Abstract
Gene duplication is thought to provide raw material for functional divergence and innovation. Fish-specific dmrt2b has been identified as a duplicated gene of the dmrt2a/terra in fish genomes, but its function has remained unclear. Here we reveal that Dmrt2b knockdown zebrafish embryos display a downward tail curvature and have U-shaped somites. Then, we demonstrate that Dmrt2b contributes to a divergent function in somitogenesis through Hedgehog pathway, because Dmrt2b knockdown reduces target gene expression of Hedgehog signaling, and also impairs slow muscle development and neural tube patterning through Hedgehog signaling. Moreover, the Dmrt2b morphants display defects in heart and visceral organ asymmetry, and, some lateral-plate mesoderm (LPM) markers expressed in left side are randomized. Together, these data indicate that fish-specific duplicated dmrt2b contributes to a divergent function in somitogenesis through Hedgehog pathway and maintains the common function for left-right asymmetry establishment.
Introduction
Gene duplication is thought to be the primary source of new genes. Many teleost fish, including zebrafish, experience an additional genome wide duplication event [1]. Since then, many of the duplicated genes have been lost, but a substantial percentage of the duplicates have been retained, and functional divergence has occurred in some duplicates [1], [2]. Recently, to identify some differentially expressed genes in early embryogenesis, two kinds of SMART cDNAs were respectively synthesized from the mature eggs and gastrula embryos, and the gastrula embryo SMART cDNA library was constructed in Carassius auratus gibelio [3], [4], [5], [6], [7]. Following this program, many differentially expressed genes at gastrula stage were screened [5], and some of them were characterized and functionally analyzed [8], [9]. Significantly, a fish-specific duplicated gene dmrt2b was identified in dmrt gene family from Carassius auratus gibelio [4].
dmrt2, dsx and mab-3 related transcription factor 2, is a member of a gene family of putative transcription factors. These transcription factors share a highly conserved zinc-finger-like DNA-binding domain (DM domain) which is implicated in sex determination [10]. However, recent studies show that the family genes function not only in sex determination, but also in embryonic development [11]. In zebrafish, dmrt2 was originally called terra [12]. dmrt2b is another duplicated copy of the dmrt2 in the genome, and dmrt2a/terra and dmrt2b have been designated for distinguishing them. Interestingly, they share similar expression pattern in the muscle-related tissues, but dmrt2b is also expressed in branchial arches, which is different from dmrt2a [13]. The preliminary data suggested that dmrt2b and dmrt2a might have both common functions and divergent functions. In zebrafish, dmrt2a was found to be a left-right (LR) asymmetry gene required for left-right synchronization of the segmentation clock [14], but the LR asymmetry defect has not been observed in mouse embryos lacking Dmrt2 function. In contrast with the LR asymmetry requirement of dmrt2a in zebrafish, the loss of Dmrt2 in mouse leads to embryonic somite patterning defects [15]. Intriguingly, a previous study in zebrafish observed that overexpression of dmrt2a induced rapid apoptosis in the mesoderm, and suggested that Dmrt2a might be involved in somitogenesis of vertebrates [12]. The seemingly contradictive data propose that functional divergence might have occurred between the two duplicated genes of dmrt2a and dmrt2b. However, exact function of the fish-specific duplicated gene dmrt2b has remained unknown. This study aims to reveal the biological functions of dmrt2b and to examine the possible underlying mechanism and signal pathway by using morpholino-mediated knockdown strategy in the model animal zebrafish.
Results
Molecular characterization and expression pattern of Dmrt2b during embryogenesis
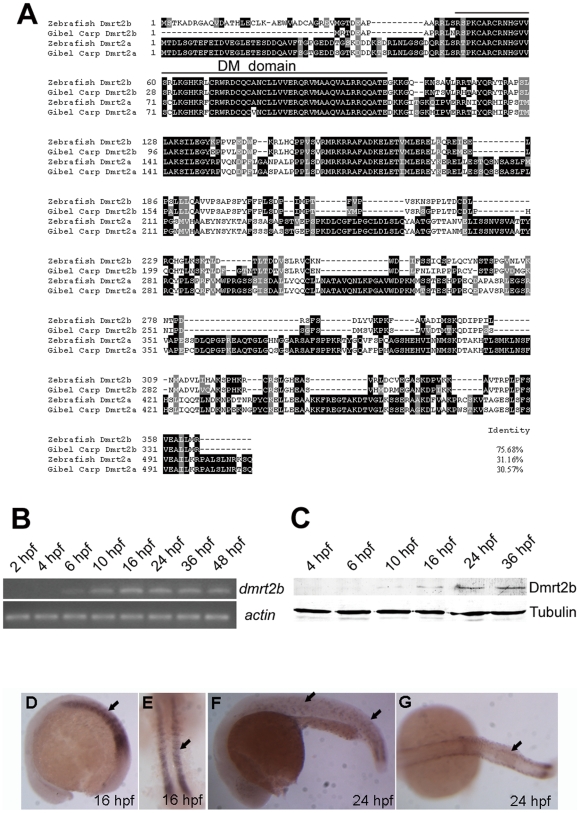
As a new member of dmrt gene family, dmrt2b was firstly cloned in gibel carp (Carassius auratus gibelio) (GenBank accession number: EF029082). Database searches revealed the closest homologue in zebrafish (Danio rerio) (GenBank accession number: NM_001079976). The DM domain of zebrafish Dmrt2b is 100% identical to that of gibel carp Dmrt2b, and has 94.7% and 93.0% identities to that of zebrafish and gibel carp Dmrt2a respectively. Significantly, the full-length amino acid sequence of zebrafish Dmrt2b has only 31.16% and 30.57% identities to zebrafish and gibel carp Dmrt2a, although it has 75.68% identity to gibel carp Dmrt2b (Figure 1A). The significant sequence divergence between Dmrt2b and Dmrt2a implicates the potential occurrence of functional divergence.
Figure 1. Molecular characterization and expression pattern of zebrafish Dmrt2b during embryogenesis.
(A) Amino acid alignment of zebrafish Dmrt2b with gibel carp Dmrt2b, gibel carp Dmrt2a and zebrafish Dmrt2a. Similar and identical amino acids are highlighted in grey and black boxes. The line indicates the DM domain. (B) RT-PCR detection of dmrt2b in zebrafish embryonic development stages, and beta-actin mRNA as the control. (C) Western blot detection of Dmrt2b during the zebrafish embryo development, and Tubulin was used for the control. (D-H) Whole-mount in situ hybridization detection of dmrt2b on somitogenesis embryos as indicated stage. The arrows indicate positive signals in the somites.
Subsequently, the expression pattern of zebrafish dmrt2b during embryogenesis was analyzed by RT-PCR, Western blotting and whole-mount in situ hybridization. As shown in Figure 1B and Figure 1C, the Dmrt2b transcription is initiated from around shield stage at 6 hpf, and kept at a basic stable level from bud stage at 16 hpf to the hatched larvae stage at 48 hpf. And, the Dmrt2b protein is expressed after bud stage at 10 hpf, and the abundant expression level is detected after 24 hpf of embryogenesis. Figure 1D–G shows in situ mRNA distribution of dmrt2b during embryogenesis. Significantly, dmrt2b is specifically expressed in somites during somitogenesis.
