) | 87.86 (2.69) | <0.001 |
| LPS IgG | |||
| Day 2 | 87.63 (51.37) | 88.92 (57.52) | 0.96 |
| Day 7 | 144.92 (155) | 195.44 (102) | <0.001 |
| Day 21 | 157.46 (89) | 214.96 (65) | <0.001 |
| TcpA IgA | |||
| Day 2 | 7.46 (6.48) | 8.63 (10.41) | 0.822 |
| Day 7 | 9.13 (4.29) | 18.91 (3.83) | 0.001 |
| Day 21 | 8.73 (2.47) | 13.11 (3.03) | 0.004 |
| TcpA IgG | |||
| Day 2 | 60.37 (32.16) | 46.67 (33.59) | 0.001 |
| Day 7 | 82.90 (63.84) | 88.74 (73.32) | 0.881 |
| Day 21 | 106.67 (70.18) | 98.56 (83.53) | 0.090 |
Vibriocidal antibody titers are shown for the subset of patients infected with V. cholerae O1. Magnitude of responses to CTB, to homologous V. cholerae LPS (O1/O139), and to TcpA by kinetic ELISA (mAb/min) are shown; CTB IgA responses in feces were corrected for total fecal IgA. Geometric mean and SD are shown.
Statistical analyses were carried out using the Mann-Whitney U test for continuous variables.
FIGURE 1.
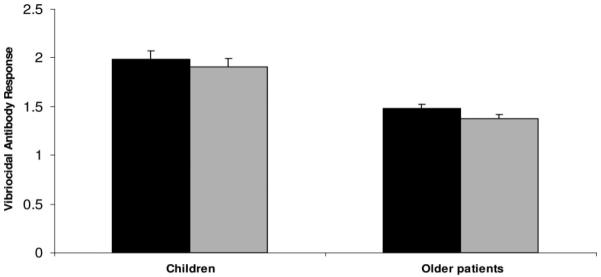
Fold increases in serum vibriocidal antibodies (log10 transformed) in children compared with those seen in older patients in the subset with infection with V. cholerae O1. (Bars) represent the mean (and 95% CI of the mean) increase in vibriocidal responses >16-fold in children and older patients measured as a ratio between convalescence at day 7 (black bars) or day 21 (shaded bars), compared with titers at the acute stage on day 2. *P = 0.01 and 0.0004 for the magnitude of differences between children and older patients at days 7 and 21, respectively.
RESULTS
Clinical Features
In this study, hospitalized children with cholera were mostly males, while hospitalized adolescents and older patients were mostly females (Table 2). An evaluation of the clinical characteristics of children (median, 6.4 years) and older patients (median, 28 years) with cholera showed that severe dehydration was seen more often in older patients than in children. The number of diarrheal stools before hospitalization was higher in older patients, with a longer interval between onset of disease and admission. Fifty-two percent of children were infected with the Ogawa serotype of V. cholerae O1 compared with only 30% of older patients. Over 93% of all V. cholerae O139 cases were seen in older patients. Retinol deficiency was more common in children than in older patients (47% versus 16%), while the rate of zinc deficiency was similar (27% versus 24%). Zinc and retinol deficiency reduced immune responses to CTB but no effect was seen on other immunologic parameters reported below.
TABLE 2.
Microbiological and Clinical Features of Patients With V. cholerae O1/O139 Induced Acute Watery Diarrhea
| Characteristics | Patients ≤12 Yrs (N = 121) | Patients >12 Yrs (N = 276) | Odds Ratio (95% CI) | P |
|---|---|---|---|---|
| Demographics | ||||
| Median age (mo) | 77 | 331 | ND | |
| Female | 54 (45)* | 164 (59) | ND | |
| Clinical | ||||
| Dehydration | ||||
| Severe | 104 (86) | 258 (94) | 0.43 (0.20-0.91) | 0.02 |
| Moderate | 17 (14) | 18 (7) | 2.34 (1.10-4.98) | 0.02 |
| No. stool 15 at presentation | 96 (79) | 254 (92) | 0.33 (0.17-0.64) | 0.0005 |
| Duration of diarrhea (h) at presentation (median; 25th, 75th percentile) | 10 (6, 22) | 14 (8, 23) | ND | 0.04 |
| Abdominal pain | 94 (78) | 205 (74) | 1.21 (0.71-2.07) | 0.55 |
| Fever (≥37.5°C)† | 25 (21) | 38 (14) | 1.63 (0.9-2.95) | 0.11 |
| Vomiting | 120 (99) | 264 (96) | 5.45 (0.73-113) | 0.12 |
| Microbiological | ||||
| V. cholerae O1, Ogawa | 63 (52) | 83 (30) | 2.53 (1.59-4.02) | <0.001 |
| V. cholerae O1, Inaba | 54 (45) | 135 (49) | 0.49 | |
| V. cholerae O139 | 4 (3) | 58 (21) | 0.13 (0.04-0.38) | <0.001 |
| Micronutrients | ||||
| Retinol deficiency‡ | 35 (47) | 12 (16) | 4.76 (2.04-10.82) | <0.001 |
| Zinc deficiency‡ | 20 (27) | 18 (24) | 1.17 (0.53-2.61) | 0.81 |
Percentage of number of patients with indicated finding.
