Summary
The transcription factor Nuclear Factor-Erythroid 2 (NF-E2) is overexpressed in the vast majority of patients with polycythaemia vera (PV). In murine models, NF-E2 overexpression increases proliferation and promotes cellular viability in the absence of erythropoietin (EPO). EPO-independent growth is a hallmark of PV. We therefore hypothesized that NF-E2 overexpression contributes to erythrocytosis, the pathognomonic feature of PV. Consequently, we investigated the effect of NF-E2 overexpression in healthy CD34+ cells. NF-E2 overexpression led to a delay in erythroid maturation, manifested by a belated appearance of glycophorin A-positive erythroid precursors. Maturation delay was similarly observed in primary PV patient erythroid cultures compared to healthy controls. Protracted maturation led to a significant increase in the accumulated number of erythroid cells both in PV cultures and in CD34+ cells overexpressing NF-E2. Similarly, NF-E2 overexpression altered erythroid colony formation, leading to an increase in BFU-E formation. These data indicate that NF-E2 overexpression delays the early phase of erythroid maturation, resulting in an expansion of erythroid progenitors, thereby increasing the number of erythrocytes derived from one CD34+ cell. These data propose a role for NF-E2 in mediating the erythrocytosis of PV.
Keywords: myeloproliferative disorders, transcription factors, CD34+ cells
Introduction
The description of a point mutation in the gene encoding Janus kinase 2 (JAK2V617F) in the vast majority of patients with polycythaemia vera (PV) as well as around 50% of patients with essential thrombocythaemia (ET) and primary myelofibrosis (PMF) underscores the interrelatedness of the three Ph—-myeloproliferative disorders (MPDs) (Baxter et al, 2005, James et al, 2005, Kralovics et al, 2005a, Levine et al, 2005). In a murine transplantation model, the JAK2V617F mutation recapitulated many features of PV, including erythrocytosis, in some cases thrombocytosis, and the development of myelofibrosis (Lacout et al, 2006, Wernig et al, 2006, Zaleskas et al, 2006). These data demonstrate that the JAK2V617F allele is sufficient to induce polycythaemia in a murine model and suggest that it plays a pivotal role in causing erythrocytosis in PV patients.
However, recently, compelling evidence has arisen that the JAK2V617F mutation is not the disease-initiating alteration in MPDs. Firstly, in families with an inherited predisposition to the development of MPDs, JAK2V617F is not inherited through the germline (Bellanné-Chantelot et al, 2006, Cario et al, 2005). Secondly, in some female patients, the percentage of JAK2V617F–alleles is significantly lower than would be predicted from the fraction of clonal granulocytes present (Kralovics et al, 2006, Nussenzveig et al, 2007). Thirdly, in around 50% of JAK2V617F–positive MPD patients who develop leukaemia, the leukaemic blasts are negative for JAK2V617F (Campbell et al, 2006, Theocharides et al, 2007). In selected patients who, in addition to the JAK2V617F mutation, carry a clonal chromosomal abnormality, marking the progeny of the MPD stem cell, it was possible to show that the JAK2V617F–negative leukaemic blasts retained the chromosomal abnormality and thus arise from the MPD stem cell (Theocharides et al, 2007). It is therefore likely that an initial molecular event precedes acquisition of the JAK2V617F mutation (Kralovics et al, 2006).
We recently described overexpression of the transcription factor NF-E2 in over 90% of PV patients tested (Goerttler et al, 2005). The degree of NF-E2 overexpression correlated with the JAK2 genotype and the percentage of JAK2V617F mutant allele expressed (Kralovics et al, 2005b, Vannucchi et al, 2006). Whether NF-E2 overexpression is mediated by the JAK2V617F mutation, or whether it is the result of independent alterations is currently being investigated in our laboratory.
Several published observations make NF-E2 an exceptionally promising candidate for the molecular aetiology of PV. It is expressed in haematopoietic precursors as well as in erythroid, megakaryocytic and granulocytic cells (Andrews et al, 1993, Toki et al, 1996). As a stem cell disorder in which trilineage hyperplasia is frequently observed, PV affects precisely these cell types. Moreover, using antisense oligonuceotides, Labbaye et al. (1995) have demonstrated that NF-E2 is required for erythroid colony formation.
Sayer et al. (2000) have previously shown that overexpression of NF-E2 in a murine erythroid cell line increases cell proliferation and promotes spontaneous morphological erythroid maturation in the absence of erythropoietin (EPO). Moreover, in murine fetal liver cells NF-E2 overexpression led to the development of EPO-independent erythroid colonies (Sayer et al, 2000). EPO-independent erythroid colonies, termed “endogenous erythroid colonies (EECs)”, are characteristically and specifically observed in PV patients (Prchal and Axelrad 1974) (reviewed in (Westwood and Pearson 1996)). In addition, ectopic expression of NF-E2 in the murine myeloid cell line M1 led to the spontaneous emergence of erythroid cells (Sayer et al, 2000). Taken together, these data demonstrate that, in murine cells, augmenting the level of NF-E2 can promote erythroid maturation in the absence of EPO and can reprogram precursor cells towards erythroid differentiation.
We therefore hypothesized that NF-E2 overexpression contributes to erythrocytosis, the pathognomonic feature of PV. To test this hypothesis, we investigated the effect of NF-E2 overexpression on the erythroid differentiation and maturation of primary human CD34+ cells.
Patients, Materials and Methods
Patients
Peripheral blood (PB) samples were obtained from therapeutic phlebotomies of PV patients who fulfilled the World Health Organization (WHO) criteria for diagnosis (WHO 2001), either from the University Hospital Freiburg, the Department of Haematology, Careggi Hospital, Florence, Italy or of the University of Chicago, Chicago, IL. Buffy coats of healthy volunteer blood donors were obtained either from the University Hospital Freiburg or from the transfusion centre at “La Sapienza” University, Rome, Italy. The study protocol was approved by the local ethics committees and informed consent was obtained from all patients. Each patient was assigned a unique patient number (UPN), which was used thereafter for the protection of privacy. In total, 41 healthy controls and 26 PV patients were analysed. All PV patients were tested for the presence of the JAK2V617F mutation by quantitative reverse transcription polymerase chain reaction (qRT-PCR) as previously described (Steimle et al, 2007).
Isolation of CD34+ Cells
CD34+ cells were purified from peripheral blood samples by dextran sedimentation followed by Ficoll-Paque (Pharmacia, Freiburg, Germany) separation (Kruisbeck et al, 1991) and antibody-based magnetic bead separation (MACS Miltenyi, Bergisch Gladbach, Germany). Prior to purification, the percentage of CD34+ cells in phlebotomies of PV patients ranged from 0.13% to 0.65%.
NFE2 mRNA Quantification and Western Blotting
NFE2 mRNA levels were determined as previously described (Goerttler et al, 2005). NF-E2 protein was detected with a polyclonal rabbit antibody directed against a peptide comprising amino acids 133 to 146 of the NF-E2 protein, generated in our laboratory, and a horseradish peroxidase (HRP)-coupled donkey-anti-rabbit secondary antibody (Amersham, München, Germany). GAPDH and beta-actin were detected using commercial antibodies (CSA-335, Stressgen, Hamburg, Germany and Cat.-No: A5441, Sigma-Aldrich, Taufkirchen Germany, respectively) and a HRP-coupled sheep-anti-mouse secondary antibody.
Erythroid Differentiation Medium
Erythroid differentiation medium consisted of StemSpan® Serum-Free Expansion Medium (SFEM) (09600, StemCell Technologies, Vancouver, BC, Canada) supplemented with 50 ng/ml recombinant human (rh) stem cell factor (SCF) (300-07, PeproTech, Rocky Hill, NJ), 1 IU/ml EPO (Erypo FS 4000, Ortho Biotech, Bridgewater, NJ), 50 IU/ml rh interleukin 3 (rhIL3) (200-03, PeproTech), 40 ng/ml Human Low Density Lipoprotein (4004, Harbor Bio-Products, Norwood, MA), 100 IU/ml Penicillin, 100 mg/ml Streptomycin (DE17-602E, Cambrex, North Brunswick, NJ) and 2 μl/ml Primocin (VZA-1021, Amaxa Biosystems, Cologne, Germany). Cultures were initially started in volumes ranging from 500 μl to 1 ml. Cells were maintained at a concentration of 2 × 105/ml, verified by daily counting and addition of medium.
Retroviral Transduction of CD34+ Cells
NFE2 cDNA was cloned into the pMYSIG-GFP retroviral vector (Kitamura et al, 2003), a kind gift of Dr. C. Stocking, Heinrich-Pette-Institut, Hamburg. Retroviral pseudotypes were prepared with the envelope protein of the feline endogenous retrovirus RD114. Human CD34+ cells were infected as previously described (Schiedlmeier et al, 2003). Briefly, between 1 and 3 × 105 cells (2 × 105 cells/ml) were prestimulated for 2 days in serum-free medium (StemSpan® SFEM, 09650, Stem Cell Technologies) with 100 ng/ml rhSCF (300-07, PeproTech), 100 ng/ml rhFLT-3Ligand (300-19, PeproTech) and 20 ng/ml each rhIL6 (200-06, PeproTech) and rh thrombopoetin (TPO) (300-18, PeproTech). Infections were performed in 6-well plates coated with retronectin® (T100B, Takara, Otsu, Shiga, Japan). Two cycles of retroviral infection were performed over a 48-h period using a multiplicity of infection (MOI) between 8 and 20. Transduction efficiencies ranged from 40 – 60% with the empty pMYSIG-GFP vector and 25 – 60% with pMYSIG-NF-E2-GFP. Following transduction, GFP-positive cells were sorted by fluorescent-activated cell sorting (FACS) and analysed as described.
