Abstract
The stem cell therapy for treating ischemic diseases is promising; however, the limited availability and compromised quality of progenitor cells in aged and diseased patients limit its therapeutic use. Here we report a nanofiber-based ex vivo stem cell expansion technology and proangiogenic growth factors overexpression of human umbilical cord blood (UCB)-derived progenitor cells to enhance angiogenic potential of therapeutic stem cells. The progenitor cells were expanded ~225-fold on nanofiber-based serum-free ex vivo expansion culture technique without inducing differentiation. The expanded cells express high levels of stem cell homing receptor, CXCR4, and adhesion molecule, LFA-1. The nanofiber-expanded stem cells uptake AcLDL effectively, and migrate efficiently in an in vitro transmigration assay. These expanded cells can also differentiate into endothelial and smooth muscle cells in vitro. In a NOD/SCID mouse hind limb vascular injury model, nanofiber-expanded cells were more effective in blood flow restoration and this effect was further augmented by VEGF164 and PDGF-BB, growth factor overexpression. The data indicate that nanofiber-based ex vivo expansion technology can provide an essential number of therapeutic stem cells. Additionally, proangiogenic growth factors overexpression in progenitor cells can potentially improve autologous or allogeneic stem cell therapy for ischemic diseases.
Keywords: Human umbilical cord blood, Hematopoietic progenitor stem cells, Proangiogenic growth factors, Nanofibers, Limb ischemia
INTRODUCTION
The limited availability and the compromised quality of endothelial progenitor cells (EPC) in bone marrow and peripheral circulation (10) and adults in disease states (7) limit its therapeutic use for successful cell- based therapy for a variety of ischemic diseases. To overcome this obstacle, an ex vivo repair of a patient’s stem cells through growth factor-enriched culture and/or genetic modification prior to autologous or allogeneic transplantation has been attempted with limited success (23). Here we report a nanofiber-based ex vivo stem cell expansion technology and proangiogenic growth factor transfection of the human umbilical cord blood (UCB)-derived progenitor cells to enhance angiogenic potential of therapeutic stem cells.
The self-renewal, proliferation, and differentiation of hematopoietic stem/progenitor cells (HSPCs) is tightly regulated in vivo by an array of signals emanating from their microenvironment, termed the hematopoietic stem cell (HSC) niche (26). Ex vivo expansion of human stem cells has been carried out either by a biological or bio-materials approach (27). In a biological approach, less defined stromal layers were used for expansion, which may also produce negative regulators of hematopoiesis (27).
A stromal-free suspension culture has been rapidly adopted as an alternative to stromal layer culture for HSC expansion due to their chemically defined nature. This method involves the use of various combinations of growth factors and cytokines to substitute for the regulatory signals provided by stromal cells (11,29). In our earlier studies using stromal-free culture, we have shown that the human umbilical cord blood (UCB)-derived CD34+ cells can be expanded on aminated nanofibers for 10 days using serum-free medium (4). However, their functionality and effectiveness have yet to be clearly defined.
Recent study with plasmid vectors of VEGF-A164 or PDGF-BB, or a combination of the two (12), indicated that VEGF-A164 increased capillary density more than PDGF-BB, and PDGF-BB preferentially stimulated arteriolar growth. The above combination increased both capillaries and arterioles. Furthermore, combined stimulation with VEGF-A, fibroblast growth factor 2 (FGF-2), or PDGF-BB has emerged as a potent strategy for therapeutic angiogenesis (15).
In the present study, with the aim of increasing neo-vascularization as a treatment strategy for myocardial ischemia and peripheral vascular disease, we examined the feasibility of expanding UCB-derived CD133+ pro-genitor cell population using nanofiber scaffold-based ex vivo expansion culture technique. We also utilized a bicistronic vector, in which both VEGF-A164 and PDGF-BB genes with internal ribosomal entry site (IRES) under CMV promoter (coupled VIP) are overexpressed in ex vivo-expanded UCB-derived progenitor stem cells.
MATERIALS AND METHODS
CD133+ Cell Isolation
Fresh human cord blood was obtained from University Hospitals Case Medical Center after IRB approval and written consent from donors. Cord blood was processed following the similar protocol published earlier (5). The heparinized cord blood was diluted with PBS and carefully layered over 10 ml of Ficoll. After 30-min centrifugation in a swinging bucket rotor at 14000 rpm, the upper layer was aspirated and the mononuclear cell layer was collected. Following labeling with magnetic bead conjugated anti-CD133 (CD133) monoclonal antibody (Miltenyi Biotec Inc, Bergisch Gladbach, Germany), two cell separation cycles (with different columns) were performed using the AutoMACS cell sorter (Miltenyi Biotec) according to the manufacturer’s protocol and reagents. After separation, purity of the cell product was determined by flow cytometry.
Electrospinning, Surface Grafting, and Amination of PES Nanofiber Mesh
All chemicals were purchased from Sigma-Aldrich (USA) unless otherwise stated. PES granules (MW 55,000) were purchased from Goodfellow Cambridge Limited (14). Electrospinning, PAAc grafting, and amination of PES nanofibers were carried out according to the procedure described elsewhere in detail (4).
