Abstract
We determined whether higher levels of physical activity in daily life are associated with better brachial artery flow-mediated dilation (FMD) among individuals with lower extremity peripheral arterial disease (PAD). Participants were 111 men and women with PAD (ankle–brachial index (ABI) ≤ 0.95) who completed baseline testing in the Study to Improve Leg Circulation (SILC). We evaluated FMD of the brachial artery at baseline and at 60 seconds following 4 minutes of suprasystolic blood pressure cuff inflation. Physical activity was measured continuously over 7 days using a vertical accelerometer (Caltrac) and a pedometer (Digiwalker). Adjusting for age, sex, race, ABI, cardiovascular risk factors and other potential confounders, higher levels of physical activity were associated with a greater percent change in brachial artery FMD at 60 seconds post cuff deflation for both Caltrac (1st tertile of activity +4.81% change; 2nd tertile +4.60% change; 3rd tertile +7.23% change; p-trend = 0.018) and the Digiwalker (1st tertile of activity +3.76% change; 2nd tertile +6.25% change; 3rd tertile +7.25% change; p-trend = 0.001). Similar findings were observed for absolute change in brachial artery FMD 60 seconds after cuff deflation. In conclusion, higher levels of physical activity during daily life are associated significantly and independently with better brachial artery FMD among individuals with PAD, even after adjusting for confounders.
Keywords: endothelial reactivity, intermittent claudication, peripheral arterial disease, physical activity
Introduction
Lower extremity peripheral arterial disease (PAD) affects eight million men and women in the USA. In the primary care setting alone, an estimated 18–23% of patients aged 55 years or older have PAD.1,2 Non-Hispanic blacks and older individuals are disproportionately affected by PAD, which is associated with traditional cardiovascular risk factors such as tobacco use, diabetes, hypertension and hypercholesterolemia.3 PAD is expected to be increasingly common as the US population survives longer with chronic disease.
Compared to individuals without PAD, those with PAD have higher rates of cardiovascular morbidity and mortality even after adjusting for known cardiovascular disease risk factors. A systematic review of 11 population-based cohort studies showed that an ankle–brachial index (ABI) (< 0.90) is significantly associated with an increased risk of all-cause mortality, coronary heart disease mortality, and death from stroke, even after adjustment for age, sex, traditional cardiovascular risk factors and underlying cardiovascular disease.4
A previous study demonstrates that endothelial function is impaired in individuals with PAD compared to individuals without PAD.5 Endothelial reactivity is a measure of the ability of a vessel to vasodilate in response to a stimulus that promotes nitric oxide formation, such as shear stress. In addition, among individuals with PAD, poorer endothelial function is associated with higher rates of cardiovascular morbidity and mortality.6,7
Prior study in individuals without PAD demonstrates that lower physical activity levels are associated with greater impairment in endothelial function.8 However, it is unknown whether lower physical activity levels are associated with greater impairment in endothelial function among those with PAD. Associations of physical activity with endothelial function may differ between individuals with versus without PAD because those with PAD have both lower physical activity levels and poorer endothelial reactivity than individuals without PAD.5,9
This study explored associations of physical activity levels during daily life with brachial artery flow-mediated dilation (FMD) in individuals with PAD. We hypothesized that higher levels of physical activity would be associated with better FMD in individuals with PAD.
Methods
Study overview
The study protocol was approved by the institutional review boards of Northwestern University, Catholic Health Partners Hospital, Evanston Northwestern Hospital, Rush Medical Center, University of Illinois-Chicago, the Jesse Brown Veterans Medical Center in Chicago, and Mt Sinai Hospital in Chicago. Participants gave written informed consent. The funding source for this study played no role in the design, conduct, or reporting of the study, or the decision to submit the manuscript.
Participants were enrolled in a randomized controlled clinical trial, the Study to Improve Leg Circulation (SILC), designed to determine whether a supervised resistance training program and a supervised walking exercise program, respectively, improved functional performance in individuals with PAD with and without classical symptoms of intermittent claudication. Participants were randomized to one of three conditions (resistance training, treadmill training, or nutrition control). Data on brachial artery FMD were collected at baseline and used for the present analyses.
Participant identification
Participants were identified using multiple methods including lists of consecutive patients diagnosed with PAD at Chicago area hospitals, newspaper advertisements, radio advertisements, bulk mailings to older men and women in the Chicago area, posted fliers, and additional community outreach.
