Abstract
Leydig cells are the primary source of testosterone in adult males. Recently, a growing body of evidence has shown that testicular innervation functions as a major regulator in Leydig cell steroidogenesis. The question then arises whether this novel regulatory pathway also plays an important role in other biological behaviors of this cell type. In the present study, we selectively resected the superior spermatic nerves (SSNs) or the inferior spermatic nerves (ISNs) to investigate the effects of testicular denervation on survival of Leydig cells. After testicular denervation, Leydig cells displayed morphological characteristics of apoptosis, such as chromatin condensation, cell shrinkage and apoptotic body formation. Flow cytometry combined with TUNEL labeling demonstrated dramatic and persistent apoptosis of Leydig cells in the denervated testes 14 and 21 days after operation. Meanwhile, serum T concentrations in the SSN- or ISN-denervated rats dramatically decreased on day 14 and declined further on day 21. Plasma LH levels underwent a remarkable rise, while serum FSH levels remained unchanged. Immunofluorescent staining and flow cytometry further demonstrated that testicular denervation activated caspase-3 and caspase-8, but not caspase-9 in Leydig cells. Our data indicate that testicular innervation functions as an important survival factor for Leydig cells in vivo.
Keywords: Testicular innervation, Leydig cells, Apoptosis
Introduction
Leydig cells are the primary source of testosterone in adult males, which is critical for sperm production and sexual function maintenance, besides its effects on kidney, liver, skin, muscles, skeleton, bone marrow and central nervous system [1,2]. In past decades, numerous studies have been carried out to investigate regulation of Leydig cell steroidogenesis, and the hypothalamic–pituitary–gonadal axis and intratesticular paracrine and autocrine factors are known to be the chief regulators [3,4]. However, the physiological mechanisms of maintaining the survival of Leydig cells remain largely unknown.
Recently, several other regulatory systems have been postulated to be involved in Leydig cell steroidogenesis. One of such candidates is testicular innervation, which reaches the testis via the superior spermatic nerves (SSNs) and the inferior spermatic nerves (ISNs) [5,6]. In the testes of most mammalians investigated so far, abundance of autonomic nerve fibers have been found in their capsules and/or parenchyma [7–10]. The receptors for neurotransmitters, such as α- and β2-adrenergic receptors, have also been detected on the surface of Leydig cells [11,12]. Furthermore, involvement of testicular innervation in Leydig cell steroidogenesis has been directly evidenced in the previous studies. In the rat, for instance, bilateral section of SSNs reduces the number of testicular luteinizing hormone (LH) receptors, blunts the human chorionic gonadotropin-stimulated testosterone production [13] and inhibits the acute stress-induced rise of serum testosterone [14], while electrical stimulation of SSNs induces an elevation of testosterone levels in the spermatic veins of cats [15]. ISN section also partly suppresses increase of testosterone in response to hemicastration [16]. More importantly, the neuronal pathway from brain through spinal cord and spermatic nerves to the testis has recently been depicted anatomically [17–19]. This pathway has been found to regulate Leydig cell steroidogenesis independently from the pituitary [17–19].
Those results, taken together, reveal that testicular innervation acts as a major regulator in the steroidogenesis of Leydig cells. The question then arises whether this novel regulatory pathway also plays an important role in other fundamental biological behaviors, especially survival, of this cell type. To answer this question, the present study was designed to investigate the effects of nerve system on Leydig cell survival. SSNs or ISNs were selectively resected to evaluate influence of testicular denervation on Leydig cell apoptosis. When persistent apoptosis of Leydig cells occurred after denervation of SSNs or ISNs, the apoptotic signaling pathway was further investigated. Our data showed that the caspase-8-dependent extrinsic pathway was involved in testicular denervation-induced apoptosis in Leydig cells.
