Abstract
Leydig cells are the testosterone-producing cells of the testis. The adult Leydig cell population ultimately develops from undifferentiated mesenchymal-like stem cells present in the interstitial compartment of the neonatal testis. Four distinct stages of adult Leydig cell development have been identified and characterized: stem Leydig cells, progenitor Leydig cells, immature Leydig cells and adult Leydig cells. The stem Leydig cells are undifferentiated cells that are capable of indefinite self-renewal, differentiation, and replenishment of the Leydig cell niche. Progenitor Leydig cells are derived from the stem Leydig cells. These spindle-shaped cells are luteinizing hormone (LH) receptor positive, have high mitotic activity, and produce little testosterone but rather testosterone metabolites. The progenitor Leydig cells give rise to immature Leydig cells which are round, contain large amounts of smooth endoplasmic reticulum, and produce some testosterone but also very high levels of testosterone metabolites. A single division of these cells produces adult Leydig cells, which are terminally differentiated cells that produce high levels of testosterone. As men age, serum testosterone levels decline, and this is associated with alterations in body composition, energy level, muscle strength, physical, sexual and cognitive functions, and mood. In the Brown Norway rat, used extensively as a model for male reproductive aging, age-related reductions in serum testosterone result from significant decline in the ability of aged Leydig cells to produce testosterone in response to LH stimulation. This review describes Leydig cell development and aging. Additionally, the molecular mechanisms by which testosterone synthesis declines with aging are discussed.
Keywords: Leydig cell, Stem cell, Testosterone, Aging
1. Introduction
In the rat, two distinct generations of Leydig cells have been identified, namely fetal Leydig cells and adult Leydig cells. The fetal Leydig cells develop in utero. These cells become competent to produce testosterone by gestational day (GD) 15.5, with production increasing markedly thereafter (Habert and Picon, 1984). Peak steroidogenic activity is reached just prior to birth, on GD19 (Habert and Brignaschi, 1991). Testosterone secreted by fetal Leydig cells is required for the differentiation of the male urogenital system late in gestation (Huhtaniemi and Pelliniemi, 1992). Fetal Leydig cells also play a role in the scrotal descent of the testis through their synthesis of insulin-like growth factor 3 (Zimmermann et al., 1999). Although fetal Leydig cells express luteinizing hormone receptor (LHR) and respond to LH stimulation (Baker and O’Shaughnessy, 2001; Migrenne et al., 2001), these cells do not require LH for their development. This has been made evident by analyses of LHR null (LHRKO) mice in which testosterone levels do not differ relative to wild-type controls during prenatal period (Zhang et al., 2001).
Adult Leydig cells, which are distinct from the fetal Leydig cells, form during puberty and supply the testosterone required for the onset of spermatogenesis, among other functions. Distinct stages of adult Leydig cell development have been identified and characterized. The stem Leydig cells are undifferentiated cells that are capable of indefinite self-renewal but also of differentiation to steroidogenic cells. These cells give rise to progenitor Leydig cells, cells that proliferate, continue to differentiate, and give rise to the immature Leydig cells. The immature Leydig cells synthesize high levels of testosterone metabolites. The adult Leydig cells are terminally differentiated cells that are derived from immature Leydig cells. These cells are characterized by their production of high levels of testosterone. With aging, both serum and testicular testosterone concentrations progressively decline (Harman et al., 2001). Testosterone decline in the human is associated with alterations in body composition, diminished energy, muscle strength and physical function, reduced sexual function, depressed mood, and decreased cognitive function (Matsumoto, 2002). The age-related decline in testosterone is complex, involving factors intrinsic and extrinsic to the Leydig cells. The ability to experimentally manipulate rodent testes as well as to isolate and culture rodent Leydig cells and their precursor cells have made it possible to address the endocrine, paracrine, cellular and molecular changes that accompany the development of the cells during puberty, and the reduced steroidogenic function during late stages of life.
This paper reviews our current understanding of the formation of adult Leydig cells in the rat and how these cells change with aging. We discuss the identification of stem Ledyig cells, the commitment of the stem cells to the Leydig cell lineage, and a four-stage model of Leydig cell differentiation from stem to progenitor to immature to adult. We also review the literature investigating the age-related changes in adult Leydig cell testosterone production and their possible causes.
2. Development of the adult Leydig cell population
The early postnatal rat testicular interstitium contains a population of spindle-shaped, undifferentiated cells that are “mesenchymal-like.” From postnatal day 14 to day 28, these cells actively proliferate while their numbers diminish, suggesting transformation of the mesenchymal cells to a new cell type. Leydig cells, which are morphologically distinct from the mesenchymal cells, increase in number as the mesenchymal cell numbers diminish, suggesting that the mesenchymal cells may differentiate to become the Leydig cells. These observations first led to the suggestion that the adult Leydig cell population is derived not from the proliferation/differentiation of the fetal Leydig cells present in the neonatal testis, but from mesenchymal-like cells (Hardy et al., 1989). It is still unclear whether fetal Leydig cells persist in the adult testis. But even if they do, they are unlikely to contribute significantly to the testosterone production in the adult (Benton et al., 1995).
2.1. Four stage model of adult Leydig cell differentiation
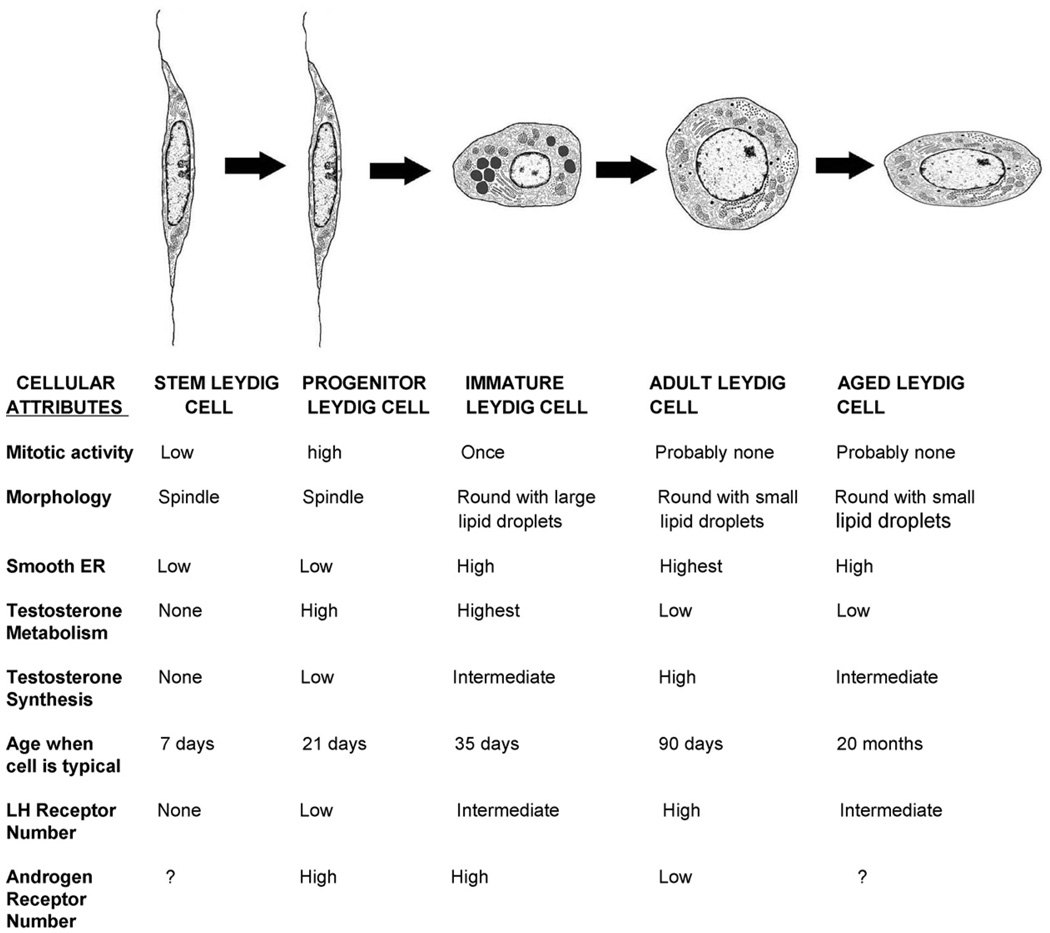
Four distinct types of cells have been identified and characterized as involved in the sequence of events leading to adult Leydig cells: the stem Leydig cells (SLCs), progenitor Leydig cells (PLCs), immature Leydig cells (ILCs), and adult Leydig cells (ALCs) (Fig. 1). Commitment of some of the SLCs to the Leydig cell lineage occurs prior to postnatal days 10–14, giving rise to the PLCs. The PLCs proliferate and continue to differentiate to produce the ILCs. This transition, completed by day 28, accounts for half of the 25 million Leydig cells in the adult rat testis. ILCs then undergo further differentiation and a single cell division, producing the full complement of 25 million adult Leydig cells by day 56. Fig. 1 depicts these transitions, and summarizes the distinguishing characteristics of SLCs, PLCs, ILCs and ALCs.
