To the Editor:
F2-isoprostanes have been advocated as a specific and reliable marker of oxidative damage (1) despite a lack of data on their biological and analytical variation. Concurrent with a longitudinal study of oxidative stress during the menstrual cycle [the BioCycle Study (2)], we have characterized the components of biological and analytical variation of free plasma F2-isoprostanes in the context of the menstrual cycle. We describe several of the key characteristics of F2-isoprostanes as a biomarker.
Samples were collected from 9 healthy volunteers who met the major inclusion criteria of being a premenopausal woman 18-44 years of age and having regular menstrual cycles. Major exclusion criteria included use of hormonal preparations or contraceptive devices, pregnancy, a history of gynecologic abnormalities, recent infectious disease, routine intake of prescription and over-the-counter medications (including vitamin and mineral supplements), and a history of certain chronic diseases or conditions. Fasting blood samples were collected between 0700 and 0830 on days 2, 7, 12, 13, 14, 18, 22, and 27 of a single menstrual cycle. Collection and handling protocols were designed to minimize preanalytical variation. Samples were frozen at -80 °C and then analyzed, in duplicate, as a single set of consecutive samples by GC-MS (1) at the Molecular Epidemiology and Biomarker Research Laboratory at the University of Minnesota. Samples were analyzed within 6 months of collection.
The SAS software package (version 9.1.3; SAS Institute) was used for all analyses. We log transformed F2-isoprostane data and used the following nested ANOVA model (3): Yijk = μ + subi + dayj(i) + ek(ji), where Yijk describes the value for the kth replicate (k = 1, 2) on the jth day (j = 1, 2, 3, . . . , 8) for the ith individual (i = 1, 2, 3, . . . , 9), μ is the grand mean, subi is the random effect of the ith individual, dayj(i) is the random effect of the jth day nested in the ith individual, and ek(ji) is the random effect of the kth replicate nested in the jth day and the ith individual. Proportions of total variation from sources between (subi) and within (dayj(i)) individuals and from analytical factors (ek(ji)) were estimated from the relevant variance components , , and , respectively. We determined CVs for each variance component and calculated the acceptability index (AI)1 for quality specification.
The intraclass correlation coefficient (ICC) was defined as:
the index of individuality (II) was defined as:
Assuming homogeneity of variances, we defined the critical difference (CD) as:
Finally, we defined the number of samples, k, necessary to estimate the individual-specific mean as:
where D represents the acceptable deviation from the mean.
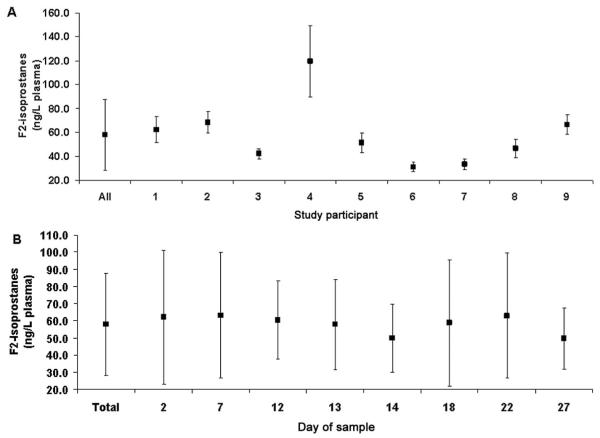
Participants had a mean (SD) age of 38.5 (4.7) years, and the mean menstrual cycle length was 28.4 (1.3) days. One individual was African American, and the remaining 8 (88.9%) were white. F2-isoprostanes were measured in 120 of 144 possible samples (9 participants × 8 time points × 2 replicate measurements). A duplicate sample was unavailable for 1 individual on days 2, 7, and 12; for 1 individual on days 2 and 22; and for 1 individual on days 7, 18, and 22. Fig. 1A presents F2-isoprostane concentrations for each study participant, and Fig. 1B presents the data with respect to each day in the menstrual cycle.
