Abstract
Human cocaine addicts show altered function within the basal ganglia and the medial prefrontal cortex (mPFC) and altered glutamate function within these areas is postulated to be critical for cocaine addiction. The present project utilized a highly valid animal model of cocaine addiction, to test whether excessive use of cocaine alters glutamate function within these brain areas. Rats were trained to lever-press for IV saline vehicle or cocaine (0.25 mg/infusion) over seven 1-hr daily sessions, after which, saline controls and half of cocaine self-administering animals (brief access condition) received 10 more 1-hr daily sessions, while the remaining cocaine animals received 10 additional 6-hrs daily sessions (extended access condition). One, 14, or 60 days after the last self-administration session, animals were sacrificed. Tissue samples from the ventral tegmental area (VTA), nucleus accumbens (N.Acc) core and shell, and mPFC were analyzed by immunoblotting for expression of Homer1b/c, Homer2a/b, mGluR1, mGluR5, NR2a, and NR2b subunits of the NMDA receptor. Brief and extended access to cocaine failed to alter protein levels within the VTA, and produced transient and similar changes in N.Acc protein expression, which were more pronounced in the core subregion. In contrast, extended access to cocaine resulted in distinct and long lasting alterations of protein expression within the mPFC that included: increased levels of Homer1b/c at 1 day, NR2b at 14 days, and NR2a at 60 days, of withdrawal. These data support the notion that altered NMDA function within the mPFC may contribute, in part, to the transition to excessive uncontrolled consumption of cocaine.
Keywords: self-administration, immunoblotting, Homers, group 1 mGluRs, rat
Introduction
In humans, the transition from recreational use of cocaine to addiction involves extended sessions of cocaine self-administration, the subsequent consumption of high doses of cocaine, and loss of control over drug intake (Gawin & Kleber, 1985, 1988; Gawin, 1991). Rats given extended (6 hrs) daily access to cocaine self-administration will also consume high doses of the drug. Moreover, these extended access rats will escalate their drug consumption over days and exhibit heightened motivation to seek cocaine, consistent with the expression of loss of control over drug use observed in humans (Ahmed & Koob, 1998; Ahmed & Cador, 2006; Ben-Shahar et al., 2008; Kippin et al. 2006; Mantsch et al. 2004; Paterson & Markou, 2003). Thus, the extended access condition models critical aspects of cocaine addiction as defined for humans. Importantly, rats given extended access to cocaine show a distinct profile of neural adaptations, as well as distinct neural responses to a cocaine challenge, compared to rats given limited access to self-administered cocaine or experimenter-administered cocaine (Ahmed & Koob, 2004; Ben-Shahar et al., 2004, 2005, 2006, 2007; Ferrario et al., 2005; Mantsch et al., 2007; O'Dell et al., 2006; Seiwell et al., 2007). What then are the neuroadaptations that underlie the transition from controlled drug use to addiction?
The human literature points to altered function within the nucleus accumbens (N.Acc) and medial prefrontal cortex (mPFC) and to a dysregulation in glutamate transmission as critical mediators the transition to cocaine addiction (Dackis & O'Brien, 2003, 2005; Volkow, 2004; Volkow et al., 1992, 1997; Volkow & Li, 2005). Indeed, glutamate in the N.Acc and mPFC is critical for cocaine-induced reinforcement and neuronal adaptations associated with repeated experimenter-administered cocaine (Kalivas & Volkow, 2005; Pierce et al., 1997; Pulvirenti et al., 1992; White et al., 1995). We reported previously that brief (1-hr) and extended (6 hrs) cocaine-access subjects differ in both their c-Fos immunoreactive response to a cocaine challenge and DAT binding within the N.Acc and the mPFC (Ben-Shahar et al. 2004, 2006). Moreover, cocaine-stimulated locomotor behavior that is tied to glutamate function within these brain regions (for reviews see: Pierce & Kalivas, 1997; Robinson & Kolb, 2004; Steketee, 2005; Vanderschuren & Kalivas, 2000; Wolf, 1998), is differentially altered in extended access animals, as compared to both saline controls and limited/short access subjects (Ben-Shahar et al., 2004, 2005). These data suggest that altered glutamate function within the N.Acc and mPFC may underlie the transition from stable to compulsive cocaine-use.
The ionotropic NMDA glutamate receptors are critical for bursting activity in mPFC neurons in response to reinforcing stimuli (Zhang & Shi, 1999). Furthermore, dopamine projections from the ventral tegmental area (VTA) inhibit input to the mPFC projecting onto NMDA receptors (Pirot et al., 1996), while chronic administration of cocaine resulted in decreased binding of NMDA receptors in the cortex (Bhargava & Kumar, 1999). Thus, NMDA receptors seem to be critical for both cocaine reinforcement and cocaine-induced neuronal adaptations. However, it is important to note that the data pertaining to alterations in NMDA receptor function were collected primarily from subjects receiving experimenter-administered cocaine and very little is known about such alterations in animals with cocaine self-administration experience, particularly under extended access conditions.
NMDA receptor are connected via PSD-95, GKAP, and the scaffold protein Shank to Homer proteins, that in turn bind to the cytoplasmic end of Group 1 metabotropic glutamate receptors (mGluRs; i.e. mGluR1 and mGluR5; Kennedy, 2000; Sheng & Kim, 2002). Evidence suggests that mGluR5 and Homer proteins also play an important role in cocaine reinforcement as mGluR5 knock-out mice do not self-administer cocaine (Chiamulera et al., 2001), and blockade of mGluR5 receptors attenuates cocaine self-administration (Tessari et al., 2004) and decreases breakpoints for cocaine in the progressive-ratio procedure (Paterson & Markou, 2005). Additionally, while the deletion of Homer1 or Homer2 in mice facilitates the acquisition of cocaine self-administration and cocaine-conditioned place preference (Szumlinski et al., 2004), studies of Homer mutant mice indicated that these proteins regulate not only Group1 mGluR, but also NMDA receptor function or expression in vivo (Szumlinski et al., 2004, 2005a) and repeated, experimenter-injected cocaine co-regulates the protein expression of Homers and these glutamate receptors within the N.Acc and mPFC (Ary & Szumlinski, 2007). We therefore sought to examine the expression of these glutamate receptors (i.e. the NR2a & NR2b subunits of the NMDA receptor and mGluR1 and mGluR5) and Homer proteins in animals self-administering cocaine under short- and long-access conditions to assess whether cocaine-induced changes in these receptors and proteins might relate to the development of addictive behaviors.
Materials and Methods
Subjects
The subjects (n=89) were male albino Sprague-Dawley rats weighing 275-325g at the beginning of each experiment and obtained from Charles River Laboratories (Hollister, CA). The animals were housed in pairs in plastic hanging cages located within a temperature-controlled (22°C), 12/12 h light/dark cycle (lights on at 2000) environment in the Psychology Department vivarium at UCSB. Subjects had ad libitum access to food and water, except during operant training for food reinforcement (see Food Training below).
Surgery
Rats were implanted with a chronic silastic catheter (13.5 cm long; 0.3 mm inner diameter, 0.64mm outer diameter; Dow Corning Corporation, Midland, MI) into the right jugular vein under isoflurane gas anesthesia (Abbott Laboratories, North Chicago, IL; 4% for induction; 2.0 - 2.5% for maintenance) as previously described (Ben-Shahar et al., 2004). Atropine (0.04 mg/kg IM) was administered to minimize respiratory congestion during anesthesia and banamine (2 mg/kg SC; a non-opiate analgesic) was provided to treat post-surgical pain. Each catheter ran subcutaneously around the shoulder to back where it was secured to a threaded 22 gauge metal guide cannula (Plastics One, Roanoke, VA), which emerged from the midline of the animal's back perpendicular to the dorsal surface. An obdurator covered the open end of the cannula to protect from contamination and the cannula was held in place via a small swatch of Bard Mesh (C.R. Bard Inc., Cranston, RI) to which it was cemented. The mesh was in turn laid flat subcutaneously on the animal's back. Animals were allowed 7 days for recovery and catheter patency was maintained by flushing the IV system with 0.1 ml of sterile heparin+timentin/saline (60 IU/ml and 100mg/ml, respectively) solution each day.