Fig. 1.
Stages of adult Leydig cell development and aging in the rat. Stem Leydig cells, progenitor Leydig cells, immature Leydig cells, adult Leydig cells and aged Leydig cells are depicted as they appear on days 7, 21, 35 and 90, and at 20 months, respectively. The characteristics of each cell type are presented. Modified from Benton et al. (1995).
2.1.1. Stem Leydig cells (SLCs)
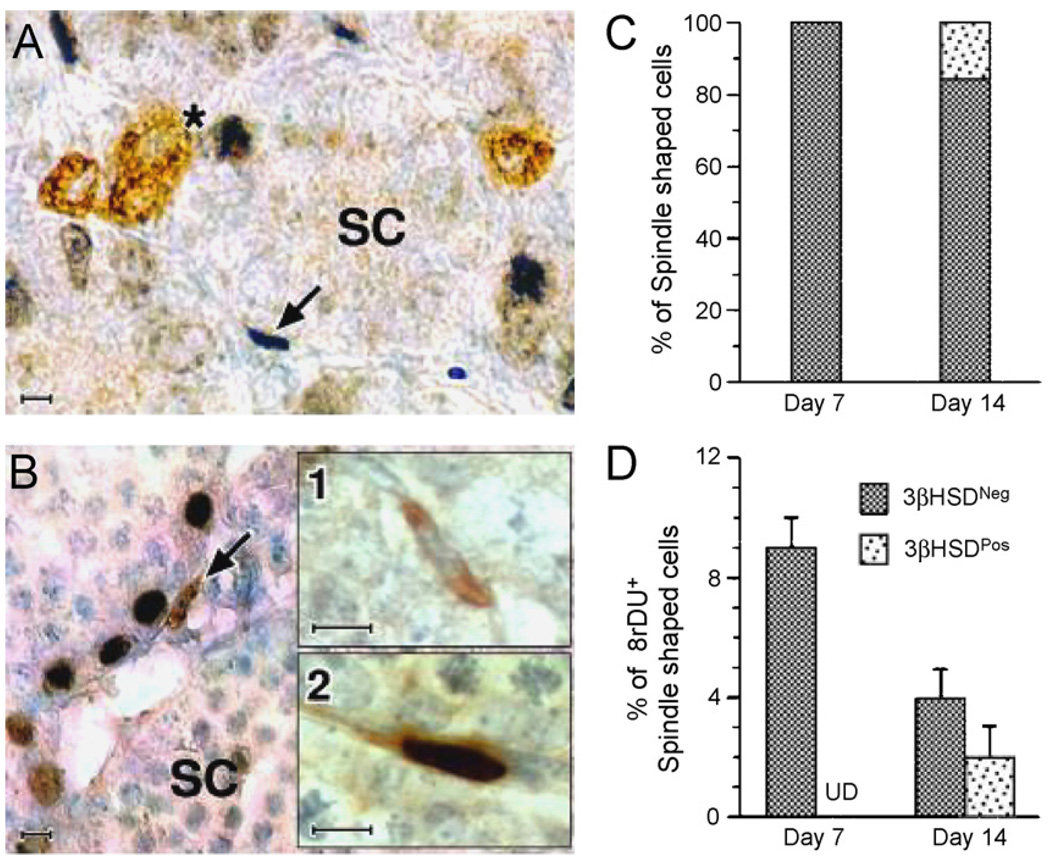
At postnatal day 7, spindle-shaped cells are seen in the testicular interstitium, primarily in the peritubular layer (Ge et al., 2006). As seen in Fig. 2, these cells do not express any of the Leydig cell-specific markers, including 3β-hydroxysteroid dehydrogenase (3β-HSD) or luteinizing hormone receptor (LHR), indicating that the cells have not entered the Leydig cell lineage. BrdU staining indicates that at least some of these cells divide. By postnatal day 14, many of the spindle-shaped cells stain for 3β-HSD, and some among these cells also are BrdU-positive. These 3β-HSD-positive cells are the PLCs.
Fig. 2.
Double immunolabeling of testicular cells for 3β-HSD and BrdU in sections of testes from day 7 (A) and day 14(B) rats. A cluster of 3β-HSD-positive cells, presumed to be fetal Leydig cells is immunolabeled (brown staining, indicated by “*”) on day 7 (A). At this age, spindle-shaped interstitial cells, presumed to be stem Leydig cells (SC, indicated by arrow), often were BrdUrd-labeled (dark blue). One week later (day 14, B), spindle-shaped progenitor Leydig cells (brown stained, indicated by arrow) are seen. 3β-HSD-positive spindle-shaped cells were either negative (B1) or positive (B2) for BrdUrd staining. (C and D) The percentages of spindle-shaped cells that were 3β-HSD-positive on days 7 and 14 (C) and BrdUrd-labeled on those days (D). Scale bars, 10 µm. (From Ge et al., 2006 with permission.)
2.1.2. Progenitor Leydig cells (PLCs)
As a direct result of the proliferation of stem cells in the testicular interstitium and their subsequent commitment to the Leydig cell lineage, Leydig cell progenitors (PLCs) are generated. The PLCs are small, spindle-shaped cells that are similar in appearance to the stem cells in the postnatal testis. In contrast to the stem cells, however, the PLCs are recognizable as members of the Leydig cell lineage by virtue of their expression of Leydig cell markers including P450 side-chain cleavage enzyme (P450scc), 3β-HSD, 17α-hydroxylase (P450c17) and LHR, and by their production of androgen (Shan et al., 1993). The PLCs contain only small amounts of smooth endoplasmic reticulum (SER), the organelle that houses several steroidogenic enzymes, but nonetheless are competent to produce steroids, secreting mainly androsterone (Shan et al., 1993). PLCs gradually enlarge, become round and reduce their proliferative capacity.
2.1.3. Immature Leydig cells (ILCs)
By postnatal day 28, the PLCs transform from spindle-shaped to round and acquire numerous lipid inclusions, forming ILCs. At this time, the number of such cells is approximately 13–14 million (Hardy et al., 1989). During the transformation from PLC to ILC, the smooth ER complement of the cells expands greatly, conferring an ultrastructure with similarities to that of adult Leydig cells. Concurrent with the expansion of smooth ER, the levels of 3β-HSD (Haider et al., 1986; Dupont et al., 1993), P450scc, and P450c17 (Shan et al., 1993) increases, and the cells develop a capacity for steroidogenesis (Zirkin and Ewing, 1987). Testosterone is not the major steroid produced, however. The ability to produce testosterone involves not only an increase in biosynthetic activity, but also a decrease in the activities of testosterone-metabolizing enzymes (Inano and Tamaoki, 1966; Steinberger and Ficher, 1969). The ILCs possess high levels of androgen-metabolizing enzyme activities (3α-hydroxysteroid dehydrogenase, 3α-HSD; and 5α-reductase), and thus their primary product is the androgen metabolite, 5α-androstane-3α, 17β-diol (ADIOL). Consistent with the enzyme activities, mRNA levels for 3α-HSD (Shan et al., 1993) and 5α-reductase (Murono, 1989) are higher in ILCs than the ALCs.