Materials and methods
Animals and experimental procedures
Adult male Sprague–Dawley rats, weighing 325–350 g, were purchased from Shanghai SLAC Laboratory Animal Co. Ltd. (Shanghai, China) and maintained in the Experiment Animal Center of Nanjing Medical University (Nanjing, China). Eighty rats were assigned randomly to four groups: SSN sham-operation group (SSG), SSN denervation group (SDG), ISN sham-operation group (ISG) and ISN denervation group (IDG). Testicular denervation was carried out as described previously with a slight modification [13]. In brief, rats were anesthetized by intra-peritoneal injection of sodium pentobarbital (40 mg/kg). SSNs were bilaterally exposed through a midabdominal incision and separated from the fat tissue surrounding the spermatic artery under the assistance of a Zeiss dissection microscope. Approximately 1 cm of the SSNs was removed. For ISN denervation, testes were bilaterally exposed through a hypogastric incision and a 1-cm portion of ISNs accompanying vas deferens was cut. Sham control rats were operated without excision of the nerves. Two and three weeks after operation, 10 rats from each group were killed by carbon dioxide at each time point. Trunk bloods were collected and testes were removed. For each animal, one testis was cut into three parts for TUNEL labeling, electron microscopy and immunofluorescent staining. The other testis was used for Leydig cell isolation. All animals were treated according to protocols approved by the Animal Committee of Nanjing Medical University.
Isolation of Leydig cells by Percoll
Leydig cells were isolated as previously described [20] with a slight modification. Briefly, testes were decapsulated and incubated in collagenase (0.25 mg/ml) in dissociation buffer with shaking (80 cycles per min) for 20 min at 34 °C. After digestion, seminiferous tubules were removed by filtration through 200 μm nylon mesh. The enriched Leydig cell fractions were collected and further separated by Percoll and BSA density gradient centrifugation. Leydig cells were harvested and the purities of Leydig cell fractions were evaluated by histochemical staining for 3β-HSD activity. Enrichment of Leydig cell was typically to 93%. The Leydig cell suspension was divided into two parts for quantitative analysis of apoptotic rates and caspase activities in Leydig cells.
Terminal deoxyribonucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) staining
In the late stage of apoptosis, nuclear DNA is cleaved into smaller fragments with exposed 3′-hydroxyl ends, which can be labeled in situ by TUNEL [21]. Testicular tissues were fixed in Bouin’s solution and cut into sections in 5 μm thickness. Apoptotic cell detection was performed using TUNEL kits (Roche Diagnostics, Mannhein, Germany) according to the manufacturer’s instructions. The TUNEL-stained cells were visualized with bromochloroindolyl phosphate (BCIP) and nitro-blue tetrazolium (NBT) salts (Sigma, St. Louis, MO, USA), and counterstained with methyl green. Negative controls were prepared by using distilled water instead of TUNEL reaction mixture.
Electron microscopic analysis
Testicular tissues were fixed overnight in 2.5% glutaraldehyde and postfixed with 1.0% osmium tetroxide. After embedding in EPON, ultrathin sections were cut and mounted on 200-mesh grids, stained with uranyl acetate followed by lead citrate, and examined under a transmission electron microscope (JEM-1010, JEOL, Japan).
Flow cytometry analysis of apoptosis in Leydig cells
For quantitative analysis of apoptotic Leydig cells, flow cytometry using Annexin-V-FITC (fluorescein isothiocyanate) and PI (propidium iodide) double staining was used in this study. After washing with PBS, Leydig cell suspension was incubated with Annexin-FITC and PI, using Annexin-V kits (BD Biosciences, San Diego, CA) following the manufacturer’s instructions. Ten thousand cells were analyzed by a FACS Vantage flow cytometer (Becton Dickinson, Franklin Lakes, NJ). Bivariant analysis of FITC-fluorescence and PI-fluorescence allows differentiation of Leydig cells into four subpopulations: (1) nonstaining viable cells, (2) early apoptotic cells (Annexin-V positive), (3) late apoptotic cells (Annexin-V + PI positive), and (4) necrotic cells (PI positive) [22,23].
Radioimmunoassay (RIA) for hormones
After clotting at room temperature, trunk bloods were centrifuged at 1000g for 30 min at 4 °C. The sera were stored at −20 °C for assay. Testosterone (T), luteinizing hormone (LH), and follicle-stimulating hormone (FSH) concentrations were determined with RIA kits (Beijing North Institute of Biological Technology, China).