FACS Analysis
Cells were stained with a phycoerythrin (PE)-conjugated anti-CD235a/GpA antibody (clone GA-R2 (HIR2)) as well as an allophycocyanin (APC)-conjugated anti-CD36 antibody (clone CB38), or their isotype controls, IgG2bk-PE (clone 27-35) and IgMk-APC (clone G155-228), all from BD Biosciences, Franklin Lakes, NJ, and analysed on a FACS Calibur (BD Biosciences) using both CellQuest (BD Biosciences) and FlowJo softwares (FlowJo, Ashland, OR).
Morphological Analysis
Cell smears were obtained by cyto-centrifugation (Shandon, Astmoor, England) and cell morphology revealed by May-Grünwald-Giemsa (Sigma-Aldrich) staining.
Benzidine Staining
Cytospin preparations were fixed in 100% Methanol for 15 s, and subsequently stained for 5 min with 1% 3,3′-Dimethoxybenizidine (o-Dianisidine, D9143, Sigma-Aldrich) followed by an incubation in 30% H2O2 (diluted 1:12 with 70% Ethanol) for 2.5 min. After washing, the slides were counter-stained with Giemsa (Accustain® Giemsa Stain, GS500-500ML Sigma-Aldrich) for 30 min, washed again and air-dried.
RNA Interference (RNAi)
Suppression of NF-E2 expression was achieved both by siRNA and by shRNA interference. Both approaches gave identical results. For suppression of NF-E2 by siRNA, between 1 and 2 × 105 CD34+ cells were cultured in erythroid differentiation medium (see above) at a concentration of 5 × 104 cells / ml for 6 days prior to nucleofection with either a negative control siRNA (Cat No 4611, Ambion, Austin, TX) or an siRNA directed against NF-E2 at a concentration of 1 μM (Cat. No. 16804, ID 3346, Ambion). Nucleofection was performed with the Nucleofector 2 (Amaxa, Koeln, Germany) using the U-008 program and the CD34+ nucleofection solution (Cat. No. 51332, ID 602, Amaxa). Twenty-four hours after nucleofection, cells were seeded in methylcellulose (1000 cells/ml) to assess colony formation.
For suppression of NF-E2 by shRNA, a lentiviral vector, pLEGO-G (Weber et al, 2008), was engineered to express either a 19 bp nucleotide shRNA against NF-E2, 5′ GGA TTA TCC CTC AAC TAT A 3′ or a scrambled version of the same nucleotides, 5′ CAC GGA TGT AAT TCC AAT T 3′ which was used as a control. Lentiviruses were produced from these constructs by transient CaPO4-mediated transfection of 293T cells with the lentiviral backbone plasmid, a vsv.g-glycoprotein encoding plasmid and a gag/pol plasmid. Titres of 5 × 106 IU/ml were regularly achieved and concentrated by ultracentrifugation to 1 × 108 IU/ml. Lentiviral infection of CD34+ cells was achieved by prestimulating between 1 and 3 × 105 cells (5 × 104 cells/ml) for 4 h in serum-free medium (StemSpan® SFEM, 09650, Stem Cell Technologies), 100 ng/ml rhSCF (300-07, PeproTech), 100 ng/ml rhFLT-3 ligand (300-19, PeproTech) and 20 ng/ml each rhIL6 (200-06, PeproTech) and rhTPO (300-18, PeproTech). Infections were performed in 6-well plates without the addition of a transduction facilitator. Two cycles of lentiviral infection were performed over a 48 hour period using MOIs between 5 and 20. Transduction efficiencies ranged from 20 – 40% with both the control and the NF-E2-shRNA vector. Following transduction, GFP-positive cells were sorted by FACS and analysed as described.
Colony Assays
Cells were seeded in methylcellulose media (1000 cells/ml) containing either SCF, IL-3, granulocyte-macrophage colony-stimulating factor (GM-CSF) and EPO (H4434, Stem Cell Technologies) or containing only SCF, IL-3 and GM-CSF but no EPO (H4534, Stem Cell Technologies) and incubated for 14 days at 37°C, 5% CO2. Erythroid colony-forming unit (CFU-E) growth was scored on day 8, erythroid burst-forming unit (BFU-E) growth on day 14. Endogenous erythroid colony (EEC) growth was scored on day 14. Colonies were counted by independent observers, blinded to the experimental conditions.
MNC Expansion Culture
10 ×106 - 20 ×106light density blood cells were separated by Ficoll (Hypaque, Amersham Pharmacia Biotec, Uppsala, Sweden) and cultured at a density of 1 × 106/ml in Iscove's Modified Dulbecco's Medium (IMDM; Invitrogen, Carlsbad, CA) containing fetal bovine serum (FBS, 20% v/v, Hyclone, Logan, UT) and Bovine Serum Albumin (BSA, 4% w/v, Sigma-Aldrich). The cultures were stimulated with optimal concentrations of SCF (10ng/mL, Amgen, Thousand Oaks, CA) and EPO (3U/mL, Epoetina alfa, Dompè Biotec, Milan, Italy), and sub-optimal concentrations of IL3 (1ng/mL, Bouty, Milan, Italy) as described (Migliaccio et al, 2002). The cultures were replenished with fresh medium and growth factors every 3 - 4 days to maintain a concentration of 1 × 106/ ml. After 13 days, cells were collected for morphological and molecular analysis.
Statistical Analysis
Statistical differences between groups were determined by Kruskal-Wallis One Way Analysis of Variance (ANOVA) on Ranks. A p value < 0.05 was considered statistically significant.
Results
NF-E2 is overexpressed in PV erythroid cells
In order to test whether PV erythroid cells display increased levels of NF-E2, we isolated CD34+ cells from PV patients and healthy controls. In freshly isolated CD34+ cells, which constitute a heterogeneous population of stem cells, there was no difference in NF-E2 expression between patients with PV and healthy controls (data not shown). Subsequently, we cultured the CD34+ cells in an erythroid differentiation medium containing EPO, IL-3 and SCF (Ugo et al, 2004). After 5 days of culture, RNA was harvested and NFE2 expression quantitated by qRT-PCR. In cultured erythroid precursor cells, PV patients displayed 2- to 4-fold higher NFE2 levels than healthy controls (Fig. 1A). In order to ensure that elevated NFE2 expression in PV patients was not merely a reflection of modified erythroid differentiation or maturation in PV cultures, the percentage of early and late erythroid progenitors (CD36+/GpA- and CD36+/GpA+ respectively, see Fig. 2) was determined. Healthy controls and PV patients contained similar percentages of both early and late erythroid progenitors in these cultures (Fig. 1B). Hence, NFE2 overexpression is not a reflection of altered maturation, but represents an intrinsic feature of PV erythroid cells.
Figure 1. NFE2 mRNA expression and erythroid maturation in cultures of PV patients and healthy controls.
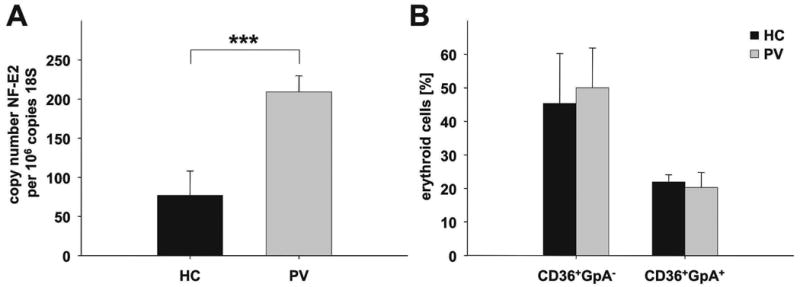
CD34+ cells were isolated from PV patients and healthy controls (HC) (n = 3 each) and cultured in erythroid differentiation medium (50 ng/ml SCF, 50 IU/ml IL-3, 1IU/ml EPO). On day 5, cells were harvested and (A) NFE2 mRNA expression quantitated by qRT-PCR. Each sample was measured in duplicate, data are depicted as copy number NF-E2 per 106 copies of 18S rRNA. The mean and standard deviation are shown, *** p = 0.004. (B) Erythroid differentiation assessed by CD36 and glycophorin A (GpA, CD235a) staining. The percentage of immature erythroid precursors (CD36+/GpA-) and mature erythroid precursor cells (CD36+/GpA+) was determined. Mean and standard deviation of the mean are shown.
Figure 2. Assessment of erythroid maturation by FACS analysis.