Ex Vivo CD133+ Hematopoietic Cell Expansion Cultures
Purified recombinant human stem cell factor (SCF), Flt-3 ligand (Flt3), TPO, and IL-3 were purchased from Peprotech Inc. (Rocky Hill, NJ, USA). The StemSpan SFEM medium was purchased from StemCell Technologies (Vancouver, BC, Canada). Nanofiber meshes were securely glued to the bottoms of wells of a 24-well tissue culture plate. Eight hundred CD133+ cells were seeded onto each scaffold in 0.6 ml StemSpanTM serum-free expansion medium, which consists of 1% BSA, 0.01 mg/ml recombinant human insulin, 0.2 mg/ml human transferrin, 0.1 mM 2-mercaptoethanol, and 2 mM L-glutamine in Iscove’s MDM, supplemented with 0.04 mg/ml low-density lipoprotein (Athens Research and Technology Inc., USA), 100 ng/ml SCF, 100 ng/ml Flt3, 50 ng/ml TPO, and 20 ng/ml IL-3. Cells were cultured at 37° C in an atmosphere containing 5% CO2 for 10 days without medium change. Cells were harvested after 10 days of expansion. All substrates were washed once with non-trypsin cell dissociation solution and twice with 2% FBS Hanks’ buffer at 5–10 min intervals between each wash. The cell suspensions collected were then concentrated through centrifugation at 500 × g for 10 min. Aliquots of the concentrated cells were then used for cell counting by a hemocytometer, flow cytometry analysis, as well as for further studies.
Flow Cytometry
For flow cytometric analysis cell surface markers were blocked with FCR Blocking Reagent (1:5; Miltenyi Biotec Inc.) and incubated for 20 min at 4° C with the following antibodies: anti-CD34-PE, and anti-CD133/2 FITC (all from Miltenyi Biotec Inc). Isotype controls were purchased from BD Pharmingen. After incubation cells were washed with MACS sorting buffer and analyzed using a FACS Calibur flow cytometer (Becton Dickinson, Heidelberg, Germany). Dead cells were excluded via propidium iodide staining. Data analysis was performed with BD Cell Quest software. The Milan-Mulhouse gating method was used for cell enumeration, where a double gating (CD133 and CD34+) strategy was used to identify the primitive hematopoietic progenitor cell population in the ex vivo expansion cultures.
Fluorescently labeled antibodies for other cell surface markers (CXCR4, von Willebrand Factor, CD31, CD14, MHC class I, MHC class II, CD69, CD3, Mac-I, LFA-1, and CD86) were purchased from BD Biosciences (USA). The cell samples were incubated at 4° C for > 30 min in 2% FBS Hanks’ buffer in the presence of various antibody combinations. After antibody staining, cells were washed twice using Hanks’ buffer and fixed in 1% paraformaldehyde. Cells were analyzed by two-color flow cytometry on a FACS Calibur analyzer (BD Biosciences). Relevant isotype controls were also included to confirm specificity and for compensation setting. At least 20,000 events were acquired.
Genetic Manipulation of Stem Cells
Freshly isolated human CD133+ MACS sorted cells or nanofiber-expanded cells were transfected with either GFP containing vector (pmaxGFP) or VIP vectors (VEGF IRES and PDGF in pAMFG vector, generous gift from Dr. Blau, Stanford University, CA) using a human CD34 cell Nucleofector kit (Amaxa Inc.) following the manufacturer’s protocol. In brief, 1–3 × 106 cells were transfected with 2–4 μg of plasmid DNA in 100 μl of CD34 cell Nucleofector solution and using Amaxa Electroporator programs: U-008 or U-001 (Amaxa Inc.). After transfection cells were cultured with DMEM complete media or as stated for further studies.
Enzyme-Linked Immunosorbent Assay (ELISA)
One million nanofiber-expanded cells transfected with coupled VIP vector or empty vector were cultured on a 24-well plate and cell culture supernatant was collected at 24 or 48 h time points for ELISA. The levels of PDGF in the cell culture supernatants were quantitated using Quantikine human PDGF-BB ELISA kit from R&D Systems (Minneapolis, MN).
Dil-Ac-LDL Uptake Assay
Dil-Ac-LDL uptake was performed following standard protocol. After expansion of CD133+ cells on nanofiber mesh for 10 days, cells were plated in glass bottom chamber slides for another 7 days with RPMI-1640 complete media. After 7 days, cells were washed with PBS and a serum-free RPMI-1640 containing 10 μg/ml Dil-Ac-LDL was added to the culture and incubated for 4 h at 37° C. Medium was aspirated and cells were washed twice with PBS to remove free Dil-Ac-LDL. Cells were then fixed with 3% formalin in PBS for 10 min followed by washing with PBS. Slides were mounted with Vectashield includes DAPI. Slides were visualized under fluorescence microscope and digital photographs were recorded.
Transwell Migration Assay
Thirty thousand freshly isolated CD133+ cells or nanofiber-expanded cells were plated on the transwell upper chamber and migratory capacity was assessed in the presence or absence of stromal-derived factor (SDF) in lower chamber, after 4 h of incubation at 37° C with 5% CO2 in a humid chamber. Migrated cells to the lower chambers were stained with Giemsa and counted under microscope.
Nanofiber-Expanded Progenitor Cell-Induced Neovascularization in a NOD/SCID Mice Hind Limb Vascular Injury Model
All animal experiments were performed in accordance with the guidelines published in the Guide for the Care and Use of Laboratory Animals (NRC publication), and under the protocols approved by the Institutional Animal Care and Use Committee at Case Western Reserve University. Female NOD/SCID mice (7 weeks old) were purchased from Jackson laboratory (Bar Harbor, ME). Mice were anesthetized with an IP injection of a cocktail of ketamine, xylazine, and acepromazine, the proximal right femoral artery was ligated at two points 3 mm apart, and the artery between the ligatures was transected. Studies were performed with four groups of mice. Each group (6–9 mice) was injected with either media alone, freshly isolated CD133+ cells (5 × 105 cells/mouse), 10-day nanofiber-expanded cells, or VIP transfected nanofiber-expanded cells via intra-ventricular delivery in 300 μl volume. At baseline, post-ligation, and days 7, 14, 21, and 28, mice were assessed for functional recovery and blood flow. At day 28, the mice were sacrificed and both right and left gastrocnemius muscles were excised. Half of each sample was snap frozen and the other half was formalin fixed for further investigation.