Immunofluorescent staining for caspase-3, -8 and -9
Caspase activation is known as an event occurring during the early stage of cellular apoptosis [24,25]. Our preliminary study using flow cytometry showed that the denervated testes contained more early-stage apoptotic Leydig cells on day 21 than day 14 after testicular denervation. Therefore, caspase assay was performed on the samples obtained on 21 day in the present study. For immunofluorescent staining of caspases in Leydig cells, cryostat blocks of testicular tissues were cut into 10-μm-thick sections. The sections were fixed in 3.7% formaldehyde overnight and then permeabilized with 0.1% Triton X-100 for 5 min. Following rehydration with 1% BSA for 30 min at room temperature, the sections were incubated with rabbit antihuman IgG against caspase-3 (1:100, Santa Cruz, USA), caspase-8 (1:50, Santa Cruz, USA) and caspase-9 (1:50, Santa Cruz, USA), which were conjugated with FITC. Fluorescent Hoechst (Molecular Probes, OR, USA) was applied to visualize the cell nuclei. The slides were observed with Axioskop 2 plus (Carl Zeiss, Thornwood, NY, USA). Exposure times during photography were uniform to enable optimal comparison of fluorescence among the test samples.
Flow cytometry analysis of activities of caspase-3, -8, and -9
The activities of caspase-3, -8 and -9 were determined by flow cytometry using fluorescein active caspase staining kits (BioVision, Mountain View, CA, USA) according to the manufacturer’s instructions. Briefly, 1 × 106 Leydig cells were suspended in 300 μl of culture medium, and 1 μl of appropriate substrates (FITC-DEVD-FMK for caspase-3, FITC-IETD-FMK for caspase-8, and FITC-LEHD-FMK for caspase-9) were added. The cells were incubated for 60 min at 37 °C in an incubator with 5% CO2. After centrifugation at 3000 rpm for 5 min, the cells were washed twice in wash buffer and analyzed by a FACS Vantage flow cytometer (Becton Dickinson, Franklin Lakes, NJ).
Statistical analysis
Results are presented as means ± SEM. The data were analyzed for statistical significance by two-way ANOVA, followed by Tukey’s multiple comparisons test. Levels of statistical significance were fixed for P values <0.05.
Results and discussion
Testicular denervation induces apoptosis of Leydig cells
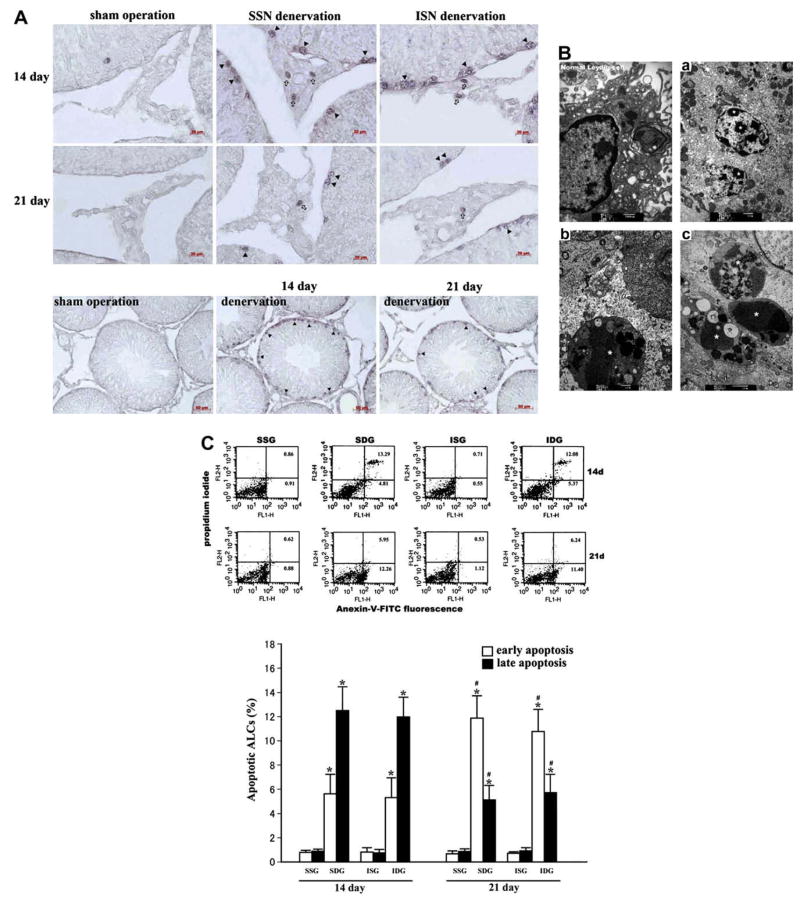
On post-operation day 14 and 21, apoptotic Leydig cells as well as spermatogonia (not discussed here), revealed by TUNEL, appeared in the SSN- or ISN-denervated testes (Fig. 1A). Meanwhile, morphological characteristics of apoptosis, such as chromatin condensation, cell shrinkage and apoptotic body formation, were also observed in Leydig cells of all the denervated testes (Fig. 1B). For further quantitative analysis, we isolated Leydig cells and utilized flow cytometry to access their apoptotic rates. In the sham-operated rats, <2% of Leydig cells were positively stained with Annexin-V (early apoptosis) or Annexin-V + PI (late apoptosis) (Fig. 1C). However, the total percentage of apoptotic Leydig cells increased by approximately more than 10 folds after the removal of SSNs or ISNs (Fig. 1C). We noted that the percentage of late apoptotic Leydig cells was 12.8% and 11.9% in the SSN- or ISN-denervated testes, respectively, on day 14, while the rates decreased to 5.6% and 5.3% on day 21. By contrast, the rates of early apoptotic Leydig cells increased from 5.1% and 5.9% on day 14 to 11.6% and 10.7% on day 21 (Fig. 1C), implying that another new group of Leydig cells underwent apoptosis on day 21. All of these results, taken together, provide convincing evidence that Leydig cells underwent dramatic and persistent apoptosis when testicular innervation was deprived, suggesting that the neuronal input to the male gonad functions as an important survival factor for this cell type in vivo.