Dmrt2b knockdown leads to defects in somitogenesis and reduces target gene expression of Hedgehog signaling
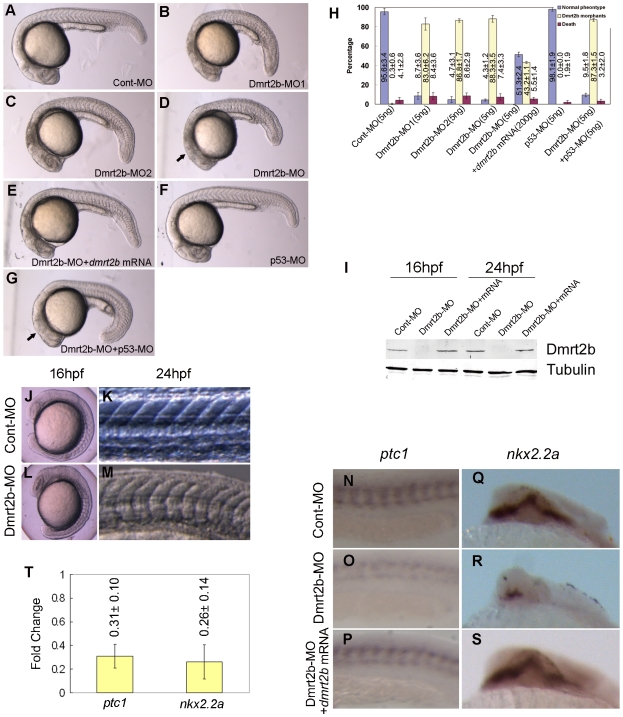
Abundant expression of dmrt2b in somites implies its significant functions in zebrafish embryo development. To assess the functions, we undertook loss-of-function experiments in zebrafish by morpholinos. We utilized two non-overlapping antisense morpholino oligonucleotides (MOs), Dmrt2b-MO1 and Dmrt2b-MO2, which designed to block translation by binding to 25 bases of the 5′ UTR upstream of the start codon (Supplementary Information, Figure S1A), because the translation-blocking morpholino was demonstrated to be a powerful tool for studying the effects of near-total loss of function during early stages of development [16]. Western blot detection showed a predicted size protein band in the Cont-MO embryos at 24 hpf, whereas the corresponding protein band was notably reduced in the embryos injected with Dmrt2b-MO1(5 ng per embryo) and Dmrt2b-MO2 (5 ng per embryo) respectively, and no any signal was observed in the embryos co-injected with Dmrt2b-MO1 (2.5 ng) and Dmrt2b-MO2 (2.5 ng) (Supplementary Information, Figure S1B). Morphological observation displayed obvious embryonic development defects in the Dmrt2b morphants. As shown in Figure 2, in comparison with normal embryos injected with 5 ng Cont-MO (Figure 2A), the morphant embryos injected with 5 ng Dmrt2b-MO1 or 5 ng Dmrt2b-MO2 exhibit an abnormal ventrally curved body shape, shot trunk and U-shaped somites at 24 hpf (Figure 2B,C), and when the two non-overlapping antisense MOs (2.5 ng each, totally 5 ng) were jointly injected, the morphant embryos exhibit similar phenotypes and even more severe defects (Figure 2D). Because co-injection of multiple targeting MOs was known to exert maximal effects [17], [18], [19], we combined Dmrt2b-MO1 and Dmrt2b-MO2 as Dmrt2b-MO for subsequent analysis. To confirm specificity of the embryonic defects, we performed rescue experiments in which we co-injected the Dmrt2b-MO with the in vitro transcribed dmrt2b mRNA. Significantly, the co-injected dmrt2b mRNA successfully rescued the embryonic defects in the morphants (Figure 2E). Since some off-targeting effects of morpholinos were found to be mediated through p53 activation, and p53-MO can attenuate the off-targeting effects [20], [21], we also checked the embryonic effects of Dmrt2b-MO and p53-MO co-injection. In comparison with normal embryos injected with p53-MO (Figure 2F), the co-injection embryos still exhibited the ventrally curved body shape, shot trunk and U-shaped somites, identically to the Dmrt2b morphants, whereas only cell death was reduced in the anterior and trunk regions (Figure 2G) compared with the Dmrt2b morphant (Figure 2D). The data indicated that the cell death observed in anterior and trunk regions of the Dmrt2b morphant appeared to be due to the off-target effects, because p53-MO co-injection could rescue the defect, and the defects in somitogenesis is specific to Dmrt2b knockdown, because they could not be rescued by p53-MO co-injection, which confirmed the specificity of the embryonic defects in the Dmrt2b morphant embryos. Figure 2H shows the statistical data of three independent experiments on the Dmrt2b knockdown, dmrt2b mRNA rescue and p53-MO co-injection.
Figure 2. Dmrt2b morphants display defects in somitogenesis and Hedgehog signaling.
(A–G) Morphology of 24 hpf embryos injected with Cont-MO (A), Dmrt2b-MO1 (B), Dmrt2b-MO2 (C), Dmrt2b-MO (D), Dmrt2b-MO+dmrt2b mRNA (E), p53-MO (F), Dmrt2b-MO+p53-MO (G). The arrows indicate the off-target cell death in Dmrt2b-MO morphant and the reduced cell death in the Dmrt2b-MO+p53-MO morphant. (H) The statistical data of three independent experiments on Dmrt2b knockdown, dmrt2b mRNA rescue and p53 MO co-injection. Results are represented as mean±SD of three separate experiments. (I) Western blot detection of Dmrt2b knockdown during embryogenesis. The protein extracts from embryos (16 hpf and 24 hpf) were analyzed by Western blot using the polyclonal anti-Dmrt2b antibody. A band of about 41 KD was not detected in Dmrt2b morphants. The picture represents typical result from three separate experiments. (J–M) Dmrt2b morphant exhibits U-shape somites. Morphology of embryos injected with Cont-MO display the typical ‘chevron’ shape (J, K). Morphology of embryos injected with Dmrt2b-MO display the U-shape (L, M). Whole-mount in situ hybridization of ptc1(N, O, P) (Anterior is left) and nkx2.2a(Q, R, S) (Anterior is top) in embryos injected with Cont-MO (N, Q) or Dmrt2b-MO (O, R) and embryos co-injected with Dmrt2b-MO with dmrt2b mRNA (P, S) at 24 hpf. (T) qPCR analysis of the expression changes of ptc1 and nkx2.2a in 24 hpf embryos injected with Cont-MO or Dmrt2b-MO. Results represent mean±SD of three separate experiments.
Furthermore, the ability of these MOs to block the endogenous dmrt2b translation was examined by Western blot. As shown in Figure 2I, the Dmrt2b protein was not detected in the morphants at 16 hpf and 24 hpf, whereas a about 41kD Dmrt2b protein band was observed in the Cont-MO and mRNA rescue embryos. The data indicate that the Dmrt2b-MO specifically blocks the dmrt2b mRNA translation, and that the morphant defects are resulted from the specific translation blocking of dmrt2b mRNA.
A detailed morphological observation for Dmrt2b morphants revealed similar common traits in Hedgehog pathway mutants, such as a curled tail, U-shaped somite boundaries and slow muscle defect [22], [23], [24], [25], [26], [27]. For example, in comparison to the typical ‘chevron’ shape (Figure 2J, K) in control embryos, the Dmrt2b morphants display severe U-shaped somites at 16 hpf (Figure 2L) and at 24 hpf (Figure 2M). To find further evidence that Dmrt2b is involved in Hedgehog signal pathway, we first checked the expression affection of patched1 (ptc1), a direct transcriptional target of the Hh pathway [28]. In comparison with high expression in somites of Cont-MO embryos (Figure 2N), an obvious down-regulation of ptc1 transcript was observed in the Dmrt2b morphants (23/27 embryos) at 24 hpf (Figure 2O). In embryos co-injected with Dmrt2b-MO and dmrt2b mRNA, low expression of ptc1 was rescued (21/25 embryos) at 24 hpf (Figure 2P). To reveal the signal point at which Dmrt2b exerts its activity on the Hh signaling cascade, we also examined the expression of the Hh target gene nkx2.2a in the developing brain [29]. As shown in Figure 2Q–S, Dmrt2b-MO causes a notable reduction of nkx2.2a in the brain (22/25 embryos), and co-injection with dmrt2b mRNA could rescue the phenotype (19/24 embryos). Quantification analysis through qPCR further confirmed the down-regulated changes of nkx2.2a and ptc1 expression (Figure 2T).