Apparatus
Eleven (29 cm wide × 25 cm long × 30 cm high) operant chambers (Med Associates Inc., St. Albans, VT) were used for all behavioral training and testing. Each chamber was equipped with two retractable levers, each positioned 7.0 cm above the grid floor on either side of a food pellet trough situated 2 cm above the grid floor. Food dispensers and syringe pumps were located outside the chambers. A center house light (2.8 W) was situated 28 cm above the grid floor in the center of the back panel. Two cue lights (2.8 W) were located 6-7 cm above each lever. In the current study, only the right cue light was used. All behavioral testing equipment and data acquisition were controlled by a desktop personal computer running Med Associates software (MED-PC for Windows, Version 1.17). A liquid swivel (375-22PS, Instech Laboratories Inc.) was located above the center of each operant chamber permitting the animals to freely move about the chamber during testing. The inlet of the liquid swivel was connected with polyethylene tubing (Plastics One; outer diameter 0.127 cm, inner diameter 0.058 cm) to a 10-ml syringe containing the self-administered solutions and seated in a syringe pump (Med Associates Inc., St. Albans, VT). An additional length of tubing passed through a cannula connector (C313CT Plastic One) from the swivel overhead to the animal where it was connected to the external cannula on the animal's back. Intravenous infusions were administered by activation of the syringe pump upon the pressing of the active lever.
Procedure
The training and testing procedures employed in this study were similar to those described previously (Ben-Shahar et al., 2008) and occurred during the dark phase of the light/dark cycle at the same time each day. To facilitate acquisition of the lever press response, rats were initially trained to lever press the right lever on a continuous reinforcement schedule for 45mg food pellet during 1-hour test sessions. For this, rats were food deprived for 24h before the initiation of training and maintained on a restricted diet for the duration of food training (one week on average). Once the lever-press operant was acquired (i.e. 100 reinforcements earned per session) food was again made available ad libitum in the animal's home cage. Surgical implantation of catheters was performed one to two days after a rat completed the food-training regimen.
Seven days after surgery, rats were divided into two self-administration (SA) training groups. One group of rats was trained to press the right lever for an infusion of 0.1 ml physiological saline, while the other group of rats was trained to press the right lever for a 0.25 mg cocaine HCl dissolved in 0.1 ml physiological saline. Training consisted of 1-h daily sessions each of which was initiated by the extension of the levers into the operant chamber and terminated by their subsequent withdrawal. Regardless of treatment, each right-lever press resulted in the 0.1 ml infusion over a 4 s period, which was accompanied by the illumination of the right cue light for 20s. During the presentation of the cue light, additional right-lever presses were recorded but produced no scheduled consequences and throughout the 1-h session, responses at the left lever were recorded but had no programmed effects. After seven days of training, cocaine self-administering rats were assigned to either a “brief access group” (1hr) or an “extended access group” (6hrs). Cocaine rats were matched for drug intake per session, such that the mean overall drug intake per session was the same in the 1h and 6h conditions prior to testing. The brief access groups experienced ten additional daily self-administration sessions during which saline (Saline group) or cocaine (Coc1h group) self-administering animals continued to earned IV infusions on a continuous reinforcement schedule over the course of 1 hr. Extended access animals experienced ten additional daily cocaine self-administration sessions but were allowed to earn their IV infusions over the course of a 6 hr session (Coc6h group). At the end of this 10-day period, rats were left alone in their home cages for the duration of the withdrawal period (i.e. 1, 14, or 60 days), and had no exposure to the operant boxes. At the end of the withdrawal period, subjects were given the fast-acting anesthetic brevital (2 mg/kg IV) via their catheters and decapitated immediately. The brains were removed, rapidly frozen in isopentane on dry ice, and then transferred to dry ice. Brains were then stored at -80°C until processing by immunoblotting.
Immunoblotting
Brains were sliced in 500 micron sections through the mPFC, the N.Acc, and the ventral tegmental area (VTA). As illustrated in Figure 1, tissue punches were obtained from the mPFC at 3.20-2.20 mm anterior to Bregma (corresponding to Figures 8-10 in the rat brain atlas of Paxinos and Watson, 1998); from the N.Acc core and shell using a 16 G tissue punch at 1.70-0.70 mm anterior to Bregma (corresponding to Figures 11-15 in the rat brain atlas of Paxinos and Watson, 1998); and from the VTA using a 16 G tissue punch at 4.80-5.30 mm posterior to Bregma (corresponding to Figures 39-41 in the rat brain atlas of Paxinos & Watson, 1998). Dissected brain punches were homogenized with a glass hand-held tissue grinder in homogenization medium consisting of 0.32 M sucrose, 2 mM EDTA, 1% w/v sodium dodecyl sulfate, 50 μM phenyl methyl sulfonyl fluoride and 1 μg/ml leupeptin (pH=7.2), while 50 mM sodium fluoride, 50 mM sodium pyrophosphate, 20 mM 2-glycerol phosphate, 1 mM p-nitrophenyl phosphate and 2 μM microcystin LR was also included to inhibit phosphatases. Samples were then subjected to low-speed centrifugation at 10,000 g for 20 min. Protein determinations of samples from the mPFC were performed using the Bio-Rad DC protein assay (Bio-Rad, Hercules, CA), according to the manufacturer's instructions and homogenates were stored at -80°C until immunoblotting. As insufficient tissue was collected from the N.Acc subregions and the VTA, protein determinations were not performed for these regions prior to electrophoresis. The procedures employed to quantify Homer2, Homer1b/c, mGluR1, mGluR5, NR2a, and NR2b protein content in brain samples were similar to those described recently (Ary and Szumlinski, 2007; Szumlinski et al., 2008). Samples (30 μg for mPFC and 10μl of brain sample for N.Acc subregions and VTA) were subjected to a SDS-polyacrylamide gel electrophoresis using Tris-Acetate gradient gels (4-8%; Invitrogen, Carlsbad, CA). Proteins were then transferred to PVDF membranes, preblocked with phosphate-buffered saline containing 0.1% (v/v) Tween-20 and 5% (w/v) nonfat dried milk powder for 1 hr before overnight incubation with primary antibodies. The following rabbit polyclonal antibodies were used: anti-Homer2a/b and anti-Homer1b/c (P.F. Worley, Johns Hopkins University School of Medicine; 1:1000 dilution), anti-mGluR1a (Upstate, 1:1000 dilution), anti-mGluR5 (Upstate; 1:1000 dilution), anti-NR2a (Calbiochem, San Diego, CA; 1:1000), and anti-NR2b (Calbiochem; 1:1000). An anti-calnexin primary antibody (Stressgen, Victoria BC; 1:1000) was employed as a loading control throughout. Membranes were then washed and incubated with horseradish peroxidase-conjugated goat anti-rabbit (Jackson ImmunoResearch Laboratories Inc., West Grove PA; 1:20,000-40,000 dilution) for 90 min, and immunoreactive bands were detected by enhanced chemiluminescence (SuperSignal West Femto; Fisher Scientific, Pittsburgh, PA). Immunoreactive levels for the proteins of interest and for calnexin were quantified by integrating band density X area using computer-assisted densitometry (Image J, NIH). Visual inspection of the mPFC blots verified even loading and transfer and thus, it was not necessary to normalize the band densities for the proteins of interest within the mPFC (30 μg total protein/lane) to their corresponding calnexin signals. To control for even loading (10 μl/lane) in the N.Acc and VTA studies, the density X area measurements for the proteins of interest were normalized to that of their corresponding calnexin measurements. The density X area measurements (for mPFC) or the calnexin-normalized data (for N.Acc and VTA) were averaged over control samples for each gel and all bands were expressed as a percent of the mean saline control value per gel.
Figure 1.
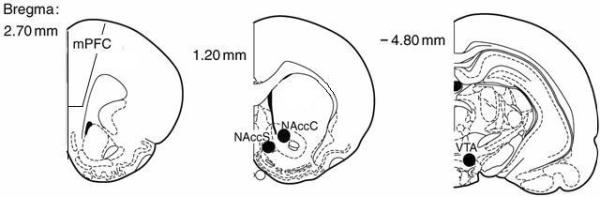
Representation of the various brain areas from which punches were taken (identified as either a closed black circle on the middle and right panels, or as open rectangle on the left panel). Numbers represent mm posterior or anterior to Bregma. mPFC = medial prefrontal cortex; NAccC = nucleus accumbens core; NAccS = nucleus accumbens shell; VTA = ventral tegmental area.
Statistical analysis
Self-administration data was analyzed by 3 (groups: Saline, Coc1h, Coc6h) × 10 (days) ANOVA, followed by simple effect analyses and post-hoc tests. Protein expression was analyzed by One Way ANOVA with main factor for group: Saline control, Coc1h, and Coc6h, followed by LSD Post Hoc Test. α=0.05 for all analyses, two-tailed.