Fig. 1.
Apoptosis of Leydig cells induced by testicular denervation. (A) TUNEL-positive Leydig cells (arrowheads) and spermatogonia (triangles) appeared in the SSN-denervated testes and ISN-denervated testes on day 14 and 21. Dramatic apoptosis of spermatids and spermatocytes was not detected in the denervated testes. (B) After testicular denervation, Leydig cells displayed morphological characteristics of apoptosis, including chromatin condensation (a), cell shrinkage (b) and apoptotic body formation (c). (C) Flow cytometry analysis demonstrated that SSN or ISN denervation-induced significant apoptosis of Leydig cells 14 days and 21 days after operation, compared to their own sham-operation control (*P < 0.05 vs control). SSN- or ISN-denervated testes contained more early apoptotic and fewer late apoptotic Leydig cells on day 21 compared to day 14 (#P < 0.05 vs day 14). No significant difference of Leydig cell apoptosis was detected between the SSN and ISN denervation groups.
In previous studies, testicular innervation removal was shown to lead to the reduced number of testicular LH receptors, the block-age of hCG-stimulated testosterone production as well as the suppression of acute stress-induced elevation of serum testosterone [13,14,16]. The underlying mechanisms, however, remain largely unknown in past decades. Based on the results of the present study, these phenomena can be explained, at least in part, by Leydig cell apoptosis. As Leydig cells die via apoptosis after spermatic nerve removal, the capacity of the denervated testes to produce testosterone and the number of LH receptors localized on Leydig cells decrease consequently.
Although SSNs and ISNs are both the providers of testicular innervation, classical study assumes that the male gonad is innervated chiefly by SSNs [5]. However, our study has demonstrated that there was no significant difference of Leydig cell apoptosis between the ISN-denervated testes and the SSN-denervated ones (Fig. 1C), indicating that ISNs and SSNs are of equal importance for Leydig cell survival.
As SSNs are adjacent to the spermatic arteries, and the testes were ever pulled up into the abdominal cavity for ISN exposure, one may then attribute Leydig cell apoptosis observed in this study to testicular ischemia resulting from injuries to the spermatic arteries during our surgical procedures or potential experimental cryptorchidism. This possibility seems unlikely, because the two operated rats developing experimental cryptorchidism have been excluded from our study. In all the other rats, no apparent pathological changes took place in their testes until three weeks after denervation. Most importantly, a growing body of evidence has shown that high temperature or ischemia, as demonstrated in the undescended testes or those undergoing testicular torsion, results in massive apoptosis of spermatocytes and spermatids, instead of Leydig cells [26–29]. So, if experimental cryptorchidism or injuries to the spermatic arteries had happened in our surgery, remarkable apoptosis of spermatocytes and spermatids should have occurred simultaneously in the denervated testes. In our study, however, dramatic apoptosis of these two cell types was not observed (Fig. 1A). These results suggest that perturbation to testicular blood supply and temperature due to the surgical procedures is, if present, negligible and also, it can be concluded that disorders of intratesticular blood flow and/or temperature caused by testicular denervation itself are slight.