Laser Doppler Perfusion Imaging
Mice were anesthetized and blood flow in the hind limbs, and functional recovery was measured with a laser Doppler perfusion image analyzer (Moor Instruments Co. Ltd., Devon, UK) at baseline, after ligation, and at days, 7, 14, 21, and 28 after stem cell transplantation.
Capillary Staining and Counting
To determine the capillary density in the border zone of each hind limb ischemia, tissues were dissected and snap frozen in liquid nitrogen. Cryosections of frozen tissues were stained using an alkaline phosphatase kit [Sigma FASTTM BCIP/NBT (5-Bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium tablets], which were used for the detection of alkaline phosphatase activity and stained as a substrate precipitant. For quantification of positively stained vessels (predominantly endothelium), four sections within the necrosis border zone of each animal were analyzed by an investigator who was blinded with respect to the cell treatment. Capillaries were counted in 12 randomly chosen high-power fields (HPFs) in four sections per tissue and five animals per group. The results were expressed as capillaries per high-power field.
Statistical Analysis
All values were presented as mean ± SEM. One-way ANOVA with Scheffe’s post hoc test for unequal sample sizes was used to compare numeric data between the four experimental groups. Datasets consisting of two groups only were compared by unpaired Student’s t-test. A level of p < 0.05 was considered as significant difference.
In Vitro Differentiation Assay, Immunocytochemistry, and Fluorescence Microscopy
To assess the angiogenesis-relevant differentiation potential of cord blood-derived CD133+ cells, which were expanded on nanofibers for 10 days, separate series of cell products (n = 4) were cultivated and induced to adopt an endothelial or smooth muscle phenotype. Ten-day expanded cells were either cultured with EGM2 medium or SMGM-2 medium (Cambrex Inc./Lonza Inc., NJ) for another 14 days on chamber slides (Labtek, Nunc Internatinal Inc.) changing media on every third day. All cultures were performed in quadruplicate, incubated at 37° C in 5% CO2 and 95% humidity, and scored after 14 days of culture by light microscopy. Immunocytochemistry/immunofluorescence staining for rhodamin conjugated F-Phalloidin (Invitrogen), smooth muscle myosin heavy chain (SM-MHC), smooth muscle α-actin, CD31, ICAM-1, VCAM-1, or von Willebrand factor (vWF) was performed (monoclonal antibody followed by either FITC-conjugated secondary goat anti-mouse IgG, or secondary reagent with DAPI). After several washing steps, samples were viewed under a fluorescence microscope (Leica, Germany). Cultured human umbilical vein endothelial cells (HUVEC) and NIH3T3 cells served as positive and negative controls, respectively.
RESULTS
Isolation of Fresh Umbilical Cord Blood-Derived Progenitor Cells and Ex Vivo Expansion
In general, via regular processing of human umbilical cord blood samples (60–80 ml sample volume), we were able to isolate 5 × 105 to 1 × 106 CD133+ cells using a magnetic bead-conjugated immunopurification system (AutoMACS). Representative flow cytometric analysis revealed (Fig. 1A) that UCB-derived CD133+ cells isolated did not yield sufficient quantity of cells for extensive ex vivo characterization. We adopted a nanofiber expansion method (4) and demonstrated the ability to successfully expand an initial population of 20,000 cells to a total of 4.5 million cells (225-fold amplification) over 10 days of culture using 24-well nanofiber-coated plates. Even at day 5, a 20–25-fold overall expansion was observed (Fig. 1B).
Figure 1.
Isolation of cord blood-derived stem cells and ex vivo expansion on nanofiber scaffold. (A) The purity of the AutoMACS separated cells was analyzed by flow cytometry. A total of six independent cord blood samples were analyzed and representative data are shown. The right panel indicates the purity is more than 90% and the left panel is the isotype control. (B) Five independent cord blood-derived CD133+ cells were expanded on nanofiber scaffold as described in detail in Materials and Methods and representative cell expansion data are shown.
Phenotypic Characteristics of the Expanded Progenitor Cells
The phenotype analysis indicated nearly 24% of the total cells retain their CD133 expression and 93% of the cells retain their CD34 expression (Fig. 2). A remarkable increase in expression of two important promigratory and proadhesive molecules (CXCR4 and LFA-1, respectively) was observed. Mild to moderate expression of other myeloid markers was observed, such as CD14, CD86, vWF, CD31, or Mac-1, indicating that these expanded cells retain their progenitor characteristics.
Figure 2.
Characterization of nanofiber scaffold-expanded cells. Flow cytometric analysis was performed for the characterization of nanofiber scaffold expanded cells (n = 3) at day 10 using various conjugated antibodies as stated in one- or two-color staining.
In Vitro Characterization of the Expanded Progenitor Cells
AcLDL Uptake Assays
AcLDL uptake property of progenitor cell characteristics was analyzed in these expanded cells (9). The data demonstrated in Figure 3A–C, CD133+ cells expanded for 10 days on nanofiber-coated plates and further cultured on a serum-containing differentiation media can uptake AcLDL efficiently.