Inclusion and exclusion criteria
In individuals without PAD, arterial pressures increase with greater distance from the heart, because of increasing impedance with increasing arterial taper,1 resulting in higher systolic pressures at the ankle compared to the brachial arteries. Thus, individuals without atherosclerosis typically have an ABI > 1.00. Therefore, the inclusion criterion for this study was an ankle–brachial index (ABI) ≤ 0.95, consistent with PAD. Exclusion criteria and the number of participants meeting each criterion are summarized in Table 1. Exclusion criteria were selected because they were likely to prevent the individual from fully participating in the exercise interventions; they interfered with our ability to collect complete data; they potentially prevented the participant from improving their functional performance in response to the intervention; they could potentially alter functional performance independently of the exercise intervention; or because they indicated that initiating a new exercise program may not be safe for the participant. Participants whose walking performance was primarily limited by a reason other than ischemic leg symptoms were excluded.
Table 1.
Reasons for exclusion among participants completing a baseline appointment in the study to improve leg circulation
| Category of exclusion criterion | Exclusion criteria |
|---|---|
| Criteria selected because they may interfere with the participant's ability to participate fully in the study interventions (n = 107) | Dementia (n = 6) |
| Above- or below-knee amputation (n = 1) | |
| Critical limb ischemia or foot ulcers (n = 6) | |
| Nursing home residence or extreme frailty (n = 8) | |
| Inability to walk on a treadmill (n = 5) | |
| Inability to speak English (n = 1) | |
| Unable/unwilling to come to the medical center three times weekly (n = 57) | |
| Failure to complete six run-in exercise sessions in 3 weeks (n = 23) | |
| Criteria selected because they may influence study outcomes independently of study participation (n =14) | Major surgery or a myocardial infarction during the previous 3 months (n = 2) |
| Major surgery planned during the next year (n = 12) | |
| Current participation in other clinical trials (n = 0) | |
| Criterion selected because it limited a potential participant's ability to respond to the intervention (n = 14) | Participant is already exercising at a level comparable to that offered by either exercise arm of the trial (n = 14) |
| Criteria selected because they indicate that study participation may be unsafe (n = 38) | Unstable angina (n = 6) |
| Abnormal baseline stress test (n = 32) | |
| Miscellaneous exclusion criterion (n = 156) | Walking limited by a condition other than leg ischemia (n = 24); baseline SPPB = 12 (n = 123); poorly controlled blood pressure (n = 9) |
SPPB, short physical performance battery.
Brachial artery flow-mediated dilation
Brachial artery flow-mediated dilation was measured by registered diagnostic cardiac sonographers trained in the use of ultrasound to evaluate FMD of the brachial artery. All studies were performed in the morning. Participants fasted for 12 hours, held all medications, and were advised not to exercise or smoke during the morning of the measurement. After participants had rested for 10 minutes in the supine position, the proximal brachial artery was located using a linear array vascular ultrasound transducer (Seimens Sequoia Model #256, frequency 8 MHz, range 5–8 MHz). Baseline B-mode and Doppler images were obtained. A blood pressure cuff on the upper arm proximal to the visualized brachial artery was inflated for 4 minutes at 50 mmHg above the systolic blood pressure. A single longitudinal image of the brachial artery and a Doppler blood flow measure were repeated at 60 and 90 seconds after cuff deflation.11 Digital images were sent to the University of Wisconsin Atherosclerosis Imaging Research Program core lab for measurement and interpretation by a single reader, blinded to treatment and study phase. Measurements were made by tracing a still frame of a 1-cm segment of the brachial artery at the R-wave, using an off-line digital analysis tool (Access Point 2004; Freeland Systems, Westfield, IN, USA). Both sonographers completed a two-day training course at the core ultrasound laboratory using standards set by the American Society of Echocardiography.12 Measurement reproducibility in the core lab was excellent, with a median (inter-quartile range) average flow-mediated dilation difference of 0.02% (−0.03 to 0.04) on blinded repeated readings.
Ankle–brachial index measurement
A hand-held Doppler probe (Nicolet Vascular Pocket Dop II; Nicolet Biomedical Inc, Golden, CO, USA) was used to obtain systolic pressures in the right and left brachial, dorsalis pedis, and posterior tibial arteries.13,14 Each pressure was measured twice: in the order listed and in reverse order. The ABI was calculated by dividing the mean of the dorsalis pedis and posterior tibial pressures in each leg by the mean of the four brachial pressures.13 Zero values for the dorsalis pedis and posterior tibial arteries were excluded from the ABI calculation. Average brachial pressures in the arm with the highest pressure were used when one brachial pressure was higher than the opposite brachial pressure in both measurement sets and the two brachial pressures differed by 10 mmHg or more in at least one measurement set, since in such cases subclavian stenosis was possible.15 The lowest leg ABI was used in the analyses.