Axillary temperature measured after rehydration.
These were measured in a subset of 75 children and 76 older patients. ND indicates not done.
Immunologic Responses in Cholera Patients
Because the magnitude of the vibriocidal response following infection with V. cholerae O1 differs from that following infection with V. cholerae O139, and because the majority of V. cholerae O139 cases occurred in the older age groups, we compared vibriocidal responses by age in the subset of patients infected with V. cholerae O1. The other immunologic responses measured did not differ between patients infected with V. cholerae O1 or O139,19 and the results for the 2 groups were analyzed together.
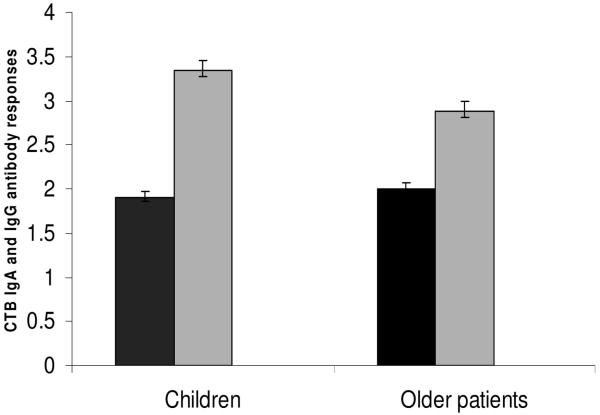
The magnitude of vibriocidal antibody responses was higher in children than in older patients at both days 7 and 21 (Table 1). More children than older patients showed >16-fold increases in vibriocidal antibody responses between days 2 and 7 (81% versus 67%, P = 0.01) and between days 2 and 21 (75% versus 53%, P = 0.0004) (Figure 1). The responses on day 2 were lower in children than those seen in the older age group (P = 0.001), perhaps reflecting less prior exposure. The magnitude of CTB-specific IgA and IgG responses were higher in children at days 7 and 21 than in older patients (Table 1). More children than older patients showed >4-fold increases in CTB-IgA antibody responses by day 7 (77% versus 60%, P = 0.002) (Fig. 2). The magnitude of fecal CTB-IgA antibody responses was higher in children at day 7 than in older patients (Table 1). More children than older patients showed >2-fold increases in fecal CTB-IgA antibody responses between days 2 and 7 (73% versus 46%, P = 0.009). The relatively longer interval between onset of disease and admission in adults compared with children (Table 2) did not have any effect on the immunologic responses seen on different study days between the 2 groups of patients (P = ns for all comparisons).
FIGURE 2.
Fold increases between days 2 and 7 in serum IgG (black bars) and IgA (shaded bars) to CTB in children compared to older patients with cholera. Error bars represent the 95% CI of the means. *P = 0.002 for the difference in the magnitude of rises in IgA between children and older patients.
