Abstract
Objective
To identify genetic modifiers of β-blocker (BB) response and long-term survival in heart failure (HF).
Background
Differences in BB treatment effect between Caucasians and African Americans with HF have been reported.
Methods
Prospective cohort study of 2,460 patients (711 African American; 1,749 Caucasian) enrolled between 1999 and 2007. 2039 (81.7%) were treated with BB. Each was genotyped for β1-adrenergic receptor (ADRB1) Arg389>Gly and G-protein receptor kinase 5 (GRK5) Gln41>Leu polymorphisms, which are more prevalent among African Americans than Caucasians. Primary endpoint was survival time from HF onset.
Results
There were 765 deaths during follow up (median 46 months). BB treatment increased survival in Caucasians (Log Rank P=0.00038) but not African Americans (Log Rank P=0.327). Among patients not taking BB, ADRB1 Gly389 was associated with decreased survival in Caucasians (HR = 1.98, 95% CI = 1.1 − 3.7, P = 0.03) while GRK5 Leu41 was associated with improved survival in African Americans (HR = 0.325, CI = 0.133 − 0.796, P = 0.01). ADRB1 Gly389 GRK5 Gln41Gln African Americans derived similar survival benefit from BB therapy (HR = 0.385 95% CI = 0.182 − 0.813, P = 0.012) as ADRB1 Gly389 GRK5 Gln41Gln Caucasians (HR = 0.529, 95% CI = 0.326 − 0.858, P=0.0098).
Conclusions
These data demonstrate that differences caused by β-adrenergic receptor signaling pathway gene polymorphisms, rather than race, are the major factors contributing to apparent differences in BB treatment effect between Caucasians and African Americans; proper evaluation of treatment response should account for genetic variance.
Introduction
Heart failure affects approximately 5 million Americans, with over half a million new cases diagnosed every year (1). Abnormalities of cardiac β-adrenergic signaling that contribute to the pathophysiology of heart failure include increased circulating epinephrine levels and down-regulation or functional uncoupling of cardio-toxic β1-adrenergic receptors (2, 3). Accordingly, β-blockers, which antagonize catecholamine-stimulated beta adrenergic receptor signaling in the heart and elsewhere, represent one of the most important non-surgical therapeutic options for this disease, reducing morbidity and mortality (4, 5). There is a class I indication for β-blocker treatment in heart failure (6). However, individual responses to β-blocker treatment vary widely and there is a need to identify non-responders within the broader clinical group that shows aggregate benefit, as well as to predict responders within groups where treatment effects are less clear.
Variability in heart failure risk and clinical course is objectively revealed by population surveys and prospective clinical trials that have identified ethnic differences in disease incidence, progression, and response to specific therapies (7-9). Accordingly, the American College of Cardiology/American Heart Association guidelines for evaluation and management of heart failure in the adult concluded that “heart failure progresses more rapidly in black than white patients” (6). The mechanisms responsible for these types of differences have not been clearly identified, and undoubtedly include social influences, access to care, and the quality of care (10). Individual genetic factors may also play a role (11). Evidence is accumulating in support of specific genetic loci that contribute to the over-representation of other complex diseases, such as hypertension and type 2 diabetes mellitus, in individuals of African heritage (12-16). A genetic or pharmacogenomic basis for differences in drug effect between individuals of African and European descent has also been proposed (17), supported by associations between variable drug clearance and functionally significant polymorphisms of genes encoding enzymes important for drug metabolism, cytochrome P450 (CYPB6) and N-acetyltransferase (NAT2) (18-20). Thus, ethnically diverse populations exhibit differences in drug response that may be due, in part, to variations within genes essential to the drug effect.
In evaluating the potential for genetic variation to influence heart failure outcome, it is notable that functional polymorphisms are common in the genes encoding the β-blocker target, β-adrenergic receptors (ADRB1 and ADRB2), and a gene that critically regulates β-adrenergic receptor signaling, G-protein receptor kinase 5 (GRK5) (21, 22). Previous pharmacogenomic studies have proposed that the β1-adrenergic receptor (ADRB1) Arg389>Gly (23) and G-protein receptor kinase 5 (GRK5) Gln41>Leu (22) polymorphisms, both of which are over-represented in African Americans, may play roles in determining individual clinical responses to β-blockade in heart failure. The biological mechanisms for the effects of both alleles were established by expressing recombinant polymorphic proteins in cultured cells and in transgenic mice (22, 24). However, studies of their impact on heart failure outcome in different ethnic groups, and comparisons of these two putative genetic risk factors to standard clinical risk factors for heart failure progression, have not been performed. Here, we report the results of a prospective longitudinal study examining the impact of ADRB1 and GRK5 genotype on β-blocker modulation of long-term outcome in subjects with systolic heart failure who presented to the specialized heart failure/transplant programs of two major United States urban medical centers.
Methods
Study Subjects
Subjects presenting to the heart failure referral programs at the University of Cincinnati or the University of Pennsylvania were prospectively recruited into one of two non-interventional longitudinal genomics studies of heart failure funded by the NHLBI (P50 HL77101 and R01 HL88577). African American inclusion at >25% of the total cohort was part of the study design approved by NHLBI, and subgroup analysis of outcomes in Caucasians and African Americans was prespecified. Human study protocols were approved by Institutional Review Boards of the University of Cincinnati and the University of Pennsylvania. All subjects provided written informed consent. Enrollment criteria were age between 18 and 80 years and documented systolic heart failure with a left ventricular ejection fraction of less than 40%. The study recruited 2,460 heart failure patients, of which 711 (29%) were African American. 1,783 subjects (1164 Caucasian Americans and 619 African Americans) were enrolled between 2000 and 2007 in Cincinnati, and 677 subjects (585 Caucasian Americans and 92 African Americans) were enrolled in Philadelphia between 2003 and 2005. The cohorts were combined to provide a sufficient number of African Americans to power an analysis of racial subgroups. Racial classification was self-reported. The study endpoints were death or cardiac transplantation. Median follow-up was 46.3 months. β-blocker use was determined by the subjects' physicians (66% carvedilol, 24% metoprolol, 10% other β-blockers) and defined as continuous therapy for at least 6 months. Medication usage was confirmed at hospital clinic visits by personal interview. Follow-up data for each study subject was obtained at least yearly, either by personal interview, by mail, or by phone conversation.
Genotyping
Genomic DNA for genotyping was isolated and extracted using the Gentra Puregene genomic DNA purification kit (Qiagen, Valencia, CA). The DNA segments containing the region of interest were amplified with the polymerase chain reaction (PCR). PCR primers were designed using Primer3 online software (http://fokker.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi) (25), and pyrosequencing primers were designed using the Pyrosequencing SNP Primer Design Version 1.01 software (http://www.pyrosequencing.com). Before use, PCR primer sequences were screened across the human genome using the NCBI Blast program to ensure their specificity for the gene of interest. PCR and pyrosequencing were performed as previously described (26). Primers and conditions are listed in Supplemental Table 1. GRK5 Gln41Leu genotyping was performed by pyrosequencing (University of Cincinnati cohort) or using a Sequenome MassArray platform (University of Pennsylvania cohort) with conservative genotype calls in 99.8% of samples. ADRB1 Arg389Gly genotyping was performed using Assays-on-Demand (Applied Biosystems, Foster City, Calif) assay number C_8898494_10 according to the manufacturer's directions.
Statistical Analysis
Student's t-tests and chi-square tests were used to assess significant differences in variables between ethnic groups and between genotype classes within ethnic groups. Hardy-Weinberg Equilibrium (HWE) was assessed in each ethnic group separately. The primary outcome was time to transplantation or all-cause mortality through 350 months. Differences in time from diagnosis to endpoint were assessed using Kaplan-Meier curves and Log Rank tests (27). Relative risks were obtained by Cox Proportional Hazards modeling using an additive genetic model after adjustment for age at diagnosis and sex. All analyses were carried out using the R Statistical Language (http://www.R-project.org) (28). An alpha level of 0.05 was used to designate significance.