Objective physical activity measures
Physical activity levels were measured objectively over 7 days using a vertical accelerometer (Caltrac; Muscle Dynamics Fitness Network, Inc., Torrence, CA, USA).16–18 Caltrac accelerometers are designed to calculate the number of kilocalories expended based on activity (vertical movement), age, weight, height, and sex. To compare activity with the Caltrac vertical accelerometer between participants irrespective of individual variation in age, weight, height, and sex, accelerometers were programmed using identical weight, height, age, and sex for each participant. 16–18 Thus, the accelerometers measured ‘activity units’. 16–18 Simultaneously with the Caltrac accelerometers, participants wore Digiwalker step counters continuously for 7 days.19,20 After wearing the Caltrac vertical accelerometer and the step counter continuously for 7 days, participants reported the number of activity units on the vertical accelerometer and the number of steps displayed on the step counter and mailed their monitors back to investigators.
Comorbidities
Comorbidities were assessed using patient report with questionnaires administered by trained, certified data collectors. For each comorbidity, participants were asked, ‘Has your doctor ever told you that you have …’. Comorbidities assessed were angina, diabetes, history of myocardial infarction, pulmonary disease, hypertension and congestive heart failure.
Other measures
Height and weight were measured and body mass index (BMI) was calculated as weight (kg)/square of height (m2). Cigarette smoking history was determined by patient report.
Statistical analyses
Associations of tertiles of vertical accelerometer-measured physical activity with participant characteristics (age, sex, race, ABI, BMI and history of angina, congestive heart failure, pulmonary disease, hypertension, diabetes and smoking) were evaluated using analyses of variance and the chi-squared test for trends. Associations of physical activity tertiles with brachial artery flow-mediated vasodilation were evaluated using analyses of co-variance and chi-squared tests for trends, adjusting for age, sex, and race. These analyses were repeated with additional adjustment for ABI, BMI, diabetes, hypertension and smoking history. The p-value for statistical significance was p < 0.05 for each analysis.
Results
Of 156 participants in SILC, 150 had Caltrac vertical accelerometer or step counter data. Of these, 111 underwent brachial artery FMD measured at baseline and were included in the present analyses. Among these 111 SILC participants with both brachial artery FMD and physical activity data, the average age was 70.6 years ± 9.7, 53.2% were men, and 38.7% were African-American. The average ABI was 0.63 ± 0.17. Male sex was the only significant difference (p = 0.03) among participants with (53.2%) versus without (33.3%) brachial artery FMD among the 150 participants who completed the 7-day physical activity measurement by Caltrac vertical accelerometer and the step counter (Table 2).
Table 2.
Characteristics of participants in SILC with and without measures of endothelial reactivity data (n = 150)
| Characteristic | Participants with brachial arterial endothelial reactivity data | Participants without brachial arterial endothelial reactivity data | p-value |
|---|---|---|---|
| n | 111 | 39 | |
| Age | 70.61 ± 9.73 | 71.59 ± 11.63 | 0.6093 |
| Male (%) | 53.15 | 33.33 | 0.0331 |
| Black race (%) | 38.74 | 35.9 | 0.7532 |
| ABI | 0.63 ± 0.17 | 0.57 ± 0.17 | 0.0695 |
| BMI (kg/m2) | 30.40 ± 6.74 | 29.57 ± 6.41 | 0.5066 |
| Myocardial infarction (%) | 20.91 | 26.32 | 0.49 |
| Angina (%) | 13.64 | 8.11 | 0.5632 |
| Heart failure (%) | 13.76 | 15.38 | 0.8031 |
| Pulmonary disease (%) | 14.55 | 7.89 | 0.4030 |
| Hypertension (%) | 83.64 | 81.58 | 0.7704 |
| Current smokers (%) | 23.42 | 23.08 | 0.9649 |
| Diabetes mellitus (%) | 39.64 | 56.41 | 0.0695 |
ABI, ankle–brachial index; BMI, body mass index.
Table 3 summarizes baseline characteristics of individuals across tertiles of physical activity measured by the Caltrac vertical accelerometer and the step counter. For the Caltrac vertical accelerometer, higher physical activity levels were associated with younger age (p-trend = 0.004), a higher ABI (p-trend = 0.005) and a lower prevalence of diabetes mellitus (p-trend = 0.024). For the step counter, higher steps walked were associated with younger age (p-trend = 0.015), lower BMI (p-trend = 0.001) and a lower prevalence of diabetes mellitus (p-trend = 0.026).
Table 3.