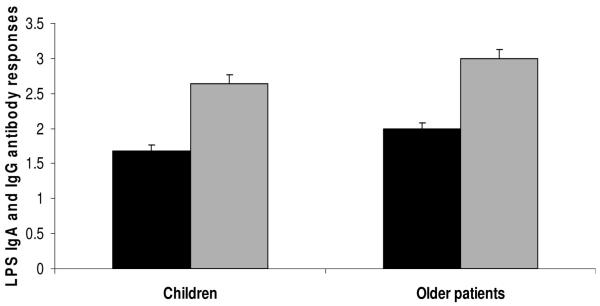
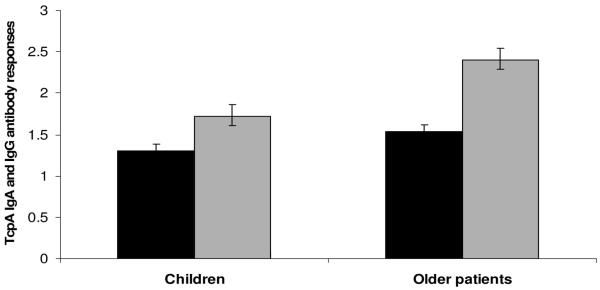
In contrast, older patients had higher LPS-specific IgA and IgG responses than children on days 7 and 21, both in magnitude and in response rates (P = 0.03) (Fig. 3; Table 1). Older patients also had a higher magnitude of TcpA-IgA antibodies at days 7 and 21, as well as fold increases of both TcpA-IgG and TcpA-IgA (Fig. 4; Table 1).
FIGURE 3.
Fold increases between days 2 and 7 in serum IgG (black bars) and IgA (shaded bars) to LPS in children compared to older patients with cholera. Error bars represent the 95% CI of the means. *P = 0.03 and **P = 0.0008 for the differences in the magnitude of rises between children and older patients, respectively.
FIGURE 4.
Fold increases between days 2 and 7 in serum IgG (black bars) and IgA antibodies (shaded bars) to TcpA children compared to older patients with cholera. Error bars represent the 95% CI of the means. *P = 0.04 and **P = 0.03 for the differences in the magnitude of rises between children and older patients, respectively.
When children were separately analyzed as groups of <5 years of age (n = 45) versus 5-12 years of age (n = 76), there were no significant differences between these 2 age strata.
Comparison of Immunologic Responses of Cholera Patients and Peru-15 Vaccine Recipients
We compared immunologic data obtained from participants in studies of the live, oral cholera vaccine, Peru-15,14,15 with those seen in the present study after natural infection in children and in older patients. Response rates for vibriocidal antibodies were higher in both children and older patients with cholera than in vaccinees (Table 3), as were the magnitude of responses (data not shown). The frequencies of responses to CTB (both IgG and IgA), LPS (both IgG and IgA), and TcpA (IgA) (Table 3), as well as the magnitude of responses to these antigens (data not shown) were also higher in children and older patients with cholera compared with vaccine recipients.
TABLE 3.
Comparison of Immune Response Rates in Children and Older Patients After Vaccination With the Live Oral Cholera Vaccine, Peru-15, to Those Seen After Natural Infection
| Immunological Parameter | Children | Older Patients | ||
|---|---|---|---|---|
| Vaccinees N = 50 Age 2-5 Yrs | Patients N = 47 Age 2-5 Yrs | Vaccinees N = 40 Age 18-45 Yrs | Patients N = 205 Age 18-45 Yrs | |
| Vibriocidal | 82% | 86% | 75%† | 90%† |
| CTB | ||||
| IgA | 20%* | 93%* | 8%† | 88%† |
| IgG | 0* | 68%* | 0† | 70%† |
| LPS | ||||
| IgA | 54% | 66% | 88% | 80% |
| IgG | 20%* | 43%* | 58%† | 69%† |
| TcpA | ||||
| IgA | 12%* | 29%* | 10%† | 58%† |
| IgG | ND | 16% | ND | 35% |
The frequency of responses were compared between children and older patients, both for vaccine recipients and for cholera patients 7 days after vaccination or onset of clinical illness. Response rates were defined as ≥4-fold responses for vibriocidal antibodies and ≥2-fold responses for the other antigens.
Significant differences in frequencies of responses between vaccinees and natural infection for children.
Significant differences between vaccinees and natural infection in older patients. ND indicates not done.
Children receiving the oral cholera vaccine showed a similar frequency of responses for vibriocidal antibodies as the older vaccinees, but the magnitude of responses were higher in the older patients than in the children (P = 0.009, data not shown); this was different than in natural infection, where children had higher vibriocidal responses than infected older patients. The frequency of IgA and IgG responses to LPS (Table 3), as well as the magnitude of responses (data not shown), were lower in children than in the older age group (P = 0.045-<0.001); this was similar to the results seen for natural infection. Both children and adult vaccine recipients responded poorly to CTB and TcpA.