Results
Extended access to IV cocaine produces an escalation in cocaine intake
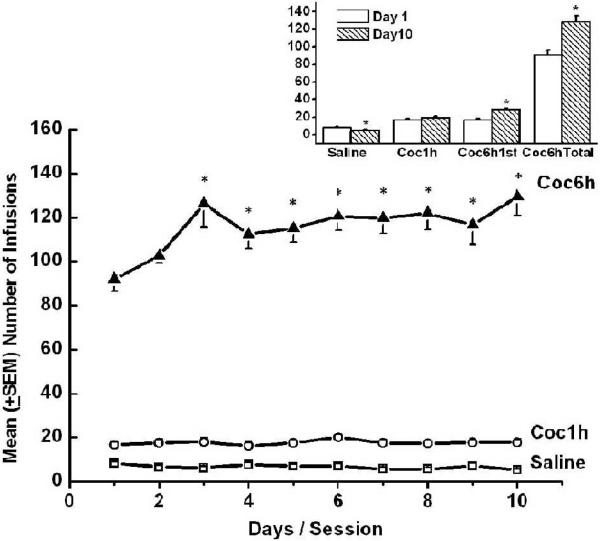
Analysis of self-administration data yielded a significant effect for Day [F(9,558)=4.647, p<0.001], a significant effect for Group [F(2,62)=52.994, p<0.001], and a significant Group x Day interaction [F(18,558)=6.341, p<0.001]. Further analysis revealed that, as expected, saline control animals exhibited low levels of self-administration that decreased significantly over time [F(1,29)=8.278, p<.007]. Brief access animals exhibited stable levels of cocaine self-administration (as can be seen in Figure 2 and confirmed by non-significant effect for days in a One Way ANOVA with repeated measurements p>0.3); and these levels were significantly lower than those exhibited by the extended access animals during the first hour of the session [F(1,39)=6.601, p<0.014]. Finally, extended access animals escalated their cocaine intake over days, an effect that was apparent both during the first hour of the session [F(9,189)=10.108, p<0.001], and over the entire session [F(9,153)=5.859, p<0.001; see Figure 2].
Figure 2.
Mean (+SEM) number of saline or cocaine self-administered infusions over days are depicted in the main graph. Significant increases in number of self-administered cocaine infusions in the first hour and over the whole session were observed in the Coc6h group by the third day. * Represents significant difference from the first day of testing (p < 0.01). Mean (+SEM) number of self-administered infusions on the first (open bars) and tenth (striped bars) day of testing for the saline, Coc1h, and Coc6h (first hour and whole session) is depicted in the inset graph. * Represents significant difference between first and tenth day of testing (p < 0.007).
Extended access to IV cocaine produces enduring changes in mPFC NR2 subunit expression
Within the mPFC of only the brief access condition there was a significant or nearly significant reduction in the expression of mGluR1 (see Figure 3) and mGluR5 (See Table 1), respectively, at 1 day withdrawal. In addition, at 14 days withdrawal there was a reduction of Homer1b/c (see Figure 3). This was evidenced by the following statistical analyses: For mGluR1 1 day withdrawal, One Way ANOVA yielded significant main effect for Group F(2,27)=5.80, p<0.01, and Post-hoc analysis yielded significant differences between the brief access condition and both the saline control (p<0.01) and extended access (p<0.01) conditions; For mGluR5 1 day withdrawal, One Way ANOVA yielded significant main effect for Group F(2,27)=3.32, p<.053, and Post-hoc analysis yielded a significant difference between the brief access and the saline control groups (p<0.02); For Homer1b/c 14 days withdrawal, One Way ANOVA yielded significant main effect for Group F(2,32)=3.66, p<0.04, and Post-hoc analysis yielded significant difference between the brief access and control animals (p<0.01).
Figure 3.
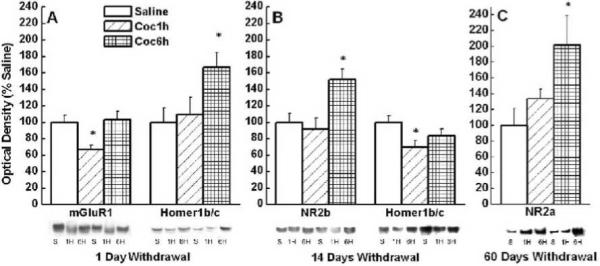
Mean (+ SEM) % of Saline optical density of mGluR1, Homer1b/c, NR2a, and NR2b in the mPFC of control, brief access and extended access animals at 1, 14, and 60 days of withdrawal. * Represents significant difference from saline control (p<.05).
Table 1.
Protein expression in the mPFC
| One Day | 14 Days | 60 Days | |
|---|---|---|---|
| mGluR1 | See Figure 3 | Sal - 100 ± 6.6 | Sal - 100 ± 14 |
| Coc1h - 89.7 ± 11 | Coc1h - 100 ± 20.6 | ||
| Coc6h - 86.6 ± 14.6 | Coc6h - 101.8 ± 10.4 | ||
|
| |||
| mGluR5 | Sal - 100 ± 11 | Sal - 100 ± 10 | Sal - 100 ± 9.6 |
| Coc1h - 63 ± 6.5 | Coc1h - 75.8 ± 13.5 | Coc1h - 79 ± 3.3 | |
| Coc6h - 76.5 ± 12 | Coc6h - 94 ± 22.4 | Coc6h - 76 ± 10 | |
|
| |||
| Homer1b/c | See Figure 3 | See Figure 3 | Sal - 100 ± 8 |
| Coc1h - 93 ± 10 | |||
| Coc6h - 113.5 ± 14 | |||
|
| |||
| Homer2a/b | Sal - 100 ± 13.5 | Sal - 100 ± 8.4 | Sal - 100 ± 9.8 |
| Coc1h - 117 ± 20 | Coc1h - 131.6 ± 17 | Coc1h - 99 ± 7.7 | |
| Coc6h - 106 ± 17 | Coc6h - 130.6 ± 12.7 | Coc6h - 95.3 ± 7 | |
|
| |||
| NR2a | Sal - 100 ± 15 | Sal - 100 ± 11 | See Figure 3 |
| Coc1h - 75 ± 8.7 | Coc1h - 103.4 ± 15.5 | ||
| Coc6h - 99.7 ± 9.7 | Coc6h - 85 ± 17 | ||
|
| |||
| NR2b | Sal - 100 ± 12 | See Figure 3 | Sal - 100 ± 22.5 |
| Coc1h - 118 ± 26 | Coc1h - 122.7 ± 24 | ||
| Coc6h - 90 ± 20 | Coc6h - 97 ± 14.5 | ||
In contrast, in the extended access condition, there was a significant increase in the expression of Homer1b/c at 1 day withdrawal, NR2b at 14 days withdrawal, and NR2a at 60 days withdrawal (see Figure 3). This was evidenced by the following analyses: For Homer1b/c 1 day withdrawal, One Way ANOVA yielded significant main effect for Group F(2,23)=4.01, p<0.03, and Post-hoc analysis yielded significant differences between the extended access condition and both the saline control (p<0.02) and brief access (p<0.04) conditions; For NR2b 14 days withdrawal, One Way ANOVA yielded significant main effect for Group F(2,29)= 4.89, p<0.02, and Post-hoc analysis yielded significant differences between the extended access condition and both the saline control (p<0.01) and brief access (p<0.01) conditions; For NR2a 60 days withdrawal, One Way ANOVA yielded significant main effect for Group F(2,23)=3.63, p<0.04, and Post-hoc analysis yielded a significant difference between the extended access and control animals (p<0.02).
As summarized in Table 1, we failed to detect group differences in the mPFC expression of: mGluR1 and mGluR5 at 14 or 60 days withdrawal; Homer1b/c at 60 days withdrawal; NR2b at 1 or 60 days withdrawal; NR2a at 14 and 60 days withdrawal; and Homer2a/b after any of the withdrawal periods (1, 14, or 60 days), within the mPFC between groups (p>0.12).
