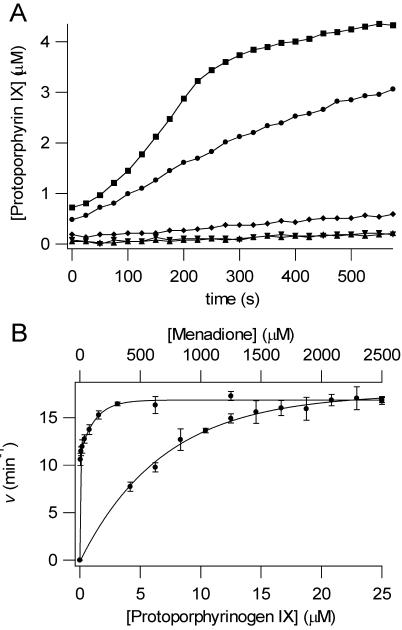
Figure 3. Enzymatic conversion of protoporphyrinogen IX to protoporphyrin IX by HemG.
A. PPO assays are shown for human PPO (■), 100 nM HemG + 1 mM menadione (●), 100 nM HemG (◆), 1 mM menadione (▼), and protoporphyrinogen alone (▲). In the absence of menadione HemG is capable of generating only minimal amounts of product when compared to autooxidation. In the presence of saturating menadione concentrations, enzymatic activity is greatly increased to levels that are comparable to human PPO. B. Determination of apparent Michaelis constants for HemG. 100 nM HemG was assayed using varying concentrations of protoporphyrinogen IX (bottom curve) and menadione (top curve). Data were fitted to single rectangular hyperbolae (Eq. 2). Km and Kcat for protoporphyrinogen IX = 7.0 μM and 17.52 min-1; Km and Kcat for menadione = 3.76 μM and 16.87 min-1.