DISCUSSION
The present analyses show that older patients present with more severe disease with V. cholerae infection than children, with higher purging rates and more dehydration; this is consistent with the findings of previous analyses.23,24 This difference in disease severity between older patients and children has also been documented in enterotoxigenic Esch-erichia coli (ETEC) diarrhea.25 The difference in clinical severity could be because of a longer delay of older patients reaching hospital facilities for treatment than that for children, as older patients may be reluctant to seek care because of missing work or may have childcare responsibilities. We did observe a longer duration of diarrhea in older patients compared with children before presentation at the hospital, suggesting that children were brought by their caregivers to clinical attention earlier in the course of illness than older patients who self-present and this may have affected the time course of their measured immune responses.
Ogawa was the main infecting serotype of V. cholerae O1 in the children in this study, while older patients had similar rates of Ogawa and Inaba infections. The reason for seeing more Ogawa infections in children might reflect differences in the prevalent serotype during enrollment.
The higher incidence of cholera caused by V. cholerae O1 in children in areas where the disease is endemic likely reflects the development of specific immunity over time, such that older patients are relatively protected. On the other hand, cholera caused by V. cholerae O139 is reported more frequently in older patients than in young children,3 and we confirmed this finding in our study. The persistence of V. cholerae O139 in older patients suggests either that this strain has not yet become sufficiently endemic that herd immunity has developed as the population ages, or that infection with this serogroup is less immunogenic than infection with V. cholerae O1.
Children with dehydrating cholera demonstrated higher vibriocidal and cholera toxin-specific antibody responses than older patients, suggesting that children in this population may be able to respond satisfactorily to these antigens. CT antibodies cross react with the related heat-labile enterotoxin, LT of ETEC, a common infection of children in the developing world; the prominent CTB-specific responses seen in the children in this study could reflect either the intrinsic immunogenicity of CT or boosting of pre-existing anti-CT or anti-LT immunity.
Interestingly, in comparison to the vibriocidal and CTB responses, infected children had lower antibody responses to LPS and TcpA than older patients. These latter antigens may be critical for mounting a protective response to cholera. The reasons for these lowered immune responses are not clear, but may reflect lack of prior priming or other factors, including age-related differences in the immune system. Nutritional deficiencies, including micronutrient deficiency, may also impact on these antibacterial responses.26 In a recent analysis, it was shown that vitamin A supplementation given every 6 months reduced the severity of diarrhea, but not the overall diarrhea-associated morbidity.27 We observed in our study that 47% of children with cholera were vitamin A deficient, compared with only 16% of older patients. Our analysis suggests that although children under 5 years of age receive vitamin A supplementation every 6 months under a government program in Bangladesh, children presenting with cholera often still lack adequate levels of vitamin A in their systemic circulation. Interestingly, previous studies showed that supplementation with vitamin A did not impact significantly on the immune responses to an oral cholera vaccine, at least for vibriocidal and anti-CTB responses,17,28 suggesting that the differences in retinol deficiency seen here between children and older patients may not fully explain the differences in immune responses.
In contrast to vitamin A deficiency, approximately 30% of both children and older patients with cholera in our study had reduced serum levels of zinc, even though zinc is an acute phase reactant and thus can be elevated with infection. Zinc deficiency is associated with reduced absorption of water and electrolytes and increased secretion after stimulation by cholera toxin.29 The observation that zinc deficiency was equally common regardless of age is of public health importance since zinc is given as treatment to children with acute diarrhea, but has not been previously considered for older patients.30,31 Zinc supplementation has also been shown to increase vibriocidal responses and fecal antibody responses to CTB, but decrease serum antibody responses to CTB after administration of an oral, killed, whole cell-cholera toxin B subunit vaccine in both children and older patients.17,28,32
The use of oral cholera vaccines is gaining acceptance as a public health control strategy, and their effectiveness in generating herd protection has also been assessed in epidemic settings.8,33 Cholera vaccines have generally been less effective in children in developing countries compared with results in naive European or U.S. subjects.7,9 The present study gave us the opportunity to compare data from patients with natural infection to similar data in vaccine recipients in the same area and time period, and to compare separately the results for older patients and for children. We found that vaccinated children responded less well than older patients in generating vibriocidal antibodies and antibodies to LPS, and that both children and older vaccinees responded poorly to CTB and TcpA. Children with natural infection had higher immune responses to all of these cholera antigens than vaccinated children. However, there may be other unrecognized differences between patients and vaccinees, such as nutritional status, micronutrient levels, or other parameters that may be important in the differing immune responses between the 2 groups.