Associations of clinical characteristics of peripheral arterial disease participants with physical activity tertiles
| (A) Caltrac physical activity data | ||||
|---|---|---|---|---|
| Participant characteristic | Tertile 1 (<410 activity units) (n = 35) | Tertile 2 (≥410 to <706 activity units) (n = 37) | Tertile 3 (≥706 activity units) (n = 36) | Trend p-value |
| Age | 72.50 (8.8) | 73.80 (9.4) | 66.10 (9.3) | 0.0044 |
| Male sex (%) | 54.29 | 48.65 | 52.78 | 0.902 |
| Black race (%) | 45.71 | 32.43 | 38.89 | 0.5612 |
| ABI | 0.58 (0.16) | 0.62 (0.15) | 0.69 (0.20) | 0.0054 |
| BMI | 31.83 (7.17) | 30.00 (7.04) | 28.74 (5.41) | 0.0498 |
| Myocardial infarction (%) | 17.65 | 21.62 | 25 | 0.4557 |
| Angina (%) | 17.14 | 11.11 | 13.89 | 0.697 |
| Heart failure (%) | 8.82 | 16.67 | 13.89 | 0.5412 |
| Hypertension (%) | 82.86 | 88.89 | 77.78 | 0.5619 |
| Current smoker (%) | 28.57 | 21.62 | 22.22 | 0.5346 |
| Diabetes mellitus (%) | 48.57 | 45.95 | 22.22 | 0.0242 |
| Brachial artery flow-mediated dilation | ||||
| Baseline diameter (mm) | 4.54 (0.72) | 4.51 (0.83) | 4.18 (0.63) | 0.042 |
| Flow stimulus (mm3/s) | 667.10 (286.2) | 607.10 (389.1) | 733.44 (324.9) | 0.446 |
| Velocity time integral ratio (reactive hyperemia to baseline) | 5.29 (2.80) | 5.26 (3.19) | 6.54 (5.12) | 0.184 |
| Diameter 60 s after cuff release (mm) | 4.74 (0.71) | 4.67 (0.84) | 4.51 (0.61) | 0.188 |
| Absolute change in brachial artery diameter (mm) | 0.20 (0.12) | 0.19 (0.16) | 0.33 (0.17) | 0.0008 |
| Percent change in brachial flow-mediated diameter (%) | 4.65 (3.17) | 4.32 (3.79) | 8.16 (4.39) | 0.0003 |
| (B) Digiwalker physical activity data | ||||
| Participant characteristic | Tertile 1 (< 8419 steps) (n = 36) | Tertile 2 (≥ 8419 to < 21,624 steps) (n = 37) | Tertile 3 (≥ 21,624 steps) (n = 37) | Trend p-value |
| Age | 72.20 (9.2) | 73.30 (8.1) | 66.70 (10.6) | 0.0147 |
| Male sex (%) | 47.22 | 56.76 | 54.05 | 0.5624 |
| Black race (%) | 44.44 | 29.73 | 40.54 | 0.7393 |
| ABI | 0.59 (0.15) | 0.65 (0.17) | 0.65 (0.19) | 0.1248 |
| BMI | 32.44 (7.61) | 31.55 (5.43) | 27.30 (6.14) | 0.0009 |
| Myocardial infarction (%) | 25.71 | 16.22 | 21.62 | 0.6814 |
| Angina (%) | 19.44 | 13.89 | 8.11 | 0.1662 |
| Heart failure (%) | 17.14 | 13.89 | 10.81 | 0.4396 |
| Hypertension (%) | 88.89 | 86.11 | 75.68 | 0.1331 |
| Current smoker (%) | 22.22 | 29.73 | 16.22 | 0.5346 |
| Diabetes mellitus (%) | 50 | 43.24 | 24.32 | 0.0263 |
| Brachial artery flow-mediated dilation | ||||
| Baseline diameter (mm) | 4.65 (0.66) | 4.49 (0.78) | 4.18 (0.76) | 0.0066 |
| Flow stimulus (mm3/s) | 626.60 (276.9) | 738.10 (430.2) | 688.20 (383.7) | 0.4755 |
| Velocity time integral ratio (reactive hyperemia to baseline) | 4.27 (1.94) | 5.87 (3.40) | 6.73 (5.01) | 0.005 |
| Diameter 60 s after cuff release (mm) | 4.79 (0.67) | 4.74 (0.79) | 4.50 (0.75) | 0.0985 |
| Absolute change in brachial artery diameter (mm) | 0.16 (0.17) | 0.25 (0.13) | 0.32 (0.18) | <0.0001 |
| Percent change in brachial flow-mediated diameter (%) | 3.53 (3.85) | 5.75 (3.23) | 8.02 (4.48) | <0.0001 |
Data shown are means (standard deviations).
ABI, ankle–brachial index; BMI, body mass index.
Table 4 summarizes the characteristics of individuals across resting brachial artery diameter size and percent change in the brachial arterial diameter 60 seconds after cuff occlusion and release. A larger baseline brachial artery diameter was associated with higher prevalences of male sex (p-trend < 0.001) and hypertension (p-trend = 0.029), and with a higher BMI (p-trend = 0.009). Greater percent increases in the brachial artery diameter at 60 seconds post cuff deflation were associated with lower prevalences of prior myocardial infarction (p-trend = 0.007), hypertension (p-trend = 0.025) and diabetes mellitus (p-trend = 0.003). There were no significant associations of ABI with brachial artery flow-mediated brachial dilation.
