Abstract
This study investigated whether the retention interval after an aversive learning experience influences the return of fear after extinction training. After fear conditioning, participants underwent extinction training either 5 min or 1 day later and in either the same room (same context) or a different room (context shift). The next day, conditioned fear was tested in the original room. When extinction took place immediately, fear renewal was robust and prolonged for context-shift participants, and spontaneous recovery was observed in the same-context participants. Delayed extinction, by contrast, yielded a brief form of fear renewal that reextinguished within the testing session for context-shift participants, and there was no spontaneous recovery in the same-context participants. The authors conclude that the passage of time allows for memory consolidation processes to promote the formation of distinct yet flexible emotional memory traces that confer an ability to recall extinction, even in an alternate context, and minimize the return of fear. Furthermore, immediate extinction can yield spontaneous recovery and prolong fear renewal. These findings have potential implications for ameliorating fear relapse in anxiety disorders.
Keywords: fear renewal, spontaneous recovery, memory consolidation, delayed extinction, immediate extinction
Determining how to minimize the return of fear after therapeutic intervention is important for the effective treatment of anxiety disorders. Here we investigate how the timing of extinction training (akin to therapeutic intervention in clinical populations) after acquiring a specific fear affects subsequent return of the extinguished fear in healthy humans. The context, or constellation of environmental cues that are the setting for an aversive experience, often disrupts the ability to maintain fear extinction when reencountered after successful extinction training or therapeutic intervention. Such context-dependent return of fear is observed, for instance, when there is a context shift from extinction training to a subsequent extinction retest, a procedure called fear renewal (Bouton, 2004). Extinguished fear can also return over time in the absence of an overt context change, a phenomenon known as spontaneous recovery (Bouton, 1993; Bouton, Westbrook, Corcoran, & Maren, 2006; Rescorla, 2004). Recent rodent and human studies have reported conflicting evidence regarding the most beneficial time for intervention to occur after an aversive experience to prevent the return of extinguished fear (Alvarez, Johnson, & Grillon, 2007; Everly & Mitchell, 1999; Gray & Litz, 2005; Myers, Ressler, & Davis, 2006; Norrholm et al., 2008; Schiller et al., 2008). The goal of this study was to bridge the experimental and theoretical literature on fear return in rodents and humans within the framework of memory consolidation, which is assumed to occur during the passage of time after a learning experience (McGaugh, 2000), to better understand the mechanisms that contribute to the maintenance of fear extinction. This study uniquely contributes to the current debate by manipulating both time and context as independent variables while measuring skin conductance response (SCR) in a human fear renewal paradigm.
Fear renewal can be studied in the laboratory in both humans and nonhuman animals with adaptation of a classical conditioning paradigm. After acquiring fear to a conditioned stimulus (CS) that reliably predicts an aversive reinforcer (unconditioned stimulus, or US), the reinforcer is removed and the fear response to the CS extinguishes. Subjects who experience a context shift after extinction training exhibit an increase in fear to the CS relative to subjects who remain in the extinction context (Alvarez et al., 2007; Bouton et al., 2006; Milad, Orr, Pitman, & Rauch, 2005; Vansteenwegen et al., 2005). The learning that takes place during extinction training is hence context bound, which enables the latent fear memories to be expressed when subjects confront conditioned reminders of the original fear experience outside of the context of extinction (Bouton, 2004; Corcoran & Maren, 2004; Myers et al., 2006; Schmajuk, Larrauri, & LaBar, 2007). Moreover, when fear acquisition, extinction, and recovery testing are all experienced in the same context, spontaneous recovery of fear also emerges under some circumstances (e.g., Corcoran & Maren, 2004; Rescorla, 2004; Schiller et al., 2008). Thus, by comparing the amount of fear recovery in participants who experienced context shifts with that of those who remained in the same context, measures of fear renewal, spontaneous recovery, and extinction maintenance can be extracted.
Since Pavlov’s original observations of spontaneous recovery (Pavlov, 1927), it has long been understood that extinction training does not “erase” the original CS–US association, but rather yields additional plastic changes in frontolimbic circuits to suppress the expression of conditioned emotional responses that are no longer appropriate (Maren & Quirk, 2004; Sotres-Bayon, Bush, & LeDoux, 2004). However, the notion that extinction does not cause fear erasure was recently challenged by Myers et al. (2006), who reported that fear renewal in rodents was abolished when extinction training immediately followed fear learning without a delay. This result was interpreted to suggest that immediate extinction blocks memory consolidation of the original fear episode, resulting in erasure of the memory trace via disruption of synaptic processing (Cain, Godsil, Jami, & Baradt, 2005; Myers et al., 2006). One inference that may be drawn from this study is that immediate extinction has the best therapeutic outcome for treating anxiety disorders. For example, very early “debriefings” that consist of recalling trauma-related cues in a safe environment can ameliorate the long-term psychological effects of the trauma (Campfield & Hills, 2001; Everly & Mitchell, 1999). Yet, immediate therapeutic intervention after a trauma is not successful in other studies, and some researchers have advocated delaying behavioral treatment therapy until trauma-related stress has dissipated (Gray & Litz, 2005; McNally, Bryant, & Ehlers, 2003; Rothbaum & Davis, 2003). Consistent with these latter clinical reports, several recent rodent studies have demonstrated that immediate extinction prevents the long-term maintenance of fear extinction, yields spontaneous recovery and reinstatement of fear, and does not ameliorate the renewal of appetitive conditioning (Maren & Chang, 2006; Schiller et al., 2008; Woods & Bouton, 2008). Moreover, a recent study using acoustic startle in humans compared how immediate (10-min delay) and delayed (72-hr delay) extinction influenced spontaneous recovery of fear (Norrholm et al., 2008). Norrholm et al. (2008) reported that immediate extinction weakens spontaneous recovery of acoustic startle in a single-cue fear paradigm, but not if contextual conditioning is controlled for, and that expectancy measures indicate a return of fear across groups. With a differential fear conditioning procedure, however, they did report reduced spontaneous recovery of acoustic startle in the immediate extinction participants. In sum, the findings do not fully support Myers et al.’s (2006) rodent findings, which were interpreted as indicating fear erasure after immediate extinction. Nonetheless, the phenomenon of conditioned fear renewal as a function of the acquisition-to-extinction time interval has not yet been parametrically examined in humans using distinct physical context manipulations, nor has fear renewal been directly compared with spontaneous recovery. Given the theoretical and clinical importance of characterizing fear return that is often mediated by contextual cues (Bouton, 2000; Bouton et al., 2006), it is important to further examine this issue.
Learning theorists have postulated that ambiguous contextual cues or temporal weighting of competing associations (Bouton, 2002; Devenport, 1998) may contribute to fear recovery effects. However, a complementary interpretation takes into consideration the role of memory consolidation. Specifically, fear conditioning and extinction training induce neurobiological cascades that require a period of several hours to stabilize the synaptic and cellular changes underlying learning, a process called synaptic memory consolidation (McGaugh, 2004; Myers & Davis, 2002; Santini et al., 2004; Schafe & LeDoux, 2000). Disruption of these molecular cascades with pharmacological manipulation immediately after fear conditioning, or just before or after extinction training, all have modulatory effects on the recall of fear extinction memories in rodents (Cai, Blundell, Han, Greene, & Powell, 2006; McGaugh & Roozendaal, 2002; Quirk, 2002; Roozendaal, 2002). For example, Cai et al. (2006) reported that injection of the stress hormone corticosterone in rats suppresses contextual fear memory recall immediately after a single reactivation trial and enhances fear memory suppression immediately after extinction. Corticosterone also augments contextual fear extinction, and its blockade before extinction yields fear renewal and spontaneous recovery, presumably by interfering with consolidation of contextual fear associations (Barrett & Gonzalez-Lima, 2004; Bohus & Lissak, 1968; Micheau, Destrade, & Soumireu-Mourat, 1982). These findings have implications for the treatment of anxiety disorders because it is possible to weaken the fear memory or augment the extinction memory with appropriate manipulations during the critical memory consolidation time period. It is unknown whether similar consolidation manipulations are effective in dampening fear renewal and spontaneous recovery in humans.