2.1.4. Adult Leydig cells (ALCs)
The ILC population doubles only once from day 28 to day 56, producing the total adult population of approximately 25 million Leydig cells per testis. The activities of androgen metabolizing enzymes decline by day 56 (Inano and Tamaoki, 1966; Steinberger and Ficher, 1969) as ILCs differentiate into ALCs. The decrease in androgen metabolism and continued increase in levels of testosterone biosynthetic enzymes culminate in the predominance of testosterone over ADIOL production in the ALCs. In Leydig cells of 90-day-old adults, testosterone production is 150 times greater than that by PLCs at 21 days of age, and 5 times greater than that by ILCs at 35 days of age (Shan et al., 1993). Compared to ILCs, the ALCs have a greater abundance of smooth ER and fewer and smaller lipid inclusions (Zirkin and Ewing, 1987). ALCs do not normally proliferate (Keeney et al., 1988), but can regenerate if the original population is eliminated. Thus, the adult population of Leydig cells is completely regenerated within 7 weeks of its elimination by ethane dimethanesulfonate (EDS), an agent that kills ALCs specifically (Sharpe et al., 1990). This regeneration may involve the same progression as occurs during normal development of the adult population of Leydig cells. Thus, as in normal development, there is a period during which ADIOL predominates before testosterone becomes the major product (Chen et al., 1996).
2.2. Identification and culture of stem Leydig cells from the neonatal rat testis
Hardy and his colleagues hypothesized that at least some among the spindle-shaped cells in the neonatal rat testis are SLCs (Ge et al., 2006). The putative SLCs were isolated, along with fetal Leydig cells, from the testes of rat pups on postnatal day 7 by Percoll gradient centrifugation (Ge et al., 2006). By immunoselecting for LHR-negative cells, the fetal Leydig cells could be removed. The remaining cells, putative SLCs, were further purified by immunos-election for the stem cell marker platelet-derived growth factor receptor α (PDGFRα). Over 99% of the cells thus selected were found to be 3β-HSD negative, LHR negative, and PDGFRα positive. The cells also were c-kit and leukemia inhibitory factor receptor positive. When cultured in expansion medium, the cells maintained a stable 3β-HSD-/LHR-/PDGFRα+ phenotype for more than 6 months. However, when the SLCs were switched to differentiation medium containing PDGFβ homodimer (PDGF-BB), LH, thyroid hormone, and IGF-1, the cells were induced to express the Leydig cell steroidogenic enzymes P450scc, 3β-HSD and P450c17, as well as LHR and steroidogenic acute regulatory protein (StAR). These cells also began to produce testosterone (Ge et al., 2006).
In addition to the ability to self-renew and differentiate, another important characteristic of stem cells is the ability to replenish their niche. To examine the potential of the putative SLCs to differentiate into Leydig cells in vivo, the cells were first tagged with a fluorescent tracking dye, carboxyfluorescein diacetate, and then injected into the adult rat testis from which Leydig cells had been eliminated by administering the Leydig cell toxin ethane dimethane sulfonate (EDS) to the rats. As seen in Fig. 3, 10 days after the putatative SLCs were injected into the Leydig cell-depleted testes, significant numbers of fluorescently labeled cells were found in the interstitial compartment. Many among the injected cells became 3β-HSD positive, indicating that the injected SLCs had begun to differentiate in the testis. These results, taken together, indicated that the 3β-HSD−/LHR−/PDGFRα+ cells that had been isolated from postnatal day 7 testes indeed were SLCs because they are capable of self-renewal in vitro without showing signs of differentiation, differentiation into testosterone-producing Leydig cells in vitro, and replenishment of their niche in vivo (Ge et al., 2006).
Fig. 3.
Differentiation of putative stem Leydig cells in vivo in adult rat testes that had been depleted of adult Leydig cells by EDS injection of the rats. (A and C) Testis sections from a control rat 4 days after the rat received an injection of vehicle under epifluorescent illumination (A) and after histochemical staining for 3β-HSD enzyme activity (C). (B) Fluorescent interstitial staining in rat testis 10 days after implantation of labeled donor stem Leydig cells into the testes of EDS-injected rats. (D) Many of the labeled donor cells were positively stained for 3β-HSD (arrow). (From Ge et al., 2006 with permission.)
3. Steroidogenesis and Leydig cell function in aging rodents
In many species, including both rat and man, reduced serum testosterone concentrations occur with aging (Harman et al., 2001; Ruffoli et al., 2001; Swerdloff and Wang, 2004; Perrot-Sinal et al., 1998; Zirkin and Chen, 2000). In the human, the reduced serum testosterone levels result from primary gonadal failure rather than from, or in addition to, changes in the hypothalamic-pituitary axis (Gray et al., 1991; Veldhuis et al., 2005). This also is true of Brown Norway rats in which, as in the human, age-related reductions in serum testosterone are not secondary to reduced LH levels, but rather occur in face of unchanging LH and increasing FSH (Chen et al., 1994, 1996; Gruenewald et al., 1994). Additionally, the long life span of Brown Norway rats, the absence of tumors and relative good health make it possible to study aging apart from disease in this strain.
3.1. Leydig cell deficits in aged Leydig cells
Enumeration of Leydig cells in the testes of young and old rats indicated that loss of Leydig cells does not explain age-related reductions in serum testosterone levels (Wang et al., 1993; Ichihara et al., 1993; Chen et al., 1994). Rather, changes in Leydig cell function appear to be responsible. Thus, in vitro studies have shown that, in response to LH, Leydig cells isolated from the testes of aged rats produce less testosterone than cells from young adults (Chen et al., 1994; Zirkin et al., 1993; Liao et al., 1993). Among the age-related changes in the steroidogenic pathway that could be responsible for the reduced ability of the aged cells to production testosterone compared to young cells are reduced LHR number, cAMP production, and/or PKA activity (Chen et al., 2002; Lin et al., 1983). There is evidence that cholesterol transport mechanisms also are compromised in aged Leydig cells (Liao et al., 1993; Culty et al., 2002). For example, reductions have been reported in the cholesterol transport proteins steroidogenic acute regulatory protein (StAR) (Luo et al., 2005; Leers-Sucheta et al., 1999) and in translocator protein (TSPO, also known as peripheral benzodiazepine receptor, PBR) (Culty et al., 2002). The activities of the steroidogenic enzymes P450scc, 3β-HSD, P450c17, and 17β-hydroxysteroid dehydrogenase (17β-HSD) also are reduced in aged Leydig cells (Luo et al., 1996, 2005). Any of these defects might explain the reduced ability of aged Leydig cells to produce testosterone in response to LH.
3.2. Mechanisms responsible for steroidogenic deficits in aged Leydig cells
3.2.1. Luteinizing hormone (LH) and cAMP production
A number of studies have addressed the issue of whether changes in the steroidogenic ability of Leydig cells from aged rats is caused by extrinsic factors, intrinsic factors, or both. The most obvious possible explanation, that reduced testosterone production is the result of reduced serum levels of LH, is not the case because serum LH levels do not change significantly with age (Chen et al., 1994, 1996; Gruenewald et al., 1994), because the in vivo administration of exogenous LH to old rats (Grzywacz et al., 1998; Wang et al., 1999) and because the in vitro, long-term culture of old cells with LH (Chen et al., 2002) failed to raise the relatively low levels of testosterone production by these cells to the significantly higher levels of the young. The response of aged Leydig cells to LH clearly is reduced, resulting in reduced cAMP production by old cells in response to LH (Chen et al., 2002). The importance of reduced cAMP became apparent when aged cells cultured with dibutyryl cAMP for 3 days were found to produce testosterone at the levels of young cells (Chen et al., 2004a). This result suggested that the reduced steroidogenic ability of old cells results from reduced cAMP production, which presumably occurs as a consequence of the relative insensitivity of aged cells to LH. Although the molecular mechanisms by which cAMP is reduced in aged cells has not been determined, there is evidence that decreased cAMP production in old cells results from changes in cAMP synthesis, not cAMP metabolism; that adenylyl cyclase and adenylyl cyclase-Gs protein interaction are maintained in the old cells; and that changes in Gi protein are unlikely to be the cause of old cells producing less cAMP than young cells (Chen et al., 2004a). Interestingly, culturing old cells with cholera toxin, which by-passes the LH receptor and activates Gs proteins directly, was found to increase cAMP production by old cells to the approximate levels of young cells. This suggests that Gs protein is maintained in old cells, and thus that defects in the coupling of the LH receptor to adenylyl cyclase through Gs proteins may be the cause of reduced cAMP production (Chen et al., 2004a).