Table 4.
Unadjusted associations of participant characteristics with brachial artery flow-mediated dilatation
| Participant characteristic | Percent flow-mediated dilation in the brachial artery at 60 s post cuff deflation | |||
|---|---|---|---|---|
| Tertile 1 (<4.04% change) (n = 37) |
Tertile 2 (≥4.04 to <7.37% change) (n = 37) |
Tertile 3 (≥7.37% change) (n = 36) |
Trend p-value | |
| Age | 71.8 (10.4) | 70.3 (8.6) | 69.4 (10.3) | 0.3008 |
| Male sex (%) | 56.76 | 51.35 | 50 | 0.5624 |
| Black race (%) | 32.43 | 48.65 | 36.11 | 0.7387 |
| ABI | 0.62 (0.17) | 0.64 (0.19) | 0.64 (0.17) | 0.6403 |
| BMI (kg/m2) | 31.56 (7.05) | 30.19 (6.67) | 29.55 (6.55) | 0.2033 |
| Myocardial infarction (%) | 37.84 | 13.89 | 11.11 | 0.007 |
| Angina (%) | 24.32 | 8.11 | 8.57 | 0.0576 |
| Heart failure (%) | 22.86 | 2.7 | 16.67 | 0.4647 |
| Hypertension (%) | 94.59 | 81.08 | 74.29 | 0.0247 |
| Current smoker (%) | 21.62 | 29.73 | 19.44 | 0.8334 |
| Diabetes mellitus (%) | 59.46 | 35.14 | 25 | 0.0033 |
Data shown are means (standard deviations).
ABI, ankle–brachial index; BMI, body mass index.
Physical activity and brachial artery flow-mediated dilation
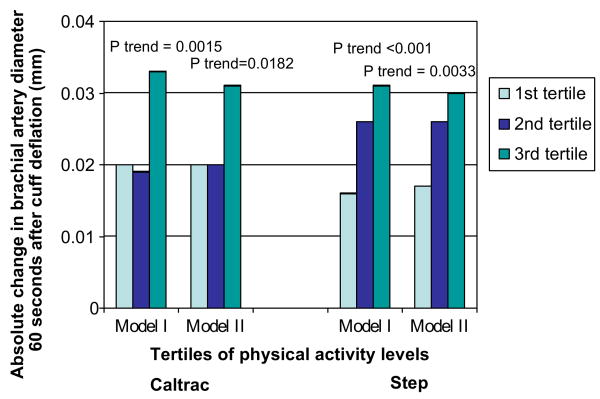
Absolute changes in flow-mediated brachial artery dilation at 60 seconds after cuff deflation across tertiles of physical activity as measured by the Caltrac vertical accelerometer and the step counter are presented in Figure 1. Adjusting for age, sex and race, higher levels of physical activity as measured by the Caltrac vertical accelerometer were associated significantly with a greater absolute change in brachial artery diameter at 60 seconds post cuff deflation (1st tertile of activity +0.020 mm change, 2nd tertile of activity +0.019 mm change, 3rd tertile of activity +0.033 mm change, p-trend = 0.002) (Figure 1, Model I). Adjusting for the same potential confounders, a significant association was also observed between physical activity as measured by the step counter and absolute change in brachial artery diameter at 60 seconds post cuff deflation (1st tertile of activity +0.016 mm change, 2nd tertile of activity +0.026 mm change, 3rd tertile of activity +0.031 mm change, p-trend < 0.001) (Figure 1, Model I).
Figure 1.
Adjusted absolute changes in brachial arterial flow-mediated dilation according to physical activity level in individuals with peripheral arterial disease (n = 111). Model I: adjusts for age, sex, and race. Model II: adjusts for age, sex, race, ankle–brachial index, body mass index, diabetes mellitus, hypertension, cigarette smoking.
Associations of absolute change in brachial artery flow-mediated dilation with physical activity levels measured by both the Caltrac vertical accelerometer and the step counter remained statistically significant even after additional adjustment for BMI, ABI, hypertension, diabetes, and smoking (Figure 1, Model II).