According to Bouton (1993; Bouton et al., 2006), spontaneous recovery can be thought of as being context bound because it occurs when the CS is reexperienced outside of the time frame in which extinction originally occurred, and thus the original fear association is recalled because of the novel temporal context. Of note, this theory predicts that the longer the interval between conditioning and extinction, the more likely it is that spontaneous recovery will occur, consistent with one study reporting recovery of fear occurring only in rats with a long (48-hr) acquisition-to-extinction interval (Myers, Ressler, & Davis, 2006). In contrast, other theorists have argued that spontaneous recovery is related to the recency of training (Devenport, 1998; Rescorla, 2004). According to Devenport’s (1998) temporal weighting model, the difference in memory strength between two competing associations diminishes as a function of their relative time in a hyperbolic manner. When fear memories and extinction memories are acquired close in time, they are approximately equally weighted compared with when they are separated in time. This model predicts the opposite of Bouton’s (1993; Bouton et al., 2006) temporal context argument: More spontaneous recovery takes place when there is little time between fear acquisition and extinction, which has been reported in rodents and pigeons in Pavlovian and instrumental conditioning (Rescorla, 2004). The experiments in this study further test the predictions of these competing theories in relation to spontaneous recovery in humans to understand how they may support or contrast with a memory consolidation framework.
The primary goal of this study was to determine how retention intervals interact with physical context shifts to alter the behavioral profile of fear return in healthy adults. By concurrently manipulating both the retention interval between fear acquisition and extinction and the physical context of extinction training in a fully crossed between-groups design, it is possible to determine their conjoint influence over fear expression. A secondary goal was to provide a more direct translational bridge between the animal research on fear renewal and the observations of fear relapse in clinical disorders. Although prior behavioral research in humans has shown fear renewal effects (Alvarez et al., 2007; Kalisch et al., 2006; Milad et al., 2005; Vansteenwegen et al., 2005), these studies differed from the animal literature in two important ways. First, only a small subset of contextual features have been altered, such as turning a room light on or off or presenting the CS in an alternate background scene on a standard computer screen. These unimodal sensory changes contrast with the global, physical context changes that take place in animal studies and are unlikely to involve similar neural mechanisms because the hippocampus preferentially encodes configural rather than elemental features of the environment (Rudy, Huff, & Matus-Amat, 2004; Rudy & O’Reilly, 1999). Furthermore, whereas most animal studies permit a 24-hr period of memory consolidation between each phase of training (acquisition, extinction, and renewal test), human studies have been conducted continuously within a single training session, which makes comparisons difficult and has less clinical relevance than spaced training.
Taking into account the memory consolidation findings and the behavioral literature on fear and extinction memory expression (Cai et al., 2006; McGaugh & Roozendaal, 2009; Quirk, 2002; Rescorla, 2004; Schiller et al., 2008), we predicted that extinction immediately after fear conditioning would have detrimental effects on long-term retention of extinction memory, resulting in a protracted form of fear renewal and robust spontaneous recovery. In contrast, when each phase of training is separated by 24 hr, consolidation of both the differential fear memory and the new extinction memory should take place, yielding a mild, transient renewal of fear and minimal spontaneous recovery (but see Norrholm et al., 2008). The outcome of this study should thus provide further insight into how putative memory processes interact with contextual cues and the passage of time to yield differing profiles of fear return.
Method
Participants
We recruited 66 young adults from the local Duke University community to participate (immediate extinction condition: n = 35 [17 women and 18 men], mean age (±SD) = 21.1 (±4.1); delayed extinction condition: n = 31 [14 women and 17 men], mean age (±SD) = 22.2 (±5.0). Participants completed a questionnaire assessing attitudes toward snakes and spiders (Klorman, Hastings, Weerts, Melamed, & Lang, 1974). Individuals scoring within 1 standard deviation of the mean of patients with specific phobia (Fredrikson, 1983) were excluded from participation. Additional exclusion criteria included current psychotropic medication use and self-reported history of psychiatric or neurological disorders, alcoholism, or substance abuse. Participants received either psychology course credit or were compensated at a rate of $10/hr. All participants provided written informed consent, and the experimental procedures were approved by the Duke University Institutional Review Board.
Stimuli and Task Design
Participants underwent a differential fear conditioning paradigm in which only one of two discrete images was partially reinforced with an aversive wrist shock (see Figure 1), as previously described (Zorawski, Blanding, Kuhn, & LaBar, 2006). Briefly, a picture of a snake and a picture of a spider obtained from the International Affective Picture System (Lang, Bradley & Cuthbert, 2001) served as the CSs; only one of these images (the CS+) was partially reinforced by the US (randomly assigned). Stimuli were presented centrally on a 17-in. computer screen. Biologically prepared stimuli were used as CSs to more closely model the specific fears found in phobic populations and thus render the study more relevant for bridging the findings to the treatment of anxiety disorders. The US was a mild electric shock to the wrist of the nondominant hand (200-ms duration, coterminating with the CS), which was set to be annoying but not painful for each participant using an ascending staircase procedure, beginning at 30 V (Zorawski et al., 2006). CSs were presented for 4 s, and the interstimulus interval was 11 ± 4 s.
Figure 1.
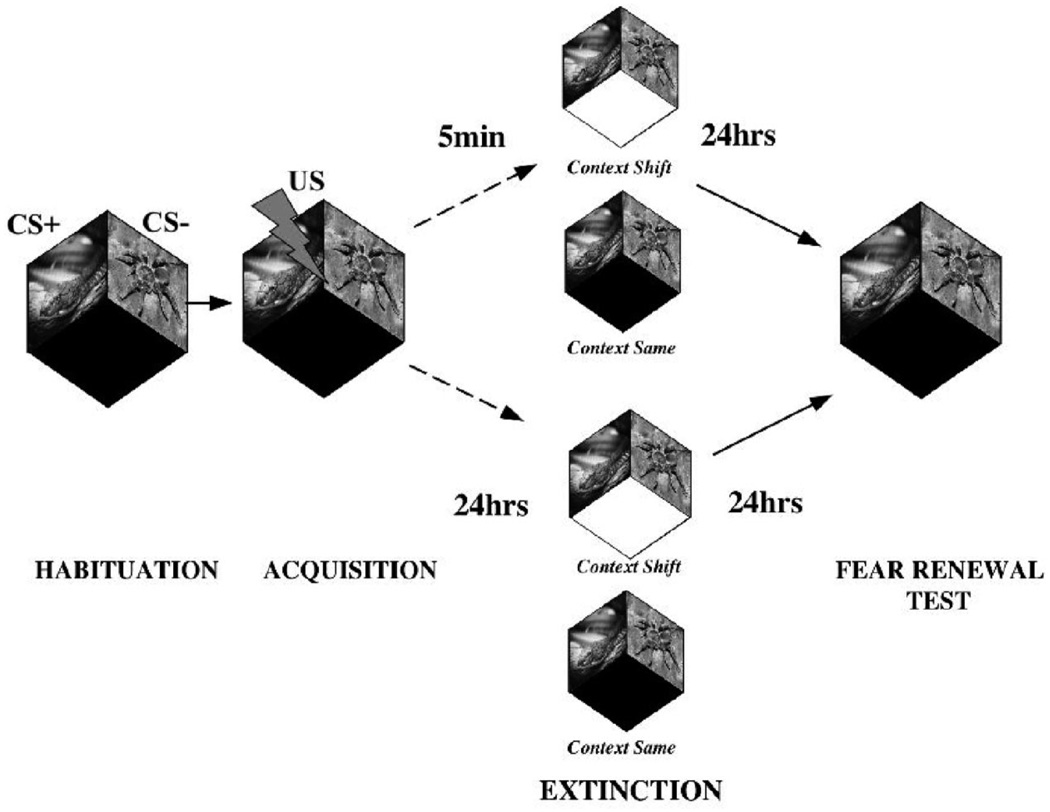
Experimental design and timeline. Participants were habituated to visual images of snakes and spiders (the conditioned stimuli [CSs]). During acquisition training, one stimulus (in this example, the snake) was partially reinforced (CS+) with a mild wrist shock (the unconditioned stimulus [US]), and the other stimulus (e.g., spider) was unreinforced (CS−). Next, participants received extinction training (unreinforced presentations of all CSs) either 5 min later (immediate condition) or 24 hr later (delayed condition). Extinction occurred in either the acquisition context (same context) or a novel context (context shift). The context manipulation and the temporal delay manipulation were fully crossed between participants to form four experimental groups. Twenty-four hours after extinction training, all participants returned to the acquisition context to test for renewal of fear to the CSs.