Results
Clinical Characteristics of the Study Population
Clinical characteristics of the heart failure study cohort, grouped by race, are in Table 1. The two racial groups were well matched in terms of age, height and weight, sex, and severity of left ventricular dysfunction. As has been noted previously (29, 30) hypertension, renal dysfunction, and cerebrovascular events are more common among African Americans with heart failure, but in this cohort, diabetes was only slightly more prevalent. Coronary artery disease and ischemic cardiomyopathy were more common in Caucasians with heart failure. As might be expected at tertiary referral centers specializing in heart failure, pharmacological treatment of heart failure was similar between the two ethnic groups, with ∼80-85% of subjects receiving a β-blocker (approximately two-thirds treated with carvedilol, one fourth with metoprolol, and 10% another agent), ∼75-80% receiving an ACE inhibitor, ∼22% receiving an angiotensin receptor blocker, and slightly over 30% receiving an aldosterone antagonist. (Fewer than 50 subjects in each group were treated with hydralazine/isosorbide dinitrate.) Similar proportions of both ethnic groups received automatic implanted defibrillators (24-29%). However, only half as many (∼7%) of African Americans underwent cardiac transplantation (averaging 74.7 months after diagnosis) as did Caucasians (∼15%; averaging 67.3 months after diagnosis). Average survival time from first objective heart failure diagnosis was 79.9 +/- 78.1 months (mean +/- S.D.) for Caucasians, compared to 69.8 +/- 68.6 months for African Americans (P = 0.17). Together, these data demonstrate that this population of heart failure study subjects recruited from the comprehensive heart failure/transplantation programs of two metropolitan medical schools has similar clinical characteristics and ethnic differences as observed in previous large heart failure trials (1, 6, 9, 29).
Table 1. Clinical characteristics of heart failure study population.
| Variable | Combined | Caucasian American | African American | |||
|---|---|---|---|---|---|---|
| N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | |
| Age at Enrollment (yrs) | 2440 | 53.4 (14.1) | 1698 | 54.4 (14.0) | 702 | 51.4 (14.1) |
| Height (in) | 2498 | 67.6 (4.2) | 1746 | 67.8 (4.2) | 710 | 67.2 (4.3) |
| Weight (lbs) | 2498 | 193.3 (50.7) | 1746 | 191.8 (48.7) | 710 | 197.7 (55.6) |
| Ejection Fraction (%) | 1911 | 31.2 (15.3) | 1545 | 31.4 (15.4) | 335 | 30.8 (14.7) |
| Follow-up Time (months) | 2421 | 67.6 (70.4) | 1682 | 70.9 (75.3) | 699 | 60.5 (57.0) |
| N | % | N | % | N | % | |
| Heart Failure Etiology | ||||||
| Ischemic | 1216 | 48.80% | 894 | 51.30% | 304 | 43.10% |
| Non-Ischemic | 1275 | 51.20% | 849 | 48.70% | 402 | 56.90% |
| Female | 896 | 35.80% | 555 | 31.70% | 323 | 45.40% |
| Diabetes | 870 | 35.00% | 585 | 33.40% | 272 | 38.40% |
| Hypertension | 1591 | 63.90% | 998 | 56.80% | 591 | 83.60% |
| Coronary disease | 1312 | 58.40% | 924 | 60.00% | 372 | 54.80% |
| Cerebrovascular event | 260 | 12.10% | 150 | 10.30% | 106 | 15.90% |
| Dyslipidemia | 1269 | 52.00% | 907 | 52.80% | 354 | 50.90% |
| Renal insufficiency | 592 | 25.90% | 355 | 22.50% | 233 | 34.10% |
| β-blocker use | 2039 | 81.70% | 1392 | 79.80% | 611 | 86.20% |
| carvedilol | 1348 | 66.00% | 926 | 66.50% | 396 | 64.60% |
| metoprolol | 488 | 23.90% | 327 | 23.50% | 154 | 25.10% |
| other | 206 | 10.10% | 140 | 10.10% | 63 | 10.30% |
| ACE inhibitor use | 1896 | 76.30% | 1302 | 74.40% | 572 | 80.90% |
| ARB use | 467 | 21.60% | 313 | 21.20% | 146 | 22.10% |
| Aldosterone antag use | 708 | 32.20% | 490 | 32.40% | 206 | 31.10% |
| Statin use | 1216 | 57.80% | 890 | 59.30% | 311 | 53.80% |
| AICD | 628 | 27.90% | 461 | 29.30% | 157 | 24.00% |
| Transplanted | 330 | 13.20% | 273 | 15.40% | 49 | 6.90% |
The impact of standard clinical risk factors on heart failure survival in the study cohort is shown in Table 2. All of the analyses presented in this table are univariate Cox proportional hazards models. Among Caucasians, increasing age, hypertension, coronary artery disease, and diabetes each approximately doubled the mortality risk, whereas among the smaller African American cohort only age and hypertension achieved significance as a risk factor for increased mortality, although the clear trend was for all three clinical factors to decrease survival. Male sex was a significant risk factor among African Americans, but not Caucasians. In contrast, the relationship between left ventricular ejection fraction at presentation and long-term clinical outcome was not consistent, as previously observed (29). The Cox proportional hazards models presented in the following sections are adjusted for age at heart failure onset and sex. Although other clinical factors were significantly associated with survival univariately, including age and sex in the Cox proportional hazards model greatly reduced or eliminated the significance of these variables' relationship with survival.
Table 2. Clinical factors affecting heart failure survival.
| Variable | Combined | Caucasian American | African American | |||
|---|---|---|---|---|---|---|
| HR | P value | HR | P value | HR | P value | |
| Age at Study Entry | 1.03 | < 0.0001 | 1.03 | < 0.0001 | 1.03 | 2.8E-9 |
| Female | 0.783 | 0.019 | 0.812 | 0.12 | 0.623 | 0.007 |
| Diabetes | 1.95 | 6.9E-12 | 2.19 | 3.9E-11 | 1.27 | 0.17 |
| Hypertension | 2.53 | < 0.0001 | 2.35 | 9.1E-12 | 2.09 | 0.0034 |
| Coronary disease | 1.69 | 4.2E-6 | 1.93 | 9.6E-6 | 1.42 | 0.055 |
| EF<25% | 0.85 | 0.11 | 0.98 | 0.87 | 0.634 | 0.03 |
β-blocker Treatment Effects on Mortality in Heart Failure
The group mean benefit of β-blocker treatment in heart failure is not disputable (6). However, results of individual studies have varied, particularly regarding the effects of β-blocker treatment in African Americans (31, 32). Factors that may have contributed to differing results include under-representation of African Americans in some trials (33), different effects of β-blockers with unique pharmacological characteristics (32), and real mechanistic differences in the magnitude of treatment effect between ethnic groups, as has been reported with ACE inhibitors versus other vasodilator therapy (34, 35). To determine if there were differences in apparent β-blocker treatment effect between Caucasians and African Americans within our study population, we examined mortality as a function of β-blocker treatment status in the overall heart failure cohort, and then separately in Caucasians and African Americans. A summary of the clinical characteristics stratified by β-blocker and race ethnicity is given in Table 3. In the combined cohort, β-blocker treatment significantly increased survival time (β-blocker Untreated N=455, β-blocker Treated N=2038, Log Rank P=0.00074, Figure 1a) and reduced mortality risk (age- and sex- adjusted Hazard Ratio = 0.71, 95% CI = 0.566 − 0.887, P=0.003). The effects of metoprolol and carvedilol appeared similar (not shown). When the survival data were analyzed in the Caucasian subgroup, increased survival with β-blocker treatment (β-blocker Untreated N=351, β-blocker Treated N=1391, Log Rank P=0.00038, Figure 1a top inset) and reduced risk of death or transplant (age- and sex-adjusted HR = 0.679, 95% CI = 0.519 − 0.888, P=0.005) were again statistically significant. However, a β-blocker treatment effect on survival was not as clear in the African American subgroup (β-blocker Untreated N=98, β-blocker Treated N=611, Log Rank P=0.327, Figure 1a bottom inset), with a trend toward reduced risk (age- and sex-adjusted HR=0.698, 95% CI = 0.453 − 1.08, P = 0.1). These data show that the HR for β-blocker treatment effect on mortality between the two races are similar, but the effects did not achieve statistical significance in African Americans because the confidence intervals are much broader, notwithstanding adequate numbers of subjects and experimental endpoints. Therefore, we further examined survival as a function of β-blocker treatment status in the two ethnic groups by comparing mortality within each treatment group. Survival times were not significantly different between Caucasians and African Americans in β-blocker untreated subjects (Figure 1b; Caucasian N=351, African American N=98, 82.8 +/- 69.3 months for African Americans versus 71.0 +/- 73.4 months in Caucasians, P=0.46, Log Rank P=0.5213), whereas among subjects taking β-blockers, African Americans had shorter survival times than Caucasians (Figure 1c; Caucasian N=1391, African American N=611, 66.8 +/- 68.4 months in African Americans versus 82.2 +/- 79.5 months in Caucasians, P=0.057, Log Rank P=0.0005). Thus, the mortality benefit from β-blockade appears greater in Caucasians than African Americans from these clinical populations.