Testicular denervation results in decrease of serum T level and increase of plasma LH concentration, with serum FSH level unaffected
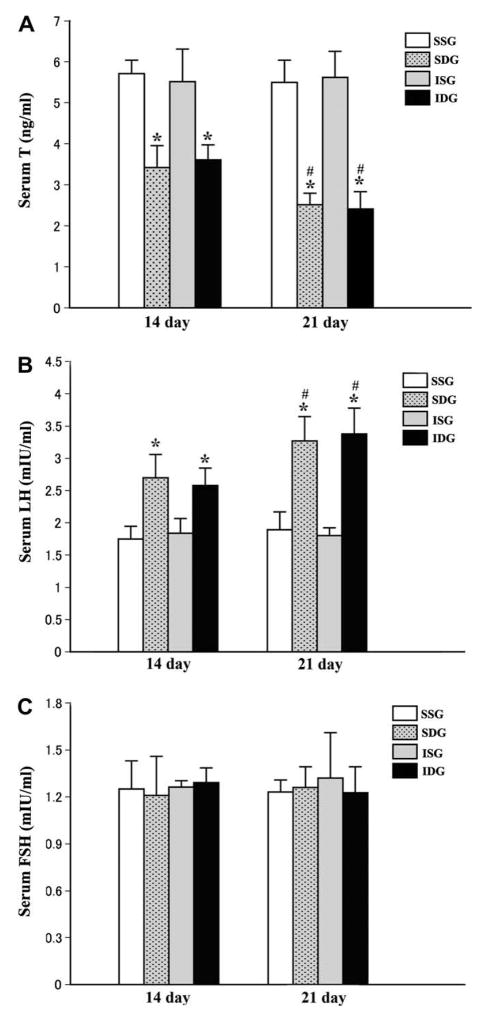
Parallel to apoptosis of Leydig cells, plasma testosterone levels in the SSN- or ISN-denervated rats dramatically decreased on day 14 and declined further on day 21 (Fig. 2A). In clinics, significant decrease of testosterone levels is also observed in the patients undergoing spinal cord injury who often have elevated serum LH levels [30,31]. Although some hypotheses have been proposed to explain this clinical phenomena, the underlying mechanisms remain unclear. Based on the present study, Leydig cell apoptosis resulting from disrupt of neuronal input to the testes of these patients seems to be one of the leading causes.
Fig. 2.
Serum T, LH and FSH levels after testicular denervation. (A) Plasma T concentrations in the SSN- or ISN-denervated rats dramatically decreased 14 days after surgery compared to their own sham-operation control (*P < 0.05 vs control), and declined further on day 21 (#P < 0.05 vs day 14). (B) Serum LH levels underwent a progressive rise after testicular denervation (*P < 0.05 vs control; #P < 0.05 vs day 14). (C) Serum FSH levels remained unchanged after operation.
It is known that LH secretion is regulated predominantly by serum testosterone via its negative feedback to the pituitary. With serumandrogen concentration dropping markedly, a progressive rise of plasma LH levels was noticed in all the denervated rats on day 14 and 21 (Fig. 2B). The levels of FSH, another gonadotropin secreted by the pituitary, however, remained unchanged following denervation (Fig. 2C), indicating that testicular denervation has no direct effect on the pituitary gonadotropin secretion. This also lends further support to the latest findings that the neuronal circuit between the brain and the testis acts independently from the pituitary [18].