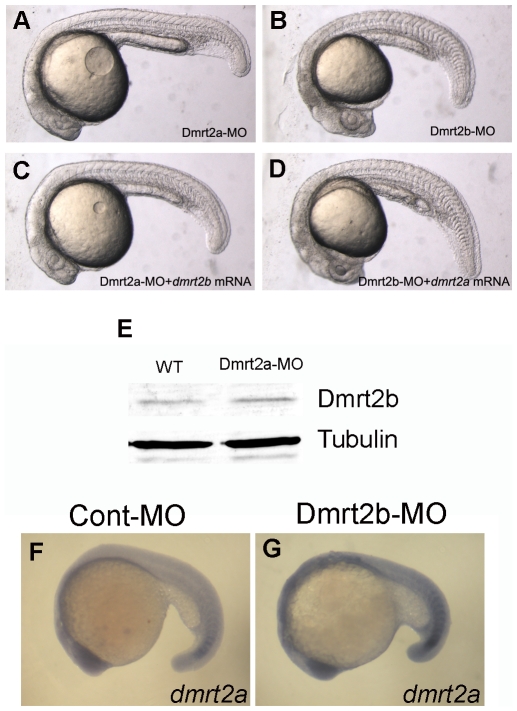
Considering sequence similarity and functional redundancy of Dmrt2a and Dmrt2b, we additionally tested the ability whether Dmrt2a could rescue Dmrt2b knockdown and vice versa. As shown in Figure 3, both Dmrt2a and Dmrt2b morphant defects (Figure 1A, B) could not be rescued by dmrt2b mRNA (Figure 3C) and dmrt2a mRNA respectively (Figure 3D). Then we analyzed the protein level of Dmrt2b in the Dmrt2a-MO injected embryos, and no any notable change of Dmrt2b expression was observed in the Dmrt2a morphants (Figure 3E). We also examined the expression of dmrt2a in the Dmrt2b morphants by whole-mount in situ hybridization, and no any change was not detected (Figure 3F and G). The data indicate that the loss-of-function of Dmrt2b leads to notable defects in somitogenesis and reduces target gene expression of Hedgehog signaling. And, the defects do not affect the expression of dmrt2a and Dmrt2a and Dmrt2b can not compensate the function for each other when they are knocked down.
Figure 3. Dmrt2a and Dmrt2b can not compensate for each other.
(A–D)Morphology of 24 hpf embryos injected with Dmrt2a-MO (A), Dmrt2b-MO (B), Dmrt2a-MO+dmrt2b mRNA (C), Dmrt2b-MO+dmrt2a mRNA (D). (E) Western blot assay showing Dmrt2b expression level in the embryos injected with wild type (WT) and Dmrt2a-MO. (F, G) The expression of dmrt2a(terra) was not affected in Dmrt2b morphants. Lateral views of embryos at 18 somites stage.
Dmrt2b is involved in slow muscle development through Hedgehog signaling
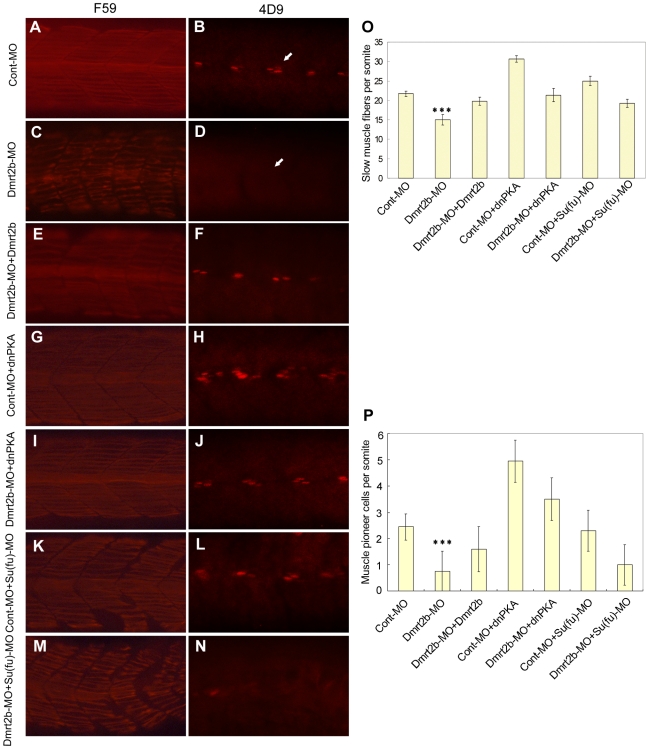
To assess the role of Dmrt2b in slow muscle development, we further analyzed the expression affect of Dmrt2b knockdown on slow muscle markers by using F59 antibody which labels slow myosin heavy clain (MyHC) in zebrafish [30] and 4D9 antibody which recognizes the engrailed protein in the nuclei of muscle pioneer cells. As shown in Figure 4, in comparison with normal superficial slow muscle fibers visualized by F59 antibody (Figure 4A) and Engrailed-expressing muscle pioneers stained by 4D9 antibody (Figure 4B) in Cont-MO embryos, the differentiation of slow muscle cells is severely perturbed in the morphant embryos. Especially, the numbers of superficial slow muscle fibers are obviously reduced (Figure 4C), and the Engrailed-expressing muscle pioneers are inhibited (Figure 4D) in the morphant embryos at 26 hpf. Significantly, the perturbed phenotypes can be rescued by co-injection of dmrt2b mRNA (Figure 4E, F). These data demonstrate that embryos with reduced Dmrt2b expression are unable to respond effectively to Hh signals during zebrafish segmentation, resulting in significant impairment in slow muscle development.
Figure 4. Dmrt2b is involved in slow muscle development through Hedgehog signaling.
Analysis of slow muscle development by whole-mount immunostaining of 26 hpf embryos with antibodies labeling slow MyHC (F59) (A, C, E, G, I, K, M) and engrailed muscle pioneer cells (4d9) (B, D, F, H, J, L, N). Lateral view of embryos injected with Cont-MO (A, B), Dmrt2b-MO (C, D), embryos co-injected with Dmrt2b-MO+dmrt2b (E, F), Cont-MO+dnPKA (G, H), Dmrt2b-MO+dnPKA (I, J), Cont-MO+Su(fu)-MO (K, L), Dmrt2b-MO+Su(fu)-MO (M, N). White arrows in 4d9 panel indicate reduction of engrailed staining in the knockdown embryos compared to controls. All images show the somites over the yolk extension. Anterior is left in all images. (O) Quantitative analysis of slow muscle fiber number per somite in embryos at 26 hpf, injected as indicated. Data represent average±SD. *** indicates significance of p<0.0001 for Dmrt2b-MO vs. Cont-MO, Dmrt2b-MO+dmrt2b, Dmrt2b-MO+dnPKA and Dmrt2b-MO+Su(fu)-MO. n = 20 to 38 embryos per condition. (P) Quantitative analysis of muscle pioneer cells number per somite in embryos at 26 hpf, injected as indicated. Data represent average±SD. *** indicates significance of p<0.0001 for Dmrt2b-MO vs. Cont-MO, Dmrt2b-MO+dmrt2b and Dmrt2b-MO+dnPKA. n = 20 to 36 embryos per condition.
PKA is a negative regulator of the Hh pathway. As described previously [31], [32], when the dominant negative PKA (dnPKA) mRNA is injected into Cont-MO embryos, the numbers of slow muscle fibers and muscle pioneers are notably increased (Figure 4G, H). Interestingly, when dnPKA mRNA and Dmrt2b-MO are co-injected, the dnPKA can efficiently rescue the development of slow muscle fibers and muscle pioneers in the morphant embryos (Figure 4I, J). Su(fu) acts as a weak inhibitor of the Hh signaling pathway [32], [33]. When Su(fu)-MO is injected into Cont-MO embryos, it leads to number increase of slow muscle fibers, but has little effect on the numbers of muscle pioneers (Figure 4K, L). When Su(fu)-MO and Dmrt2b-MO are co-injected, the Su(fu)-MO can also rescue the development of slow muscle fibers in the morphant embryos (Figure 4M), but the number of muscle pioneer cells can not be increased (Figure 4N). In these experiments, a total of 20 to 38 embryos were contributed to each analysis. Figure 4O and 4P respectively show the quantitative data of slow muscle fibers and muscle pioneer cells per somite. The data indicate that Dmrt2b knockdown does appear to affect slow muscle fibers, and therefore, Dmrt2b is involved in slow muscle development.