Table 3. Characteristics of Heart Failure Subjects by β-blocker Use.
| Variables | Caucasians | African American | ||
|---|---|---|---|---|
| β-blocker Untreated | β-blocker Treated | β-blocker Untreated | β-blocker Treated | |
| n=351 | n=1391 | n=98 | n=611 | |
| Age at Enrollment (yrs) | 52.3 | 52.6 | 47.5 | 51.6 |
| Height (in) | 67.8 | 67.9 | 67.2 | 67.0 |
| Weight (lbs) | 179.8 | 194.6 | 197.0 | 198.0 |
| Ejection Fraction (%) | 32.0 | 31.1 | 32.2 | 30.6 |
| Follow-up (months) | 60.5 | 73.2 | 70.2 | 58.6 |
| % | % | % | % | |
| Heart Failure Etiology | ||||
| Ischemic | 51.6% | 48.1% | 64.3% † | 56.1% ‡ |
| Non-Ischemic | 48.4% | 51.9% | 35.7% | 43.9% |
| Female | 31.3% | 31.9% | 50.0% † | 44.7% ‡ |
| Diabetes | 37.8% | 38.7% | 30.6% | 34.6% |
| Hypertension | 75.5% | 84.9% | 53.7% *† | 57.1% ‡ |
| Coronary disease | 42.1% * | 56.8% | 54.2% * | 61.4% |
| Cerebrovascular event | 9.6% * | 17.0% | 15.4% | 9.2% ‡ |
| Dyslipidemia | 38.1% * | 53.1% | 43.6% * | 55.3% |
| Renal insufficiency | 36.5% * | 33.7% | 32.2% | 20.5% ‡ |
| ACE inhibitor use | 65.3% * | 83.4% | 63.3% | 77.4% ‡ |
| ARB use | 18.5% | 22.7% | 20.9% | 21.5% |
| Aldosterone antag use | 21.3% * | 32.7% | 24.3% * | 34.0% |
| Statin use | 41.0% * | 55.9% | 50.2% * | 61.6% ‡ |
| AICD | 8.1% * | 26.5% | 22.4% *† | 31.2% ‡ |
| Transplanted | 18.4% * | 5.1% | 39.3% *† | 9.6% ‡ |
| Achieved endpoint | 45.9% * | 23.8% | 59.8% *† | 25.3% |
P<0.05 versus β-blocker treated subjects of the same race
P<0.05 versus β-blocker untreated Caucasians
P<0.05 versus β-blocker treated Caucasians
Figure 1.
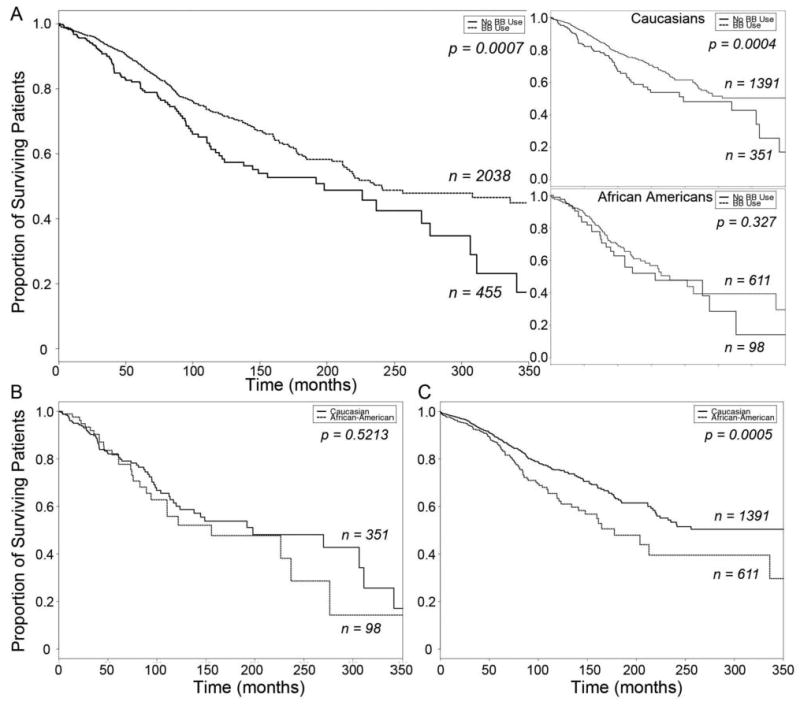
Kaplan-Meier Curves of β-blocker treatment effect on heart failure survival A. Combined heart failure cohort, stratified by β-blocker usage (top inset, Caucasian Americans; bottom inset, African Americans). Solid line, β-blocker untreated; dashed line, β-blocker treated. B. Survival of β-blocker untreated heart failure subjects, stratified by race. C. Survival of β-blocker treated heart failure subjects, stratified by race. Solid line, Caucasian; dashed line, African American.
ADRB1 Gly389 in Heart Failure
We considered that the broader confidence intervals for β-blocker treatment effect in African Americans might indicate a greater degree of inter-individual variability in this subgroup, possibly as a consequence of genetic factors. We further noted that the only two functionally significant β-receptor pathway gene polymorphisms reported to affect the response to β-blocker therapy in heart failure are both over-represented in African Americans (23, 36). The first reported such polymorphism substitutes Gly for Arg at position 389, within the signal transduction domain of the major cardiac β-blocker target, β1-adrenergic receptors (37). Therefore, we genotyped our cohort for this polymorphism and examined whether it was associated with difference in β-blocker treatment effect. Allele frequencies of the minor ADRB1 Gly389 allele were 0.298 in Caucasians and 0.392 in African Americans. Genotypes were in Hardy-Weinberg equilibrium (African American P = 0.67; Caucasian American P = 0.98) and similar to the reported allele frequencies in normal subjects (Caucasians, 0.12-0.25 and African Americans, 0.23-0.38, respectively (21, 36). Thus, as previously reported, this allelic variant is more common among African Americans than Caucasians, but its prevalence within racial sub-groups is similar between heart failure subjects and non-affected controls, supporting previous conclusions that this polymorphism does not, by itself, modify the risk of developing heart failure (38).
Functional allelic variants that are not independent risk factors for disease may nevertheless modify the course of that disease or its response to specific therapies. This may particularly apply to polymorphisms of β-receptor pathway genes in heart failure, because hyper-activation of cardiac catecholaminergic signaling occurs early in the course of the disease. In our study cohort, there were no differences in the clinical characteristics of heart failure subjects stratified by ADRB1 genotype (Table 3). Before adjusting for clinical risk modifiers, the ADRB1 Gly389 polymorphism tended to be associated with decreased heart failure survival times in subjects not taking β-blockers when examined for the overall cohort (Arg389 Homozygous N=543, Gly389 Carriers N=597, Log Rank P=0.0587; Figure 2a), or separately for Caucasians (Arg389 Homozygous N=422, Gly389 Carriers N=369, Log Rank P=0.0436; Figure 2a top inset), but not in African Americans (Arg389 Homozygous N=119, Gly389 Carriers N=223, Log Rank P=0.546; Figure 2a bottom inset). After adjusting for age- and sex-, however, the Gly389 association with increase mortality risk in β-blocker untreated subjects was clearly significant in the entire cohort (HR = 1.76, 95% CI = 1.09 − 2.85, P = 0.02) as well as in Caucasian Americans analyzed as a separate sub-group (HR = 1.98, 95% CI = 1.07 − 3.65, P = 0.03). (The number of African Americans not taking β-blockers and carrying the minor allele [N=34] was insufficient to properly power this subgroup analysis.) These results suggest a modest benefit for being homozygous (“wild-type”) Arg389 among β-blocker non-treated subjects, as has previously been reported (39).
Figure 2.