Figure 3.
In vitro functional evaluation of the expanded cells. (A–C) The 10-day nanofiber-expanded and recultured cells were incubated with Dil-AcLDL for 4 h at 37° C for uptake; cells were washed, fixed, and mounted with DAPI. (A) Nucleus stained with DAPI (blue) and uptaken Dil-AcLDL [red in (B)]. (C) Merged pictures. (D) Stem cell migration was evaluated on transwell membrane in the presence or absence of stromal-derived factor (SDF) after 4 h of incubation. Migrated cells were stained with Giemsa and counted under a microscope.
Transwell Migration Assays
The invasive potential of expanded CD133+ cells in the presence or absence of stromal-derived factor (SDF)-1 was examined. After 4 h of incubation followed by Giemsa staining, a statistically significant (p < 0.01) twofold increase in migration activity was observed for nanofiber-expanded cells compared to freshly isolated cells in the presence or absence of SDF-1 (Fig. 3D).
In Vitro Differentiation of the Expanded Progenitor Cells
We assessed the ability of nanofiber-expanded cells to differentiate to smooth muscle and endothelial lineages upon culturing in differentiation media for an additional 14 days on chamber slides. Expressions of endothelial specific markers (CD31, vWF, ICAM-1, VCAM-1) were assessed after an additional 14-day incubation in endothelial cell growth medium (EGM2) (Cambrex Inc./Lonza Inc., NJ). These cells were positive for the early endothelial markers (Fig. 4A–D). Isotype controls were evaluated for the respective experiments (Fig. 4E). Cells were also cultured with smooth muscle growth medium (SMGM-2, Cambrex Inc./Lonza Inc., NJ) for another 14 days for differentiation towards the smooth muscle lineage. Under these conditions, early smooth muscle differentiated markers such as F-Phalloidin, smooth muscle myosin heavy chain (SM-MHC), and smooth muscle α-actin were expressed on the differentiated cells (Fig. 5A–I). Respective isotype controls were also included and evaluated in the studies (Fig. 5G, H).
Figure 4.
In vitro differentiation of expanded stem cells to endothelial lineage. The 10-day nanofiber-expanded cells were recultured for another 14 days on chamber slides in EGM2 media. Early endothelial markers, such as CD31, vWF, and ICAM-1 and VCAM-1 (A–D) were studied using immunofluorescence staining; isotype-matched IgG were also used for control staining (E).
Figure 5.
In vitro differentiation of expanded stem cells to smooth muscle cells. Expanded cells were cultured with SMGM complete media for another 14 days. Early smooth muscle differentiated marker such as F-Phalloidin, smooth muscle myosin heavy chain (SM-MHC), and smooth muscle α-actin staining were performed (A–I). Respective isotype controls were also evaluated for representative assays (Fig. 5G, H).
Genetic Modification of Expanded Stem Cells
We transfected pmaxGFP vector to the nanofiber-expanded progenitor stem cells. GFP expression was verified after 24-h posttransfection under a fluorescence microscope. We observed approximately 90% transfection efficiency using this approach (Fig. 6A, B). We subsequently cultured these transfected cells under endothelial differentiation conditions for an additional 14 days. Microscopic examination showed that these cells appeared elongated in shape and endothelial-like in appearance (Fig. 6C, D).
Figure 6.
Genetic modification of expanded stem cells. At 24-h posttransfection of pmaxGFP vector, expanded cells were visualized under fluorescent microscope for green fluorescence protein (GFP) expression (A) and under normal light for cellular morphology (B). Subsequent culture of GFP transfected cells in complete DMEM media for another 14 days showed an endothelial-like morphology (C, D). Expanded cells were transfected with VIP vector (see Materials and Methods for details) and after cytospin cells were stained for PDGF expression using immunofluorescence technique (E, F). (G) Cell culture supernatants were collected at 24 and 48 h after transfection, and PDGF secretion was measured by ELISA.
Transfection of Expanded Progenitor Cells With Coupled VEGF and PDGF
We transfected nanofiber-expanded HSPCs, using the transfection protocol described above, with a coupled bicistronic vector containing VEGF and PDGF (obtained from Dr. Blau, Stanford University, CA). Using immunohistochemical staining observed for PDGF, more than 60% transfection efficiency was demonstrated (Fig. 6E, F). To evaluate PDGF secretion by these expanded and transfected HSPC, ELISA was performed from the culture supernatants after 24 and 48 h post-transfection. After 48 h of posttransfection, we observed approximately fourfold induction of PDGF secretion compared to control vector-transfected cells (Fig. 6G). These results demonstrate that the nanofiber-expanded HSPC can be successfully transfected with the coupled VIP containing plasmid vector.
In Vivo Functional Evaluation of Coupled VIP-Transfected Progenitor Cells in a NOD/SCID Hind Limb Vascular Injury Model
We next sought to evaluate whether these nanofiber-expanded and proangiogenic growth factors genes over-expressed progenitor stem cells have reparative capacity through angiogenesis in a hind limb vascular injury (ligated the femoral artery) model of immunodeficient NOD/SCID mice. Four groups of animals were used (6–9 animals per group): 1) expanded untransfected, 2) expanded transfected with coupled VEGF and PDGF vector, 3) freshly isolated UCB-CD133+ cells, or 4) media only (no cells). Each group of animals was treated with stem/progenitor cell therapy (5 × 105 cells/animal, intraventricular delivery).