Natural V. cholerae infection resulted in a quick onset of immunologic responses in children, similar to those seen in older patients. This suggests that the children had already been primed by at least some previous exposure; this is not surprising since cholera incidence rates are quite high in young children in endemic areas,2,11,34,35 giving ample opportunity for multiple exposures. Both natural infection and immunization with Peru-15 resulted in poorer responses to LPS and TcpA in children. The reduced response to LPS in children is probably because young children and infants are relatively less responsive to polysaccharide antigens than adults.36 The lower responses to LPS and TcpA may yield a shorter period of protection in children than in older patients after both natural infection and vaccination, although this has not yet been directly demonstrated. It is possible that nutritional interventions may improve immune responses in children to these antigens and improve protection following disease, as has been shown in cholera vaccination.17,37 Large scale use of zinc in acute diarrhea is already being advocated in Bangladesh and other developing countries.31,37
More effort needs to be given to creating awareness of the impact of cholera on children and to improving the immunogenicity of cholera vaccines among children in developing countries, particularly in children under 5 years of age.
ACKNOWLEDGMENTS
The authors thank the study participants as well as the dedicated field and laboratory workers of the Cholera Immune Response Study at the ICDDR, B.
Supported by the ICDDR, B and by the following grants: U01 A0I58935 (to S.B.C.); RO3 AI063079 (to F.Q.); RO1 AI40725 (to E.T.R.); DOMI funds from the Bill and Melinda Gates Foundations and International Vaccine Institute (to F.Q.); International Research Scientist Development Award KO1 TW07144 (to R.C.L.); International Research Scientist Development Award KO1 TW07409 (to J.B.H.); A.I. Khan is a recipient of the Fogarty/Ellison Fellowship in Global Health awarded by the Fogarty International Center at the National Institutes of Health (D43 TW005572).
REFERENCES
- 1.Harris JB, Khan AI, LaRocque RC, et al. Blood group, immunity, and risk of infection with Vibrio cholerae in an area of endemicity. Infect Immun. 2005;73:7422–7427. doi: 10.1128/IAI.73.11.7422-7427.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cholera Working Group. International Centre for Diarrhoeal Diseases Research Large epidemic of cholera-like disease in Bangladesh caused by Vibrio cholerae O139 synonym Bengal. Lancet. 1993;342:387–390. Bangladesh. [PubMed] [Google Scholar]
- 3.Sack RB, Siddique AK, Longini IM, Jr, et al. A 4-year study of the epidemiology of Vibrio cholerae in four rural areas of Bangladesh. J Infect Dis. 2003;187:96–101. doi: 10.1086/345865. [DOI] [PubMed] [Google Scholar]
- 4.Faruque AS, Fuchs GJ, Albert MJ. Changing epidemiology of cholera due to Vibrio cholerae O1 and O139 Bengal in Dhaka, Bangladesh. Epidemiol Infect. 1996;116:275–278. doi: 10.1017/s0950268800052572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albert MJ. Epidemiology & molecular biology of Vibrio cholerae O139 Bengal. Indian J Med Res. 1996;104:14–27. [PubMed] [Google Scholar]
- 6.Clemens JD, Sack DA, Harris JR, et al. Field trial of oral cholera vaccines in Bangladesh: results from three-year follow-up. Lancet. 1990;335:270–273. doi: 10.1016/0140-6736(90)90080-o. [DOI] [PubMed] [Google Scholar]
- 7.Suharyono Simanjuntak C, Witham N, et al. Safety and immunogenicity of single-dose live oral cholera vaccine CVD 103-HgR in 5-9-year-old Indonesian children. Lancet. 1992;340:689–694. doi: 10.1016/0140-6736(92)92231-4. [DOI] [PubMed] [Google Scholar]