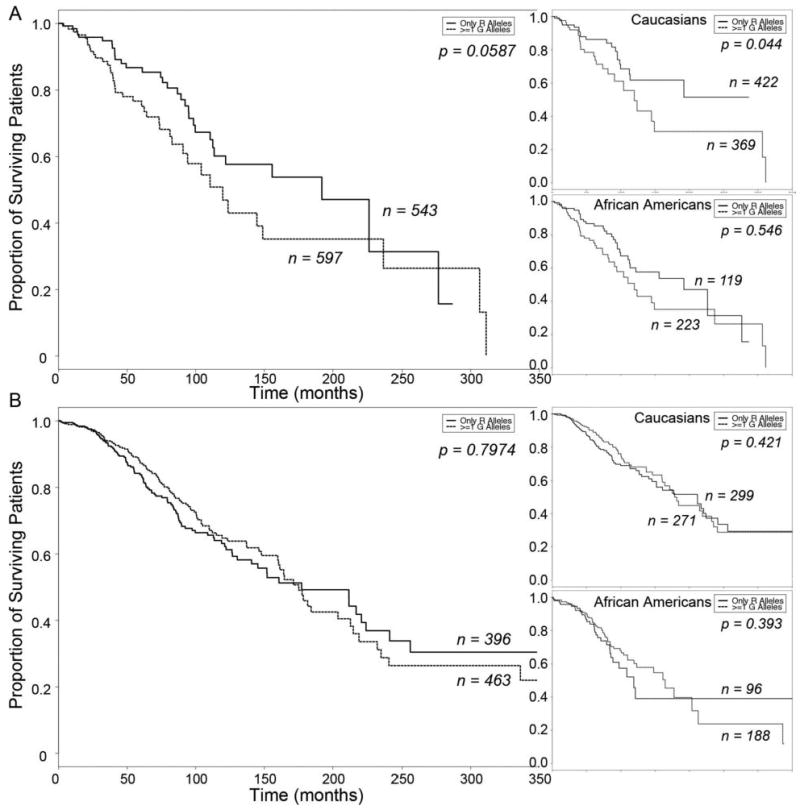
Kaplan-Meier Curves of ADRB1 Arg/Gly 389 effect on heart failure survival A. β-blocker untreated subjects stratified by ADRB1 389Gly carrier status (top inset, Caucasian Americans; bottom inset, African Americans). B. β-blocker treated subjects stratified by ADRB1 389Gly carrier status (top inset, Caucasian Americans; bottom inset, African Americans). Solid line, Arg/Arg 389; dashed line, Gly 389 carriers.
Among subjects treated with the β-blockers carvedilol or metoprolol (as were approximately 90% of the treated subjects in our study), there was no significant association between survival and ADRB1 Arg389Gly genotype in the entire cohort (Arg389 Homozygous N=396, Gly389 Carriers N=463, Log Rank P=0.7974, Figure 2b; age- and sex- adjusted HR = 0.953, 95% CI = 0.736 − 1.24, P = 0.501), or when ethnic groups were analyzed separately (for Caucasian Americans: Arg389 Homozygous N=299, Gly389 Carriers N=271, Log Rank P=0.421, Figure 2b top inset; adjusted HR = 0.884, 95% CI = 0.638 − 1.23, P = 0.46; for African Americans: Arg389 Homozygous N=96, Gly389 Carriers N=188, Log Rank P=0.3928, Figure 2b bottom inset; adjusted HR = 0.813, 95% CI = 0.515 − 1.28, P = 0.37). These data suggest that the Arg389 ADRB1 genotype does not predict a treatment effect for carvedilol or metoprolol.
GRK5 Leu41 in Heart Failure
The second adrenergic signaling polymorphism reported to affect the response to β-blocker therapy in heart failure substitutes Leu for Gln at position 41 of GRK5, one of the G-protein receptor kinases that desensitize cardiac β-adrenergic receptors (22, 40). The GRK5 Leu41 variant exhibits greater desensitization than the more common “wild-type” GRK5 Gln41 (41). For this reason, we genotyped the heart failure cohort and examined whether possessing this polymorphism was associated with any difference in heart failure outcome or β-blocker treatment effect. Allele frequencies of the minor GRK5 Leu41 allele were 0.017 for Caucasians and 0.231 for African Americans. Genotypes were in Hardy-Weinberg equilibrium (African American P = 0.63; Caucasians P =0.39) and are similar to the reported allele distributions in normal subjects (0.013 and 0.23 respectively (22). Except for slight differences in age at enrollment, there were no differences in the clinical characteristics between subjects homozygous for wild-type GRK5 Leu41 polymorphism verses those carrying at least one polymorphic GRK5 Gln41 allele (Table 4).
Table 4. Characteristics of Heart Failure Subjects by ADRB1 and GRK5 Genotype.
| Variable | ADRB1 | ADRB1 | GRK5 | GRK5 | ||||
|---|---|---|---|---|---|---|---|---|
| Caucasians | African American | Caucasians | African American | |||||
| Gly389 | Arg389 | Gly389 | Arg389 | Gln41 | Leu41 | Gln41 | Leu41 | |
| n=369 | n=422 | n=223 | n=119 | n=1587 | n=53 | n=374 | n=263 | |
| Age at Enrollment (yrs) | 51.2 | 50.5 | 48.4 | 50.1 | 52.5 | 46.5* | 51.6 | 45.5* |
| Height (in) | 67.9 | 67.9 | 67.8 | 67.0 | 67.9 | 68.0 | 67.5 | 67.0 |
| Weight (lbs) | 191.1 | 192.1 | 204.9 | 194.7 | 191.2 | 191.9 | 199.9 | 197.5 |
| Ejection Fraction (%) | 32.1 | 31.0 | 30.8 | 34.8 | 31.4 | 29.7 | 33.2 | 30.2 |
| Follow-up (months) | 79.2 | 80.0 | 75.5 | 79.7 | 73.7 | 60.3* | 63.8 | 61.8 |
| % | % | % | % | % | % | % | % | |
| Heart Failure Etiology | ||||||||
| Ischemic | 50.0% | 54.7% | 45.4% | 40.4% | 49.6% | 56.6% | 41.7% | 42.6% |
| Non-Ischemic | 50.0% | 45.3% | 54.6% | 59.6% | 50.4% | 43.4% | 58.3% | 57.4% |
| Female | 47.1% | 47.5% | 31.5% | 32.0% | 31.6% | 26.4% | 43.9% | 48.3% |
| Diabetes | 36.4% | 39.6% | 29.0% | 42.2% | 32.6% | 45.3% | 39.3% | 33.7% |
| Hypertension | 66.1% | 59.9% | 86.6% | 84.7% | 54.6% | 60.4% | 81.7% | 83.2% |
| Coronary disease | 58.0% | 60.6% | 48.2% | 40.1% | 57.7% | 64.3% | 51.0% | 52.6% |
| Cerebrovascular event | 16.4% | 16.0% | 17.5% | 19.1% | 10.6% | 5.7% | 14.5% | 17.8% |
| Dyslipidemia | 56.4% | 51.1% | 37.9% | 43.9% | 51.1% | 56.9% | 50.4% | 48.2% |
| Renal insufficiency | 35.7% | 42.3% | 39.1% | 42.7% | 23.1% | 28.9% | 39.3% | 31.1% |
| β-blocker use | ||||||||
| carvedilol | 50.7% | 56.6% | 57.1% | 63.7% | 54.8% | 67.9% | 57.4% | 55.7% |
| metoprolol | 16.1% | 13.3% | 19.3% | 17.0% | 20.1% | 11.3% | 23.3% | 23.3% |
| other | 4.0% | 3.8% | 4.2% | 4.0% | 4.2% | 0% | 4.3% | 7.6% |
| ACE inhibitor use | 81.0% | 81.3% | 84.0% | 86.6% | 74.5% | 77.4% | 80.9% | 82.1% |
| ARB use | 29.6% | 24.4% | 25.7% | 26.0% | 21.8% | 19.5% | 22.2% | 22.3% |
| Aldosterone antag use | 33.7% | 33.6% | 34.6% | 35.0% | 33.1% | 44.4% | 29.3% | 36.5% |
| Statin use | 62.5% | 64.5% | 50.0% | 46.9% | 58.2% | 59.5% | 53.5% | 51.2% |
| AICD | 35.9% | 35.9% | 25.5% | 24.7% | 30.0% | 37.8% | 25.4% | 25.1% |
| Transplanted | 24.9% | 26.8% | 10.9% | 8.5% | 16.4% | 18.9% | 7.2% | 7.6% |
| Achieved endpoint | 49.1% | 51.7% | 44.5% | 39.9% | 33.6% | 45.3% | 30.2% | 27.5% |
P<0.05 versus major allele within same sub-group.