On post-cell delivery days 7, 14, 21, and 28, Doppler imaging studies were performed to evaluate blood flow in the hind limbs (both ligated and nonligated). Data were analyzed using a ratio of flow between the ischemic limb and the contralateral normal limb. Results from this Doppler study indicated that blood flow was much higher in mice treated with expanded cells compared to those treated with media alone. Moreover, in mice treated with expanded cells transfected with coupled VIP, blood flow was further augmented (from 43% to 62% recovery at day 28) (Fig. 7A, B). Capillary counts were performed as an anatomical marker of induced angiogenesis. Neovascularization was much more prominent and significantly increased in the group that received expanded cells compared to the media-alone group, and most prominent in the group that received genetically modified expanded cells, verified either by immunostaining or total capillary counts (media; fresh CD133; nanofiber expanded; genetically modified expanded cells: 62, 73, 118, 156 capillaries/hpf, respectively) (Fig. 7C). Taken together these data demonstrated that genetically modified CD133+ cells with proangiogenic growth factors were significantly more potent than that of unmodified cells or freshly isolated cells.
Figure 7.
In vivo effect of manipulated stem cells in hind limb ischemic mouse model. Each group of ischemic mice was inoculated with stem cells or media as stated. (A) Doppler images for blood flow were taken for every 7 days, up to 28 days, and representative images are depicted. (B) Total images were analyzed and presented in a graphical format. Paired t-tests were performed for statistical evaluation. (C) Four sections of each sample were counted for alkaline phosphatase staining and five high-power fields (HPF) were counted for each section. Paired t-tests were performed to evaluate statistical significance.
Immunohistochemical Detection of Injected Stem/Progenitor Cells in the Ischemic Region of Mouse Hind Limb Vascular Injury Model
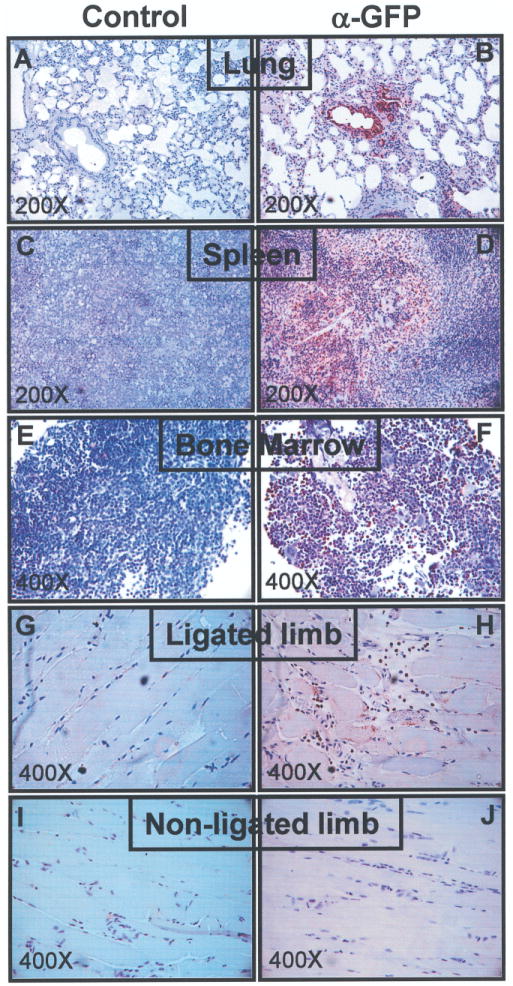
Next, we sought to verify that nanofiber-expanded stem cells could physically migrate and respond to ischemic signals to facilitate local therapeutic benefit. We transfected a GFP-containing vector (as described in the previous section) to the cells. After 16 h of transfection, GFP vector transfected cells (5 × 105 cells/mouse) were injected into hind limb ischemic mice via intraventricular delivery. Thirty-six hours after cell delivery, mice were sacrificed and organs were harvested. Immunohistochemical analysis was performed on fixed and paraffin-embedded tissue sections using an anti-GFP Ab (Fig. 8A–J). Appropriate controls were also evaluated. We detected GFP-positive stem cells in the ischemic tissue area, indicating the homing of these stem cells in the target area of interest. In addition, GFP-positive cells were also detected in the lung, spleen, and bone marrow. GFP-positive cells were not found in the kidney, liver, or brain tissues (data not shown).
Figure 8.
Detection of stem cell homing. After 36 h of GFP vector or empty vector-transfected nanofiber-expanded stem cells injection, mice were sacrificed and organs were harvested. Immunohistochemical detection was performed with the fixed and paraffin-embedded tissue sections using anti-GFP Ab, keeping appropriate controls.
In summary, we have demonstrated that nanofibers could serve as an effective substrate to promote the expansion of functional hematopoietic stem/progenitor cells that retain progenitor cell phenotype of their freshly derived counterparts. We have also demonstrated that the expanded cells maintain the potential to differentiate into endothelial or smooth muscle lineages and can migrate to distant ischemic zones to provide local beneficial effects. Nanofiber-expanded cells are more potent than freshly isolated CD133+ cells in mediating neovascularization in a mouse model of hind limb ischemia. Furthermore, expanded cells genetically modified with proangiogenic growth factors showed dramatic improvement in blood flow restoration and neovascularization.
DISCUSSION
In the present study, the feasibility of ex vivo expansion of HSPCs on nanofiber-coated plates and further genetic modification of expanded HSPCs with proangiogenic growth factors, and their enhanced vasculogenic potential is demonstrated. Recent studies have shown that HSPCs, including autologous BM-derived CD133+ cells, injected either via intracoronary infusion (2) or via intramyocardial injection (28) augmented vasculogenesis in patients with coronary artery ischemia (6). However, the limited availability of progenitor cell population in bone marrow and peripheral circulation (32) and compromised potential of these cells in aged patients and adults in disease states (8,13) has created problems for studying the mechanism of successful cell-based therapy in these patients.