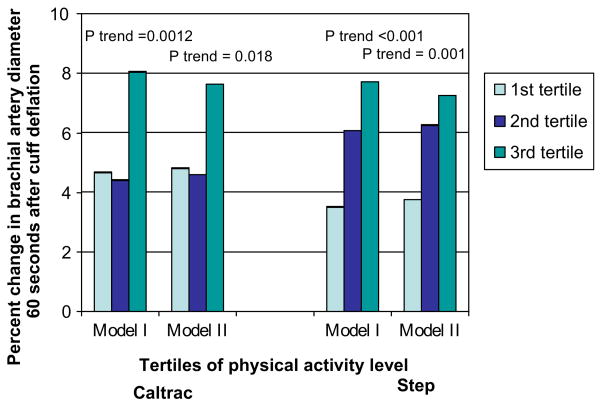
Percent changes in flow-mediated brachial artery dilation at 60 seconds after cuff deflation across tertiles of physical activity are presented in Figure 2. Adjusting for age, sex, and race, higher physical activity levels are associated with a greater percent change in brachial artery diameter at 60 seconds post deflation for both the Caltrac vertical accelerometer (1st tertile of activity +4.67% change, 2nd tertile of activity +4.41% change, 3rd tertile of activity +8.05% change, p-trend = 0.001) and the step counter (1st tertile of activity +3.51% change, 2nd tertile of activity +6.06% change, 3rd tertile of activity +7.71% change, p-trend < 0.001) (Figure 2, Model I). Even after additional adjustment for BMI, ABI, hypertension, diabetes, and smoking history, higher levels of physical activity remained significantly associated with a greater percent change in brachial artery diameter at 60 seconds for both measures of physical activity (Figure 2, Model II).
Figure 2.
Percent change in brachial arterial flow-mediated dilation according to physical activity level in individuals with peripheral arterial disease (n = 111). Model I: adjusts for age, sex, and race. Model II: adjusts for age, sex, race, ankle–brachial index, body mass index, diabetes mellitus, hypertension, cigarette smoking.
Results in Figures 1 and 2 were not substantially changed when analyses were repeated among the subset of participants without diabetes mellitus (data not shown). Similarly, results in Figures 1 and 2 were not substantially changed when analyses were repeated among participants with an ABI < 0.90 (data not shown).
Discussion
Results reported here show that higher levels of physical activity during daily life are associated with greater brachial artery FMD among individuals with PAD. This relationship remained statistically significant after adjusting for age, sex, race, BMI, ABI, hypertension, diabetes mellitus and smoking history. Results were not substantially changed when analyses were repeated among PAD participants without diabetes mellitus. To our knowledge, this is the first study to assess the associations of physical activity with brachial artery FMD in individuals with PAD.
A previous study by Harris et al. established that endothelial function is impaired in individuals with PAD compared to those without PAD.5 Harris et al. evaluated endothelial function in 26 healthy controls compared to 25 individuals with a history of intermittent claudication, peripheral bypass surgery or lower extremity amputation. Participants were divided into three categories: individuals without PAD (mean age 30.7 years), older individuals without PAD (mean age 70.4 years) and older individuals with PAD (mean age 64.3 years). Sixty percent of the PAD participants but none of the participants without PAD had diabetes. Results showed a significantly higher baseline brachial artery diameter and attenuated responses to ischemia and hyperemia among the PAD individuals compared to the healthy controls. However, this study did not adjust for potential confounders including age, race, sex, diabetes, or other differences in baseline characteristics between the participants with versus without PAD.
Previous studies demonstrated a beneficial effect of exercise training on endothelial function in individuals with hypertension, congestive heart failure, coronary artery disease and polymetabolic syndrome.21–24 To our knowledge, only one study assessed whether supervised exercise activity improved brachial artery FMD in individuals with PAD.25 In an uncontrolled clinical trial involving 19 participants with PAD and intermittent claudication, Brendle et al. showed that 6 months of supervised exercise training improved treadmill time to onset of claudication pain, treadmill time to maximal claudication pain, and brachial artery FMD.25
In the current observational, cross-sectional study, we demonstrated that higher levels of physical activity during daily life are associated with greater brachial artery FMD in individuals with PAD. Physical activity during daily life is typically less intensive than supervised treadmill exercise training. Despite this, we observed significant associations of higher levels of physical activity during daily life with greater brachial artery FMD. Further study is indicated to determine whether an intervention that increases daily physical activity levels in individuals with PAD improves brachial arterial FMD. If interventions that increase physical activity result in better brachial arterial FMD, increasing physical activity during daily life may be more easily adopted than a supervised exercise program three times weekly. Currently, most individuals with PAD do not participate in supervised walking exercise programs, in part because these programs are not covered by health insurance plans in the United States.26,27
Two prior studies demonstrate that better brachial artery FMD is associated with higher event-free survival rates in PAD individuals undergoing vascular surgery.6,7 In a prospective study, Huang et al. examined the predictive value of brachial arterial FMD for cardiovascular events in 267 PAD individuals undergoing vascular surgery.6 Outcomes included cardiac death, myocardial infarction, unstable angina, congestive heart failure and non-hemorrhagic stroke. Across tertiles of endothelial function, individuals with higher hyperemic velocity and flow-mediated dilation had better event-free survival than those with poorer endothelial function. Similar findings were observed by Gokce et al., who prospectively followed 187 PAD individuals undergoing vascular surgery and demonstrated that impaired FMD was an independent predictor for postoperative cardiac events.7 Of these two studies, only the study by Gokce et al. included administration of nitroglycerin, so that the endothelium-dependent brachial artery FMD response could be compared with the endothelium-independent dilator response to nitroglycerin. Further study is needed to determine whether interventions that improve FMD are associated with lower cardiovascular morbidity and mortality rates among individuals with PAD.