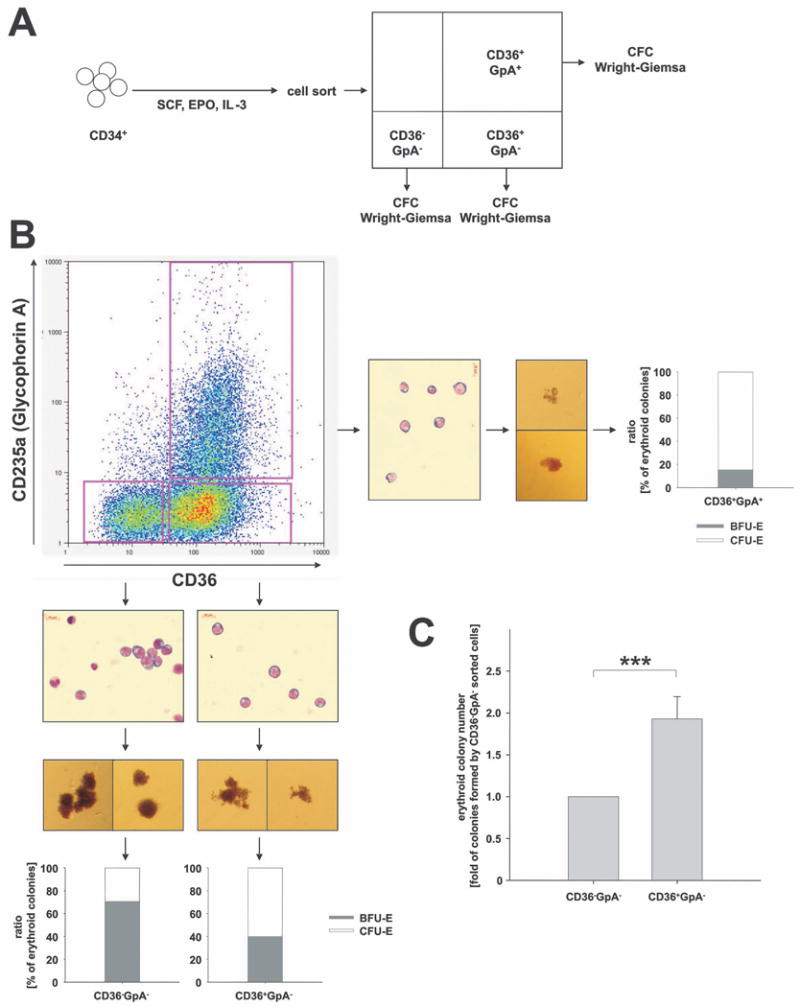
(A) Experimental design: Peripheral blood CD34+ cells from healthy volunteers were cultured in erythroid differentiation medium (SCF, IL-3 and EPO) and subsequently FACS sorted according to CD36 and GpA surface expression. Sorted populations were assayed for colony formation (CFC assay) and cell morphology (Wright Giemsa). (B) Representative FACS analysis of cells stained for CD36 and GpA expression on day 3. In order to characterize cells at different maturation stages, 3 phenotypic populations were sorted: CD36-/GpA-, CD36+/GpA- and CD36+/GpA+ cells. Inserts depict cell morphology by Wright Giemsa staining, typical colony formation, and the percentages of BFU-E-derived bursts and CFU-E-derived colonies formed. Data are representative of 4 independent experiments. (C) Effect of differentiation stage on erythroid colony formation. Two phenotypic populations, CD36-/GpA- and CD36+/GpA-, representing different maturation stages, were sorted and analysed for colony formation. Depicted is the fold difference in the numbers of colonies formed per 1000 cells plated. Because of inter-individual differences in absolute colony numbers, the number of colonies obtained with CD36-/GpA- was normalized to 1. Mean and standard deviation of the fold increase in colony numbers from CD36+/GpA- cells in three independent experiments are depicted, ***p < 0.03.
NF-E2 overexpression delays erythroid maturation
We subsequently used FACS analysis to assess erythroid maturation in the liquid culture system. Both the culture conditions used and the assessment of erythroid maturation using CD36 and GpA (Glycophorin A, CD235a) were established by Dr. Vainchenker's laboratory (Garcon et al, 2005, Ugo et al, 2004). In order to characterize the cell population delineated by these two markers, initial experiments were conducted with untreated peripheral blood CD34+ cells from healthy volunteers. CD34+ cells were cultured in erythroid differentiation medium and analysed daily for CD36 and GpA expression by FACS. While freshly isolated CD34+ cells displayed neither CD36 nor GpA on their surface (CD36-/GpA-), cultivation in erythroid differentiation medium lead to the appearance of CD36-positive cells within 2 - 3 days. However, these cells initially did not express GpA (CD36+/GpA-). Following further cultivation, CD36+/GpA+ double positive cells began to appear. The percentage of CD36+/GpA+ double positive cells increased steadily, while, at the same time, the population of immature CD36-/GpA- double negative cells declined. Following 8 – 10 days in culture, all cells were CD36+/GpA+ double positive (data not shown).
We characterized the three cell populations, CD36-/GpA- double negative, CD36+/GpA- single positive and CD36+/GpA+ double positive cells by plating in methylcellulose and by Wright Giemsa staining of cytospins (Fig. 2). While the CD36-/GpA- double negative fraction contained mostly BFU-E-derived bursts (Fig. 2B, inserts), the CD36+/GpA- single positive cells appeared functionally more mature, giving rise to some CFU-E-derived colonies (20 – 40%) in addition to BFU-E-derived bursts (60 – 80%). CD36+/GpA+ double positive cells almost exclusively gave rise to CFU-E colonies. Morphologically, all three cell populations were indistinguishable (Fig 2B, inserts).
We enumerated the colony forming potential of different erythroid maturation stages delineated by CD36 and GpA. In three separate donors the 1000 CD36-/GpA- double negative cells gave rise to only half the number of colonies seen in the 1000 CD36+/GpA- single positive cells (Fig. 2C). These data suggest that, during the very early phases of differentiation, more immature erythroid precursors display a lower plating efficiency in methylcellulose than more mature precursors.
We subsequently used this system to assess the effect of NF-E2 overexpression on erythroid differentiation and maturation (Fig. 3). Peripheral blood (PB) CD34+ cells from healthy human volunteers were transduced with the pMYSIG-GFP retrovirus, a kind gift of Dr. Carol Stocking, or with pMYSIG-NF-E2-GFP. GFP-positive cells were sorted and NF-E2-overexpressing as well as control PB CD34+ cells were cultured in erythroid differentiation medium. NFE2 mRNA and NF-E2 protein expression was assessed by qRT-PCR and Western Blot, respectively, after 8 days in culture (Fig. 3A). Compared to empty vector-transduced cells, NF-E2 transduced cells contained 2- to 10-fold higher levels of NFE2 mRNA and substantially elevated levels of NF-E2 protein (Fig. 3A). High endogenous levels of NFE2 mRNA expression in erythroid precursor cells probably prevented a higher degree of overexpression by the retroviral transgene. The degree of overexpression achieved (a mean of 5.5-fold, Fig. 3A) closely mirrored that observed in PV patients (mean 7-fold (Goerttler et al, 2005)).
Figure 3. The effect of NF-E2 overexpression on erythroid differentiation and maturation.
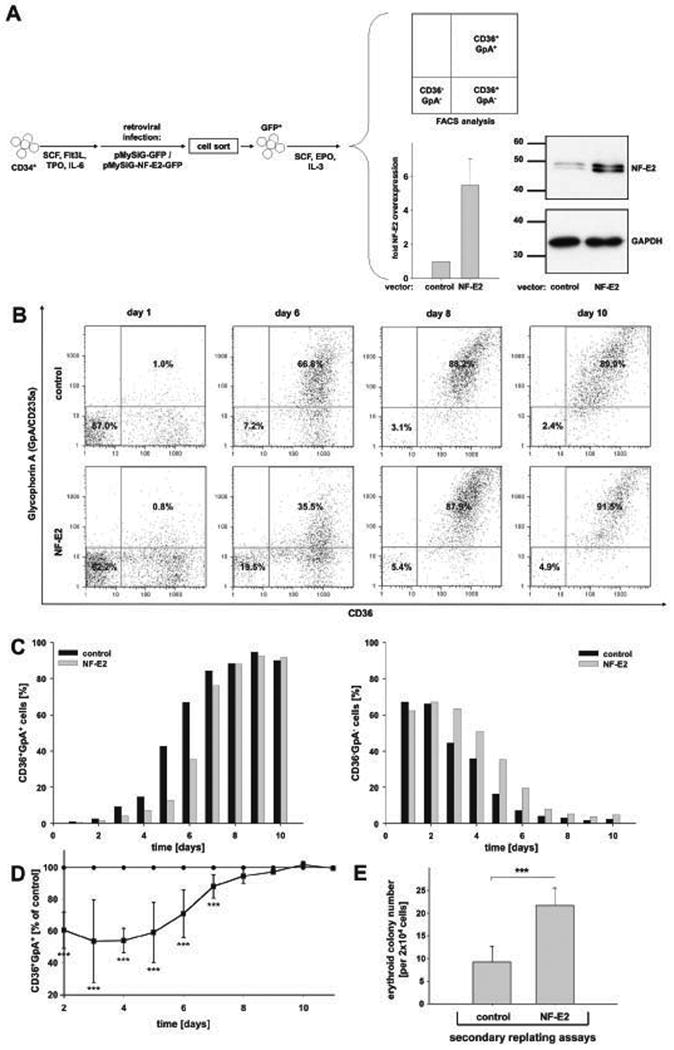
(A) Experimental design and NF-E2 overexpression: Peripheral blood CD34+ cells were transduced with pMYSIG-NF-E2-GFP (NF-E2) or with a empty pMYSIG-GFP (control), sorted for GFP expression and cultured in erythroid differentiation medium (SCF, IL-3 and EPO). Cells were analysed for CD36 and GpA expression by FACS at various time points. Inserts: On day 8 following retroviral transduction, NFE2 mRNA expression was determined by qRT-PCR (see legend to Fig. 1). Mean and standard deviation of 4 independent experiments are shown. Likewise, on day 8, NF-E2 protein expression was analysed by Western Blot, and assessed for equal protein loading using an antibody against GAPDH. (B) FACS analysis of CD36 and GpA expression. Representative results of control transduced (top) and NF-E2 transduced (bottom) CD34+ cells on days 1 (left), 6 (middle left), 8 (middle right) and 10 (right), analysed for CD36 and GpA expression, are shown. (C) Time course of the accumulation of CD36+/GpA+ double positive and depletion of CD36-/GpA- double negative cells. The percentage of CD36+/GpA+ cells (left graph) and CD36-/GpA- cells (right graph) in NF-E2 overexpressing (grey) and control (black) cells is depicted over time. The experiment was conducted 5 times with very similar results. (D) Statistical analysis of 5 independent experiments. Due to the inter-individual differences between separate buffy coats, representative results are shown in panels B and C. Here, statistical analysis of all 5 experiments is depicted. The graph displays the difference in the presence of CD36+/GpA+ mature erythroid precursors over time. For each buffy coat and each time point, the percentage of mature erythroid precursors in the empty virus transduced control cells was set at 100% (control). The relative percentage of mature erythroid precursors in the corresponding NF-E2 transduced cells is shown as a mean and standard deviation of five independent experiments, using five independent blood donors, *** p < 0.05. (E) Replating potential of NF-E2 transduced and control erythroid cells. NF-E2 transduced and control cells were cultured in erythroid differentiation medium (SCF, IL-3, EPO) for 10 days. Subsequently, 2 × 104 cells were seeded in methylcellulose with SCF, IL-3, GM-CSF and EPO to determine colony forming potential. Mean and standard deviation of three independent experiments are shown, *** p < 0.05.