In an aggregate analysis of β-blocker untreated subjects there was no evidence for an effect of GRK5 Leu41 genotype on heart failure survival (Gln41 Homozygous N=390, Leu41 Carriers N=47, Log Rank P=0.8211; Figure 3a) or for decreased mortality risk (age- and sex-adjusted HR for death = 0.755, 95% CI = 0.399 − 1.43, P = 0.39). When the data were analyzed according to ethnic group, we found only 3 subjects in the Caucasian subgroup that carried a Leu41 allele, were not taking β-blockers, and achieved the mortality endpoint during the study period (Figure 3a top inset). Thus, we are underpowered for this analysis. Among β-blocker untreated African Americans however, in which nearly equal numbers of subjects carry a GRK5 Leu41 allele verses are homozygous Gln41 “wild-type”, the presence of at least one GRK5 Leu41 was associated with longer survival (Gln41 Homozygous N=56, Leu41 Carriers N=35, Log Rank P=0.0181; Figure 3a, bottom inset), and with a significant decrease in mortality risk after adjustment for age and sex (HR = 0.325, CI = 0.133 − 0.796, P = 0.01).
Figure 3.
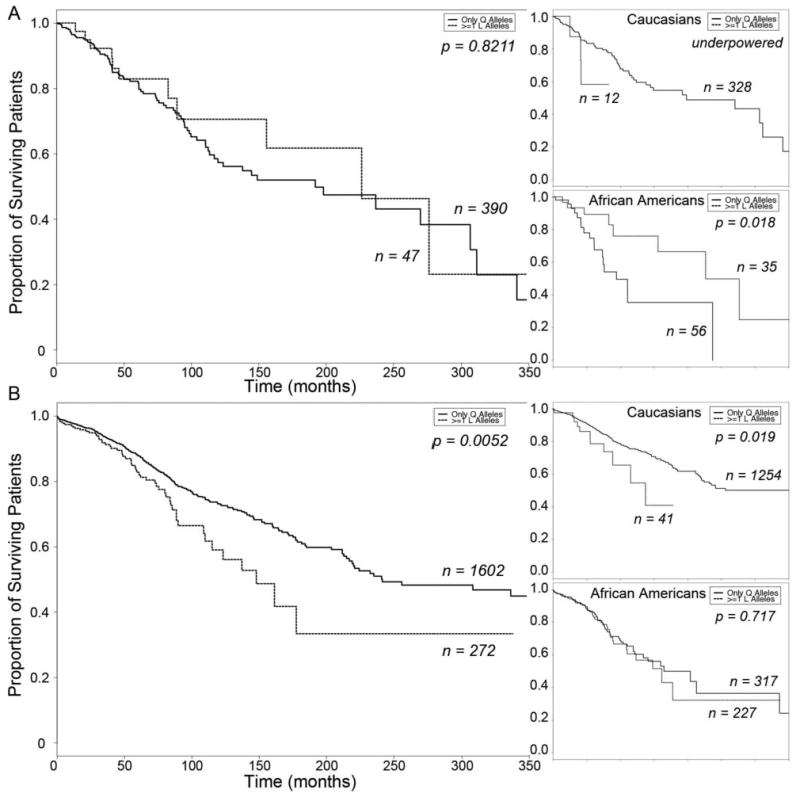
Kaplan-Meier Curves of GRK5 Gln/Leu 41 effect on heart failure survival. A. β-blocker untreated subjects stratified by GRK5 41Leu carrier status (top inset, Caucasian Americans; bottom inset, African Americans). B. β-blocker treated subjects stratified by GRK5 41Leu carrier status (top inset, Caucasian Americans; bottom inset, African Americans). Solid line, Gln/Gln 41; dashed line, Leu 41 carriers.
Interestingly, when β-blocker treated subjects within the cohort were analyzed as a whole for consequences of the GRK5 polymorphism, a significant detrimental effect of GRK5 Leu41 was detected (Gln41 Homozygous N=1602, Leu41 Carriers N=272, Log Rank P=0.0052, Figure 3b). However, the apparent difference by genotype is actually attributable to the general difference in outcome observed between β-blocker treated Caucasians and African Americans (compare Figure 3b with Figure 1c), because the Leu41 variant is ten-times more common among African Americans, and there were only 10 Caucasian subjects that carried a Leu41 allele, were taking β-blockers, and reached the study endpoint (Figure 3b, top inset). In contrast, among β-blocker treated African American subjects (in whom there was roughly equal distribution of homozygous Gln41 wild-type versus Leu41 carriers), there was no association between GRK5 Gln41 polymorphism genotype and heart failure outcome (Gln41 Homozygous N=317, Leu41 Carriers N=227, Log Rank P=0.7166, Figure 3b bottom inset).
Heart Failure Risk Profiling Using Clinical and Genetic Factors
Genotyping of our study cohort for two functional β-AR signaling polymorphisms revealed one genotype, ADRB1 Arg389, that is associated with significantly decreased mortality risk in β-blocker untreated Caucasians, and another, GRK5 Leu41, that is associated with decreased mortality risk in β-blocker untreated African Americans, with no evidence for a multiplicative interaction between these ADRB1 and GRK5 variants. We considered that these genetic factors might contribute to the apparent differences in heart failure outcomes and β-blocker response observed between Caucasians and African Americans in our cohort, and re-examined outcome in subjects matched for these two polymorphisms, i.e. in ADRB1 Gly389 GRK5 Gln41Gln individuals. When ADRB1 Gly389 GRK5 Gln41Gln patients were analyzed, β-blocker treatment significantly increased survival time (β-blocker Untreated N=110, β-blocker Treated N=371, Log Rank P = 0.00058, Figure 4a) and reduced mortality risk (age- and sex- adjusted Hazard Ratio = 0.530, 95% CI = 0.357 − 0.788, P=0.0017) in the combined cohort. The same was true for β-blocker treatment in Caucasians (β-blocker Untreated N=87, β-blocker Treated N=256, Log Rank P = 0.0053, Figure 4a top inset; age- and sex- adjusted HR = 0.529, 95% CI = 0.326 − 0.858, P=0.0098). When this analysis was applied to African Americans without considering genotype, it had failed to show a β-blocker treatment effect in this cohort (see Figure 1a, right inset). However, when the ADRB1 Gly389 GRK5 Gln41Gln subgroup of African Americans was analyzed, β-blocker treatment enhancement of survival was evident (β-blocker Untreated N=22, β-blocker Treated N=111, Log Rank P = 0.0373, Figure 4a bottom inset), as was a significant reduction in mortality risk (age- and sex- adjusted HR = 0.385 95% CI = 0.182 − 0.813, P = 0.012). Survival times were similar in genotype-matched Caucasian and African American β-blocker untreated subjects (Caucasian N=87, African American N=22, Log Rank P= 0.2368, Figure 4b; age- and sex- adjusted HR = 1.46, 95% CI = 0.611 − 3.51, P= 0.39) and β-blocker treated subjects (Caucasian N=256, African American N=111, Log Rank P= 0.2283, Figure 4c), although there was still a trend toward shorter survival times in β-blocker treated African Americans after adjusting for age and sex (adjusted HR = 1.55, 95% CI = 0.992 − 2.43, P= 0.054). Another way to conceptualize the interaction of genotype and β-blocker treatment is to examine the effect of genotype within treatment class. By comparing the ADRB1 Gly389 GRK5 Gln41Gln genotype to all other genotypes pooled, we found that genotype had no effect among β-blocker treated subjects (Caucasians: HR=0.854, P=0.35; African Americans: HR=0.829, P=0.39) but did have a significant effect among β-blocker untreated Caucasians (Caucasians: HR=2.09, P=0.02; African Americans: HR=1.77, P=0.19). These data suggest that in this heart failure population differences caused by β-adrenergic receptor signaling pathway gene polymorphisms, rather than race, are the major factor contributing to apparent differences in β-blocker treatment effect between Caucasians and African Americans.
Figure 4.