3.2.2. Free radicals and redox environment of aging cells
A number of “aging theories” might explain inefficiency in the coupling of the LH receptor to adenylyl cyclase in old Leydig cells, including free radical-induced damage to components of signal transduction (Beckman and Ames, 1998; Finkel and Holbrook, 2000). There is evidence that reactive oxygen species (ROS) are produced in Leydig cells both by the mitochondrial electron transport chain as in other cells (Georgiou et al., 1987; Chen et al., 2001) and additionally by the P450 enzymes (Peltola et al., 1996; Hanukoglu, 2006). Culturing Leydig or luteal cells with hydrogen peroxide has been shown to result in reduced steroid production, and it has been suggested that this occurs via reduced cAMP production and/or cholesterol transport (Stocco et al., 1993; Diemer et al., 2003; Tsai et al., 2003; Behrman and Aten, 1991). In vitro studies with kidney cells showed that hydrogen peroxide treatment results in a redox shift to an oxidizing environment, and suggested that this might be linked to uncoupling of the dopamine receptor-cyclase interaction (Asghar et al., 2006).
Using microarray technology, we reported that there is agerelated down-regulation of a number of genes that scavenge and/or repair free radical-induced damage in aged Leydig cells, including Cu–Zn superoxide dismutase (SOD1), microsomal glutathione S-transferase (MGST1), and glutathione S-transferase (GSTM2) (Chen et al., 2004b). Realizing that the reduced expression of these genes could be of importance to Leydig cell aging because of the significant roles played by scavenging enzymes and glutathione in protecting cells from ROS-induced damage, we and others also measured the activities of a number of antioxidant molecules in young adult and old rat Leydig cells. The activities of the antioxidants SOD1, SOD2 and glutathione peroxidase-1 (GPX-1) were found to be reduced, as is glutathione (Cao et al., 2004; Luo et al., 2006). Additionally, both ROS production and lipid peroxidation were found to be elevated (Chen et al., 2001; Cao et al., 2004). Lipid peroxidation, through its effects on membrane structure and/or fluidity, can reduce testosterone because of the dependence of steroidogenesis on the integrity of cell membranes. Indeed, perturbation of membrane fluidity has been shown to affect cAMP production in a number of cell types, including Leydig cells (Kolena et al., 1986; Thomas et al., 1978; Wu et al., 1993).
With the understanding that reactive oxygen produced over time by Leydig cell steroidogenesis itself might have deleterious effects on steroidogenesis, we asked whether the chronic suppression of steroidogenesis in Brown Norway rats would affect normal age-related reductions in Leydig cell steroidogenic function (Chen and Zirkin, 1999). Indeed, we found that after administering contraceptive doses of testosterone to rats from middle age to old age, subsequent LH stimulation resulted in testosterone production at the level of young rats. Thus, suppression of steroidogenesis through the administration of testosterone prevented the age-related reduction in the ability of the Leydig cells to produce testosterone that occurred in control rats. Long-term administration of the antioxidant vitamin E delayed age-related decreases in steroidogenesis, while long-term vitamin E deficiency had the opposite effect (Chen et al., 2005; Abidi et al., 2004). Finally, depletion of the antioxidant glutathione was found to reduce Leydig cell steroidogenic function both in vitro and in vivo, while the antioxidants vitamin E, N-tert-butyl-α-phenylnitrone and Trolox all prevented this effect of reduced glutathione in vitro (Chen et al., 2008). These observations, taken together, support the hypothesis that reactive oxygen plays an important role in age-related reductions in Leydig cell testosterone production. The mechanism by which it does so remains uncertain.
3.2.3. Cyclooxygenase 2
Besides stimulating steroidogenesis via effects on cholesterol transport and the steroidogenic enzymes, LH stimulates arachidonic acid release from Leydig cells (Ronco et al., 2002; Wang et al., 2002; Castilla et al., 2004; Cornejo Maciel et al., 2005). Arachidonic acid, in turn, can modulate the acute effects of LH on steroidogenesis (Ronco et al., 2002; Wang et al., 2002; Castilla et al., 2004; Cornejo Maciel et al., 2005). The inhibition of cyclooxygenase 2 (COX2), an inducible enzyme involved in metabolizing arachidonic acid, was shown in MA-10 Leydig tumor cells to result in increased progesterone production in response to dbcAMP stimulation (Wang et al., 2002). Is this applicable to aging? In Brown Norway rat Leyidg cells, COX2 mRNA and protein levels were shown to increase with age (Syntin et al., 2001; Wang et al., 2005; Chen et al., 2007). Considering the inhibitory effect of COX2 on progesterone production by MA-10 cells, it seemed possible that increased COX2 may play a role in the reduction in testosterone production of old cells, and therefore that inhibition of COX2 should increase testosterone production. Indeed, this is what was found (Wang et al., 2005; Chen et al., 2007). However, inhibition of COX2 also was found to increase testosterone production by young cells (Chen et al., 2007). To establish COX2 as a causal factor in age-related reductions in Leydig cell testosterone production, the stimulatory effect of COX2 should be specific to old cells, or should occur to a greater extent in old than in young cells. As yet, this has not been addressed. Nonetheless, it is possible that increase in the metabolism of arachidonic acid by COX2 may play a role in age-related decline in testosterone production.
3.2.4. Mitogen-activated protein kinase (MAPK)
Age-related increases in oxidative stress may damage cellular DNA, protein and lipids. As importantly, however, recent studies have indicated that ROS may affect cellular function through modifying redox-sensitive cellular signaling molecules (Jones, 2008). Mitogen-activated protein kinase signaling molecules respond to a variety of stimuli including growth factors, cytokines, oxidative stress, and environmental/toxic chemical insults (McCubrey et al., 2006). In cells of the adrenal gland, as in Leydig cells, steroid production (corticosterone in the case of the adrenal) decreases with age, accompanied by increase in oxidative stress (Abidi et al., 2008a). Inhibition of p38 MAPK activity through suppression of its phosphorylation in aged rat adrenal cells was shown to partially reverse the age-related loss of steroidogenic function (Abidi et al., 2008a), strongly suggesting that phosphorylation of p38 MAPK may be a mediator between increased oxidative stress and decreased steroidogenesis in the adrenal (Abidi et al., 2008b). Although the exact role that p38 MAPK plays remains unclear, one possibility is that phosphorylation of p38 MAPK increases COX2 protein synthesis, and that this, in turn, reduces StAR protein and steroidogenic function. This possibility is supported by studies in other cell systems that COX-2 is induced by oxidants such as superoxide, hydrogen peroxide and 4-hydroxynonenal (Nakamura and Sakamoto, 2001; Kiritoshi et al., 2003; Kumagai et al., 2004; Yang et al., 2005, 2006), and that p38 MAPK is necessary for the oxidant-dependent induction of COX-2 (Guan et al., 1998; Lasa et al., 2000; Hendrickx et al., 2003; Zarrouki et al., 2007). At this point, however, whether p38 MAPK plays any role in age-related reductions in Leydig cell steroidogenesis is still unclear.
3.2.5. Pituitary adenylate cyclase-activating peptide
Pituitary adenylate cyclase-activating peptide (PACAP) is a member of the vasoactive intestinal peptide (VIP) neuropeptide family which functions in the central nervous system to regulate pituitary LH secretion (Osuga et al., 1992). PACAP also is expressed in the testis (Arimura et al., 1991). PACAP has been shown to stimulate cAMP production in many cell types, including gonadotropes and Leydig, germ, and Sertoli cells (Vaudry et al., 2000) and to stimulate testosterone production by Leydig cells (El-Gehani et al., 2000; Rossato et al., 1997; Romanelli et al., 1997). Interestingly, PACAP is expressed in germ cells but not in Sertoli or Leydig cells (Shioda et al., 1994), suggesting that its effect on Leydig cell steroidogenesis might result from a paracrine effect of germ cell PACAP on Leydig cells through PACAP receptors expressed in Leydig cells.