- 8.Lucas ME, Deen JL, von Seidlein L, et al. Effectiveness of mass oral cholera vaccination in Beira, Mozambique. N Eng J Med. 2005;352:757–767. doi: 10.1056/NEJMoa043323. [DOI] [PubMed] [Google Scholar]
- 9.Richie EE, Punjabi NH, Sidharta YY, et al. Efficacy trial of single-dose live oral cholera vaccine CVD 103-HgR in North Jakarta, Indonesia, a cholera-endemic area. Vaccine. 2000;18:2399–2410. doi: 10.1016/s0264-410x(00)00006-2. [DOI] [PubMed] [Google Scholar]
- 10.Glass RI, Becker S, Huq MI, et al. Endemic cholera in rural Bangladesh, 1966-1980. Am J Epidemiol. 1982;116:959–970. doi: 10.1093/oxfordjournals.aje.a113498. [DOI] [PubMed] [Google Scholar]
- 11.Agtini MD, Soeharno R, Lesmana M, et al. The burden of diarrhoea, shigellosis, and cholera in North Jakarta, Indonesia: findings from 24 months surveillance. BMC Infect Dis. 2005;5:89. doi: 10.1186/1471-2334-5-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mosley WH, Benenson AS, Barui R. A serological survey for cholera antibodies in rural East Pakistan. 2. A comparison of antibody titres in the immunized and control population of a cholera-vaccine field-trial area and the relation of antibody titre to cholera case rate. Bull WHO. 1968;38:335–346. [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor DN, Cardenas V, Perez J, Puga R, Svennerholm AM. Safety, immunogenicity, and lot stability of the whole cell/recombinant B subunit (WC/rCTB) cholera vaccine in Peruvian adults and children. Am J Trop Med Hyg. 1999;61:869–873. doi: 10.4269/ajtmh.1999.61.869. [DOI] [PubMed] [Google Scholar]
- 14.Qadri F, Chowdhury MI, Faruque SM, et al. Peru-15, a live attenuated oral cholera vaccine, is safe and immunogenic in Bangladeshi toddlers and infants. Vaccine. 2007;25:231–238. doi: 10.1016/j.vaccine.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 15.Qadri F, Chowdhury MI, Faruque SM, et al. Randomized, controlled study of the safety and immunogenicity of Peru-15, a live attenuated oral vaccine candidate for cholera, in adult volunteers in Bangladesh. J Infect Dis. 2005;192:573–579. doi: 10.1086/432074. [DOI] [PubMed] [Google Scholar]
- 16.Diarrhoeal diseases control programme Global activities, 1988-1989. Releve epidemiologique hebdomadaire/Section d’hygiene du Secretariat de la Societe des Nations = Weekly epidemiological record/Health Section of the Secretariat of the League of Nations. 1990;65:289–292. [PubMed] [Google Scholar]
- 17.Albert MJ, Qadri F, Wahed MA, et al. Supplementation with zinc, but not vitamin A, improves seroconversion to vibriocidal antibody in children given an oral cholera vaccine. J Infect Dis. 2003;187:909–913. doi: 10.1086/368132. [DOI] [PubMed] [Google Scholar]
- 18.Qadri F, Azim T, Chowdhury A, et al. Production, characterization, and application of monoclonal antibodies to Vibrio cholerae O139 synonym Bengal. Clin Diagn Lab Immunol. 1994;1:51–54. doi: 10.1128/cdli.1.1.51-54.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qadri F, Mohi G, Hossain J, et al. Comparison of the vibriocidal antibody response in cholera due to Vibrio cholerae O139 Bengal with the response in cholera due to Vibrio cholerae O1. Clin Diagn Lab Immunol. 1995;2:685–688. doi: 10.1128/cdli.2.6.685-688.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asaduzzaman M, Ryan ET, John M, et al. The major subunit of the toxin-coregulated pilus TcpA induces mucosal and systemic immuno-globulin A immune responses in patients with cholera caused by Vibrio cholerae O1 and O139. Infect Immun. 2004;72:4448–4454. doi: 10.1128/IAI.72.8.4448-4454.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rollenhagen JE, Kalsy A, Cerda F, et al. Transcutaneous immunization with toxin-coregulated pilin A induces protective immunity against Vibrio cholerae O1 El tor challenge in mice. Infecti Immun. 