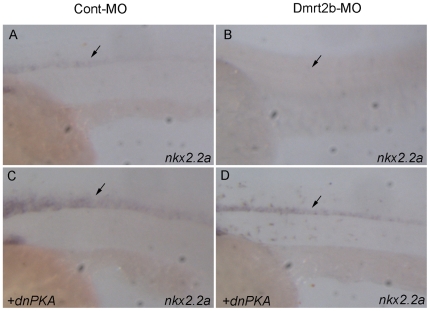
Dmrt2b is involved in neural tube patterning through Hedgehog signaling
Hh signaling has been identified as a key morphogen in patterning of the ventral neural tube [34]. To assess the contribution of Dmrt2b to Hh-mediated signaling in the neural tube, we analyzed the expression of nkx2.2a. In non-injected and control-injected embryos, nkx2.2a is expressed in lateral floor plate of the neural tube at 24 hpf (Figure 5A). In the Dmrt2b morphant embryos, the nkx2.2a expression is severely suppressed (22/25 embryos) (Figure 5B). Moreover, we observed that dnPKA mRNA injection could lead to ectopic expression of nkx2.2a in the neural tube of Cont-MO embryo (19/25 embryos) (Figure 5C) as described previously [31], [35]. Consistently with the epistatic analysis using slow muscle fiber and muscle pioneer markers (Figure 4I, J), co-injection of Dmrt2b-MO with dnPKA normalizes the ectopic expression of nkx2.2a (18/26 embryos) (Figure 5D). These data indicate a requirement of Dmrt2b during the development of neural tube for the appropriate cellular response to the Hh signals. To determine whether Dmrt2b is required for transcription of Hedgehog genes, we further examined the expression of three Hedgehog genes shha, ihhb and shhb in Dmrt2b morphant embryos and control embryos. Whole mount in situ hybridization analysis showed that shha, ihhb and shhb expression appeared normal in Dmrt2b morphant embryos at 10 hpf (bud stage) and at 24 hpf (Supplementary Figure S2). These data suggest that Dmrt2b should act downstream of Hedgehog genes and upstream of Gli protein, the core components of the Hh pathway [36], because three Hedgehog genes shha, ihhb and shhb are not affected in the Dmrt2b morphants, but dnPKA and Su(fu)-MO can counteract the Dmrt2b morphant phenotypes [22], [31].
Figure 5. Patterning defects in the neural tube of Dmrt2b morphant embryos.
Lateral views of embryos at 24 hpf, anterior to the left, dorsal to the top. (A) Wild phenotype of Cont-MO injected embryo; (B) Severe suppression of nkx2.2a in the neural tube of Dmrt2b-MO injected embryo; (C) Ectopic expression of nkx2.2a in the neural tube of dnPKA injected embryo; (D) The normalized expression of nkx2.2a in the neural tube of dnPKA and Dmrt2b-MO coinjected embryo. Black arrows indicate the expression position of nkx2.2a in the neural tube. All images show the somites over the yolk extension.
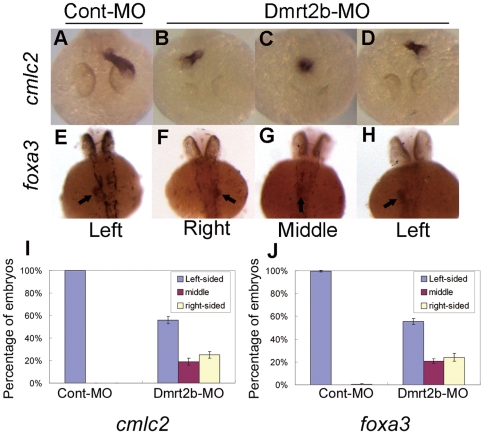
Dmrt2b morphants display defects in heart and visceral organs asymmetry
As described previously, Dmrt2a is essential for establishment of LR asymmetry in zebrafish development [14], and shh regulates the establishment of LR asymmetry in zebrafish development [37]. We also examined heart asymmetry defects in Dmrt2b morphant embryos by using specific marker of heart, cardiac myosin light chain 2 (cmlc2) [38]. In embryos injected with Cont-MO, 100% of the embryos show the heart tube on the left side, at 48 hpf (Figure 6A, I). By contrast, in the Dmrt2b marphant embryos, 24.9% of the heart tubes shift rightward and 19% remain in the middle (Figure 6B, C, D and I) (n = 86 to 113 in three separate experiments).
Figure 6. Dmrt2b morphant embryos display defects in heart and visceral organs asymmetry.
(A–D) Expression of cmlc2 in 48 hpf embryos detected by whole mount in situ hybridization with antisense RNA probe. (E–H) Expression of foxa3 in 48 hpf embryos detected by whole mount in situ hybridization. Arrows in foxa3 panel indicate the liver. Graphical representation of the percentage of embryos exhibiting the expression patterns for cmlc2 (I) and for foxa3 (J). Results are represented as mean±SD of three separate experiments.
In addition to the heart, morphological LR asymmetry of the visceral organs was also observed in zebrafish embryos [37]. To determine if LR asymmetry of the visceral organs was affected in dmrt2b knockdown embryos, we checked the expression of foxa3 which marks the liver. As expected, 100% of embryos injected with Cont-MO exhibit a leftward budding of the liver, at 48 hpf (Figure 6E, J). By contrast, 24.0% of the liver shift rightward, and 20.7% remain in the middle in the Dmrt2b marphant embryos (Figure 6F, G, H and J) (n = 83 to 100 in three separate experiments).
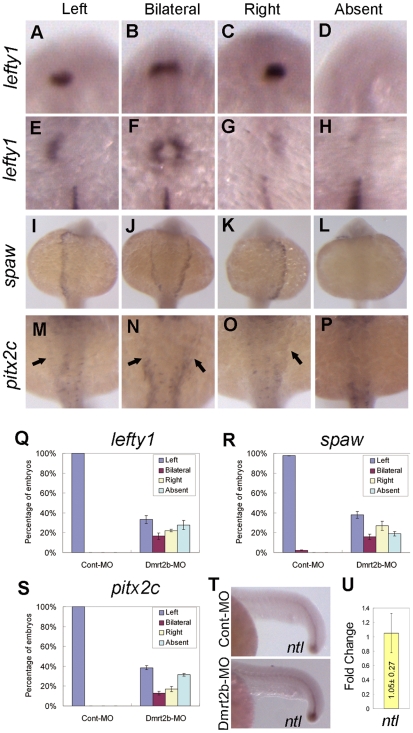
Dmrt2b activity is important for establishment of left-right asymmetry in lateral plate mesoderm
Left-sided expression of several genes such as lefty1, spaw and pitx2c in the lateral plate mesoderm (LPM) is important to heart and visceral organs asymmetry [39], [40], [41]. To determine whether the randomized heart and visceral organs asymmetry is preceded by alteration of underlying molecular cues, we analyzed these LR markers. lefty1 is a Nodal signaling antagonist, which expresses in the left dorsal diencephalon and left LPM at 22 somites stage (overlapping the prospective heart field) (Figure 7A, E). However only 33.3% of the Dmrt2b-MO injected embryos show the normal lefty1 patterns, the rest exhibit right-sided, bilateral, or absent expression (Figure 7B–D, F–H and Figure 7Q) (n = 36). spaw, which encodes a nodal-related protein in zebrafish, is normally expressed in the left LPM at 20 somites stage and regulates left-right asymmetry (Figure 7I). However, the later asymmetric spaw expression in the LPM is dramatically altered in Dmrt2b morphant embryos with only 38.1% showing left-sided expression, compared with embryos injected with Cont-MO (Figure 7I–L and Figure 7R) (n = 63). pitx2c encoding a bicoid-related transcription factor is also expressed in the left LPM at 22 somite stage (Figure 7M). As expected, in Dmrt2b-MO injected embryos (Figure 7M–P), only 38.6% showing normal left-sided expression compared with embryos injected with Cont-MO (Figure 7S) (n = 70).
Figure 7. Dmrt2b knockdown disrupts L–R identity in lateral plate mesoderm.
(A–D) lefty1 normal expression in left dorsal diencephalon at 22 somites stage were disrupted embryos injected with Dmrt2b-MO. (E–H) lefty1 normal expression in left LPM at 22 somites stage were disrupted embryos injected with Dmrt2b-MO. (Q) Graphical representation of the percentage of embryos exhibiting the expression patterns for lefty1 in Dmrt2b knockdown embryos and control embryos. (I–L) spaw normal expression in left LPM at 20 somites stage were disrupted embryos injected with Dmrt2b-MO. (R) Graphical representation of the percentage of embryos exhibiting the expression patterns for spaw in Dmrt2b knockdown embryos and control embryos. (M–P) pitx2c normal expression in left LPM at 22 somites stage were disrupted embryos injected with Dmrt2b-MO. (S) Graphical representation of the percentage of embryos exhibiting the expression patterns for pitx2c in Dmrt2b knockdown embryos and control embryos. (T) Lateral view of the embryos at 22 somites stage. Expression of the ntl in embryos injected with Dmrt2b-MO is similar to expression of ntl in control embryos. Results are represented as mean±SD of three separate experiments. (U) qPCR analysis of the expression changes of ntl in 24 hpf embryos injected with Cont-MO or Dmrt2b-MO. The data represents mean±SD of three separate experiments.