Testicular denervation induces Leydig cell apoptosis via activating caspase-8-not caspase-9-dependent pathway
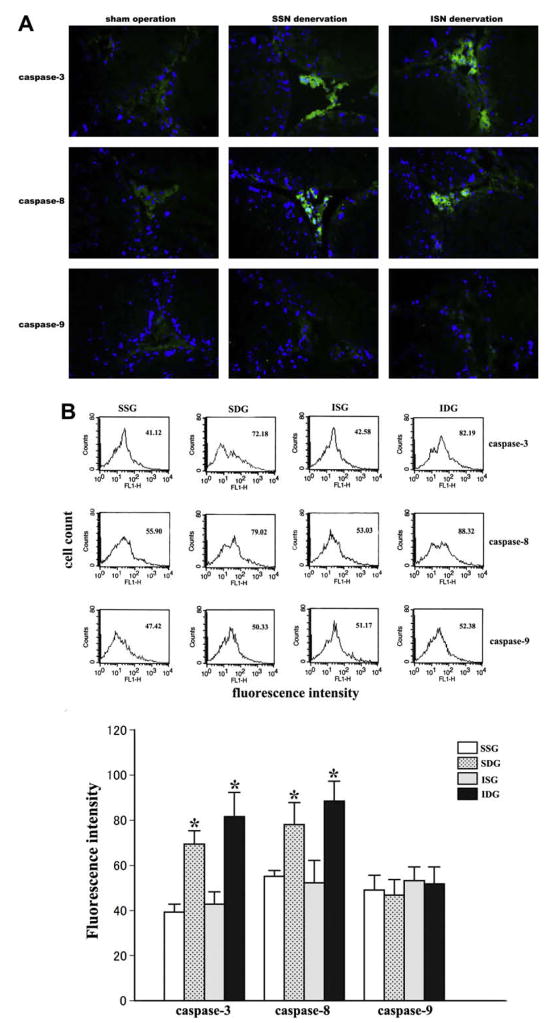
Caspases have been identified as center mediators of apoptosis, exerting their effects in a cascade involving both death receptor-dependent (extrinsic) and mitochondria-dependent (intrinsic) pathways. Caspase-8 and -9 are the initiators of these two pathways, respectively, and induce apoptosis commonly via activating caspase-3, a down-stream effector caspase [32,33]. In this study, immunofluorescent staining and flow cytometry analysis showed that the expression and the activity of caspase-3 in Leydig cells dramatically increased after SSN or ISN removal (Fig. 3), indicating that testicular denervation triggers Leydig cell apoptosis through activating caspase-dependent pathways. Further analysis demonstrated significantly increased expression and activity of caspase-8 not caspase-9 in Leydig cells after testicular denervation (Fig. 3), suggesting that Leydig cell apoptosis induced by testicular denervation is mediated by the death receptor-dependent pathway instead of the mitochondria-dependent one.
Fig. 3.
Expression and activities of caspases in Leydig cells after testicular denervation on day 21. (A) Representative merged pictures of Hoechst (blue) and caspase-3, -8, -9 (green) double staining. On day 21, the expression of caspase-3 and caspase-8 was significantly elevated in Leydig cells after denervation of SSNs or ISNs, but caspase-9 expression was not affected (magnification, 400×). (B) Flow cytometry showed that after SSN or ISN cutting, caspase-3 and caspase-8 activities increased significantly compared to their own sham-operation control (*P < 0.05 vs control), but the activity of caspase-9 remained unchanged. (For interpretation of color mentioned in this figure legend the reader is referred to the web version of the article.)
In conclusion, the present study has showed that the deprival of testicular innervation results in significant apoptosis of Leydig cells, followed by the decrease of serum T level and the increase of plasma LH concentration. Apparently, testicular denervation induces Leydig cell apoptosis through activating the caspase-8-dependent pathway. These findings may have important implications in deepening understanding male reproductive physiology and clinical conditions, such as spinal cord injury, in which impaired steroidogenesis in Leydig cells takes place due to disrupt of the neuronal input to the male gonad.
Acknowledgments
This work was supported by the grant from National Natural Science Foundation of China (30400161) and in part by NIH R01 HD050570 and R01 AG030598.