In 4-month-old PACAP knockout mice (C57BL/6, PACAP−/−), serum testosterone concentration and Leydig cell steroidogenic function (StAR, 3β-HSD, P450c17 expression) were significantly reduced compared to age-matched wild-type controls (Lacombe et al., 2006). By age 15 months, however, when steroidogenesis in the wild-type mice was significantly decreased, serum testosterone and Leydig cell steroidogenic function in the knockout mice were at significantly higher levels than those in age-matched controls, and there was less germ cell depletion (Lacombe et al., 2006). The observation that germ cell depletion in old mice was associated with a higher content of peroxynitrites, a marker of oxidative stress, suggests there might be reduced ROS-induced damage in the testes of PACAP−/− animals, and therefore lends support to the hypothesis that reactive oxygen in some way contributes to the aging of Leydig cells.
3.2.6. Other factors
Macrophages as well as Leydig cells are present in the interstitial compartment of the testis. There is good evidence that two cell types are functionally related. Local inflammation and infection can activate macrophages to produce cytokines (e.g. interleukin1, tumor necrosis factor-α), which have been shown to affect Leydig cell steroidogenesis (Hales, 2002). Aging, in general, has been shown to be associated with increases in pro-inflammatory cytokines (Krabbe et al., 2004). Although testicular macrophages have been shown to change ultrastructurally with aging (accumulation of lipofuscin granules, for example) (Giannessi et al., 2005), it is not known whether their function and intratesticular cytokine concentration change. Macrophages also are major sources of ROS (Nagata, 2005). The possible contribution of ROS to age-related reductions in Leydig cell function has been discussed above. Recently, lipopolysaccharide endotoxemia was shown to affect Leydig cell mitochondrial function and steroidogenesis by inducing ROS production by testicular macrophages (Allen et al., 2004). Nitric oxide (NO), produced by macrophages, inhibits Leydig cell steroidogenesis (Weissman et al., 2005). Whether or not there is a prominent endogenous NO-generating system in adult Leydig cells is in dispute, but it is possible that aging affects this system (Ruffoli et al., 2001; Hikim et al., 2005).
4. Summary
Adult Leydig cells originate within the rat testis by day 56 postnatally. Their formation is the product of active proliferation and differentiation of undifferentiated stem cells to form progenitor Leydig cells, the differentiation of these cells to form steroidogenically active immature Leydig cells that produce primarily 5α-reduced androgens rather than testosterone, and finally the production of testosterone-producing adult cells from the immature cells. The adult cells rarely divide. With aging (by about 20 months), the steroidogenic capacity of the Leydig cells is reduced by about 50%. There is evidence that ROS, derived from the mitochondrial electron transport chain, steroidogenesis and/or macrophages, by altering the redox environment of the aging Leydig cells, might cause damage to Leydig cell membrane lipids and proteins and that this, in turn, might result in the reduced LH signaling that characterizes aged Leydig cells. Reduced LH signaling would be expected to affect cAMP production, cholesterol transport via StAR and TSPO, and the steroidogenic enzymes, all of which are seen in aged cells. Age-dependent increases in COX2, which might result from ROS-induced phosphorylation of p38 MAPK, also might contribute to reduced StAR and thus in reduced steroidogenesis. Additionally, other hormones and/or growth factors, including PACAP and cytokines, might be involved in age-related reductions in Leydig cell steroidogenesis.
References
- Abidi P, Leers-Sucheta S, Azhar S. Suppression of steroidogenesis and activator protein-1 transcription factor activity in rat adrenals by vitamin E deficiency-induced chronic oxidative stress. J. Nutr. Biochem. 2004;15:210–219. doi: 10.1016/j.jnutbio.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Abidi P, Leers-Sucheta S, Cortez Y, Han J, Azhar S. Evidence that age-related changes in p38 MAP kinase contribute to the decreased steroid production by the adrenocortical cells from old rats. Aging Cell. 2008a;7:166–178. doi: 10.1111/j.1474-9726.2007.00364.x. [DOI] [PubMed] [Google Scholar]
- Abidi P, Zhang H, Zaidi SM, Shen WJ, Leers-Sucheta S, Cortez Y, Han J, Azhar S. Oxidative stress-induced inhibition of adrenal steroidogenesis requires participation of p38 mitogen-activated protein kinase signaling pathway. J. Endocrinol. 2008b;198:193–207. doi: 10.1677/JOE-07-0570. [DOI] [PubMed] [Google Scholar]
- Allen JA, Diemer T, Janus P, Hales KH, Hales DB. Bacterial endotoxin lipopolysaccharide and reactive oxygen species inhibit Leydig cell steroidogenesis via perturbation of mitochondria. Endocrine. 2004;25:265–275. doi: 10.1385/ENDO:25:3:265. [DOI] [PubMed] [Google Scholar]
- Arimura A, Somogyvari-Vigh A, Miyata A, Mizuno K, Coy DH, Kitada C. Tissue distribution of PACAP as determined by RIA: highly abundant in the rat brain and testes. Endocrinology. 1991;129:2787–2789. doi: 10.1210/endo-129-5-2787. [DOI] [PubMed] [Google Scholar]
- Asghar M, Banday AA, Fardoun RZ, Lokhandwala MF. Hydrogen peroxide causes uncoupling of dopamine D1-like receptors from G proteins via a mechanism involving protein kinase C and G-protein-coupled receptor kinase 2. Free Radic. Biol. Med. 2006;40:13–20. doi: 10.1016/j.freeradbiomed.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Baker PJ, O’Shaughnessy PJ. Role of gonadotrophins in regulating numbers of Leydig and Sertoli cells during fetal and postnatal development in mice. Reproduction. 2001;122:227–234. doi: 10.1530/rep.0.1220227. [DOI] [PubMed] [Google Scholar]
- Beckman KB, Ames BN. The free radical theory of aging matures. Physiol. Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- Behrman HR, Aten RF. Evidence that hydrogen peroxide blocks hormone-sensitive cholesterol transport into mitochondria of rat luteal cells. Endocrinology. 1991;128:2958–2966. doi: 10.1210/endo-128-6-2958. [DOI] [PubMed] [Google Scholar]
- Benton L, Shan LX, Hardy MP. Differentiation of adult Leydig cells. J. Steroid Biochem. Mol. Biol. 1995;53:61–68. doi: 10.1016/0960-0760(95)00022-r. [DOI] [PubMed] [Google Scholar]
- Cao L, Leers-Sucheta S, Azhar S. Aging alters the functional expression of enzymatic and non-enzymatic antioxidant defense systems in testicular rat Leydig cells. J. Steroid Biochem. Mol. Biol. 2004;88:61–67. doi: 10.1016/j.jsbmb.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Castilla R, Maloberti P, Castillo F, Duarte A, Cano F, Maciel FC, Neuman I, Mendez CF, Paz C, Podesta EJ. Arachidonic acid regulation of steroid synthesis: new partners in the signaling pathway of steroidogenic hormones. Endocr. Res. 2004;30:599–606. doi: 10.1081/erc-200043765. [DOI] [PubMed] [Google Scholar]