The experiment consisted of three phases: habituation–acquisition, extinction, and fear renewal test (see Figure 1). In the immediate extinction condition, fear conditioning and extinction were conducted on Day 1 and fear renewal testing took place on Day 2. In the delayed extinction condition, fear conditioning, extinction, and renewal testing were each separated by 24 hr. Orthogonal to the extinction delay manipulation, participants were also randomly assigned to either the same-context group or the context-shift group. The same-context group remained in the same physical context for all phases of the study. The context-shift group was placed in a novel context during extinction training and then placed back into the original training context during the renewal test. Context A was a standard physiological testing room with overhead fluorescent lighting, a small rectangular desk and chair, and a computer screen to view the stimuli. Context B was a slightly larger room that was transformed into a domestic setting. Dim blue lighting was provided by a faux antique silver lamp placed in the corner. A motorized waterfall, bowl of scented potpourri, and vase of dried eucalyptus were located on an L-shaped desk adjacent to the computer screen. Walls were decorated with artwork. Contexts were counterbalanced across participants.
The habituation phase consisted of four nonreinforced trials of each CS type (snake or spider). The acquisition phase consisted of 16 trials of each CS type. Five of the 16 CS+ trials were reinforced by cotermination with the US, whereas CS− trials were always unreinforced. The 11 nonreinforced CS+ trials were interspersed among the 5 reinforced CS+ trials. The extinction phase consisted of 16 nonreinforced presentations of each CS type. Fear renewal testing also consisted of 16 nonreinforced presentations of each CS type. Trials were pseudorandomized so that no more than 2 trials of each CS type were presented consecutively. Participants were unaware that they would not receive shocks during the habituation, extinction, or fear renewal sessions. At the end of the session, participants were verbally debriefed about the purpose of the experiment.
Partial reinforcement of the CS+ was used to delay the normally rapid extinction that occurs in human participants after 100% reinforcement (LaBar et al., 1998; Phelps et al., 2004). Partial reinforcement also provides a more lifelike contingency in which aversive events do not always occur after a feared stimulus. Such reinforcement schedules may make it more likely to evoke fear renewal when encountered in a novel context (Grillon, 2002), thus providing more opportunity to observe contextual control over fear expression to an ambiguous cue.
Task Instructions
Before each training phase, participants were informed that they would be shown pictures of a snake and a spider and that they might receive occasional electrical stimulation. They were instructed to classify each picture as a snake or a spider by pressing the number pad of the keyboard with their dominant hand.
Physiological Measurements
SCR was used as the dependent measure of conditioning, as described previously (LaBar, Cook, Torpey, & Welsh-Bohmer, 2004; LaBar, Gatenby, Gore, LeDoux, & Phelps, 1998). SCRs were monitored from the participants’ nondominant hand, using Ag–AgCl electrodes attached to the middle phalanges of the second and third digits by Velcro straps (BIOPAC Systems, Goleta, CA). A saline-based gel (Sigma Gel) was used as a conductive electrolyte. Skin conductance was monitored at 250 Hz and was stored offline for analysis, using AcqKnowledge software (BIOPAC Systems, Goleta, CA). The physiologic data were scored in response to the onset of each CS and US according to conventional criteria, as previously described (LaBar & Phelps, 2005; LaBar et al., 2004, 1998). For inclusion in the data analysis, the following criteria were established: SCR latency = 1–4 s, SCR duration = 0.5–5 s, and minimum SCR amplitude = 0.02 micro-siemens. Responses that did not meet these criteria were scored as zero. The CS–US interval used is sufficient to separate SCRs to the cues and reinforcers, as previously shown (e.g., LaBar et al., 2004; LaBar & Phelps, 2005).
Data Analysis
We averaged trial-by-trial changes in SCR into early (first half) and late (second half) blocks for each trial type (CS+ or CS−) and phase of the experiment before statistical analyses. Each of these blocks consisted of a mean of two trials per CS type for the habituation phase and a mean of eight trials per CS type (early or late) for the acquisition, extinction, and fear renewal phases. We hypothesized that learning-related changes would be found in the late acquisition phase, as had been reported previously (LaBar et al., 2004; LaBar, LeDoux, Spencer, & Phelps, 1995). Before statistical analysis, conditioned SCRs were square-root transformed to reduce skewness and were range corrected (LaBar et al., 2004).
To derive a dependent measure of fear acquisition, we compared SCR data from the late acquisition phase with those from the late habituation phase as a baseline for each CS type. Likewise, we initially measured extinction by comparing SCR data from the late acquisition phase with those from the late extinction phase, a between-session assessment. Fear renewal and spontaneous recovery were calculated as difference scores from late extinction as follows (see also Norrholm et al., 2008; Schiller et al., 2008): (Late Extinction – Late Acquisition) – (Early or Late Fear Renewal). Notably, with this approach we account for relative differences in responding at both late acquisition and late extinction because they can influence detection of fear return. Moreover, this calculation also provides us with a between-session extinction performance index, an important measure that is often discussed in the extinction literature (e.g., Corcoran & Quirk, 2007; Quirk & Meuller, 2008). We also performed statistical analyses (t tests) on the raw data points by CS type at early and late fear renewal tests as an additional demonstration of the basic findings. We also conducted t tests to confirm within-session extinction learning by comparing early and late extinction time points. Otherwise, we analyzed data by means of mixed repeated measures analyses of variance (ANOVAs) with context (same or shift) and extinction interval (immediate or 24-hr delayed) as between-groups factors and phase (e.g., late acquisition or late extinction) and CS type (CS+ or CS−) as within-subjects factors. Post hoc Bonferroni-corrected t tests were conducted as appropriate. Pearson correlations were conducted on differential SCR scores (CS+ minus CS−) to determine the relationship between extinction learning (reverse coded so that high values reflected low SCRs) and early or late fear renewal. We also calculated differential SCR scores as an index of learning by subtracting responses to the CS+ from those to the CS− across trial blocks. According to this measure, scores of zero reflect no differential learning, whereas differential scores above zero do (LaBar et al., 2004, 1995). We used this measure only in correlational analysis to attain a single data point that reflected the magnitude of extinction learning for each participant. The alpha level was set at .05 in all analyses.
Results
Acquisition of Differential Fear
An ANOVA conducted on fear acquisition revealed a significant main effect of CS type, F(1, 61) = 40.15, p < .001, reflecting stronger responding to the CS+ than to the CS− (see Figure 2). There was also a main effect of phase, which reflected higher SCRs overall during late acquisition than during both late habituation, F(1, 61) = 7.11, p < .02, and early acquisition, F(1, 61) = 5.97, p < .02. Finally, we observed a significant interaction of CS Type × Phase between early and late acquisition, F(1, 61) = 9.47, p < .01, which implies that differential fear learning to the CS+ emerged during late acquisition, as in prior reports (e.g., Zorawski et al., 2006). As anticipated, extinction interval or context manipulations showed no effects on fear acquisition because these manipulations took place after acquisition training.
Figure 2.