Cell counts were monitored during the 48 h of retroviral transduction (Table 1). CD34+ cells transduced with empty virus and those transduced with NF-E2 expressing virus yielded similar absolute numbers of cells at the end of the transduction cycle. Hence, transmission of NF-E2 did not impact proliferation during the 48 h of retroviral transduction.
Table 1. Cell numbers of nine individual preparations of healthy control CD34+ cells prior to and post retroviral infection.
Column 1: unique patient number (UPN), here the “patients” are healthy controls. Column 2: type of retrovirus used, empty (E) or NF-E2 (N). Column 3: cell number prior to retroviral infection. Column 4: cell number following 48 h of retroviral infection, the total cell number is given prior to sorting for GFP-positive cells. Column 5: relationship between cell number in NF-E2 transduced cells and control cells. (N=E: NF-E2 and control cells expanded equally, N>E: NF-E2 transduced cells expanded more than control cells, N<E: NF-E2 transduced cells expanded less than control cells).
| UPN | retrovirus:empty [E] or NF-E2 [N] | cell count before infection*** | total cell count after infection, before sort*** | relation N/E |
|---|---|---|---|---|
| 1660 | E | 2.50 × 105 | 1.84 × 105 | N = E |
| N | 1.90 × 105 | |||
| 1668 | E | 1.44 × 105 | 5.41 × 104 | N = E |
| N | 5.15 × 104 | |||
| 1669 | E | 3.80 × 105 | 2.75 × 105 | N > E |
| N | 3.13 × 105 | |||
| 1699 | E | 8.50 × 104 | 9.53 × 104 | N = E |
| N | 9.49 × 104 | |||
| 1701 | E | 2.21 × 105 | 2.24 × 105 | N = E |
| N | 2.15 × 105 | |||
| 1723 | E | 4.97 × 105 | 7.72 × 105 | N < E |
| N | 6.84 × 105 | |||
| 1724 | E | 9.90 × 104 | 7.39 × 104 | N > E |
| N | 9.36 × 104 | |||
| 1744 | E | 3.49 × 105 | 6.52 × 105 | N < E |
| N | 5.71 × 105 | |||
total cell numbers were sometimes greater prior to infection than after infection, since many cells were lost by three different harvesting and centrifugation steps (for transferral of CD34+ cells onto new, retronectin-coated plates)
Erythroid maturation was assessed by CD36 and GpA staining and FACS analysis (Fig. 3B - D). NF-E2 overexpression was accompanied by a delay in erythroid maturation, manifested by a belated appearance of CD36+/GpA+ double positive late erythroid precursors (Fig. 3B - D). At the same time, at any given time point, increased proportions of early erythroid progenitors (CD36-/GpA-) remained in NF-E2 overexpressing cultures (Fig. 3B and C). Importantly, NF-E2 overexpressing cells on day 10 of liquid culture retained significantly elevated numbers of progenitors capable of colony formation in methylcellulose (Fig. 3E). Morphological evaluation similarly revealed more immature cells and fewer benzidine positive, haemoglobinized cells in NF-E2 overexpressing cultures (Fig. 4A and B).
Figure 4. The effect of NF-E2 overexpression on erythroid maturation and erythroid cell accumulation.
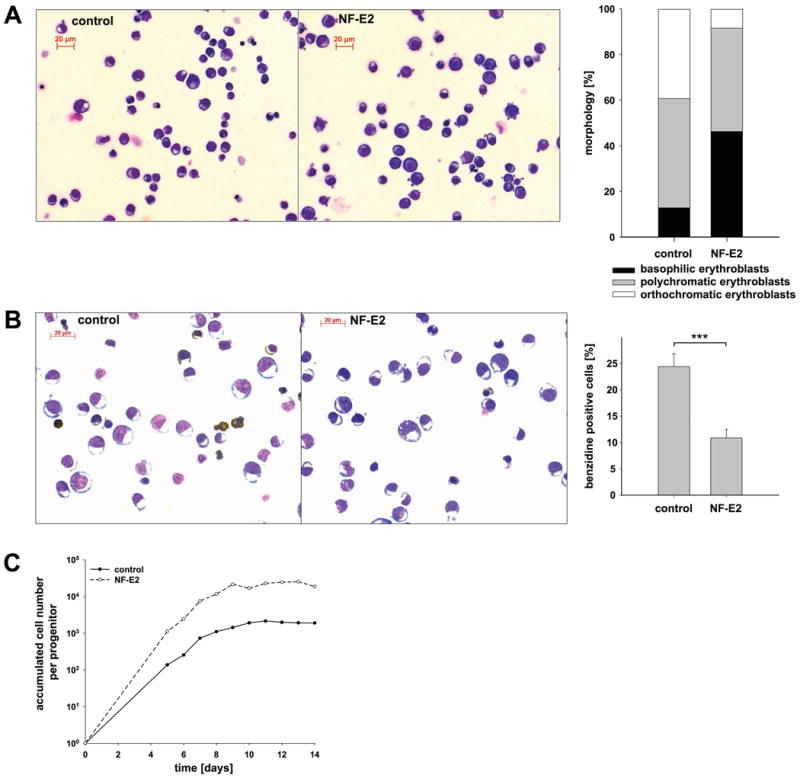
The experimental design is as described in Fig. 3A. Instead of FACS analysis, cultures were assessed by Wright Giemsa and benzidine staining. (A) Morphology. Representative Wright Giemsa stain of day 12 cells transfected with an empty vector control (left) or NF-E2 (middle), cumulative cell frequencies (right graph). (B) Benzidine staining. Representative benzidine stain of day 12 cells transfected with empty vector (left) or NF-E2 (middle), mean and standard deviation of three independent experiments (right graph), *** p < 0.003. (C) Accumulation of mature erythroid cells in culture. On the indicated days, cells were counted and the accumulated cell number determined. The graph displays the accumulated cell number per initially present erythroid precursor. The number of initially present erythroid precursors was enumerated by colony assays seeded on day 0. (A – C) The experiment was conducted 5 times with very similar results. Due to the inter-individual differences between buffy coats, typical results are shown.
Over time, delayed maturation led to a significant increase in the accumulated number of erythroid cells per progenitor cell in NF-E2 overexpressing cultures (Fig. 4C).
NF-E2 overexpression alters colony formation
To further test the hypothesis that NF-E2 overexpression delays erythroid maturation, we next transduced PB CD34+ cells and analysed colony formation in methylcellulose (Fig. 5). NF-E2 overexpression led to a drastic reduction in the number of erythroid colonies (Fig. 5B), while the number of myeloid colonies (CFU-G, CFU-M and CFU-GM) was unaffected. Initially, these results appeared paradoxical as NF-E2 overexpression led to increased cell proliferation in the liquid culture system. However, proliferation and colony forming ability are distinct features of erythroid progenitors. We had previously demonstrated that, in our culture system, early erythroid precursors display a lower cloning efficiency than more mature progenitors (Fig. 2C). Moreover, colony morphology was strikingly altered by NF-E2 overexpression. While empty vector transduced cells mainly formed CFU-E-derived colonies (70% +/- 20%) and, to a small degree, BFU-E-derived bursts (30% +/- 20%), NF-E2 overexpressing cells almost exclusively formed bursts (80% +/- 5%) (Fig. 5C). In addition, NF-E2 overexpressing bursts were larger and more dispersed than control bursts. Therefore, both the decrease in colony number and the more immature colony type observed in NF-E2 overexpressing cells were consistent with our observations that NF-E2 overexpression delays erythroid maturation (Figs. 3 and 4). Xu et al. (2005) have previously reported a similar reduction in cloning efficiency in CD34+ cells from patients with idiopathic myelofibrosis, which have been shown to overexpress NF-E2 (Guglielmelli et al, 2007).