References
- 1.Schleich F, Legros JJ. Effects of androgen substitution on lipid profile in the adult and aging hypogonadal male. Eur J Endocrinol. 2004;151:415–424. doi: 10.1530/eje.0.1510415. [DOI] [PubMed] [Google Scholar]
- 2.Gao W, Bohl CE, Dalton JT. Chemistry and structural biology of androgen receptor. Chem Rev. 2005;105:3352–3370. doi: 10.1021/cr020456u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huhtaniemi I, Toppari J. Endocrine, paracrine and autocrine regulation of testicular steroidogenesis. Adv Exp Med Biol. 1995;377:33–54. doi: 10.1007/978-1-4899-0952-7_3. [DOI] [PubMed] [Google Scholar]
- 4.Zirkin BR, Chen H. Regulation of Leydig cell steroidogenic function during aging. Biol Reprod. 2000;63:977–981. doi: 10.1095/biolreprod63.4.977. [DOI] [PubMed] [Google Scholar]
- 5.Kuntz A, Morris RE. Components and distribution of the spermatic nerves and the nerves of the vas deferens. J Comp Neurol. 1946;85:33–44. doi: 10.1002/cne.900850104. [DOI] [PubMed] [Google Scholar]
- 6.Setchell BP, Maddocks S, Brooks DE. The Physiology of Reproduction. 2. Raven Press; New York: 1994. Anatomy, vasculature, innervation, and fluids of the male reproductive tract. [Google Scholar]
- 7.Zhu BC, Chiocchio SR, Suburo AM, Tramezzani JH. Monoaminergic and peptidergic contributions of the superior and the inferior spermatic nerves to the innervation of the testis in the rat. J Androl. 1995;16:248–258. [PubMed] [Google Scholar]
- 8.Suburo AM, Chiocchio SR, Cantó Soler MV, Nieponice A, Tramezzani JH. Peptidergic innervation of blood vessels and interstitial cells in the testis of the cat. J Androl. 2002;23:121–134. doi: 10.1002/j.1939-4640.2002.tb02605.x. [DOI] [PubMed] [Google Scholar]
- 9.Tainio H. Peptidergic innervation of the human testis and epididymis. Acta Histochem. 1994;96:415–420. doi: 10.1016/S0065-1281(11)80028-0. [DOI] [PubMed] [Google Scholar]
- 10.Wrobel KH, Moustafa MN. On the innervation of the donkey testis. Ann Anat. 2000;82:13–22. doi: 10.1016/S0940-9602(00)80116-8. [DOI] [PubMed] [Google Scholar]
- 11.Anakwe OO, Murphy PR, Moger WH. Characterization of beta-adrenergic binding sites on rodent Leydig cells. Biol Reprod. 1985;33:815–826. doi: 10.1095/biolreprod33.4.815. [DOI] [PubMed] [Google Scholar]
- 12.Mayerhofer A, Steger RW, Gow G, Bartke A. Catecholamines stimulate testicular testosterone release of the immature golden hamster via interaction with alpha-and beta-adrenergic receptors. Acta Endocrinol. 1992;127:526–530. doi: 10.1530/acta.0.1270526. [DOI] [PubMed] [Google Scholar]
- 13.Campos MB, Chiocchio SR, Calandra RS, Ritta MN. Effect of bilateral denervation of the immature rat testis on testicular gonadotropin receptors and in vitro androgen production. Neuroendocrinology. 1993;57:189–194. doi: 10.1159/000126359. [DOI] [PubMed] [Google Scholar]
- 14.Frankel AI, Ryan EL. Testicular innervation is necessary for the response of plasma testosterone levels to acute stress. Biol Reprod. 1981;124:491–495. doi: 10.1095/biolreprod24.3.491. [DOI] [PubMed] [Google Scholar]
- 15.Chiocchio SR, Suburo AM, Vladucic E, Zhu BC, Charreau E, Décima EE, Tramezzani JH. Differential effects of superior and inferior spermatic nerves on testosterone secretion and spermatic blood flow in cats. Endocrinology. 1999;140:1036–1043. doi: 10.1210/endo.140.3.6569. [DOI] [PubMed] [Google Scholar]
- 16.Frankel AI, Mock EJ, Chapman JC. Hypophysectomy and hemivasectomy can inhibit the testicular hemicastration response of the mature rat. Biol Reprod. 1984;30:804–808. doi: 10.1095/biolreprod30.4.804. [DOI] [PubMed] [Google Scholar]
- 17.Lee S, Miselis R, Rivier C. Anatomical and functional evidence for a neural hypothalamic–testicular pathway that is independent of the pituitary. Endocrinology. 2002;143:4447–4454. doi: 10.1210/en.2002-220392. [DOI] [PubMed] [Google Scholar]