Since defects in midline integrity can also lead to abnormal LR asymmetry. We examined the midline structure of these embryos by using in situ hybridization of ntl. Expression of the nlt was not affected in embryos injected with Dmrt2b-MO (Figure 7T and 7U). The above data demonstrate dmrt2b importance in the establishment of left-right asymmetry of the body plan.
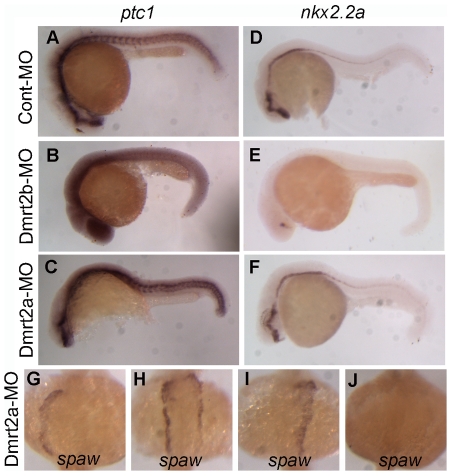
Dmrt2a does not contribute to Hedgehog pathway during zebrafish somitogenesis
As previously report, Dmrt2a is also involved in somitogenesis in zebrafish [12]. To examine whether Dmrt2a is contribute to hedgehog pathway as Dmrt2b, we performed comparative studies on two marker genes between Dmrt2a and Dmrt2b. As shown in Figure 8, in comparison with normal expression in Cont-MO embryos at 24 hpf (Figure 8A), the down-regulation of ptc1 transcripts in Dmrt2b morphant embryos (Figure 8B) is not observed in Dmrt2a-MO injected embryos (Figure 8C). Similar results are also observed in checking nkx2.2a expression. In contrast with the severely suppression in Dmrt2b morphant embryos (Figure 8E), the expression level of nkx2.2a does not reduce in the Dmrt2a morphant embryos (Figure 8F). Moreover, we also detected abnormal spaw expression in the Dmrt2a morphant embryos as described previously [14]. Figure 8G–J show the spaw expression alteration in left LPM in the 43 Dmrt2a-MO injected embryos with only 39.5% left-sided expression, 20.9% bilateral expression, 23.3% right-sided expression and 16.3% absent expression. The data indicate that Dmrt2a and Dmrt2b play similar rules in the establishment of left-right asymmetry of the body plan, but Dmrt2a does not contribute to Hedgehog pathway during zebrafish somitogenesis.
Figure 8. Dmrt2a does not contribute to Hedgehog pathway during zebrafish somitogenesis.
(A–C) The expression of ptc1 in embryos injected with Cont-MO, Dmrt2b-MO and Dmrt2a-MO at 24 hpf. (D–F) The expression of nkx2.2a in embryos injected with Cont-MO, Dmrt2b-MO and Dmrt2a-MO at 24 hpf. (G–J) spaw normal expression in left LPM at 20 somites stage were disrupted embryos injected with Dmrt2b-MO.
Dmrt2a has been described to be involved in synchronizing left-right somitogenesis in zebrafish. To clarify whether Dmrt2b has similar role for synchronizing left-right somitogenesis, we further analyzed expression changes of the presomitic mesoderm marker genes her1 and deltaC in the Dmrt2b morphants. As shown in Supplementary Figure S3, the expression of her1 and deltaC are not affected in the Dmrt2b-MO injected embryos. The data confirm that Dmrt2b is not involved in synchronizing left-right somitogenesis in zebrafish.
Discussion
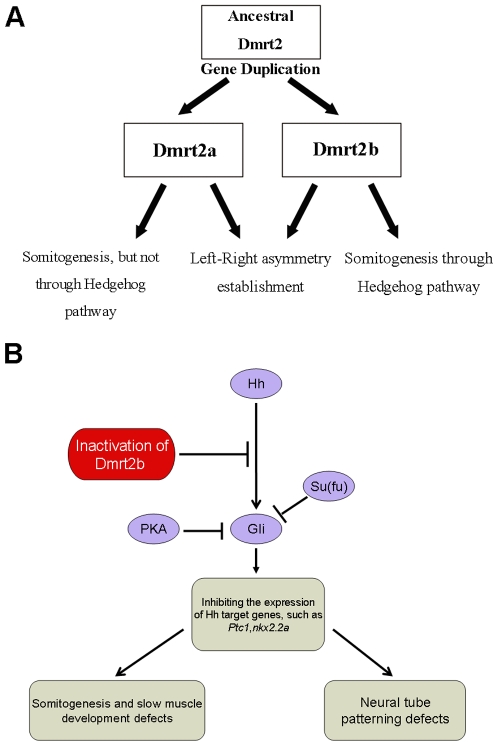
Gene duplication provides raw material for functional divergence and innovation [42], and neofunctionalization and subfunctionalization hypotheses have been proposed. The neofunctionalization hypothesis argues that after duplication one daughter gene retains the ancestral function while the other acquires new functions. In contrast, the subfunctionalization hypothesis asserts that the functions of the ancestral gene are partitioned between the duplicated genes [43]. Recent advances have suggested a new model termed subneofunctionalization, because neither neofunctionalization nor subfunctionalization can alone be explained in a large proportion of duplicate genes, and rapid subfunctionalization is often accompanied by prolonged and substantial neofunctionalization. This new model proposes that a large part of duplicated genes might play both overlapping functions and divergent functions [44]. In this study, we found significant sequence divergence between the fish-specific duplicated gene dmrt2b and dmrt2a, observed severe defects in somitogenesis, slow muscle development and neural tube patterning in the Dmrt2b loss-of-function zebrafish embryos by using morpholino-mediated knockdown strategy, and revealed the underlying mechanism that is involved in Hedgehog signaling pathway. Moreover, we also found the overlapping functions between dmrt2b and dmrt2a in LR asymmetry establishments, including heart and visceral organ asymmetry establishment and lateral plate mesoderm asymmetry establishment. Additionally, we further verified that Dmrt2a and Dmrt2b play common overlapping functions in the establishment of left-right asymmetry of the body plan, but Dmrt2a does not contribute to Hedgehog pathway (Figure 9A). Therefore, the current study clarifies exact function of the fish-specific duplicated gene dmrt2b, and provides a significant and interesting case for functional divergence of duplicate genes.
Figure 9. A schematic diagram illustrating the duplication and subsequent functional diversification of zebrafish Dmrt2a and Dmrt2b (A), and a hypothesized acting position that Dmrt2b is involved in Hedgehog pathway (B).
Another significant advance in this study was to find similar defect phenotypes in the Dmrt2b morphants to that in the so called “you-type mutants” which are demonstrated to be inactivated in particular genes in the Hedgehog signaling pathway [22], [23], [24], [25], [26], [27], [45]. The defects include the curled tail and the U-shaped somite boundaries. Subsequently, we have confirmed that the expression of Hedgehog target genes ptc1 and nkx2.2a are severely inhibited in the Dmrt2b morphants, and both slow muscle development and neural tube development are also disrupted by Hedgehog pathway impairment. Moreover, we have observed normal expression of three Hedgehog genes shha, ihhb and shhb in Dmrt2b morphants, indicating that Dmrt2b mophant defects are in the response to Hedgehog signals not in Hedgehog transcript expressed at low level. The data suggest that Dmrt2b might act downstream of Hedgehog gene transcription. To assess what level in the pathway Dmrt2b is active, we further used two rescue experiments. Co-injection of dnPKA and Su(fu)-MO could rescue some defects of the Dmrt2b morphants in slow muscle development, implicating that Dmrt2b should act downstream of Hedgehog genes and upstream of Gli protein, the core components of the Hh pathway [36], and therefore contribute to somitogenesis, slow muscle development and neural tube patterning by inhibiting the expression of Hh target genes (Figure 9B). In addition, we found that Dmrt2a expression was not affected in the Dmrt2b morphants (Figure 3F and G), and overexpression of dmrt2a was previously observed to induce rapid apoptosis in the mesoderm [12]. Moreover, no any Hedgehog signals responding defects were observed in zebrafish Dmrt2a morphants in our experiment (Figure 8). Therefore, the new function of Dmrt2b during somitogenesis and the underlying mechanism that is involved in Hedgehog signaling pathway are different from the function of Dmrt2a during somitogenesis which is linked to apoptosis.