Daily access to IV cocaine results in transient changes in N.Acc Shell mGluR1 and NR2b expression
At 14, but not 1 or 60 days withdrawal, there was a significant increase in the N.Acc shell expression of mGluR1 receptors in both the brief access and extended access animals (see Figure 4 and Table 2). In addition, there was an increase that approached significance in N.Acc shell NR2b expression observed only in brief access animals. This was evidenced by the following analyses: For mGluR1, a One Way ANOVA yielded significant main effect for Group F(2,31)=3.58, p<0.04, while Post-hoc analysis yielded significant differences between saline control and both brief access animals (p< 0.04), and extended access animals (p< 0.04); For NR2b, a One Way ANOVA yielded significant main effect for Group F(2,32)=3.28, p<.052, while Post-hoc analysis yielded significant differences between brief access and both saline controls (p<0.02), extended-access animals (p<0.04). As summarized in Table 2, there was no significant difference in the expression of mGluR5, Homer 1b/c, Homer 2, NR2a, or NR2b, after any of the withdrawal periods (1, 14, or 60 days) in the N.Acc Shell between groups. Thus, in contrast to recent data for experimenter-injected cocaine (Ary and Szumlinski, 2007), these data demonstrate very few and modest effects of IV cocaine self-administration history upon glutamate receptor/Homer expression within the N.Acc shell.
Figure 4.
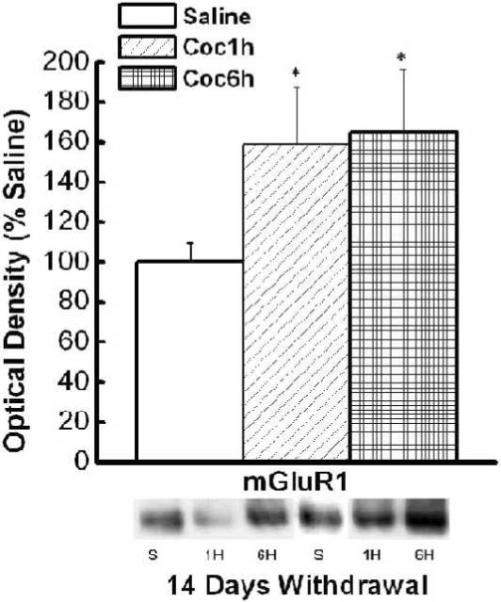
Mean (+ SEM) % of Saline optical density of mGluR1 in the N.Acc shell of control, brief access and extended access animals at 14 days of withdrawal. * Represents significant difference from saline control (p<.05).
Table 2.
Protein expression in the N.Acc Shell.
| One Day | 14 Days | 60 Days | |
|---|---|---|---|
| mGluR1 | Sal - 100 ± 25.7 | See Figure 4 | Sal - 100 ± 14 |
| Coc1h - 105 ± 41.5 | Coc1h - 93.4 ± 9.4 | ||
| Coc6h - 77.9 ± 28.9 | Coc6h - 113 ± 14.5 | ||
|
| |||
| mGluR5 | Sal - 100 ± 4.3 | Sal - 100 ± 15 | Sal - 100 ± 14.6 |
| Coc1h - 84 ± 10 | Coc1h - 83.8 ± 11.4 | Coc1h - 113 ± 22 | |
| Coc6h - 76.4 ± 11 | Coc6h - 113.4 ± 26.8 | Coc6h - 104.8 ± 19 | |
|
| |||
| Homer1b/c | Sal - 100 ± 7 | Sal - 100 ± 6.2 | Sal - 100 ± 16.4 |
| Coc1h - 97.6 ± 12.8 | Coc1h - 123.8 ± 12.3 | Coc1h - 110.9 ± 19 | |
| Coc6h - 84 ± 18 | Coc6h - 99 ± 12.7 | Coc6h - 110.5 ± 20 | |
|
| |||
| Homer2a/b | Sal - 100 ± 12.5 | Sal - 100 ± 16.2 | Sal - 100 ± 25 |
| Coc1h - 77.6 ± 10 | Coc1h - 113 ± 29 | Coc1h - 138.5 ± 21 | |
| Coc6h - 98.6 ± 9.3 | Coc6h - 125.6 ± 42.5 | Coc6h - 117 ± 15.6 | |
|
| |||
| NR2a | Sal - 100 ± 13.4 | Sal - 100 ± 17 | Sal - 100 ± 14.6 |
| Coc1h - 86.4 ± 14 | Coc1h - 141 ± 30 | Coc1h - 165.6 ± 46.6 | |
| Coc6h - 81 ± 17 | Coc6h - 102.5 ± 15 | Coc6h - 149.4 ± 48.8 | |
|
| |||
| NR2b | Sal - 100 ± 12 | Sal - 100 ± 11 | Sal - 100 ± 10 |
| Coc1h - 78 ± 9 | Coc1h - 137 ± 7* | Coc1h - 109 ± 21.9 | |
| Coc6h - 65 ± 10 | Coc6h - 99 ± 14 | Coc6h - 159.8 ± 38.5 | |
signify difference from Saline controls p<0.05 - Main effect for group p < 0.052
Cocaine self-administration significantly reduces N.Acc Core levels of Homer proteins and mGluR5 during short-term withdrawal
In the N.Acc core there was a significant reduction in the expression of Homer1b/c at 1 day withdrawal, and of Homer2a/b and mGluR5 at 14 days withdrawal, in both the brief access and extended access animals (see Figure 5). This was evidenced by the following analyses: For Homer1b/c 1 day withdrawal, One Way ANOVA yielded significant main effect for Group F(2,23)=6.41, p<0.01, and Post-hoc analysis yielded significant different between saline control and both the brief access (p< 0.01) and extended access (p< 0.02) conditions; For mGluR5 14 days withdrawal, One Way ANOVA yielded significant main effect for Group F(2,28)=4.23, p<0.03, and Post-hoc analysis yielded significant different between saline control and both the brief access (p<0.02) and extended access (p<0.03) conditions; For Homer2a/b 14 days withdrawal One Way ANOVA yielded significant main effect for Group F(2,33)=3.61, p<0.04, and Post-hoc analysis yielded significant different between saline control and both the brief access (p<0.03) and extended access (p<0.03) conditions. As summarized in Table 3, there was no significant difference in the expression of Homer1b/c in the N.Acc core at 14 or 60 days withdrawal or Homer2a/b and mGluR5 at 1 and 60 days withdrawal. Likewise, there were no significant differences in the expression of mGluR1, NR2a, or NR2b, after any of the withdrawal periods (1, 14, or 60 days) in the N.Acc Core between groups (p>0.09). These data for the N.Acc Core are also in contrast to a recent report for experimenter-injected cocaine (Ary and Szumlinski, 2007) and indicate that a history of IV cocaine self-administration produces short-term (1 to 14 days) reductions in mGluR5/Homer expression within the N.Acc Core, regardless of the length of the daily session.
Figure 5.
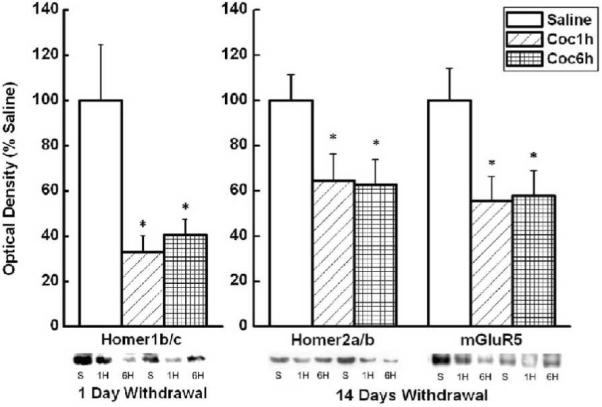
Mean (+ SEM) % of Saline optical density of mGluR5, Homer1b/c, and Homer2a/b in the N.Acc core of control, brief access and extended access animals at 1 and 14 days of withdrawal. * Represents significant difference from saline control (p<.05).
Table 3.