This study has limitations. First, data are cross-sectional. Results showing that higher levels of physical activity during daily life are associated with poorer brachial artery FMD cannot be construed as causal. Second, participants were those eligible for the exercise trial, SILC. Generalizability of the findings is limited to PAD individuals who meet SILC inclusion criteria. Third, not all participants in SILC completed an assessment of brachial arterial FMD. Fourth, because we did not measure change in brachial artery diameter after administration of nitroglycerin, we cannot determine the degree to which observed changes in brachial artery FMD were specific to endothelial function.
In conclusion, higher levels of physical activity are associated independently and significantly with better brachial artery FMD in response to reactive hyperemia in individuals with PAD. Further study should address whether interventions that increase levels of physical activity during daily life improve brachial artery FMD in individuals with PAD, and whether this improvement in brachial artery FMD correlates independently with lower cardiovascular morbidity and mortality rates in those with PAD.
Acknowledgments
Supported by R01-HL073351 from the National Heart Lung and Blood Institute, NIH.
Contributor Information
Laila Payvandi, Northwestern University Feinberg School of Medicine.
Alan Dyer, Northwestern University Feinberg School of Medicine.
David McPherson, University of Texas.
Philip Ades, University of Vermont.
James Stein, University of Wisconsin.
Kiang Liu, Northwestern University Feinberg School of Medicine.
Luigi Ferrucci, National Institute on Aging.
Michael H Criqui, University of California at San Diego School of Medicine.
Jack M Guralnik, National Institute on Aging.
Donald Lloyd-Jones, Northwestern University Feinberg School of Medicine.
Melina R Kibbe, Northwestern University Feinberg School of Medicine.
Susan T Liang, University of Texas.
Bonnie Kane, Northwestern University Feinberg School of Medicine.
William H Pearce, Northwestern University Feinberg School of Medicine.
Michael Verta, Evanston Northwestern Hospital.
Walter J McCarthy, Rush School of Medicine.
Joseph R Schneider, DuPage Hospital.
Adhir Shroff, University of Illinois.
Mary M McDermott, Northwestern University Feinberg School of Medicine.
References
- 1.Farkouh MA, Oddone EZ, Simel DL. International Cooperative Group for Clinical Examination Research. Improving the clinical examination for a low ankle–brachial index. Int J Angiol. 2002;11:41–45. For the. [Google Scholar]
- 2.Fowkes FG, Housley E, Cawood EH, Macintyre CC, Ruckley CV, Prescott RJ. Edinburgh Artery Study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. Int J Epidemiol. 1991;20:384–392. doi: 10.1093/ije/20.2.384. [DOI] [PubMed] [Google Scholar]
- 3.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States; results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation. 2004;110:738–743. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 4.Heald CL, Fowkes FG, Murray GD, Price JF. the Ankle Brachial Index Collaboration. Risk of mortality and cardiovascular disease associated with the ankle–brachial index: systematic review. Atherosclerosis. 2006;189:61–69. doi: 10.1016/j.atherosclerosis.2006.03.011. On behalf of. [DOI] [PubMed] [Google Scholar]
- 5.Harris LM, Faggioli GL, Shah R, et al. Vascular reactivity in patients with peripheral vascular disease. Am J Cardiol. 1995;76:207–212. doi: 10.1016/s0002-9149(99)80066-6. [DOI] [PubMed] [Google Scholar]
- 6.Huang AL, Silver AE, Shvenke E, et al. Predictive value of reactive hyperemia for cardiovascular events in patients with peripheral arterial disease undergoing vascular surgery. Arterioscler Thromb Vasc Biol. 2007;27:2113–2197. doi: 10.1161/ATVBAHA.107.147322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Menzoian JO, Vita JA. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: a prospective study. Circulation. 2002;105:1567–1572. doi: 10.1161/01.cir.0000012543.55874.47. [DOI] [PubMed] [Google Scholar]
- 8.McKechnie R, Rubenfire M, Mosca L. Association between self-reported physical activity and vascular reactivity in postmenopausal women. Atherosclerosis. 2001;159:483–490. doi: 10.1016/s0021-9150(01)00529-9. [DOI] [PubMed] [Google Scholar]
- 9.McDermott MM, Greenland P, Liu K, et al. The ankle brachial index is associated with leg function and physical activity: the Walking and Leg Circulation Study. Ann Intern Med. 2002;136:I–32. doi: 10.7326/0003-4819-136-12-200206180-00008. [DOI] [PubMed] [Google Scholar]
