Abstract
Glucocorticoid hormones are critical to respond and adapt to stress. Genetic variations in the glucocorticoid receptor (GR) gene alter hypothalamic-pituitary-adrenal (HPA) axis activity and associate with hypertension and susceptibility to metabolic disease. Here we test the hypothesis that reduced GR density alters blood pressure and glucose and lipid homeostasis and limits adaption to obesogenic diet. Heterozygous GRβgeo/+ mice were generated from embryonic stem (ES) cells with a gene trap integration of a β-galactosidase-neomycin phosphotransferase (βgeo) cassette into the GR gene creating a transcriptionally inactive GR fusion protein. Although GRβgeo/+ mice have 50% less functional GR, they have normal lipid and glucose homeostasis due to compensatory HPA axis activation but are hypertensive due to activation of the renin-angiotensin-aldosterone system (RAAS). When challenged with a high-fat diet, weight gain, adiposity, and glucose intolerance were similarly increased in control and GRβgeo/+ mice, suggesting preserved control of intermediary metabolism and energy balance. However, whereas a high-fat diet caused HPA activation and increased blood pressure in control mice, these adaptions were attenuated or abolished in GRβgeo/+ mice. Thus, reduced GR density balanced by HPA activation leaves glucocorticoid functions unaffected but mineralocorticoid functions increased, causing hypertension. Importantly, reduced GR limits HPA and blood pressure adaptions to obesogenic diet.—Michailidou, Z., Carter, R. N., Marshall, E., Sutherland, H. G., Brownstein, D. G., Owen, E., Cockett, K., Kelly, V., Ramage, L., Al-Dujaili, E. A. S., Ross, M., Maraki, I., Newton, K., Holmes, M. C., Seckl, J. R., Morton, N. M., Kenyon, C. J., Chapman, K. E. Glucocorticoid receptor haploinsufficiency causes hypertension and attenuates hypothalamic-pituitary-adrenal axis and blood pressure adaptions to high-fat diet.
Keywords: HPA axis, diet-induced obesity, NR3C1, glucose and lipid homeostasis
Glucocorticoids coordinately regulate gene pathways in response to external (stress) and internal (circadian) cues and form part of important homeostatic control mechanisms, critical in adaption to environmental stressors. They are required for the integrity of central nervous system function, for the response to infection and injury, and for cardiovascular and metabolic homeostasis. They regulate blood pressure (1), energy intake and expenditure, and glucose and lipid homeostasis (2). In excess, glucocorticoids cause hypertension, visceral obesity, insulin resistance/diabetes, and disordered mood and cognition (Cushing’s syndrome), whereas glucocorticoid deficiency causes hypotension, fatigue, weight loss, and anorexia.
Most actions of glucocorticoids are mediated through the widely distributed glucocorticoid receptor (GR; refs. 3,4,5), which belongs to the superfamily of nuclear receptor transcription factors (6). Studies in vitro (7, 8) and in vivo (9, 10) have shown the importance of receptor density in determining cellular glucocorticoid sensitivity. Complete loss of GR is incompatible with postnatal survival (11, 12), but partial loss of GR function in humans causes the rare familial/sporadic glucocorticoid resistance syndrome (13), characterized by hypercortisolism without other features of Cushing’s syndrome. Polymorphisms in the human Nr3c1 gene (encoding GR; here abbreviated to GR) are associated with hyper- or hyposensitivity to exogenous glucocorticoid (dexamethasone) suppression of the hypothalamic-pituitary-adrenal (HPA) axis and a range of metabolic and cardiovascular parameters. Thus, polymorphisms associated with glucocorticoid hypersensitivity (BclI, N363S) are typically (dependent on age, gender, and ethnic background) associated with hypertension, obesity, decreased lean mass, insulin resistance, and increased risk of cardiovascular disease despite reduced plasma cortisol levels, whereas the ER22/23EK polymorphism, associated with glucocorticoid hyposensitivity, is associated with beneficial metabolic outcomes (reviewed in ref. 14).
Several mouse lines with altered GR expression or function have been generated to investigate the role of GR in HPA axis regulation and its role in mood regulation and in the immune system (reviewed in refs. 15,16,17). Fewer studies have investigated the effects of altered GR density on cardiovascular and metabolic outcomes. Reduced GR density through antisense GR transgene expression (central nervous system targeted, although widely expressed) lowered energy intake but also caused obesity (18, 19). However, neuron-specific deletion of GR caused a small-lean phenotype postweaning and increased energy expenditure, due to the peripheral effects of high glucocorticoid levels coupled with normal levels of peripheral GR (20). Liver-specific GR gene deletion led to hypoglycemia, but only after prolonged starvation, and ameliorated hyperglycemia in streptozotocin-induced diabetes (21). Recently, transgenic mice have been described with cardiomyocyte-specific GR overexpression. These mice display conduction defects, reduced heart rate, atrioventricular block, altered calcium homeostasis, and ion channel remodeling in isolated cardiomyocytes (22). Although these studies have provided important information on the tissue-specific functions of GR, the cardiovascular and metabolic consequences of globally altered GR density, and hence glucocorticoid sensitivity, have not been described, although this is the clinically relevant situation.
Here we have generated a novel line of mice with reduced GR density, heterozygous for a null mutation of the GR gene (GRβgeo/+), to investigate whether global reduction in GR density alters cardiovascular (blood pressure), fat distribution, and metabolic (glucose and lipid homeostasis) parameters. A high-fat (HF) diet was introduced to determine whether GR haploinsufficiency altered the adaptive hormonal and metabolic changes that accompany dietary-induced obesity.
MATERIALS AND METHODS
Generation of GRβgeo/+ mice
A mouse ES cell line (ESKN92) generated by gene-trap mutagenesis in which a β-galactosidase-neomycin phosphotransferase (βgeo) reporter cassette integrated within the GR gene, generating a translational fusion between GR and βgeo, has been described previously (23). 5′-Rapid amplification of cDNA ends (RACE) confirmed integration of the β-galactosidase-neomycin phosphotransferase (βgeo) reporter cassette between exons 3 and 4 of the GR gene. Fluorescence in situ hybridization (FISH) confirmed a single integration site on chromosome 18 at the GR locus. Chimeric mice were generated by injection of ESKN92 cells into C57BL/6J blastocysts. Chimeras were mated to C57BL/6J female mice to achieve germ line transmission and further backcrossed to generate a congenic line (full name, Nr3c1gtESK92MRCHGU; here abbreviated to GRβgeo). Heterozygotes were identified by polymerase chain reaction (PCR) for the presence of lacZ (in the βgeo cassette) using the following primers: forward, 5′-GTTGCGCAGCCTGAATGGCG-3′; and reverse, 5′-GCCGTCACTCCAACGCAGCA-3′. All the experiments described were performed on adult male (5–6 months) GRβgeo/+ and GR+/+ littermates, backcrossed for five generations (F5) to C57BL/6J. Experimental groups were 8 to 9 per group, unless otherwise stated. To generate homozygous GRβgeo/βgeo mice, F7 GRβgeo/+ mice were intercrossed.
Animal maintenance and diets
All animal experimentation was conducted in strict accord with the accepted standards of humane animal care under the auspices of the Animal (Scientific Procedures) Act UK 1986 after prior approval by the local ethical committee. Mice were housed in standard cages under controlled lighting (12:12-h light-dark cycle, lights on at 7 AM) and were fed standard chow from weaning unless specified otherwise. For measurement of unstressed plasma corticosterone ACTH and glucose in tail nick blood samples, animals were acclimatized to single housing and tail nicks were performed within 1 min of disturbing the cage. For diet-induced obesity experiments, mice were weaned onto either HF/low carbohydrate diet (58% kcal as fat, D12331; Research Diets, New Brunswick, NJ, USA) or low-fat (LF)/high-carbohydrate diet (11% kcal as fat, D12328; Research Diets) and remained on the diet for 22 wk with ad libitum access to water and diet. Body weight and food intake were monitored weekly and for 3 wk, respectively.