Figure 5. The effect of NF-E2 overexpression on colony formation in healthy peripheral blood CD34+ cells.
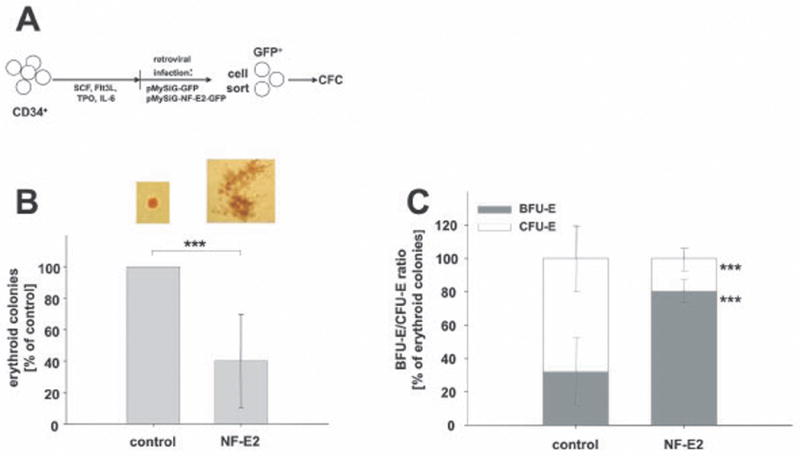
(A) Experimental design: Peripheral blood CD34+ cells were transduced with pMYSIG-NF-E2-GFP (NF-E2) or with empty pMYSIG-GFP (empty), sorted for GFP expression and 1000 GFP-positive cells were plated in methylcellulose and assayed for colony formation. CFU-E were scored on day 8, BFU-E on day 14. (B) Total erythroid colony formation: The total number of erythroid colonies (BFU-E plus CFU-E) is depicted. Because of inter-individual variation in colony formation from different blood donors, the number of colonies formed by empty vector transduced cells is set at 100%. Data depict average and standard deviation of 6 independent experiments, *** p < 0.03. (C) Percentage of BFU-E and CFU-E in CD34+ cells transduced with empty vector or with NF-E2. Data depict average and standard deviation of 5 independent experiments. Note that the data are depicted as the percentage of total erythroid colonies (BFU-E and CFU-E); the absolute number of colonies was drastically reduced in NF-E2 overexpressing cells, see B, *** p = 0.001
In the converse experiment, we examined the effect of decreasing NF-E2 expression by RNA interference (RNAi) (Fig. 6). In healthy control CD34+ cells, decreased NF-E2 expression increased erythroid colony formation (Fig. 6B). In contrast to NF-E2 overexpression, which led to an increase in BFU-E-derived bursts, inhibition of NF-E2 by RNAi led to an increase in more mature, CFU-E-derived colonies (Fig. 6C). Thus, both NF-E2 overexpression and NF-E2 depletion modulate erythroid colony formation in a consistent fashion.
Figure 6. The effect of NF-E2 depletion by RNAi on colony formation in peripheral blood CD34+ cells from healthy controls and PV patients.
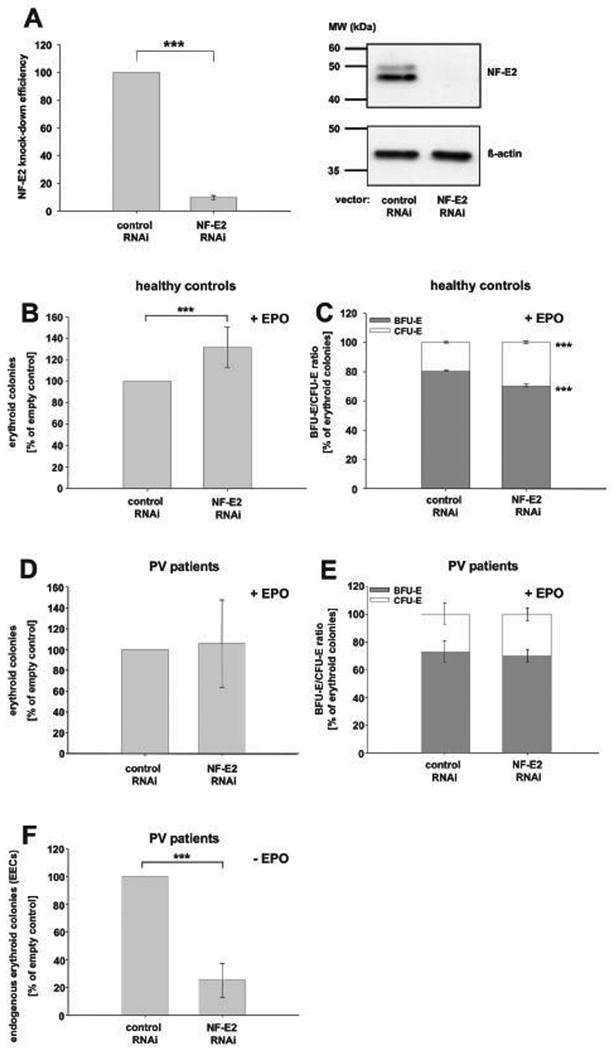
(A) Effect of NF-E2 RNAi on NFE2 mRNA and NF-E2 protein expression. Left Panel: CD34+ cells were either nucleofected with a control siRNA (control RNAi) or an siRNA against NF-E2 (NF-E2 RNAi) and cultured in erythroid differentiation medium for 8 days prior to analysis. Subsequently, RNA was isolated. NFE2 mRNA expression was quantitated by qRT-PCR. Each sample was measured in duplicate and a copy number NF-E2 per 106 copies of 18S rRNA determined. Data from 5 independent experiments are depicted as a percentage of control, which was set to 100%. The mean and standard deviation of the mean are shown, *** p < 0.001. Right panel: CD34+ cells were lentivirally transduced with either a control shRNA (control RNAi) or an shRNA against NF-E2 (NF-E2 RNAi) and subsequently cultured in erythroid expansion medium for 8 days prior to analysis. Protein extracts were analysed for NF-E2 expression by Western Blot (top) and assessed for equal protein loading using an antibody against beta-actin (bottom). A representative experiment is depicted. (B and C) The effect of NF-E2 depletion on colony formation in healthy peripheral blood CD34+ cells. (B) Erythroid colony formation. Peripheral blood CD34+ cells were nucleofected with a control siRNA (control RNAi) or an siRNA against NF-E2 (NF-E2 RNAi). Erythroid colony formation was assessed by plating in methylcellulose medium containing SCF, IL-3, GM-CSF and EPO and assayed for colony formation. CFU-E were scored on day 8, BFU-E on day 14. The total number of erythroid colonies (BFU-E plus CFU-E) is depicted. Because of inter-individual variation in colony formation from different blood donors, the number of colonies formed by control siRNA transduced cells is set at 100%. Data depict mean and standard deviation of 5 independent experiments, *** p < 0.05. (C) Percentage of BFU-E and CFU-E. CD34+ cells were lentivirally transduced with either a control shRNA (control RNAi) or an shRNA against NF-E2 (NF-E2 RNAi). Erythroid colony formation was assessed by plating in methylcellulose medium containing SCF, IL-3, GM-CSF and EPO and assayed for colony formation. CFU-E were scored on day 8, BFU-E on day 14. Data depict mean and standard deviation of 4 independent experiments. Note that the percentage of each colony type, BFU-E-derived burst or CFU-E-derived colony, of the total erythroid colonies (BFU-E plus CFU-E) is depicted; the absolute number of colonies was increased in NF-E2 depleted cells, see B, *** p = 0.001. (D - E) The effect of NF-E2 depletion on colony formation in peripheral blood CD34+ cells from PV patients in the presence of EPO. (D) Erythroid colony formation. CD34+ cells from PV patients were lentivirally transduced with either a control shRNA (control RNAi) or an shRNA against NF-E2 (NF-E2 RNAi). Erythroid colony formation was assessed by plating in methylcellulose medium containing SCF, IL-3, GM-CSF and EPO and assayed for colony formation. CFU-E were scored on day 8, BFU-E on day 14. The total number of erythroid colonies (BFU-E plus CFU-E) is depicted. Because of inter-individual variation in colony formation from different PV patients, the number of colonies formed by control shRNA transduced cells is set at 100%. Data depict mean and standard deviation of 5 independent experiments. (E) Percentage of BFU-E and CFU-E. Data is depicted as the percentage of each colony type, BFU-E-derived burst or CFU-E-derived colony, of the total erythroid colonies (BFU-E plus CFU-E). The mean and standard deviation of 4 independent experiments is shown. (F) The effect of NF-E2 depletion on endogenous erythroid colony (EEC) formation in peripheral blood CD34+ cells from PV patients in the absence of EPO. CD34+ cells from PV patients were lentivirally transduced with either a control shRNA (control RNAi) or an shRNA against NF-E2 (NF-E2 RNAi). EPO-independent erythroid colony formation was assessed by plating in methylcellulose medium containing only SCF, IL-3 and GM-CSF. EECs were scored on day 14. Because of inter-individual variation in colony formation from different PV patients, the number of colonies formed by control shRNA transduced cells is set at 100%. Data depict mean and standard deviation of 5 independent experiments, *** p < 0.001.