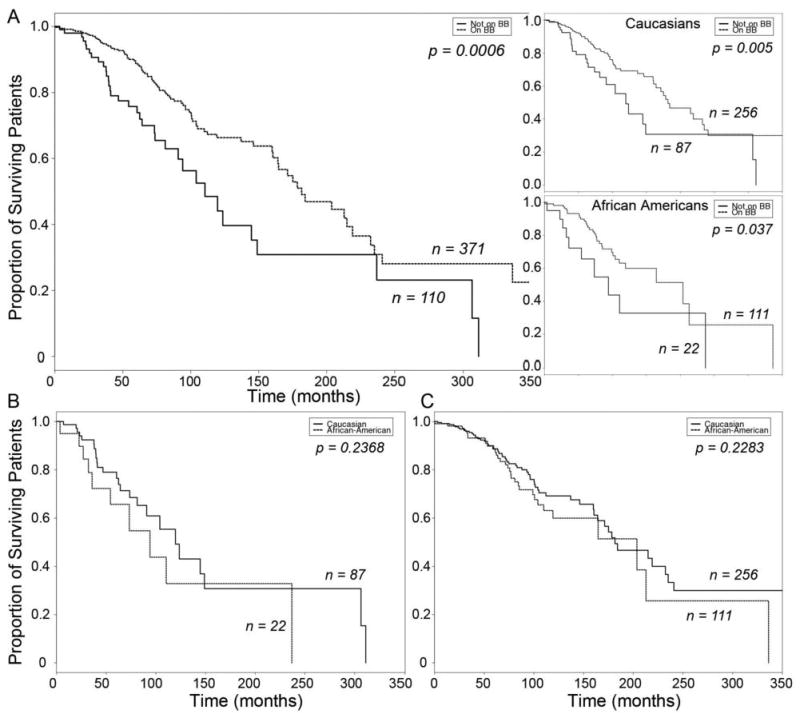
Kaplan-Meier Curves of β-blocker treatment effect on heart failure survival in subjects matched for ADRB1 Gly 389 and GRK5 Gln 41 homozygous genotype. A. Combined heart failure cohort, stratified by β-blocker usage (top inset, Caucasian Americans; bottom inset, African Americans). Solid line, β-blocker untreated; dashed line, β-blocker treated. B. Survival of β-blocker untreated heart failure subjects, stratified by race. C. Survival of β-blocker treated heart failure subjects, stratified by race. Solid line, Caucasian; dashed line, African American.
Discussion
The major finding of this study is that genetic polymorphisms of the major cardiac β-blocker target, the β1-adrenergic receptor, and a kinase that terminates its signaling, GRK5, can significantly impact heart failure outcomes, and that adjusting for these gene variants abrogates the apparent ethnic differences in β-blocker treatment effect on heart failure survival.
In heart failure patients with similar clinical presentations, some will have a more rapid disease course, whereas disease progression is less aggressive in others (42, 43), and the benefits of recent advances in pharmacological and mechanical therapies for heart failure have not been equally shared among all patient groups. In particular, management of heart failure remains a challenge for the African American population. African Americans have a 50% higher prevalence of heart failure, with earlier and more severe onset of disease and decreased survival (9, 44). There are many possible reasons for racial differences in health care outcome, including differences in heart failure etiology, clinical risk factors or management practices, as well as the absence of pre-specified analysis of African Americans as a separate sub-group in many large clinical trials and a tendency for African Americans to be under-represented in these studies (45). Our study was specifically designed to have proportional representation of African Americans and to minimize differences in clinical risk factors and management practices that may contribute to ethnic differences in heart disease. Thus, heart failure patients were recruited from two large municipal tertiary referral heart failure/transplant programs, affording a consistent standard of care that has been shown to reduce or eliminate ethnic differences in mortality (46). The two ethnic cohorts were well-matched for ventricular function, ACE inhibitor and β-blocker treatment, and AICD use. Nevertheless, African Americans had significantly higher mortality rates, a less clear response to β-blocker therapy, and were transplanted only half as often as Caucasians. For this reason, the endpoint for these studies was all-cause mortality, and our multi-variate risk factor analysis adjusted for differences in sex and age.
The mortality from treated heart failure exceeds that of most cancers, (47) and β-blockers are one of only two drug classes that increase survival (the other being ACE inhibitors). β-blockers are in general highly effective in prolonging survival in heart failure patients, but inter-individual differences in treatment effect continue to complicate disease management. Therefore, our focus in these studies was on two critical components of the signaling pathway targeted by β-blockers, the β1-adrenergic receptor itself (48), and GRK5, an abundant kinase in myocardium that terminates β-adrenergic receptor signaling (40, 41, 49). The β1-adrenergic receptor, encoded by the ADRB1gene, is the major cardiac target of β-blockers. Unique to humans is a common polymorphic variation of ADRB1 encoding either Gly or Arg at amino acid 389, located within the intracellular G-protein coupling domain (23). This region of β1-adrenergic receptor is highly conserved between species, with the human Gly variant being the only known instance of divergence from Arg at this position. The functional consequence of introducing Gly is diminished coupling between the β1-adrenergic receptor variant and Gαs (50) and decreased responsiveness to β-blockers in genetic mouse models (51). Human studies have been inconsistent as to whether there are meaningful associations between this polymorphism and heart failure outcome (24, 38, 39, 52-58). The more common Arg389 β1-adrenergic receptor exhibits enhanced functional coupling, is less prevalent in African Americans, and is associated with a favorable response to the experimental β-blocker, bucindolol, whereas carriers of the Gly389 minor allele reportedly shows no survival benefit from bucindolol (23). An investigation of adrenergic receptor gene polymorphisms on cardiovascular outcomes in the Woman's Ischemia Syndrome Evaluation (39) found that carriers of the Gly389 allele were at increased risk for heart failure and all cause mortality. The current findings of a trend for improved heart failure survival in Caucasians homozygous for Arg389, and the experimental findings of Akhter, et al that Arg389 transgenic mice recover from ischemic insults faster than Gly389 mice (59), also suggest a more complex role for this β-receptor variant in primary myocardial disease than has generally been recognized.
The other polymorphism we evaluated is ten-times more common in African Americans than Caucasian Americans, substituting Leu for Gln at position 41 of GRK5. GRK5 helps to terminate β-adrenergic receptor signaling by phosphorylating and uncoupling agonist-occupied receptors from their Gαs signal transducers (40, 49). Amino acid 41 is in a putative regulatory domain for the kinase, and (as with the β1-adrenergic receptor 389 polymorphism) GRK5 amino acid sequence is conserved at this position across human, bovine, mouse, rat, dog, and zebrafish homologs, except for the human variant (22). Compared to the “wild-type” GRK5, the Leu41 variant promotes more rapid agonist-mediated desensitization, phosphorylation, and internalization of β1-adrenergic receptor (41), and mimics β-blockers in genetic mouse models (22). In a previous report, which is the only report to date of this polymorphism in a human study, we described a pharmacogenomic interaction with β-blockers for the combined endpoint of cardiac transplant or death in a small cohort of African Americans with heart failure (22). Here, we have greatly expanded the number of heart failure subjects studies, doubling the number of African American study subjects to 711, and compared outcomes to almost 1,800 Caucasians recruited from the same two academic centers. In so doing, we demonstrate a significant increase in heart failure survival in African Americans carrying at least one GRK5 Leu41 allele.
Our findings that the gene modifier effects for the ADRB1 389 polymorphism have greater significance for Caucasians, and for the GRK5 41 polymorphism are significant only in African Americans do not necessarily indicate different disease mechanisms between these ethnic groups. Both the ADRB1 and GRK5 SNPs exhibit significant differences in prevalence between populations of European and African descent, and natural variations in polymorphism allele frequency between different ethnic groups can result in correspondingly different pathological roles for these genetic events in different study populations. For example, the 8q24 locus associated with prostate cancer is associated with a higher prevalence of this disease among African Americans not because of a greater individual gene effect in African American individuals, but because the risk allele occurs with greater frequency in this population, and thereby contributes to increased incidence (60). Thus, the most important aspect of the current results is not what impact specific adrenergic signaling pathway polymorphisms have on heart failure, because these effects have been shown to vary between different populations, with different treatment practices, and in different cardiovascular conditions (24, 38, 39, 52-58). Instead, the take-home message is that SNPs such as these two that clearly alter functional pathways in experimental systems have the potential to produce variability of disease prognosis and outcome in the same manner as clinical risk modifiers. Therefore, proper evaluation of disease risk and treatment response should account for genetic variance, either by matching subjects by relevant genotype as herein, or by including relevant genotypes in multi-variate models.