Tissue collection, metabolic parameters, and liver triglyceride levels
Experimental mice were killed by decapitation between 8 and 10 AM. Trunk blood samples were collected into EDTA-coated tubes (Sarstedt, Nümbrecht, Germany) and centrifuged (6000 g, 10 min), and plasma was stored at −80°C before assay. Tissues were rapidly frozen in dry ice for RNA or Western blot analysis or fixed in formalin (left adrenal, kidney) for histology. Evening plasma corticosterone levels were determined from blood sampled (by tail nick) at 7 PM. Plasma corticosterone was measured by an in-house radioimmunoassay as described previously (24). Plasma ACTH levels were measured by ELISA (Biomerica, Newport Beach, CA, USA). For the glucose tolerance test (GTT), animals were deprived of food for 6 h, 2 mg/g body weight of 25% glucose was injected intraperitoneally, and tail nick blood sampling was performed at time 0 (before injection) and 15, 30, 60, and 120 min after injection. Plasma glucose was measured using a glucose monitoring system (One Touch Ultra, Lifescan, Johnson & Johnson, Langhorne, PA, USA), insulin by ELISA (Crystalchem, Downers Grove, IL, USA), nonesterified fatty acids (NEFAs) with a NEFA C kit (Wako Chemicals GmbH, Nuess, Germany), and triglycerides with an L-type triglyceride kit (Wako Chemicals GmbH). Insulin, NEFAs, and triglycerides were measured after 24 h of food deprivation. Hepatic triglycerides were extracted after homogenization of 100 mg liver in isopropanol (10 vol) and then incubation at 37°C for 45 min. After centrifugation (3000 g, 10 min), 10 μl supernatant was incubated at 37°C for 5 min with 1 ml Thermotrace triglyceride reagent (Alpha Laboratories, Eastleigh, UK) and absorbance at 500 nm was measured. Plasma renin activity and angiotensinogen concentration were measured by radioimmunoassay as described previously (25). Plasma aldosterone concentration was measured by in-house ELISA as described previously (26, 27). Formalin-fixed tissues were processed for histopathology, sectioned (4 μm), and stained with hematoxylin and eosin for histopathological examination.
Blood pressure measurements
Systolic blood pressure was measured on 2 separate days in conscious mice by tail cuff plethysmography (Harvard Apparatus, Edenbridge, UK) as described previously (28). Before measurements were recorded, all mice underwent three periods of training to acclimatize them to the procedure. Mice were kept at 37°C for 10 min before measurements were initiated. Mean systolic blood pressure was calculated from the mean of 12 measurements per mouse.
RNA extraction and real-time PCR
Total RNA was extracted from frozen tissues as described previously (25). One microgram of RNA was pretreated with DNaseI (Invitrogen, Paisley, UK) and then reverse transcribed into cDNA using oligo(dT) primer and Superscript III first strand cDNA synthesis kit (Invitrogen). Real-time PCR was carried out on cDNA using a LightCycler 480 (Roche Diagnostics, Burgess Hill, UK) with a commercial master mix (FAM-hydrolysis probe, Roche Diagnostics) and the following primer/probe sets (Applied Biosystems, Foster City, CA, USA): β-actin, Mm00607939_s1; GR, Mm01260497_m1 (amplicon spanning exons 5–6) and Mm00433832_m1 (amplicon spanning exons 2–3); 11β-HSD1, Mm00476182_m1; angiotensinogen, Mm00599662; 11β-HSD2, Mm00492541_g1; and mineralocorticoid receptor (MR), Mm01241597_m1. Renin primers were in-house primers as follows: forward, 5′-GGTGCCCTCCACCAAGTG-3′; reverse 5′-TCAGAGGACTCATAGAGGCTGTGA-3′; and probe, 5′-AGCCGCCTCTACCTTGCTTGTGGG-3′. Negative controls omitting Superscript III or RNA were included. Annealing temperature was 60°C. Data were analyzed using the second derivative maximum method. The ratio of levels of the transcript of interest to levels of β-actin mRNA was determined for each sample.
In situ mRNA hybridization
Brain and pituitary GR mRNA levels were determined by in situ mRNA hybridization histochemistry, as described previously (29). Briefly, coronal brain sections (10 μm) were hybridized overnight at 55°C to 35S-labeled GR cRNA probe complementary to exons 5–9 of GR (29; absent from ESKN92-encoded GR-βgeo mRNA). Sections were then treated with RNase at 37°C for 1 h and washed at 60–70°C. Hybridized sections were exposed to autoradiographic film for 7 days. For densitometry, autoradiographs were imaged on a lightbox fitted with a coolsnap photometrics camera and analyzed using MCID software (InterFocus Imaging, Cambridge, UK).
Western blotting
Fifty milligrams of epididymal fat tissue was homogenized in 600 μl protein extraction buffer (Invitrogen). Proteins (25 μg homogenate) were resolved on 4–12% Bris-Tris Novex precast Gels (Invitrogen), transferred to a nitrocellulose membrane, and then incubated with a GR-specific antibody (M-20, Santa Cruz Biotechnology, Santa Cruz, CA, USA). Immunoreactive bands were visualized by chemiluminesense (ECL kit; Amersham Biosciences, Little Chalfont, UK). An anti-β-tubulin monoclonal antibody (Sigma Aldrich, St. Louis, MO, USA) or membrane staining with Ponceau red were used to verify equal protein loading between samples.
X-gal staining
X-gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) staining to detect β-galactosidase activity was carried out on frozen coronal brain sections (10 μm). Sections were transferred directly from −80°C into fixative (4% paraformaldehyde, 0.02% Nonidet P-40, 0.01% sodium deoxycholate, 5 mM EGTA, and 2 mM MgCl2) for 15 min at 4°C; washed twice in PBS containing 2 mM MgCl2, 0.02% Nonidet P-40, and 0.01% sodium deoxycholate; and stained for 6 h in PBS containing 2 mM MgCl2, 0.02% Nonidet P-40, 0.01% sodium deoxycholate, 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, and 1 mg/ml X-gal.
Transfection assays
Human embryonic kidney (HEK293) cells were maintained in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin, at 37°C, 5% CO2. For transfection, 2.5 × 105 cells were seeded per well in 6-well plates coated with poly-d-lysine in DMEM supplemented with 10% charcoal-stripped fetal calf serum and transfected the following day using Lipofectamine 2000 (Invitrogen) with 500 ng reporter plasmid, 200 ng expression plasmid and/or empty vector, pcDNA3.1(−) (Invitrogen), and 50 ng pRL-CMV (Promega, Southampton, UK) encoding renilla luciferase, used as internal control. Dexamethasone (1 μM) was added 1 h after transfection, and the cells were harvested 48 h later for luciferase assays. Reporter plasmids were MMTV-LTR-luciferase (30) or rPNMT-998/-466 Luc (31). Expression plasmids encoded wild-type (WT) mouse GR or GR-βgeo. WT GR was subcloned from pSV2wrec (32). A polymorphism within the DNA binding domain of the pSV2wrec-encoded GR (encoding Val437) (33) was changed to Gly437, to be identical to the C57BL/6J and 129 mouse strain-encoded GR, by site-directed mutagenesis. The GR-βgeo cDNA was assembled from the WT GR cDNA, the 5′-RACE product, and the pGT3 vector in the gene trap (23) by subcloning of appropriate DNA fragments. All constructs were verified by DNA sequencing.
Statistical analysis
The effects of genotype and diet were assessed by two-way ANOVA followed by post hoc Tukey’s tests for group differences. Significance was set at P < 0.05. To evaluate significant differences in GR mRNA levels between GR+/+ and GRβgeo/+ mice, a Student’s t test was used. For GTT comparisons, the area under the curve was calculated for each animal and then group means compared with 2-way ANOVA. For transfection assays, data were analyzed by ANOVA, followed by post hoc tests. Values are means ± se.
RESULTS
GR-βgeo allele is a null allele that decreases functional GR levels in heterozygous mice
Generation of GRβgeo/+ mice
GRβgeo/+ mutant mice were generated from an ES cell line (ESKN92) in which a βgeo reporter cassette had integrated into the GR gene, generating a transcriptional and translational fusion (23). 5′-RACE carried out on ESKN92 RNA and PCR analysis of genomic DNA demonstrated the site of integration between exons 3 and 4 (data not shown). The resulting fusion protein lacks part of the DNA binding domain and the entire ligand binding domain (Fig. 1A).
Figure 1.
The GR-βgeo fusion protein is transcriptionally inactive. A) Schematic view of the functional domains of GR (top), exon structure of GR cDNA (middle), and predicted structure of the GR-βgeo chimeric mRNA (bottom). The N-terminal domain (NTD) of GR is encoded by exon 2 (exon 1 is noncoding), the DNA binding domain (DBD) by exons 3–4, and the ligand binding domain (LBD) by exons 5–9. The βgeo cassette replaces exons 4–9. B, C) HEK293 cells were transiently transfected with WT mouse GR, GR-βgeo, or “empty” vector together with MMTV LTR-luciferase (B) or PMNT-998/-466-luciferase (C) reporters and then incubated without (white bars) or with (black bars) 1 μM dexamethasone. Data are means ± se of 3 independent experiments, each performed in triplicate. Values are expressed relative to vector, arbitrarily set to 1. ***P < 0.001 vs. untreated mice. Note the log scale for promoter activity. A.U., arbitrary units.