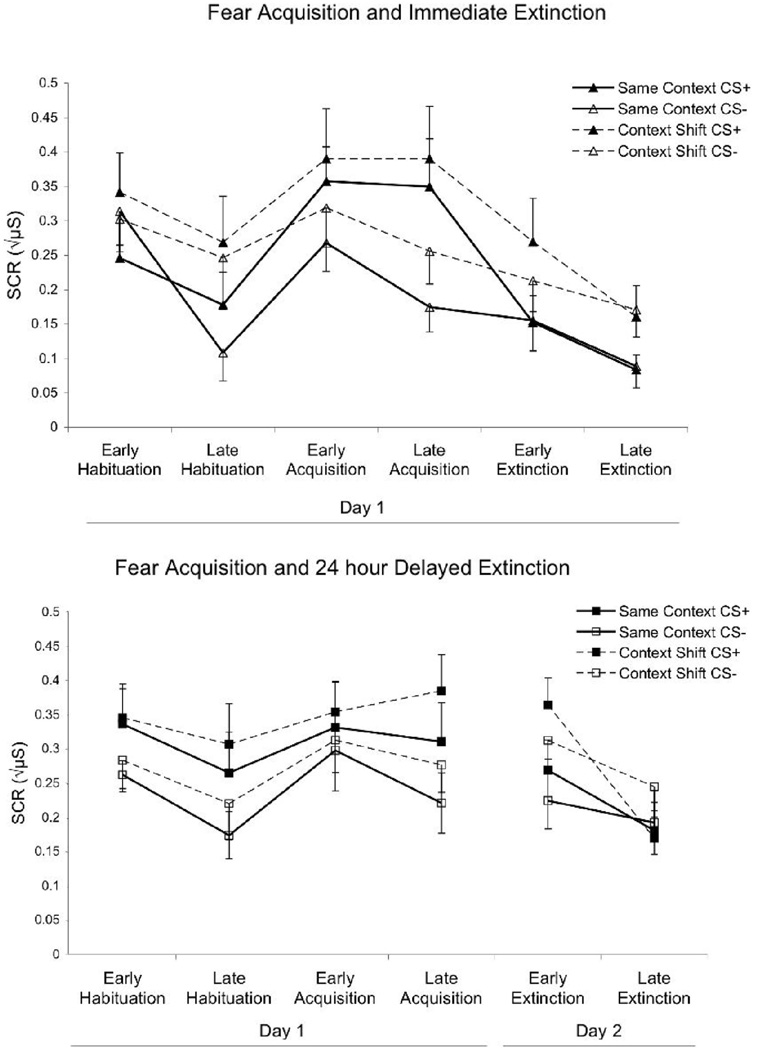
Acquisition and extinction of fear conditioning. Depicts skin conductance responses (SCRs) to the reinforced stimulus (CS+) and the unreinforced stimulus (CS−) at early and late training phases of habituation, fear acquisition and extinction (bars indicate M ± SEM). Top: Extinction was conducted 5 min after fear acquisition (immediate extinction). Bottom: Extinction was conducted approximately 24 hr after fear acquisition (delayed extinction). As demonstrated in both figures, there is greater differential learning (more responding to CS+ than CS−) during late acquisition compared with late habituation training, indicating successful fear conditioning in all participants. There is a reduction in differential fear response by the end of extinction training, indicating successful extinction to the CS+ in all participants.
Extinction of Fear
To determine between-session extinction learning, we compared SCR at late acquisition with that at late extinction. An ANOVA revealed a significant main effect of phase, F(1, 61) = 28.92, p < .001, and CS type, F(1, 61) = 21.60, p < .001, and a Phase × CS Type interaction, F(1, 61) = 23.56, p < .001. These results indicate that successful extinction took place overall, with a selective decrease in responding to the CS+ during late extinction.
We performed t tests on early and late extinction scores to assess within-session extinction performance between the immediate and delayed extinction groups. The t tests on early extinction revealed a significant difference in SCR between groups (Ns = 25 and 22 for immediate and delayed extinction, respectively) to CS+ and CS− ( ps = .012 and .015, respectively). However, at late extinction a t test found no significant difference in SCR to CS+ ( p = .09), although there was a difference in SCR to CS− ( p = .014). The results suggest that the immediate extinction group had more rapid extinction early on, but that by late extinction all participants had equivalently extinguished their SCR to the CS+.
Fear Renewal and Spontaneous Recovery
An ANOVA conducted on the fear renewal difference scores (reflecting between- session performance) revealed a main effect of extinction interval, F(1, 61) = 6.93, p < .01, highlighting overall higher levels of fear relapse after immediate extinction relative to delayed extinction (see Figure 3). Furthermore, an Phase × Extinction Interval interaction, F(1, 61) = 9.65, p < .01, indicates a more protracted time course of fear return after immediate extinction relative to delayed extinction. However, there was no interaction between extinction interval and context type ( p > .05) because in the immediate extinction condition both context-shift and same-context participants displayed an increase in SCR to the CS+ at fear renewal testing, whereas in the delayed extinction condition only the context-shift participants displayed an increase in SCR at fear renewal testing. Consistent with the traditional learning theory framework, the response of the immediate extinction context-shift participants is interpreted as fear renewal, whereas the response of same-context participants is interpreted as reflecting spontaneous recovery. Thus, regardless of physical context at extinction learning, both groups show a return of fear to the CS+ on Day 3 relative to SCR at late extinction on Day 2.
Figure 3.
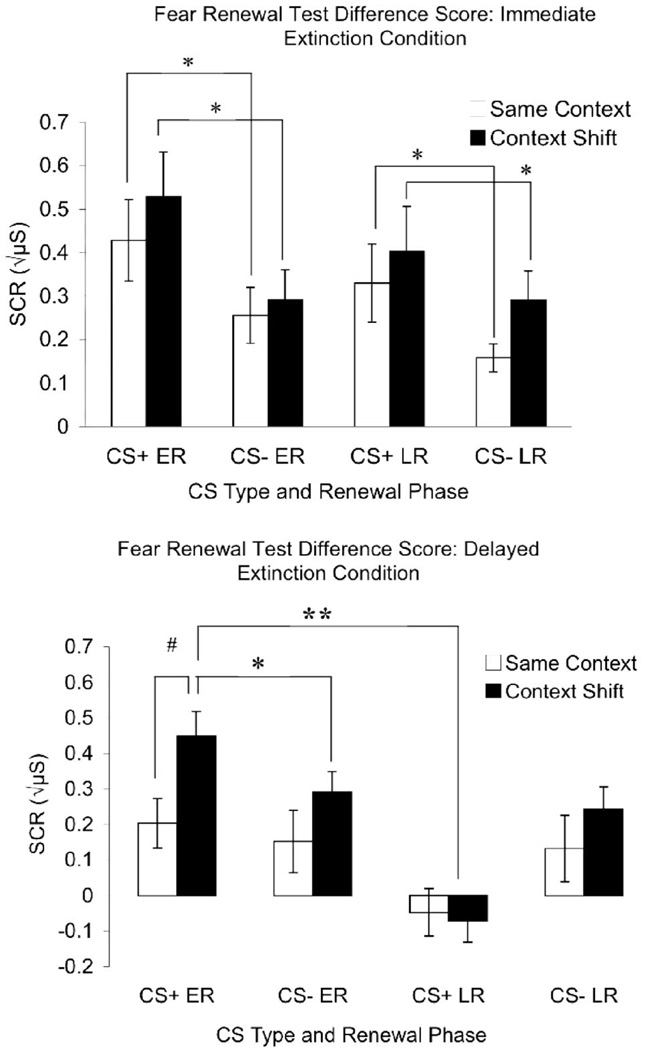
Fear renewal test difference scores. Bars indicate mean (±SEM) skin conductance responses (SCRs) to the previously reinforced stimulus (CS+) and the unreinforced stimulus (CS−). The SCRs represent return-of-fear difference scores that reflect between-session performance, (late extinction–late acquisition) – (early or late renewal). Top (immediate extinction): When extinction occurred immediately after acquisition, both the same-context and context-shift groups exhibited increased SCR to the CS+ at fear renewal test, as calculated by the fear renewal difference score. We interpret these effects as spontaneous recovery in same-context participants and fear renewal in context-shift participants. Bottom (delayed extinction): When extinction training was delayed, context-shift participants displayed enhanced SCR to the CS+ at early fear renewal as calculated by the fear renewal difference score. At late-phase fear renewal testing, this response was diminished (reextinguished) in the context-shift participants. After delayed extinction, the same-context group did not display enhanced SCR to CS+, as calculated by fear renewal difference score, suggesting maintained extinction. ER = early renewal difference score, LR = late renewal difference score. * p < .05 between CS+ and CS−. ** p < .05 between early and late test blocks. # p < .05 between same-context and context-shift groups.