- Chen H, Hardy MP, Huhtaniemi I, Zirkin BR. Age-related decreased Leydig cell testosterone production in the brown Norway rat. J. Androl. 1994;15:551–557. [PubMed] [Google Scholar]
- Chen H, Huhtaniemi I, Zirkin BR. Depletion and repopulation of Leydig cells in the testes of aging brown Norway rats. Endocrinology. 1996;137:3447–3452. doi: 10.1210/endo.137.8.8754773. [DOI] [PubMed] [Google Scholar]
- Chen H, Zirkin BR. Long term suppression of Leydig cell steroidogenesis prevents Leydig cell aging. Proc. Natl. Acad. Sci. U.S.A. 1999;96:14877–14881. doi: 10.1073/pnas.96.26.14877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Cangello D, Benson S, Folmer J, Zhu H, Trush MA, Zirkin BR. Age-related increase in mitochondrial superoxide generation in the testosterone-producing cells of Brown Norway rat testes: relationship to reduced steroidogenic function? Exp. Gerontol. 2001;36:1361–1373. doi: 10.1016/s0531-5565(01)00118-8. [DOI] [PubMed] [Google Scholar]
- Chen H, Hardy MP, Zirkin BR. Age-related decreases in Leydig cell testosterone production are not restored by exposure to LH in vitro. Endocrinology. 2002;143:1637–1642. doi: 10.1210/endo.143.5.8802. [DOI] [PubMed] [Google Scholar]
- Chen H, Liu J, Luo L, Zirkin BR. Dibutyryl cyclic adenosine monophosphate restores the ability of aged Leydig cells to produce testosterone at the high levels characteristic of young cells. Endocrinology. 2004a;145:4441–4446. doi: 10.1210/en.2004-0639. [DOI] [PubMed] [Google Scholar]
- Chen H, Irizarry RA, Luo L, Zirkin BR. Leydig cell gene expression: effects of age and caloric restriction. Exp. Gerontol. 2004b;39:31–43. doi: 10.1016/j.exger.2003.09.021. [DOI] [PubMed] [Google Scholar]
- Chen H, Liu J, Luo L, Baig MU, Kim JM, Zirkin BR. Vitamin E, aging and Leydig cell steroidogenesis. Exp. Gerontol. 2005;40:728–736. doi: 10.1016/j.exger.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Chen H, Luo L, Liu J, Zirkin BR. Cyclooxygenases in rat Leydig cells: effects of luteinizing hormone and aging. Endocrinology. 2007;148:735–742. doi: 10.1210/en.2006-0925. [DOI] [PubMed] [Google Scholar]
- Chen H, Pechenino AS, Liu J, Beattie MC, Brown TR, Zirkin BR. Effect of glutathione depletion on Leydig cell steroidogenesis in young and old brown Norway rats. Endocrinology. 2008;149:2612–2619. doi: 10.1210/en.2007-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornejo Maciel F, Maloberti P, Neuman I, Cano F, Castilla R, Castillo F, Paz C, Podesta EJ. An arachidonic acid-preferring acyl-CoA synthetase is a hormone-dependent and obligatory protein in the signal transduction pathway of steroidogenic hormones. J. Mol. Endocrinol. 2005;34:655–666. doi: 10.1677/jme.1.01691. [DOI] [PubMed] [Google Scholar]
- Culty M, Luo L, Yao ZX, Chen H, Papadopoulos V, Zirkin BR. Cholesterol transport, peripheral benzodiazepine receptor, and steroidogenesis in aging Leydig cells. J. Androl. 2002;23:439–447. [PubMed] [Google Scholar]
- Diemer T, Allen JA, Hales ICH, Hales DB. Reactive oxygen disrupts mitochondria in MA-10 tumor Leydig cells and inhibits steroidogenic acute regulatory (StAR) protein and steroidogenesis. Endocrinology. 2003;144:2882–2891. doi: 10.1210/en.2002-0090. [DOI] [PubMed] [Google Scholar]
- Dupont E, Labrie F, Luu-The V, Pelletier G. Ontogeny of 3 beta-hydroxysteroid dehydrogenase/delta 5-delta 4 isomerase (3 beta-HSD) in rat testis as studied by immunocytochemistry. Anat. Embryol. 1993;187:583–589. doi: 10.1007/BF00214437. [DOI] [PubMed] [Google Scholar]
- El-Gehani F, Tena-Sempere M, Huhtaniemi I. Evidence that pituitary adenylate cyclase-activating polypeptide is a potent regulator of fetal rat testicular steroidogenesis. Biol. Reprod. 2000;63:1482–1489. doi: 10.1095/biolreprod63.5.1482. [DOI] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Ge RS, Dong Q, Sottas CM, Papadopoulos V, Zirkin BR, Hardy MP. In search of rat stem Leydig cells: identification, isolation, and lineage-specific development. Proc. Natl. Acad. Sci. U.S.A. 2006;103:2719–2724. doi: 10.1073/pnas.0507692103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiou M, Perkins LM, Payne AH. Steroid synthesis-dependent, oxygen-mediated damage of mitochondrial and microsomal cytochrome P-450 enzymes in rat Leydig cell cultures. Endocrinology. 1987;121:1390–1399. doi: 10.1210/endo-121-4-1390. [DOI] [PubMed] [Google Scholar]
- Giannessi F, Giambelluca MA, Scavuzzo MC, Ruffoli R. Ultrastructure of testicular macrophages in aging mice. J. Morphol. 2005;263:39–46. doi: 10.1002/jmor.10287. [DOI] [PubMed] [Google Scholar]
- Gray A, Feldman HA, McKinlay JB, Longcope C. Age, disease, and changing sex hormone levels in middle-aged men: results of the Massachusetts Male Aging Study. J. Clin. Endocrinol. Metab. 1991;73:1016–1025. doi: 10.1210/jcem-73-5-1016. [DOI] [PubMed] [Google Scholar]
- Gruenewald DA, Naai MA, Hess DL, Matsumoto AM. The Brown Norway rat as a model of male reproductive aging: evidence for both primary and secondary testicular failure. J. Gerontol. 1994;49:B42–B50. doi: 10.1093/geronj/49.2.b42. [DOI] [PubMed] [Google Scholar]
- Grzywacz FW, Chen H, Allegretti J, Zirkin BR. Does age-associated reduced Leydig cell testosterone production in Brown Norway rats result from under-stimulation by luteinizing hormone? J. Androl. 1998;19:625–630. [PubMed] [Google Scholar]
- Guan Z, Buckman SY, Miller BW, Springer LD, Morrison AR. Interleukin-1beta-induced cyclooxygenase-2 expression requires activation of both c-Jun NH2-terminal kinase and p38 MAPK signal pathways in rat renal mesangial cells. J. Biol. Chem. 1998;273:28670–28676. doi: 10.1074/jbc.273.44.28670. [DOI] [PubMed] [Google Scholar]
- Habert R, Brignaschi P. Developmental changes in in vitro testosterone production by dispersed Leydig cells during fetal life in rats. Arch. Androl. 1991;27:65–71. doi: 10.3109/01485019108987654. [DOI] [PubMed] [Google Scholar]
- Habert R, Picon R. Testosterone, dihydrotestosterone and estradiol-17 beta levels in maternal and fetal plasma and in fetal testes in the rat. J. Steroid biochem. 1984;21:193–198. doi: 10.1016/0022-4731(84)90383-2. [DOI] [PubMed] [Google Scholar]
- Haider SG, Passia D, Overmeyer G. Studies on the fetal and postnatal development of rat Leydig cells employing 3 beta-hydroxysteroid dehydrogenase activity. Acta Histochem. Suppl. 1986;32:197–202. [PubMed] [Google Scholar]
- Hales DB. Testicular macrophage modulation of Leydig cell steroidogenesis. J. Reprod. Immunol. 2002;57:3–18. doi: 10.1016/s0165-0378(02)00020-7. [DOI] [PubMed] [Google Scholar]
- Hanukoglu I. Antioxidant protective mechanisms against reactive oxygen species (ROS) generated by mitochondrial P450 systems in steroidogenic cells. Drug Metab. Rev. 2006;38:171–196. doi: 10.1080/03602530600570040. [DOI] [PubMed] [Google Scholar]
- Hardy MP, Zirkin BR, Ewing LL. Kinetic studies on the development of the adult population of Leydig cells in testes of the pubertal rat. Endocrinology. 1989;124:762–770. doi: 10.1210/endo-124-2-762. [DOI] [PubMed] [Google Scholar]