The encoded protein is transcriptionally inactive
The ability of the GR-βgeo fusion protein to activate transcription was tested in transfected HEK293 cells, which lack functional endogenous GR. In contrast to WT GR, the GR-βgeo fusion protein had no effect on promoter activity either in the presence or absence of dexamethasone (Fig. 1B, C), demonstrating that it is transcriptionally inactive and that the exon 2–3 encoded portion of GR, present in the fusion protein, has no constitutive activity. Importantly, cotransfection of GR-βgeo with WT GR had no effect on dexamethasone-mediated WT GR transactivation of either MMTV-LTR (Fig. 1B) or the PNMT promoter (Fig. 1C), demonstrating that the fusion protein does not exert a dominant-negative activity. Thus, although the fusion protein is expressed in GRβgeo/+ mice (e.g., Fig. 2), these properties predict that the GR-βgeo allele is a null allele.
Figure 2.
GRβgeo/+ mice have reduced GR mRNA levels in the brain, with normal distribution of GR-βgeo expression. A) Representative X-gal stained GRβgeo/+ brain section showing strong staining in a pattern identical to GR mRNA distribution in hippocampal subfields CA1/2, dentate gyrus (DG), and paraventricular nucleus of the hypothalamus (PVN), with weaker staining in cortex and thalamus. B, C) Representative autoradiographs showing in situ hybridization of GR mRNA in brain coronal sections of GR+/+ mice (B) and GRβgeo/+ mice (C). D) Quantification of GR mRNA levels [optical density (OD)] in the PVN of GR+/+ (white bars) and GRβgeo/+ mice (black bars) (n=6/group; *P<0.05).
Homozygous GRβgeo/βgeo mice die postnatally
Previous data have shown that homozygosity for a null allele of GR is lethal (34), whereas ∼10–15% of mice homozygous for a hypomorphic allele survive (11). To test the lethality of the GR-βgeo allele, heterozygous GRβgeo/+ mice were intercrossed. Of 145 offspring, none were homozygous for the GR-βgeo allele, suggesting that homozygous mice die before adulthood, consistent with previous data showing the lethality of a null allele around birth (11, 34). In contrast, examination of embryos from heterozygous intercrosses showed the presence of homozygous GRβgeo/βgeo embryos at the expected frequency (26.6%).
Functional GR levels are reduced in GRβgeo/+ mice
X-gal staining of brain sections from GRβgeo/+ mice showed expression of GR-βgeo throughout the brain, mirroring normal GR mRNA expression (Fig. 2A, B). Moreover, quantitative PCR (qPCR) measurements of GR mRNA levels in GR+/+ and GRβgeo/+ mice using primers that span exons 2–3 (present in both normal GR and GR-βgeo alleles) did not differ between genotypes (data not shown). However, in situ mRNA hybridization histochemistry of GR mRNA, using a probe complementary to exons 5–9 of GR (absent from mRNA encoding GR-βgeo; Fig. 1A) showed a halving in the full-length GR mRNA levels in the brain (hippocampus and paraventricular nucleus of the hypothalamus) and pituitary of GRβgeo/+ mice compared with GR+/+ littermates (Fig. 2B–D; Table 1). Similarly, qPCR to detect exons 5–6 showed that GRβgeo/+ mice had ∼50% reduced full-length GR mRNA levels in adipose tissue, muscle, liver, and adrenal gland (Table 1). Interestingly, the decrease measured in the adrenal gland was significantly greater than that in adipose tissue and liver (P<0.05; repeated one-way ANOVA), possibly reflecting developmental effects. Western blot analysis showed similar reductions in a 95 kDa GR protein in GRβgeo/+ mice (Fig. 3). A 191 kDa immunoreactive protein, corresponding to the predicted mass of the GR-βgeo fusion protein, was detected in GRβgeo/+ but not in GR+/+ mouse tissues (Fig. 3).
TABLE 1.
GRβgeo/+ mice have half of the normal GR mRNA levels in the brain and peripheral tissues
| Tissue | GR mRNA level (AU)
|
Reduction (%) | P value | |
|---|---|---|---|---|
| GR+/+ | GRβgeo/+ | |||
| Brain | ||||
| Hippocampus | ||||
| CA1 | 0.4 ± 0.05 | 0.2 ± 0.03 | 50 | 0.002 |
| CA2 | 0.5 ± 0.06 | 0.2 ± 0.05 | 60 | 0.003 |
| CA3 | 0.14 ± 0.01 | 0.06 ± 0.02 | 57 | 0.002 |
| DG | 0.4 ± 0.03 | 0.2 ± 0.03 | 50 | 0.002 |
| PVN | 0.4 ± 0.1 | 0.2 ± 0.05 | 50 | 0.05 |
| Pituitary | 0.5 ± 0.07 | 0.2 ± 0.04 | 60 | 0.002 |
| Periphery | ||||
| Adrenal gland | 1.7 ± 0.3 | 0.6 ± 0.08 | 65 | 0.002 |
| Liver | 1.0 ± 0.05 | 0.7 ± 0.06 | 30 | 0.002 |
| Epididymal fat | 1.3 ± 0.09 | 0.7 ± 0.05 | 46 | 0.0002 |
| Inguinal fat | 1.1 ± 0.06 | 0.6 ± 0.1 | 45 | 0.006 |
| Mesenteric fat | 0.6 ± 0.07 | 0.3 ± 0.08 | 50 | 0.05 |
| EDL muscle | 1.4 ± 0.1 | 0.6 ± 0.1 | 57 | 0.004 |
| Soleus muscle | 1.0 ± 0.1 | 0.5 ± 0.04 | 50 | 0.002 |
Levels of GR mRNA in brain were measured by in situ mRNA hybridization using a cRNA probe complementary to exons 5–9 of GR, absent from mRNA encoding the GR-βgeo fusion protein. Levels of full-length GR mRNA in peripheral tissues were measured by qPCR using a primer-probe set spanning exons 5–6. Student’s t test was performed for comparisons between genotypes (n=6/group). Significance was set at P < 0.05. AU, arbitrary units; CA, cornu ammonis; DG, dentate gyrus; PVN, paraventricular nucleus of the hypothalamus; EDL, extensor longus digitalis.
Figure 3.
GRβgeo/+ mice have reduced functional GR protein levels. Representative Western blot analysis showing decreased normal GR (95 kDa) in epididymal fat of GRβgeo/+ (+/−) mice compared with GR+/+ (+/+) mice (top). The GR-βgeo fusion protein (191 kDa) was apparent only in GRβgeo/+ mice. Tubulin (middle) and ponceau red staining (bottom) demonstrate equivalent protein loading.