To overcome the limitations of low numbers and compromised functional BM- or cord blood-derived HSPCs, we tested a functional nanofiber expansion method that was introduced recently (4). We have achieved 225-fold expansion of HSPCs in 10 days of culture in a defined serum-free medium. The expanded cells retained their progenitor cell phenotype. Nevertheless, over the course of nanofiber expansion, there was a significant decrease in CD133+ expression, but CD34+ expression was maintained at 93%. Our data indicate that nanofiber-expanded cord blood-derived HSPCs are functionally more efficient in restoring tissue blood flow in the ischemic hind limb muscle compared with freshly enriched CD133+ cells. Injection of proangionenic growth factor-transfected HSPCs further increased the regenerative capacity of peripheral ischemia. Histological analysis of the hind limb muscle samples 4 weeks after stem cell treatment revealed improved vascular regeneration. Even though all animal groups that received cell injections showed increased vascualrization compared to the control group, nanofiber-expanded and proangiogenic growth factor-transfected cells showed a much higher rate of vascualrization compared to either freshly isolated CD133+ cells or controls.
The mechanisms for HSPC-mediated vasculogenesis remain to be fully elucidated. Recent studies have questioned the capacity of HSPCs to transdifferentiate into matured cells of injured tissues (22). Mechanisms other than myogenesis have also been suggested, such as angioblast-mediated vasculogenesis (16) and antiapoptotic effects of the paracrine factors secreted by HSPCs (31). Paracrine mechanisms have been reported to mediate the therapeutic effects of HSPCs in skeletal muscle ischemia. In a mouse model of hind limb ischemia, mouse bone marrow stromal cells were shown to enhance collateral flow recovery and remodeling, and to attenuate muscle atrophy (17). The effect was explained by the release of cytokines, such as VEGF and fibroblast growth factor (FGF), from the progenitor cells. A variety of recombinant angiogenic growth factors have induced angiogenesis and collateral artery growth leading to improved regional blood flow and tissue recovery (1). A combination of VEGF and FGF-2 has also been shown to promote both angiogenesis and lymphangio-genesis in several animal models (14). However, as a novel approach of combining angiogenic growth factors and HSPC-based cell therapy, we have shown a significant enhanced neovascularization effect, and a better recovery of blood flow in murine ischemic muscle than HSPC infusion alone. In addition to the proangiogenic effect of VEGF, this markedly improved neovascularization may be enhanced by the maturation effect of PDGF (3). PDGF has at least two distinct functions in proangiogenic signaling. On one hand, PDGF increases survival and proliferation of endothelial cells and, on the other, PDGF regulates vessel growth via pericyte recruitment and association to newly formed vessels (18).
In addition to paracrine function of the HSPCs, the locally retained HSPCs may differentiate into cells of interest and integrated into host tissue. Our findings (Fig. 8) indicate that a small number of HSPCs home to the ischemic region as well as to the lungs, spleen, and bone marrow. This is supported by the fact that HSPCs have the ability to bi-potentially differentiated into endothelial and smooth muscle cells (Fig. 4 and 5), hence contributing to vasculogenesis.
The chemokine stromal-derived factor (SDF)-1 and its unique receptor CXCR4 are essential for normal cardiovascular development but also play a critical role in postnatal vasculogenesis (25). The CXCR4 receptor, which is highly expressed on both endothelial and hematopoietic progenitor cells (21), has been shown to be essentially involved in mobilization and homing of hematopoietic stem cells (24,33). Additional studies on the interaction of SDF1/CXCR4 with VEGF has also suggested that SDF-1 and CXCR4 contribute to the involvement of bone marrow-derived cells and collaborate with VEGF in the development of several types of neovascularization (19). In this study, the substantial increase in neovascularization potential of nanofiber-expanded and proangiogenic growth factor-transfected cells suggests that the interaction between upregulated CXCR4 expressed by the expanded cells with VEGF is important. However, the mechanism involved in the upregulation of the nanofiber-expanded progenitor population is not clear. Further definitive studies are necessary to delineate the above signaling mechanism.
Leukocyte function associated antigen 1 (LFA-1) is considered as a marker of late stage stem cell maturation when expressed on CD34+ bone marrow cells (20,30). We observed that nanofiber-expanded UCB-derived CD133+ cells express high levels of LFA-1. In addition, Torensma et al. also showed that CD34+ LFA-1− cells expressed LFA-1 within 24 h in culture (30). These in vitro findings indicate that LFA-1 is upregulated by default due to the lack of negative regulating signals from stromal cells. The biological significance of elevated levels of LFA-1 expression in nanofiber-expanded cells is not clear at this point and warrants further detailed study.
In summary, we have shown that functional nanofibers can substantially expand cord blood-derived HSPCs in serum-free culture. The expanded cells retain their progenitor cell phenotype and provide an opportunity for genetic manipulation prior to HSPC infusion. We have provided evidence that nonviral delivery of proangiogenic factors VEGF and PDGF markedly enhanced the angiogenesis effect of HSPCs compared to untransfected cells. This study has demonstrated the feasibility of a combined HSPC-based cell therapy with a proangiogenic gene therapy approach, and propose the therapeutic potential of the nanofiber-expanded HSPCs in treating ischemic diseases.