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men, Baltimore Longitudinal Study of Aging. J. Endocrinol. Metab. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- Hendrickx N, Volanti C, Moens U, Seternes OM, de Witte P, Vandenheede JR, Piette J, Agostinis P. Up-regulation of cyclooxygenase-2 and apoptosis resistance by p38 MAPK in hypericin-mediated photodynamic therapy of human cancer cells. J. Biol. Chem. 2003;26:52231–52239. doi: 10.1074/jbc.M307591200. [DOI] [PubMed] [Google Scholar]
- Hikim AP, Vera Y, Vernet D, Castanares M, Diaz-Romero M, Ferrini M, Swerdloff RS, Gonzalez-Cadavid NF, Wang C. Involvement of nitric oxide-mediated intrinsic pathway signaling in age-related increase in germ cell apoptosis in male Brown-Norway rats. J. Gerontol. A Biol. Sci. Med. Sci. 2005;60:702–708. doi: 10.1093/gerona/60.6.702. [DOI] [PubMed] [Google Scholar]
- Huhtaniemi I, Pelliniemi LJ. Fetal Leydig cells: cellular origin, morphology, life span, and special functional features. Proc. Soc. Exp. Biol. Med. 1992;201:125–140. doi: 10.3181/00379727-201-43493. [DOI] [PubMed] [Google Scholar]
- Ichihara I, Kawamura H, Pelliniemi LJ. Ultrastructure and morphometry of testicular Leydig cells and the interstitial components correlated with testosterone in aging rats. Cell Tissue Res. 1993;271:241–255. doi: 10.1007/BF00318610. [DOI] [PubMed] [Google Scholar]
- Inano H, Tamaoki BI. Bioconversion of steroids in immature rat testes in vitro. Endocrinology. 1966;79:579–590. doi: 10.1210/endo-79-3-579. [DOI] [PubMed] [Google Scholar]
- Jones DP. Radical-free biology of oxidative stress. Am. J. Physiol. Cell. Physiol. 2008;295:C849–C868. doi: 10.1152/ajpcell.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeney DS, Mendis-Handagama SM, Zirkin BR, Ewing LL. Effect of long term deprivation of luteinizing hormone on Leydig cell volume, Leydig cell number, and steroidogenic capacity of the rat testis. Endocrinology. 1988;123:2906–2915. doi: 10.1210/endo-123-6-2906. [DOI] [PubMed] [Google Scholar]
- Kiritoshi S, Nishikawa T, Sonoda K, Kukidome D, Senokuchi T, Matsuo T, Matsumura T, Tokunaga H, Brownlee M, Araki E. Reactive oxygen species from mitochondria induce cyclooxygenase-2 gene expression in human mesan gial cells: potential role in diabetic nephropathy. Diabetes. 2003;52:2570–2577. doi: 10.2337/diabetes.52.10.2570. [DOI] [PubMed] [Google Scholar]
- Kolena J, Blazicek P, Horkovics-Kovats S, Ondrias K, Sebokova E. Modulation of rat testicular LH/hCG receptors by membrane lipid fluidity. Mol. Cell. Endocrinol. 1986;44:69–76. doi: 10.1016/0303-7207(86)90107-3. [DOI] [PubMed] [Google Scholar]
- Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp. Gerontol. 2004;39:687–699. doi: 10.1016/j.exger.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Kumagai T, Matsukawa N, Kaneko Y, Kusumi Y, Mitsumata M, Uchida K. A lipid peroxidation-derived inflammatory mediator: identification of 4-hydroxy-2-nonenal as a potential inducer of cyclooxygenase-2 in macrophages. J. Biol. Chem. 2004;279:48389–48396. doi: 10.1074/jbc.M409935200. [DOI] [PubMed] [Google Scholar]
- Lacombe A, Lelievre V, Roselli CE, Salameh W, Lue YH, Lawson G, Muller JM, Waschek JA, Vilain E. Delayed testicular aging in pituitary adenylate cyclase-activating peptide (PACAP) null mice. Proc. Natl. Acad. Sci. U.S.A. 2006;103:3793–3798. doi: 10.1073/pnas.0505827103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasa M, Mahtani KR, Finch A, Brewer G, Saklatvala J, Clark AR. Regulation of cyclooxygenase 2 mRNA stability by the mitogen-activated protein kinase p38 signaling cascade. Mol. Cell. Biol. 2000;20:4265–4274. doi: 10.1128/mcb.20.12.4265-4274.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leers-Sucheta S, Stocco DM, Azhar S. Down-regulation of steroidogenic acute regulatory (StAR) protein in rat Leydig cells: implications for regulation of testosterone production during aging. Mech. Ageing Dev. 1999;107:197–203. doi: 10.1016/s0047-6374(98)00149-3. [DOI] [PubMed] [Google Scholar]
- Liao C, Reaven E, Azhar S. Age-related decline in the steroidogenic capacity of isolated rat Leydig cells: a defect in cholesterol mobilization and processing. J. Steroid Biochem. Mol. Biol. 1993;46:39–47. doi: 10.1016/0960-0760(93)90207-d. [DOI] [PubMed] [Google Scholar]
- Lin T, Vinson NE, Murono EP, Osterman J, Nankin HR. The aging Leydig cell. VIII. Protein kinase activity. J. Androl. 1983;4:324–330. doi: 10.1002/j.1939-4640.1983.tb02380.x. [DOI] [PubMed] [Google Scholar]
- Luo L, Chen H, Zirkin BR. Are Leydig cell steroidogenic enzymes differentially regulated with aging? J. Androl. 1996;17:509–515. [PubMed] [Google Scholar]
- Luo L, Chen H, Zirkin BR. Temporal relationships among testosterone production, steroidogenic acute regulatory protein (StAR), and P450 side-chain cleavage enzyme (P450scc) during Leydig cell aging. J. Androl. 2005;26:25–31. [PubMed] [Google Scholar]
- Luo L, Chen H, Trush MA, Show MD, Anway MD, Zirkin BR. Aging and the brown Norway rat Leydig cell antioxidant defense system. J. Androl. 2006;27:240–247. doi: 10.2164/jandrol.05075. [DOI] [PubMed] [Google Scholar]
- Matsumoto AM. Andropause: clinical implications of the decline in serum testosterone levels with aging in men. J. Gerontol. A Biol. Sci. Med. Sci. 2002;57:M76–M99. doi: 10.1093/gerona/57.2.m76. [DOI] [PubMed] [Google Scholar]
- McCubrey JA, Lahair MM, Franklin RA. Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid. Redox Signal. 2006;8:1775–1789. doi: 10.1089/ars.2006.8.1775. [DOI] [PubMed] [Google Scholar]
- Migrenne S, Pairault C, Racine C, Livera G, Celoso A, Habert R. Luteinizing hormone-dependent activity and luteinizing hormone-independent differentiation of rat fetal Leydig cells. Mol. Cell. Endocrinol. 2001;172:193–202. doi: 10.1016/s0303-7207(00)00339-7. [DOI] [PubMed] [Google Scholar]
- Murono EP. Maturational changes in steroidogenic enzyme activities metabolizing testosterone and dihydrotestosterone in two populations of testicular interstitial cells. Acta Endocrinol. 1989;121:477–483. doi: 10.1530/acta.0.1210477. [DOI] [PubMed] [Google Scholar]
- Nagata M. Inflammatory cells and oxygen radicals. Curr. Drug Targets Inflamm. Allergy. 2005;4:503–504. doi: 10.2174/1568010054526322. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Sakamoto K. Reactive oxygen species upregulates cyclooxygenase-2, p53, and Bax mRNA expression in bovine luteal cells. Biochem. Biophys. Res. Commun. 2001;284:201–210. doi: 10.1006/bbrc.2001.4927. [DOI] [PubMed] [Google Scholar]
- Osuga Y, Mitsuhashi N, Mizuno M. In vivo effect of pituitary adenylate cyclase activating polypeptide 38 (PACAP 38) on the secretion of luteinizing hormone (LH) in male rats. Endocrinol. Jpn. 1992;39:153–156. doi: 10.1507/endocrj1954.39.153. [DOI] [PubMed] [Google Scholar]
- Peltola V, Huhtaniemi I, Metsa-Ketela T, Ahotupa M. Induction of lipid peroxidation during steroidogenesis in the rat testis. Endocrinology. 1996;137:105–112. doi: 10.1210/endo.137.1.8536600. [DOI] [PubMed] [Google Scholar]
- Perrot-Sinal TS, Kavaliers M, Ossenkopp KP. Spatial learning and hippocampal volume in male deer mice: relations to age, testosterone and adrenal gland weight. Neuroscience. 1998;86:1089–1099. doi: 10.1016/s0306-4522(98)00131-6. [DOI] [PubMed] [Google Scholar]