GRβgeo/+ mice show altered HPA adaption to HF diet
Differing HPA axis alterations in response to HF diet in GRβgeo/+ mice
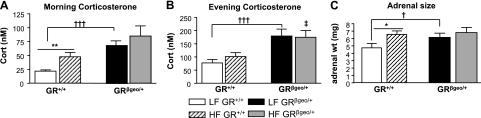
HF diets stimulate the HPA axis (35) and induce hypertension (36, 37). It is well known that GR manipulations affect the HPA axis (15). To establish that HPA axis activity is increased in GRβgeo/+ mice and to determine whether reduced GR levels alter the metabolic adaption to an HF diet, GRβgeo/+ mice were weaned onto a defined HF or LF diet and maintained on the diets for 22 wk. Although on the LF diet GRβgeo/+ mice had elevated plasma corticosterone levels (basal and peak) compared with GR+/+ mice (Fig. 4), consistent with the predicted increase in basal HPA axis activity in GR deficiency (15), they resisted the HF diet-induced increase in basal (morning) plasma corticosterone levels (Fig. 4). Thus, while the HF diet increased basal corticosterone levels in GR+/+ mice by ∼2-fold, it had no significant effect on basal corticosterone levels in GRβgeo/+ mice, resulting in no difference between mice of the two genotypes fed the HF diet (Fig. 4). Peak circulating corticosterone levels remained higher in GRβgeo/+ mice compared with their GR+/+ littermates and were unaffected by diet (Fig. 4B). There was no significant difference between genotypes in basal (morning) plasma ACTH levels in either LF-fed (data not shown) or HF-fed mice (GR+/+ vs. GRβgeo/+: 38±8 and 19±7 pg/ml, respectively), consistent with the similar basal plasma corticosterone levels in the latter. Similarly, there were no significant effects of either genotype or diet on pituitary POMC mRNA levels, although there was a trend for the HF diet to increase POMC mRNA levels, only in GR+/+ mice (LF- vs. HF-fed GR+/+ mice: 0.7±0.1 and 1.3±0.3, n=2 and 5, respectively, P=0.3; LF- vs. HF-fed GRβgeo/+ mice: 0.9±0.2 and 0.9±0.2, respectively, n=5/group). Strikingly, and similar to basal plasma corticosterone levels, adrenal weight was significantly higher in LF-fed GRβgeo/+ mice compared with their GR+/+ littermates, but, in contrast to GR+/+ mice, in which adrenal weight was increased by the HF diet, the HF diet had no effect on adrenal weight in GRβgeo/+ mice (Fig. 4C). Adrenal morphology also differed between genotypes, and this was particularly apparent on the LF diet (Fig. 5). The morphology suggests stimulation of corticosterone- and aldosterone-producing cells of GRβgeo/+ mice in the zona fasciculata and glomerulosa, respectively. In adrenal glands from GRβgeo/+ mice, the columns of endocrine cells in the zona fasciculata are longer than in GR+/+ mice, with individual cells having homogeneous eosinophilic cytoplasm (Fig. 5A–D). The zona glomerulosa of GRβgeo/+ adrenal glands appears thicker than in GR+/+ mice, with more glomeruli evident and containing hypertrophied epithelium (Fig. 5C, D). This adrenal phenotype was also apparent in mice fed the HF diet (Fig. 5E, F), but the morphological differences between LF and HF groups were more pronounced in GR+/+ mice than in GRβgeo/+ mice (Fig. 5). Measurements of adrenal cell size in the different zones (glomerulosa, fascilulata, and reticularis) did not differ between genotypes (data not shown), indicating that the larger adrenal glands in GRβgeo/+ mice reflect hyperplasia rather than cellular hypertrophy.
Figure 4.
HPA hyperactivity in GRβgeo/+ mice. A, B) Plasma corticosterone (cort) levels in LF-fed GR+/+ (white bars), HF-fed GR+/+ (hatched bars), LF-fed GRβgeo/+ (black bars) and HF-fed GRβgeo/+ mice (gray bars) in the morning (A) and evening (B). C) Left adrenal weights in LF or HF-fed GR+/+ and GRβgeo/+ mice (n=8–9/group). *,†P < 0.05; **P < 0.01; †††P < 0.001. ‡P < 0.01 vs. HF-fed GR+/+ mice.
Figure 5.
GRβgeo/+ mice have larger adrenal glands. Representative images of hematoxylin and eosin stained sections of adrenal glands from LF-fed GR+/+ mice (A, C); LF-fed GRβgeo/+ mice (B, D); HF-fed GR+/+ mice (E); and HF-fed GRβgeo/+ mice (F). ZF, zona fasciculate; ZG, zona glomerulosa; MED, medulla. n = 8–9/group. Scale bars = 25 μm.
GRβgeo/+ mice have normal adipose tissue distribution and glucose homeostasis but higher liver triglyceride levels on HF diet
Although GR polymorphisms in humans are associated with alterations in body weight and/or composition (reviewed in ref. 14), there were no differences in body weight (Fig. 6A) or food intake (data not shown) between GRβgeo/+ mice and their GR+/+ littermates, on either the LF or HF diet. There were also no differences between genotypes on either diet in weights of adipose tissue (epididymal, inguinal, and mesenteric) and muscle (extensor digitorum longus) or in plasma levels of insulin, NEFAs, and triglyceride (Table 2). However, while the HF diet caused the expected increase in liver triglyceride levels in both genotypes, levels were higher in HF-fed GRβgeo/+ than in GR+/+ mice (Table 2). Both genotypes fed the LF diet showed similar responses in GTTs with an identical impaired response in mice fed HF diet (Fig. 6B).
Figure 6.
Unaltered body composition and glucose homeostasis in GRβgeo/+ mice after HF diet. Body weight (A) and plasma glucose levels (B) after GTT in LF-fed GR+/+ mice (open squares, dashed line); HF-fed GR+/+ mice (open circles, dashed line); LF-fed GRβgeo/+ mice (black squares, solid line) and HF-fed GRβgeo/+ mice (black circles, solid line) (n=6/group). ***P < 0.001.
TABLE 2.
Similar regional adiposity, lean mass, and plasma insulin and lipid levels in GRβgeo/+ and GR+/+ mice
| Variable | LF diet
|
HF diet
|
||
|---|---|---|---|---|
| GR+/+ | GRβgeo/+ | GR+/+ | GRβgeo/+ | |
| Epididymal fat mass | ||||
| % Body weight | 2.08 ± 0.36 | 1.66 ± 0.38 | 4.42 ± 0.22*** | 4.90 ± 0.38*** |
| Absolute wt (mg) | 657 ± 140 | 489 ± 126 | 1972 ± 110 | 2476 ± 285 |
| Inguinal fat mass | ||||
| % Body weight | 1.79 ± 0.31 | 1.36 ± 0.07 | 4.22 ± 0.27*** | 4.5 ± 0.3.5*** |
| Absolute wt (mg) | 559 ± 119 | 387 ± 23 | 1914 ± 186 | 2230 ± 274 |
| Mesenteric fat mass | ||||
| % Body weight | 1.31 ± 0.15 | 1.07 ± 0.07 | 2.16 ± 0.13** | 2.00 ± 0.17** |
| Absolute wt (mg) | 408 ± 67 | 301 ± 31 | 979 ± 92 | 984 ± 121 |
| Brown adipose mass | ||||
| % Body weight | 0.38 ± 0.03 | 0.38 ± 0.03 | 0.62 ± 0.05** | 0.79 ± 0.05** |
| Absolute wt (mg) | 118 ± 15 | 107 ± 8 | 282 ± 31 | 381 ± 34 |
| Muscle (EDL) mass | ||||
| % Body weight | 0.24 ± 0.02 | 0.22 ± 0.02 | 0.16 ± 0.01** | 0.15 ± 0.01** |
| Absolute wt (mg) | 70.3 ± 4 | 60.8 ± 5 | 68.6 ± 3 | 68.8 ± 3 |
| Plasma insulin (ng/ml) | 0.2 ± 0.01 | 0.3 ± 0.1 | 0.9 ± 0.2* | 1.0 ± 0.2* |
| Plasma NEFA (mEq/l) | 0.8 ± 0.1 | 0.6 ± 0.1 | 0.65 ± 0.1 | 0.8 ± 0.1 |
| Plasma TG (mmol/l) | 0.44 ± 0.05 | 0.37 ± 0.06 | 0.52 ± 0.03 | 0.47 ± 0.04 |
| Liver TG (μmol/g) | 5.4 ± 0.7 | 8.0 ± 1.6 | 14.8 ± 0.7*** | 18±0.8*** |
Plasma NEFA, plasma triglyceride (TG), and plasma insulin levels were measured after 24 h fast.
P < 0.05;
P < 0.01;
P < 0.001 vs. LF diet. Values in italics indicate a significant difference between genotypes. n = 8/group.
GRβgeo/+ mice are hypertensive and show an attenuated blood pressure response to HF diet
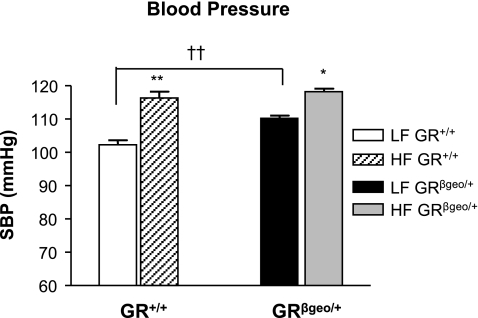
In LF-fed mice, systolic blood pressure was significantly elevated (8 mmHg) in GRβgeo/+ mice compared with their GR+/+ littermates (Fig. 7). Furthermore, blood pressure was increased in both genotypes by the HF diet, although interestingly the magnitude of the increase was lower in GRβgeo/+ mice (8 mmHg) than in GR+/+ mice (14 mmHg), resulting in no difference in blood pressure between genotypes in mice fed the HF diet (Fig. 7).
Figure 7.