Additionally, an interesting aspect of the Dmrt2b morphants is that it encompasses only a subset of defects observed from other zebrafish Hedgehog pathway mutants. For example, pectoral fins, which are disrupted in mutants syu and smu [22], [24], appear normal in Dmrt2b knockdown embryos (some Dmrt2b morphant embryos have smaller pectoral fins). We also observed incomplete absence of the slow muscle phenotype. These phenotypic characteristics may be a result of the functionally redundant components in Hh pathway. Indeed, similar varying levels of slow muscle development defects have been described in the different Hh pathway genetic mutants [46], potentially indicating compensatory effects by other genes in the Hedgehog pathway.
In conclusion, we have endeavored to elucidate the roles of Dmrt2b in the developing zebrafish embryo, and compared the Dmrt2b function to the Dmrt2a function. Through morpholino loss-of-function approach, we demonstrated that Dmrt2b contributes to multiple developmental processes, including zebrafish somitogenesis through Hedgehog pathway and establishment of left-right asymmetry. We have uncovered important new roles for Dmrt2b in zebrafish, which were unanticipated by studies of this molecule's duplicated gene Dmrt2a in zebrafish and orthologous gene Dmrt2 in mouse. The data suggest fish-specific duplicated genes Dmrt2b and Dmrt2a play overlapping roles in establishment of Left-Right asymmetry and divergent functions in somitogenesis, which Dmrt2b contributes to Hedgehog pathway.
Materials and Methods
Maintenance of zebrafish
A breeding colony of zebrafish (Danio rerio) were maintained at 28.5°C on a 14 h light/10 h dark cycle [47]. All embryos used were collected by natural spawning and staged according to standard procedures [48].
Antisense morpholino and mRNA microinjection
Two non-overlapping translation-blocking morpholino oligonucleotides (MOs) [16] Dmrt2b-MO1 and Dmrt2b-MO2, standard control MO against the zebrafish β-globin intron, p53 MO, and Su(fu) MO, were obtained from Gene Tools, LLC. The morpholino sequences were as follows: Dmrt2b-MO1, 5′-TTCTCACGAACCCACGACGCTCATC-3′; Dmrt2b-MO2, 5′-CCGCTTTAGTGGACATTTATGAGCT-3′; stand control Mo(Cont-MO), 5′-CCTCCTACCTCAGTTACAATTTATA-3′; p53 MO (p53-MO), 5′- GCGCCATTGCTTTGCAAGAATTG-3′ [20], [21]; Su(fu) MO (Su(fu)-MO), 5′-GCTGCTAGGCCGCATCTCATCCATC-3′ [49]; Dmrt2a MO (Dmrt2a-MO), 5′ - AGATCCGTCATTTTCTGGCCGCGTA – 3′ [14]. Dmrt2b ORF was subcloned into the pCS2+ vector for in vitro transcription. dnPKA in pCS2+ were kind gifts from Dr. Whitfield (University of Sheffield). Capped sense RNAs were synthesized using the mMESSAGE mMACHINE system (Ambion) from the linearized dnPKA-bGFP-pCS2+, and dmrt2b-pCS2+ plasmids, re-suspended in 0.1 M KCl. MOs and capped mRNA injections were done into 1-cell-stage embryos according to standard methods. For injection, MOs were diluted at a concentration of about 5 ng each embryo. Dmrt2b mRNA were injected at a concentration of about 200pg each embryo. dnPKA mRNA were injected at a concentration of about 100 pg each embryo. For co-injection experiment, we injected each embryo twice, first with the MO and then with the mRNA or another MO. After injection, embryos were incubated at 28.5°C in Embryo Medium [47].
Antibody production
The cDNA fragment coding amino acids 136–364 peptide was subcloned into pET-32a expression vector. A fusion protein was expressed in BL21 (DE3), and detected only in inclusion body. The protein was prepared and immunized mouse as described previously [50].
Western blotting
Zebrafish embryos were manually dechorionated, deyolked [51] before homogenized. Deyolked samples were dissolved in 2 µl of 2× sodium dodecyl sulfate (SDS) sample buffer per embryo and incubated for 5 min at 95°C. After full-speed centrifugation for 1 min in a microcentrifuge to remove insoluble particles, samples were loaded on a 12% SDS gel. Western blot analysis was performed according to the previous report [50] using the anti-Dmrt2b antibody and anti-tubulin antibody.
Whole-mount in situ hybridization
Antisense probes for ptc1 [35], nkx2.2a [29], pax6a [52], lefty1 [41], spaw [39], cmlc2 [38], foxa3 [53] and ntl were prepared as described previously. Probe for pitx2c and echidna Hedgehog (ihhb) were generated directly from PCR products that included T7 of T3 RNA polymerase binding sequence at the 3′ end. Primer sequences used were: pitx2c forward, 5′-ACTGCCGCAAACTTGCATCA-3′ reverse, 5′-CATTAACCCTCACTAAAGGGAAGTTGCTTGGCTTTCAGTCTC-3′; ihhb forward, 5′-ATGAGACTCTCCACGGCGGC-3′ reverse, 5′-CATTAACCCTCACTAAAGGGAAATCTCTCAGTTGCCTCTAAG-3′. Probes for sonic Hedgehog (shha) and tiggywinkle Hedgehog (shhb) were prepared using shh-pT7Ts and twhh-pT7Ts respectively (kind gifts from Dr. Randall Moon, University of Washington). Embryos were fixed at the stages indicated and processed essentially as previously described [21].
Immunohistochemistry
Embryos were grown to the indicated stages and processed essentially as described previously. Immunostainings of whole zebrafish embryos were performed following standard protocols. The 4d9 antibody used 1∶100 dulution, which recognizes the engrailed protein in the nuclei of muscle pioneer cells, the F59 antibody used 1∶100 dilution, which labels slow myosin heavy clain (MyHC) in zebrafish [30], were obtained from the Developmental Studies Hybridoma Bank. Fluorescent secondary antibodies against mouse used for detection were FITC or Cy3 conjugated. Stained embryos were photographed on fluorescence optics of Confocal Microscope LEICA DMIRE2 (Leica, Germany).
Quantification of slow muscle defects
As previous report [33], 26 hpf embryos were fixed and stained using F59 antibody. Numbers of slow muscle fibers were counted in 5 somites over the yolk extension per embryo.
Supporting Information
Dmrt2b translation is blocked by non-overlapping morpholinos. (A) The Dmrt2b-MO1 and Dmrt2b-MO2 target sequences are shown in relation to the 5′UTR region of the Dmrt2b mRNA sequence. (B) Western blot assay showing Dmrt2b translation in the embryos injected with Cont-MO, Dmrt2b-MO1, Dmrt2b-MO2 and Dmrt2b-MO (Dmrt2b-MO1+Dmrt2b-MO2). The signal of Dmrt2b protein were significant reduced in Dmrt2b-MO1, Dmrt2b-MO2 and Dmrt2b-MO injected embryos.
(0.72 MB TIF)
Dmrt2b is not required for shha, ihhb and shhb transcription. Dorsal views of embryos at the bud stage (10 hpf) (A, C, E, G, I and K). Lateral views of embryos at 24 hpf (B, D, F, H, J and L). Expression of shha in embryos injected with Dmrt2b-MO (C and D) is similar to expression of shha in control embryos (A and B). Expression of ihhb in embryos injected with Dmrt2b-MO (G and H) is similar to expression of ihhb in control embryos (E and F). Expression of shhb in embryos injected with Dmrt2b-MO (K and L) is similar to expression of shhb in control embryos (I and J).