- 18.Selvage DJ, Rivier C. Importance of the paraventricular nucleus of the hypothalamus as a component of a neural pathway between the brain and the testes that modulates testosterone secretion independently of the pituitary. Endocrinology. 2003;144:594–598. doi: 10.1210/en.2002-220781. [DOI] [PubMed] [Google Scholar]
- 19.Selvage DJ, Parsons L, Rivier C. Role played by brainstem neurons in regulating testosterone secretion via a direct neural pathway between the hypothalamus and the testes. Endocrinology. 2006;147:3070–3075. doi: 10.1210/en.2005-1358. [DOI] [PubMed] [Google Scholar]
- 20.Kim JM, Luo L, Zirkin BR. Caspase-3 activation is required for Leydig cell apoptosis induced by ethane dimethanesulfonate. Endocrinology. 2000;141:1846–1853. doi: 10.1210/endo.141.5.7444. [DOI] [PubMed] [Google Scholar]
- 21.Paula-Lopes FF, Hansen PJ. Heat shock-induced apoptosis in preimplantation bovine embryos is a developmentally regulated phenomenon. Biol Reprod. 2002;66:1169–1177. doi: 10.1093/biolreprod/66.4.1169. [DOI] [PubMed] [Google Scholar]
- 22.Chiloeches A, Sánchez-Pacheco A, Gil-Araujo B, Aranda A, Lasa M. Thyroid hormone-mediated activation of the ERK/dual specificity phosphatase 1 pathway augments the apoptosis of GH4C1 cells by down-regulating nuclear factor-kappaB activity. Mol Endocrinol. 2008;22:2466–2480. doi: 10.1210/me.2008-0107. [DOI] [PubMed] [Google Scholar]
- 23.Ueda EK, Lo HL, Bartolini P, Walker AM. S179D prolactin primarily uses the extrinsic pathway and mitogen-activated protein kinase signaling to induce apoptosis in human endothelial cells. Endocrinology. 2006;147:4627–4637. doi: 10.1210/en.2006-0348. [DOI] [PubMed] [Google Scholar]
- 24.Bantel H, Ruck P, Gregor M, Schulze-Osthoff K. Detection of elevated caspase activation and early apoptosis in liver diseases. Eur J Cell Biol. 2001;80:230–239. doi: 10.1078/0171-9335-00154. [DOI] [PubMed] [Google Scholar]
- 25.Telford WG, Komoriya A, Packard BZ. Detection of localized caspase activity in early apoptotic cells by laser scanning cytometry. Cytometry. 2002;47:81–88. doi: 10.1002/cyto.10052. [DOI] [PubMed] [Google Scholar]
- 26.Ishikawa T, Kondo Y, Goda K, Fujisawa M. Overexpression of endothelial nitric oxide synthase in transgenic mice accelerates testicular germ cell apoptosis induced by experimental cryptorchidism. J Androl. 2005;26:281–288. doi: 10.1002/j.1939-4640.2005.tb01096.x. [DOI] [PubMed] [Google Scholar]
- 27.Wang ZQ, Todani T, Watanabe Y, Toki A, Ogura K, Miyamoto O, Toyoshima T, Itano T. Germ-cell degeneration in experimental unilateral cryptorchidism role of apoptosis. Pediatr Surg Int. 1998;14:9–13. 35. doi: 10.1007/s003830050424. [DOI] [PubMed] [Google Scholar]
- 28.Lysiak JJ, Turner SD, Turner TT. Molecular pathway of germ cell apoptosis following ischemia/reperfusion of the rat testis. Biol Reprod. 2000;63:1465–1472. doi: 10.1095/biolreprod63.5.1465. [DOI] [PubMed] [Google Scholar]
- 29.Turner TT, Tung KS, Tomomasa H, Wilson LW. Acute testicular ischemia results in germ cell-specific apoptosis in the rat. Biol Reprod. 1997;57:1267–1274. doi: 10.1095/biolreprod57.6.1267. [DOI] [PubMed] [Google Scholar]
- 30.Clark MJ, Schopp LH, Mazurek MO, Zaniletti I, Lammy AB, Martin TA, Thomas FP, Acuff ME. Testosterone levels among men with spinal cord injury relationship between time since injury and laboratory values. Am J Phys Med Rehabil. 2008;87:758–767. doi: 10.1097/PHM.0b013e3181837f4f. [DOI] [PubMed] [Google Scholar]
- 31.Celik B, Sahin A, Caglar N, Erhan B, Gunduz B, Gultekin O, Karabulut M. Sex hormone levels and functional outcomes a controlled study of patients with spinal cord injury compared with healthy subjects. Am J Phys Med Rehabil. 2007;86:784–790. doi: 10.1097/PHM.0b013e318151fa70. [DOI] [PubMed] [Google Scholar]
- 32.Li J, Yuan J. Caspases in apoptosis and beyond. Oncogene. 2008;20:6194–6206. doi: 10.1038/onc.2008.297. [DOI] [PubMed] [Google Scholar]
- 33.Salvesen GS, Riedl SJ. Caspase mechanisms. Adv Exp Med Biol. 2008;615:13–23. doi: 10.1007/978-1-4020-6554-5_2. [DOI] [PubMed] [Google Scholar]