GRβgeo/+ mice have elevated blood pressure. Systolic blood pressure (SBP) in LF-fed GR+/+ mice (white bars), HF-fed GR+/+ mice (hatched bars), LF-fed GRβgeo/+ mice (black bars), and HF-fed GRβgeo/+ mice (gray bars). *P < 0.05; **,††P < 0.01.
GRβgeo/+ mice have an activated RAAS
Increased systolic blood pressure in GRβgeo/+ mice was associated with a 2-fold increase in plasma renin activity, which was unaffected by diet in either genotype, remaining higher in GRβgeo/+ mice (Fig. 8A). Similarly, plasma aldosterone levels were increased in GRβgeo/+ mice, irrespective of diet (Fig. 8B). Plasma angiotensinogen levels were comparable between LF diet-fed GRβgeo/+ and GR+/+ mice but were markedly increased (3-fold) by the HF diet only in GRβgeo/+ mice (Fig. 8C). Hepatic angiotensinogen mRNA levels mirrored the pattern of plasma angiotensinogen (Fig. 8E). Increased adipose tissue expression of angiotensinogen, although much lower than hepatic expression, has been postulated to contribute to or even underlie hypertension in diet-induced obesity (38). However, although we observed higher expression of angiotensinogen mRNA in epididymal adipose tissue of LF-fed GRβgeo/+ mice compared with GR+/+ mice, levels were lower in HF-fed mice and did not differ between genotypes (Fig. 8E). Neither kidney nor adrenal renin mRNA levels differed between genotypes on the LF diet nor were they altered by the HF diet (Table 3). Similarly, renal 11β-HSD2 and MR mRNA levels, both critical determinants of blood pressure (39), were identical between genotypes and they did not differ with diet (Table 3). Thus, activation of the RAAS in GRβgeo/+ mice is likely to underlie their hypertension but does not account for changes in blood pressure after the HF diet. The activation of RAAS in GRβgeo/+ mice was not accompanied by grossly abnormal kidney function, since kidney morphology was similar in both genotypes (data not shown).
Figure 8.
Activation of the RAAS in GRβgeo/+ mice. Plasma renin activity (A), plasma aldosterone concentration (B), plasma angiotensinogen concentration (C), epididymal fat angiotensinogen (AGT) mRNA levels (D), and hepatic AGT mRNA levels (E) in LF-fed GR+/+ mice (white bars), HF-fed GR+/+ mice (hatched bars), LF-fed GRβgeo/+ mice (black bars), and HF-fed GRβgeo/+ mice (gray bars). For plasma measurements, n = 5/group. For mRNA levels, n = 8–9/group. *,†P < 0.05; **,††P < 0.01; ***,†††P < 0.001.
TABLE 3.
Similar expression of mRNAs encoding renin, 11β-HSD2, and MR in GRβgeo/+ and GR+/+ mice
| mRNA | Tissue | LF diet
|
HF diet
|
||
|---|---|---|---|---|---|
| GR+/+ | GRβgeo/+ | GR+/+ | GRβgeo/+ | ||
| Renin | Kidney | 0.87 ± 0.07 | 0.95 ± 0.20 | 0.83 ± 0.09 | 0.75 ± 0.06 |
| Adrenal | 0.98 ± 0.20 | 1.0 ± 0.10 | ND | ND | |
| 11β-HSD2 | Kidney | 0.85 ± 0.03 | 0.86 ± 0.09 | 0.94 ± 0.04 | 0.77 ± 0.09 |
| MR | Kidney | 0.80 ± 0.08 | 0.96 ± 0.20 | 0.98 ± 0.07 | 0.99 ± 0.10 |
Levels of specific mRNAs were measured by qPCR and are expressed in arbitrary units. There were no significant effects of either genotype or diet upon levels of any of the mRNAs measured (n=6/group).
DISCUSSION
Although GR polymorphisms are associated with hypertension and differences in body mass index and body composition in humans, few animal studies have addressed the effects of altered GR density on body weight and composition and none have examined the effect on metabolic adaption to the HF diet. Here we found that reduced tissue GR density has no effect on body weight, adipose tissue distribution or glucose homeostasis, basally, on a chow diet (unpublished observations) or an LF diet or after an HF diet. However, like most humans heterozygous for mutations in the GR gene, GRβgeo/+ mice have an activated HPA axis and, as we have now shown, elevated blood pressure. Interestingly, GRβgeo/+ mice failed to show the full extent of the HPA and blood pressure adaptions to the HF diet seen in control mice, suggesting that tissue GR density limits the adaptive response to chronic dietary stress.
As expected, GRβgeo/+ mice display a hyperactive HPA axis, with elevated plasma corticosterone and larger adrenal glands. The latter results from hyperplasia rather than cellular hypertrophy, since adrenal cell size was not different in GRβgeo/+ mice in any of the zones. This is consistent with previous data showing increased plasma corticosterone levels in mice with reduced GR density (11, 18), although measurements in these previous studies are likely to have been stress levels. It differs, however, from a previous study in mice heterozygous for a null allele of GR (GRnull/+) in which nonstressed plasma corticosterone levels, both morning and evening, were the same as in control mice, although the mice did show an increased plasma corticosterone response to stress (40), similar to GRβgeo/+ mice (our unpublished data). The discrepancy in nonstressed plasma corticosterone levels between the two models may be related to environmental or strain differences: here, backcrossed to C57BL/6J for five generations, whereas Ridder et al. (40) examined F1 offspring of a C57BL/6J X FVB/N cross. We (41) have noted higher basal plasma corticosterone levels in FVB/N mice.
GRβgeo/+ mice have completely normal body weight, regional adiposity, and lean mass. Glucose homeostasis (measured by fasting plasma glucose) and insulin levels, as well as glucose tolerance, were also entirely normal in GRβgeo/+ mice. This metabolic phenotype contrasts with the obesity seen in chow-fed transgenic mice with globally reduced GR density because of antisense GR RNA expression (18, 19). These mice, similar to GRβgeo/+ mice, also show an activated HPA axis (18). The reason for the discrepancy between these two models, each with reduced GR and elevated corticosterone, is not clear but may relate to the unknown effects of the GR transgene in the antisense GR-expressing mice in which knockdown of the GR is achieved through use of a neurofilament gene promoter, which may be differentially expressed within the central nervous system. Mice with a conditional deletion of the GR gene in neurons have very high plasma corticosterone levels due to a lack of negative feedback at the hypothalamus (42). However, these mice have normal peripheral GR levels, are growth retarded, and have proportionately more body fat before weaning, but less after (20). In GRβgeo/+ mice, reduced peripheral GR density may compensate for the increased plasma corticosterone levels, normalizing body fat distribution. The lack of effect of genotype on glucose homeostasis is not surprising, as selective GR gene deletion in hepatocytes showed that GR is essential for glucose homeostasis only after prolonged fasting or in a diabetic state (21). Moreover, antisense GR mice also show normal glucose homeostasis (19).
Like most humans heterozygous for inactivating mutations in the GR gene (43), GRβgeo/+ mice are hypertensive. In GRβgeo/+ mice, the hypertension results from elevated activity of the renin-angiotensin-aldosterone system. Plasma aldosterone levels were increased in GRβgeo/+ mice, as was plasma renin activity. The latter, however, was not associated with increased expression of renin mRNA in either the kidney or adrenal gland. A similar discrepancy between plasma renin and mRNA levels has previously been reported and postulated to be due to the more rapid secretion and turnover of newly synthesized renin compared with the long half-life of renin mRNA (44). Alternatively, renin clearance may be increased in GRβgeo/+ mice. Despite reduced GR density and elevated plasma corticosteroid levels in GRβgeo/+ mice, there were no compensatory changes, at least in the kidney, in the expression of MR or 11β-HSD2, which protects MR from illicit occupation by glucocorticoids (39). Similarly, MR levels in the brain are normal in mice with forebrain-specific deletion of GR (45), suggesting that MR density is not altered to compensate for reduced GR expression. The increased plasma aldosterone levels in GRβgeo/+ mice were consistent with the adrenal histopathology, suggesting high synthetic activity of aldosterone producing cells. This was predicted from the phenotype of GRhypo/hypo mice, which have elevated levels of aldosterone synthase in the zona glomerulosa (11), and is probably a consequence of elevated plasma ACTH levels in these mice. Indeed, Pomc−/− mice, which lack circulating ACTH, have small adrenal glands and dramatically reduced plasma aldosterone levels (46).