There was also a main effect of CS type, F(1, 61) = 39.11, p < .001, indicating more fear return overall to the CS+ than to the CS−. The main effect of CS type was qualified, however, by three interactions that confirm the main predictions. First, fear return was more selective to the CS+ for the context-shift groups relative to the same-context groups, F(1, 61) = 4.94, p = .03. Second, fear return was stronger to the CS+ for the immediate extinction participants than for the delayed extinction participants, F(1, 61) = 7.71, p < .007, in part because of its persistence. Finally, when collapsed across groups, fear return was strongest to the CS+ during the early phase of testing than it was later on, F(1, 61) = 9.28, p = .003.
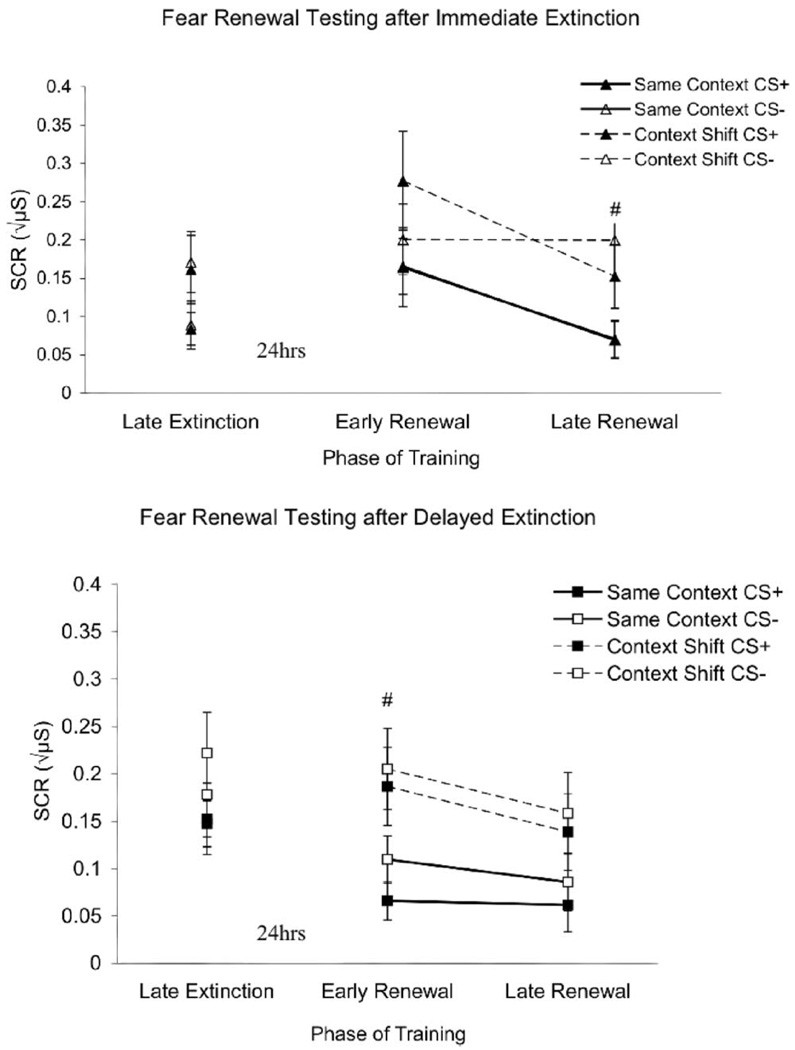
The t tests conducted on the raw data points at early and late fear renewal further confirmed the difference score results reported earlier (see Figure 4). The t tests comparing SCR at early fear renewal test for immediate extinction participants (n = 25) revealed no differences to CS+ ( p = .07) or CS− ( p = .30). We interpret this to reflect renewal of fear in context-shift participants and spontaneous recovery in same-context participants. At late fear renewal testing of immediate extinction participants, t tests revealed a significant difference in SCR to CS+ ( p = .05) and CS− ( p = .013) because fear renewal was prolonged in the context-shift participants only, whereas fear renewal was reextinguished in the same-context participants. In contrast, t tests for delayed extinction participants (n = 22) at early renewal testing report differences in SCR to CS+ ( p = .028), but not to CS− ( p = .07), reflecting fear renewal to CS+ only in context-shift participants and maintained extinction in same-context participants. At late fear renewal testing, t tests on delayed extinction performance found no differences in SCR to CS+ or CS− ( ps = .18 and .20, respectively) because both displayed extinction processes.
Figure 4.
Fear renewal test primary data points. Bars indicate mean (±SEM) skin conductance responses (SCRs) to the previously reinforced stimulus (CS+) and the unreinforced stimulus (CS−) at late extinction and early and late fear renewal testing. Top (immediate extinction): When extinction occurred immediately after acquisition, fear renewal and spontaneous recovery were displayed at early fear renewal testing (early renewal) as evidenced by enhanced SCR to CS+ in context-shift and same-context participants. Elevated SCR to CS+ and CS− was maintained at late fear renewal testing (late renewal) in context-shift participants. Bottom (delayed extinction): When extinction training was delayed, context-shift participants displayed fear renewal with elevated SCR to CS+ at early fear renewal testing (early renewal). At late fear renewal testing, both context-shift and same-context participants displayed a decrease in SCR to CS+, reflecting extinction processes. # p < .05 between same-context and context-shift groups.