Given that NF-E2 overexpression is consistently observed in PV patients (Goerttler et al, 2005), we subsequently examined the result of decreasing NF-E2 in peripheral blood CD34+ cells from PV patients (Fig. 6D - F). In the presence of EPO, NF-E2 depletion did not increase erythroid colony formation in PV CD34+ cells (Fig. 6D), contrary to the observation in healthy control cells (Fig. 6B). Likewise, the percentages of BFU-E and CFU-E remained constant (Fig. 6E). Since formation of EPO-independent colonies, named endogenous erythroid colonies (EECs), is pathognomonic for PV patients, we examined the effect of NF-E2 depletion on EEC formation (Fig. 6F). Decreasing NF-E2 expression significantly reduced EEC formation (Fig. 6F), implicating this transcription factor in facilitating EPO-independent growth.
Cultures of primary PV cells display delayed erythroid maturation
Because our data suggest that NF-E2 overexpression delays erythroid maturation, we examined erythroid differentiation in primary cultures of PV patients and compared this to healthy controls. Peripheral blood mononuclear cells (MNC) from PV patients or healthy controls were cultured in MNC expansion medium and analysed morphologically on day 13. There was a striking difference in the cell lineages elaborated by the two sources. While PV patient cultures gave rise almost exclusively to erythroid cells, healthy control MNCs formed erythroid cells only with an average frequency of 62% and non-erythroid cells (lymphocytes, monocytes and neutrophils) with an average frequency of 38%, (Fig. 7A and B). The marked heterogeneity observed in healthy controls appeared to reflect physiological inter-individual variation and was contrasted by the uniform response of PV patients, who carry the JAK2V617F mutation.
Figure 7. Differentiation and maturation of healthy control and PV MNCs in culture.
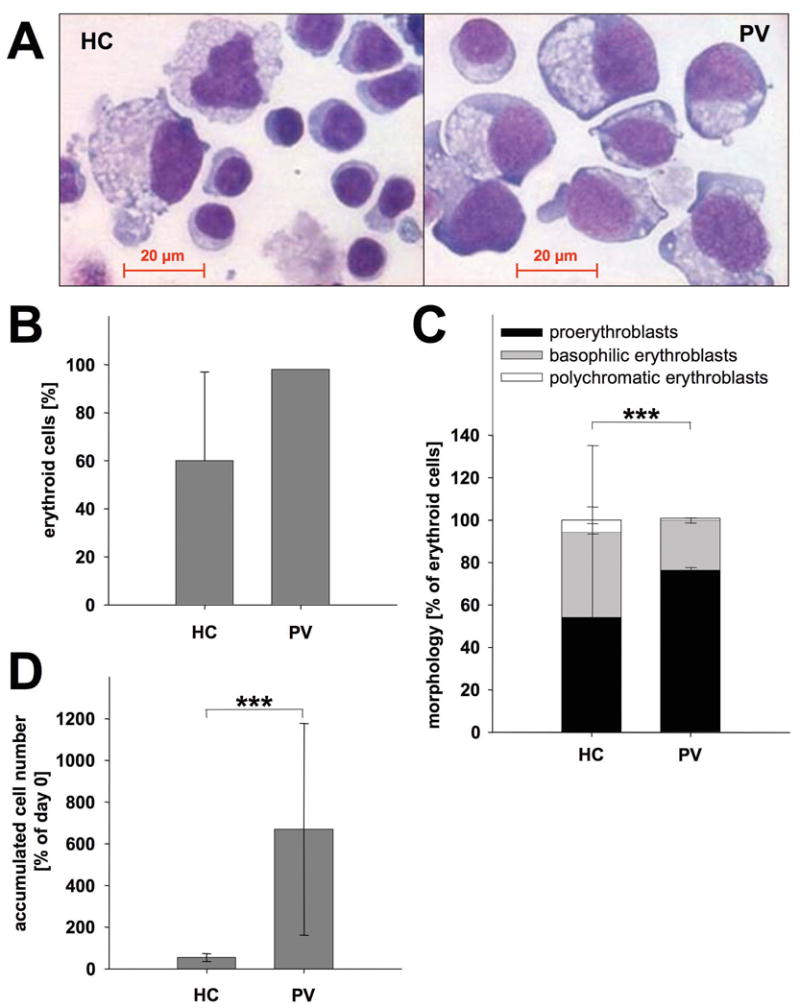
(A-D) Peripheral blood light density cells of healthy controls (HC) (n = 8) and PV patients (n = 15) were cultured in MNC expansion medium. On day 13, cells were counted, cytospins were prepared, stained with May-Grünwald Giemsa and cell morphologies assessed. (A) May-Grünwald Giemsa staining: Representative cytospins of a healthy donor (left) and a PV patient (right). Cell morphology is presented at 40× original magnification. (B) Percentage of erythroid cells elaborated by healthy control and PV samples: Mean and standard deviation of the percentage of erythroid cells (pro-erythroblasts, basophilic erythroblasts and polychromatic erythroblasts taken together) formed are depicted. The standard deviation in PV patients is too small to be visible. (C) Differential counts: for each sample, 400 cells were enumerated. Data depict the mean and standard deviation of the percentage of pro-erythroblasts, basophilic erythroblasts and polychromatic erythroblasts in controls and PV cultures, ***p < 0.01. (D) Accumulated cell numbers in MNC cultures of healthy controls and PV patients: Data are plotted as mean and standard deviation of accumulated cell number on day 13 relative to the number of initially present erythroid precursors, enumerated by colony assays seeded on day 0, ***p < 0.01.
Within the erythroid lineage, PV cultures contained increased numbers of immature proerythroblasts compared to control cultures (Fig. 7C). Concomitantly, the number of more mature polychromatic erythroblasts was decreased in comparison to controls (Fig. 7C). PV MNC cultures therefore displayed a delay in erythroid maturation similar to that observed in NF-E2 overexpressing CD34+ cells.
We calculated the number of cells accumulated in PV and healthy control cultures. PV MNC gave rise to between 7- and 8-fold higher cell numbers than healthy control cultures (Fig. 7D).
Discussion
We investigated the role that overexpression of the transcription factor NF-E2 may play in the pathophysiology of PV. Our data suggest that NF-E2 overexpression delays erythroid maturation and retains erythroid cells in the proliferative stage of development for an extended time. Because erythroid precursors are maintained in the proliferative phase, each progenitor undergoes more cell divisions and more progeny are generated from each precursor cell. Therefore, more mature erythroid cells are formed from one NF-E2 overexpressing precursor cell than from a healthy precursor (Fig. 4C). Consistent with this observation, Eaves and Eaves (1984) previously demonstrated an “increase in the cycling activity of primitive BFU-E in the marrow of PV patients”.
We similarly observed delayed maturation and protracted presence of immature cells in primary PV cells, when erythroid cultures of PV MNC were compared to healthy controls (Fig. 7C). In addition, both the number of erythroid cells and the number of total cells produced from PV cultures was significantly greater than in healthy controls (Fig. 7D). Contrary to our data, Ugo et al. (2004) have previously described accelerated maturation of PV cells compared to healthy controls. However, these authors used bone marrow-derived, isolated CD34+ cells, whereas we used peripheral blood MNC. In addition, the EPO concentration in our MNC cultures was higher, while the SCF concentration was lower than that used by Ugo et al (2004) (3 IU/ml vs. 1 IU/ml EPO and 10 ng/ml vs. 50 ng/ml SCF, respectively).
Importantly, the isolated overexpression of NF-E2 in CD34+ cells investigated here does not completely recapitulate the situation in PV patients, in which both NF-E2 overexpression and presence of the JAK2V617F mutation coincide. We propose that increased NF-E2 expression and augmented JAK2 activity synergize, perhaps by enhancing proliferation as well as delaying and accelerating differentiation at different stages of erythroid maturation. We are currently investigating this hypothesis by examining the effect of concurrent presence of JAK2V617F and augmented NF-E2 levels.
The observation that NF-E2 overexpression led to a reduction in erythroid colony formation (Fig. 5B) was unexpected. However, note that the number of immature BFU-E-derived bursts was not decreased, only the more mature CFU-E-derived colonies were reduced in NF-E2 overexpressing cells. In summary, this led to a large increase in the percentage of bursts present in NF-E2 overexpressing cultures (Fig. 5C). Again, a delayed maturation appears to be responsible. CD34+ cells plated following the 2 days of prestimulation, but prior to retroviral transfer, almost exclusively formed BFU-E colonies (data not shown). During the following 48 h of retroviral transduction, empty vector transduced cells matured, and subsequently formed predominantly CFU-E-derived colonies, whereas NF-E2 overexpressing cells were delayed in maturation, and retained a more immature phenotype, forming predominantly BFU-E-derived bursts (Fig. 5C). In colony assays NF-E2 overexpression thus likewise caused a shift towards the less mature stage, capable of forming BFU-E-derived bursts, but largely prevented maturation to the CFU-E stage.