HF diets chronically stress the HPA axis in rodents, increasing basal corticosterone levels and causing adrenal enlargement (35, 47), although negative feedback efficiency is unaffected by HF diet (35). Consistent with this, in GR+/+ mice, the HF diet from weaning increased adrenal size and increased morning plasma corticosterone levels. In contrast, in GRβgeo/+ mice, the HF diet had no effect on either of these parameters. Thus, differences between genotypes in these parameters were eliminated by the diet. Evening (nonstressed) plasma corticosterone levels were unaffected by the HF diet and remained higher in GRβgeo/+ mice. These results suggest that the HPA axis response to the HF diet is limited by tissue GR density, this adaptive response being abolished by a 50% reduction in GR levels.
Surprisingly, given the role of glucocorticoids in energy homeostasis (2), GRβgeo/+ mice showed an entirely normal body weight gain, altered glucose homeostasis and insulin sensitivity on the HF diet, and did not differ from GR+/+ controls. Similarly, mice of both genotypes fed the HF diet doubled their hepatic accumulation of triglyceride compared with mice on the control diet, although hepatic triglyceride levels were significantly higher in HF-fed GRβgeo/+ mice than in GR+/+ mice. Although plasma NEFAs did not differ between LF- and HF-fed mice, these were measured after a 24 h fast, when NEFAs release from adipose tissue constitutes the major energy source and is maximal. Glucocorticoids increase hepatic lipogenesis and secretion (48,49,50). Furthermore, the consequences of altered glucocorticoid status on hepatic triglyceride metabolism are dependent on diet composition (51), but only when the diet is fed ad libitum (52). Thus, if lipid flux is low, when animals are maintained on an LF diet or with the restricted HF diet, then glucocorticoids minimally affect hepatic triglyceride storage and secretion (52). Whether the increased hepatic triglyceride levels in GRβgeo/+ mice are a consequence of the elevated peak corticosterone levels in these mice (morning levels did not differ after the HF diet) or a direct consequence of reduced hepatic GR density remains to be determined. 11β-HSD1 levels are unlikely to contribute to elevated hepatic triglycerides, since adipose or liver mRNA levels were unaltered (unpublished observations).
Blood pressure was increased by the HF diet in both genotypes, consistent with previous data (36, 37, 53). However, the magnitude of the HF-induced increase in blood pressure was smaller in GRβgeo/+ mice so that blood pressure in HF-fed GRβgeo/+ mice did not differ from that in HF-fed GR+/+ mice. The mechanism underlying the blood pressure increase with the HF diet remains unclear but may differ between genotypes. In GRβgeo/+ mice, further RAAS activation may contribute. Plasma angiotensinogen levels were increased by the HF diet in GRβgeo/+ mice, probably as a result of increased hepatic expression of angiotensinogen, but were unaffected in GR+/+ mice in which the diet-induced increase in blood pressure was greater. The HF diet had no effect on plasma aldosterone levels or plasma renin activity in either genotype. Thus, activation of the systemic RAAS by the HF diet in GR+/+ mice is ruled out. Elevated plasma glucocorticoid levels drive hypertension in both humans and rodents (1, 54). However, the underlying mechanisms remain controversial but are likely to include changes in the sympathetic nervous system. In this regard, it is of note that adrenal catecholamines, implicated in the HF diet-induced hypertension (53), are reduced in GR+/− mice (11, 34). It will be of interest, in future work, to determine whether sympathetic nervous system activity is altered in GRβgeo/+ mice.
In humans, polymorphisms in the GR gene associated with hypersensitivity or hyposensitivity to glucocorticoids in a dexamethasone suppression test have been variably associated with differences in body mass index, waist-hip ratio, altered body composition, and metabolic parameters (reviewed in ref. 14) as well as differences in blood pressure (55,56,57,58,59). However, the association studies (14) are controversial and may depend on age, gender, and ethnic background. The data reported here provide insights into that heterogeneity. In our model of glucocorticoid resistance due to ∼50% reduction in GR density, the observed physiological differences between genotypes depend on environment. Thus, several of the differences between genotypes are abolished or reduced after the HF diet. If the same applies in humans, then (for example) obesity or other chronic stress may mask differences in blood pressure between populations with different GR polymorphisms.
Glucocorticoids form part of important homeostatic control mechanisms that are critical in adapting to environmental challenges. Thus, they maintain stability through change over time (allostasis; reviewed in refs. 60, 61). GRβgeo/+ mice do not show the normal adaptions to the HF diet; they have an attenuated blood pressure response and do not show the alterations in adrenal size and HPA axis activity seen in WT mice. Consequently, basal corticosterone levels are normalized between genotypes after the HF diet. Moreover, GRβgeo/+ mice accumulated more hepatic triglyceride, possibly because of inadequate adaption to excessive dietary fat/energy intake and the need to protect vital organs. Continued excessive lipid accumulation in the liver may, in turn, lead to insulin resistance and metabolic disease. GR density is likely to be limiting in other systems in adaption to environmental stressors. Mice heterozygous for inactivating mutations in GR show reduced coping behavior in a mouse correlate of depression, with compromised indicators of neural plasticity (40), whereas overexpression of GR results in resistance to stress and robust protection against endotoxins. These adaptive or “remodeling” effects of glucocorticoids in the adult response to the HF diet are reminiscent of the prenatal “programming” effects of glucocorticoids (reviewed in ref. 62) where exposure of the late fetus to glucocorticoids permanently programs tissue systems throughout life, including blood pressure, glucose/lipid homeostasis, and tissue GR density itself. It will be of great interest to determine whether GR density influences the prenatal programming effects of glucocorticoids in the same way that it alters the postnatal blood pressure and HPA adaptions to a chronic HF diet.
Acknowledgments
We thank Mark Danielsen (Georgetown University School of Medicine, Washington, DC, USA) for kindly providing the SV2wrec plasmid encoding mouse GR cDNA and David Pearce (University of California, San Francisco, Department of Medicine, San Francisco, CA, USA) for providing the rPNMT-998/-466 Luc plasmid. We thank the Biomedical Research Resources facility, University of Edinburgh, facility for assistance with animal care and members of the Endocrinology Unit for helpful discussions and advice. This work was supported by a Wellcome Trust PhD Studentship (to Z.M.), a British Heart Foundation Project Grant (to K.E.C., C.J.K., N.M.M., and J.R.S.), a Wellcome Trust Programme Grant (to J.R.S. and K.E.C.), a Wellcome Trust Project Grant (to M.C.H and J.R.S.), and a Medical Research Council Programme Grant (to C.J.K.). N.M.M. is supported by a Wellcome Trust Research Career Development Fellowship.