Fig. 1.
Mean (SD) concentrations of F2-isoprostanes measured in plasma by individual (A) and by day of the menstrual cycle when the sample was collected (B).
Most of the variation in F2-isoprostane concentrations occurred between study individuals (approximately 85%; ), whereas within-individual variation (approximately 13%; ) and analytical variation (approximately 2%; ) were smaller. Within-individual imprecision (CVW = 3.7%) exceeded that for analytical factors (CVA = 1.5%), for an AI value of 0.41; thus analytical variation increased within-individual variation by only 8% (i.e., total ), thereby meeting “desirable” performance specifications (4). The high ICC value of 0.97 (95% CI, 0.96-0.98) ensured that decreases in study power due to analytical factors would necessitate a compensatory increase in sample size of 11%, at most.
The II value of 0.42 suggests that population reference limits for the detection of “abnormal” values are likely to be of limited utility. Use of a CD criterion suggests that a minimum difference of 1.0 ng/L between serial samples might be nonstochastic in nature. Estimation of the individual-specific mean F2-isoprostane concentration within 5%, 10%, or 20% of the actual mean value will require 13.8, 3.5, or 0.9 simultaneously assayed samples, respectively.
The number of participants in this study was small; however, studies on biological variation have demonstrated that estimates of intraindividual and interindividual variation are similar regardless of the number of individuals studied (5). Our estimates of variation agree with those of other studies that did not span the menstrual cycle and suggest that plasma F2-isoprostanes are sufficiently reliable for use as a biomarker of oxidative damage in epidemiologic studies of women.
Acknowledgments
Role of Sponsor: The funding organizations played a direct role in the design of the study, in the choice of enrolled patients, in the review and interpretation of data, and in the preparation and final approval of the manuscript.
Footnotes
Authors’ Disclosures of Potential Conflicts of Interest: Upon manuscript submission, all authors completed the Disclosures of Potential Conflict of Interest form. Potential conflicts of interest:
Employment or Leadership: None declared.
Consultant or Advisory Role: M. Trevisan, Johnson & Johnson.
Stock Ownership: None declared.
Honoraria: M. Trevisan, Johnson & Johnson.
Research Funding: National Institute of Child Health and Human Development (Contract no. ADB-N01-HD-4-3394).
Expert Testimony: None declared.
- AI
- acceptability index
- ICC
- intraclass correlation coefficient
- II
- index of individuality
- CD
- critical difference
References
- 1.Milne GL, Yin H, Brooks JD, Sanchez S, Jackson Roberts L, 2nd, Morrow JD. Quantification of F2-isoprostanes in biological fluids and tissues as a measure of oxidant stress. Methods Enzymol. 2007;433:113–26. doi: 10.1016/S0076-6879(07)33006-1. [DOI] [PubMed] [Google Scholar]
- 2.Wactawski-Wende J, Schisterman EF, Hovey KM, Howards PP, Browne RW, Hediger M, et al. the BioCycle Study Group BioCycle study: design of the longitudinal study of the oxidative stress and hormone variation during the menstrual cycle. Paediatr Perinat Epidemiol. 2009;23:171–84. doi: 10.1111/j.1365-3016.2008.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fraser CG. Biological variation: from principles to practice. AACC Press; Washington (DC): 2001. p. 151. [Google Scholar]
- 4.Cotlove E, Harris EK, Williams GZ. Biological and analytic components of variation in long-term studies of serum constituents in normal subjects. 3. Physiological and medical implications. Clin Chem. 1970;16:1028–32. [PubMed] [Google Scholar]
- 5.Sebastián-Gámbaro MA, Lirón-Hernández FJ, Fuentes-Arderiu X. Intra- and inter-individual biological variability data bank. Eur J Clin Chem Clin Biochem. 1997;35:845–52. [PubMed] [Google Scholar]