(3.61 MB TIF)
Whole-mount in situ hybridization of persomitic mesoderm genes her1 and deltaC in the Dmrt2b morphants. Expression patterns of her1 in the Dmrt2b-MO (A) and Cont-MO (B) embryos. Expression patterns of deltaC in the Dmrt2b-MO (C) and Cont-MO (D) embryos. All the embryos are at 10 somites stage. Panels show dorsal views, anterior to the top.
(4.94 MB TIF)
Acknowledgments
We thank Dr. Whitfield for providing us dnPKA plasmid, Dr. Randall Moon for providing us twhh-pT7Ts and shh-pT7Ts plasmid.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by grants from the National Major Basic Research Program (2004CB117401, 2010CB126301), the national natural science foundation of China (30630050), the Innovation Project of Chinese Academy of Sciences (KSCX2-YW-N-020), the Open Project of State Key Laboratory of Freshwater Ecology and Biotechnology (2008FB007), and the Innovation Project of Institute of Hydrobiology, Chinese Academy of Sciences (075A011301). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Postlethwait J, Amores A, Cresko W, Singer A, Yan YL. Subfunction partitioning, the teleost radiation and the annotation of the human genome. Trends Genet. 2004;20:481–490. doi: 10.1016/j.tig.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Taylor JS, Braasch I, Frickey T, Meyer A, Van de Peer Y. Genome duplication, a trait shared by 22000 species of ray-finned fish. Genome Res. 2003;13:382–390. doi: 10.1101/gr.640303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong CH, Yang ST, Yang ZA, Zhang L, Gui JF. A C-type lectin associated and translocated with cortical granules during oocyte maturation and egg fertilization in fish. Dev Biol. 2004;265:341–354. doi: 10.1016/j.ydbio.2003.08.028. [DOI] [PubMed] [Google Scholar]
- 4.Liu J, Shi YH, Yin J, Gui JF. Screen of differentially expressed genes between gastrula embryos and tail bud embryos in gynogenetic gibel carp (Carassius auratus gibelio). J Genet Genomics. 2005;32:253–263. [PubMed] [Google Scholar]
- 5.Liu JX, Shi YH, Gui JF. Screen of differentially expressed genes at gastrula stage during embryogenesis of gibel carp. Acta Hydrobiologica Sinica. 2005;29:359–365. [PubMed] [Google Scholar]
- 6.Xie J, Wen JJ, Chen B, Gui JF. Differential gene expression in fully-grown oocytes between gynogenetic and gonochoristic crucian carps. Gene. 2001;271:109–116. doi: 10.1016/s0378-1119(01)00491-7. [DOI] [PubMed] [Google Scholar]
- 7.Zhou L, Wang Y, Gui JF. Genetic evidence for gonochoristic reproduction in gynogenetic silver crucian carp (Carassius auratus gibelio bloch) as revealed by RAPD assays. J Mol Evol. 2000;51:498–506. doi: 10.1007/s002390010113. [DOI] [PubMed] [Google Scholar]
- 8.Xia JH, Liu JX, Zhou L, Li Z, Gui JF. Apo-14 is required for digestive system organogenesis during fish embryogenesis and larval development. Int J Dev Biol. 2008;52:1089–1098. doi: 10.1387/ijdb.072519jx. [DOI] [PubMed] [Google Scholar]
- 9.Yin J, Xia JH, Du XZ, Liu J, Zhou L, et al. Developmental expression of CagMdkb during gibel carp embryogenesis. Int J Dev Biol. 2007;51:761–769. doi: 10.1387/ijdb.072346jy. [DOI] [PubMed] [Google Scholar]
- 10.Raymond CS, Shamu CE, Shen MM, Seifert KJ, Hirsch B, et al. Evidence for evolutionary conservation of sex-determining genes. Nature. 1998;391:691–695. doi: 10.1038/35618. [DOI] [PubMed] [Google Scholar]
- 11.Hong C, Park B, Saint-Jeannet J. The function of Dmrt genes in vertebrate development: it is not just about sex. Dev Biol. 2007;310:1–9. doi: 10.1016/j.ydbio.2007.07.035. [DOI] [PubMed] [Google Scholar]
- 12.Meng A, Moore B, Tang H, Yuan B, Lin S. A Drosophila doublesex-related gene, terra, is involved in somitogenesis in vertebrates. Development. 1999;126:1259–1268. doi: 10.1242/dev.126.6.1259. [DOI] [PubMed] [Google Scholar]
- 13.Zhou X, Li Q, Lu H, Chen H, Guo Y, et al. Fish specific duplication of Dmrt2: characterization of zebrafish Dmrt2b. Biochimie. 2008;90:878–887. doi: 10.1016/j.biochi.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 14.Saúde L, Lourenço R, Gonçalves A, Palmeirim I. terra is a left-right asymmetry gene required for left-right synchronization of the segmentation clock. Nat Cell Biol. 2005;7:918–920. doi: 10.1038/ncb1294. [DOI] [PubMed] [Google Scholar]
- 15.Seo K, Wang Y, Kokubo H, Kettlewell J, Zarkower D, et al. Targeted disruption of the DM domain containing transcription factor Dmrt2 reveals an essential role in somite patterning. Dev Biol. 2006;290:200–210. doi: 10.1016/j.ydbio.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 16.Nasevicius A, Ekker SC. Effective targeted gene ‘knockdown’ in zebrafish. Nat Genet. 2000;26:216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- 17.Ekker SC. Morphants: a new systematic vertebrate functional genomics approach. Yeast. 2000;17:302–306. doi: 10.1002/1097-0061(200012)17:4<302::AID-YEA53>3.0.CO;2-#. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ekker S, Larson J. Morphant technology in model developmental systems. Genesis. 2001;30:89–93. doi: 10.1002/gene.1038. [DOI] [PubMed] [Google Scholar]
- 19.Schlueter PJ, Royer T, Farah MH, Laser B, Chan SJ, et al. Gene duplication and functional divergence of the zebrafish insulin-like growth factor 1 receptors. FASEB J. 2006;20:1230–1232. doi: 10.1096/fj.05-3882fje. [DOI] [PubMed] [Google Scholar]
- 20.Robu M, Larson J, Nasevicius A, Beiraghi S, Brenner C, et al. p53 activation by knockdown technologies. PLoS Genet. 2007;3:e78. doi: 10.1371/journal.pgen.0030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mei J, Zhang QY, Li Z, Lin S, Gui JF. C1q-like inhibits p53-mediated apoptosis and controls normal hematopoiesis during zebrafish embryogenesis. Dev Biol. 2008;319:273–284. doi: 10.1016/j.ydbio.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 22.Barresi MJ, Stickney HL, Devoto SH. The zebrafish slow-muscle-omitted gene product is required for Hedgehog signal transduction and the development of slow muscle identity. Development. 2000;127:2189–2199. doi: 10.1242/dev.127.10.2189. [DOI] [PubMed] [Google Scholar]
- 23.Woods I, Talbot W. The you gene encodes an EGF-CUB protein essential for Hedgehog signaling in zebrafish. PLoS Biol. 2005;3:e66. doi: 10.1371/journal.pbio.0030066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schauerte HE, van Eeden FJ, Fricke C, Odenthal J, Strahle U, et al. Sonic hedgehog is not required for the induction of medial floor plate cells in the zebrafish. Development. 1998;125:2983–2993. doi: 10.1242/dev.125.15.2983. [DOI] [PubMed] [Google Scholar]
- 25.Karlstrom RO, Talbot WS, Schier AF. Comparative synteny cloning of zebrafish you-too: mutations in the Hedgehog target gli2 affect ventral forebrain patterning. Genes Dev. 1999;13:388–393. doi: 10.1101/gad.13.4.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karlstrom RO, Tyurina OV, Kawakami A, Nishioka N, Talbot WS, et al. Genetic analysis of zebrafish gli1 and gli2 reveals divergent requirements for gli genes in vertebrate development. Development. 2003;130:1549–1564. doi: 10.1242/dev.00364. [DOI] [PubMed] [Google Scholar]