Supplementary Material
Acknowledgments
This work was funded by R01s HL087871 and HL088577, and NIH Specialized Center for Clinically-Oriented Research (SCCORs) in Cardiac Dysfunction and Disease P50 HL077101 and P50 HL077113, and the Penn Cardiovascular Institute. This publication was also made possible by Grant Number UL1 RR024992 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Footnotes
Author contributions: Drs. Cresci, Cappola, Kelly, Kardia and Dorn had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Financial Disclosure: None reported.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.American Heart Association. Dallas, Texas: American Heart Association; 2001. 2002 Heart and Stroke Statistical Update. Available at: http://heartnet.bjmu.edu.cn/epi/usa/2002update.htm. [Google Scholar]
- 2.Kaye DM, Smirk B, Finch S, Williams C, Esler MD. Interaction between cardiac sympathetic drive and heart rate in heart failure: modulation by adrenergic receptor genotype. J Am Coll Cardiol. 2004;44:2008–15. doi: 10.1016/j.jacc.2004.07.058. [DOI] [PubMed] [Google Scholar]
- 3.Lefkowitz RJ, Rockman HA, Koch WJ. Catecholamines, cardiac beta-adrenergic receptors, and heart failure. Circulation. 2000;101:1634–7. doi: 10.1161/01.cir.101.14.1634. [DOI] [PubMed] [Google Scholar]
- 4.Bristow MR. beta-adrenergic receptor blockade in chronic heart failure. Circulation. 2000;101:558–69. doi: 10.1161/01.cir.101.5.558. [DOI] [PubMed] [Google Scholar]
- 5.Domanski MJ, Krause-Steinrauf H, Massie BM, et al. A comparative analysis of the results from 4 trials of beta-blocker therapy for heart failure: BEST, CIBIS-II, MERIT-HF, and COPERNICUS. J Card Fail. 2003;9:354–63. doi: 10.1054/s1071-9164(03)00133-7. [DOI] [PubMed] [Google Scholar]
- 6.Hunt SA, Baker DW, Chin MH, et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to revise the 1995 Guidelines for the Evaluation and Management of Heart Failure) Journal of American College of Cardiology. 2001;38:2101–13. doi: 10.1016/s0735-1097(01)01683-7. [DOI] [PubMed] [Google Scholar]
- 7.Gillum RF. Heart failure in the United States 1970-1985. Am Heart J. 1987;113:1043–5. doi: 10.1016/0002-8703(87)90077-9. [DOI] [PubMed] [Google Scholar]
- 8.Gillum RF. The epidemiology of cardiovascular disease in black Americans. N Engl J Med. 1996;335:1597–9. doi: 10.1056/NEJM199611213352110. [DOI] [PubMed] [Google Scholar]
- 9.Dries DL, Exner DV, Gersh BJ, Cooper HA, Carson PE, Domanski MJ. Racial differences in the outcome of left ventricular dysfunction. N Engl J Med. 1999;340:609–16. doi: 10.1056/NEJM199902253400804. [DOI] [PubMed] [Google Scholar]
- 10.Smedley BD, Stith AY, Nelson AR. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care (IOM 2002 Report) National Academies Press; 2003. [PubMed] [Google Scholar]
- 11.Yancy CW. Executive summary of the African-American Initiative. MedGenMed. 2007;9:28. [PMC free article] [PubMed] [Google Scholar]
- 12.Young JH, Chang YP, Kim JD, et al. Differential susceptibility to hypertension is due to selection during the out-of-Africa expansion. PLoS Genet. 2005;1:e82. doi: 10.1371/journal.pgen.0010082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deo RC, Patterson N, Tandon A, et al. A high-density admixture scan in 1,670 African Americans with hypertension. PLoS Genet. 2007;3:e196. doi: 10.1371/journal.pgen.0030196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu X, Cooper RS. Admixture mapping provides evidence of association of the VNN1 gene with hypertension. PLoS ONE. 2007;2:e1244. doi: 10.1371/journal.pone.0001244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Helgason A, Páisson S, Thorleifsson G, et al. Refining the impact of TCF7L2 gene variants on type 2 diabetes and adaptive evolution. Nat Genet. 2007;39:218–25. doi: 10.1038/ng1960. [DOI] [PubMed] [Google Scholar]
- 16.Leak TS, Keene KL, Langefeld CD, et al. Association of the proprotein convertase subtilisin/kexin-type 2 (PCSK2) gene with type 2 diabetes in an African American population. Mol Genet Metab. 2007;92:145–50. doi: 10.1016/j.ymgme.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campbell M, Tishkoff S. African Genetic Diversity: Implications for Human Demographic History, Modern Human Origins, and Complex Disease Mapping. Annual Review of Genomics and Human Genetics. 2008;9:403–33. doi: 10.1146/annurev.genom.9.081307.164258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehlotra RK, Bockarie MJ, Zimmerman PA. CYP2B6 983T>C polymorphism is prevalent in West Africa but absent in Papua New Guinea: implications for HIV/AIDS treatment. Br J Clin Pharmacol. 2007;64:391–5. doi: 10.1111/j.1365-2125.2007.02884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nyakutira C, Röshammar D, Chiqusta E, et al. High prevalence of the CYP2B6 516G-->T(*6) variant and effect on the population pharmacokinetics of efavirenz in HIV/AIDS outpatients in Zimbabwe. Eur J Clin Pharmacol. 2008;64:357–65. doi: 10.1007/s00228-007-0412-3. [DOI] [PubMed] [Google Scholar]
- 20.Patin E, Barreiro LB, Sabeti PC, et al. Deciphering the Ancient and Complex Evolutionary History of Human Arylamine N-Acetyltransferase Genes. Am J Hum Genet. 2006;78:423–36. doi: 10.1086/500614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brodde OE. β-1 and β-2 adrenoceptor polymorphisms: Functional importance, impact on cardiovascular diseases and drug responses. Pharmacology & Therapeutics. 2008;117:1–29. doi: 10.1016/j.pharmthera.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Liggett SB, Cresci S, Kelly RJ, et al. A GRK5 polymorphism that inhibits β-adrenergic receptor signaling is protective in heart failure. Nat Med. 2008;14:510–7. doi: 10.1038/nm1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liggett SB, Mialet-Perez J, Thaneemit-Chen S, et al. A polymorphism within a conserved beta(1)-adrenergic receptor motif alters cardiac function and beta-blocker response in human heart failure. Proc Natl Acad Sci U S A. 2006;103:11288–93. doi: 10.1073/pnas.0509937103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mialet PJ, Rathz DA, Petrashevskaya NN, et al. Beta 1-adrenergic receptor polymorphisms confer differential function and predisposition to heart failure. Nat Med. 2003;9:1300–5. doi: 10.1038/nm930. [DOI] [PubMed] [Google Scholar]
- 25.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–86. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 26.Marsh S, King CR, Garsa AA, McLeod HL. Pyrosequencing of clinically relevant polymorphisms. Methods Mol Biol. 2005;311:97–114. doi: 10.1385/1-59259-957-5:097. [DOI] [PubMed] [Google Scholar]
- 27.Parmar M, Machin D. Survival Analysis: a practical approach. New York: John Wiley and Sons; 1995. [Google Scholar]
- 28.R Development Core Team. A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria: 2008. [Google Scholar]
- 29.Rathmore SS, Foody JM, Wang Y, et al. Race, quality of care, and outcomes of elderly patients hospitalized with heart failure. Journal of American Medical Association. 2003;289:2517–24. doi: 10.1001/jama.289.19.2517. [DOI] [PubMed] [Google Scholar]
- 30.Yancy CW, Strong M. The natural history, epidemiology, and prognosis of heart failure in African Americans. Congest Heart Fail. 2004;10:15–8. doi: 10.1111/j.1527-5299.2004.02026.x. [DOI] [PubMed] [Google Scholar]