- Romanelli F, Filio S, Isidori A, Conte D. Pituitary adenylate cyclase-activating polypeptide regulates rat Leydig cell function in vitro. Neuropeptides. 1997;31:311–317. doi: 10.1016/s0143-4179(97)90064-0. [DOI] [PubMed] [Google Scholar]
- Ronco AM, Moraga PF, Llanos MN. Arachidonic acid release from rat Leydig cells: the involvement of G protein, phospholipase A2 and regulation of cAMP production. J. Endocrinol. 2002;172:95–104. doi: 10.1677/joe.0.1720095. [DOI] [PubMed] [Google Scholar]
- Rossato M, Nogara A, Gottardello F, Bordon P, Foresta C. Pituitary adenylate cyclase activating polypeptide stimulates rat Leydig cell steroidogenesis through a novel transduction pathway. Endocrinology. 1997;138:3228–3235. doi: 10.1210/endo.138.8.5314. [DOI] [PubMed] [Google Scholar]
- Ruffoli R, Giambelluca MA, Giannessi F, Soldani P, Grasso L, Gasperi M, Giannessi F. Ultrastructural localization of the NADPH-diaphorase activity in the Leydig cells of aging mice. Anat. Embryol. 2001;203:383–391. doi: 10.1007/s004290100168. [DOI] [PubMed] [Google Scholar]
- Shan LX, Phillips DM, Bardin CW, Hardy MP. Differential regulation of steroidogenic enzymes during differentiation optimizes testosterone production by adult rat Leydig cells. Endocrinology. 1993;133:2277–2283. doi: 10.1210/endo.133.5.8404681. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, Maddocks S, Kerr JB. Cell-cell interactions in the control of spermatogenesis as studied using Leydig cell destruction and testosterone replacement. Am. J. Anat. 1990;188:3–20. doi: 10.1002/aja.1001880103. [DOI] [PubMed] [Google Scholar]
- Shioda S, Legradi G, Leung WC, Nakajo S, Nakaya K, Arimura A. Localization of pituitary adenylate cyclase-activating polypeptide and its messenger ribonucleic acid in the rat testis by light and electron microscopic immunocyto-chemistry and in situ hybridization. Endocrinology. 1994;135:818–825. doi: 10.1210/endo.135.3.8070375. [DOI] [PubMed] [Google Scholar]
- Steinberger E, Ficher M. Differentiation of steroid biosynthetic pathways in developing testes. Biol. Reprod. 1969;1:119–133. doi: 10.1095/biolreprod1.supplement_1.119. [DOI] [PubMed] [Google Scholar]
- Stocco DM, Wells J, Clark BJ. The effects of hydrogen peroxide on steroidogenesis in mouse Leydig tumor cells. Endocrinology. 1993;133:2827–2832. doi: 10.1210/endo.133.6.8243310. [DOI] [PubMed] [Google Scholar]
- Swerdloff RS, Wang C. Androgens and the ageing male. Best Pract. Res. Clin. Endocrinol. Metab. 2004;18:349–362. doi: 10.1016/j.beem.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Syntin P, Chen H, Zirkin BR, Robaire B. Gene expression in Brown Norway rat Leydig cells: effects of age and of age-related germ cell loss. Endocrinology. 2001;142:5277–5285. doi: 10.1210/endo.142.12.8526. [DOI] [PubMed] [Google Scholar]
- Thomas JP, Dorflinger LJ, Behrman HR. Mechanism of the rapid antigo-nadotropic action of prostaglandins in cultured luteal cells. Proc. Natl. Acad. Sci. U.S.A. 1978;75:1344–1348. doi: 10.1073/pnas.75.3.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SC, Lu CC, Lin CS, Wang PS. Antisteroidogenic actions of hydrogen peroxide on rat Leydig cells. J. Cell. Biochem. 2003;90:1276–1286. doi: 10.1002/jcb.10738. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol. Rev. 2000;52:269–324. [PubMed] [Google Scholar]
- Veldhuis JD, Keenan DM, Iranmanesh A. Mechanisms of ensemble failure of the male gonadal axis in aging. J. Endocrinol. Invest. 2005;28:8–13. [PubMed] [Google Scholar]
- Wang C, Leung A, Sinha-Hikim AP. Reproductive aging in the male Brown-Norway rat: a model for human. Endocrinology. 1993;133:2773–2781. doi: 10.1210/endo.133.6.8243304. [DOI] [PubMed] [Google Scholar]
- Wang C, Sinha Hikim AP, Lue YH, Leung A, Baravarian S, Swerdloff RS. Reproductive aging in the Brown Norway rat is characterized by accelerated germ cell apoptosis and is not altered by luteinizing hormone replacement. J. Androl. 1999;20:509–518. [PubMed] [Google Scholar]
- Wang XJ, Dyson MT, Mondillo C, Patrignani Z, Pignataro O, Stocco DM. Interaction between arachidonic acid and cAMP signaling pathways enhances steroidogenesis and StAR gene expression in MA-10 Leydig tumor cells. Mol. Cell. Endocrinol. 2002;188:55–63. doi: 10.1016/s0303-7207(01)00748-1. [DOI] [PubMed] [Google Scholar]
- Wang X, Shen CL, Dyson MT, Eimerl S, Orly J, Hutson JC, Stocco DM. Cyclooxygenase-2 regulation of the age-related decline in testosterone biosynthesis. Endocrinology. 2005;146:4202–4208. doi: 10.1210/en.2005-0298. [DOI] [PubMed] [Google Scholar]
- Weissman BA, Niu E, Ge R, Sottas CM, Holmes M, Hutson JC, Hardy MP. Paracrine modulation of androgen synthesis in rat leydig cells by nitric oxide. J. Androl. 2005;26:369–378. doi: 10.2164/jandrol.04178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Yao K, Carlson JC. Plasma membrane changes in the rat corpus luteum induced by oxygen radical generation. Endocrinology. 1993;133:491–495. doi: 10.1210/endo.133.2.8344194. [DOI] [PubMed] [Google Scholar]
- Yang T, Zhang A, Honeggar M, Kohan DE, Mizel D, Sanders K, Hoidal JR, Briggs JP, Schnermann JB. Hypertonic induction of COX-2 in collecting duct cells by reactive oxygen species of mitochondrial origin. J. Biol. Chem. 2005;280:34966–34973. doi: 10.1074/jbc.M502430200. [DOI] [PubMed] [Google Scholar]
- Yang T, Zhang A, Pasumarthy A, Zhang L, Warnock Z, Schnermann JB. Nitric oxide stimulates COX-2 expression in cultured collecting duct cells through MAPkinases and superoxide but not cGMP. Am. J. Physiol. Renal Physiol. 2006;291:F891–F895. doi: 10.1152/ajprenal.00512.2005. [DOI] [PubMed] [Google Scholar]
- Zarrouki B, Soares AF, Guichardant M, Lagarde M, Géloën A. The lipid peroxidation end-product 4-HNE induces COX-2 expression through p38MAPK activation in 3T3-L1 adipose cell. FEBS Lett. 2007;581:2394–2400. doi: 10.1016/j.febslet.2007.04.048. [DOI] [PubMed] [Google Scholar]
- Zhang FP, Poutanen M, Wilbertz J, Huhtaniemi I. Normal prenatal but arrested postnatal sexual development of luteinizing hormone receptor knockout (LuRKO) mice. Mol. Endocrinol. 2001;15:172–183. doi: 10.1210/mend.15.1.0582. [DOI] [PubMed] [Google Scholar]
- Zimmermann S, Steding G, Emmen JM, Brinkmann AO, Nayernia K, Holstein AF, Engel W, Adham IM. Targeted disruption of the Insl3 gene causes bilateral cryptorchidism. Mol. Endocrinol. 1999;13:681–691. doi: 10.1210/mend.13.5.0272. [DOI] [PubMed] [Google Scholar]
- Zirkin BR, Chen H. Regulation of Leydig cell steroidogenic function during aging. Biol. Reprod. 2000;63:977–981. doi: 10.1095/biolreprod63.4.977. [DOI] [PubMed] [Google Scholar]
- Zirkin BR, Ewing LL. Leydig cell differentiation during maturation of the rat testis: a stereological study of cell number and ultrastructure. Anat. Rec. 1987;219:157–163. doi: 10.1002/ar.1092190208. [DOI] [PubMed] [Google Scholar]
- Zirkin BR, Santulli R, Strandberg JD, Wright WW, Ewing LL. Testicular steroidogenesis in the aging brown Norway rat. J. Androl. 1993;14:118–123. [PubMed] [Google Scholar]