2006;74:5834–5839. doi: 10.1128/IAI.00438-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qadri F, Ryan ET, Faruque AS, et al. Antigen-specific immunoglobulin A antibodies secreted from circulating B cells are an effective marker for recent local immune responses in patients with cholera: comparison to antibody-secreting cell responses and other immunological markers. Infect Immun. 2003;71:4808–4814. doi: 10.1128/IAI.71.8.4808-4814.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rabbani GH, Greenough WB., III . Pathophysiology and Clinical Aspects of Cholera. Plenum; New York, NY: 1992. [Google Scholar]
- 24.Longini IM, Jr, Yunus M, Zaman K, et al. Epidemic and endemic cholera trends over a 33-year period in Bangladesh. J Infect Dis. 2002;186:246–251. doi: 10.1086/341206. [DOI] [PubMed] [Google Scholar]
- 25.Qadri F, Svennerholm AM, Faruque AS, et al. Enterotoxigenic Esche-richia coli in developing countries: epidemiology, microbiology, clinical features, treatment, and prevention. Clin Microbiol Rev. 2005;18:465–483. doi: 10.1128/CMR.18.3.465-483.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krishnan S, Bhuyan UN, Talwar GP, et al. Effect of vitamin A and protein-calorie undernutrition on immune responses. Immunology. 1974;27:383–392. [PMC free article] [PubMed] [Google Scholar]
- 27.Fischer Walker CL, Black RE. Micronutrients and diarrheal disease. Clin Infect Dis. 2007;45(Suppl 1):S73–S77. doi: 10.1086/518152. [DOI] [PubMed] [Google Scholar]
- 28.Qadri F, Ahmed T, Wahed MA, et al. Suppressive effect of zinc on antibody response to cholera toxin in children given the killed, B subunit-whole cell, oral cholera vaccine. Vaccine. 2004;22:416–421. doi: 10.1016/j.vaccine.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Roy SK, Tomkins AM, Ara G, et al. Impact of zinc deficiency on Vibrio cholerae enterotoxin-stimulated water and electrolyte transport in animal model. J Health Popul Nutr. 2006;24:42–47. [PubMed] [Google Scholar]
- 30.Khan AM, Larson CP, Faruque AS, et al. Introduction of routine zinc therapy for children with diarrhoea: evaluation of safety. J Health Popul Nutr. 2007;25:127–133. [PMC free article] [PubMed] [Google Scholar]
- 31.Baqui AH, Black RE, Fischer Walker CL, et al. Zinc supplementation and serum zinc during diarrhea. Indian J Pediatr. 2006;73:493–497. doi: 10.1007/BF02759893. [DOI] [PubMed] [Google Scholar]
- 32.Karlsen TH, Sommerfelt H, Klomstad S, et al. Intestinal and systemic immune responses to an oral cholera toxoid B subunit whole-cell vaccine administered during zinc supplementation. Infect Immun. 2003;71:3909–3913. doi: 10.1128/IAI.71.7.3909-3913.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ali M, Emch M, von Seidlein L, et al. Herd immunity conferred by killed oral cholera vaccines in Bangladesh: a reanalysis. Lancet. 2005;366:44–49. doi: 10.1016/S0140-6736(05)66550-6. [DOI] [PubMed] [Google Scholar]
- 34.Sharma NC, Mandal PK, Dhillon R, Jain M. Changing profile of Vibrio cholerae O1, O139 in Delhi and its periphery (2003-2005) Indian J Med Res. 2007;125:633–640. [PubMed] [Google Scholar]
- 35.Sur D, Deen JL, Manna B, et al. The burden of cholera in the slums of Kolkata, India: data from a prospective, community based study. Arch Dis Child. 2005;90:1175–1181. doi: 10.1136/adc.2004.071316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rijkers GT, Dollekamp I, Zegers BJ. 8-Mercaptoguanosine overcomes unresponsiveness of human neonatal B cells to polysaccharide antigens. J Immunol. 1988;141:2313–2316. [PubMed] [Google Scholar]
- 37.Glass RI, Svennerholm AM, Stoll BJ, et al. Effects of undernutrition on infection with Vibrio cholerae O1 and on response to oral cholera vaccine. Pediatr Infect Dis J. 1989;8:105–109. [PubMed] [Google Scholar]