- 10.Guralnik JM, Ferrucci L, Simonsick E, Salive ME, Wallace RB. Lower extremity function in persons over 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 12.Gottdiener JS, Bednarz J, Devereux R, et al. Recommendations for use of echocardiography in clinical trials: a report from the American Society of Echocardiography's Nomenclature and Standards Committee and The Task Force on Echocardiography in Clinical Trials. J Am Soc Echocardiogr. 2004;17:1086–1119. doi: 10.1016/j.echo.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 13.McDermott MM, Greenland P, Liu K, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286:1599–1606. doi: 10.1001/jama.286.13.1599. [DOI] [PubMed] [Google Scholar]
- 14.McDermott MM, Criqui MH, Liu K, et al. Lower ankle/brachial index, as calculated by averaging the dorsalis pedis and posterior tibial arterial pressures, and association with leg functioning in peripheral arterial disease. J Vasc Surg. 2000;32:1164–1171. doi: 10.1067/mva.2000.108640. [DOI] [PubMed] [Google Scholar]
- 15.Shadman R, Criqui MH, Bundens WP, et al. Subclavian artery stenosis: prevalence, risk factors, and association with cardiovascular diseases. J Am Coll Cardiol. 2004;44:618–623. doi: 10.1016/j.jacc.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 16.McDermott MM, Liu K, O'Brien E, et al. Measuring physical activity in peripheral arterial disease: a comparison of two physical activity questionnaires with an accelerometer. Angiology. 2000;51:91–100. doi: 10.1177/000331970005100201. [DOI] [PubMed] [Google Scholar]
- 17.McDermott MM, Ohlmiller SM, Liu K, et al. Gait alterations associated with walking impairment in people with peripheral arterial disease with and without intermittent claudication. J Am Geriatr Soc. 2001;49:747–754. doi: 10.1046/j.1532-5415.2001.49151.x. [DOI] [PubMed] [Google Scholar]
- 18.Garg PK, Tian L, Criqui MH, et al. Physical activity during daily life and mortality in patients with peripheral arterial disease. Circulation. 2006;114:242–248. doi: 10.1161/CIRCULATIONAHA.105.605246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crouter SE, Schneider PL, Karabulut M, Bassey DR., Jr Validity of 10 electronic pedometers for measuring steps, distance, and energy cost. Med Sci Sports Exerc. 2003;35:1455–1460. doi: 10.1249/01.MSS.0000078932.61440.A2. [DOI] [PubMed] [Google Scholar]
- 20.Welk GJ, Differding JA, Thompson RW, Blair SN, Dxiura J, Hart P. The utility of the Digi-Walker step counter to assess daily physical activity patterns. Med Sci Sports Exerc. 2000;32(9 suppl):S481–S488. doi: 10.1097/00005768-200009001-00007. [DOI] [PubMed] [Google Scholar]
- 21.Higashi Y, Sasaki S, Kurisu S, et al. Regular aerobic exercise augments endothelium-dependent vascular relaxation in normotensive as well as hypertensive subjects: role of endothelium-derived nitric oxide. Circulation. 1999;100:1194–1202. doi: 10.1161/01.cir.100.11.1194. [DOI] [PubMed] [Google Scholar]
- 22.Hornig B, Maier V, Drexler H. Physical training improves endothelial function in patients with chronic heart failure. Circulation. 1996;93:210–214. doi: 10.1161/01.cir.93.2.210. [DOI] [PubMed] [Google Scholar]
- 23.Hambrecht R, Wolf A, Gielen S, et al. Effect of exercise on coronary endothelial function in patients with coronary artery disease. N Engl J Med. 2000;342:454–460. doi: 10.1056/NEJM200002173420702. [DOI] [PubMed] [Google Scholar]
- 24.Lavrencic A, Salobir BG, Keber I. Physical training improves flow-mediated dilation in patients with the poly-metabolic syndrome. Arterioscler Thromb Vasc Biol. 2000;20:551–555. doi: 10.1161/01.atv.20.2.551. [DOI] [PubMed] [Google Scholar]
- 25.Brendle DC, Joseph LJ, Corretti MC, Gardner AW, Katzel LI. Effects of exercise rehabilitation on endothelial reactivity in older patients with peripheral arterial disease. Am J Cardiol. 2001;87:324–329. doi: 10.1016/s0002-9149(00)01367-9. [DOI] [PubMed] [Google Scholar]
- 26.Falcone RA, Hirsch AT, Regensteiner JG, et al. J Cardiopulm Rehabil. 2003;23:170–175. doi: 10.1097/00008483-200305000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Regensteiner JG. Exercise rehabilitation for the patient with intermittent claudication: a highly effective yet underutilized treatment. Curr Drug Targets Cardiovasc Haematol Disord. 2004;4:233–239. doi: 10.2174/1568006043336195. [DOI] [PubMed] [Google Scholar]