The initially surprising overall reduction in colony numbers (Fig. 5B) was explained by the observation that less mature cells had a lower plating efficiency than more mature cells (Fig. 2C). Xu et al. (2005) previously described that CD34+ cells from idiopathic myelofibrosis (IMF) patients display lower plating efficiencies than CD34+ cells from healthy peripheral blood. By FACS analysis IMF CD34+ cells were similarly shown to be phenotypically more immature than control cells. The ability to form colonies in methylcellulose may thus characterize slightly more mature cells. By definition, CFU-E and BFU-E were scored on day 8 and day 14 of culture, respectively. More immature cells, which require a longer period of time in culture to allow maturation of the cells within the colony, would not have been scored in these assays. We have to consider the alternative possibility that CD36-/GpA- cells may be depleted of GFU-E. We consider this alternative less likely, as, under the culture conditions used, 90% of the cells were erythroid (CD36+/GpA+) on day 10. This observation would argue that many of the 45 – 60% CD36-/GpA- cells on day 3 still harboured erythroid potential.
NF-E2 overexpression caused the formation of very large bursts, similar to those described by Dupont et al. (2007) in PV patients.
We have previously shown that the degree of NF-E2 overexpression varies greatly between individual PV patients (Goerttler et al, 2005). It is tempting to speculate that the level of NF-E2 overexpression may influence the degree of erythrocytosis and hence, disease severity. Whether NF-E2 expression is regulated by JAK2, directly or indirectly is currently being examined in our laboratory. Several transcription factors have been shown to regulate NF-E2 expression. These include GATA-1 (Moroni et al, 2000) and SCL/TAL1 (McCormack et al, 2006). Elevated GATA-1 levels have recently been reported in MPD patients (Geron et al, 2008, Rinaldi et al, 2008). As GATA-1 activity is regulated via the phosphoinositide-3 kinase/AKT pathway (Zhao et al, 2006), and the phosphoinositide-3 kinase inhibitor LY294002 has been shown to inhibit EPO-independent growth of PV cells (Ugo et al, 2004), it is likely that this signal transduction pathway is aberrantly activated in MPD patients and may contribute to NF-E2 overexpression.
Several lines of evidence suggest that NF-E2 overexpression can result independent of the presence of the JAK2V617F mutation. For example, in ET, NF-E2 levels do not correlate with the JAK2V617F allele burden (Vannucchi et al, 2006). Moreover, PV and ET patients who do not carry the JAK2V617F mutation nonetheless display increased NF-E2 levels ((Kralovics et al, 2005b) and Goerttler and Pahl, unpublished observations). We are currently examining whether these individuals carry the described JAK2 exon 12 mutations (Scott et al, 2007), novel mutations in the JAK2 kinase or perhaps alterations in other signal transduction pathways.
Taken together, the data presented here corroborate our hypothesis that NF-E2 overexpression delays erythroid maturation and retains erythroid progenitors in an immature stage with increased proliferation capacity. In summary, this causes the production of excess erythroid elements from a given number of precusor cells. Based on their seminal observations in PV patients, Eaves and Eaves (1984) proposed that PV may result from an increased output of red cells per progenitor cell. NF-E2 overexpression provides one possible molecular mechanism explaining this hypothesis.
Acknowledgments
Our first and foremost thanks go to the patients who consented to be included in this study. Our gratitude extends to their physicians and nurses, especially Drs. Hoffman and Vannucchi, whose contribution is pivotal to this endeavor. We are indebted to Dr. Carol Stocking and her co-workers, especially to Ulla Bergholtz for advice and support in establishing the retroviral transduction of human CD34+ cells and to Dr. Kristoff Weber for the generous gift of pLEGO-G prior to publication. Drs. Ugo, James and Delhommeau gave much valued advice on the culture and erythroid differentiation of CD34+ cells. Sincere thanks to Dr. Katija Jelicic for conducting the PV MNC cultures and to Drs. M. Carroll and D. Tenen for insightful comments on the manuscript. Thanks also go to Klaus Geiger of the Core Facility for his help in FACS sorting. We sincerely thank Prof. Dr. K. K. Geiger for his continued support.
This work was supported by grants from the National Cancer Institute (PO1 CA108671 to ARM and HLP), the Else-Kröner-Fresenius-Stiftung (P 42-01 to HLP) and the Roche Foundation for Anemia Research (RoFAR) (5530613349 to HLP).ARM and HLP are members of the MPD Research Consortium.
References
- Andrews N, Erdjument-Bromage H, Davidson MB, Tempst P, Orkin SH. Erythroid transcription factor NF-E2 is a hematopoietic-specific basic-leucine zipper protein. Nature. 1993;362:722–728. doi: 10.1038/362722a0. [DOI] [PubMed] [Google Scholar]
- Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, Vassiliou GS, Bench AJ, Boyd EM, Curtin N, Scott MA, Erber WN, Green AR. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- Bellanné-Chantelot C, Chaumarel I, Labopin M, Bellanger F, Barbu V, De Toma C, Delhommeau F, Casadevall N, Vainchenker W, Thomas G, Najman A. Genetic and clinical implications of the Val617Phe JAK2 mutation in 72 families with myeloproliferative disorders. Blood. 2006;108:346–352. doi: 10.1182/blood-2005-12-4852. [DOI] [PubMed] [Google Scholar]
- Campbell P, Baxter E, Beer P, Scott L, Bench A, Huntly B, Erber W, Kusec R, Larsen T, Giraudier S, Le Bousse-Kerdilès M, Griesshammer M, Reilly J, Cheung B, Harrison C, Green A. Mutation of JAK2 in the myeloproliferative disorders: timing, clonality studies, cytogenetic associations and role in leukemic transformation. Blood. 2006;108:3548–3555. doi: 10.1182/blood-2005-12-013748. [DOI] [PubMed] [Google Scholar]
- Cario H, Goerttler PS, Steimle C, Levine RL, Pahl HL. The JAK2V617F mutation is acquired secondary to the predisposing alteration in familial polycythaemia vera. British Journal of Haematology. 2005;130:800–801. doi: 10.1111/j.1365-2141.2005.05683.x. [DOI] [PubMed] [Google Scholar]
- Dupont S, Masse A, James C, Teyssandier I, Lecluse Y, Larbret F, Ugo V, Saulnier P, Koscielny S, Le Couedic JP, Casadevall N, Vainchenker W, Delhommeau F. The JAK2 V617F mutation triggers erythropoietin hypersensitivity and terminal erythroid amplification in primary cells from patients with polycythemia vera. Blood. 2007;110:1013–1021. doi: 10.1182/blood-2006-10-054940. [DOI] [PubMed] [Google Scholar]
- Eaves AC, Eaves CJ. Erythropoiesis in culture. Clinical Haematology. 1984;13:371–391. [PubMed] [Google Scholar]
- Garcon L, Lacout C, Svinartchouk F, Le Couedic JP, Villeval JL, Vainchenker W, Dumenil D. Gfi-1B plays a critical role in terminal differentiation of normal and transformed erythroid progenitor cells. Blood. 2005;105:1448–1455. doi: 10.1182/blood-2003-11-4068. [DOI] [PubMed] [Google Scholar]
- Geron I, Abrahamsson AE, Barroga CF, Kavalerchik E, Gotlib J, Hood JD, Durocher J, Mak CC, Noronha G, Soll RM, Tefferi A, Kaushansky K, Jamieson CH. Selective inhibition of JAK2-driven erythroid differentiation of polycythemia vera progenitors. Cancer Cell. 2008;13:321–330. doi: 10.1016/j.ccr.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Goerttler PS, Kreutz C, Donauer J, Faller D, Maiwald T, Marz E, Rumberger B, Sparna T, Schmitt-Graff A, Wilpert J, Timmer J, Walz G, Pahl HL. Gene expression profiling in polycythaemia vera: overexpression of transcription factor NF-E2. British Journal of Haematology. 2005;129:138–150. doi: 10.1111/j.1365-2141.2005.05416.x. [DOI] [PubMed] [Google Scholar]
- Guglielmelli P, Zini R, Bogani C, Salati S, Pancrazzi A, Bianchi E, Mannelli F, Ferrari S, Le Bousse-Kerdiles MC, Bosi A, Barosi G, Migliaccio AR, Manfredini R, Vannucchi AM. Molecular profiling of CD34+ cells in idiopathic myelofibrosis identifies a set of disease-associated genes and reveals the clinical significance of Wilms' tumor gene 1 (WT1) Stem Cells. 2007;25:165–173. doi: 10.1634/stemcells.2006-0351. [DOI] [PubMed] [Google Scholar]