- 31.Yancy CW, Fowler MB, Colucci WS, et al. Race and the Response to Adrenergic Blockade with Carvedilol in Patients with Chronic Heart Failure. N Engl J Med. 2001;344:1358–65. doi: 10.1056/NEJM200105033441803. [DOI] [PubMed] [Google Scholar]
- 32.Eichhorn EJ, Domanski MJ, Krause-Steinrauf H, Bristow MR, Lavori PW. A Trial of the Beta-Blocker Bucindolol in Patients with Advanced Chronic Heart Failure. N Engl J Med. 2001;344:1659–67. doi: 10.1056/NEJM200105313442202. [DOI] [PubMed] [Google Scholar]
- 33.The Merit HF Investigators. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF) Lancet. 1999;353:2001–7. [PubMed] [Google Scholar]
- 34.Carson P, Ziesche S, Johnson G, Cohn JN. Racial differences in response to therapy for heart failure: analysis of the vasodilator-heart failure trials. Vasodilator-Heart Failure Trial Study Group. Journal of Cardiac Failure. 1999;5:178–87. doi: 10.1016/s1071-9164(99)90001-5. [DOI] [PubMed] [Google Scholar]
- 35.Exner DV, Dries DL, Domanski MJ, Cohn JN. Lesser Response to Angiotensin-Converting–Enzyme Inhibitor Therapy in Black as Compared with White Patients with Left Ventricular Dysfunction. N Engl J Med. 2009;344:1351–7. doi: 10.1056/NEJM200105033441802. [DOI] [PubMed] [Google Scholar]
- 36.Dorn GW, II, Liggett SB. Pharmacogenomics of beta-adrenergic receptors and their accessory signaling proteins in heart failure. Clinical and Translational Medicine. 2008;1:255–62. doi: 10.1111/j.1752-8062.2008.00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Podlowski S, Wenzel K, Luther HP, et al. Beta1-adrenoceptor gene variations: a role in idiopathic dilated cardiomyopathy? Journal of Molecular Medicine. 2000;78:87–93. doi: 10.1007/s001090000080. [DOI] [PubMed] [Google Scholar]
- 38.Liggett SB, Mialet-Perez J, Thaneemit-Chen S, et al. A polymorphism within a conserved beta(1)-adrenergic receptor motif alters cardiac function and beta-blocker response in human heart failure. Proc Natl Acad Sci U S A. 2006;103:11288–93. doi: 10.1073/pnas.0509937103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pacanowski MA, Zineh I, Li H, et al. Adrenergic gene polymorphisms and cardiovascular risk in the NHLBI-sponsored Women's Ischemia Syndrome Evaluation. Journal of Translational Medicine. 2008;6:11. doi: 10.1186/1479-5876-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kohout TA, Lefkowitz RJ. Regulation of G protein-coupled receptor kinases and arrestins during receptor desensitization. Mol Pharmacol. 2003;63:9–18. doi: 10.1124/mol.63.1.9. [DOI] [PubMed] [Google Scholar]
- 41.Wang WC, Mihlbachler KA, Bleecker ER, Weiss ST, Liggett SB. A polymorphism of G-protein coupled receptor kinase5 alters agonist-promoted desensitization of beta2-adrenergic receptors. Pharmacogenet Genomics. 2008;18:729–32. doi: 10.1097/FPC.0b013e32830967e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diaz RA, Obasohan A, Oakley CM. Prediction of outcome in dilated cardiomyopathy. Br Heart J. 1987;58:393–9. doi: 10.1136/hrt.58.4.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Felker GM, Thompson RE, Hare JM, et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342:1077–84. doi: 10.1056/NEJM200004133421502. [DOI] [PubMed] [Google Scholar]
- 44.Yancy CW. Heart failure in African Americans: a cardiovascular engima. J Card Fail. 2000;6:183–6. doi: 10.1054/jcaf.2000.17610. [DOI] [PubMed] [Google Scholar]
- 45.Hall WD. Representation of blacks, women, and the very elderly (aged > or = 80) in 28 major randomized clinical trials. Ethn Dis. 1999;9:333–40. [PubMed] [Google Scholar]
- 46.Pamboukian SV, Costanzo MR, Meyer P, Bartlett L, Mcleod M, Heroux A. Influence of race in heart failure and cardiac transplantation: Mortality differences are eliminated by specialized, comprehensive care. Journal of Cardiac Failure. 2003;9:80–6. doi: 10.1054/jcaf.2003.11. [DOI] [PubMed] [Google Scholar]
- 47.Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 48.Bristow MR. Changes in myocardial and vascular receptors in heart failure. J Am Coll Cardiol. 1993;22:61A–71A. doi: 10.1016/0735-1097(93)90465-d. [DOI] [PubMed] [Google Scholar]
- 49.Premont RT, Koch WJ, Inglese J, Lefkowitz RJ. Identification, purification, and characterization of GRK5, a member of the family of G protein-coupled receptor kinases. J Biol Chem. 1994;269:6832–41. [PubMed] [Google Scholar]
- 50.Mason DA, Moore JD, Green SA, Liggett SB. A gain-of-function polymorphism in a G-protein coupling domain of the human beta1-adrenergic receptor. J Biol Chem. 1999;274:12670–4. doi: 10.1074/jbc.274.18.12670. [DOI] [PubMed] [Google Scholar]
- 51.Mialet PJ, Rathz DA, Petrashevskaya NN, et al. Beta 1-adrenergic receptor polymorphisms confer differential function and predisposition to heart failure. Nat Med. 2003;9:1300–5. doi: 10.1038/nm930. [DOI] [PubMed] [Google Scholar]
- 52.White HL, de Boer RA, Magbool A, et al. An evaluation of the beta-1 adrenergic receptor Arg389Gly polymorphism in individuals with heart failure: a MERIT-HF sub-study. European Journal of Heart Failure. 2003;5:463–8. doi: 10.1016/s1388-9842(03)00044-8. [DOI] [PubMed] [Google Scholar]
- 53.De Groote P, Helbecque N, Lamblin N, et al. Association between beta-1 and beta-2 adrenergic receptor gene polymorphisms and the response to beta-blockade in patients with stable congestive heart failure. Pharmacogenet Genomics. 2005;15:137–42. doi: 10.1097/01213011-200503000-00001. [DOI] [PubMed] [Google Scholar]
- 54.Terra SG, Hamilton KK, Pauly DF, et al. Beta1-adrenergic receptor polymorphisms and left ventricular remodeling changes in response to beta-blocker therapy. Pharamcogenet Geonomics. 2005;15:227–34. doi: 10.1097/01213011-200504000-00006. [DOI] [PubMed] [Google Scholar]
- 55.Chen L, Meyers D, Javorsky G, et al. Arg389Gly-beta1-adrenergic receptors determine improvement in left ventricular systolic function in nonischemic cardiomyopathy patients with heart failure after chronic treatment with carvedilol. Pharmacogenet Genomics. 2007;17:941–9. doi: 10.1097/FPC.0b013e3282ef7354. [DOI] [PubMed] [Google Scholar]
- 56.Biolo A, Clausell N, Santos KG, et al. Impact of beta1-adrenergic receptor polymorphisms on susceptibility to heart failure, arrhythmogenesis, prognosis, and response to beta-blocker therapy. Am J Cardiol. 2008;102:726–32. doi: 10.1016/j.amjcard.2008.04.070. [DOI] [PubMed] [Google Scholar]
- 57.Sehnert AJ, Daniels SE, Elashoff M, et al. Lack of association between adrenergic receptor genotypes and survival in heart failure patients treated with carvedilol or metoprolol. Journal of American College of Cardiology. 2008;52:644–51. doi: 10.1016/j.jacc.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 58.Kurnik PB, Li C, Sofowora GG, et al. Beta-1-adrenoceptor genetic variants and ethnicity independently affect response to beta-blockade. Pharamcogenet Geonomics. 2008;18:895–902. doi: 10.1097/FPC.0b013e328309733f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Akhter SA, D'Souza KM, Petrashevskaya NN, Mialet-Perez J, Liggett SB. Myocardial beta1-adrenergic receptor polymorphisms affect functional recovery after ischemic injury. American Journal of Physiology Heart and Circulatory Physiology. 2006;290:H1427–H1432. doi: 10.1152/ajpheart.00908.2005. [DOI] [PubMed] [Google Scholar]
- 60.Haiman CA, Patterson N, Freedman ML, et al. Multiple regions within 8q24 independently affect risk for prostate cancer. Nat Genet. 2007;39:638–44. doi: 10.1038/ng2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