Protein expression in the N.Acc Core
| One Day | 14 Days | 60 Days | |
|---|---|---|---|
| mGluR1 | Sal - 100 ± 27.4 | Sal - 100 ± 13.7 | Sal - 100 ± 14.9 |
| Coc1h - 194.5 ± 152.3 | Coc1h - 59.7 ± 10.7 | Coc1h - 100 ± 9.9 | |
| Coc6h - 56.7 ± 15 | Coc6h - 151.4 ± 42.4 | Coc6h - 93.8 ± 8 | |
|
| |||
| mGluR5 | Sal - 100 ± 21.5 | See Figure 5 | Sal - 100 ± 14 |
| Coc1h - 157 ± 45.9 | Coc1h - 89.5 ± 12 | ||
| Coc6h - 98.3 ± 32.6 | Coc6h - 103.8 ± 16 | ||
|
| |||
| Homer1b/c | See Figure 5 | Sal - 100 ± 7.9 | Sal - 100 ± 12.6 |
| Coc1h - 86.4 ± 10 | Coc1h - 83.3 ± 14 | ||
| Coc6h - 87.8 ± 11 | Coc6h - 84.6 ± 5 | ||
|
| |||
| Homer2a/b | Sal - 100 ± 24.9 | See Figure 5 | Sal - 100 ± 14 |
| Coc1h - 63.8 ± 14 | Coc1h - 72.9 ± 10.4 | ||
| Coc6h - 55.7 ± 10.7 | Coc6h - 75 ± 4.5 | ||
|
| |||
| NR2a | Sal - 100 ± 15.8 | Sal - 100 ± 11.4 | Sal - 100 ± 16.5 |
| Coc1h - 99.8 ± 26 | Coc1h - 200.7 ± 55 | Coc1h - 97.8 ± 14 | |
| Coc6h - 59.5 ± 11 | Coc6h - 95.9 ± 21.7 | Coc6h - 117.6 ± 14.6 | |
|
| |||
| NR2b | Sal - 100 ± 27.7 | Sal - 100 ± 7.6 | Sal - 100 ± 14 |
| Coc1h - 72.4 ± 19.9 | Coc1h - 108 ± 20.7 | Coc1h - 72.9 ± 10.4 | |
| Coc6h - 57.4 ± 8 | Coc6h - 64 ± 7 | Coc6h - 80.8 ± 6.7 | |
Neither brief nor extended daily access to cocaine alters glutamate receptor/Homer protein expression in the VTA
Table 4 summarizes the effects of cocaine self-administration upon VTA protein expression. As indicated in the table, neither brief nor extended access to cocaine significantly affected protein expression at any of the withdrawal time points (p>0.2). However, it should be noted that at 1 day withdrawal, Homer1b/c expression was marginally reduced in both brief and extended access animals, compared to controls [F(2,22)=2.989 p<.073; saline vs. Coc1h p<.042, saline vs. Coc6h p<.057.
Table 4.
Protein expression in the VTA
| One Day | 14 Days | 60 Days | |
|---|---|---|---|
| mGluR1 | Sal - 100 ± 17.3 | Sal - 100 ± 12.9 | Sal - 100 ± 25.5 |
| Coc1h - 69.5 ± 16 | Coc1h - 117.4 ± 22.8 | Coc1h - 69.6 ± 12.4 | |
| Coc6h - 87.6 ± 17 | Coc6h - 83 ± 7.8 | Coc6h - 75 ± 11 | |
|
| |||
| mGluR5 | Sal - 100 ± 22 | Sal - 100 ± 9.7 | Sal - 100 ± 13.5 |
| Coc1h - 62.8 ± 3 | Coc1h - 101.6 ± 11 | Coc1h - 102 ± 10.5 | |
| Coc6h - 72.5 ± 12.5 | Coc6h - 99 ± 13 | Coc6h - 99.7 ± 13.4 | |
|
| |||
| Homer1b/c | Sal - 100 ± 17.2 | Sal - 100 ± 7.4 | Sal - 100 ± 19.4 |
| Coc1h - 61 ± 3.7 | Coc1h - 111 ± 15 | Coc1h - 79.9 ± 15 | |
| Coc6h - 70.1 ± 7.9 | Coc6h - 86 ± 24 | Coc6h - 108.6 ± 18.4 | |
|
| |||
| Homer2a/b | Sal - 100 ± 28.3 | Sal - 100 ± 11 | Sal - 100 ± 18 |
| Coc1h - 138.9 ± 48.6 | Coc1h - 144 ± 23 | Coc1h - 118.6 ± 16 | |
| Coc6h - 73.6 ± 15.8 | Coc6h - 113 ± 13 | Coc6h - 120 ± 12 | |
|
| |||
| NR2a | Sal - 100 ± 15.7 | Sal - 100 ± 11 | Sal - 100 ± 9 |
| Coc1h - 92.5 ± 38.4 | Coc1h - 115 ± 15 | Coc1h - 104 ± 15 | |
| Coc6h - 105.8 ± 17.7 | Coc6h - 99 ± 21.6 | Coc6h - 104 ± 11.7 | |
|
| |||
| NR2b | Sal - 100 ± 13.5 | Sal - 100 ± 4.8 | Sal - 100 ± 24 |
| Coc1h - 183.1 ± 41.5 | Coc1h - 122 ± 26 | Coc1h - 96.7 ± 13 | |
| Coc6h - 186.8 ± 52.3 | Coc6h - 137 ± 23 | Coc6h - 106.6 ± 26.7 | |
Discussion
Extended access to cocaine resulted in escalated cocaine consumption while brief access animals maintained stable levels of self-administration, as we and others have shown previously (Ahmed & Koob, 1998; Ben-Shahar et al., 2004, 2005, 2006, 2007, 2008). Moreover, alterations in protein expression within the mPFC dramatically differed between the extended and brief access conditions. Thus, at 1 day withdrawal mGluR1 expression was significantly reduced only in the brief access condition, while Homer1b/c expression was significantly increased only in the extended access condition. At 14 days withdrawal, Homer1b/c expression was significantly reduced only in the brief access condition, while NR2b expression was significantly increased only in the extended access condition. Finally, at 60 days withdrawal NR2a expression was significantly increased only in the extended access condition. Protein expression within the N.Acc was similarly altered after brief or extended access to cocaine. Within the N.Acc core of both brief and extended access animals, Homer1b/c expression was significantly decreased at 1 day withdrawal, Homer2a/b and mGluR5 expression was reduced at 14 days withdrawal, while in the N.Acc shell of brief and extended access animals mGluR1 expression was significantly elevated, however, at this withdrawal period NR2b was only elevated in the N.Acc shell of brief access animals. Finally, in the VTA, neither brief nor extended access to cocaine altered expression of any of the proteins assessed (i.e. mGluR1, mGluR5, Homer1b/c, Homer2a/b, NR2a, and NR2b) at any withdrawal period examined. In short, only within the mPFC altered proteins expression paralleled the behavioral differences observed between the brief and extended access conditions.
As stated above, protein alterations within the mPFC were significantly different in the brief and extended access conditions. Moreover, only in the extended access condition and only within the mPFC were alterations in proteins expression present for up to 60 days of withdrawal. Our data is consistent with other reports showing increased expression of NR2b proteins in the mPFC of animals self-administering cocaine under extended access conditions (i.e. 8 -24 hrs/day) after 2 weeks (Tang et al., 2004), but not at 15-16 hrs (Hemby et al., 2005a), of withdrawal. Changes in the subunit composition of NMDA receptors result in altered function of these receptors (Loftis & Janowsky, 2003; Paoletti & Neyton, 2007; Tang et al., 2004). These data are therefore consistent with the notion that changes in the composition of NMDA receptors (i.e. increased expression of either NR2a or NR2b) and therefore in NMDA receptor function, within the mPFC may be involved in the transition to escalated cocaine use. We opted not to employ a yoked control group, as previous research suggest that the levels of self-administration exhibited by our extended access animals will be lethal for a yoked control animals (Dworkin et al., 1995). It is therefore possible that the changes we observed in the expression of Homer1b/c and NMDA subunits in the mPFC resulted from mere exposure to higher doses of cocaine. Additional studies are needed to confirm that alterations in NMDA receptor function within the mPFC mediate the transition to compulsive consumption of cocaine. Finally, Homer proteins link to NR2 subunits via a Shank-GKAP-PSD95 scaffold (Tu et al. 1999; Lim et al. 1999) and regulate the expression of NR2a/b subunits, at least within the N.Acc (Szumlinski et al., 2005b). Thus, it is tempting to speculate that the latent and enduring rise in mPFC NR2a/b expression might relate to the transient increase in mPFC Homer1b/c expression observed during early withdrawal from cocaine self-administration in extended access animals.
The mPFC is especially important for the inhibition of impulses or inappropriate behavior (Clark et al., 2004; Clarke et al., 2004, 2005; Damasio, 1996; Kawashima et al., 1996; Konishi et al., 1998; Rolls et at., 1994). Interestingly, one persistent neuronal abnormality found in human cocaine addicts is decreased function in this area (Fein et al., 2002; Franklin et al., 2002; Lim et al., 2002; Matochik et al., 2003; Volkow et al., 1992, 1993). It was therefore suggested that the observed inability of cocaine addicts to inhibit drug-taking behavior stems at least in part from deterioration in the function of this brain area, or more specifically from glutamatergic dysfunction in the mPFC (Dackis & O'brien 2003; Jentch & Taylor, 1999; Kelley et al., 2004; Kalivas & Volkow, 2005). Consistent with this hypothesis are the numerous studies showing that the specific aspects of impulsive behavior shown after damage to the mPFC are also exhibited by cocaine addicts (e.g. Bechara et al., 2001, 2002; Bechara & Damasio, 2002; Bornovalova et al., 2005; Coffey et al., 2003; Grant et al., 2000; Monterosso et al., 2001). Our data, though correlational in nature, suggest that shifts in NR2a/b subunit content and consequent effects upon NMDA receptor function may be critical for the loss of control over cocaine-taking observed in the extended access condition.