- James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, Garcon L, Raslova H, Berger R, Bennaceur-Griscelli A, Villeval JL, Constantinescu SN, Casadevall N, Vainchenker W. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Koshino Y, Shibata F, Oki T, Nakajima H, Nosaka T, Kumagai H. Retrovirus-mediated gene transfer and expression cloning: powerful tools in functional genomics. Experimental Hematology. 2003;31:1007–1014. [PubMed] [Google Scholar]
- Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, Tichelli A, Cazzola M, Skoda RC. A gain-of-function mutation of JAK2 in myeloproliferative disorders. New England Journal of Medicine. 2005a;352:1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- Kralovics R, Teo SS, Buser AS, Brutsche M, Tiedt R, Tichelli A, Passamonti F, Pietra D, Cazzola M, Skoda RC. Altered gene expression in myeloproliferative disorders correlates with activation of signaling by the V617F mutation of Jak2. Blood. 2005b;106:3374–3376. doi: 10.1182/blood-2005-05-1889. [DOI] [PubMed] [Google Scholar]
- Kralovics R, Teo SS, Li S, Theocharides A, Buser AS, Tichelli A, Skoda RC. Acquisition of the V617F mutation of JAK2 is a late genetic event in a subset of patients with myeloproliferative disorders. Blood. 2006;108:1377–1380. doi: 10.1182/blood-2005-11-009605. [DOI] [PubMed] [Google Scholar]
- Kruisbeck AM, Margulies DH, Shevach EM, Strober W. Current Protocols in Immunology. John Wiley & Sons; New York: 1991. [Google Scholar]
- Labbaye C, Valtieri M, Barberi T, Meccia E, Masella B, Pelosi E, Condorelli GL, Testa U, Peschle C. Differential expression and functional role of GATA-2, NF-E2, and GATA-1 in normal adult hematopoiesis. Journal of Clinical Investigation. 1995;95:2346–2358. doi: 10.1172/JCI117927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacout C, Pisani DF, Tulliez M, Moreau Gachelin F, Vainchenker W, Villeval JL. JAK2V617F expression in murine hematopoietic cells leads to MPD mimicking human PV with secondary myelofibrosis. Blood. 2006;108:1652–1660. doi: 10.1182/blood-2006-02-002030. [DOI] [PubMed] [Google Scholar]
- Levine R, Wadleigh M, Cools J, Ebert B, Wernig G, Huntley B, Boggon T, Wlodarska I, Clark J, Moore S, Adelsperger J, Koo S, Lee J, Gabriel G, Mercher T, D'Andrea A, Fröhling S, Döhner K, Marynen P, Vandenberghe P, Mesa R, Tefferi A, Griffin J, Eck M, Sellers W, Meyerson M, Golub T, Lee S, Gilliland D. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- McCormack MP, Hall MA, Schoenwaelder SM, Zhao Q, Ellis S, Prentice JA, Clarke AJ, Slater NJ, Salmon JM, Jackson SP, Jane SM, Curtis DJ. A critical role for the transcription factor Scl in platelet production during stress thrombopoiesis. Blood. 2006;108:2248–2256. doi: 10.1182/blood-2006-02-002188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio G, Di Pietro R, di Giacomo V, Di Baldassarre A, Migliaccio AR, Maccioni L, Galanello R, Papayannopoulou T. In vitro mass production of human erythroid cells from the blood of normal donors and of thalassemic patients. Blood Cells, Molecules, and Diseases. 2002;28:169–180. doi: 10.1006/bcmd.2002.0502. [DOI] [PubMed] [Google Scholar]
- Moroni E, Mastrangelo T, Razzini R, Cairns L, Moi P, Ottolenghi S, Giglioni B. Regulation of mouse p45 NF-E2 transcription by an erythroid-specific GATA-dependent intronic alternative promoter. Journal of Biological Chemistry. 2000;275:10567–10576. doi: 10.1074/jbc.275.14.10567. [DOI] [PubMed] [Google Scholar]
- Nussenzveig RH, Swierczek SI, Jelinek J, Gaikwad A, Liu E, Verstovsek S, Prchal JF, Prchal JT. Polycythemia vera is not initiated by JAK2V617F mutation. Experimental Haematology. 2007;35:32–38. doi: 10.1016/j.exphem.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Prchal JF, Axelrad AA. Bone-marrow responses in polycythemia vera. New England Journal of Medicine. 1974;290:1382. doi: 10.1056/nejm197406132902419. [DOI] [PubMed] [Google Scholar]
- Rinaldi CR, Martinelli V, Rinaldi P, Ciancia R, del Vecchio L. GATA1 is overexpressed in patients with essential thrombocythemia and polycythemia vera but not in patients with primary myelofibrosis or chronic myelogenous leukemia. Leukaemia and Lymphoma. 2008;49:1416–1419. doi: 10.1080/10428190802087462. [DOI] [PubMed] [Google Scholar]
- Sayer MS, Tilbrook PA, Spadaccini A, Ingley E, Sarna MK, Williams JH, Andrews NC, Klinken SP. Ectopic expression of transcription factor NF-E2 alters the phenotype of erythroid and monoblastoid cells. Journal of Biological Chemistry. 2000;275:25292–25298. doi: 10.1074/jbc.M908695199. [DOI] [PubMed] [Google Scholar]
- Schiedlmeier B, Klump H, Will E, Arman-Kalcek G, Li Z, Wang Z, Rimek A, Friel J, Baum C, Ostertag W. High-level ectopic HOXB4 expression confers a profound in vivo competitive growth advantage on human cord blood CD34+ cells, but impairs lymphomyeloid differentiation. Blood. 2003;101:1759–1768. doi: 10.1182/blood-2002-03-0767. [DOI] [PubMed] [Google Scholar]
- Scott LM, Tong W, Levine RL, Scott MA, Beer PA, Stratton MR, Futreal PA, Erber WN, McMullin MF, Harrison CN, Warren AJ, Gilliland DG, Lodish HF, Green AR. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. New England Journal of Medicine. 2007;356:459–468. doi: 10.1056/NEJMoa065202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steimle C, Lehmann U, Temerinac S, Goerttler PS, Kreipe H, Meinhardt G, Heimpel H, Pahl HL. Biomarker analysis in polycythemia vera under interferon-alpha treatment: clonality, EEC, PRV-1, and JAK2 V617F. Annals of Hematology. 2007;86:239–244. doi: 10.1007/s00277-006-0214-1. [DOI] [PubMed] [Google Scholar]
- Theocharides A, Boissinot M, Girodon F, Garand R, Teo SS, Lippert E, Talmant P, Tichelli A, Hermouet S, Skoda RC. Leukemic blasts in transformed JAK2-V617F positive myeloproliferative disorders are frequently negative for the JAK2-V617F mutation. Blood. 2007;110:375–379. doi: 10.1182/blood-2006-12-062125. [DOI] [PubMed] [Google Scholar]
- Toki T, Itoh J, Arai K, Kitazawa J, Yokoyama M, Igarashi K, Yamamoto M, Ito E. Abundant expression of erythroid transcription factor P45 NF-E2 mRNA in human peripheral granurocytes. Biochemical Biophysical Research Communications. 1996;219:760–765. doi: 10.1006/bbrc.1996.0307. [DOI] [PubMed] [Google Scholar]
- Ugo V, Marzac C, Teyssandier I, Larbret F, Lecluse Y, Debili N, Vainchenker W, Casadevall N. Multiple signaling pathways are involved in erythropoietin-independent differentiation of erythroid progenitors in polycythemia vera. Experimental Hematology. 2004;32:179–187. doi: 10.1016/j.exphem.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Vannucchi AM, Pancrazzi A, Bogani C, Antonioli E, Guglielmelli P. A quantitative assay for JAK2(V617F) mutation in myeloproliferative disorders by ARMS-PCR and capillary electrophoresis. Leukemia. 2006;20:1055–1060. doi: 10.1038/sj.leu.2404209. [DOI] [PubMed] [Google Scholar]
- Weber K, Bartsch U, Stocking C, Fehse B. A multicolor panel of novel lentiviral “gene ontology” (LeGO) vectors for functional gene analysis. Molecular Therapy. 2008;16:698–706. doi: 10.1038/mt.2008.6. [DOI] [PubMed] [Google Scholar]
- Wernig G, Mercher T, Okabe R, Levine RL, Lee BH, Gilliland DG. Expression of Jak2V617F causes a polycythemia vera-like disease with associated myelofibrosis in a murine bone marrow transplant model. Blood. 2006;107:4274–4281. doi: 10.1182/blood-2005-12-4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westwood NB, Pearson TC. Diagnostic applications of haemopoietic progenitor culture techniques in polycythaemias and thrombocythaemias. Leukaemia and Lymphoma. 1996;22:95–103. doi: 10.3109/10428199609074366. [DOI] [PubMed] [Google Scholar]
- WHO. Tumours of the hemopoietic and lymphoid tissues. IARC Press; Lyon: 2001. [Google Scholar]
- Xu M, Bruno E, Chao J, Ni H, Lindgren V, Nunez R, Mahmud N, Finazzi G, Fruchtman SM, Popat U, Liu E, Prchal JT, Rondelli D, Barosi G, Hoffman R. The constitutive mobilization of bone marrow-repopulating cells into the peripheral blood in idiopathic myelofibrosis. Blood. 2005;105:1699–1705. doi: 10.1182/blood-2004-06-2485. [DOI] [PubMed] [Google Scholar]
- Zaleskas VM, Krause DS, Lazarides K, Patel N, Hu Y, Li S, Van Etten RA. Molecular Pathogenesis and Therapy of Polycythemia Induced in Mice by JAK2 V617F. PLoS ONE. 2006;1:e18. doi: 10.1371/journal.pone.0000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Kitidis C, Fleming MD, Lodish HF, Ghaffari S. Erythropoietin stimulates phosphorylation and activation of GATA-1 via the PI3-kinase/AKT signaling pathway. Blood. 2006;107:907–915. doi: 10.1182/blood-2005-06-2516. [DOI] [PMC free article] [PubMed] [Google Scholar]