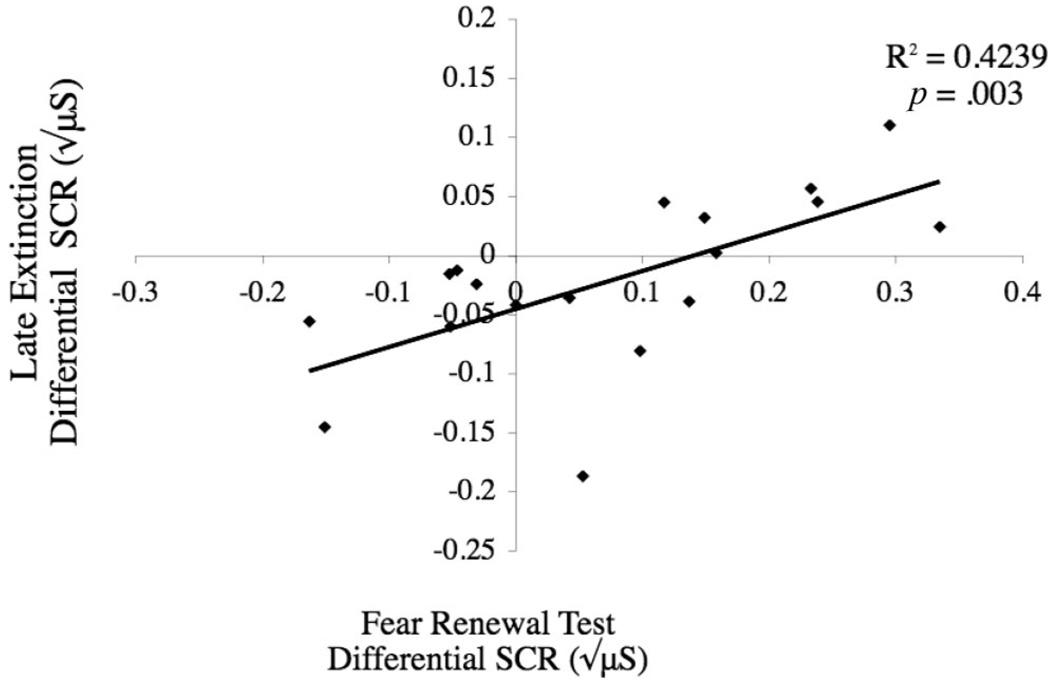
We computed Pearson correlation coefficients to further investigate the relationship between fear return and the strength of extinction learning across individual participants. In particular, it was important to rule out the possibility that fear return was because of incomplete extinction. For these analyses, we averaged the raw differential SCR (CS+ minus CS−) across all fear renewal trials and correlated it with the raw differential SCR during late extinction expressed as an inverted score (so that higher values indicate strong extinction learning; see Figure 5). In the delayed extinction condition, there was a significant positive correlation between differential fear renewal and extinction magnitude in the context-shift group, r(17) = .65, p = .003, but not in the same-context group, r(13) = .37, p = .20. These results further suggest that after delayed extinction, fear renewal is not the result of incomplete extinction and is not generalized across CSs, which would have yielded a negative correlation. By contrast, in the immediate extinction condition, there was no systematic relation-ship between differential fear renewal and the magnitude of extinction learning for either the context-shift or same-context groups, r(17) = .23, p = .36, and r(18) = .12, p = .65, respectively. Taken together, the main findings provide evidence that a 24-hr retention interval between acquisition and extinction training (a) reduces the duration and strength of fear renewal, (b) attenuates spontaneous recovery, and (c) creates a tighter coupling between the strength of the extinction memory and return of fear when the conditioned stimuli are subsequently reencountered in the original acquisition context.
Figure 5.
The relationship between late extinction responses and fear relapse. Skin conductance responses (SCRs) are expressed as a differential score (CS+ minus CS−) for the late extinction phase and the relapse testing phase. Extinction data are reverse coded so that higher numbers indicate more extinction. Individual differences in extinction magnitude predicted later fear renewal, but only for participants who experienced a context shift after a 24-hr delay (nonsignificant data from the other groups not shown).
Discussion
In this study, when the interval between the initial fear acquisition and subsequent extinction training session was minimal, both the context-shift and same-context participants displayed long-lasting and robust responses to the CS+ 24 hr after extinction learning. In this case, it may be that spontaneous recovery is masking the renewal effect because no context effects are statistically reported. However, when 24 hr intervened between acquisition and extinction training, fear renewal levels were lower and reextinguished rapidly within the testing session in the context-shift group. Furthermore, participants who remained in the same context displayed little fear (i.e., they maintained extinction) to the CS+. Thus, delayed extinction does not appear to yield spontaneous recovery to the CS+, whereas immediate extinction does, and delayed extinction elicits an attenuated and transient form of fear renewal. We interpret these findings as reflecting alterations in memory consolidation processes, but without a direct biological assay of memory consolidation this remains an assumption, and therefore the most parsimonious explanation is that manipulation of the passage of time after fear conditioning and before extinction influences the return of fear. Specifically, delaying extinction after fear conditioning appears to be most effective in minimizing fear renewal and spontaneous recovery.
In addition, the participants given delayed extinction training showed a positive correlation between extinction learning and return of fear. In other words, those individuals who extinguished the most later showed a CS+-specific (but transient) fear renewal when placed back in the original context. This novel result argues against the possibility that fear renewal was related to inadequate within-session extinction in the delayed extinction participants. Although it may seem counterintuitive that extinction strength predicts later renewal, as discussed earlier this form of fear renewal is transient, specific to the CS+ in the original context, and lower in magnitude than with immediate extinction. In this sense, it can be considered an attenuated (or more adaptive) return of fear. Indeed, it appears that both the fear and extinction memories are intact after spaced training and that the behavioral response is mediated by context memory (Bouton, 2004; Ji & Maren, 2007). It is likely that with repeated extinction training over a spaced interval, expression of fear renewal may diminish altogether. Regardless, these findings present the strongest human evidence to date that both the acquisition and extinction memories are available and compete for expression, supporting Pavlov’s (1927) original idea, later echoed by others (Bouton, 2004; Konorski, 1948; Pearce & Hall, 1980; Wagner, 1981), that extinction does not destroy the original conditioned association.
Because spontaneous recovery occurred with a short acquisition-to-extinction interval but not a longer one, it is unlikely that the changing temporal context was the mechanism (Bouton, 1993) because this theory predicts the opposite result. However, our current finding is consistent with evidence from Rescorla (2004) in rodents and pigeons demonstrating that the magnitude of spontaneous recovery is greater with a shorter acquisition-to-extinction interval. These and the present findings are supported by the temporal weighting model (Devenport, 1998), which when applied here suggests that when fear acquisition and extinction memories are acquired at virtually the same time, they maintain nearly equal weight in guiding behavior. However, traces of the more recent extinction experience degrade more quickly in this case, such that recall is biased toward the initial fear memory, resulting in spontaneous recovery rather than extinction.
Theoretical models have proposed several psychological mechanisms to explain fear renewal and spontaneous recovery, such as the influence of contextual cues or the relative recency of memory acquisition (e.g., Bouton, 2004, 2000; Rescorla, 2004), whereas neurobiological work has suggested that memory consolidation processes at the molecular level interact with internal (e.g., stress hormones) and external (e.g., aversive experiences) factors to determine fear or extinction recall (Cai et al., 2006; Cain et al., 2005; McGaugh & Roozendaal, 2009; Myers & Davis, 2002). In this study, we crossed two different physical context manipulations with two acquisition-to-extinction retention intervals to investigate how memory consolidation parameters might influence the return of fear in healthy adults. Taken together, one can argue that the internal state of the organism at the time of extinction learning is critical to the recall of extinction or adaptive expression of fear (Barrett & Gonzalez-Lima, 2004; Bouton, 2000; Cai et al., 2006; McGaugh & Roozendaal, 2002). Future studies should address hormonal influences or other time-varying effects (mood state, arousal levels, or anxiety) on retention of extinction in humans.
Our goal in this study was to vary the acquisition-to-extinction interval while keeping constant the extinction-to-renewal test interval because we were primarily interested in examining consolidation of the fear acquisition and extinction episodes. Future studies should also vary the interval between extinction and renewal testing to determine effects on performance because there was a different temporal window between initial conditioning and renewal testing across groups. Rodent studies have investigated this issue using similar procedures and did not find time from conditioning to be the critical factor determining long-term extinction maintenance (Maren & Chang, 2006). In addition, Rescorla (2004) controlled for the conditioning-to-recovery test interval in rodents across instrumental and Pavlovian conditioning paradigms and still reported that the acquisition-to-extinction interval had an important effect on spontaneous recovery magnitude.
A controversial report by Myers et al. (2006) presented rodent findings that were interpreted as demonstrating that extinction training immediately after fear conditioning results in erasure of the fear memory. In the present study, there was no evidence of fear erasure after immediate extinction. Our results are consistent with another human study showing intact renewal of startle when extinction was given immediately after fear acquisition (Alvarez et al., 2007) and with a recent study demonstrating fear reinstatement and spontaneous recovery after immediate extinction in humans (Schiller et al., 2008), although one recent article has reported reduced spontaneous recovery of fear-potentiated acoustic startle after immediate extinction, but only in a differential cue paradigm (Norrholm et al., 2008). Additionally, other rodent studies have demonstrated that immediate extinction fails to yield long-term fear suppression (Maren & Chang, 2006). Thus, there is convergence in the data between animal and human models. However, this study is the first to directly compare immediate and delayed extinction training in humans with substantial contextual manipulations as a second critical independent variable and, in this regard, is most analogous to the rodent study by Myers et al. (2006). Closer inspection of the Myers et al. data suggest that the apparent erasure may have been the result of lack of extinction in the control subjects, which was remedied with a 72-hr delay, rather than of an increase in fear renewal in the context-shift group. Therefore, our study not only calls into question the conclusion that fear is erased with immediate extinction but also implies that extinction training is most effective when given after a delay.