Cocaine self-administration also caused several changes in Homer/glutamate receptor expression within the core and shell subregions of the N.Acc; however, in contrast to the mPFC, neither the direction nor the magnitude of the observed effects differed as a function of cocaine access (with the exception of the increase of NR2b expression within the N.Acc shell of brief access animals at 14 days of withdrawal). As cocaine-induced neuronal adaptation within the N.Acc core (i.e. c-Fos response to self-administered cocaine challenge, and binding of the dopaminergic transporter; Ben-Shahar et al., 2004 and 2006, respectively) differ between brief and extended access animals, and as the motivation to administer cocaine is much greater in the extended access animals (Ahmed & Cador, 2006; Ben-Shahar et al., 2008; Kippin et al. 2006; Mantsch et al. 2004; Paterson & Markou, 2003), these data for Homer/glutamate receptors protein expression suggest that alterations in these proteins within the N.Acc are involved in cocaine reinforcement, rather than the transition from stable to escalated cocaine self-administration. Consistent with this are the data of Kenny et al. (2005) showing that blockade of mGluR5 by systemic injections of antagonist to these receptors affected cocaine self-administration equally in brief and extended access animals.
Interestingly, the present data for animals self-administering cocaine as pertains to changes in N.Acc protein expression are not entirely consistent with the changes in N.Acc Homer/glutamate receptor protein expression reported previously for animals injected repeatedly with IP cocaine. More specifically, following cocaine self-administration, alterations in protein expression were found mainly in the N.Acc core, while following experimenter-administered IP cocaine, reductions in protein expression were observed exclusively in the N.Acc shell. Thus, following three weeks withdrawal from five or seven daily IP injections of 30 mg/kg cocaine resulted in reduced levels of Homer1b/c (Swanson et al., 2001; Ary & Szumlinski, 2007), mGluR5 (Swanson et al., 2001), or mGluR1 (Ary & Szumlinski, 2007), and Homer 2a/b, NR2a, and NR2b (Ary & Szumlinski, 2007) in the N.Acc Shell, but no alterations in the N.Acc Core. However, two other studies (Loftis & Janowsky, 2000; Zhang et al., 2007) using slightly different regimens of injections (i.e. 7 daily injections of 20mg/kg and 4 daily injections of 40 mg/kg, respectively) found increases rather than decreases in protein expression of NR2b within the N.Acc Shell, at 2 or 3 weeks of withdrawal, respectively. Such data suggests that differences in the number of daily injections and the dose of cocaine used, as well as in length of the withdrawal period, results in differential changes in protein expression of mGluR1, mGluR5, and NR2b. Nevertheless, altered expression of mGluR1, mGluR5, Homer1b/c, Homer 2a/b, NR2a, and NR2b in any of the studies using daily IP injections of cocaine were found mostly or only in the N.Acc shell. In contrast, cocaine self-administration altered protein expression mainly in the N.Acc core. The subregional differences in the effects of passive experimenter-administered vs. self-administered cocaine are very intriguing. Indeed, it was suggested that cocaine-induced changes in the N.Acc shell are associated with alterations related to classical or Pavlovian conditioning, alterations that are more likely to occur during passive exposure to cocaine, while cocaine-induced changes in the N.Acc core are associated with alterations related to operant conditioning that are more likely to occur during active self-administration (Di Chiara, 2002). Consistent with such an explanation are the demonstrations that: (1) inactivation of the N.Acc core, but not shell, blocked CS-induced reinstatement of lever-pressing for food (Floresco et al., 2008); (2) cocaine prime-induced reinstatement of self-administration is blocked by reversible lesion of the N.Acc core but not shell (McFarland & Kalivas, 2001, McFarland et al., 2003); and (3) that it is the N.Acc core and not shell that is the final common pathway for reinstatement of cocaine-seeking behavior by stress, cocaine-paired cue, or cocaine prime (Kalivas & McFarland, 2003).
Finally, the lack of changes in protein expression of group I mGluRs-Homers-NMDA subunits within the VTA after brief or extended access to self-administered IV cocaine is consistent with previous reports. Although repeated IP cocaine administration protocols that are known to induce behavioral sensitization result in increased responsiveness of VTA neurons to glutamate administration, this increased responsiveness was mediated via AMPA receptors (White et at., 1995; Zhang et at., 1997), and such cocaine administration protocols fail to alter NR2a, NR2b (Fitzgerald et al., 1996), mGluR1, mGluR5, or Homer1b/c (Swanson et al., 2001) protein expression within the VTA. More relevant to the current study, Hemby et al. (2005a) demonstrated that prolonged access to cocaine self-administration (i.e., 8 hrs a day, up to 60 infusions of 0.5 mg/kg) also failed to alter NR2a or NR2b expression within the VTA. While increases in NMDA receptor mRNA in the VTA were reported in human cocaine addicts that died from overdose of cocaine (Hemby et al., 2005b; Tang et al., 2003), no studies explored specific changes in subunits of the NMDA receptor in human cocaine addicts. Moreover, neuroadaptations found after lethal overdose of cocaine might be specific to this condition and different than those resulting from non-lethal, binge cocaine-use. Together, these data suggest that cocaine-induced changes in the VTA expression of NR2a, NR2b, mGluR1, mGluR5, or Homer proteins mediate neither the stable pattern of self-administration exhibited by brief access animals nor the escalation in cocaine intake exhibited by animals with extended access to cocaine.
In summary, cocaine self-administration caused no observable changes in protein expression within the VTA. In the N.Acc, the brief and extended access conditions altered protein expression similarly and these changes were apparent mostly within the core subregion, implicating Homer-Group1 mGluRs within the N.Acc core in cocaine reinforcement. Finally, extended access to self-administered cocaine resulted in distinct changes in Homer1b/c and NR2a/b within the mPFC, suggesting that NMDA function within the mPFC may be involved in the transition to compulsive and excessive cocaine use. Additional studies are needed to confirm such a role for NMDA receptors within the mPFC.
Acknowledgment
We would like to thank Dr. Paul Worley (Johns Hopkins University School of Medicine) for his generous gift of the Homer1b/c and Homer2a/b antibodies. This work was supported by a NARSAD Young Investigator Award to KKS, and NIDA grant DA017104 to OBS.