- 27.Nakano Y, Kim HR, Kawakami A, Roy S, Schier AF, et al. Inactivation of dispatched 1 by the chameleon mutation disrupts Hedgehog signalling in the zebrafish embryo. Dev Biol. 2004;269:381–392. doi: 10.1016/j.ydbio.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 28.Alexandre C, Jacinto A, Ingham PW. Transcriptional activation of hedgehog target genes in Drosophila is mediated directly by the cubitus interruptus protein, a member of the GLI family of zinc finger DNA-binding proteins. Genes Dev. 1996;10:2003–2013. doi: 10.1101/gad.10.16.2003. [DOI] [PubMed] [Google Scholar]
- 29.Barth KA, Wilson SW. Expression of zebrafish nk2.2 is influenced by sonic hedgehog/vertebrate hedgehog-1 and demarcates a zone of neuronal differentiation in the embryonic forebrain. Development. 1995;121:1755–1768. doi: 10.1242/dev.121.6.1755. [DOI] [PubMed] [Google Scholar]
- 30.Mei J, Li Z, Gui JF. Cooperation of Mtmr8 with PI3K regulates actin filament modeling and muscle development in zebrafish. PLoS ONE. 2009;4:e4979. doi: 10.1371/journal.pone.0004979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hammerschmidt M, Bitgood MJ, McMahon AP. Protein kinase A is a common negative regulator of Hedgehog signaling in the vertebrate embryo. Genes Dev. 1996;10:647–658. doi: 10.1101/gad.10.6.647. [DOI] [PubMed] [Google Scholar]
- 32.Wolff C, Roy S, Ingham P. Multiple muscle cell identities induced by distinct levels and timing of hedgehog activity in the zebrafish embryo. Curr Biol. 2003;13:1169–1181. doi: 10.1016/s0960-9822(03)00461-5. [DOI] [PubMed] [Google Scholar]
- 33.Wilbanks AM, Fralish GB, Kirby ML, Barak LS, Li YX, et al. Beta-arrestin 2 regulates zebrafish development through the hedgehog signaling pathway. Science. 2004;306:2264–2267. doi: 10.1126/science.1104193. [DOI] [PubMed] [Google Scholar]
- 34.Ruiz i Altaba A, Nguyen V, Palma V. The emergent design of the neural tube: prepattern, SHH morphogen and GLI code. Curr Opin Genet Dev. 2003;13:513–521. doi: 10.1016/j.gde.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 35.Concordet JP, Lewis KE, Moore JW, Goodrich LV, Johnson RL, et al. Spatial regulation of a zebrafish patched homologue reflects the roles of sonic hedgehog and protein kinase A in neural tube and somite patterning. Development. 1996;122:2835–2846. doi: 10.1242/dev.122.9.2835. [DOI] [PubMed] [Google Scholar]
- 36.Ruiz i Altaba A. Gli proteins and Hedgehog signaling: development and cancer. Trends Genet. 1999;15:418–425. doi: 10.1016/s0168-9525(99)01840-5. [DOI] [PubMed] [Google Scholar]
- 37.Schilling TF, Concordet JP, Ingham PW. Regulation of left-right asymmetries in the zebrafish by Shh and BMP4. Dev Biol. 1999;210:277–287. doi: 10.1006/dbio.1999.9214. [DOI] [PubMed] [Google Scholar]
- 38.Yelon D, Horne SA, Stainier DY. Restricted expression of cardiac myosin genes reveals regulated aspects of heart tube assembly in zebrafish. Dev Biol. 1999;214:23–37. doi: 10.1006/dbio.1999.9406. [DOI] [PubMed] [Google Scholar]
- 39.Long S, Ahmad N, Rebagliati M. The zebrafish nodal-related gene southpaw is required for visceral and diencephalic left-right asymmetry. Development. 2003;130:2303–2316. doi: 10.1242/dev.00436. [DOI] [PubMed] [Google Scholar]
- 40.Tsukui T, Capdevila J, Tamura K, Ruiz-Lozano P, Rodriguez-Esteban C, et al. Multiple left-right asymmetry defects in Shh(−/−) mutant mice unveil a convergence of the shh and retinoic acid pathways in the control of Lefty-1. Proc Natl Acad Sci U S A. 1999;96:11376–11381. doi: 10.1073/pnas.96.20.11376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bisgrove BW, Essner JJ, Yost HJ. Regulation of midline development by antagonism of lefty and nodal signaling. Development. 1999;126:3253–3262. doi: 10.1242/dev.126.14.3253. [DOI] [PubMed] [Google Scholar]
- 42.Conant GC, Wolfe KH. Turning a hobby into a job: how duplicated genes find new functions. Nat Rev Genet. 2008;9:938–950. doi: 10.1038/nrg2482. [DOI] [PubMed] [Google Scholar]
- 43.Zhang J. Evolution by gene duplication: an update. Trends Ecol Evol. 2003;18:292–298. [Google Scholar]
- 44.He X, Zhang J. Rapid subfunctionalization accompanied by prolonged and substantial neofunctionalization in duplicate gene evolution. Genetics. 2005;169:1157–1164. doi: 10.1534/genetics.104.037051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Eeden F, Granato M, Schach U, Brand M, Furutani-Seiki M, et al. Mutations affecting somite formation and patterning in the zebrafish, Danio rerio. Development. 1996;123:153–164. doi: 10.1242/dev.123.1.153. [DOI] [PubMed] [Google Scholar]
- 46.Wolff C, Roy S, Lewis KE, Schauerte H, Joerg-Rauch G, et al. iguana encodes a novel zinc-finger protein with coiled-coil domains essential for Hedgehog signal transduction in the zebrafish embryo. Genes Dev. 2004;18:1565–1576. doi: 10.1101/gad.296004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Westerfield M. Eugene: Univ. of Oregon Press; 2000. The zebrafish book. [Google Scholar]
- 48.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 49.Koudijs MJ, den Broeder MJ, Keijser A, Wienholds E, Houwing S, et al. The zebrafish mutants dre, uki, and lep encode negative regulators of the hedgehog signaling pathway. PLoS Genet. 2005;1:e19. doi: 10.1371/journal.pgen.0010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xia W, Zhou L, Yao B, Li CJ, Gui JF. Differential and spermatogenic cell-specific expression of DMRT1 during sex reversal in protogynous hermaphroditic groupers. Mol Cell Endocrinol. 2007;263:156–172. doi: 10.1016/j.mce.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 51.Link V, Shevchenko A, Heisenberg C. Proteomics of early zebrafish embryos. BMC Dev Biol. 2006;6:1. doi: 10.1186/1471-213X-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krauss S, Johansen T, Korzh V, Moens U, Ericson JU, et al. Zebrafish pax[zf-a]: a paired box-containing gene expressed in the neural tube. EMBO J. 1991;10:3609–3619. doi: 10.1002/j.1460-2075.1991.tb04927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Odenthal J, Nusslein-Volhard C. fork head domain genes in zebrafish. Dev Genes Evol. 1998;208:245–258. doi: 10.1007/s004270050179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dmrt2b translation is blocked by non-overlapping morpholinos. (A) The Dmrt2b-MO1 and Dmrt2b-MO2 target sequences are shown in relation to the 5′UTR region of the Dmrt2b mRNA sequence. (B) Western blot assay showing Dmrt2b translation in the embryos injected with Cont-MO, Dmrt2b-MO1, Dmrt2b-MO2 and Dmrt2b-MO (Dmrt2b-MO1+Dmrt2b-MO2). The signal of Dmrt2b protein were significant reduced in Dmrt2b-MO1, Dmrt2b-MO2 and Dmrt2b-MO injected embryos.
(0.72 MB TIF)
Dmrt2b is not required for shha, ihhb and shhb transcription. Dorsal views of embryos at the bud stage (10 hpf) (A, C, E, G, I and K). Lateral views of embryos at 24 hpf (B, D, F, H, J and L). Expression of shha in embryos injected with Dmrt2b-MO (C and D) is similar to expression of shha in control embryos (A and B). Expression of ihhb in embryos injected with Dmrt2b-MO (G and H) is similar to expression of ihhb in control embryos (E and F). Expression of shhb in embryos injected with Dmrt2b-MO (K and L) is similar to expression of shhb in control embryos (I and J).
(3.61 MB TIF)
Whole-mount in situ hybridization of persomitic mesoderm genes her1 and deltaC in the Dmrt2b morphants. Expression patterns of her1 in the Dmrt2b-MO (A) and Cont-MO (B) embryos. Expression patterns of deltaC in the Dmrt2b-MO (C) and Cont-MO (D) embryos. All the embryos are at 10 somites stage. Panels show dorsal views, anterior to the top.
(4.94 MB TIF)