Under some circumstances, the hippocampus mediates context-dependent fear renewal (Corcoran & Maren, 2001; Ji & Maren, 2005, 2007), likely through interactions with the amygdala and ventromedial prefrontal cortex (Hobin, Goosens, & Maren, 2003; Kalisch et al., 2006; Maren & Quirk, 2004; Sotres-Bayon, Bush, & LeDoux, 2004), whereas spontaneous recovery appears to involve a lack of coordinated cellular changes in the basolateral amygdala and prefrontal cortex that are necessary to express extinction in rodents (Herry & Mons, 2004). More important, in rodents lesions of prefrontal cortex or disruption of hippocampus during extinction learning can impair subsequent extinction recall without affecting within-session performance (LeBron, Milad, & Quirk, 2004; Farinelli, Deschaux, Hugues, Thevenet, & Garcia, 2006). Thus, from a neurobiological view, our findings of rapid within-session extinction but subsequent strong return of fear in the immediate extinction group may reflect impaired recall of extinction training resulting from disruption of memory consolidation processes within the prefrontal cortex–hippocampus–amygdala fear extinction circuitry specifically (Corcoran & Quirk, 2007; Maren & Quirk, 2004; Quirk & Meuller, 2008). Future studies with functional MRI may confirm this hypothesis.
This investigation is important for providing a more direct bridge from animal studies of fear renewal to clinical treatment. In anxiety, depression, and drug addiction, individuals are particularly prone to relapse when they return to settings that resemble those previously associated with stressful or drug-taking experiences. To model these effects and to be consistent with the extant animal literature, we implemented context shifts that involved physically moving individuals between different experimental testing rooms during extinction training. This manipulation was lacking in the human experimental studies to date, which have instead focused on changing single environmental features within a given physical context (Alvarez et al., 2007; Vansteenwegen et al., 2005; but see LaBar & Phelps, 2005; Schiller et al., 2008). Moreover, our two extinction contexts were made up of different configurations of multiple sensory cues in an attempt to elicit hippocampal-dependent contextual processing, as demonstrated in prior human (Kalisch et al., 2006; LaBar & Phelps, 2005) and rodent (e.g., Ji & Maren, 2005) studies.
In summary, a period of time that permits synaptic memory consolidation after an aversive experience might provide a more stable neurobiological state in which to acquire a new extinction memory and thus reduce the likelihood of fear return. Whether this paradigm would also serve to reduce the duration and magnitude of fear renewal in clinical populations remains to be determined. Nonetheless, these findings may help explain why some human fears return after efforts to suppress them, with implications for determining optimal therapeutic strategies to control unwanted fear memories in affective illness.
Acknowledgments
This work was supported in part by National Science Foundation CAREER award 0239614 to Kevin S. LaBar and National Institutes of Health Grants 2 P01 NS041328 to Kevin S. LaBar and F32 MH078471 to Nicole C. Huff. We thank Nestor Schmajuk for helpful comments on the data reported in the article and Matthew Fecteau for assistance with running participants.
References
- Alvarez RP, Johnson L, Grillon C. Contextual-specificity of short-delay extinction in humans: Renewal of fear-potentiated startle in a virtual environment. Learning & Memory. 2007;14:247–253. doi: 10.1101/lm.493707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett D, Gonzalez-Lima F. Behavioral effects of metyrapone on Pavlovian extinction. Neuroscience Letters. 2004;371:91–96. doi: 10.1016/j.neulet.2004.08.046. [DOI] [PubMed] [Google Scholar]
- Bohus B, Lissak K. Adrenocortical hormones and avoidance behaviour of rats. International Journal of Neuropharmacology. 1968;4:301–306. doi: 10.1016/0028-3908(68)90012-9. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychological Bulletin. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Bouton ME. A learning theory perspective on lapse, relapse, and the maintenance of behavior change. Health Psychology. 2000;19:57–63. doi: 10.1037/0278-6133.19.suppl1.57. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, ambiguity, and unlearning: Sources of relapse after behavioral extinction. Biological Psychiatry. 2002;10:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral process in extinction. Learning & Memory. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Bouton M, Westbrook F, Corcoran K, Maren S. Contextual and temporal modulation of extinction: Behavioral and biological mechanisms. Biological Psychiatry. 2006;60:352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Cai W, Blundell J, Han J, Greene R, Powell C. Postreactivation glucocorticoids impair reactivation of established fear memory. Journal of Neuroscience. 2006;26:9560–9566. doi: 10.1523/JNEUROSCI.2397-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain C, Godsil B, Jami S, Baradt M. The L-type calcium channel blocker nifedipine impairs extinction, but not reduced contingency effects, in mice. Learning & Memory. 2005;12:277–284. doi: 10.1101/lm.88805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campfield K, Hills A. Effect of timing of critical incident stress debriefing (CISD) on posttraumatic symptoms. Journal of Trauma Stress. 2001;14(2):327–340. doi: 10.1023/A:1011117018705. [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Maren S. Hippocampal inactivation disrupts contextual retrieval of fear memory after extinction. Journal of Neuroscience. 2001;21:1720–1726. doi: 10.1523/JNEUROSCI.21-05-01720.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Maren S. Factors regulating the effects of hippocampal inactivation on renewal of conditional fear after extinction. Learning & Memory. 2004;11:598–603. doi: 10.1101/lm.78704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ. Recalling safety: cooperative functions of the ventromedial prefrontal cortex and the hippocampus in extinction. CNS Spectrums. 2007;12:200–206. doi: 10.1017/s1092852900020915. [DOI] [PubMed] [Google Scholar]
- Devenport L. Spontaneous recovery without interference: Why remembering is adaptive. Animal Learning & Behavior. 1998;26:172–181. [Google Scholar]
- Everly GS, Jr, Mitchell JT. The debriefing “controversy” and crisis intervention: A review of lexical and substantive issues. International Journal on Emerging Mental Health. 1999;4:211–225. [PubMed] [Google Scholar]
- Farinelli M, Deschaux O, Hugues S, Thevenet A, Garcia R. Hippocampal train stimulation modulates recall of fear extinction independently of prefrontal cortex synaptic plasticity and lesions. Learning & Memory. 2006;13:329–334. doi: 10.1101/lm.204806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrikson M. Reliability and validity of some specific fear questionnaires. Scandinavian Journal of Psychology. 1983;24:331–334. doi: 10.1111/j.1467-9450.1983.tb00507.x. [DOI] [PubMed] [Google Scholar]
- Gray MJ, Litz BT. Behavioral interventions for recent trauma: Empirically informed practice guidelines. Behavioral Modification. 2005;29:189–215. doi: 10.1177/0145445504270884. [DOI] [PubMed] [Google Scholar]
- Grillon C. Startle reactivity and anxiety disorders: Aversive conditioning, context, and neurobiology. Biological Psychiatry. 2002;52:958–975. doi: 10.1016/s0006-3223(02)01665-7. [DOI] [PubMed] [Google Scholar]
- Herry C, Mons N. Resistance to extinction is associated with impaired immediate early gene induction in medial prefrontal cortex and amygdala. European Journal of Neuroscience. 2004;20:781–790. doi: 10.1111/j.1460-9568.2004.03542.x. [DOI] [PubMed] [Google Scholar]