Acknowledgments
The VIP plasmid expression vector was provided by Prof. Helen Blau at Stanford University, CA. Financial support was provided by the Center for Stem Cell and Regenerative Medicine, Cleveland, OH; Arteriocyte, Inc., Cleveland, OH; and the Wolfe Family Foundation. This work was supported in part by National Institutes of Health grant K01 AR054114 (to H.D.). Dr. Vincent Pompili has equity interest with Arteriocyte, Inc. Dr. Hai-Quan Mao has licensed a patent technology to Arteriocyte, Inc.
References
- 1.Abo-Auda W, Benza RL. Therapeutic angiogenesis: Review of current concepts and future directions. J Heart Lung Transplant. 2003;22(4):370–382. doi: 10.1016/s1053-2498(02)00665-4. [DOI] [PubMed] [Google Scholar]
- 2.Bartunek J, Vanderheyden M, Vandekerckhove B, Mansour S, De Bruyne B, De Bondt P, Van Haute I, Lootens N, Heyndrickx G, Wijns W. Intracoronary injection of CD133-positive enriched bone marrow progenitor cells promotes cardiac recovery after recent myocardial infarction: Feasibility and safety. Circulation. 2005;112(9 Suppl):I178–183. doi: 10.1161/CIRCULATIONAHA.104.522292. [DOI] [PubMed] [Google Scholar]
- 3.Cao R, Bjorndahl MA, Religa P, Clasper S, Garvin S, Galter D, Meister B, Ikomi F, Tritsaris K, Dissing S, Ohhashi T, Jackson DG, Cao Y. PDGF-BB induces intratumoral lymphangiogenesis and promotes lymphatic metastasis. Cancer Cell. 2004;6(4):333–345. doi: 10.1016/j.ccr.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 4.Chua KN, Chai C, Lee PC, Tang YN, Ramakrishna S, Leong KW, Mao HQ. Surface-aminated electrospun nanofibers enhance adhesion and expansion of human umbilical cord blood hematopoietic stem/progenitor cells. Biomaterials. 2006;27(36):6043–6051. doi: 10.1016/j.biomaterials.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 5.Das H, Sugita M, Brenner MB. Mechanisms of Vdelta1 gammadelta T cell activation by microbial components. J Immunol. 2004;172(11):6578–6586. doi: 10.4049/jimmunol.172.11.6578. [DOI] [PubMed] [Google Scholar]
- 6.Erbs S, Linke A, Adams V, Lenk K, Thiele H, Diederich KW, Emmrich F, Kluge R, Kendziorra K, Sabri O, Schuler G, Hambrecht R. Transplantation of blood-derived progenitor cells after recanalization of chronic coronary artery occlusion: first randomized and placebo-controlled study. Circ Res. 2005;97(8):756–762. doi: 10.1161/01.RES.0000185811.71306.8b. [DOI] [PubMed] [Google Scholar]
- 7.Fadini GP, Sartore S, Albiero M, Baesso I, Murphy E, Menegolo M, Grego F, Vigili de Kreutzenberg S, Tiengo A, Agostini C, Avogaro A. Number and function of endothelial progenitor cells as a marker of severity for diabetic vasculopathy. Arterioscler Thromb Vasc Biol. 2006;26(9):2140–2146. doi: 10.1161/01.ATV.0000237750.44469.88. [DOI] [PubMed] [Google Scholar]
- 8.Fadini GP, Schiavon M, Cantini M, Baesso I, Facco M, Miorin M, Tassinato M, de Kreutzenberg SV, Avogaro A, Agostini C. Circulating progenitor cells are reduced in patients with severe lung disease. Stem Cells . 2006;24(7):1806–1813. doi: 10.1634/stemcells.2005-0440. [DOI] [PubMed] [Google Scholar]
- 9.Finney MR, Greco NJ, Haynesworth SE, Martin JM, Hedrick DP, Swan JZ, Winter DG, Kadereit S, Joseph ME, Fu P, Pompili VJ, Laughlin MJ. Direct comparison of umbilical cord blood versus bone marrow-derived endothelial precursor cells in mediating neovascularization in response to vascular ischemia. Biol Blood Marrow Transplant. 2006;12(5):585–593. doi: 10.1016/j.bbmt.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 10.Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed BS, Szapary PO, Rader DJ, Edman JS, Klein S. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med. 2003;348(21):2082–2090. doi: 10.1056/NEJMoa022207. [DOI] [PubMed] [Google Scholar]
- 11.Hackney JA, Charbord P, Brunk BP, Stoeckert CJ, Lemischka IR, Moore KA. A molecular profile of a hematopoietic stem cell niche. Proc Natl Acad Sci USA. 2002;99(20):13061–13066. doi: 10.1073/pnas.192124499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hao X, Mansson-Broberg A, Blomberg P, Dellgren G, Siddiqui AJ, Grinnemo KH, Wardell E, Sylven C. Angiogenic and cardiac functional effects of dual gene transfer of VEGF-A165 and PDGF-BB after myocardial infarction. Biochem Biophys Res Commun. 2004;322(1):292–296. doi: 10.1016/j.bbrc.2004.07.101. [DOI] [PubMed] [Google Scholar]
- 13.Heeschen C, Lehmann R, Honold J, Assmus B, Aicher A, Walter DH, Martin H, Zeiher AM, Dimmeler S. Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation. 2004;109(13):1615–1622. doi: 10.1161/01.CIR.0000124476.32871.E3. [DOI] [PubMed] [Google Scholar]