References
- Ahmed SH, Cador M. Dissociation of psychomotor sensitization from compulsive cocaine consumption. Neuropsychopharmacology. 2006;31:563–571. doi: 10.1038/sj.npp.1300834. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set-point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Changes in response to a dopamine receptor antagonist in rats with escalating cocaine intake. Psychopharmacology. 2004;172:450–454. doi: 10.1007/s00213-003-1682-9. [DOI] [PubMed] [Google Scholar]
- Ary AW, Szumlinski KK. Regional differences in the effects of withdrawal from repeated cocaine upon Homer and glutamate receptor expression: a two-species comparison. Brain Res. 2007;1184:295–305. doi: 10.1016/j.brainres.2007.09.035. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H. Decision-making and addiction (part I): impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia. 2002;40:1675–1689. doi: 10.1016/s0028-3932(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Hindes A. Decision-making and addiction (part II): myopia for the future or hypersensitivity to reward? Neuropsychologia. 2002;40:1690–1705. doi: 10.1016/s0028-3932(02)00016-7. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar O, Ahmed SH, Koob GF, Ettenberg A. The transition from controlled to compulsive drug use is associated with a loss of sensitization. Brain Res. 2004;995:46–54. doi: 10.1016/j.brainres.2003.09.053. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar O, Moscarello JM, Jacob B, Roarty MP, Ettenberg A. Prolonged daily exposure to IV cocaine results in tolerance to its stimulant effects. Pharmacol Biochem Behav. 2005;82:411–416. doi: 10.1016/j.pbb.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar O, Moscarello JM, Ettenberg A. One hour, but not six hours, of daily access to self-administered cocaine results in elevated levels of the dopamine transporter. Brain Res. 2006;1095:148–153. doi: 10.1016/j.brainres.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar O, Keeley P, Cook M, Brake W, Joyce M, Nyffeler M, Heston R, Ettenberg A. Changes in levels of D1, D2, or NMDA receptors during withdrawal from brief or extended daily access to IV cocaine. Brain Res. 2007;1131:220–228. doi: 10.1016/j.brainres.2006.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar O, Posthumus EJ, Waldroup SA, Ettenberg A. Heightened drug-seeking motivation following extended daily access to self-administered cocaine. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2008;32:863–869. doi: 10.1016/j.pnpbp.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava HN, Kumar S. Sensitization to the locomotor stimulant effect of cocaine modifies the binding of [3H]MK-801 to brain regions and spinal cord of the mouse. Gen Pharmacol. 1999;32:359–363. doi: 10.1016/s0306-3623(98)00107-4. [DOI] [PubMed] [Google Scholar]
- Bornovalova MA, Lejuez CW, Daughters SB, Zachary Rosenthal M, Lynch TR. Impulsivity as a common process across borderline personality and substance use disorders. Clin Psychol Rev. 2005;25:790–812. doi: 10.1016/j.cpr.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Chiamulera C, Epping-Jordan MP, Zocchi A, Marcon C, Cottiny C, Tacconi S, Corsi M, Orzi F, Conquet F. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat Neurosci. 2001;4:873–874. doi: 10.1038/nn0901-873. [DOI] [PubMed] [Google Scholar]
- Clark L, Cools R, Robbins TW. The neuropsychology of ventral prefrontal cortex: decision-making and reversal learning. Brain Cogn. 2004;55:41–53. doi: 10.1016/S0278-2626(03)00284-7. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Dalley JW, Crofts HS, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion. Science. 2004;304:878–880. doi: 10.1126/science.1094987. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Walker SC, Crofts HS, Dalley JW, Robbins TW, Roberts AC. Prefrontal serotonin depletion affects reversal learning but not attentional set shifting. J Neurosci. 2005;25:532–538. doi: 10.1523/JNEUROSCI.3690-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey SF, Gudleski GD, Saladin ME, Brady KT. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Exp Clin Psychopharmacol. 2003;11:18–25. doi: 10.1037//1064-1297.11.1.18. [DOI] [PubMed] [Google Scholar]
- Dackis C, O'Brien C. Glutamatergic agents for cocaine dependence. Ann N Y Acad Sci. 2003;1003:328–345. doi: 10.1196/annals.1300.021. [DOI] [PubMed] [Google Scholar]
- Dackis C, O'Brien C. Neurobiology of addiction: treatment and public policy ramifications. Nat Neurosci. 2005;8:1431–1436. doi: 10.1038/nn1105-1431. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci. 1996;351:1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behavioural Brain Res. 2002;137:75–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- Dworkin SI, Mirkis S, Smith JE. Response-dependent versus response-independent presentation of cocaine: differences in the lethal effects of the drug. Psychopharm. 1995;117:262–266. doi: 10.1007/BF02246100. [DOI] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Meyerhoff DJ. Prefrontal cortical volume reduction associated with frontal cortex function deficit in 6-week abstinent crack-cocaine dependent men. Drug Alcohol Depend. 2002;68:87–93. doi: 10.1016/s0376-8716(02)00110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, Robinson TE. Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biol Psychiatry. 2005;58:751–759. doi: 10.1016/j.biopsych.2005.04.046. [DOI] [PubMed] [Google Scholar]
- Fitzgerald L, Ortiz J, Hamedani A, Nestler E. Drugs of abuse and stress increase the expression of GluR1 and NMDAR1 glutamate receptor subunits in the rat ventral tegmental area: common adaptations among cross-sensitizing agents. J Neurosci. 1996;16:274–282. doi: 10.1523/JNEUROSCI.16-01-00274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, McLaughlin RJ, Haluk DM. Opposing roles for the nucleus accumbens core and shell in cue-induced reinstatement of food-seeking behavior. Neuroscience. 2008;154:877–884. doi: 10.1016/j.neuroscience.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, O'Brien CP, Childress AR. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry. 2002;51:134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- Gawin FH. Cocaine addiction: psychology and neurophysiology. Science. 1991;251:1580–1586. doi: 10.1126/science.2011738. [DOI] [PubMed] [Google Scholar]
- Gawin FH, Kleber HD. Cocaine use in a treatment population: patterns and diagnostic distinctions. NIDA Res Monogr. 1985;61:182–192. [PubMed] [Google Scholar]
- Gawin FH, Kleber HD. Evolving conceptualizations of cocaine dependence. Yale J Biol Med. 1988;61:123–136. [PMC free article] [PubMed] [Google Scholar]
- Grant S, Contoreggi C, London ED. Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia. 2000;38:1180–1187. doi: 10.1016/s0028-3932(99)00158-x. [DOI] [PubMed] [Google Scholar]
- Hemby SE, Tang W, Muly EC, Kuhar MJ, Howell L, Mash DC. Cocaine-induced alterations in nucleus accumbens ionotropic glutamate receptor subunits in human and non-human primates. J Neurochem. 2005b;95:1785–1793. doi: 10.1111/j.1471-4159.2005.03517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemby SE, Horman B, Tang W. Differential regulation of ionotropic glutamate receptor subunits following cocaine self-administration. Brain Res. 2005a;1064:75–82. doi: 10.1016/j.brainres.2005.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology. 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kawashima R, Satoh K, Itoh H, Ono S, Furumoto S, Gotoh R, Koyama M, Yoshioka S, Takahashi T, Takahashi K, Yanagisawa T, Fukuda H. Functional anatomy of GO/NO-GO discrimination and response selection—a PET study in man. Brain Res. 1996;728:79–89. [PubMed] [Google Scholar]
- Kelley AE, Schochet T, Landry CF. Risk taking and novelty seeking in adolescence: introduction to part I. Acad Sci. 2004;1021:27–32. doi: 10.1196/annals.1308.003. [DOI] [PubMed] [Google Scholar]
- Kennedy MB. Signal-processing machines at the postsynaptic density. Science. 2000;290:750–754. doi: 10.1126/science.290.5492.750. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Boutrel B, Gasparini F, Koob GF, Markou A. Metabotropic glutamate 5 receptor blockade may attenuate cocaine self-administration by decreasing brain reward function in rats. Psychopharmacology. 2005;179:247–254. doi: 10.1007/s00213-004-2069-2. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Fuchs RA, See RE. Contributions of prolonged contingent and noncontingent cocaine exposure to enhanced reinstatement of cocaine seeking in rats. Psychopharmacology. 2006;187:60–67. doi: 10.1007/s00213-006-0386-3. [DOI] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Sekihara K, Miyashita Y. No-go dominant brain activity in human inferior prefrontal cortex revealed by functional magnetic resonance imaging. Eur J Neurosci. 1998;10:1209–1213. doi: 10.1046/j.1460-9568.1998.00167.x. [DOI] [PubMed] [Google Scholar]
- Lim KO, Choi SJ, Pomara N, Wolkin A, Rotrosen JP. Reduced frontal white matter integrity in cocaine dependence: a controlled diffusion tensor imaging study. Biol Psychiatry. 2002;51:890–895. doi: 10.1016/s0006-3223(01)01355-5. [DOI] [PubMed] [Google Scholar]
- Lim S, Naisbitt S, Yoon J, Hwang JI, Suh PG, Sheng M, Kim E. Characterization of the Shank family of synaptic proteins. Multiple genes, alternative splicing, and differential expression in brain and development. J Biol Chem. 1999;274:29510–29518. doi: 10.1074/jbc.274.41.29510. [DOI] [PubMed] [Google Scholar]