- Hobin JA, Goosens KA, Maren S. Context-dependent neuronal activity in the lateral amygdala represents fear memories after extinction. Journal of Neuroscience. 2003;23:8410–8416. doi: 10.1523/JNEUROSCI.23-23-08410.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, Maren S. Electrolytic lesions of the dorsal hippocampus disrupt renewal of conditional fear after extinction. Learning & Memory. 2005;12:270–276. doi: 10.1101/lm.91705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, Maren S. Hippocampal involvement in contextual modulation of fear extinction. Hippocampus. 2007;17:749–758. doi: 10.1002/hipo.20331. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Korenfeld E, Stephan KE, Weiskopf N, Seymour B, Dolan RJ. Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. Journal of Neuroscience. 2006;26:9503–9511. doi: 10.1523/JNEUROSCI.2021-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klorman R, Hastings E, Weerts T, Melamed B, Lang P. Psychometric description of some specific fear questionnaires. Behavior Therapy. 1974;5:401–409. [Google Scholar]
- Konorski J. Conditioned reflexes and neuron organization. Cambridge, England: Cambridge University Press; 1948. [Google Scholar]
- LaBar KS, Cook CA, Torpey DC, Welsh-Bohmer KA. Impact of healthy aging on awareness and fear conditioning. Behavioral Neuroscience. 2004;5:905–915. doi: 10.1037/0735-7044.118.5.905. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;5:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- LaBar KS, LeDoux JE, Spencer DD, Phelps EA. Impaired fear conditioning following unilateral temporal lobectomy in humans. Journal of Neuroscience. 1995;10:6846–6855. doi: 10.1523/JNEUROSCI.15-10-06846.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Phelps EA. Reinstatement of conditioned fear in humans is context dependent and impaired in amnesia. Behavioral Neuroscience. 2005;3:677–686. doi: 10.1037/0735-7044.119.3.677. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Instruction manual and affective ratings (Tech. Rep. No. A-5) Gainesville: University of Florida; 2001. [Google Scholar]
- Lebrón K, Milad MR, Quirk GJ. Delayed recall of fear extinction in rats with lesions of ventral medial prefrontal cortex. Learning & Memory. 2004;11:544–548. doi: 10.1101/lm.78604. [DOI] [PubMed] [Google Scholar]
- Maren S, Chang CH. Recent fear is resistant to extinction. Proceedings of the National Academy of Sciences, USA. 2006;103:18020–18025. doi: 10.1073/pnas.0608398103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Quirk G. Neuronal signalling of fear memory. Nature Reviews Neuroscience. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Drug facilitation of learning and memory. Annual Review of Pharmacology. 1973;13:229–241. doi: 10.1146/annurev.pa.13.040173.001305. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory—A century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual Reviews of Neuroscience. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Roozendaal B. Role of adrenal stress hormones in forming lasting memories in the brain. Current Opinion in Neurobiology. 2002;2:205–210. doi: 10.1016/s0959-4388(02)00306-9. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Roozendaal B. Drug enhancement of memory consolidation: Historical perspective and neurobiological implications. Psychopharmacology (Berlin) 2009;202:3–14. doi: 10.1007/s00213-008-1285-6. [DOI] [PubMed] [Google Scholar]
- McNally RJ, Bryant RA, Ehlers A. Does early psychological intervention promote recovery from post-traumatic stress disorder? Psychology Science in the Public Interest. 2003;4:45–79. doi: 10.1111/1529-1006.01421. [DOI] [PubMed] [Google Scholar]
- Micheau J, Destrade C, Soumireu-Mourat B. Posttrial injections of corticosterone in dorsal hippocampus of the BALB/c mouse facilitate extinction of appetitive operant conditioning in the Skinner box. Comptes Rendus des Séances de l’Académie des Sciences Serie III: Science de la Vie. 1982;294:1109–1112. [PubMed] [Google Scholar]
- Milad MR, Orr SP, Pitman RK, Rauch SL. Context modulation of memory for fear extinction in humans. Psychophysiology. 2005;42:456–464. doi: 10.1111/j.1469-8986.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biological Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;4:567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- Myers KM, Ressler K, Davis M. Different mechanisms of fear extinction dependent on length of time since fear acquisition. Learning and Memory. 2006;2:216–223. doi: 10.1101/lm.119806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Vervliet B, Jovanovic T, Boshoven W, Myers KM, Davis M, et al. Timing of extinction relative to acquisition: A parametric analysis of fear extinction in humans. Behavioral Neuroscience. 2008;122:1016–1030. doi: 10.1037/a0012604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov I. Conditioned reflexes. London: Oxford University Press; 1927. [Google Scholar]
- Pearce JM, Hall G. A model for Pavlovian conditioning: Variations in the effectiveness of conditioned but not unconditioned stimuli. Psychological Review. 1980;87:332–352. [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: Role of the amygdala and vmPFC. Neuron. 2004;6:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Quirk GJ. Extinction: New excitement for an old phenomenon. Biological Psychiatry. 2006;60:317–318. doi: 10.1016/j.biopsych.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Meuller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA. Spontaneous recovery varies inversely with the training–extinction interval. Learning and Behavior. 2004;32:401–408. doi: 10.3758/bf03196037. [DOI] [PubMed] [Google Scholar]
- Roozendaal B. Stress and memory: Opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiology of Learning Memory. 2002;78:578–595. doi: 10.1006/nlme.2002.4080. [DOI] [PubMed] [Google Scholar]
- Rothbaum B, Davis M. Applying learning principles to the treatment of post-trauma reactions. Annals of the New York Academy of Sciences. 2003;1008:112–121. doi: 10.1196/annals.1301.012. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Huff NC, Matus-Amat P. Understanding contextual fear conditioning: Insights from a two-process model. Neuroscience and Biobehavioral Reviews. 2004;28:675–685. doi: 10.1016/j.neubiorev.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Rudy JW, O’Reilly R. Contextual fear conditioning, conjunctive representations, pattern completion, and the hippocampus. Behavioral Neuroscience. 1999;113:867–880. doi: 10.1037//0735-7044.113.5.867. [DOI] [PubMed] [Google Scholar]
- Santini E, Ge H, Ren K, Peña de Ortiz S, Quirk GJ. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. Journal of Neuroscience. 2004;25:5704–5710. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe G, LeDoux J. Memory consolidation of auditory Pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. Journal of Neuroscience. 2000;20:RC96. doi: 10.1523/JNEUROSCI.20-18-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Cain C, Curley N, Schwartz J, Stern S, Ledoux J, Phelps E. Evidence for recovery of fear following immediate extinction in rats and humans. Learning & Memory. 2008;6:394–402. doi: 10.1101/lm.909208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmajuk N, Larrauri J, LaBar KS. Reinstatement of conditioned fear and the hippocampus: An attentional-associative model. Behavioral Brain Research. 2007;177:242–253. doi: 10.1016/j.bbr.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Bush D, LeDoux J. Emotional perseveration: An update on prefrontal-amygdala interactions in fear extinction. Learning & Memory. 2004;11:525–535. doi: 10.1101/lm.79504. [DOI] [PubMed] [Google Scholar]
- Vansteenwegen D, Hermans D, Vervliet B, Francken G, Beckers T, Beayens F, et al. Return of fear in human differential conditioning paradigm caused by a return to the original acquisition context. Behavior Research and Therapy. 2005;42:323–336. doi: 10.1016/j.brat.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Wagner AR. SOP: A model of automatic memory processing in animal behavior. In: Spear NE, Miller RR, editors. Information processing in animals: Memory mechanisms. Hillsdale, NJ: Erlbaum; 1981. pp. 5–47. [Google Scholar]
- Woods A, Bouton ME. Immediate extinction causes a less durable loss of performance than delayed extinction following either fear or appetitive conditioning. Learning & Memory. 2008;15:909–920. doi: 10.1101/lm.1078508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorawski M, Blanding NQ, Kuhn C, LaBar KS. Effects of sex on acquisition and consolidation of human fear conditioning. Learning & Memory. 2006;13:441–450. doi: 10.1101/lm.189106. [DOI] [PMC free article] [PubMed] [Google Scholar]