- 14.Ibukiyama C. Angiogenesis. Angiogenic therapy using fibroblast growth factors and vascular endothelial growth factors for ischemic vascular lesions. Jpn Heart J. 1996;37(3):285–300. doi: 10.1536/ihj.37.285. [DOI] [PubMed] [Google Scholar]
- 15.Kano MR, Morishita Y, Iwata C, Iwasaka S, Watabe T, Ouchi Y, Miyazono K, Miyazawa K. VEGF-A and FGF-2 synergistically promote neoangio-genesis through enhancement of endogenous PDGF-B-PDGFRbeta signaling. J Cell Sci. 2005;118(Pt 16):3759–3768. doi: 10.1242/jcs.02483. [DOI] [PubMed] [Google Scholar]
- 16.Kawamoto A, Gwon HC, Iwaguro H, Yamaguchi JI, Uchida S, Masuda H, Silver M, Ma H, Kearney M, Isner JM, Asahara T. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation . 2001;103(5):634–637. doi: 10.1161/01.cir.103.5.634. [DOI] [PubMed] [Google Scholar]
- 17.Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, Fuchs S, Epstein SE. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109(12):1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 18.Li M, Jendrossek V, Belka C. The role of PDGF in radiation oncology. Radiat Oncol. 2007;2:5. doi: 10.1186/1748-717X-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lima e Silva R, Shen J, Hackett SF, Kachi S, Akiyama H, Kiuchi K, Yokoi K, Hatara MC, Lauer T, Aslam S, Gong YY, Xiao WH, Khu NH, Thut C, Campochiaro PA. The SDF-1/CXCR4 ligand/receptor pair is an important contributor to several types of ocular neovascularization. FASEB J. 2007;21(12):3219–3230. doi: 10.1096/fj.06-7359com. [DOI] [PubMed] [Google Scholar]
- 20.Mendez-Ferrer S, Frenette PS. Hematopoietic stem cell trafficking: regulated adhesion and attraction to bone marrow microenvironment. Ann NY Acad Sci. 2007;1116:392–413. doi: 10.1196/annals.1402.086. [DOI] [PubMed] [Google Scholar]
- 21.Mohle R, Bautz F, Rafii S, Moore MA, Brugger W, Kanz L. The chemokine receptor CXCR-4 is expressed on CD34+ hematopoietic progenitors and leukemic cells and mediates transendothelial migration induced by stromal cell-derived factor-1. Blood. 1998;91(12):4523–4530. [PubMed] [Google Scholar]
- 22.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA, Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428(6983):664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 23.Parson AB. Stem cell biotech: Seeking a piece of the action. Cell. 2008;132(4):511–513. doi: 10.1016/j.cell.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, Nagler A, Ben-Hur H, Many A, Shultz L, Lider O, Alon R, Zipori D, Lapidot T. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283(5403):845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 25.Salvucci O, Yao L, Villalba S, Sajewicz A, Pittaluga S, Tosato G. Regulation of endothelial cell branching morphogenesis by endogenous chemokine stromal-derived factor-1. Blood. 2002;99(8):2703–2711. doi: 10.1182/blood.v99.8.2703. [DOI] [PubMed] [Google Scholar]
- 26.Sauvageau G, Iscove NN, Humphries RK. In vitro and in vivo expansion of hematopoietic stem cells. Oncogene. 2004;23(43):7223–7232. doi: 10.1038/sj.onc.1207942. [DOI] [PubMed] [Google Scholar]
- 27.Sorrentino BP. Clinical strategies for expansion of haematopoietic stem cells. Nat Rev Immunol. 2004;4(11):878–888. doi: 10.1038/nri1487. [DOI] [PubMed] [Google Scholar]
- 28.Stamm C, Kleine HD, Westphal B, Petzsch M, Kittner C, Nienaber CA, Freund M, Steinhoff G. CABG and bone marrow stem cell transplantation after myocardial infarction. Thorac Cardiovasc Surg. 2004;52(3):152–158. doi: 10.1055/s-2004-817981. [DOI] [PubMed] [Google Scholar]
- 29.Stevens MM, George JH. Exploring and engineering the cell surface interface. Science. 2005;310(5751):1135–1138. doi: 10.1126/science.1106587. [DOI] [PubMed] [Google Scholar]
- 30.Torensma R, Nelissen JM, van Kooyk Y, Raymakers RA, Pennings AH, de Witte T, Figdor CG. Regulation of LFA-1 expression by CD34 positive cells and inducible growth factor production by stroma enable formation of bone marrow compartments. Hematology. 2000;5(4):295–302. doi: 10.1080/10245332.2000.11746521. [DOI] [PubMed] [Google Scholar]
- 31.Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res. 2006;98(11):1414–1421. doi: 10.1161/01.RES.0000225952.61196.39. [DOI] [PubMed] [Google Scholar]
- 32.Van Zant G, Liang Y. The role of stem cells in aging. Exp Hematol. 2003;31(8):659–672. doi: 10.1016/s0301-472x(03)00088-2. [DOI] [PubMed] [Google Scholar]
- 33.Walter DH, Haendeler J, Reinhold J, Rochwalsky U, Seeger F, Honold J, Hoffmann J, Urbich C, Lehmann R, Arenzana-Seisdesdos F, Aicher A, Heeschen C, Fichtlscherer S, Zeiher AM, Dimmeler S. Impaired CXCR4 signaling contributes to the reduced neovascularization capacity of endothelial progenitor cells from patients with coronary artery disease. Circ Res. 2005;97(11):1142–1151. doi: 10.1161/01.RES.0000193596.94936.2c. [DOI] [PubMed] [Google Scholar]