- Loftis JM, Janowsky A. Regulation of NMDA receptor subunits and nitric oxide synthase expression during cocaine withdrawal. J Neurochem. 2000;75:2040–2050. doi: 10.1046/j.1471-4159.2000.0752040.x. [DOI] [PubMed] [Google Scholar]
- Loftis JM, Janowsky A. The N-methyl-D-aspartate receptor subunit NR2B: localization, functional properties, regulation, and clinical implications. Pharmacol Ther. 2003;97:55–85. doi: 10.1016/s0163-7258(02)00302-9. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Yuferov V, Mathieu-Kia AM, Ho A, Kreek MJ. Effects of extended access to high versus low cocaine doses on self-administration, cocaine-induced reinstatement and brain mRNA levels in rats. Psychopharmacology. 2004;175:26–36. doi: 10.1007/s00213-004-1778-x. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Cullinan WE, Tang LC, Baker DA, Katz ES, Hoks MA, Ziegler DR. Daily cocaine self-administration under long-access conditions augments restraint-induced increases in plasma corticosterone and impairs glucocorticoid receptor-mediated negative feedback in rats. Brain Res. 2007;1167:101–111. doi: 10.1016/j.brainres.2007.05.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matochik JA, London ED, Eldreth DA, Cadet JL, Bolla KI. Frontal cortical tissue composition in abstinent cocaine abusers: a magnetic resonance imaging study. Neuroimage. 2003;19:1095–1102. doi: 10.1016/s1053-8119(03)00244-1. [DOI] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monterosso J, Ehrman R, Napier KL, O'Brien CP, Childress AR. Three decision-making tasks in cocaine-dependent patients: do they measure the same construct? Addiction. 2001;96:1825–1837. doi: 10.1046/j.1360-0443.2001.9612182512.x. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Manzardo AM, Polis I, Stouffer DG, Parsons LH. Biphasic alterations in serotonin-1B (5-HT1B) receptor function during abstinence from extended cocaine self-administration. J Neurochem. 2006;99:1363–1376. doi: 10.1111/j.1471-4159.2006.04163.x. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Neyton J. NMDA receptor subunits: function and pharmacology. Curr Opin Pharmacol. 2007;7:39–47. doi: 10.1016/j.coph.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Markou A. Increased motivation for self-administered cocaine after escalated cocaine intake. Neuroreport. 2003;14:2229–2232. doi: 10.1097/00001756-200312020-00019. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Markou A. The metabotropic glutamate receptor 5 antagonist MPEP decreased break points for nicotine, cocaine and food in rats. Psychopharmacology. 2005;179:255–261. doi: 10.1007/s00213-004-2070-9. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. fourth edition Academic Press, An Imprint of Elsevier; 1998. [Google Scholar]
- Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Meil WM, Kalivas PW. The NMDA antagonist, dizocilpine, enhances cocaine reinforcement without influencing mesoaccumbens dopamine transmission. Psychopharmacology. 1997;133:188–195. doi: 10.1007/s002130050390. [DOI] [PubMed] [Google Scholar]
- Pirot S, Glowinski J, Thierry AM. Mediodorsal thalamic evoked responses in the rat prefrontal cortex: influence of the mesocortical DA system. Neuroreport. 1996;7:1437–1441. doi: 10.1097/00001756-199605310-00023. [DOI] [PubMed] [Google Scholar]
- Pulvirenti L, Maldonado-Lopez R, Koob GF. NMDA receptors in the nucleus accumbens modulate intravenous cocaine but not heroin self-administration in the rat. Brain Res. 1992;594:327–330. doi: 10.1016/0006-8993(92)91145-5. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Hornak J, Wade D, McGrath J. Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. J Neurol Neurosurg Psychiatry. 1994;57:1518–1524. doi: 10.1136/jnnp.57.12.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiwell AP, Reveron ME, Duvauchelle CL. Increased accumbens Cdk5 expression in rats after short-access to self-administered cocaine, but not after long-access sessions. Neurosci Lett. 2007;417:100–105. doi: 10.1016/j.neulet.2007.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Kim MJ. Postsynaptic signaling and plasticity mechanisms. Science. 2002;298:776–780. doi: 10.1126/science.1075333. [DOI] [PubMed] [Google Scholar]
- Steketee JD. Cortical mechanisms of cocaine sensitization. Crit Rev Neurobiol. 2005;17:69–86. doi: 10.1615/critrevneurobiol.v17.i2.20. [DOI] [PubMed] [Google Scholar]
- Swanson CJ, Baker DA, Carson D, Worley PF, Kalivas PW. Repeated cocaine administration attenuates group I metabotropic glutamate receptor-mediated glutamate release and behavioral activation: a potential role for Homer. J Neurosci. 2001;21:9043–9052. doi: 10.1523/JNEUROSCI.21-22-09043.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Ary AW, Lominac KD, Klugmann M, Kippin TE. Accumbens Homer2 overexpression facilitates alcohol-induced neuroplasticity in C57BL/6J mice. Neuropsychopharmacology. 2008;33:1365–1378. doi: 10.1038/sj.npp.1301473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Dehoff MH, Kang SH, Frys KA, Lominac KD, Klugmann M, Rohrer J, Griffin W, 3rd, Toda S, Champtiaux NP, Berry T, Tu JC, Shealy SE, During MJ, Middaugh LD, Worley PF, Kalivas PW. Homer proteins regulate sensitivity to cocaine. Neuron. 2004;43:401–413. doi: 10.1016/j.neuron.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Kalivas PW, Worley PF. Homer proteins: implications for neuropsychiatric disorders. Curr Opin Neurobiol. 2006;16:251–257. doi: 10.1016/j.conb.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Lominac KD, Kleschen MJ, Oleson EB, Dehoff MH, Schwarz MK, Seeburg PH, Worley PF, Kalivas PW. Behavioral and neurochemical phenotyping of Homer1 mutant mice: possible relevance to schizophrenia. Genes Brain Behav. 2005a;4:273–288. doi: 10.1111/j.1601-183X.2005.00120.x. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Lominac KD, Oleson EB, Walker JK, Mason A, Dehoff MH, Klugmann M, Cagle S, Welt K, During M, Worley PF, Middaugh LD, Kalivas PW. Homer2 is necessary for EtOH-induced neuroplasticity. J Neurosci. 2005b;25:7054–7061. doi: 10.1523/JNEUROSCI.1529-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Wesley M, Freeman WM, Liang B, Hemby SE. Alterations in ionotropic glutamate receptor subunits during binge cocaine self-administration and withdrawal in rats. J Neurochem. 2004;89:1021–1033. doi: 10.1111/j.1471-4159.2004.02392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W-X, Fasulo WH, Mash DC, Hemby SE. Molecular profiling of midbrain dopamine regions in cocaine overdose victims. J Neurochem. 2003;85:911–924. doi: 10.1046/j.1471-4159.2003.01740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessari M, Pilla M, Andreoli M, Hutcheson DM, Heidbreder CA. Antagonism at metabotropic glutamate 5 receptors inhibits nicotine- and cocaine-taking behaviours and prevents nicotine-triggered relapse to nicotine-seeking. Eur J Pharmacol. 2004;499:121–133. doi: 10.1016/j.ejphar.2004.07.056. [DOI] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P, Doan A, Aakalu VK, Lanahan AA, Sheng M, Worley PF. Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron. 1999;23:583–592. doi: 10.1016/s0896-6273(00)80810-7. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology. 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Volkow ND. Imaging the addicted brain: from molecules to behavior. J Nucl Med. 2004;45:13N–16N. 19N–20N, 22N. passim. [PubMed] [Google Scholar]
- Volkow ND, Hitzemann R, Wang GJ, Fowler JS, Wolf AP, Dewey SL, Handlesman L. Long-term frontal brain metabolic changes in cocaine abusers. Synapse. 1992;11:184–190. doi: 10.1002/syn.890110303. [DOI] [PubMed] [Google Scholar]
- Volkow N, Li TK. The neuroscience of addiction. Nat Neurosci. 2005;8:1429–1430. doi: 10.1038/nn1105-1429. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386:830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Hitzemann R, Fowler JS, Wolf AP, Pappas N, Biegon A, Dewey SL. Decreased cerebral response to inhibitory neurotransmission in alcoholics. Am J Psychiatry. 1993;150:417–422. doi: 10.1176/ajp.150.3.417. [DOI] [PubMed] [Google Scholar]
- White FJ, Hu XT, Zhang XF, Wolf ME. Repeated administration of cocaine or amphetamine alters neuronal responses to glutamate in the mesoaccumbens dopamine system. J. Pharmacol. Exp. Ther. 1995;273:445–454. [PubMed] [Google Scholar]
- Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol. 1998;54:679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]
- Zhang XF, Hu XT, White FJ, Wolf ME. Increased responsiveness of ventral tegmental area dopamine neurons to glutamate after repeated administration of cocaine or amphetamine is transient and selectively involves AMPA receptors. J Pharmacol Exp Ther. 1997;281:699–706. [PubMed] [Google Scholar]
- Zhang X, Lee TH, Davidson C, Lazarus C, Wetsel WC, Ellinwood EH. Reversal of cocaine-induced behavioral sensitization and associated phosphorylation of the NR2B and GluR1 subunits of the NMDA and AMPA receptors. Neuropsychopharmacology. 2007;32:377–387. doi: 10.1038/sj.npp.1301101. [DOI] [PubMed] [Google Scholar]
- Zhang XX, Shi WX. Dendritic glutamate-induced bursting in prefrontal pyramidal cells: role of NMDA and non-NMDA receptors. Zhongguo Yao Li Xue Bao. 1999;20:1125–1131. [PubMed] [Google Scholar]