References
- Whitworth J A, Brown M A, Kelly J J, Williamson P M. Mechanisms of cortisol-induced hypertension in humans. Steroids. 1995;60:76–80. doi: 10.1016/0039-128x(94)00033-9. [DOI] [PubMed] [Google Scholar]
- Dallman M F, Strack A M, Akana S F, Bradbury M J, Hanson E S, Scribner K A, Smith M. Feast and famine–critical role of glucocorticoids with insulin in daily energy-flow. Front Neuroendocrinol. 1993;14:303–347. doi: 10.1006/frne.1993.1010. [DOI] [PubMed] [Google Scholar]
- Ballard P L, Baxter J D, Higgins S J, Rousseau G G, Tomkins G M. General presence of glucocorticoid receptors in mammalian tissues. Endocrinology. 1974;94:998–1002. doi: 10.1210/endo-94-4-998. [DOI] [PubMed] [Google Scholar]
- Hollenberg S M, Weinberger C, Ong E S, Cerelli G, Oro A, Lebo R, Thompson E B, Rosenfeld M G, Evans R M. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature. 1985;318:635–641. doi: 10.1038/318635a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesfeld R, Rusconi S, Godowski P J, Maler B A, Okret S, Wikström A C, Gustafsson J-Å, Yamamoto K R. Genetic complementation of a glucocorticoid receptor deficiency by expression of cloned receptor cdna. Cell. 1986;46:389–399. doi: 10.1016/0092-8674(86)90659-8. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D J, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans R M. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderbilt J N, Miesfeld R, Maler B A, Yamamoto K R. Intracellular receptor concentration limits glucocorticoid-dependent enhancer activity. Mol Endocrinol. 1987;1:68–74. doi: 10.1210/mend-1-1-68. [DOI] [PubMed] [Google Scholar]
- Ramdas J, Liu W, Harmon J M. Glucocorticoid-induced cell death requires autoinduction of glucocorticoid receptor expression in human leukemic T cells. Cancer Res. 1999;59:1378–1385. [PubMed] [Google Scholar]
- Reichardt H M, Umland T, Bauer A, Kretz O, Schütz G. Mice with an increased glucocorticoid receptor gene dosage show enhanced resistance to stress and endotoxic shock. Mol Cell Biol. 2000;20:9009–9017. doi: 10.1128/mcb.20.23.9009-9017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazirandeh A, Xue Y, Prestegaard T, Jondal M, Okret S. Effects of altered glucocorticoid sensitivity in the T-cell lineage on thymocyte and T-cell homeostasis. FASEB J. 2002;16:727–729. doi: 10.1096/fj.01-0891fje. [DOI] [PubMed] [Google Scholar]
- Cole T J, Blendy J A, Monaghan A P, Krieglstein K, Schmid W, Aguzzi A, Fantuzzi G, Hummler E, Unsicker K, Schutz G. Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev. 1995;9:1608–1621. doi: 10.1101/gad.9.13.1608. [DOI] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Reichardt H M, Schütz G. Genetic dissection of glucocorticoid receptor function in mice. Curr Opin Genet Dev. 1998;8:532–538. doi: 10.1016/s0959-437x(98)80007-5. [DOI] [PubMed] [Google Scholar]
- Kino T, Chrousos G P. Glucocorticoid and mineralocorticoid resistance/hypersensitivity syndromes. J Endocrinol. 2001;169:437–445. doi: 10.1677/joe.0.1690437. [DOI] [PubMed] [Google Scholar]
- van Rossum E F, Lamberts S W. Polymorphisms in the glucocorticoid receptor gene and their associations with metabolic parameters and body composition. Recent Prog Horm Res. 2004;59:333–357. doi: 10.1210/rp.59.1.333. [DOI] [PubMed] [Google Scholar]
- Gass P, Reichardt H M, Strekalova T, Henn F, Tronche F. Mice with targeted mutations of glucocorticoid and mineralocorticoid receptors: models for depression and anxiety? Physiol Behav. 2001;73:811–825. doi: 10.1016/s0031-9384(01)00518-2. [DOI] [PubMed] [Google Scholar]
- Howell M P, Muglia L J. Effects of genetically altered brain glucocorticoid receptor action on behavior and adrenal axis regulation in mice. Front Neuroendocrinol. 2006;27:275–284. doi: 10.1016/j.yfrne.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Kleiman A, Tuckermann J P. Glucocorticoid receptor action in beneficial and side effects of steroid therapy: lessons from conditional knockout mice. Mol Cell Endocrinol. 2007;275:98–108. doi: 10.1016/j.mce.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Pepin M-C, Pothier F, Barden N. Impaired type II glucocorticoid receptor function in mice bearing antisense RNA transgene. Nature. 1992;355:725–728. doi: 10.1038/355725a0. [DOI] [PubMed] [Google Scholar]
- Richard D, Chapdelaine S, Deshaies Y, Pepin M C, Barden N. Energy balance and lipid metabolism in transgenic mice bearing an antisense GCR gene construct. Am J Physiol Regul Integr Comp Physiol. 1993;265:R146–R150. doi: 10.1152/ajpregu.1993.265.1.R146. [DOI] [PubMed] [Google Scholar]
- Kellendonk C, Eiden S, Kretz O, Schutz G, Schmidt I, Tronche F, Simon E. Inactivation of the GR in the nervous system affects energy accumulation. Endocrinology. 2002;143:2333–2340. doi: 10.1210/endo.143.6.8853. [DOI] [PubMed] [Google Scholar]
- Opherk C, Tronche F, Kellendonk C, Kohlmuller D, Schulze A, Schmid W, Schutz G. Inactivation of the glucocorticoid receptor in hepatocytes leads to fasting hypoglycemia and ameliorates hyperglycemia in streptozotocin-induced diabetes mellitus. Mol Endocrinol. 2004;18:1346–1353. doi: 10.1210/me.2003-0283. [DOI] [PubMed] [Google Scholar]
- Sainte-Marie Y, Nguyen Dinh Cat A, Perrier R, Mangin L, Soukaseum C, Peuchmaur M, Tronche F, Farman N, Escoubet B, Benitah J P, Jaisser F. Conditional glucocorticoid receptor expression in the heart induces atrio-ventricular block. FASEB J. 2007;21:3133–3141. doi: 10.1096/fj.07-8357com. [DOI] [PubMed] [Google Scholar]
- Sutherland H G, Mumford G K, Newton K, Ford L V, Farrall R, Dellaire G, Caceres J F, Bickmore W A. Large-scale identification of mammalian proteins localized to nuclear sub-compartments. Hum Mol Genet. 2001;10:1995–2011. doi: 10.1093/hmg/10.18.1995. [DOI] [PubMed] [Google Scholar]
- Harris H J, Kotelevtsev Y, Mullins J J, Seckl J R, Holmes M C. Intracellular regeneration of glucocorticoids by 11b-hydroxysteroid dehydrogenase (11b-HSD)-1 plays a key role in regulation of the hypothalamic-pituitary-adrenal axis: analysis of 11b-HSD-1 deficient mice. Endocrinology. 2001;142:114–120. doi: 10.1210/endo.142.1.7887. [DOI] [PubMed] [Google Scholar]
- Morton N M, Densmore V, Wamil M, Ramage L, Nichol K, Bunger L, Seckl J R, Kenyon C J. A polygenic model of the metabolic syndrome with reduced circulating and intra-adipose glucocorticoid action. Diabetes. 2005;54:3371–3378. doi: 10.2337/diabetes.54.12.3371. [DOI] [PubMed] [Google Scholar]
- Al-Dujaili E A, Edwards C R. Development and application of a simple radioimmunoassay for urinary aldosterone. Clin Chim Acta. 1981;116:277–287. doi: 10.1016/0009-8981(81)90047-4. [DOI] [PubMed] [Google Scholar]
- Al-Dujaili E A. Development and validation of a simple and direct ELISA method for the determination of conjugated (glucuronide) and non-conjugated testosterone excretion in urine. Clin Chim Acta. 2006;364:172–179. doi: 10.1016/j.cccn.2005.06.019. [DOI] [PubMed] [Google Scholar]
- Evans A L, Brown W, Kenyon C J, Maxted K J, Smith D C. Improved system for measuring systolic blood pressure in the conscious rat. Med Biol Eng Comput. 1994;32:101–102. doi: 10.1007/BF02512487. [DOI] [PubMed] [Google Scholar]
- Seckl J R, Dickson K L, Fink G. Central 5,7-dihydroxytryptamine lesions decrease hippocampal glucocorticoid and mineralocorticoid receptor messenger ribonucleic acid expression. J Neuroendocrinol. 1990;2:911–916. doi: 10.1111/j.1365-2826.1990.tb00659.x. [DOI] [PubMed] [Google Scholar]
- Lefebvre P, Barard D S, Cordingley M G, Hager G L. Two regions of the mouse mammary tumor virus long terminal repeat regulate the activity of its promoter in mammary cell lines. Mol Cell Biol. 1991;11:2529–2537. doi: 10.1128/mcb.11.5.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams M, Meijer O C, Wang J, Bhargava A, Pearce D. Homodimerization of the glucocorticoid receptor is not essential for response element binding: activation of the phenylethanolamine N-methyltransferase gene by dimerization-defective mutants. Mol Endocrinol. 2003;17:2583–2592. doi: 10.1210/me.2002-0305. [DOI] [PubMed] [Google Scholar]
- Danielsen M, Northrop J P, Ringold G M. The mouse glucocorticoid receptor: mapping of functional domains by cloning, sequencing and expression of wild-type and mutant receptor proteins. EMBO J. 1986;5:2513–2522. doi: 10.1002/j.1460-2075.1986.tb04529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai Y. Two naturally-occurring isoforms and their expression of a glucocorticoid receptor gene from an androgen-dependent mouse tumor. FEBS Lett. 1990;274:99–102. doi: 10.1016/0014-5793(90)81339-p. [DOI] [PubMed] [Google Scholar]
- Finotto S, Krieglstein K, Schober A, Deimling F, Lindner K, Bruhl B, Beier K, Metz J, Garcia-Arraras J E, Roig-Lopez J L, Monaghan P, Schmid W, Cole T J, Kellendonk C, Tronche F, Schutz G, Unsicker K. Analysis of mice carrying targeted mutations of the glucocorticoid receptor gene argues against an essential role of glucocorticoid signalling for generating adrenal chromaffin cells. Development. 1999;126:2935–2944. doi: 10.1242/dev.126.13.2935. [DOI] [PubMed] [Google Scholar]
- Tannenbaum B M, Brindley D N, Tannenbaum G S, Dallman M F, McArthur M D, Meaney M J. High-fat feeding alters both basal and stress-induced hypothalamic- pituitary-adrenal activity in the rat. Am J Physiol Endocrinol Metab. 1997;273:E1168–E1177. doi: 10.1152/ajpendo.1997.273.6.E1168. [DOI] [PubMed] [Google Scholar]
- Mills E, Kuhn C M, Feinglos M N, Surwit R. Hypertension in CB57BL/6J mouse model of non-insulin-dependent diabetes mellitus. Am J Physiol Regul Integr Comp Physiol. 1993;264:R73–R78. doi: 10.1152/ajpregu.1993.264.1.R73. [DOI] [PubMed] [Google Scholar]
- Williams T D, Chambers J B, Roberts L M, Henderson R P, Overton J M. Diet-induced obesity and cardiovascular regulation in C57BL/6J mice. Clin Exp Pharmacol Physiol. 2003;30:769–778. doi: 10.1046/j.1440-1681.2003.t01-1-03808.x. [DOI] [PubMed] [Google Scholar]
- Frederich R C, Jr, Kahn B B, Peach M J, Flier J S. Tissue-specific nutritional regulation of angiotensinogen in adipose tissue. Hypertension. 1992;19:339–344. doi: 10.1161/01.hyp.19.4.339. [DOI] [PubMed] [Google Scholar]
- Seckl J R, Brown R W. 11β-hydroxysteroid dehydrogenase: on several roads to hypertension. J Hypertension. 1994;12:105–112. [PubMed] [Google Scholar]
- Ridder S, Chourbaji S, Hellweg R, Urani A, Zacher C, Schmid W, Zink M, Hortnagl H, Flor H, Henn F A, Schutz G, Gass P. Mice with genetically altered glucocorticoid receptor expression show altered sensitivity for stress-induced depressive reactions. J Neurosci. 2005;25:6243–6250. doi: 10.1523/JNEUROSCI.0736-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau J L, Noble J, Thomas S, Kerwin R, Morgan P E, Lightman S, Seckl J R, Pariante C M. The antidepressant desipramine requires the ABCB1 (Mdr1)-type p-glycoprotein to upregulate the glucocorticoid receptor in mice. Neuropsychopharmacology. 2007;32:2520–2529. doi: 10.1038/sj.npp.1301389. [DOI] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban P C, Bock R, Klein R, Schutz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- Kino T, Vottero A, Charmandari E, Chrousos G P. Familial/sporadic glucocorticoid resistance syndrome and hypertension. Ann N Y Acad Sci. 2002;970:101–111. doi: 10.1111/j.1749-6632.2002.tb04416.x. [DOI] [PubMed] [Google Scholar]
- Nakamura N, Soubrier F, Menard J, Panthier J J, Rougeon F, Corvol P. Nonproportional changes in plasma renin concentration, renal renin content, and rat renin messenger RNA. Hypertension. 1985;7:855–859. doi: 10.1161/01.hyp.7.6.855. [DOI] [PubMed] [Google Scholar]
- Boyle M P, Brewer J A, Funatsu M, Wozniak D F, Tsien J Z, Izumi Y, Muglia L J. Acquired deficit of forebrain glucocorticoid receptor produces depression-like changes in adrenal axis regulation and behavior. Proc Natl Acad Sci U S A. 2005;102:473–478. doi: 10.1073/pnas.0406458102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll A P, Challis B G, Yeo G S, Snell K, Piper S J, Halsall D, Thresher R R, O'Rahilly S. The effects of proopiomelanocortin deficiency on murine adrenal development and responsiveness to adrenocorticotropin. Endocrinology. 2004;145:4721–4727. doi: 10.1210/en.2004-0491. [DOI] [PubMed] [Google Scholar]
- Carroll K K, Noble R L. Effects of feeding rape oil on some endocrine functions of the rat. Endocrinology. 1952;51:476–486. doi: 10.1210/endo-51-6-476. [DOI] [PubMed] [Google Scholar]
- Klausner H, Heimberg M. Effect of adrenalcortical hormones on release of triglycerides and glucose by liver. Am J Physiol. 1967;212:1236–1246. doi: 10.1152/ajplegacy.1967.212.6.1236. [DOI] [PubMed] [Google Scholar]
- Kirk C J, Verrinder T R, Hems D A. Fatty acid synthesis in the perfused liver of adrenalectomized rats. Biochem J. 1976;156:593–602. doi: 10.1042/bj1560593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett S M, Gibbons G F. Short- and longer-term regulation of very-low-density lipoprotein secretion by insulin, dexamethasone and lipogenic substrates in cultured hepatocytes. A biphasic effect of insulin. Biochem J. 1988;249:37–43. doi: 10.1042/bj2490037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantha L, Palacios E, Deshaies Y. Modulation of triglyceride metabolism by glucocorticoids in diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 1999;277:R455–R464. doi: 10.1152/ajpregu.1999.277.2.R455. [DOI] [PubMed] [Google Scholar]
- Mantha L, Deshaies Y. Energy intake-independent modulation of triglyceride metabolism by glucocorticoids in the rat. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1424–R1432. doi: 10.1152/ajpregu.2000.278.6.R1424. [DOI] [PubMed] [Google Scholar]
- Kaufman L N, Li H Y, Peterson M M, Gilardy A K. Adrenal medulla as a mediator of diet-induced hypertension. Am J Physiol Regul Integr Comp Physiol. 1993;265:R1–R6. doi: 10.1152/ajpregu.1993.265.1.R1. [DOI] [PubMed] [Google Scholar]
- Magiakou M A, Smyrnaki P, Chrousos G P. Hypertension in Cushing’s syndrome. Best Pract Res Clin Endocrinol Metab. 2006;20:467–482. doi: 10.1016/j.beem.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Watt G C, Harrap S B, Foy C J, Holton D W, Edwards H V, Davidson H R, Connor J M, Lever A F, Fraser R. Abnormalities of glucocorticoid metabolism and the renin-angiotensin system: a four-corners approach to the identification of genetic determinants of blood pressure. J Hypertens. 1992;10:473–482. doi: 10.1097/00004872-199205000-00011. [DOI] [PubMed] [Google Scholar]
- Panarelli M, Holloway C D, Fraser R, Connell J M C, Ingram M C, Anderson N H, Kenyon C J. Glucocorticoid receptor polymorphism, skin vasoconstriction, and other metabolic intermediate phenotypes in normal human subjects. J Clin Endocrinol Metab. 1998;83:1846–1852. doi: 10.1210/jcem.83.6.4828. [DOI] [PubMed] [Google Scholar]
- Takami S, Wong Z Y, Stebbing M, Harrap S B. Linkage analysis of glucocorticoid and beta2-adrenergic receptor genes with blood pressure and body mass index. Am J Physiol Heart Circ Physiol. 1999;276:H1379–H1384. doi: 10.1152/ajpheart.1999.276.4.H1379. [DOI] [PubMed] [Google Scholar]
- Rosmond R, Chagnon Y C, Holm G, Chagnon M, Pérusse L, Lindell K, Carlsson B, Bouchard C, Björntorp P. A glucocorticoid receptor gene marker is associated with abdominal obesity, leptin, and dysregulation of the hypothalamic-pituitary- adrenal axis. Obes Res. 2000;8:211–218. doi: 10.1038/oby.2000.24. [DOI] [PubMed] [Google Scholar]
- Di Blasio A M, van Rossum E F, Maestrini S, Berselli M E, Tagliaferri M, Podesta F, Koper J W, Liuzzi A, Lamberts S W. The relation between two polymorphisms in the glucocorticoid receptor gene and body mass index, blood pressure and cholesterol in obese patients. Clin Endocrinol (Oxf) 2003;59:68–74. doi: 10.1046/j.1365-2265.2003.01798.x. [DOI] [PubMed] [Google Scholar]
- Korte S M, Koolhaas J M, Wingfield J C, McEwen B S. The Darwinian concept of stress: benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neurosci Biobehav Rev. 2005;29:3–38. doi: 10.1016/j.neubiorev.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Chapman K E, Seckl J R. 11beta-HSD1, inflammation, metabolic disease and age-related cognitive (dys)function. Neurochem Res. 2007 doi: 10.1007/s11064-007-9504-9. [DOI] [PubMed] [Google Scholar]
- Seckl J R. Glucocorticoid programming of the fetus; adult phenotypes and molecular mechanisms. Mol Cell Endocrinol. 2001;185:61–71. doi: 10.1016/s0303-7207(01)00633-5. [DOI] [PubMed] [Google Scholar]